Abstract
Age-related macular degeneration (AMD) is the primary cause of blindness among the elderly worldwide. To date, no cure is available, and the available palliative treatments only showed limited efficacy in improving visual acuity. The etiology of AMD remains elusive but research over the past decade has uncovered characteristic features of the disease. Known as the hallmarks of AMD, these features include (A) oxidative stress and RPE cytotoxicity; (B) loss of macromolecular permeability and hydraulic conductivity: (C) inflammation; (D) choroidal neovascularization and vascular leakage; and (E) loss of neuroprotection. Recent breakthrough in understanding the pathogenesis of AMD has spawned an array of novel therapeutic agents designed to address these hallmarks. Here we review the features of AMD and highlight the most promising therapeutic and diagnostic approaches based on the patents published from 2008 to 2011. Most likely, a next generation treatment for AMD will be developed from these emerging efforts.
Introduction
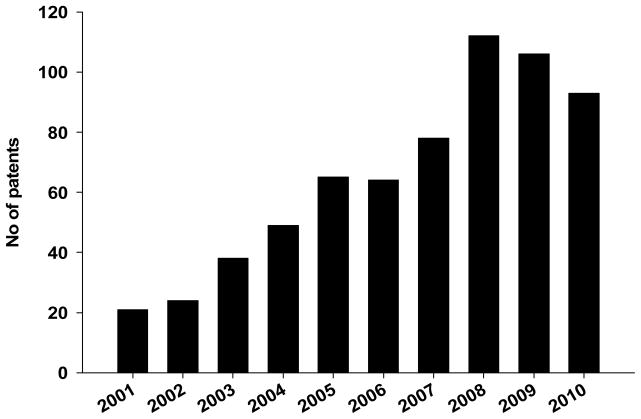
Age-related macular degeneration (AMD) is the primary cause of blindness among people over 50 years old (1). Globally, 33 million individuals are affected by AMD with the direct cost estimated at $255 billion (2). In the United States alone, 1.8 million individuals are afflicted by AMD and the number is estimated to increase to an epidemic level of almost 3 million by 2020 (3). Early AMD is characterized by drusen (yellow spots) and hypopigmention or hyperpigmentation in the choroid/retinal pigment epithelium (RPE) layers in the macula (4–5). Late AMD has interconvertible “dry” and “wet” forms. The advanced form of dry AMD, also called geographic atrophy (GA), is characterized by extensive loss of the RPE, as well as its neighboring photoreceptors (PR) and choriocapillaris. Choroidal neovascularization (CNV), which involves abnormal growth of blood vessels from the choroid into the retina, is a hallmark of wet (or neovascular) AMD. Although, its etiology remains unknown, AMD is suggested to be a multifactorial disease. A mixture of sustained oxidative stress, chronic inflammation and genetic predispositions appear to alter the architecture and the health of the retina, which ultimately leads to dry and wet AMD. To date, no cure is available for dry AMD, and the palliative treatments for wet AMD are restricted to anti-neovascularization agents, photodynamic therapy and thermal laser (6). Encouragingly, there has been a fivefold increase in the number of patents for therapeutic agents targeting the disease hallmarks over the past decade (Fig. 1). The current review highlights the recent patents describing novel therapeutic approaches to address the hallmarks of AMD.
Figure 1.
Chronological increase in patent publication involving age-related macular degeneration. [source: World Intellectual Property Organization (WIPO) database]
Hallmarks of AMD and their genetic basis
Recent genome-wide association studies (GWAS) together with cell culture or animal models are starting to unravel the genetic and pathogenic mechanisms of AMD. These extensive studies have suggested that the following hallmarks tend to drive the disease progression, which include: (A) oxidative stress and RPE cytotoxicity; (B) loss of macromolecular permeability and hydraulic conductivity: (C) inflammation; (D) choroidal neovascularization and vascular leakage; and (E) loss of neuroprotection. Accordingly, numerous patents aiming at controlling these critical processes using novel therapeutic approaches have been filed in recent years.
(A) Oxidative stress and RPE cytotoxicity
Oxidative stress is a critical component in the pathogenesis of AMD. Cigarette smoking, which poses systemic oxidative stress, has been shown to be a significant risk factor for AMD (7). PR cells in the retina are continuously bathed with light and oxygen, and therefore need to be constantly replaced due to the sustained oxidative damage. RPE cells are required for PR phagocytosis, survival, function and renewal (8). Over a lifetime, the ability of the RPE cells to perform their task decreases and recycling of the damaged PR cells is impaired. Accumulation of the debris, in the form of drusen, is thought to ensue, leading to further damage and death of the RPE and PR cells. The importance of the RPE functional integrity in AMD has been highlighted by GWAS. Polymorphisms within the age-related maculopathy susceptibility 2 gene (ARMS2) increase susceptibility to AMD presumably by increasing mitochondrial RPE production of super oxide radicals which react with proteins, DNA, lipids and eventually cause cell death (9–10). Similarly, mutations within the ATP-binding cassette transporter 4 (ABCA4) gene are thought to cause accumulation of the phospholipid conjugate of the all-trans-retinaldehyde, N-retinylidene-N-retinylethanolamine (A2E) in the lysosomes of the RPE cells (11–13). Although tolerated at low levels, excessive amounts of A2E can lead to lysosomal dysfunction, the production of lipofuscin, and potentially drusen under the macula (14). Additionally, low wavelength light can oxidize A2E into toxic forms; making the compound cytotoxic to the RPE and the retinal as a whole (15). Besides oxidative stress, other factors may also lead to RPE cytotoxicity. In a recent report, pathogenic RNA species (Alu RNA) were shown to trigger RPE cytotoxicity and cause GA (16). On the other hand, oxidative stress not only induces RPE death, but also leads to immune response in AMD. Modified oxidative products, such as carboxyethylpyrrole (CEP) (an oxidation fragment of docosahexaenoic acid) and Malondialdehyde (a common lipid peroxidation product), are believed to cause inflammatory response in AMD (17–18).
RPE lysosomal dysfunction is also directly associated with AMD. Polymorphisms in the CST3 gene which encodes Cystatin C (a potent inhibitor of lysosomal proteinases) has also been shown to be highly prevalent amongst patients with AMD (19). The mutations appear to lead to an impairment of lysosomal enzyme present in RPE cells where it is required for rod outer segments recycling. Inhibition of cathepsin S, a lysosomal cysteine protease, has also been shown to lead to accumulation of debris, further reinforcing the importance of lysosomal integrity for RPE function (20).
(B) Loss of macromolecular permeability and hydraulic conductivity
Bruch’s membrane (BrM) is the inner layer of the choroid, separating it from the RPE layer. Functional integrity of the BrM is critical to a healthy retina. The BrM maintains the blood retinal barrier and caters to the nutritional needs of the RPE and outer part of the sensory retina. It maintains a two way traffic where metabolic waste from the RPE is discarded across into choroid and nutrients from choriocapillaris diffuse across to the RPE (21). With age, this hydraulic conductivity of BrM is thought to be impaired due to the clogging and thickening of the membrane. Several polymorphisms that precipitate the structure’s decline and increase susceptibility to AMD have been identified. Mutations in the ApoE gene which encodes a lipid transport protein involved in low-density cholesterol modulation are thought to result in the accumulation of lipoproteins between the RPE and the BrM thereby disrupting the functional integrity of the structure (22). Similarly, missense mutations in fibulin 5 gene which encodes an extracellular protein required elastin fiber assembly may lead to increased thickening of BrM that occurs in AMD (23).
(C) Inflammation
Chronic inflammation is hypothesized to play an important role in AMD pathogenesis (24). Accumulation of drusen at the base of the retina appears to elicit an inflammatory response which causes bystander damage to RPE cells. This leads to further drusen production and with it, an amplified and sustained inflammatory response that exacerbates PR degeneration and results in severe loss of visual acuity. Several clues as to the importance of sustained inflammation in the pathogenesis have been unraveled. A number of inflammation-related genes that predispose individuals to AMD have been identified (25). Most prominent among them are polymorphisms in several members of the complement pathway including complement factor H (CFH), complement factor B, complement components 2, 3 and 7 and complement factor I (26–30). The complement pathway is an evolutionary ancient defense mechanism against pathogens. Activation of the complement pathway leads to the formation of the membrane attack complex (MAC) which causes target cell lysis and chemokine release, which in turn can recruit inflammatory cells and increase vascular permeability (31). Among the complement factors, CFH was recently discovered as a major peroxidation product Malondialdehyde binding protein which protects against oxidative stress-induced inflammatory response (32). Toll-like receptors (TLRs) regulate activation of the innate immune response in response to bacterial, viral and parasitic ligands (33). TLR3 has been recently implicated in GA with specific loss-of-function single-nucleotide polymorphisms (SNPs) in TLR3 shown to provide protection against AMD (34). Another large association study has implicated variants of toll-like receptor 4 (TLR4) to AMD susceptibility. TLR4 has been demonstrated to play a significant role in pro-inflammatory signaling pathways, likely contributing to the inflammation observed in AMD pathogenesis (35). Mutations in these genes and additional pro-inflammatory genes such as IL-8 predispose individuals to acute and chronic forms of inflammation, which renders those individuals higher risk for AMD (36).
(D) Choroidal neovascularization and vascular leakage
As the BrM and RPE cells slowly degenerate, apoptotic RPE cells and infiltrating inflammatory cells produce several angiogenic factors that cause abnormal sprouting of blood vessels from the choroid into the retina through a process called CNV, which disrupts the retinal architecture and leads to the breakdown of the blood retinal barrier. Indeed, although affecting only 20% of the AMD population, CNV is responsible for 80% of all blindness resulting from AMD. Vascular leakage in CNV further exacerbates the trauma to the retina and RPE, leading to accelerated PR apoptosis and activation of inflammatory cascades which can irreversibly impair vision.
Several factors that promote novel blood vasculature have been implicated in the pathogenesis of AMD. Prime among them, vascular endothelial growth factor (VEGF) has been shown by several clinical and animal studies to play a pivotal role in the development of wet AMD. Several population studies have also uncovered significant association between polymorphisms in the VEGFA gene and the risk of AMD (37–39). The ability of VEGF to induce vascular leakage and inflammation by triggering increased production and permeability of capillary endothelial cells has been established in a variety of human pathological situations. In AMD, increased levels of VEGF are thought to induce retinal vascular leakage and neovascularization. Anti-VEGF agents, including bevacizumab and ranibizumab, have been approved by FDA and demonstrated efficacy in treating CNV in neovascular AMD. Because of its crucial role in the pathogenesis of wet AMD and promising results from therapies targeting this factor, close to 50% of the novel AMD therapies aim to regulate the expression of angiogenic molecules in AMD.
(E) Loss of neuroprotection
In addition to providing the PRs with nourishment and discarding waste, the RPE cells express several neurotrophic factors that nourish and sustain the neurosensory retina. Several of those factors have been identified including platelet-derived growth factor (PDGF), pigment-derived epithelial factor (PEDF), VEGF and brain-derived neurotrophic factor (BDNF) (40–43). BDNF is expressed by the RPE and Müller glia cells in the retina and has been shown to play an important role in retinal neuron survival. BDNF promotes PR survival following experimental retinal detachment or light-induced oxidative damage (44). PEDF is also expressed by RPE cells, and has both neuroprotective and anti-angiogenic properties (45). Production of PEDF is thought to be initiated by the action of L-DOPA, an intermediate product of melanin synthesis on the ocular albinism type 1 (OA1) receptor (46). Similar to BDNF, PEDF limits PR damage induced by oxidative stress and also maintains retinal structural integrity after retinal detachment. A Met72Thr (rs1136287) variant in the PEDF gene has been associated with increased susceptibility to AMD in a Taiwanese cohort (47). More recently, nicotine has been shown to increase the VEGF/PEDF ratio in RPEs, suggesting a possible mechanism for CNV in smokers with AMD (48).
Therapies to target AMD
The current review summarizes the recent inventions designed for AMD treatment available through the World Intellectual Property Organization (WIPO) (http://www.wipo.int/patentscope/search/en/search.jsf), an agency of the United Nations dedicated to developing a balanced and accessible international intellectual property (IP) system (Table 1). The WIPO database lists the patents of the 184 member states including the United States of America. Encompassing patents published from 2008 to 2011, our current research screened for patents that selectively remedied to one or more of the hallmarks of AMD. For inclusion in the current report, the invention had to meet one of three criteria. The invention had to show efficacy in a stringent in vitro model of AMD using RPE or endothelial cell lines. Alternatively, the invention had to demonstrate in vivo efficacy in established animal models of AMD. Finally, if the invention had been tested in phase I clinical trials with promising results, its patent was included. Since abundant literature is currently available for therapeutic agents undergoing clinical trials, the current review primarily focuses on novel preclinical therapeutic agents emerging from recent cutting edge scientific developments. For further reading on the current and completed clinical trials on wet and dry AMD, please refer to the articles listed (49–52).
Table 1.
Highlighted potential therapeutic agents for age-related macular degeneration.
| Table 1A | |||
|---|---|---|---|
| Hallmark of AMD | Agent class | Agent | PCT or APP # |
| Oxidative stress and RPE cytotoxicity | Small molecule | Primary amine compounds | PCT/US2010/059426 [101] |
| Compound A | PCT/US2010/059719 [102] | ||
| Fenretinide | App#: 10181656 [103] | ||
| Zinc N-acetyltaurinate | PCT/FR2009/050297[104] | ||
| Compound 4 | PCT/US2008/011421[105] | ||
| Adenosine receptor agonist | PCT/US2007/021211[107] | ||
| siRNA | RHO opsin | PCT/US2010/033107 [106] | |
| Loss of macromolecular permeability and hydraulic conductivity | Small molecule | Activators of CD36 | PCT/CA2009/000200 [108] |
| Decorin | PCT/US2010/058856 [109] | ||
| Syk (RTK inhibitor) | PCT/IB2008/000486 [110] | ||
| Antibody | Anti-Aβ plaque antibody | PCT/US2009/036119 [111] | |
| Antagomirs | Anti-miR-204 and -211 | PCT/US2009/055000 [112] | |
| Gene therapy | Gluconeogenesis | PCT/US2010/031211 [113] | |
| Inflammation | Small molecule | Recombinant protein, CR2-FH | PCT/US2007/014602 [118] |
| TLR3 antagonists | PCT/US2010/032964 [121] | ||
| PCT/US2009/059383 [122] | |||
| PCT/US2009/001106[123] | |||
| Copaxone | PCT/IL2007/000798 [124] | ||
| Naltrexone | PCT/IB2008/002412[125] | ||
| Tryptase beta-like inhibitor | PCT/US2009/068625 [126] | ||
| STAT3 inhibitors | PCT/US2008/077510 [127] | ||
| Lysyl oxidase inhibitors | PCT/US2010/023359 [128] | ||
| Antibody | MASP-2 inhibitory agent | PCT/US2010/052954 [114] | |
| Anti C5 | PCT/US2010/039448 [115] | ||
| C3b | PCT/EP2010/056129 [116] | ||
| Factor D | PCT/US2008/064526 [117] | ||
| CD25 | PCT/US2010/053734 [120] | ||
| Gene therapy | CD59 | PCT/US2009/000947 [119] | |
| Loss of neuroprotection | ECT devices | PEDF | PCT/US2010/051602 [164] |
| Glucagon-like peptide and endostatin | PCT/EP2010/002899 [165] | ||
| Small molecule | OA1 receptor agonists | PCT/US2009/041021 [166] | |
| BDNF | PCT/IB2010/003220 [167] | ||
| Benzopyran derivative, BP3 | PCT/IE2009/000055 [168] | ||
| 7-β-hydroxyethyltheophylline | PCT/IE2009/000054 [169] | ||
| Norgestrel | PCT/IE2009/000053 [170] | ||
| Gene therapy | HDAC4 | PCT/US2009/053730 [171] | |
| Table 1B | |||
|---|---|---|---|
| Hallmark of AMD: Choroidal neovascularization and vascular leakage | Agent class | Agent | PCT or APP # |
| VEGF production and signaling | Small molecule | Sirolimus | PCT/US2010/033639[131] |
| Temsirolimus | PCT/JP2010/056498[132] | ||
| Everolimus | PCT/US2008/073520[133] | ||
| pazopanib | PCT/US2010/042211[134] | ||
| Axl antagonists | PCT/US2010/043248[135] | ||
| Sorafenib | App # 08425559[136] | ||
| Hedgehog inhibitor | App # 09163654[137] | ||
| Wnt inhibitor | PCT/US2008/076255[138] | ||
| siRNA | VEGF | PCT/US2010/059090[129] | |
| PCT/IN2009/000671[130] | |||
| Vaccine | VEGFR1 vaccine | PCT/JP2010/003871[139] | |
| Vascular sprouting | Antibody | Dll4 | PCT/EP2010/064695[140] |
| PCT/US2007/017546[141] | |||
| CD44v6 | PCT/US2010/028291[142] | ||
| Fibromodulin | PCT/US2010/033724[144] | ||
| Small molecule | Fïbronectin receptor α5βl inhibitors | PCT/EP2008/065596[145] | |
| Disintegrins | PCT/US2010/061738[146] | ||
| α-2-glycoprotein 1 Antagonists | PCT/GB2010/001681[147] | ||
| Aspirin-triggered lipoxins | App#10003357[148] | ||
| Bizelesin | PCT/US2009/005744[149] | ||
| Curcumin-analogs | PCT/US2009/060726[150] | ||
| Carboxyamidotriazole | PCT/US2007/025041[151] | ||
| Angiotensin II receptor blockers | PCT/JP2008/073504[152] | ||
| Inorganic selenium | PCT/AU2008/001469[153] | ||
| Polyoxyethylene/polyoxypropylene copolymers | PCT/US2008/009603[154] | ||
| Antagomirs/miRNA mimic | miR-27 and miR-23 | PCT/AU2010/000698[155] and patent pending | |
| Macular edema and vascular repair | Cell therapy | Mesenchymal precursor cells | PCT/US2009/003902[156] |
| CD33/CD14 monocytes | PCT/US2010/000477[157] | ||
| Small molecule | IFNγ | PCT/US2009/053808[158] | |
| Modified glucocorticoids | PCT/EP2009/058572[159] | ||
| Rimcazole | PCT/GB2009/002700[160] | ||
| Danazol | PCT/US2010/039461[161] | ||
| Alpha connexin c-terminal peptide | PCT/US2008/067944[162] | ||
| Inhibitors to the lysl oxidase | PCT/US2010/023359[163] | ||
(A) Limiting oxidative stress and RPE damage
As noted above, excessive formation of A2E can lead to RPE and PR cytotoxicity. Several compounds have been tested for their ability to either abate or reverse the process. Primary amine compounds administrated directly to the eye were shown to effectively reduce the formation of A2E in a light-induced model of macular degeneration, (PCT/US2010/059426) [101]. Optical coherence tomography analysis demonstrated that the primary amines significantly alleviated retinal degeneration as described by the limited disruption in retinal morphology and a 30% increase in 11-cis-retinal in the treated mice. The primary amines are thought to sequester free all-trans-retinal that escaped via the photoreceptor outer segments of the retina without adversely affecting normal retinoid cycle. By eliminating the excess all-trans-retinal, the primary amines prevent its conversion to pathogenic derivatives such as A2E, thereby mitigating retinal degeneration. Similar compounds targeting precursors of A2E include Compound A by NEURON SYSTEMS (PCT/US2010/059719) [102], Fenretinide by Revision therapeutics (App#: 10181656) [103], Zinc N-acetyltaurinate by TRI-INOV (PCT/FR2009/050297) [104], compound 4 by ACUCELA INC (PCT/US2008/011421) [105], and hammerhead ribozymes or short hairpin RNAs against RHO opsin (PCT/US2010/033107) [106]. In addition, an intriguing therapeutic approach aimed at restoring an optimal acidic pH to compromised lysosomes in the RPE is also being investigated. The agent is thought to slow the progress of AMD by lowering lysosomal pH and thereby enhancing the activity of degradative enzymes (PCT/US2007/021211) [107].
(B) Enhancing BrM hydraulic conductivity and macromolecular permeability
Functional integrity of the BrM is essential to retinal homeostasis. With age, the BrM thickens and gets clogged. The ensuing loss in hydraulic conductivity leads to decreased choroidal perfusion and loss of RPE and PR cells. Accordingly, several approaches are being devised to maintain the BrM structural and functional integrity. One current pathway currently being investigated involves the use of activators of CD36, a type B scavenger receptor that is expressed in RPE cells (PCT/CA2009/000200) [108]. Stimulation of CD36 is thought to enhance the phagocytosis of oxidized lipids and consequently inhibit their deposition in the BrM. Using ApoE−/− mice which show impaired lipid metabolism and develop clogging of the BrM and impaired functionality under high lipid diet, the authors of the patent demonstrated that treatment with the CD36 activator EP80317 could prevent BrM thickening (53). Similar therapeutic modalities being considered include intravitreal injection of small leucine-rich repeat proteoglycans such as decorin which stabilize and reorganize the extracellular matrix of BrM (PCT/US2010/058856) [109].
Other avenues being explored to enhance hydraulic conductivity of the Bruch’s membrane involve direct unclogging of the BrM. For instance, elimination of Aβ amyloid plaque associated with the pathogenesis of AMD is currently being tested (PCT/IB2008/000486) [110]. Using aged human apolipoprotein E4 knock-in mice on a high-fat cholesterol enriched diet, the authors show that treatment with anti-Aβ amyloid antibody immunotherapy confers visual protection and prevents thickening of the BrM (54). Likewise, a peroral therapy using inhibitors to the protein tyrosine kinase, Syk, have been used to cause a significant reduction in Aβ amyloid plaque formation (PCT/US2009/036119) [111].
As the BrM and RPE fail, the transport of metabolites to PRs is drastically diminished. To maintain PR homeostasis, agents aiming at improving RPE transepithelial transport are also being considered. The use of antagomiRs to miR-204 and miR-211 (PCT/US2009/055000) [112] to increase epithelial permeability and gene therapy to promote RPE survival in a microenvironment with limited nutritional content (PCT/US2010/031211) [111] is also being explored (55).
(C) Anti-inflammatory and immunosuppressive agents
Polymorphisms in the complement cascade are thought to confer heightened susceptibility to AMD due to chronic retinal inflammation. Accordingly several therapeutic agents targeting complement proteins are currently being developed. One of the most promising agents involves a MASP-2 inhibitory agent (PCT/US2010/052954) [114]. MASP-2 is the protease responsible for activating C4 and C2 to generate the C3 convertase C4b2b (56). Using a laser-induced model of AMD, the authors of the invention report a significant inhibition of CNV in mice pre-treated with a single injection of anti-MASP-2 monoclonal antibody. Additional antibodies targeting C5 (PCT/US2010/039448) [115], C3b (PCT/EP2010/056129) [116] and factor D (PCT/US2008/064526) [117] that mitigate the adverse effects of the complement system are being pursued and have demonstrated promising preliminary results. In addition to depleting antibodies, endogenous inhibitors of the complement pathway are also being used. For instance, a recombinant form of complement factor H is currently being tested as a treatment for AMD (PCT/US2007/014602) [118]. The recombinant protein, CR2-FH, comprises two domains. The CR2 fraction allows for targeted delivery of the molecule to the sites of complement activation, and the FH portion is responsible for specifically inhibiting complement activation of the alternative pathway. Using laser photocoagulation in a mouse model of AMD, the inventors showed that CR2-fH delivered directly to the eye reduces the spread of retinal lesion (57). The use of gene therapy to curtail complement-mediated inflammation is another avenue being considered. CD59 is a membrane-bound glycoprotein that blocks the assembly of functional MACs and thus protects cells from complement-mediated lysis (58). Using subretinal injections of an adenoviral vector encoding human CD59, the inventors were able to achieve expression of the protein by the mouse RPE cells, and more importantly the protection of those RPE cells from human MAC deposition in vivo (PCT/US2009/000947) [119].
In addition to curtailing chronic inflammation arising from constitutive activation of the complement cascade, novel approaches to temper the cellular immune responses that exacerbate AMD are being developed. Among the most interesting is the use of Daclizumab, a humanized monoclonal antibody to IL-2 Rα is currently undergoing clinical trials for wet AMD (PCT/US2010/053734) [120]. The antibody selectively inhibits T cell mediated immune responses and has demonstrated promising results when used in combination with anti-VEGF agents (59). In addition to the adaptive immune system, additional agents targeting the innate immune system are being considered. Loss-of-function polymorphisms in the toll-like receptor 3 (TLR3) have been shown to protect against geographic atrophy. Accordingly, antibody antagonists to TLR3 have been developed and have been shown to significantly decrease the production of inflammatory cytokines when used with human umbilical vein endothelial cells subjected to TLR3 ligand poly(I:C) (PCT/US2010/032964, PCT/US2009/059383) [121,122]. Additional RNA-based TLR3 antagonists are also being tested for efficacy in GA (PCT/US2009/001106) [123].
Immunomodulation is also being considered as a possible avenue for limiting retinal damage leading to AMD. Three compounds notably copaxone, naltrexone, and tryptase beta type inhibitor have demonstrated significant efficacy. Copaxone is a random chain (polymer) of four amino acids, namely glutamic acid, lysine, alanine and tyrosine which has been shown to downregulate the immune responses and was particularly successful at mitigating multiple sclerosis (60). In a trial involving dry AMD study patients, systemic injections of copaxone led to an average reduction of 53.6% in total drusen area (PCT/IL2007/000798) [124]. Similarly, other immune modulators including the opioid antagonist, naltrexone (PCT/IB2008/002412) [125], and a tryptase β-like inhibitor (PCT/US2009/068625) [126] by SANOFI have been shown to curtail the progression of AMD in patients and animal models respectively. Compounding the rest, agents targeting global mediators of inflammatory responses are also currently being targeted. For instance, STAT3, a transcription factor required for the expression of several inflammatory molecules including tumor necrosis factor α (TNF-α), IL-6, IL-12, and MCP-1 is currently being investigated as a therapeutic target in a diabetic retinopathy model (61). Using a diabetic mouse model, the inventors were able to show that intravitreal delivery of the STAT3 inhibitors could decrease expression of pro-inflammatory and pro-angiogenic genes in a diabetic model of retinal edema (PCT/US2008/077510) [127]. In the same line, inhibitors to lysyl oxidase (LOX), proteins responsible for the modification of the extracellular matrix have been shown to significantly decrease infiltration of immune cells and the degree of neovascularization in laser-induced CNV mouse model (PCT/US2010/023359) [128]. In contrast, a separate study found increased laser-induced CNV in mice lacking lysyl oxidase-like1 gene, highlighting the need for additional studies exploring the role of the protein in the pathogenesis of AMD (62).
(D) Inhibiting choroidal neovascularization and vascular leakage
As PR and RPE cells degenerate with increasing age, the retinal microenvironment is thought to become hypoxic and inflammation prone. CNV occurs in response to these insults, when the RPE cells secrete several angiogenic factors such as VEGF that promote the migration and proliferation of vascular endothelial cells. These cells form vascular tubes which invade and disrupt the base of the retina as they give rise to new blood vessels. The infiltrating blood vessels often leak and bleed, causing hemorrhage, swelling and eventually the development of scar tissue. Because these events namely (a) the production and response to VEGF; (b) the sprouting of new blood vessels from vascular endothelial cells; and (c) vascular leakage may distort central vision most severely and cause blindness, a lot of effort is currently being poured into inhibiting CNV as exemplified by the fact that nearly half of the agents being developed are geared at curtailing this process.
(a) Restricting VEGF production and signaling
Several agents are currently being pursued to selectively target VEGF. Short interfering RNAs (siRNAs) to downregulate the expression of the molecule have demonstrated beneficial effects in animal models of AMD (PCT/US2010/059090, PCT/IN2009/000671) [129, 130]. In addition, several small molecule inhibitors selectively blocking VEGFR signaling have also been tested for their efficacy at inhibiting AMD. They encompass several classes of molecules including receptor tyrosine kinase inhibitors such as sirolimus (PCT/US2010/033639) [131], Temsirolimus (PCT/JP2010/056498) [132], Everolimus (PCT/US2008/073520) [133], pazopanib (PCT/US2010/042211) [134], Axl antagonists (PCT/US2010/043248) [135], and Sorafenib (App # 08425559) [136]. Moreover, specific small molecules inhibitors to the Hedgehog pathway (App # 09163654) [137] and the Wnt pathway (PCT/US2008/076255) [138] were also shown to decrease VEGF expression in CNV animal models.
One interesting therapy currently being pursued involves the generation of a VEGFR1 vaccine (63). Using a peptide derived from human VEGF receptor 1 and 2 (PCT/JP2010/003871) [139], the inventors were able to generate cytotoxic T-lymphocytes (CTLs) against the molecule. Moreover, patients receiving the treatment have already shown significantly enhancement in visual acuity.
(b) Inhibiting vascular sprouting
The proliferation, migration and tube formation by vascular endothelial cells is essential to neovascularization. Thus, several of the agents currently under development aim to inhibit this process. Some of the agents currently being pursued include antibodies targeting several cellular molecules required for angiogenesis such as Delta like 4 (Dll4) (PCT/EP2010/064695, PCT/US2007/017546) [140, 141], α5βl integrin (PCT/US2010/028291) [142], CD44v6 (PCT/EP2010/003523) [143] and fibromodulin (PCT/US2010/033724) [144].
Other non-antibody molecules targeting vascular sprouting are also under testing and have been shown to cause significant regression of the blood vessels. They include f bronectin receptor α5βl inhibitors (PCT/EP2008/065596) [145], disintegrins (PCT/US2010/061738) [146], antagonists of Leucine-rich α-2-glycoprotein 1 (PCT/GB2010/001681) [147], aspirin-triggered lipoxins (App#10003357) [148], bizelesin (PCT/US2009/005744) [149], curcumin-analogs (PCT/US2009/060726) [150], carboxyamidotriazole (PCT/US2007/025041) [151], angiotensin II receptor blockers (PCT/JP2008/073504) [152], inorganic selenium (PCT/AU2008/001469) [153] and polyoxyethylene/polyoxypropylene copolymers (PCT/US2008/009603) [154].
microRNAs have also been shown to be effective targets for regulating the growth of new blood vessels (64). Overexpression of miR-27a has been shown to disrupt angiogenesis in both in vivo and in vitro models of capillary tube formation (PCT/AU2010/000698) [155]. In contrast, using a laser-induced CNV mouse model, our group has demonstrated that inhibition of miR-23 and miR-27 by intravitreal injection of the locked-nucleic-acid modified anti-miRs led to about 50% repression in CNV (patent application pending) (65). Thus, in addition to the agents described above, the selective modulation of miRNAs could provide a viable form of therapy for treating wet AMD.
(c) Limiting macular edema and promoting vascular repair
In addition to the growth of new vessels into the retina, macular edema characterized by the extravasation of fluids from nascent blood vessels further causes deterioration in visual acuity owing to the disruption of retinal neurons. Accordingly, several therapies to limit the disruption of the blood retinal barrier and macular leakage have been devised. The use of mesenchymal precursor cells, in combination with anti VEGF therapy, has shown significant efficacy at eliminating severely leaky vessels (PCT/US2009/003902) [156]. Mesenchymal precursor cells (MPCs) are capable of differentiating into several cell types including perivascular mesenchymal precursor cells (66). Using Cynomolgus Monkeys in a laser-induced choroidal neovascularization model, the authors demonstrated that intravitreal injection of a single dose of allogeneic MPCs in combination with the anti-VEGF monoclonal antibody, Lucentis, can functionally stabilize the new vessels and reduce the severity of leaky vessels compared to the antibody alone. Similarly, CD33/CD14 monocytes have also been tested for the ability to affect vascular repair and have shown promising results (PCT/US2010/000477) [157].
A recent surprising discovery regarding a previously considered inflammatory cytokine might further open additional avenues for treating macular edema. Interferon gamma (IFNγ) is a cytokine produced by several immune cells with inflammatory and regulatory functions in innate and adaptive immunity. Interestingly, it appears that subretinal injection of the cytokine enhances transport of fluid from the neuroretina towards the choroidal side of the RPE, thereby clearing excess fluid from the subretinal space (PCT/US2009/053808) [158] (67). The cytokine has been tested in rodent model of retinal detachment and is under clinical trial (NCT01376362). Several other molecules that showed promising results in macular edema treatment based on animal and cell culture studies include modified glucocorticoids (PCT/EP2009/058572) [159], Rimcazole (PCT/GB2009/002700) [160], Danazol (PCT/US2010/039461) [161], and alpha connexin c-terminal peptide (PCT/US2008/067944) [162].
Lastly, fibrotic damage in the wake of CNV has also been shown to severely impair retinal function. Inhibitors to the lysl oxidase enzymes which can modulate the cross-linking of collagen and elastin in the extracellular space leading to fibrosis have demonstrated significant success in a laser-induced CNV model (PCT/US2010/023359) [163].
(E) Restoring neuroprotection
Neurotrophins are molecules produced typically by nerve cells that regulate the survival, growth and development of various cells. The RPE cells produce an array of these molecules and with progressive AMD, their expression significantly decreases as the retinal cells undergo cell death. Among these molecules, PEDF appears to be essential for the prevention of AMD. Accordingly, strategies to restore the level of this molecule in the retina are currently being investigated. One approach showing promising results in visual acuity improvement involves the use of encapsulated cell technology (ECT) where ECT devices embedded with PEDF expressing cells are implanted in the vitreous cavity (PCT/US2010/051602) [164] (68). Similarly, ECT devices in the form of microbeads embedded with cells producing glucagon-like peptide 1 (neuroprotector) and endostatin (anti-angiogenic agent) are also being tested for neuronal protection against AMD (PCT/EP2010/002899) [165]. In addition to directly implanting PEDF secreting cells in the vitreous cavity, other avenues for increasing the local levels of PEDF are being explored. One of the ways involves the use of OA1 receptor agonists which have been shown to elevate the production of PEDF (PCT/US2009/041021) [166].
BDNF is also produced by the retina and is similarly essential to retinal homeostasis. Its use as a therapeutic agent has been explored using a light-induced model of retinal degeneration in mice. In this model, retinal degeneration is induced by light damage to retinal PRs following a lengthy exposure to a strong source of light. Excessive absorption of photons by the visual pigment rhodopsin leads to PR cell death (69). The patent stated that topical application of this neurotrophin can significantly promote survival of retinal cells and decrease light-induced impairment of the retinal as measured by electroretinogram (ERG) (PCT/IB2010/003220) [167].
Additional compounds have been screened for their protection of light-induced retinal degeneration. Systemic treatment with the Benzopyran derivative, BP3, was shown to prevent apoptosis or degeneration of retinal PR cells. (PCT/IE2009/000055) [168]. In rdlO mice which display autosomal recessive retinal degeneration, daily intravitreal injections of BP3 was further shown to lead to enhanced survival of PR cells. Additional compounds notably 7-β-hydroxyethyltheophylline (PCT/IE2009/000054) [169] and Norgestrel (PCT/IE2009/000053) [170] showed similar protective effects using the light-induced model of retinal degeneration.
In addition to pharmacological treatments, gene therapy is also being considered as an option for neuroprotection. Recently, it was reported that histone deacetylase 4 (HDAC4) is required for normal development of rod PR and bipolar interneurons and is HIF-lα dependent (70). RNA interference to decrease the expression levels of HDAC4 led to neuronal cell death by apoptosis. In contrast, transgenic overexpression of HDAC4 in PR cells in retinal degeneration mice (rdl−/−) led to prolonged PR survival. Accordingly, the inventors suggest the use of retroviral infection with HDAC4 as a means to combat AMD (PCT/US2009/053730) [170].
Novel diagnostic tools
With respect to diagnostics, several novel agents are currently in the pipeline. The first set of diagnostic tools is designed to screen for pathogenic polymorphisms. As mentioned above, several AMD susceptibility genes have been identified and their availability to patients would allow them to adjust controllable environmental factors to decrease their disease susceptibility. One of the embodiments includes gene chips which identify nucleotide variants associated with AMD (PCT/US2010/041720, PCT/US2010/033052) [172, 173]. The next stage in therapy enhancement which is already underway involves analyzing how specific polymorphisms can specifically predict drug efficacy. One ongoing study involves examining how polymorphisms in specific genes translate to efficacy of the anti-angiogenic drug (PCT/US2010/021615) [174]. In a study examining the efficacy of Lucentis against individual polymorphisms, the authors demonstrated that the Ala69Ser polymorphism in the ARMS2 gene is potentially associated with Lucentis efficacy. A similar invention attempting to correlate attributes unique to the patients ranging from obesity and vitamin intake to drug efficacy is also underway (PCT/US2009/040919) [175].
The second set of diagnostic tools is to use serum biomarkers for predicting whether the patient is at risk for developing AMD and for evaluating progress of therapy. One of the diagnostic kits evaluates the serum levels of several complement proteins. The inventors report that low serum or plasma protein levels of complement Factor H and high serum levels of complement factor polypeptides Bb, C3a, C5a are predictive of increased susceptibility to develop AMD (PCT/US2010/043964) [176]. Similarly, other inflammatory makers namely IP-10 and exotaxin have demonstrated tremendous ability to discriminate between AMD and control individuals (PCT/US2010/032801) [177]. Interferon γ–inducible protein-10 (IP-10) serves as a chemoattractant to lymphocytes and monocytes, while eotaxin is a chemoattractant for eosinophils. The concentration of both cytokines was highly elevated in patient at very early and late stages of AMD (71). Since the cytokines offer an early predictive tool, they can provide a larger window of opportunity for treatment. Furthermore, serum levels of advanced glycation endproducts (AGEs) namely carboxyethylpyrrole (CEP) adducts, carboxymethyllysine (CML), and pentosidine are also being used as predictive biomarkers (72). Oxidative protein modifications like CEP and CML are elevated in BrM of AMD patients, and were found to induce neovascularization in vivo. The authors of the invention found that plasma levels of CML together with pentosidine discriminate between AMD and control subjects with 92% accuracy and that pentosidine in combination with CEP adducts can discriminate with 95% accuracy (PCT/US2010/034398) [178].
Novel delivery methods
One active area of research involves develop novel delivery methods for AMD drugs. Current ocular drug delivery approaches include systemic, topical, intravitreal and subretinal administration of the drug. Topical administration has limited penetration, suffering from rapid tear washout and poor patient compliance. On the other hand, intravitreal or subretinal drug delivery may lead to retinal detachment and hemorrhage. Since the drugs are rapidly dispersed at the site of injection, repetitive injections are often needed. Accordingly, inventions to allow localized delivery, increasing half-life and sustained release of the drugs are being actively sought currently. Several delivery systems are being optimized to fit these functions. Particulate delivery systems primarily involve nano and micropaticles that are loaded with the therapeutically active agents that can be released at precise locations in a sustained fashion. Such an embodiment includes currently developed ranibizumab loaded microparticles that can be directly injected into the vitreous humor of the eye. The microparticles consist of a polymeric matrix into which ranibizumab particles are embedded. Because this method leads to sustained drug release by the microparticles which average 50 microns in size, only a limited number of these particles need to be injected at intervals of up to 12 months (PCT/US2010/051068) [179]. In contrast to these drug delivering methods, a new generation of nanoparticles has been developed that can directly cause regression of retinal vascular lesions and prevent light-induced PR damage in mouse models (73–74). The nanoparticles are made of cerium oxide which has been shown to limit oxidative stress, decrease the generation of free radical production, and prolong cell longevity (PCT/US2009/041675, PCT/US2006/016050) [180, 181]. Besides microparticles, liposome mediated delivery of siRNAs to VEGFR1 have also shown significant promise in restricting CNV (PCT/US2010/059995) [182].
Ocular implants are also being tapped as novel avenues for localized and sustained drug delivery. As described above, ECT devices consist of semipermeable membranes packed with genetically modified cells that can achieve therapeutic delivery of macromolecules including PEDF and CNTF. One disadvantage with the ECT devices is their retrieval. Accordingly, other implants currently being tested include biodegradable polymeric vehicles. Consisting of hyaluronic acid or PLGA, these biodegradable implants are loaded with therapeutic agents such as bevacizumab or rapamycin that can be delivered subconjunctivally or intravitreally (PCT/US2009/050373, PCT/US2010/059995) [183, 184].
Conclusion remarks and future perspective
Although the etiology of AMD remains unclear, extensive research during the past decade is starting to uncover the mechanism of AMD pathogenesis. Accordingly, we have witnessed an explosion of novel therapeutic and diagnostic agents devised to address the diverse hallmarks of AMD. For instance, to combat the heightened oxidative stress in AMD, a set of inhibitors are being developed to selectively prevent the formation and promote the clearance of A2E and its precursors. To maintain the functional integrity of BrM, activator of scavenger receptor CD36 and anti-Aβ amyloid antibody have been effectively used to prevent BrM thickening in mouse models of AMD. To mitigate the adverse effect of complement pathways which are causative of the chronic inflammation in AMD, complement inhibitors and complement factor H recombinant protein are being tested for AMD treatment in mouse models and have shown promising preliminary results. In the wake of the success of anti-VEGF therapy in CNV in wet AMD, numerous radical approaches devised to target specific aspects of wet AMD are being developed. New anti-angiogenic small molecules and antibodies, mesenchymal progenitor cells, VEGF receptor vaccines, microRNAs, and immunomodulators promise superior outcomes in the treatment of wet AMD, either when used alone or in combination with the existing FDA-approved anti-VEGF agents. In addition, novel diagnostic tools, including gene chips to detect disease gene polymorphisms, and serum biomarkers, such as IP-10 and CEP, are being devised for early detection of AMD susceptibility and evaluation of drug efficacy. Novel delivery technologies, including nanoparticles and biodegradable ocular implants, represent the next generation delivery system to ensure localized and sustained drug effects. Considering the promising preclinical data of these novel agents and technologies, a next generation treatment for AMD is on the horizon.
Looking into the future, several therapeutic opportunities still remain untapped. The implementation of stem cell technology appears to still lag behind when it comes to treatment of AMD. Currently, we are aware of only one such study looking into the therapeutic use of stem cell technology for the treatment of AMD (PCT/US2010/057056) [185]. Moreover, it is now obvious that single drug therapies tackling only one of the hallmarks of AMD are not sufficient for long-term treatment. There is a greater need for combination therapies and the use of bispecific antibodies is breaking new ground into this emerging field (PCT/EP2010/064695, PCT/EP2010/057246) [186, 187]. Additionally, with a wider variety of therapies, it becomes important to fine tune the selection of agents to attain maximum efficacy with individual patients. In this instance, correlating efficacy of each therapeutic agent to unique polymorphisms or sets of polymorphisms would significantly improve the personalized medicine. To our knowledge, there is no study looking at the efficacy of combination therapies geared toward unique sets of polymorphisms. Finally, as with most diseases, there is still a dearth of reliable molecular biomarkers that would allow early preventive action. Still, with the momentum of a decade of intense research, it is most likely that a cure is in the works and with it, a much brighter outlook for patients with AMD.
Executive summary.
With an increasingly aging population, there is a dire need for the development of new therapies for treatment of AMD. The current table summarizes the potential therapeutic reagents covered.
Limiting oxidative stress and RPE damage
Blocking formation of A2E and its precursors.
Enhancement of RPE lysosomal activity.
Enhancing Bruch’s membrane hydraulic conductivity and macromolecular permeability
Modulation of Bruch’s membrane extracellular matrix.
Clearance of clogging molecules such as oxidized lipids and Aβ amyloid.
Enhancement of RPE transepithelial transport and response to nutritional stress.
Anti-inflammatory and immunosuppressive agents
Inhibition of complement system.
Suppression of adaptive and innate cellular immune responses.
Small molecule-mediated antagonism to inflammatory mediators.
Inhibiting choroidal neovascularization and vascular leakage
Blocking VEGF production and signaling.
Inhibiting vascular sprouting.
Limiting macular edema and promoting vascular repair.
Restoring neuroprotection
Topical release of neurotrophic factors to promote photoreceptor survival.
Inhibition of photoreceptor cell apoptosis by gene therapy.
Novel diagnostic tools
Identification of risk conferring SNPs by genome sequencing.
Identification of predictive serum biomarkers.
Novel modes of delivery
Nano and micropaticles-mediated treatment and delivery of therapeutically active agents.
Encapsulated cell technology (ECT) devices.
Acknowledgments
S.W. was supported by a Startup fund from the Department of Ophthalmology at UT Southwestern Medical Center at Dallas, President’s Research Council New Investigator Award, NIH Grants EY021862 and EY020799, and by an unrestricted grant from Research to Prevent Blindness.
Defined Key Terms
- Age-related Macular Degeneration (AMD)
A currently incurable eye disease that is the major cause of blindness and vision impairment in the elderly population. The condition primarily impairs the central portion of the retina and can present itself in “dry” and “wet” forms
- Drusen
Extracellular deposits consisting of complex waxy amyloid mix of molecules that accumulate between the RPE and Bruch membrane
- Retinal pigement epithelium (RPE)
Pigmented cell layer between neurosensory retina and choroid that is essential to photoreceptor cell homeostatsis. RPE cells provide nourishment and other essential molecules to sustain the retina, and recycle visual cycle byproducts from photoreceptor cells
- Geographic Atrophy (GA)
An advanced form of “dry” age-related macular degeneration, which involves breakdown or wasting away of RPE cells and the overlying photoreceptor cells, and loss of visual acuity
- Choroidal Neovascularization (CNV)
The invasion of retina by abnormal blood vessels originating from the choroid: a condition characteristic of the “wet” from age-related macular degeneration
- Genome wide association study (GWAS)
A genetic epidiemology approach that involves sequencing the DNA of individuals to identify nucleotide sequence variations such as single nucleotide polymorphisms (SNPs) that associate with disease
- Oxidative stress
Cellular stress resulting from exposure to reactive oxygen intermediates generated by the heightened metabolic activity or constant exposure to high levels of irradiation
- Complement system
Liver-derived small proteins that protect against pathogens by causing the lysis of the foreign organisms, and by recruiting cellular mediators of the immune system. Chronic activation of the complement system is thought to partially drive AMD
- Neurotrophins
Molecules that regulate the survival, growth and development of various cells. Brain-derived neurotrophic factor (BDNF) and pigment-derived epithelial factor (PEDF) are neurotrophins produced by the RPE that sustain and protect the neurosensory retina
Footnotes
Financial disclosure
The authors have no financial involvement in or financial conflict with the subject matter or materials discussed in the manuscript. No writing assistance was utilized in the production of the manuscript.
References
- 1.Pascolini D, Mariotti SP, Pokharel GP, Pararajasegaram R, Etya’ale D, Negrel AD, et al. 2002 global update of available data on visual impairment: a compilation of population-based prevalence studies. Ophthalmic Epidemiol. 2004;11(2):67–115. doi: 10.1076/opep.11.2.67.28158. [DOI] [PubMed] [Google Scholar]
- 2.Gordois A, Pezzullo L, Cutler H. The Global Economic Cost of Visual Impairment: AMD Alliance International. 2010. [Google Scholar]
- 3.Friedman DS, O’Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–72. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 4.Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358(24):2606–17. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- 5.de Jong PT. Age-related macular degeneration. N Engl J Med. 2006;355(14):1474–85. doi: 10.1056/NEJMra062326. [DOI] [PubMed] [Google Scholar]
- 6.Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration--emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38(7):450–71. doi: 10.1080/07853890600946724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein R, Klein BE, Linton KL, DeMets DL. The Beaver Dam Eye Study: the relation of age-related maculopathy to smoking. Am J Epidemiol. 1993;137(2):190–200. doi: 10.1093/oxfordjournals.aje.a116659. [DOI] [PubMed] [Google Scholar]
- 8.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85(3):845–81. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 9.Kanda A, Chen W, Othman M, Branham KE, Brooks M, Khanna R, et al. A variant of mitochondrial protein LOC387715/ARMS2, not HTRA1, is strongly associated with age-related macular degeneration. Proc Natl Acad Sci U S A. 2007;104(41):16227–32. doi: 10.1073/pnas.0703933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fritsche LG, Loenhardt T, Janssen A, Fisher SA, Rivera A, Keilhauer CN, et al. Age-related macular degeneration is associated with an unstable ARMS2 (LOC387715) mRNA. Nat Genet. 2008;40(7):892–6. doi: 10.1038/ng.170. [DOI] [PubMed] [Google Scholar]
- 11.Allikmets R, Shroyer NF, Singh N, Seddon JM, Lewis RA, Bernstein PS, et al. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science. 1997;277(5333):1805–7. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- 12.Shroyer NF, Lewis RA, Yatsenko AN, Wensel TG, Lupski JR. Cosegregation and functional analysis of mutant ABCR (ABCA4) alleles in families that manifest both Stargardt disease and age-related macular degeneration. Hum Mol Genet. 2001;10(23):2671–8. doi: 10.1093/hmg/10.23.2671. [DOI] [PubMed] [Google Scholar]
- 13.Mata NL, Weng J, Travis GH. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc Natl Acad Sci U S A. 2000;97(13):7154–9. doi: 10.1073/pnas.130110497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finnemann SC, Leung LW, Rodriguez-Boulan E. The lipofuscin component A2E selectively inhibits phagolysosomal degradation of photoreceptor phospholipid by the retinal pigment epithelium. Proc Natl Acad Sci U S A. 2002;99(6):3842–7. doi: 10.1073/pnas.052025899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sparrow JR, Nakanishi K, Parish CA. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Invest Ophthalmol Vis Sci. 2000;41(7):1981–9. [PubMed] [Google Scholar]
- 16.Kaneko H, Dridi S, Tarallo V, Gelfand BD, Fowler BJ, Cho WG, et al. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature. 2011;471(7338):325–30. doi: 10.1038/nature09830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, et al. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008;14(2):194–8. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki M, Kamei M, Itabe H, Yoneda K, Bando H, Kume N, et al. Oxidized phospholipids in the macula increase with age and in eyes with age-related macular degeneration. Mol Vis. 2007;13:772–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Zurdel J, Finckh U, Menzer G, Nitsch RM, Richard G. CST3 genotype associated with exudative age related macular degeneration. Br J Ophthalmol. 2002;86(2):214–9. doi: 10.1136/bjo.86.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rakoczy PE, Mann K, Cavaney DM, Robertson T, Papadimitreou J, Constable IJ. Detection and possible functions of a cysteine protease involved in digestion of rod outer segments by retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1994;35(12):4100–8. [PubMed] [Google Scholar]
- 21.Booij JC, Baas DC, Beisekeeva J, Gorgels TG, Bergen AA. The dynamic nature of Bruch’s membrane. Prog Retin Eye Res. 2010;29(1):1–18. doi: 10.1016/j.preteyeres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Baird PN, Richardson AJ, Robman LD, Dimitrov PN, Tikellis G, McCarty CA, et al. Apolipoprotein (APOE) gene is associated with progression of age-related macular degeneration (AMD) Hum Mutat. 2006;27(4):337–42. doi: 10.1002/humu.20288. [DOI] [PubMed] [Google Scholar]
- 23.Stone EM, Braun TA, Russell SR, Kuehn MH, Lotery AJ, Moore PA, et al. Missense variations in the fibulin 5 gene and age-related macular degeneration. N Engl J Med. 2004;351(4):346–53. doi: 10.1056/NEJMoa040833. [DOI] [PubMed] [Google Scholar]
- 24.Telander DG. Inflammation and age-related macular degeneration (AMD) Semin Ophthalmol. 2011;26(3):192–7. doi: 10.3109/08820538.2011.570849. [DOI] [PubMed] [Google Scholar]
- 25.Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009;28(1):1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–9. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308(5720):421–4. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 28.Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308(5720):419–21. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 29.Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AJ, Gehrs K, et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38(4):458–62. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yates JR, Sepp T, Matharu BK, Khan JC, Thurlby DA, Shahid H, et al. Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med. 2007;357(6):553–61. doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- 31.Zipfel PF, Lauer N, Skerka C. The role of complement in AMD. Adv Exp Med Biol. 2010;703:9–24. doi: 10.1007/978-1-4419-5635-4_2. [DOI] [PubMed] [Google Scholar]
- 32.Weismann D, Hartvigsen K, Lauer N, Bennett KL, Scholl HP, Charbel Issa P, et al. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature. 2011;478(7367):76–81. doi: 10.1038/nature10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–50. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Yang Z, Stratton C, Francis PJ, Kleinman ME, Tan PL, Gibbs D, et al. Toll-like receptor 3 and geographic atrophy in age-related macular degeneration. N Engl J Med. 2008;359(14):1456–63. doi: 10.1056/NEJMoa0802437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zareparsi S, Buraczynska M, Branham KE, Shah S, Eng D, Li M, et al. Toll-like receptor 4 variant D299G is associated with susceptibility to age-related macular degeneration. Hum Mol Genet. 2005;14(11):1449–55. doi: 10.1093/hmg/ddi154. [DOI] [PubMed] [Google Scholar]
- 36.Goverdhan SV, Ennis S, Hannan SR, Madhusudhana KC, Cree AJ, Luff AJ, et al. Interleukin-8 promoter polymorphism -251A/T is a risk factor for age-related macular degeneration. Br J Ophthalmol. 2008;92(4):537–40. doi: 10.1136/bjo.2007.123190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Churchill AJ, Carter JG, Lovell HC, Ramsden C, Turner SJ, Yeung A, et al. VEGF polymorphisms are associated with neovascular age-related macular degeneration. Hum Mol Genet. 2006;15(19):2955–61. doi: 10.1093/hmg/ddl238. [DOI] [PubMed] [Google Scholar]
- 38.Haines JL, Schnetz-Boutaud N, Schmidt S, Scott WK, Agarwal A, Postel EA, et al. Functional candidate genes in age-related macular degeneration: significant association with VEGF, VLDLR, and LRP6. Invest Ophthalmol Vis Sci. 2006;47(1):329–35. doi: 10.1167/iovs.05-0116. [DOI] [PubMed] [Google Scholar]
- 39.Lin JM, Wan L, Tsai YY, Lin HJ, Tsai Y, Lee CC, et al. Vascular endothelial growth factor gene polymorphisms in age-related macular degeneration. Am J Ophthalmol. 2008;145(6):1045–51. doi: 10.1016/j.ajo.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 40.Lee HT, Chang YC, Tu YF, Huang CC. VEGF-A/VEGFR-2 signaling leading to cAMP response element-binding protein phosphorylation is a shared pathway underlying the protective effect of preconditioning on neurons and endothelial cells. J Neurosci. 2009;29(14):4356–68. doi: 10.1523/JNEUROSCI.5497-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smits A, Kato M, Westermark B, Nister M, Heldin CH, Funa K. Neurotrophic activity of platelet-derived growth factor (PDGF): Rat neuronal cells possess functional PDGF beta-type receptors and respond to PDGF. Proc Natl Acad Sci U S A. 1991;88(18):8159–63. doi: 10.1073/pnas.88.18.8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steele FR, Chader GJ, Johnson LV, Tombran-Tink J. Pigment epithelium-derived factor: neurotrophic activity and identification as a member of the serine protease inhibitor gene family. Proc Natl Acad Sci U S A. 1993;90(4):1526–30. doi: 10.1073/pnas.90.4.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weibel D, Kreutzberg GW, Schwab ME. Brain-derived neurotrophic factor (BDNF) prevents lesion-induced axonal die-back in young rat optic nerve. Brain Res. 1995;679(2):249–54. doi: 10.1016/0006-8993(95)00238-l. [DOI] [PubMed] [Google Scholar]
- 44.Kano T, Abe T, Tomita H, Sakata T, Ishiguro S, Tamai M. Protective effect against ischemia and light damage of iris pigment epithelial cells transfected with the BDNF gene. Invest Ophthalmol Vis Sci. 2002;43(12):3744–53. [PubMed] [Google Scholar]
- 45.Tombran-Tink J. The neuroprotective and angiogenesis inhibitory serpin, PEDF: new insights into phylogeny, function, and signaling. Front Biosci. 2005;10:2131–49. doi: 10.2741/1686. [DOI] [PubMed] [Google Scholar]
- 46.Lopez VM, Decatur CL, Stamer WD, Lynch RM, McKay BS. L-DOPA is an endogenous ligand for OA1. PLoS Biol. 2008;6(9):e236. doi: 10.1371/journal.pbio.0060236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin JM, Wan L, Tsai YY, Lin HJ, Tsai Y, Lee CC, et al. Pigment epithelium-derived factor gene Met72Thr polymorphism is associated with increased risk of wet age-related macular degeneration. Am J Ophthalmol. 2008;145(4):716–21. doi: 10.1016/j.ajo.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Pons M, Marin-Castano ME. Nicotine increases the VEGF/PEDF ratio in retinal pigment epithelium: a possible mechanism for CNV in passive smokers with AMD. Invest Ophthalmol Vis Sci. 2011;52(6):3842–53. doi: 10.1167/iovs.10-6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ni Z, Hui P. Emerging pharmacologic therapies for wet age-related macular degeneration. Ophthalmologica. 2009;223(6):401–10. doi: 10.1159/000228926. [DOI] [PubMed] [Google Scholar]
- 50.Zarbin MA, Rosenfeld PJ. Pathway-based therapies for age-related macular degeneration: an integrated survey of emerging treatment alternatives. Retina. 2010;30(9):1350–67. doi: 10.1097/IAE.0b013e3181f57e30. [DOI] [PubMed] [Google Scholar]
- 51.Truong A, Wong TY, Khachigian LM. Emerging therapeutic approaches in the management of retinal angiogenesis and edema. J Mol Med (Berl) 2011;89(4):343–61. doi: 10.1007/s00109-010-0709-z. [DOI] [PubMed] [Google Scholar]
- 52.Meleth AD, Wong WT, Chew EY. Treatment for atrophic macular degeneration. Curr Opin Ophthalmol. 2011;22(3):190–3. doi: 10.1097/ICU.0b013e32834594b0. [DOI] [PubMed] [Google Scholar]
- 53.Picard E, Houssier M, Bujold K, Sapieha P, Lubell W, Dorfman A, et al. CD36 plays an important role in the clearance of oxLDL and associated age-dependent sub-retinal deposits. Aging (Albany NY) 2010;2(12):981–9. doi: 10.18632/aging.100218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ding JD, Johnson LV, Herrmann R, Farsiu S, Smith SG, Groelle M, et al. Anti-amyloid therapy protects against retinal pigmented epithelium damage and vision loss in a model of age-related macular degeneration. Proc Natl Acad Sci U S A. 2011;108(28):E279–87. doi: 10.1073/pnas.1100901108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang FE, Zhang C, Maminishkis A, Dong L, Zhi C, Li R, et al. MicroRNA-204/211 alters epithelial physiology. FASEB J. 2010;24(5):1552–71. doi: 10.1096/fj.08-125856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beinrohr L, Dobo J, Zavodszky P, Gal P. C1, MBL-MASPs and C1-inhibitor: novel approaches for targeting complement-mediated inflammation. Trends Mol Med. 2008;14(12):511–21. doi: 10.1016/j.molmed.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 57.Rohrer B, Long Q, Coughlin B, Renner B, Huang Y, Kunchithapautham K, et al. A targeted inhibitor of the complement alternative pathway reduces RPE injury and angiogenesis in models of age-related macular degeneration. Adv Exp Med Biol. 2010;703:137–49. doi: 10.1007/978-1-4419-5635-4_10. [DOI] [PubMed] [Google Scholar]
- 58.Cashman SM, Ramo K, Kumar-Singh R. A non membrane-targeted human soluble CD59 attenuates choroidal neovascularization in a model of age related macular degeneration. PLoS One. 2011;6(4):e19078. doi: 10.1371/journal.pone.0019078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goebel J, Stevens E, Forrest K, Roszman TL. Daclizumab (Zenapax) inhibits early interleukin-2 receptor signal transduction events. Transpl Immunol. 2000;8(3):153–9. doi: 10.1016/s0966-3274(00)00021-6. [DOI] [PubMed] [Google Scholar]
- 60.Racke MK, Lovett-Racke AE, Karandikar NJ. The mechanism of action of glatiramer acetate treatment in multiple sclerosis. Neurology. 2010;74 (Suppl 1):S25–30. doi: 10.1212/WNL.0b013e3181c97e39. [DOI] [PubMed] [Google Scholar]
- 61.Stepkowski SM, Chen W, Ross JA, Nagy ZS, Kirken RA. STAT3: an important regulator of multiple cytokine functions. Transplantation. 2008;85(10):1372–7. doi: 10.1097/TP.0b013e3181739d25. [DOI] [PubMed] [Google Scholar]
- 62.Yu HG, Liu X, Kiss S, Connolly E, Gragoudas ES, Michaud NA, et al. Increased choroidal neovascularization following laser induction in mice lacking lysyl oxidase-like 1. Invest Ophthalmol Vis Sci. 2008;49(6):2599–605. doi: 10.1167/iovs.07-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takahashi H, Ishizaki H, Tahara H, Tamaki Y, Yanagi Y. Suppression of choroidal neovascularization by vaccination with epitope peptide derived from human VEGF receptor 2 in an animal model. Invest Ophthalmol Vis Sci. 2008;49(5):2143–7. doi: 10.1167/iovs.07-0523. [DOI] [PubMed] [Google Scholar]
- 64.Wang S, Olson EN. AngiomiRs--key regulators of angiogenesis. Curr Opin Genet Dev. 2009;19(3):205–11. doi: 10.1016/j.gde.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou Q, Gallagher R, Ufret-Vincenty R, Li X, Olson EN, Wang S. Regulation of angiogenesis and choroidal neovascularization by members of microRNA-23~27~24 clusters. Proc Natl Acad Sci U S A. 2011;108(20):8287–92. doi: 10.1073/pnas.1105254108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joe AW, Gregory-Evans K. Mesenchymal stem cells and potential applications in treating ocular disease. Curr Eye Res. 2010;35(11):941–52. doi: 10.3109/02713683.2010.516466. [DOI] [PubMed] [Google Scholar]
- 67.Li R, Maminishkis A, Banzon T, Wan Q, Jalickee S, Chen S, et al. IFN{gamma} regulates retinal pigment epithelial fluid transport. Am J Physiol Cell Physiol. 2009;297(6):C1452–65. doi: 10.1152/ajpcell.00255.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Short BG. Safety evaluation of ocular drug delivery formulations: techniques and practical considerations. Toxicol Pathol. 2008;36(1):49–62. doi: 10.1177/0192623307310955. [DOI] [PubMed] [Google Scholar]
- 69.Hafezi F, Steinbach JP, Marti A, Munz K, Wang ZQ, Wagner EF, et al. The absence of c-fos prevents light-induced apoptotic cell death of photoreceptors in retinal degeneration in vivo. Nat Med. 1997;3(3):346–9. doi: 10.1038/nm0397-346. [DOI] [PubMed] [Google Scholar]
- 70.Chen B, Cepko CL. HDAC4 regulates neuronal survival in normal and diseased retinas. Science. 2009;323(5911):256–9. doi: 10.1126/science.1166226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mo FM, Proia AD, Johnson WH, Cyr D, Lashkari K. Interferon gamma-inducible protein-10 (IP-10) and eotaxin as biomarkers in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51(8):4226–36. doi: 10.1167/iovs.09-3910. [DOI] [PubMed] [Google Scholar]
- 72.Ni J, Yuan X, Gu J, Yue X, Gu X, Nagaraj RH, et al. Plasma protein pentosidine and carboxymethyllysine, biomarkers for age-related macular degeneration. Mol Cell Proteomics. 2009;8(8):1921–33. doi: 10.1074/mcp.M900127-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou X, Wong LL, Karakoti AS, Seal S, McGinnis JF. Nanoceria inhibit the development and promote the regression of pathologic retinal neovascularization in the Vldlr knockout mouse. PLoS One. 2011;6(2):e16733. doi: 10.1371/journal.pone.0016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen J, Patil S, Seal S, McGinnis JF. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat Nanotechnol. 2006;1(2):142–50. doi: 10.1038/nnano.2006.91. [DOI] [PubMed] [Google Scholar]
Patent references
- 101.Case Western Reserve University. PCT/US2010/059426 . 2011
- 102.Neuron Systems, Inc. PCT/US2010/059719 . 2011
- 103.Revision Therapeutics, Inc. App#: 10181656 . 2011
- 104.Tri-Inov. PCT/FR2009/050297 . 2009
- 105.Acuela, Inc. PCT/US2008/011421 . 2009
- 106.The Research Foundation of State University of New York. PCT/US2010/033107 . 2010
- 107.The Trustees of the University of Pennsylvania. PCT/US2007/021211 . 2008
- 108.Universite de Montréal. PCT/CA2009/000200 . 2009
- 109.Euclid Systems Corp. PCT/US2010/058856 . 2011
- 110.Rinat Neuroscience Corp. PCT/IB2008/000486 . 2008
- 111.Sanofi-Aventis. PCT/US2009/036119 . 2009
- 112.The United States of America, DHHS. PCT/US2009/055000 . 2010
- 113.President and Fellows of Harvard College. PCT/US2010/031211 . 2010
- 114.Omeros Corp. PCT/US2010/052954 . 2011
- 115.Alexion Pharmaceuticals, Inc. PCT/US2010/039448 . 2010
- 116.Novartis AG. PCT/EP2010/056129 . 2010
- 117.Genentech, Inc. PCT/US2008/064526 . 2008
- 118.Musc Foundation For Research Development. PCT/US2007/014602 . 2007
- 119.Tufts University. PCT/US2009/000947 . 2009
- 120.The United States of America, DHHS. PCT/US2010/053734 . 2011
- 121.Janssen Biotech Inc. PCT/US2010/032964 . 2010
- 122.Centocor Ortho Biotech Inc. PCT/US2009/059383 . 2010
- 123.University Of Kentucky Research Foundation. PCT/US2009/001106 . 2009
- 124.Yeda Research And Development Co., Ltd. PCT/IL2007/000798 . 2008
- 125.Ak Kimya Ithalat-Ihracat Ve Sanayii Anonim Sirketi. PCT/IB2008/002412 . 2010
- 126.Sanofi. PCT/US2009/068625 . 2010
- 127.Farjo, Rafal, A. PCT/US2008/077510 . 2009
- 128.Arresto Biosciences, Inc. PCT/US2010/023359 . 2010
- 129.Opko Ophthalmics, LLC. PCT/US2010/059090 . 2011
- 130.Reliance Life Sciences, PVT., Ltd. PCT/IN2009/000671 . 2010
- 131.Santen Pharmaceutical Co., Ltd. PCT/US2010/033639 . 2010
- 132.Santen Pharmaceutical Co., Ltd. PCT/JP2010/056498 . 2010
- 133.Macusight, Inc. PCT/US2008/073520 . 2009
- 134.Glaxo Wellcome Manufacturing, PTE., Ltd. PCT/US2010/042211 . 2011
- 135.Genentech, Inc. PCT/US2010/043248 . 2011
- 136.S I F I Societa Ind Farmaceuti. App # 08425559 . 2010
- 137.Fond Telethon. App # 09163654 . 2009
- 138.Farjo, Rafal, A. PCT/US2008/076255 . 2009
- 139.Oncotherapy Science, Inc. PCT/JP2010/003871 . 2010
- 140.Boehringer Ingelheim International GMBH. PCT/EP2010/064695 . 2011
- 141.Regeneron Pharmaceuticals, Inc. PCT/US2007/017546 . 2008
- 142.Genentech, Inc. PCT/US2010/028291 . 2010
- 143.Karlsruher Institut Für Technologie. PCT/EP2010/003523 . 2010
- 144.Children’s Medical Center Corp. PCT/US2010/033724 . 2010
- 145.ClanoTech AB. PCT/EP2008/065596 . 2009
- 146.DCB-USA LLC. PCT/US2010/061738 . 2011
- 147.UCL Business, PLC. PCT/GB2010/001681 . 2011
- 148.Brigham & Womens Hospital. App # 10003357 . 2010
- 149.Oncotx, LLC. PCT/US2009/005744 . 2010
- 150.Chen, Danyang. PCT/US2009/060726 . 2010
- 151.Tactical Therapeutics Inc. PCT/US2007/025041 . 2008
- 152.Daiichi Sankyo Co., Ltd. PCT/JP2008/073504 . 2009
- 153.Velacor Therapeutics, PTY., Ltd. PCT/AU2008/001469 . 2009
- 154.Synthrx, Inc. PCT/US2008/009603 . 2009
- 155.Centenary Institute Of Cancer Medicine And Cell Biology. PCT/AU2010/000698 . 2010
- 156.Angioblast Systems, Inc. PCT/US2009/003902 . 2010
- 157.The Scripps Research Institute. PCT/US2010/000477 . 2010
- 158.The United States of America, DHHS. PCT/US2009/053808 . 2010
- 159.Nicox S.A. PCT/EP2009/058572 . 2010
- 160.Modern Biosciences, PLC. PCT/GB2009/002700 . 2010
- 161.Dmi Acquistion Corp. PCT/US2010/039461. 2010
- 162.Musc Foundation For Research Development. PCT/US2008/067944 . 2008
- 163.Arresto Biosciences, Inc. PCT/US2010/023359 . 2010
- 164.Neurotech USA, Inc. PCT/US2010/051602 . 2011
- 165.Biocompatibles UK, Ltd. PCT/EP2010/002899 . 2010
- 166.Arizona Board Of Regents. PCT/US2009/041021 . 2009
- 167.Hmfra Hungary LLC. PCT/IB2010/003220 . 2011
- 168.University College Cork. PCT/IE2009/000055 . 2010
- 169.University College Cork. PCT/IE2009/000054 . 2010
- 170.University College Cork. PCT/IE2009/000053 . 2010
- 171.President And Fellows Of Harvard College. PCT/US2009/053730 . 2010
- 172.The Regents Of The University Of Michigan. PCT/US2010/041720 . 2011
- 173.Massachusetts Eye And Ear Infirmary. PCT/US2010/033052 . 2010
- 174.Novartis, AG. PCT/US2010/021615 . 2010
- 175.Tufts Medical Center. PCT/US2009/040919 . 2009
- 176.Tufts Medical Center, Inc. PCT/US2010/043964 . 2011
- 177.The Schepens Eye Research Institute, Inc. PCT/US2010/032801 . 2010
- 178.Cleveland Clinic Foundation. PCT/US2010/034398 . 2010
- 179.Surmodics Pharmaceuticals, Inc. PCT/US2010/051068 . 2011
- 180.The Board Of Regents Of The University Of Oklahoma. PCT/US2009/041675 . 2009
- 181.University Of Central Florida Research Foundation, Inc. PCT/US2006/016050 . 2006
- 182.Allergan, Inc. PCT/US2010/059995 . 2011
- 183.Allergan, Inc. PCT/US2009/050373 . 2010
- 184.Allergan, Inc. PCT/US2010/059995 . 2011
- 185.Advanced Cell Technology, Inc. PCT/US2010/057056 . 2011
- 186.Boehringer Ingelheim International, GMBH. PCT/EP2010/064695 . 2011
- 187.Glaxo Group Ltd. PCT/EP2010/057246 . 2010