Abstract
OBJECTIVES
To determine the incidence and risk factors associated with new onset and worsening portal hypertensive gastropathy (PHG) in patients with chronic hepatitis C (CHC).
METHODS
831 CHC patients with bridging fibrosis or cirrhosis at entry were prospectively monitored for clinical and histological liver disease progression while receiving either low dose peginterferonα2a or no antiviral therapy in the HALT-C Trial. Upper endoscopy with grading of PHG was performed at baseline and year 4 of the study. The presence and severity of PHG were determined using the NIEC criteria and worsening PHG was defined as a score increase of > 1 point.
RESULTS
During a median follow-up of 3.85 years, 50% of the 514 subjects without PHG developed new onset PHG while 26% of the 317 patients with baseline PHG had worsening PHG. Independent predictors of new onset PHG included higher alkaline phosphatase and being diabetic, while predictors of worsening PHG were Caucasian race, lower albumin, and higher serum AST/ ALT ratio and HOMA levels. New onset and worsening PHG were significantly associated with clinical as well as histological progression. New onset and worsening PHG were also associated with new onset and worsening of gastroesophageal varices.
CONCLUSIONS
New onset and worsening PHG develops at a rate of 12.9% per year and 6.7% per year, respectively, in non-responder CHC patients with advanced fibrosis. If confirmed in other studies, endoscopic surveillance for PHG may need to be tailored to individual patient risk factors.
Keywords: Endoscopy, cirrhosis, peginterferon, decompensation, varices
Introduction
Portal hypertensive gastropathy (PHG) is characterized by the presence of a mucosal mosaic pattern with focal red and brown spots as well as vascular ectasias that may be locally or diffusely distributed in the stomach of patients with chronic liver disease or portal vein thrombosis (1-3). The severity of PHG can vary from mild to severe and patients with PHG are at increased risk of acute and chronic gastrointestinal bleeding (4-6). The pathogenesis of PHG is not well understood but is believed to be related to high portal pressure and impaired gastric mucosal defenses (7, 8). However, the incidence and risk factors for new onset or worsening PHG in prospectively monitored patients with chronic liver disease have not been well described.
The Hepatitis C Antiviral Long-term Treatment against Cirrhosis (HALT-C) Trial was a large, prospective multi-center study designed to determine if prolonged low-dose peginterferon therapy would reduce the rate of histological and clinical disease progression amongst prior non-responder patients with compensated chronic hepatitis C (CHC) (9). The HALT-C Trial and other similar studies failed to demonstrate a benefit of prolonged interferon therapy compared to no treatment in reducing the rate of disease progression (9-12). Because it was hypothesized that interferon treated patients may experience a reduction in portal pressure, all HALT-C Trial patients underwent a baseline endoscopy prior to randomization and at the end of follow-up to determine the incidence of new onset and worsening gastroesophageal varices and PHG (13, 14). In a prior analysis, 34% of HALT-C Trial patients had mild PHG at baseline and these patients had clinical and laboratory evidence of more severe liver disease compared to those without PHG (15). In this paper, the incidence and risk factors associated with new onset PHG and worsening of baseline PHG in 831 HALT-C Trial patients that underwent endoscopic surveillance are reported. The relationship between changes in PHG score and clinical and histological outcomes as well as evolution of gastroesophageal varices are also reported
Methods
Prior non-responders to standard interferon with advanced fibrosis on biopsy (i.e. Ishak fibrosis score > 3) with no history of hepatic decompensation or hepatocellular carcinoma (HCC) were retreated with full dose peginterferon and ribavirin during the “lead-in” phase of the HALT-C Trial (9). All participants had a baseline Child-Turcotte-Pugh (CTP) score of 5 or 6. Patients with persistently detectable HCV RNA at week 20, were randomized to maintenance peginterferon-α2a 90 ug/ week (Pegasys; Roche Laboratories, Nutley NJ) or no treatment for the next 3.5 years. Week 20 virological responders in the lead-in phase continued combination therapy for 48 weeks. However, patients with detectable HCV RNA during treatment (breakthrough) or after stopping treatment (relapse) were also eligible for randomization. In addition, “express” patients who had received at least 24 weeks of full-dose peginterferon and ribavirin with persistent viremia were eligible for enrollment in the randomized phase. This study was approved by the Institutional Review Board at all 10 HALT-C Trial sites and all subjects provided written informed consent for the trial as well as prior to each endoscopy.
Endoscopic evaluation and endpoints
A written protocol that included standardized criteria for identifying and grading the presence of esophageal varices, gastric varices and PHG was implemented at all centers prior to study initiation. All subjects underwent a pretreatment baseline endoscopy prior to randomization and subjects with established gastroesophageal varices at baseline had a repeat endoscopy at 18 months following randomization which is deemed study year 2. In addition, all randomized patients who had not died or reached a clinical endpoint underwent a follow-up endoscopy at year 3.5 following randomization which is deemed study year 4. The presence and severity of PHG were scored by the local study endoscopist according to the New Italian Endoscopy Conference criteria (NIEC) (2). Specifically, the mucosal mosaic pattern was considered mild and scored 1 if there were pink, non-hemmorhagic polygonal, scale-like areas and severe with a score of 2 if present throughout the mucosa with red hemmorhagic foci. Focal flat or slightly bulging red marks were considered mild and scored 1 if found in isolated areas and severe with a score of 2 if present throughout the mucosa. Gastric antral vascular ectasia (GAVE) was diagnosed by the presence of flat or slightly raised, red stripe-like lesions radiating from the pylorus to the antrum and body of the stomach and given a score of 2. The presence of black-brown spots representative of old submucosal hemorrhage were not recorded. A PHG score of 0 was considered none, 1 to 3 was considered mild PHG and a score of 4 or more was considered severe PHG (4). The presence and size of esophageal varices were graded as small (F1), medium (F2), and large (F3).
De novo PHG was defined as any patient without baseline PHG who had a PHG score of 1 or more at a follow-up endoscopy or developed upper gastrointestinal bleeding. PHG progression was defined as an increase in PHG score by > 1 point at a follow-up endoscopy or development of upper gastrointestinal bleeding. PHG regression or improvement was defined as reduction in the PHG score by > 1 point. De novo varices were defined as newly identified esophageal or gastric varices or the development of variceal hemorrhage during follow-up in the patients without varices at baseline (16). Variceal progression was defined as an increase in esophageal varix size of at least 1 grade at the year 2 or year 4 endoscopy, variceal bleeding at any time in the randomized phase, or development of new gastric varices. Clinical management of gastroesophageal varices and PHG was at the discretion of the local investigator.
Laboratory and clinical assessment
Routine baseline laboratory values (i.e. serum aminotransferases, albumin, bilirubin, platelet count) were obtained at the local laboratories. HOMA-IR was calculated as HOMA= [(insulin* glucose)/ 22.5]*0.5551 where insulin is expressed in mU/mL and glucose is mg/dl. Serum HCV RNA testing was done at baseline and during the randomized phase using the quantitative COBAS Amplicor HCV Monitor Test (Roche Molecular Diagnostics, Branchburg, NJ) with a lower limit of detection of 600 IU/ml at a central laboratory. Serum iron, total iron binding capacity, total bile acids and hyaluronic acid levels were determined at a reference laboratory (WAKO Diagnostics, Richmond VA). Liver biopsies were evaluated by a panel of expert liver pathologists and scored for the degree of hepatic fibrosis and inflammation defined by the Ishak scoring system and the degree of hepatic steatosis was estimated as grade 0 to 4. Splenomegaly was defined by a spleen length > 13 cm on sonography.
All patients were seen every 3 months during the randomized phase for laboratory and clinical assessment. In addition, annual liver ultrasounds were obtained to screen for HCC and serum alpha fetoprotein levels were obtained every 6 months. Clinical endpoints for the study included an increase in CTP score to > 7 on two consecutive occasions 3 months apart, variceal bleeding, ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, HCC, or death. Histological progression was defined as an increase in the Ishak fibrosis score of > 2 points at the year 2 or year 4 liver biopsies in non-cirrhotic patients. The current analysis of clinical outcomes includes patients who were followed at the HALT-C Trial sites for a median of 6 years following randomization.
Statistical methods
Statistical analyses were performed at the data coordinating center (New England Research Institute, Watertown, MA) with the use of SAS software, 9.2 (SAS Institute, Cary, NC). Log transformation of non-normally distributed variables was undertaken when indicated. Bivariate logistic regression methods were used to explore factors associated with new PHG development and PHG progression. For multivariate models, individual factors that were associated with the outcome on bivariate analysis with a p < 0.20 were initially included. Then only variables that were significant with p < 0.05 were retained in the final multivariate model. Area under the receiver operator curve (AUROC) was calculated to quantify the predictive ability of the model, ranging from 0.5 (by chance) to 1 (perfect discrimination).
Results
Patient population
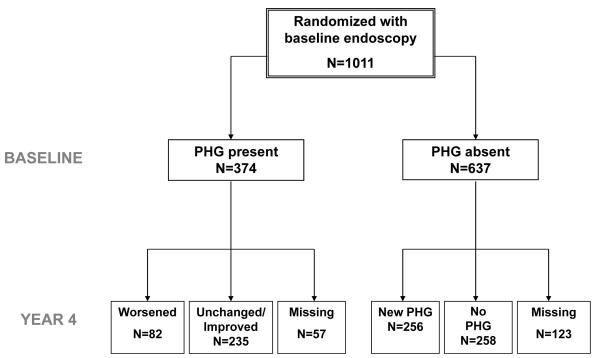
A baseline endoscopy was available in 1011 (96.3%) of the 1050 patients randomized in the HALT-C Trial. Features of PHG were absent in 637 (63%) while 374 (37%) had PHG (Figure 1). A follow-up endoscopy was available in 831 subjects including 514 subjects without baseline PHG and 317 subjects with baseline PHG. Reasons for failure to undergo repeat endoscopy included development of a clinical outcome (70) including 38 deaths, withdrawal from the study (70) or patient refusal (40). The 180 subjects who did not undergo repeat upper endoscopy had evidence of more severe liver disease at entry including higher serum AST/ ALT ratio and total bile acids and lower serum albumin levels as well as a greater likelihood of developing a clinical outcome during follow-up compared to those included (Data not shown).
Figure 1.
Description of the HALT-C Trial cohort. Among the 637 subjects without baseline portal hypertensive gastropathy (PHG), 258 continued to not have PHG during follow-up while 256 developed new onset PHG. Among the 374 subjects with baseline PHG, 235 had stable or improved PHG scores while 82 subjects had worsening PHG.
Development of new onset portal hypertensive gastropathy
During follow-up, 256 of the 514 subjects (50%) developed new onset PHG and the estimated annual rate of new onset PHG was 12.9% per year (Table 1). The PHG score at follow-up was 1 in 167 subjects, 2 in 36 subjects and 3 or more in 45 subjects and 8 had experienced gastrointestinal bleeding. The mucosal mosaic pattern was identified in 91%, red marks in 33%, and GAVE features in 9%. At baseline, those with new onset PHG had evidence of more severe liver disease and laboratory markers of more severe portal hypertension (Table 1). In addition, diabetics and subjects with higher body mass index (BMI) were more likely to develop new onset PHG. However, lifetime alcohol consumption, smoking status, and subject race were not associated with new onset PHG. Use of peginterferon therapy during the randomized phase was similar in those with and without new onset PHG (52% vs 49%, p=0.596).
Table 1. Characteristics of the 514 patients without baseline portal hypertensive gastropathy.
| All Patients | Patients with new PHG (denovo) |
Patients without PHG |
p-value | Odds Ratio (95% CI) |
|
|---|---|---|---|---|---|
| N | 514 | 256 | 258 | ||
| Age (yrs) | 50.2(7.4) | 50.2(7.0) | 50.3 (7.8) | 0.97 | 1.00 (0.98, 1.02) |
| Male (%) | 70% | 73% | 68% | 0.27 | 1.24(0.85, 1.81) |
| Race (% Caucasian) | 70% | 71% | 69% | 0.60 | 1.11(0.76, 1.61) |
| Patient treatment history | 0.30 | ||||
| % Lead-in non-responder | 62% | 65% | 60% | 1.14 (0.75, 1.76) | |
| % Lead-in breakthru/ relapser | 16% | 13% | 18% | 0.78 (0.44, 1.39) | |
| % Express | 22% | 22% | 22% | Ref | |
| Log HCV RNA | 6.48(0.52) | 6.41(0.58) | 6.55(0.45) | 0.003 | 0.58 (0.41, 0.82) |
| HCV genotype 1 (%) | 94% | 94% | 94% | 0.99 | 1.00 (0.48, 2.08) |
| Peginterferonα2a group | 50% | 52% | 49% | 0.60 | 1.10 (0.78, 1.55) |
| Medical history | |||||
| Current smoker (%) Lifetime alcohol consumption ** |
25% | 28% | 23% | 0.14 | 1.35 (0.91, 2.01) |
| 15546 (22038) | 17430 (23648) | 13684 (20195) | 0.06 | 1.08 (1.00, 1.17) | |
| Diabetes mellitus (%) | 23% | 29% | 16% | 0.0007 | 2.09 (1.36, 3.21) |
| Hypertension (%) | 35% | 40% | 29% | 0.01 | 1.59 (1.10, 2.29) |
| Medications at baseline | |||||
| Beta-blockers (%) | 8% | 10% | 6% | 0.11 | 1.71 (0.89, 3.27) |
| ACE/ Calcium channel blocker (%) |
4% | 4% | 4% | 0.64 | 1.24 (0.51, 3.05) |
| Aspirin/ non-steroidals (%) | 5% | 6% | 4% | 0.22 | 1.65 (0.74, 3.72) |
| Statins (%) | 1% | 0% | 2% | 0.99 | ND |
| Proton pump inhibitor (%) | 16% | 16% | 17% | 0.75 | 0.93 (0.58, 1.48) |
| Liver disease severity | |||||
| Cirrhosis (%) | 33% | 38% | 28% | 0.01 | 1.61 (1.11, 2.33) |
| Mean HAI baseline | 7.4(2.1) | 7.5(2.0) | 7.3(2.1) | 0.47 | 1.03 (0.95, 1.12) |
| Hepatic steatosis >=2 (%) | 41% | 45% | 36% | 0.05 | 1.42 (1.00, 2.03) |
| Varices at baseline (%) | 18% | 29% | 8% | <.0001 | 4.75 (2.79, 8.07) |
| Splenomegaly on ultrasound (%) | 31% | 38% | 25% | 0.002 | 1.86 (1.27, 2.73) |
| Body mass index (kg/m2) | 29.7(5.2) | 30.2(5.6) | 29.2(4.9) | 0.03 | 1.04 (1.00, 1.07) |
| Homeostasis model assessment (HOMA) |
14.3(18.7) | 15.4(17.9) | 13.1(19.5) | 0.23 | 1.01 (1.00, 1.02) |
| Lab parameters | |||||
| Hemoglobin (g/dl) | 15.0(1.4) | 15.1(1.4) | 15.0 (1.5) | 0.46 | 1.05 (0.93, 1.18) |
| Anemia (%)λ | 3.5% | 3.1% | 3.9% | 0.64 | 0.80 (0.31, 2.06) |
| Log Ferritin (ng/ml) | 2.34 (0.48) | 2.37 (0.47) | 2.31 (0.48) | 0.18 | 1.33 (0.88, 2.02) |
| Ferritin < 30 ng/ml (%) | 4.5% | 4.0% | 5.0% | 0.65 | 0.80 (0.31, 2.07) |
| Ferritin < 40 ng/ml (%) | 6.8% | 7.0% | 6.5% | 0.82 | 1.09 (0.50, 2.39) |
| Platelets ***(103/ml) | 1.74(0.63) | 1.65(0.65) | 1.83(0.61) | 0.002 | 0.63 (0.48, 0.84) |
| AST/ALT | 0.85(0.29) | 0.9(0.3) | 0.81(0.27) | 0.0007 | 3.06(1.61, 5.82) |
| Alk phosphatase ratio (ULN) | 0.8(0.37) | 0.87(0.44) | 0.74(0.27) | 0.0002 | 3.01 (1.70, 5.32) |
| Albumin (g/dl) | 3.9(0.35) | 3.9(0.38) | 3.97(0.32) | 0.03 | 0.57 (0.34, 0.93) |
| Total bilirubin (mg/dl) | 0.75(0.36) | 0.79(0.39) | 0.7(0.32) | 0.003 | 2.23 (1.33, 3.76) |
| INR >1.0 (%) | 68% | 66% | 71% | 0.23 | 0.80 (0.55, 1.16) |
| Total bile acids (umol/L) | 14.0(16.1) | 15.4(17.4) | 12.5(14.6) | 0.05 | 1.01 (1.00, 1.02) |
| Log hyaluronic acid (ng/ml) | 1.9(0.45) | 1.96(0.46) | 1.83(0.44) | 0.001 | 1.94 (1.30, 2.91) |
Data reported as % or mean (Standard deviation) ACE= Angiotensin converting enzyme inhibitor
Lifetime alcohol consumption/10,000 for calculation of OR
Platelets/100
Defined as hemoglobin < 12 g/dl in women and < 13 g/dl in men
On multivariate analysis, a higher baseline serum alkaline phosphatase and being diabetic were significantly associated with new onset PHG. The area under the receiver operating curve (AUROC) for this model was 0.62 (95% CI: 0.57, 0.66). There was no significant change in the model when baseline liver histology or baseline gastroesophageal varices were added or by removing patients who only developed GAVE features alone.
Progression of baseline PHG
As expected, the 317 patients with baseline PHG had more severe liver disease compared to the 514 patients without baseline PHG including more frequent cirrhosis (55% vs 33%, p < 0.0001), esophageal varices (42% vs 18%, p < 0.0001), and higher total bilirubin levels (0.87 vs 0.75 mg/dl, p < 0.001). During follow-up, the 317 patients with baseline PHG also had a higher incidence of clinical outcomes (35% vs 16%, p<0.0001). The severity of baseline PHG was mild in 92% and severe in 8% with total PHG score of 1 in 186 subjects, 2 in 76 subjects and 3 or more in 55 subjects. The mucosal mosaic pattern was identified in 92%, red marks in 42%, and GAVE features in only 11%.
During follow-up, 82 of the 317 (26%) patients with baseline PHG had a worsening of their PHG score by at least 1 point with an estimated annual rate of 6.7%/ year. Amongst these 82 patients, 27 had an increase in their PHG score of only 1 point, 16 had an increase of 2 points and 21 had an increase of 3 or more points while 18 others had gastrointestinal hemorrhage. The proportion of patients with worsening or new mosaic pattern was 93%, worsening or new red marks was 79%, and new onset GAVE was 36%. Subjects with PHG progression were more likely to be Caucasian and have underlying cirrhosis and baseline laboratory parameters suggestive of more severe liver disease with lower platelet counts and higher serum AST/ ALT ratio, hyaluronic acid, total bile acids, HOMA and total bilirubin levels (Table 2). However, lifetime alcohol consumption and receipt of peginterferon during the randomized phase were not different nor was the frequency of beta-blocker or NSAID use at baseline.
Table 2. Characteristics of the 317 patients with portal hypertensive gastropathy at baseline endoscopy.
| Variables | All Patients | Patients with Worsening PHG |
Patients with Stable PHG |
p-value | Odds Ratio (95 % CI) |
|---|---|---|---|---|---|
| N | 317 | 82 | 235 | ||
| Age (yrs) | 50.3(6.5) | 50.3(6.4) | 50.3 (6.6) | 0.99 | 1.00 (0.96, 1.04) |
| Male (%) | 73% | 74% | 72% | 0.72 | 1.11 (0.63, 1.9) |
| Caucasian (%) | 74% | 87% | 70% | 0.005 | 2.74 (1.37, 5.48) |
| Patient treatment history | 0.56 | ||||
| Lead-in non-responder (%) | 65% | 70% | 63% | 1.31(0.68, 2.51) | |
| Lead-in breakthrough/ relapser (%) |
14% | 12% | 15% | 0.94 (0.38, 2.34) | |
| Express (%) | 21% | 18% | 22% | Ref | |
| Log HCV RNA | 6.41(0.5) | 6.39(0.47) | 6.42(0.51) | 0.59 | 0.87 (0.53, 1.44) |
| HCV genotype 1 (%) | 94% | 93% | 94% | 0.68 | 0.81 (0.30, 2.19) |
| Peginterferonα2a group | 46% | 43% | 48% | 0.44 | 0.82 (0.49, 1.36) |
| Medical history | |||||
| Current smoker (%) | 31% | 37% | 29% | 0.17 | 1.45 (0.85, 2.46) |
| Lifetime drinks** | 19260(35743) | 19594(31927) | 19143(37046) | 0.92 | 1.00 (0.94, 1.08) |
| Diabetes mellitus (%) | 27% | 35% | 24% | 0.05 | 1.71 (0.99, 2.94) |
| Hypertension (%) | 37% | 42% | 35% | 0.29 | 1.32 (0.79, 2.21) |
| Medications at baseline | |||||
| Beta-blocker (%) | 12% | 10% | 13% | 0.42 | 0.71 (0.31, 1.62) |
| ACE/ Calcium channel blocker (%) |
5% | 6% | 4% | 0.50 | 1.46 (0.48, 4.41) |
| Aspirin/ non-steroidals (%) | 4% | 1% | 5% | 0.19 | 0.25 (0.03, 1.98) |
| Statins (%) | 2% | 4% | 1% | 0.11 | 4.42 (0.73, 26.96) |
| Proton pump inhibitor (%) | 16% | 20% | 15% | 0.33 | 1.39(0.72, 2.66) |
| Liver disease severity | |||||
| Cirrhosis (%) | 55% | 72% | 49% | 0.0003 | 2.72(1.58, 4.70) |
| Mean HAI baseline | 7.78(2.01) | 7.76(2.07) | 7.79(2.00) | 0.90 | 0.99 (0.88, 1.13) |
| Hepatic steatosis >=2 (%) | 43% | 38% | 45% | 0.28 | 0.75 (0.45, 1.26) |
| Varices at baseline (%) | 42% | 52% | 38% | 0.03 | 1.78(1.07, 2.95) |
| Splenomegaly on USN (%) | 39% | 50% | 35% | 0.02 | 1.89(1.13, 3.16) |
| Body mass index (kg/m2) | 29.96(5.08) | 30.15(5.60) | 29.9(4.90) | 0.71 | 1.01 (0.96, 1.06) |
| Homeostasis model assessment (HOMA) |
18.31(27.03) | 26.58(36.58) | 15.29(21.91) | 0.009 | 1.01 (1.00, 1.02) |
| Lab parameters | |||||
| Hemoglobin (g/dl) | 15.04(1.41) | 14.96(1.40) | 15.06(1.42) | 0.57 | 0.95 (0.80, 1.14) |
| Anemia (%) ^ | 3.5% | 4.9% | 3.0% | 0.42 | 1.67 (0.48, 5.86) |
| Log Ferritin (ng/ml) | 2.35 (0.45) | 2.32 (0.47) | 2.37 (0.45) | 0.61 | 0.85 (0.46, 1.58) |
| Ferritin < 30 ng/ml (%) | 4.4% | 3.8% | 6.0% | 0.46 | 0.62 (0.18, 2.20) |
| Ferritin < 40 ng/ml (%) | 6.4% | 6.0% | 7.5% | 0.67 | 0.79 (0.26, 2.36) |
| Platelets (103/ml)*** | 1.48(0.63) | 1.21(0.54) | 1.58(0.63) | <.0001 | 0.33 (0.20, 0.54) |
| Serum AST/ALT | 0.91(0.29) | 1.00(0.26) | 0.88(0.30) | 0.002 | 3.90(1.69, 9.04) |
| Alk phos ratio (ULN) | 0.89(0.42) | 0.92(0.45) | 0.88(0.42) | 0.41 | 1.27(0.72, 2.26) |
| Albumin (g/dl) | 3.80(0.42) | 3.75(0.40) | 3.82(0.42) | 0.17 | 0.66(0.36, 1.20) |
| Total bilirubin (mg/dl) | 0.87(0.46) | 0.98(0.57) | 0.83(0.41) | 0.01 | 1.97 (1.16, 3.35) |
| INR >1.0 (%) | 50% | 46% | 51% | 0.50 | 0.84(0.51, 1.39) |
| Total bile acids (umol/L) | 20.86(22.27) | 28.35(27.93) | 18.25(19.32) | 0.001 | 1.02 (1.01, 1.03) |
| Log hyaluronic acid (ng/ml) | 2.10(0.49) | 2.26(0.44) | 2.05(0.50) | 0.001 | 2.55 (1.45, 4.49) |
Data reported as % or mean (Standard deviation)
ACE= Angiotensinconverting enzyme inhibitor
Lifetime alcohol consumption/10,000 for calculation of OR
Platelets/100
Defined as hemoglobin < 12 g/dl in women and < 13 g/dl in men
On multivariate analysis, Caucasian race, lower platelets and albumin, and higher baseline serum AST/ ALT ratio and HOMA levels were significantly associated with PHG progression. The AUROC of this model was 0.78 (95% CI: 0.71, 0.84). When baseline liver histology and gastroesophageal varices were added to the model, the AUROC remained unchanged.
Evolution of PHG and clinical outcomes
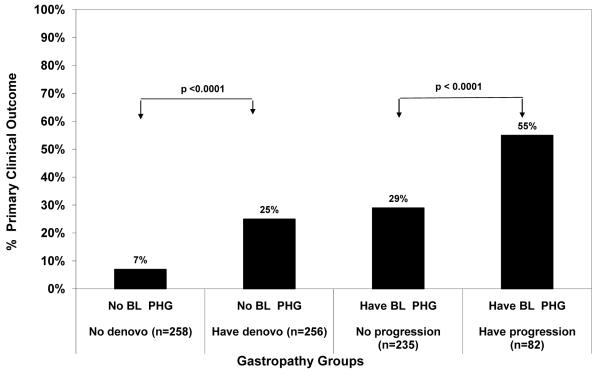
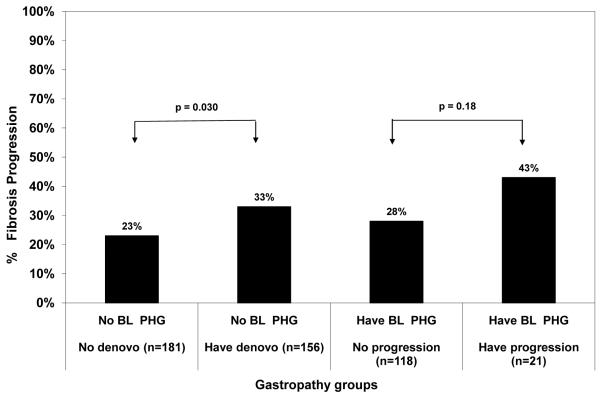
Amongst the 514 patients without PHG at baseline, 82 subjects (16%) developed one or more clinical outcomes during a median follow-up of 6 years. The specific clinical outcomes included 61 with worsening CTP, 22 HCC, 39 deaths, and 30 liver transplants. The likelihood of developing a clinical outcome was significantly higher in the 256 subjects with new onset PHG compared to the 258 CHC patients without new onset PHG (25% vs 7%, Odds ratio = 4.11 (95% CI: 2.38, 7.10), p< 0.0001) (Figure 2). We also investigated the relationship between new onset PHG and histological progression in 337 non-cirrhotic HALT-C Trial patients. As shown in Figure 3, histological progression was significantly more common in subjects with new onset PHG compared to those that did not develop PHG during follow-up (33% vs 23%, Odds ratio = 1.71 (95% CI: 1.06, 2.76) p=0.030).
Figure 2.
New or worsening PHG and clinical outcomes. During follow-up, the 256 subjects who developed new onset PHG were also significantly more likely to develop a clinical outcome compared to the 258 without PHG (p < 0.0001). Similarly, the 82 subjects who had worsening PHG were more likely to develop a clinical outcome compared to the 235 subjects with stable or improved PHG (p< 0.0001). BL PHG= baseline portal hypertensive gastropathy
Figure 3.
New or worsening PHG and histological progression. The 156 non-cirrhotic patients who developed new onset PHG were significantly more likely to experience histological fibrosis progression compared to the 181 patients without de novo PHG (p=0.030). In addition, there was a trend for the 21 non-cirrhotic patients with worsening PHG during follow-up to have histological progression compared to the 118 patients with stable or improved PHG (p=0.18). BL PHG = baseline portal hypertensive gastropathy
Amongst the 317 subjects with PHG at baseline, 112 (35%) developed one or more clinical outcomes during follow-up which included 82 with a worsening CTP score, 28 HCC, 44 deaths, and 35 liver transplants. Per Figure 2, the 82 subjects with worsening PHG were significantly more likely to also experience a clinical outcome compared to the 235 subjects without worsening PHG (55% vs 29%, Odds ratio 3.05 (95% CI: 1.81, 5.12), p < 0.0001). In addition, the 21 non-cirrhotic patients with worsening PHG were more likely to have histological fibrosis progression during follow-up compared to the 118 patients without worsening PHG (43% vs 28%, Odds ratio = 1.93 (95% CI 0.75, 5.01) p=0.18) (Figure 3).
Regression of PHG
During follow-up, 100 patients had an unchanged PHG score and 135 patients had an improved PHG score. Of the 135 with an improved PHG score, 99 improved by 1 point, 23 improved by 2 points and 13 improved by 3 or more points. On univariate analysis, subjects with an improved PHG score were more likely to have laboratory markers indicative of less severe liver disease (i.e. lower serum AST/ ALT ratio, higher platelet counts) and also less likely to have cirrhosis, advanced hepatic steatosis, and concomitant varices (Data not shown). On multivariate analysis, subjects with an improved PHG score were less likely to have advanced hepatic steatosis (36% vs 48%, Odds ratio: 0.46 (95 % CI: 0.28, 0.75), p=0.002), lower serum AST/ ALT ratio (0.86 vs 0.95, Odds ratio = 0.25 (95% CI:0.11, 0.61), p=0.002) and a lower likelihood of baseline varices (33% vs 49%, Odds ratio =0.49 (95% CI: 0.30-0.78), p=0.003). In addition, subjects with improved PHG scores were significantly less likely to experience a clinical outcome (29% vs 40%, p=0.04) and the non-cirrhotic patients were less likely to experience fibrosis progression (22% vs 35%, p=0.027) compared to those with stable or worsening PHG scores.
Changes in PHG and the development/progression of varices
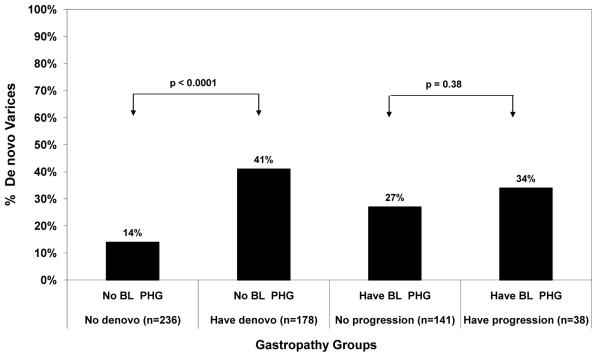
Among the 514 patients without PHG at baseline, 104 subjects (20%) developed new onset gastroesophageal varices and 22 patients (4%) had worsening of baseline varices. The likelihood of developing new varices was significantly greater in patients who also had new onset PHG during follow-up compared to subjects without new onset PHG (41% vs 14%, Odds ratio: 4.33, 95% CI: 2.69, 6.98, p < 0.0001) (Figure 4). .Similarly, worsening of pre-existing varices was significantly more frequent in those who developed new onset PHG compared to those without new onset PHG (30% vs 5%, Odds ratio: 8.14, 95% CI, 1.02, 64.83, Fisher’s p =0.023) (Figure 5).
Figure 4.
Relationship between PHG evolution and de novo varices. The 178 patients who developed new onset PHG were also more likely to develop de novo varices compared to the 236 patients without PHG during follow-up (p < 0.0001). The 38 patients who had worsening PHG were also more likely to develop de novo varices compared to the 141 patients with stable PHG but this trend was not significant (p=0.38). BL PHG= baseline portal hypertensive gastropathy
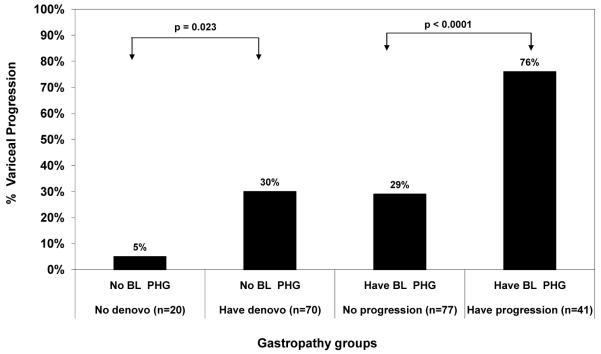
Figure 5.
Relationship of PHG evolution and variceal progression. The 70 patients with new onset PHG were also more likely to have variceal progression compared to the 20 patients without new onset PHG (p=0.023). Similarly, the 41 patients with worsening PHG were significantly more likely to have variceal progression compared to the 77 subjects with stable/ improved PHG (p< 0.0001). BL PHG = baseline portal hypertensive gastropathy
Among the 317 CHC patients with PHG at baseline, 51 subjects (16%) developed new onset gastroesophageal varices and 53 patients (18%) had enlargement of baseline varices. Per Figure 4, the likelihood of developing new varices was higher in patients who also had worsening PHG compared to subjects without worsening PHG but this trend was not significant 34% vs 27%, OR = 1.41 (95% CI: 0.66, 3.03) p =0.38). Finally, the likelihood of developing worsening varices was greater in subjects with worsening PHG compared to subjects without (76% vs 29%, OR: 7.75 (95% CI: 3.26, 18.45) p < 0.0001).
Discussion
Portal hypertensive gastropathy (PHG) is the most common gastric mucosal injury pattern reported in patients with chronic liver disease (17). The frequency and severity of PHG are greater in patients with decompensated cirrhosis compared to compensated cirrhosis (3, 5, 17). However, PHG is a dynamic lesion which can develop following variceal band ligation or other events that raise portal resistance and also spontaneously regress over time (18, 19). In the current study, we set out to determine the incidence and risk factors for new onset and worsening PHG in patients who underwent a repeat endoscopy after a mean follow-up of 4 years.
New onset PHG developed in 50% of the HALT-C Trial patients and the rate of new onset PHG was over 2 times the rate of new onset varices in this same cohort (12.9% vs 5.2%) (20). The majority of patients with new onset PHG had low PHG scores during follow-up and the mucosal mosaic pattern was the most commonly reported feature. During follow-up, only 3% of the patients with new onset PHG experienced gastrointestinal bleeding. However, a clinical outcome was significantly more likely to develop in subjects with new onset PHG compared to subjects without new onset PHG (25% vs 7%). In addition, non-cirrhotic subjects with new onset PHG were significantly more likely to develop worsening hepatic fibrosis compared to those without PHG (33% vs 23%). These data demonstrate that new onset PHG directly correlates with global worsening of liver disease status in a large cohort of well-characterized CHC patients.
On multivariate analysis, independent risk factors for the development of new onset PHG included a higher serum alkaline phosphatase level and being diabetic. Serum alkaline phosphatase levels have been previously associated with more severe liver diseasein CHC patients (21,22). The positive association with diabetes suggests that subjects with the metabolic syndrome may be at increased risk of developing new onset PHG. In support of this, the prevalenceof diabetes and hypertension was higher in subjects with new onset PHG but the proportion with significant hepatic steatosis and baseline HOMA levels were not higher compared to those without new onset PHG (Table 1). Based upon prior studies, we had anticipated that the peginterferon treated patients in HALT-C would have a lower likelihood of developing new onset PHG or varices (13,14). However, peginterferon therapy was not associated with a reduced risk of new onset PHG nor varices. The greater frequency of beta-blocker use in those with new onset PHG may be due to the greater likelihood of those patients also having baseline gastroesophageal varices (29% vs 8%, p < 0.0001).
During follow-up, 26% of the patients with baseline PHG had a worsening of their PHG score. The endoscopic feature that most commonly worsened was the mucosal mosaic pattern and most subjects had an increase in their score of only 1 point. Nonetheless, 22% of these patients with worsening PHG had a documented episode of upper gastrointestinal bleeding. These observations are consistent with prior reports of a greater short-term risk of upper gastrointestinal bleeding in patients with severe versus mild PHG (23, 24). In addition, subjects with worsening PHG were also at much higher risk of experiencing a clinical complication from their liver disease compared to those with stable or improved PHG. Similarly, the non-cirrhotic patients with worsening PHG were significantly more likely to have fibrosis progression (Figures 2 & 3). These data confirm the strong positive correlation between worsening endoscopic stigmata of portal hypertension and the clinical and histological progression of CHC.
On multivariate analysis, independent risk factors for PHG worsening included Caucasian race, lower serum albumin and higher serum AST/ ALT ratio and HOMA levels. These findings are consistent with our prior analyses demonstrating that African-Americans with CHC are less likely to have baseline PHG or varices and also less likely to develop new onset gastroesophageal varices (15, 16, 20). These data are also consistent with other studies that indicate that African-Americans with CHC tend to have less severe hepatic necroinflammation and steatosis compared to Caucasians at presentation and during follow-up (25-27). If confirmed in other studies, it is possible that endoscopic surveillance strategies in CHC patients with advanced fibrosis may not need to be as frequent in African Americans versus other groups. The positive associations of the various laboratory parameters with the risk of worsening PHG presumably reflect the patients who had more advanced disease at baseline. The absence of any beneficial effect of peginterferon therapy on the worsening of PHG or baseline varices confirms the lack of any portal hypotensive effect with the doses of peginterferon used in the HALT-C Trial (20).
Strengths of our study include the large number of well-characterized patients who underwent serial liver biopsy, frequent study visits, and protocol endoscopies in this multicenter prospective study. In addition, baseline laboratory and clinical parameters were collected in a uniform manner. Furthermore, all of these patients had the same etiology of liver disease with no prior history of gastrointestinal bleeding or banding of varices allowing us to effectively conduct a prospective natural history study of liver disease progression and evolution of endoscopic findings. Finally, overall compliance with the recommended endoscopy surveillance schedule was high with over 80% of the eligible patients completing the follow-up exam. Limitations of our study include the lack of centralized review of the endoscopic findings which was not possible due to limited resources. However, a standardized manual of procedures and training was provided to all sites prior to and during the study. In addition, it was not possible to perform baseline or serial portal pressure measurements due to the cost, risk and inconvenience. However, recent studies have failed to demonstrate a consistent relationship between portal pressure measurements and the frequency and severity of PHG (17). Finally, the criteria to define new onset or worsening of PHG have not been well-standardized. A prior study had validated the PHG scoring system used in our study with an excellent interobserver agreement and correlation with the risk of bleeding during follow-up (24). The strong association of PHG with clinical and histological outcomes (Figures 2 & 3) further confirms that a 1 point increase in the PHG score is clinically meaningful. In addition, the strong positive association between new onset or worsening PHG with new onset varices (Figure 4) and variceal progression (Figure 5) further validate the clinically utility of a 1 point increase in PHG score in CHC patients. Finally, the lower likelihood of clinical and histological disease progression noted in subjects with an improved PHG score over time further supports the utility of monitoring the severity of PHG in individual patients.
In summary, 50% of CHC patients enrolled in the HALT-C Trial developed new onset PHG during 4 years of follow-up. The majority of patients had mild PHG and only 3% bled during follow-up. In contrast, 26% of patients with baseline PHG had worsening of PHG over time and the risk of bleeding was substantially higher in these patients. In both subgroups of patients, PHG onset or worsening was strongly associated with clinical and histological progression of liver disease. Furthermore, new onset or worsening PHG was strongly associated with the development or worsening of gastroesophageal varices. A model to predict patients at high risk of new onset PHG was developed but the low AUROC may limit its clinical utility. In contrast, the model to identify patients at high risk for worsening PHG may potentially be useful to clinicians. Finally, our data support an emerging body of evidence that the initial and subsequent risk of endoscopic stigmata of portal hypertension may vary substantially by subject ethnicity in CHC patients (25-27).
Study Highlights.
What is current Knowledge
-
-
Portal gastropathy can cause significant gastrointestinal bleeding in some patients with chronic liver disease.
-
-
High portal pressure and impaired gastric mucosal defenses are involved in the etiopathogenesis of PHG but the incidence and risk factors for new onset or worsening gastropathy are unknown
What is new here
-
-
New onset portal gastropathy developed in 50% of compensated hepatitis C patients that were prospectively followed over 3.8 years.
-
-
Baseline portal gastropathy worsened in 26% of patients and nearly 20% of these developed gastrointestinal bleeding during follow-up.
-
-
Risk factors for worsening gastropathy included Caucasian race, insulin resistance, and laboratory markers of more severe liver disease while diabetes and higher alkaline phosphatase levels were associated with new onset PHG.
ACKNOWLEDGMENTS
This study was supported by the National Institute of Diabetes & Digestive & Kidney Diseases (contract numbers are listed below). Additional support was provided by the National Institute of Allergy and Infectious Diseases (NIAID), the National Cancer Institute, the National Center for Minority Health and Health Disparities and by General Clinical Research Center and Clinical and Translational Science Center grants from the National Center for Research Resources, National Institutes of Health (grant numbers are listed below). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Additional funding to conduct this study was supplied by Hoffmann-La Roche, Inc., through a Cooperative Research and Development Agreement (CRADA) with the National Institutes of Health.
In addition to the authors of this manuscript, the following individuals were instrumental in the planning, conduct and/or care of patients enrolled in this study at each of the participating institutions as follows:
University of Massachusetts Medical Center, Worcester, MA: (Contract N01-DK-9-2326) Gyongyi Szabo, MD, Barbara F. Banner, MD, Maureen Cormier, RN, Donna Giansiracusa, RN
University of Connecticut Health Center, Farmington, CT: (Grant M01RR-06192) Gloria Borders, RN, Michelle Kelley, RN, ANP
Saint Louis University School of Medicine, St Louis, MO: (Contract N01-DK-9-2324) Adrian M. Di Bisceglie, MD, Bruce Bacon, MD, Brent Neuschwander-Tetri, MD, Elizabeth M. Brunt, MD, Debra King, RN
Massachusetts General Hospital, Boston, MA: (Contract N01-DK-9-2319, Grant M01RR-01066; Grant 1 UL1 RR025758-01, Harvard Clinical and Translational Science Center) Jules L. Dienstag, MD, Raymond T. Chung, MD, Atul K. Bhan, MD, Wallis A. Molchen, David P. Lundmark
University of Colorado Denver, School of Medicine, Aurora, CO: (Contract N01-DK-9-2327, Grant M01RR-00051, Grant 1 UL1 RR 025780-01), Gregory T. Everson, MD, Thomas Trouillot, MD, Marcelo Kugelmas, MD, S. Russell Nash, MD, Jennifer DeSanto, RN, Carol McKinley, RN
University of California – Irvine, Irvine, CA: (Contract N01-DK-9-2320, Grant M01RR-00827) John C. Hoefs, MD, John R. Craig, MD, M. Mazen Jamal, MD, MPH, Muhammad Sheikh, MD, Choon Park, RN
University of Texas Southwestern Medical Center, Dallas, TX: (Contract N01-DK-9-2321, Grant M01RR-00633, Grant 1 UL1 RR024982-01, North and Central Texas Clinical and Translational Science Initiative) Thomas E. Rogers, MD, Peter F. Malet, MD, Janel Shelton, Nicole Crowder, LVN, Rivka Elbein, RN, BSN, Nancy Liston, MPH
University of Southern California, Los Angeles, CA: (Contract N01-DK-9-2325, Grant M01RR-00043) Karen L. Lindsay, MD, MMM, Sugantha Govindarajan, MD, Carol B. Jones, RN, Susan L. Milstein, RN
University of Michigan Medical Center, Ann Arbor, MI: (Contract N01-DK-9-2323, Grant M01RR-00042, Grant 1 UL1 RR024986, Michigan Center for Clinical and Health Research) Anna S. Lok, MD, Joel K. Greenson, MD, Pamela A. Richtmyer, LPN, CCRC, R. Tess Bonham, BS
Virginia Commonwealth University Health System, Richmond, VA: (Contract N01-DK-9-2322, Grant M01RR-00065) Mitchell L. Shiffman, MD, Richard K. Sterling, MD, MSc, Melissa J. Contos, MD, A. Scott Mills, MD, Charlotte Hofmann, RN, Paula Smith, RN
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD: T. Jake Liang, MD, David Kleiner, MD, PhD, Yoon Park, RN, Elenita Rivera, RN, Vanessa Haynes-Williams, RN
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: James E. Everhart, MD, Leonard B. Seeff, MD, Patricia R. Robuck, PhD, Jay H. Hoofnagle, MD, Elizabeth C. Wright, PhD
University of Washington, Seattle, WA: (Contract N01-DK-9-2318) Chihiro Morishima, MD, David R. Gretch, MD, PhD, Minjun Chung Apodaca, BS, ASCP, Rohit Shankar, BC, ASCP, Natalia Antonov, M. Ed.
New England Research Institutes, Watertown, MA: (Contract N01-DK-9-2328) Kristin K. Snow, MSc, ScD, Anne M. Stoddard, ScD, Linda Massey
Armed Forces Institute of Pathology, Washington, DC: Zachary D. Goodman, MD, PhD, Fanny Monge, Michelle Parks
Data and Safety Monitoring Board Members: (Chair) Gary L. Davis, MD, Guadalupe Garcia-Tsao, MD, Michael Kutner, PhD, Stanley M. Lemon, MD, Robert P. Perrillo, MD
Abbreviations
- AA
African-American
- AUROC
Area under the receiver operating curve
- BMI
Body Mass index
- CHC
Chronic hepatitis C
- CTP
Child-Turcotte-Pugh
- GAVE
Gastric antral vascular ectasia
- HALT-C
Hepatitis C Antiviral Long-term Treatment against cirrhosis
- HCC
Hepatocellular carcinoma
- HOMA
Homeostasis model
- NIEC
New Italian Endoscopy Conference criteria
- NSAID
Non-steroidal anti-inflammatory drug
- PHG
Portal hypertensive gastropathy
Footnotes
This is publication #60 of the HALT-C Trial.
*The HALT-C Trial was registered with clinicaltrials.gov (#NCT00006164).
Financial Disclosures Paragraph
Financial relationships of the authors with Hoffmann-La Roche, Inc., are as follows: R.J. Fontana is on the Speaker’s bureau; H.L. Bonkovsky receives research support; T.R. Morgan is on the Speaker’s bureau and receives research support; and W.M. Lee receives research support.
Authors with no financial relationships related to this project are: A.J. Sanyal, M.G. Ghany, H.J. Litman, A.E. Reid, D. Naishadham.
References
- 1.Carpinelli C, Primignani M, Preatoni P, et al. Portal hypertensive gastropathy: reproducibility of a classification, prevalence of elementary lesions, sensitivity and specificity in the diagnosis of cirrhosis of the liver. A NIEC multicenter study. Ital J Gastroenterol. 1997;29:533–540. [PubMed] [Google Scholar]
- 2.Sarin SK. Diagnostic issues: portal hypertensive gastropathy and gastric varices. In Portal Hypertension II. In: DeFranchis R, editor. Proceedings of the second Baveno International consensus workshop on definitions, methodology and therapeutic strategies. Blackwell Science; Oxford: 1996. pp. 30–55. [Google Scholar]
- 3.Payen JL, Cales P, Voigt JJ, et al. Severe portal hypertensive gastropathy and antral vascular ectasia are distinct entities in patients with cirrhosis. Gastroenterology. 1995;108:134–144. doi: 10.1016/0016-5085(95)90018-7. [DOI] [PubMed] [Google Scholar]
- 4.Merli M, Nicolini G, Angeloni S, et al. The natural history of portal hypertensive gastropathy in patients with liver cirrhosis and mild portal hypertension. Am J Gastroenterol. 2004;99:1959–1965. doi: 10.1111/j.1572-0241.2004.40246.x. [DOI] [PubMed] [Google Scholar]
- 5.Primignani M, Carpinelli L, Preatoni P, et al. Natural history of portal hypertensive gastropathy in patients with liver cirrhosis. Gastroenterology. 2000;119:181–187. doi: 10.1053/gast.2000.8555. [DOI] [PubMed] [Google Scholar]
- 6.Gostout CJ, Viggiano TR, Balm RK. Acute gastrointestinal bleeding from portal hypertensive gastropathy: Prevalence and clinical features. Am J Gastroenterol. 1993;88:2030–2033. [PubMed] [Google Scholar]
- 7.Kinjo N, Kawanaka H, Akahoshi T, et al. Significance of ERK nitration in portal hypertensive gastropathy and its therapeutic implications. Am J Physiology. 2008;295(5):G1016–1024. doi: 10.1152/ajpgi.90329.2008. [DOI] [PubMed] [Google Scholar]
- 8.Perini RF, Camara PRS, Ferraz JGP. Pathogenesis of portal hypertensive gastropathy: translating basic research into clinical practice. Nat Gastro Hepatology. 2009;6:150–158. doi: 10.1038/ncpgasthep1356. [DOI] [PubMed] [Google Scholar]
- 9.DiBisceglie AM, Shiffman ML, Everson GT, et al. Prolonged therapy of advanced chronic hepatitis C with low dose peginterferon. N Engl J Med. 2008;359:2429–2441. doi: 10.1056/NEJMoa0707615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fartoux L, Degos F, Trepo C, et al. Effect of prolonged interferon therapy on the outcome of hepatitis C virus-related cirrhosis: A randomized trial. Clinic Gastro & Hepatology. 2007;5:502–507. doi: 10.1016/j.cgh.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Afdhal NH, Levine R, Brown R, et al. Colchicine versus peginterferon alfa-2b long term therapy: results of the 4 year CoPilot Trial (Abstract) J Hepatology. 2008;48:S4. [Google Scholar]
- 12.Bruix J, Poynard T, Colombo M, et al. Final results of the EPIC3 cirrhosis maintenance Trial: Pegintron maintenance therapy in cirrhotic (METAVIR F4) HCV patients, who failed to respond to Interferon/ ribavirin therapy. Gastroenterology. 2009;136(Suppl 1):#295. (Abstract) [Google Scholar]
- 13.Roberts S, Gordon A, McLean C, et al. Effect of sustained viral response on hepatic venous pressure gradient in Hepatitis-C related cirrhosis. Clin Gastro & Hep. 2007;5:932–937. doi: 10.1016/j.cgh.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Rincon D, Ripoll C, LoIacono O, et al. Antiviral therapy decreases hepatic venous pressure gradient in patients with chronic hepatitis C and advanced fibrosis. Am J Gastroenterol. 2006;101:2269–2274. doi: 10.1111/j.1572-0241.2006.00743.x. [DOI] [PubMed] [Google Scholar]
- 15.Fontana RJ, Sanyal AJ, Mehta S, et al. Portal hypertensive gastropathy in chronic hepatitis C patients with bridging fibrosis and compensated cirrhosis: Results from the HALT-C Trial. Am J Gastroenterol. 2006;101:1–10. doi: 10.1111/j.1572-0241.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- 16.Sanyal AJ, Fontana RJ, DiBisceglie AM, et al. The prevalence and risk factors associated with esophageal varices in subjects with hepatitis C and advanced fibrosis. Gastrointest Endosc. 2006;64:855–864. doi: 10.1016/j.gie.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Kumar A, Mishra SR, Sharma P, et al. Clinical, laboratory, and hemodynamic parameters in portal hypertensive gastropathy. A study of 254 cirrhotics. J Clin Gastroenterol. 2010;44:294–300. doi: 10.1097/MCG.0b013e3181b37ea1. [DOI] [PubMed] [Google Scholar]
- 18.Sarin SK, Shahi H, Jain M, et al. The natural history of portal hypertensive gastropathy: influence of variceal eradication. Am J Gastroenterology. 2000;95:2888–99. doi: 10.1111/j.1572-0241.2000.03200.x. [DOI] [PubMed] [Google Scholar]
- 19.Sarin SK, Sreenivas DV, Lahoti D, et al. Factors influencing development of portal hypertensive gastropathy in patients with portal hypertension. Gastroenterology. 1992;101:994–999. doi: 10.1016/0016-5085(92)90188-5. [DOI] [PubMed] [Google Scholar]
- 20.Fontana RJ, Sanyal AJ, Ghany MG, et al. Factors that determine the development and progression of gastroesophageal varices in patients with chronic hepatitis C. Gastroenterology. 2010;138:2321–2331. doi: 10.1053/j.gastro.2010.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lok ASF, Ghany MG, Goodman ZD, et al. Predicting cirrhosis in patients with chronic hepatitis C based on standard laboratory tests: Results of the HALT-C cohort. Hepatology. 2005;38:518–526. doi: 10.1002/hep.20772. [DOI] [PubMed] [Google Scholar]
- 22.Sheth SG, Flamm SL, Gordon FD, et al. AST/ ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1998;93:44–48. doi: 10.1111/j.1572-0241.1998.044_c.x. [DOI] [PubMed] [Google Scholar]
- 23.Dore MP, Mura D, Deledda S, et al. Active peptic ulcer disease in patients with hepatitis C virus-related cirrhosis. The role of helicobacter pylori infection and portal hypertensive gastropathy. Can J Gastroenterol. 2004;18:521–4. doi: 10.1155/2004/150674. [DOI] [PubMed] [Google Scholar]
- 24.Stewart CA, Sanyal AJ. Grading portal gastropathy: Validation of a gastropathy scoring system. Am J Gastorenterol. 2003;98:1758–1765. doi: 10.1111/j.1572-0241.2003.07595.x. [DOI] [PubMed] [Google Scholar]
- 25.Lepe R, Layden-Almer JE, Layden TJ, et al. Ethnic differences in the presentation of chronic hepatitis C. J V Hepatitis. 2006;13:116–120. doi: 10.1111/j.1365-2893.2005.00672.x. [DOI] [PubMed] [Google Scholar]
- 26.Tencate V, Layden-Almer JE, Wolfert M, et al. Paired biopsies illustrate racial differences in fibrosis progression in HCV patients (Abstract) Hepatology. 2009;50(Suppl 1):#1629. [Google Scholar]
- 27.Kallwitz ER, Layden -Almer J, Dhamija M, et al. Ethnicity and body mass index are associated with hepatitis C presentation and progression. Clin Gastro & Hepatology. 2010;8:72–78. doi: 10.1016/j.cgh.2009.08.009. [DOI] [PubMed] [Google Scholar]