Abstract
Tsetse flies have a highly regulated and defined microbial fauna made of 3 bacterial symbionts (obligate Wigglesworthia glossinidia, commensal Sodalis glossinidius and parasitic Wolbachia pipientis) in addition to a DNA virus (Glossina pallidipes Salivary gland Hypertrophy Virus, GpSGHV). It has been possible to rear flies in the absence of either Wigglesworthia or in totally aposymbiotic state by dietary supplementation of tsetse’s bloodmeal. In the absence of Wigglesworthia, tsetse females are sterile, and adult progeny are immune compromised. The functional contributions for Sodalist are less known, while Wolbachia cause reproductive manupulations known as Cytoplasmic Incompatibility (CI). High GpSGHV virus titers result in reduced fecundity and lifespan, and have compromised efforts to colonize flies in the insectary for large rearing purposes. Here we investigated the within community effects on the density regulation of the individual microbiome partners in tsetse lines with different symbiotic compositions. We show that absence of Wigglesworthia results in loss of Sodalis in subsequent generations possibly due to nutritional dependancies between the symbiotic partners. While an initial decrease in Wolbachia and GpSGHV levels are also noted in the absence of Wigglesworthia, these infections eventually reach homeostatic levels indicating adaptations to the new host immune environment or nutritional ecology. Absence of all bacterial symbionts also results in an initial reduction of viral titers, which recover in the second generation. Our findings suggest that in addition to the host immune system, interdependencies between symbiotic partners result in a highly tuned density regulation for tsetse’s microbiome.
Keywords: symbiont, virus, intercommunity, tsetse
1. Introduction
Tsetse flies are the sole vectors responsible for cyclical transmission of protozoan trypanosomes that cause human African trypanomiasis (HAT or sleeping sickness) and African animal trypanosomiasis (AAT or nagana). HAT causes devastating effect on humans, while AAT impacts agricultural development and nutritional resources in about 37 countries in sub-Saharan Africa (Welburn et al., 2009). There are no effective vaccines for disease control and drugs available for treatment are expensive and difficult to administer (Aksoy, 2011). Control of the tsetse populations however has had considerable success for disease control. However, disease elimination requires vector control methods that are cheap and effective for sustainability. In addition to transmitting trypanosomes, tsetse harbors several symbiotic microbes. Knowledge on tsetse symbiosis, which is essential for nutrition, fecundity and immunity can lead to novel approaches for vector control (Aksoy, 2000; Aksoy et al., 2008a; Aksoy et al., 2008b; Rio et al., 2004). Tsetse females have an unusual viviparous reproductive biology. Females develop a single oocyte per gonotrophic cycle. The oocyte is ovulated, fertilized and undergoes embryonic development in-utero. The resulting larva hatches and is carried in the intrauterine environment through three larval instars before being deposited. During its intrauterine life, the larva receives all of its nutrients in the form of milk secreted by the female accessory glands, milk glands. Tsetse feed exclusively on vertebrate blood, which is limited in nutrients. To supplement its diet with metabolites missing from its diet, tsetse has established symbiosis with Wigglesworthia glossinidia (called Primary endosymbiont) (Aksoy, 1995). In addition tsetse can carry the facultative commensal Sodalis glossinidius (called Secondary endosymbiont) and parasitic Wolbachia pipientis (Dale and Maudlin, 1999; O'Neill, 1993). While Wolbachia is vertically transmitted trans-ovum, Sodalis and Wigglesworthia are maternally transmitted via mother’s milk (Attardo et al., 2008; Cheng and Aksoy, 1999). Wolbachia symbionts are wide spread intracellular bacteria that have been estimated to infect over 60% of insects (Hilgenboecker et al., 2008). Wolbachia infections have been shown to cause a number of reproductive modifications in their hosts, the most common being cytoplasmic incompatibility (CI) (Saridaki and Bourtzis, 2010; Werren, 1997). CI occurs when a Wolbachia infected male mates with an uninfected female or with a female infected with a different strain of Wolbachia (Serbus et al., 2008; Werren et al., 2008). In tsetse, Wolbachia is localized exclusively within germ-line tissue and induces strong CI (Alam et al., 2011; Cheng et al., 2000). In addition to CI, some Wolbachia infections can benefit host fitness, including nutrition provision, influencing lifespan, and conferring resistance to pathogens (Aleksandrov et al., 2007; Glaser and Meola, 2010; Hosokawa et al., 2010; Kambris et al., 2010; Moreira et al., 2009; Walker et al., 2011). Furthermore, presence of certain Wolbachia strain infections in mosquitoes have been associated with resistance to other pathogen infections, including dengue and plasmodium (Brelsfoard and Dobson, 2011a; Kambris et al., 2010; Walker et al., 2011). The facultative commensal endosymbiont Sodalis has established intra and extra-cellular infections in various diverse tissues in tsetse, including midgut, hemolymph and milk gland (Attardo et al., 2008; Cheng and Aksoy, 1999). The genome sequence of Sodalis has shown that it has reduced coding capacity (around 51%), and contains a large number of fragmented CDSs and pseudogenes, which are apparently non functional in the restricted nutritional ecology of its host. Thus, Sodalis represents an evolutionary intermediate transitioning from a free-living to a mutualistic lifestyle (Toh et al., 2006). The function of Sodalis in tsetse is unclear, but it has been suggested to play a role in vector competence by favoring trypanosome establishment (Dale and Welburn, 2001; Farikou et al., 2010). While all individuals in laboratory lines harbor Sodalis, infection prevalence in natural populations vary from 0% to 85% in the different species analyzed (Lindh and Lehane, 2011; Maudlin et al., 1990). Furthermore presence of multiple Sodalis genotypes have also been described in natural population (Geiger et al., 2005).
All tsetse individuals harbor the obligate mutualist Wigglesworthia, which has coevolved with the tsetse host over 50 million years (Chen et al., 1999). Wigglesworthia resides intracellularly in the midgut bacteriome organ, and extracellularly in mother’s milk secretions (Attardo et al., 2008; Ma and Denlinger, 1974; Pais et al., 2008). Wigglesworthia genome has been drastically reduced to about 700 kb in size and has retained functions presumably necessary for the hosts such as the vitamin coding genes, which are thought to supplement tsetse’s nutritionally restricted blood diet with vitamins (Akman et al., 2002; Rita V.M. Rio, 2012). Furthermore, Wigglesworthia has retained functional flagella and motility coding genes, which are expressed preferentially in the milk gland and early larval developmental indicating that they may be responsible for the transmission of Wigglesworthia from mother to progeny and for the colonization in early intrauterine larva (Rita V.M. Rio, 2012). It has been possible to generate Wigglesworthia free flies by maintaining fertile females on ampicillin supplemented blood diets (Pais et al., 2008). The ampicillin eliminates the extracellular Wigglesworthia population residing in the milk gland, but does not impact the intracellular Wigglesworthia within bacteriocytes. Thus, females continue to reproduce but give rise to progeny, which lack Wigglesworthia and hence which are reproductively sterile. It has also been possible to maintain flies fertile in the absence of all of their endosymbionts by supplementing their antibiotic containing diets with 1% (w/v) yeast extract (Alam et al., 2011). Thus, by supplementing the diet of fertile G. morsitans morsitans (Gmm) females by either ampicillin or tetracycline, lines have been developed that either lack only Wigglesworthia while retaining Sodalis and Wolbachia (GmmWig-) or that lack all symbionts (aposymbiotic, GmmApo). Absence of Wigglesworthia during larval progeny development has been associated with compromised immunity in emerging adults, indicating that Wigglesworthia is essential for immune maturation during development in addition to its nutrient supplementation role in adults (Pais et al., 2008; Weiss et al., 2011).
Finally, in addition to the three bacterial symbionts, laboratory flies and a number of natural populations carry a nuclear rod-shaped enveloped DNA virus (Glossina pallidipes Salivary gland Hypertrophy Virus, GpSGHV), that was first identified by Jaenson as the causative agent of salivary gland hypertrophy (Jaenson, 1978). GpSGHV can be maternally transmitted either through trans ovum or infected milk glands. In laboratory maintained colony, it can also be horizontally transmitted during blood feeding on an in vitro membrane (Abd-Alla et al., 2011). High virus titers result in reduced fecundity and lifespan, and have compromised efforts to colonize flies in the insectary for large rearing purposes (Abd-Alla et al., 2011; Sang et al., 1999).
Environmental bacteria other than the symbiotic partners have been described from G. fuscipes fuscipes in Kenya and G. palpalis palpalis in Angola (Geiger et al., 2009; Lindh and Lehane, 2011), but their relative densities, transmission and prevalence in natural populations remains to be seen. While each symbiont exhibits different levels of integration with host biology and can impact different aspects of host physiology, there may also be within community dynamics that impacts their density regulation. For example, based on genome comparative analysis, it appears that Sodalis and Wigglesworthia may show metabolic complementarity (Belda et al., 2010; Snyder et al., 2010). It appears that Sodalis is unable to complete thiamine biosynthesis pathway but encodes a thiamine transporter. In our earlier studies, we investigated symbiont density dynamics thru development (Rio et al., 2006). These results showed lack of proliferation during juvenile development, followed by a narrow window of opportunity for proliferation in young adults after hatching, but regulated growth in adulthood. We reported highly variable densities for Wolbachia, but in light of the recently discovered chromosomal insertions for the wsp gene used for analysis in G. m. morsitans, these results do not indicate the true cytoplasmic Wolbachia density measurements (Doudoumis, 2011).
In this paper, we examined the microbiome density regulation through development in tsetse. In particular, we measured cytoplasmic Wolbachia density regulation through different host developmental stages and sex. We also used the host lines we developed, GmmWig- that lacks Wigglesworthia but retains Sodalis, Wolbachia and GpSGHV, and GmmApo that lacks all symbionts but retains GpSGHV, to understand the intercommunity dynamics on symbiotic density regulation outcomes.
2. Materials and Methods
2.1 Insects and trypanosomes
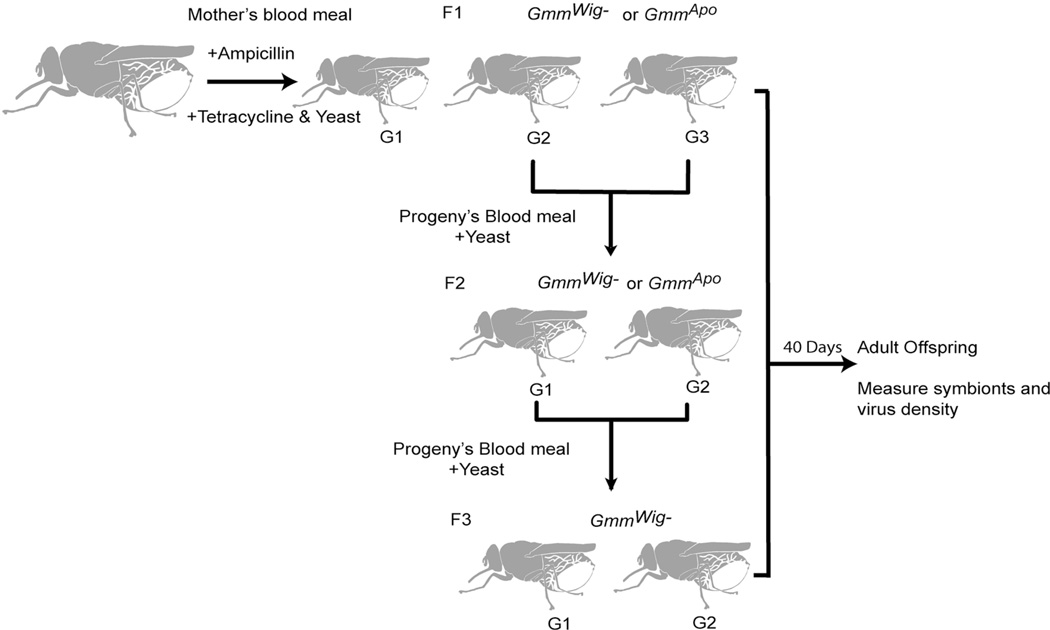
Ampicillin and tetracycline treated Glossina morsitans morsitans females were maintained as described (Alam et al., 2011; Pais et al., 2008). Briefly, females were fed on blood meal supplemented with ampicillin (50 µg/ml) or with tetracycline (20 µg/ml) and yeast extract (1 mg/ml) (BD, Franklin Lakes, NJ). Pupal progeny from the second and third gonotrophic cycles of ampicillin treated mothers were collected and reared to adulthood (denoted as GmmWig--F1). GmmWig--F1. F1 progeny were mated and their progeny (denoted as GmmWig-F2) were obtained. Similarly the progeny of GmmWig--F2 were collected (denoted as GmmWig--F3). Two generations of progeny of tetracycline treated mothers, denoted as GmmApo-F1 and GmmApo-F2 were collected similarly. Schematic protocol for rearing methods is shown in Fig. 1. All progeny received bloodmeals supplemented with yeast extract (10 mg/ml) to keep them fertile in the absence of the obligate Wigglesworthia. Normal teneral flies were fed on same yeast supplemented blood until 40 days old and were used as controls.
Fig. 1. Experimental scheme of sample collections.
Pregnant females were maintained on bloodmeals containing either ampicillin (50 µg/ml) or tetracycline (20 µg/ml) with all supplemented with yeast extract (10 mg/ml). Second and third gonotrophic cycles (G2 and G3) were collected and maintained on bloodmeal supplemented with yeast for 40 days (called F1). Two gonotrophic cycles from F1 were collected and maintained similarly (called F2). Two gonotrophic cycles from F2 were collected and maintained similarly (called F3). All flies were analyzed at 40 days post eclosion.
2.2 Wolbachia density in tsetse
For Wolbachia quantifications, we used primers specific for the heat-shock protein 60, groel, which we have confirmed to be absent from G. m. morsitans chromosomal DNA (Doudoumis, 2011). For measurements, we used same tissue and developmental DNA samples as described (Rio et al., 2006). Briefly, mothers and their four sequential offsprings (1st, 2nd 3rd and 4th deposition) were collected and DNA was extracted using Holmes-bonner method (Holmes and Bonner, 1973). DNA from larva (three stages), early pupae (24–48 hr post deposition) and late pupae (28–30 day post deposition), 1 week, 2 week and 4 week old females and males were similarly extracted.
2.3 Quantification of symbiont and SGHV densities in GmmWig- and GmmApo flies
At least four females of GmmWig- (F1, F2 and F3) and GmmApo (F1 and F2) were collected when adults were 40 days old and surface sterilized by washing twice in 1% bleach and twice in 75% ethanol, respectively. Total DNA was extracted using the Holmes-bonner method (Holmes and Bonner, 1973). Symbionts and GpSGHV were quantified by qPCR using the primers listed in Table 1. All data were normalized to host β-tubulin. All data were expressed as fold change compared to the same samples collected from normal controls (GmmWT). Values are presented as means. Proportional data was Arcsinsqrt transformed prior to statistical analysis and significance was determined using a one-way ANOVA test.
Table. 1.
Primers used for qPCR reactions.
| Primer name | Primer pair sequence (F: Forward, R: Reverse) |
|---|---|
| qGmmtub- | F: 5’ CCATTCCCACGTCTTCACTT 3’ |
| R: 5’ GACCATGACGTGGATCACAG 3’ | |
| qgroel Wolbachia | F: 5’CAGAGGATATCGAAGGTGAA 3' |
| R: 5' CCTGGAGCTTTTACTGCGG 3' | |
| qfliC Sodalis | F: 5' TGGGGACAGTACGATGGCAGAGC 3' |
| R: 5' TCATAGGCGGTCGGGGATAATTGCG 3' | |
| qSGHV | F: 5’ CAAATGATCCGTCGTGGTAGAA 3’ |
| R: 5’ AAGCCGATTATGTCATGGAAG 3’ |
3. Results
3.1 Maternal transmission of Wolbachia
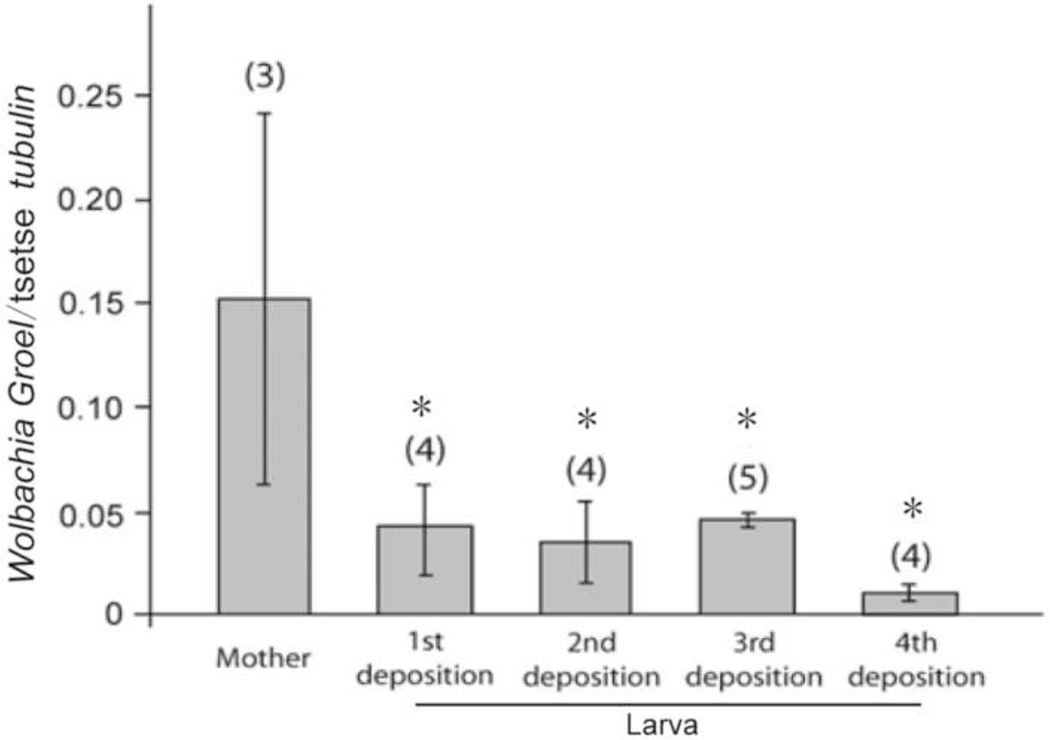
To investigate the transmission density dynamics of the three symbionts from mother to offspring, mothers and female offspring of their four depositions were collected and measured. Our prior data had indicated that there was no significant variation in the number of symbionts (Sodalis and Wigglesworthia) acquired from mother to her sequential progeny (Rio et al., 2006). Here we analyzed the same samples for cytoplasmic Wolbachia density variations using primers that do not amplify from the chromosomal insertions. Our findings show that each of the sequential progeny of the mothers acquired Wolbachia from the mother and there were no significant variations in the number of symbionts acquired between the different sequential larval progeny analyzed immediately upon deposition (Fig. 2). Significant differences in comparisons of the Wolbachia density of mothers and her offspring was observed (ANOVA; F1,14 = 5.0, P =0.01) (Fig. 2). The mothers were shown to have a significantly greater Wolbachia density than their offspring (Fig. 2), however there was high variability in the number of Wolbachia present in the mothers analyzed. Our data indicate perfect maternal inheritance of Wolbachia infection through trans-ovum transmission, and a high variability in Wolbachia densities in adult females.
Fig. 2. Wolbachia density from mothers and their 1st, 2nd, 3rd and 4th larval offsprings.
Error bars represent the standard error of the mean. Numbers in parentheses represent the sample size (n). Asterisks represent statistically significant data for the larva in comparison to the mothers according to Tukey-Kramer post-hoc comparisons (P<0.05).
3.2 Wolbachia density in immature stages
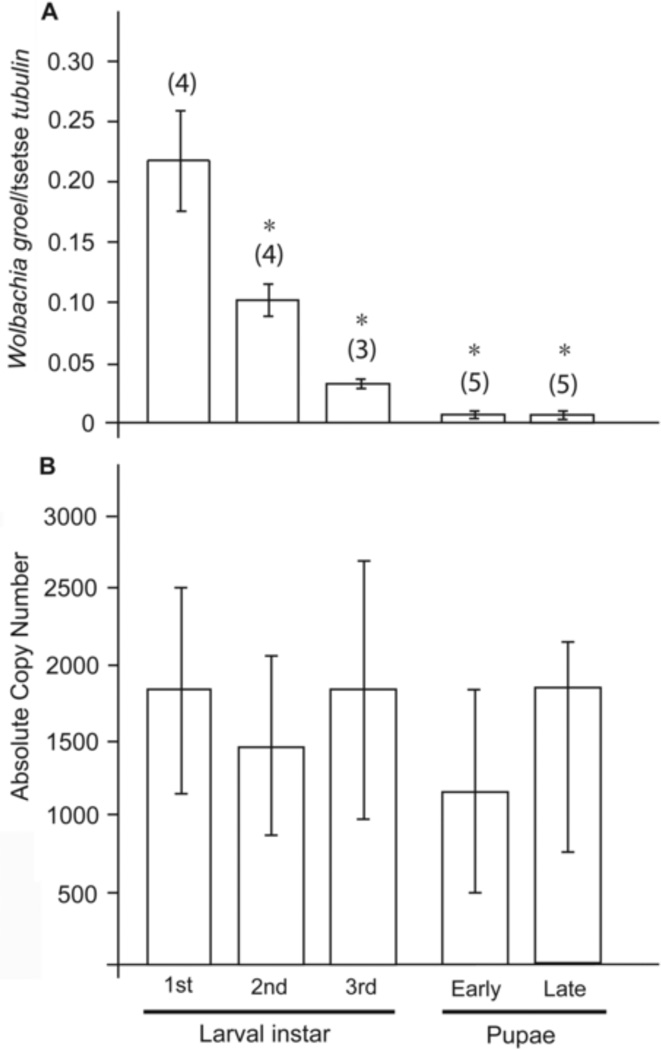
We next examined the proliferation status of Wolbachia during the three larval stages in utero obtained by microscopic dissections and during early and late pupal stages obtained post parturition. We present our data both as absolute Wolbachia numbers and as normalized to host β-tubulin levels to better gain information on symbiotic proliferation. The presence of Wolbachia was detected in the 1st larval state and its relative density during larval growth changed significantly when comparing the larval stages through the pupa stages (ANOVA, F4,16 = 5.4, P = 0.006) when normalized by the tsetse β-tubulin gene (Fig. 3A). Wolbachia density did not increase during pupal growth either (the early and late pupal stages) (Fig. 3A). However, no difference in the absolute copy number of Wolbachia was observed through development suggesting that Wolbachia does not proliferate significantly during juvenile development in tsetse (ANOVA, F4,16 = 0.16, P =0.95) (Fig 3B). This proliferation is different from the other two symbionts Sodalis and Wigglesworthia, which proliferate in parallel with host cell growth during immature stages (Rio et al., 2006).
Fig. 3. Wolbachia density through juvenile development.
(A) Wolbachia density normalized to host β-tubulin levels, (B) absolute copy number of Wolbachia present in the different developmental stages analyzed. Error bars represent the standard error of the mean. Numbers in parentheses represent the sample size (n). Asterisks above bars represent statistically significant data according to Tukey-Kramer post-hoc comparisons (P<0.05).
3.3 Wolbachia density in adults
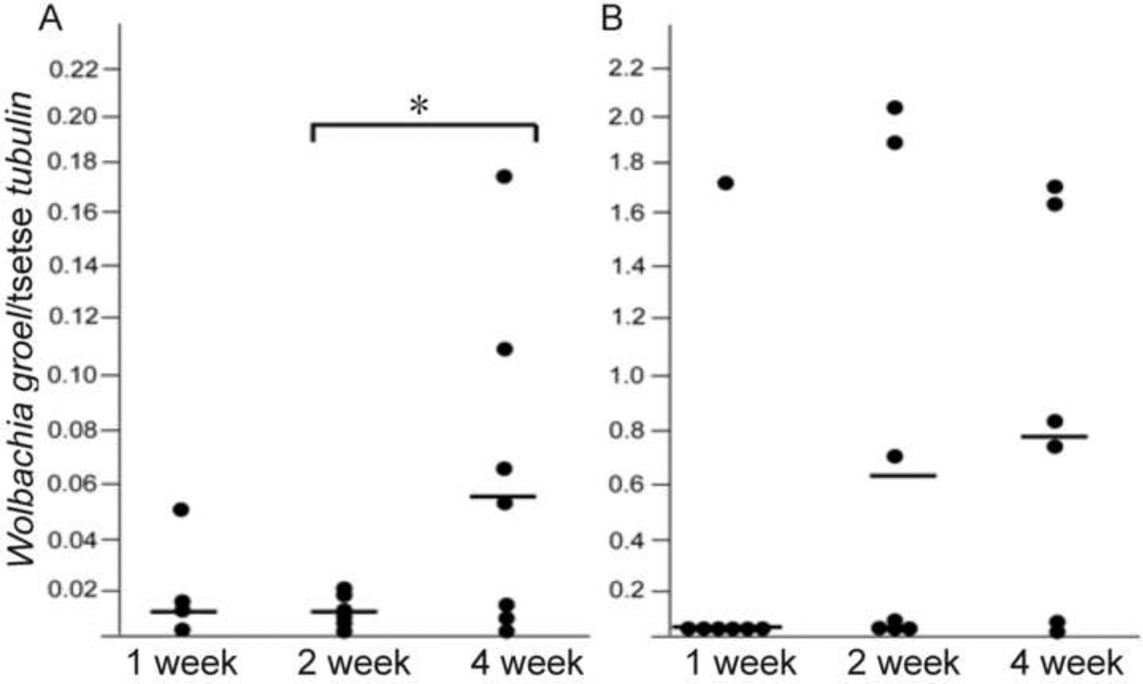
We next measured the Wolbachia density from adult male and female flies at 1, 2 and 4 weeks post eclosion. Wolbachia infection density was significantly higher in males than in females and increased with age in females (ANOVA, F2,17 = 3.80, P = 0.04) but not in males (ANOVA, F2,19 = 1.35, P = 0.28) (Fig. 4A and B, respectively). However, in older females (4 weeks old) and in males (2 and 4 weeks old), the relative Wolbachia density varied significantly (Fig. 4B), suggesting that in some individuals Wolbachia infections may proliferate without tight host regulation in the adulthood, unlike what we observed during juvenile development. It appears that particularly males carry higher loads of Wolbachia and are subject to varying proliferations in the adulthood.
Fig. 4. Sex and age specific Wolbachia density.
(A) females and (B) males analyzed at different adult ages post eclosion. Lines intersecting data points represent the median. *, P<0.05, Tukey-Kramer post-hoc comparisons.
3.4 Dynamics of symbionts density in GmmWig- flies
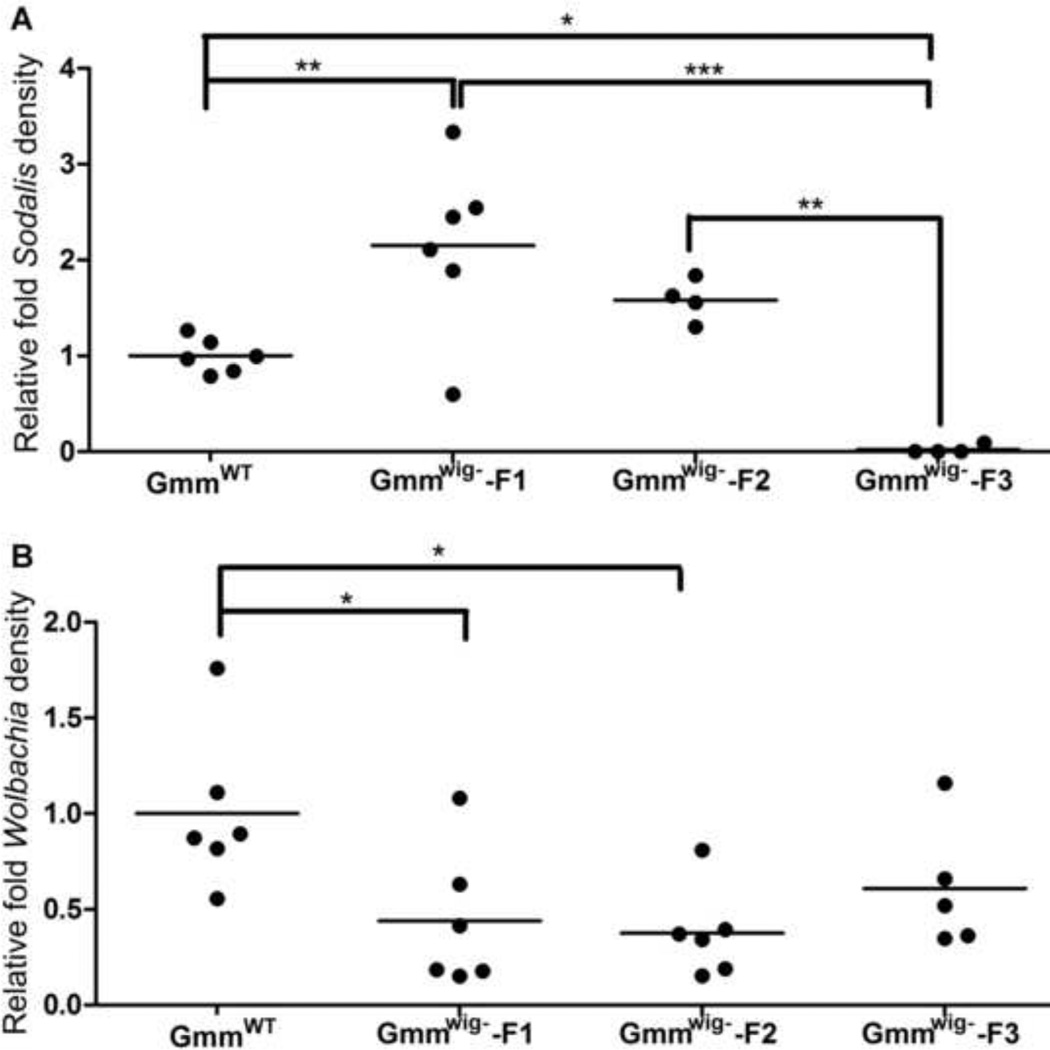
Development of Wigglesworthia free flies (GmmWig-) allowed us to study the intercommunity dynamics for symbiont density regulation in the host. We investigated density regulation of Sodalis and Wolbachia in GmmWig- from the three generations of 40 day old GmmWig-. When normalized against the levels detected in the corresponding control GmmWT flies that retained Wigglesworthia, Sodalis numbers in the first generation (GmmWig--F1) increased initially, returned to similar levels as the GmmWT controls by the second generation (GmmWig--F2), but significantly declined in the third generation (GmmWig--F3) (Fig. 5A). It is likely that there is a complementary relationship between Sodalis and Wigglesworthia given the restricted nutritional ecology they share in the tsetse midgut. At least one product, thiamine has been shown to be limiting for Sodalis growth as only the thiamine transporter is present in its genome (Toh et al., 2006). Given that Wigglesworthia can synthesize thiamine, it is possible that Sodalis may benefit and exploit Wigglesworthia metabolites for its growth alone (Belda et al., 2010; Snyder et al., 2010). Without Wigglesworthia, Sodalis levels may decline in parallel with the decreasing level of nutrition. Interestingly, Wolbachia density analyzed from the same samples showed a decrease in the first two generations (F1 and F2), but reached a stable level in the F3 revealing that mutual beneficial relationships may also exist between Wolbachia and Wigglesworthia (Fig. 5B).
Fig. 5. Symbiont density in female GmmWig-.
(A) Sodalis and (B) Wolbachia density were measured by qPCR (n≥4). Symbiont density is expressed as relative fold difference calculated as the density measured from GmmWig- state normalized to that measured from the corresponding normal state (GmmWt) *, P9003C;0.05; **, P<0.001, ***, P<0.0001.
3.5 Dynamics of GpSGHV in GmmWig- and GmmApo flies
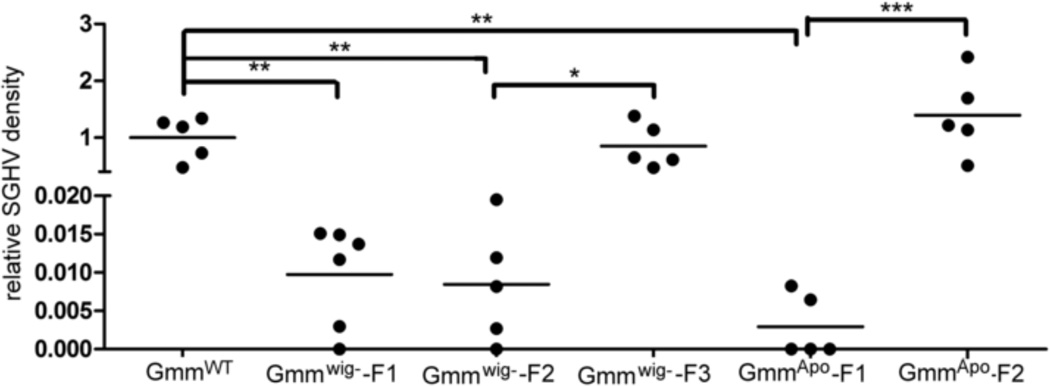
We found that the prevalence for GpSGHV infection in our laboratory tsetse colony is 100% based on PCR assay. We do not however observe any salivary gland hypertrophy phenotype in the colony flies (data not shown). To further investigate the interaction of this virus with the symbiotic fauna of the host, GpSGHV levels were examined in GmmWig- and GmmApo flies. GpSGHV load decreased significantly in the absence of Wigglesworthia by over 100 fold in the first two generations (GmmWig--F1 and GmmWig--F2), but reached normal density levels in the third generation flies (GmmWig--F3) (Fig. 6). In symbiont-free GmmApo flies, we saw the same trend, GpSGHV level was reduced in the first generation (GmmApo-F1) by over 500 fold, but increased significantly in the second (GmmApo-F2) reaching normal levels. These data suggest that tsetse’s microbiome can influence GpSGHV densities either directly or indirectly and that in the absence of the microbiome, proliferation of the virus also suffers. It remains to be seen if the higher levels of GpSGHV we noted in the aposymbiotic flies results in atrophy of salivary glands or in sterility in the males.
Fig. 6. GpSGHV density in GmmWig- and GmmApo flies.
GpSGHV was quantified in GmmWig- and GmmApo and GmmWT. Relative virus density was determined as density measured in Gmmwig- or GmmApo normalized to levels found in the corresponding control GmmWt flies (n≥5). *, P<0.05; **, P<0.001, ***, P<0.0001.
4. Discussion
All multicellular eukaryotes live in symbiotic associations with microorganisms, which can form complex communities referred to as the microbiome. While many studies have looked at the role of the individual microorganism and the one host-one microbe dynamics, host physiology and the dynamics of the individual infections can be affected and shaped by community interdependencies. In contrast to higher eukaryotes, which are colonized by hundreds to thousands of prokaryotic phylotypes representing members of five of the six kingdoms of life, insects harbor a significantly less diverse community of microbial symbionts. Our studies here describe Glossina (tsetse fly) that houses a vertically transmitted microbiome composed of two enteric symbionts (genus Sodalis and Wigglesworthia) residing in the gut, one parasitic bacterium genus Wolbachia) present in the gonadal tissues in addition to a maternally transmitted DNA virus (GpSGHV). We used this simple symbiosis to understand symbiotic density regulation and within community effects on the individual symbiont fitness traits (density regulation and vertical transmission efficiency). Our results show that there is synergy between Sodalis and Wigglesworthia such that fly lines that lack Wigglesworthia eventually loose Sodalis infections by the third generation. This was in contrast to Wolbachia infections that showed wide variability in different individuals as a function of age, and that continued to maintain infections at homeostatic levels in the absence of Wigglesworthia. The GpSGHV levels measured without symbionts showed significant initial fluctuations only to reach again homeostatic levels by the third generation. Our results suggest interdependencies between the symbiotic partners. However, lack of extensive proliferation by any one partner in the absence of another reflects the association of a tightly regulated microbiome in tsetse.
The symbiotic fauna with the exception of Wolbachia are all maternal transmitted to tsetse’s intrauterine larva in mother’s milk secretions. Wolbachia is acquired by the progeny vertically trans-ovum. Sodalis and Wigglesworthia proliferate in sink with host development during immature developmental stages (larva and pupae). In young adults post eclosion however, both symbiotic densities increase for several days before being regulated by the host (Rio et al., 2006). It is interesting to note however, that in females but not in males, Wigglesworthia continues to proliferate for several weeks, possibly reflecting its essential role in female fecundity (Rio et al., 2006). Wolbachia is transmitted to offspring trans-ovum through germ-line infections (Alam et al., 2011). Our prior studies on Wolbachia dynamics had used primers that amplified Wolbachia fragments incorporated into tsetse’s chromosomal DNA. Reanalysis of the spatial and temporal distribution of Wolbachia using primers specific for the cytoplasmic Wolbachia show that Wolbachia is localized in tsetse’s reproductive tissues only (Doudoumis, 2011). Our data also show that there are no significant differences in the levels of Wolbachia acquired by the sequential progeny from their mother when analyzed in larva immediately post deposition (Fig. 2) and there is no proliferation during juvenile growth stages (Fig. 3). In the adult stages however, males have at least ten fold higher Wolbachia density than females and Wolbachia densities begin to increase around 2-weeks post eclosion (Fig. 4). Interestingly, older adults of both sexes show wide variability in Wolbachia levels. Results are similar to wAlbA Wolbachia strain infecting the mosquito Aedes albopictus, with a higher density observed in older males and wide variability in Wolbachia level (Tortosa et al., 2010). However, unlike tsetse a rapid decay of infection in older males only was observed (Tortosa et al., 2010). In contrast the Wolbachia infections in the mosquito Culex pipiens was observed to increase with age, and was dependent upon strain type (Duron et al., 2007).
Our data from colony flies need to be validated in field populations as nutritional ecology and environmental parameters (temperature and humidity) in the field are likely to be different than the optimal conditions maintained in the insectary. Given that Wolbachia densities may affect the levels of CI expression, it remains to be seen if the increase in Wolbachia we noted in males as a function of age may contribute significantly to CI in tsetse (Brelsfoard and Dobson, 2011b; Ikeda et al., 2003; Noda et al., 2001). However, male age has not been shown to influence CI rates in Aedes and Culex mosquitoes (Brelsfoard and Dobson, 2011b; Duron et al., 2007; Noda et al., 2001) and in two planthopper species (Noda et al., 2001). Albeit there is no clear trend on the effects of Wolbachia density and the age of the insect host, it seems Wolbachia density may be host specific and influenced by environmental, nutritional and rearing conditions (Mouton et al., 2003; Mouton et al., 2006; Mouton et al., 2007; Wiwatanaratanabutr and Kittayapong, 2009).
In addition to individual host-symbiont interactions, interactions between the community of organisms have been reported, ranging from beneficial to antagonistic in nature (Oliver et al., 2010). Metabolic interdependence between Wigglesworthia and Sodalis is predicted based on their coding capacity and an in vitro empirical study (Belda et al., 2010; Snyder et al., 2010). Apparently, Sodalis may compensate Wigglesworthia for folate and Coenzyme A synthesis, while Wigglesworthia provisions thiamine to Sodalis. The reduction of Sodalis levels in GmmWig- flies by the third generation further confirms that Sodalis fitness depends on the presence of Wigglesworthia (Fig. 5A). The decrease in Wolbachia density in the absence of Wigglesworthia may also suggest co-adaptation or nutritional dependence. Thus, nutritional dependencies within the community can shape density regulation and transmission dynamics of the individual partners.
It is also possible that host immune responses in the absence of Wigglesworthia may be detrimental for commensal and parasitic microbes, including GpSGHV. Our studies have shown that to ensure optimal fecundity benefits, tsetse overexpresses an immune effector molecule peptidoglycan recognition protein (PGRP)-LB, which functions to eliminate microbe released immune elicitors such as PGN (Wang et al., 2009). PGRP-LB is also maternally transmitted to tsetse’s intrauterine larva where it apparently functions again to as a negative regulator of the host immune system. In the absence of Wigglesworthia, there is less PGRP-LB, which in turn results in the upregulation of the host immune system. Although, studies have shown that Sodalis is resistant to tsetse’s antimicrobial peptides, the effect of AMPs on Wolbachia is unknown. There may also be other effectors that may be upregulated in the absence of Wigglesworthia that may in turn damage the other symbionts. Future studies that investigate the host immune status in the absence of Wigglesworthia in larva and adult stages may shed some light on the role of the host immune system in community density regulation.
Tsetse GpSGHV at high densities can be pathogenic and adversely affect reproduction by suppressing vitellogenesis, causing testicular aberrations, and/or disrupting mating behavior (Lietze et al., 2011). It’s interesting that despite 100% prevalence in our laboratory reared flies, no adverse effects are seen on fecundity possibly due to virus density that may be maintained below a threshold. It has been reported that lipopolysaccharide of commensals may reduce host antiviral response which in this case may help virus to transfer to offspring (Kane et al., 2011). In aphids, beneficial relationship among host, commensal microbes and virus is also observed. The aphid symbiont provides habitat for a virus, which in turn synthesizes a toxin against wasps’ larva to protect the aphid host (Roossinck, 2011). Declining pattern of GpSGHV levels in GmmWig- -F1, –F2 and GmmApo –F1 may also suggest a beneficial community dynamics for virus maintenance. Recovery of virus titers to normal level in GmmWig--F3 and GmmApo-F2 however suggests either adaption of the virus in the maternal transmission process so that higher densities are transferred to progeny, or modifications in the host immune system leading to relaxed density regulation, or viral adaptations leading to evasion of the host immune regulation. While direct effects of antibiotics on viral replication have not been noted, GpSGHV levels may be adversely affected by unknown epigenetic effects antibiotic treatment may have had on host physiology. It remains to be seen whether viral pathogenesis would be observed in subsequent generations.
Results from the tsetse system presents a well-tuned intercommunity dynamics for microbes that display varying levels of integration in host biology. Perturbations to this microbial ecology result in adverse effects on the density dynamics in the different partners of the microbiome. The eventual recovery of Wolbachia and GpSGHV densities in the absence of Wigglesworthia is in line with their anticipated roles in host biology, parasitic and pathogenic respectively. This is in contrast to Sodalis’s commensal relationship with its tsetse host. The extensive genomic reduction noted in Sodalis coding capacity already indicates interdependencies on tsetse’s nutritional ecology, which may suffer in the absence of Wigglesworthia. Interestingly perturbations to the microbiome did not result in long-term unregulated proliferation of its individual members, implying strong density regulation effects likely imposed by the host immune system or by community interdependencies resulting from the highly restricted nutritional ecology of the tsetse host.
Research Highlight.
Three symbionts and a DNA virus reside in different tsetse tissues.
Availability of GmmWig- and GmmApo enables analysis of intercommunity dependencies.
The symbiont densities are tightly regulated through host development.
There is cooperative interaction between Wigglesworthia and Sodalis
DNA virus is commensal without displaying pathogenesis in the absence of symbionts.
Acknowledgements
This work was generously funded by grants to S.A. from the NIAID AI051584, AI068932, Li Foundation and Ambrose Monell Foundation. We especially thank Geoffrey Attardo for editorial assistance. This work received support from the IAEA/FAO Coordinated Research Program “Improving SIT for tsetse flies through research on their symbionts and pathogens”.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
J.W. None
C.B None
Y. W. None
S. A. None
References
- Abd-Alla AM, et al. Tsetse salivary gland hypertrophy virus: hope or hindrance for tsetse control? PLoS Negl Trop Dis. 2011;5:e1220. doi: 10.1371/journal.pntd.0001220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akman L, et al. Genome sequence of the endocellular obligate symbiont of tsetse, Wigglesworthia glossinidia. Nat. Genet. 2002;32:402–407. doi: 10.1038/ng986. [DOI] [PubMed] [Google Scholar]
- Aksoy S. Wigglesworthia gen. nov. and Wigglesworthia glossinidia sp. nov., taxa consisting of the mycetocyte-associated, primary endosymbionts of tsetse flies. Int J Syst Bacteriol. 1995;45:848–851. doi: 10.1099/00207713-45-4-848. [DOI] [PubMed] [Google Scholar]
- Aksoy S. Tsetse - A haven for microorganisms. Parasitiology Today. 2000;16:114–118. doi: 10.1016/s0169-4758(99)01606-3. [DOI] [PubMed] [Google Scholar]
- Aksoy S. Sleeping sickness elimination in sight: time to celebrate and reflect, but not relax. PLoS Negl Trop Dis. 2011;5:e1008. doi: 10.1371/journal.pntd.0001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy S, et al. Paratransgenesis applied for control of tsetse transmitted sleeping sickness. Transgenesis and the Management of Vector-Borne Disease. 2008a:35–48. doi: 10.1007/978-0-387-78225-6_3. [DOI] [PubMed] [Google Scholar]
- Aksoy S, et al. Paratransgenesis applied for control of tsetse transmitted sleeping sickness. Advanced Experimental Medical Biology. 2008b;627:35–48. doi: 10.1007/978-0-387-78225-6_3. [DOI] [PubMed] [Google Scholar]
- Alam U, et al. Wolbachia symbiont infections induce strong cytoplasmic incompatibility in the tsetse fly Glossina morsitans. PLoS Pathogens. 2011;7:e1002415. doi: 10.1371/journal.ppat.1002415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksandrov ID, et al. Removing endosymbiotic Wolbachia specifically decreases lifespan of females and competitiveness in a laboratory strain of Drosophila melanogaster. Genetika. 2007;43:1372–1378. [PubMed] [Google Scholar]
- Attardo GM, et al. Analysis of milk gland structure and function in Glossina morsitans: milk protein production, symbiont populations and fecundity. J Insect Physiol. 2008;54:1236–1242. doi: 10.1016/j.jinsphys.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belda E, et al. Mobile genetic element proliferation and gene inactivation impact over the genome structure and metabolic capabilities of Sodalis glossinidius, the secondary endosymbiont of tsetse flies. BMC Genomics. 2010;11:449. doi: 10.1186/1471-2164-11-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelsfoard CL, Dobson SL. An update on the utility of Wolbachia for controlling insect vectors and disease transmission. Asia Pacific Journal of Molecular Biology and Biotechnology. 2011a;19:85–92. [Google Scholar]
- Brelsfoard CL, Dobson SL. Wolbachia effects on host fitness and the influence of male aging on cytoplasmic incompatibility in Aedes polynesiensis (Diptera: Culicidae) Journal of Medical Entomology. 2011b;48:1008–1015. doi: 10.1603/me10202. [DOI] [PubMed] [Google Scholar]
- Chen X, et al. Concordant evolution of a symbiont with its host insect species: molecular phylogeny of genus Glossina and its bacteriome-associated endosymbiont, Wigglesworthia glossinidia. J Mol Evol. 1999;48:49–58. doi: 10.1007/pl00006444. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Aksoy S. Tissue tropism, transmission and expression of foreign genes in vivo in midgut symbionts of tsetse flies. Insect Molecular Biology. 1999;8:125–132. doi: 10.1046/j.1365-2583.1999.810125.x. [DOI] [PubMed] [Google Scholar]
- Cheng Q, et al. Tissue distribution and prevalence of Wolbachia infections in tsetse flies, Glossina spp. Med Vet Entomol. 2000;14:44–50. doi: 10.1046/j.1365-2915.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- Dale C, Maudlin I. Sodalis gen. nov. and Sodalis glossinidius sp. nov., a microaerophilic secondary endosymbiont of the tsetse fly Glossina morsitans morsitans. Int J Syst Bacteriol. 1999;49 Pt 1:267–275. doi: 10.1099/00207713-49-1-267. [DOI] [PubMed] [Google Scholar]
- Dale C, Welburn SC. The endosymbionts of tsetse flies: manipulating host-parasite interactions. Int J Parasitol. 2001;31:628–631. doi: 10.1016/s0020-7519(01)00151-5. [DOI] [PubMed] [Google Scholar]
- Doudoumis V, Tsiamis G, Wamwiri F, Brelsfoard C, Alam U, Aksoy E, Dalaperas S, Abd-Alla A, Ouma J, Takac P, Aksoy S, Bourtzis K. Detection and characterization of Wolbachia infections in laboratory and natural populations of different species of tsetse flies (genus Glossina) BMC Microbiology. 2011 doi: 10.1186/1471-2180-12-S1-S3. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duron O, et al. Influence of aging on cytoplasmic incompatibility, sperm modification and Wolbachia density in Culex pipiens mosquitoes. Heredity. 2007;98:368–374. doi: 10.1038/sj.hdy.6800948. [DOI] [PubMed] [Google Scholar]
- Farikou O, et al. Tripartite interactions between tsetse flies, Sodalis glossinidius and trypanosomes--an epidemiological approach in two historical human African trypanosomiasis foci in Cameroon. Infect Genet Evol. 2010;10:115–121. doi: 10.1016/j.meegid.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Geiger A, et al. Two Tsetse fly species, Glossina palpalis gambiensis and Glossina morsitans morsitans, carry genetically distinct populations of the secondary symbiont Sodalis glossinidius. Appl Environ Microbiol. 2005;71:8941–8943. doi: 10.1128/AEM.71.12.8941-8943.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger A, et al. First isolation of Enterobacter, Enterococcus, and Acinetobacter spp. as inhabitants of the tsetse fly (Glossina palpalis palpalis) midgut. Infect Genet Evol. 2009;9:1364–1370. doi: 10.1016/j.meegid.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Glaser RL, Meola MA. The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS ONE. 2010;5:e11977. doi: 10.1371/journal.pone.0011977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgenboecker K, et al. How many species are infected with Wolbachia? - a statistical analysis of current data. Fems Microbiology Letters. 2008;281:215–220. doi: 10.1111/j.1574-6968.2008.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes DS, Bonner J. Preparation, molecular weight, base composition and secondary structure of giant nuclear ribonucleic acid. Biochemistry. 1973;12:2330–2338. doi: 10.1021/bi00736a023. [DOI] [PubMed] [Google Scholar]
- Hosokawa T, et al. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc Natl Acad Sci U S A. 2010;107:769–774. doi: 10.1073/pnas.0911476107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T, et al. Infection density of Wolbachia and level of cytoplasmic incompatibility in the Mediterranean flour moth, Ephestia kuehniella. J Invertebr Pathol. 2003;84:1–5. doi: 10.1016/s0022-2011(03)00106-x. [DOI] [PubMed] [Google Scholar]
- Jaenson TG. Virus-like rods associated with salivary gland hyperplasia in tsetse, Glossina pallidipes. Trans R Soc Trop Med Hyg. 1978;72:234–238. doi: 10.1016/0035-9203(78)90200-6. [DOI] [PubMed] [Google Scholar]
- Kambris Z, et al. Wolbachia stimulates immune gene expression and inhibits plasmodium development in Anopheles gambiae. PLoS Pathog. 2010;6:e1001143. doi: 10.1371/journal.ppat.1001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane M, et al. Successful transmission of a retrovirus depends on the commensal microbiota. Science. 2011;334:245–249. doi: 10.1126/science.1210718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lietze VU, et al. Salivary gland hypertrophy viruses: a novel group of insect pathogenic viruses. Annu Rev Entomol. 2011;56:63–80. doi: 10.1146/annurev-ento-120709-144841. [DOI] [PubMed] [Google Scholar]
- Lindh JM, Lehane MJ. The tsetse fly Glossina fuscipes fuscipes (Diptera: Glossina) harbours a surprising diversity of bacteria other than symbionts. Antonie Van Leeuwenhoek. 2011;99:711–720. doi: 10.1007/s10482-010-9546-x. [DOI] [PubMed] [Google Scholar]
- Ma WC, Denlinger dL. Secretory discharge and microflora of milk gland in tsetse flies. Nature. 1974;247:301–303. [Google Scholar]
- Maudlin I, et al. The relationship between rickettsia-like-organisms and trypanosome infections in natural populations of tsetse in Liberia. Tropical Medicine and Parasitology. 1990;41:265–267. [PubMed] [Google Scholar]
- Moreira LA, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- Mouton L, et al. Strain-specific regulation of intracellular Wolbachia density in multiply infected insects. Mol Ecol. 2003;12:3459–3465. doi: 10.1046/j.1365-294x.2003.02015.x. [DOI] [PubMed] [Google Scholar]
- Mouton L, et al. Effect of temperature on Wolbachia density and impact on cytoplasmic incompatibility. Parasitology. 2006;132:49–56. doi: 10.1017/S0031182005008723. [DOI] [PubMed] [Google Scholar]
- Mouton L, et al. Interaction between host genotype and environmental conditions affects bacterial density in Wolbachia symbiosis. Biol Lett. 2007;3:210–213. doi: 10.1098/rsbl.2006.0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda H, et al. Infection density of Wolbachia and incompatibility level in two planthopper species, Laodelphax striatellus and Sogatella furcifera. Insec. Biochem. Mol. Bio. 2001;31:727–737. doi: 10.1016/s0965-1748(00)00180-6. [DOI] [PubMed] [Google Scholar]
- O'Neill SL, Gooding RH, Aksoy S. Phylogenetically distant symbiotic microorganisms reside in Glossina midgut and ovary tissues. Medical and Veterinary Entomology. 1993;7:377–383. doi: 10.1111/j.1365-2915.1993.tb00709.x. [DOI] [PubMed] [Google Scholar]
- Oliver KM, et al. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol. 2010;55:247–266. doi: 10.1146/annurev-ento-112408-085305. [DOI] [PubMed] [Google Scholar]
- Pais RR, et al. The obligate Mutualist Wigglesworthia glossinidia Influences Reproduction, Digestion, and Immunity Processes of Its Host, the Tsetse Fly. Applied and Environmental Microbiology. 2008:74. doi: 10.1128/AEM.00741-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio RV, et al. Strategies of the home-team: symbioses exploited for vector-borne disease control. TRENDS in Microbiology. 2004;12:325–336. doi: 10.1016/j.tim.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Rio RV, et al. Dynamics of multiple symbiont density regulation during host development: tsetse fly and its microbial flora. Proc Biol Sci. 2006;273:805–814. doi: 10.1098/rspb.2005.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio Rita VM, RES, Wang Jingwen, Lohs Claudia, Wu Yi-neng, Snyder Anna K, Bjornson Robert D, Oshima Kenshiro, Biehl Bryan S, Perna Nicole T, Hattori Masahira, Aksoy Serap. Insight into transmission biology and species-specific functional capabilities of tsetse (Diptera Glossinidae) obligate symbiont Wigglesworthia. mBio. 2012 doi: 10.1128/mBio.00240-11. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roossinck MJ. The good viruses: viral mutualistic symbioses. Nat Rev Microbiol. 2011;9:99–108. doi: 10.1038/nrmicro2491. [DOI] [PubMed] [Google Scholar]
- Sang RC, et al. The effects of a tsetse DNA virus infection on the functions of the male accessory reproductive gland in the host fly Glossina morsitans centralis (Diptera; Glossinidae) Curr Microbiol. 1999;38:349–354. doi: 10.1007/pl00006815. [DOI] [PubMed] [Google Scholar]
- Saridaki A, Bourtzis K. Wolbachia: more than just a bug in insects genitals. Curr Opin Microbiol. 2010;13:67–72. doi: 10.1016/j.mib.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Serbus LR, et al. The genetics and cell biology of Wolbachia-host interactions. Annu Rev Genet. 2008;42:683–707. doi: 10.1146/annurev.genet.41.110306.130354. [DOI] [PubMed] [Google Scholar]
- Snyder AK, et al. Nutrient provisioning facilitates homeostasis between tsetse fly (Diptera: Glossinidae) symbionts. Proc Biol Sci. 2010;277:2389–2397. doi: 10.1098/rspb.2010.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh H, et al. Massive genome erosion and functional adaptations provide insights into the symbiotic lifestyle of Sodalis glossinidius in the tsetse host. Genome Res. 2006;16:149–156. doi: 10.1101/gr.4106106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortosa P, et al. Wolbachia age-sex-specific density in Aedes albopictus: a host evolutionary response to cytoplasmic incompatibility? PLoS One. 2010;5:e9700. doi: 10.1371/journal.pone.0009700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476:450–453. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- Wang J, Wu Y, Yang G, Aksoy s. Interactions between mutualist Wigglesworthia and tsetse peptidoglycan recognition protein (PGRP-LB) influence trypanosome transmission. Proc Natl Acad Sci U S A. 2009;106:12133–12138. doi: 10.1073/pnas.0901226106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss BL, et al. Tsetse immune system maturation requires the presence of obligate symbionts in larvae. PLoS Biol. 2011;9:e1000619. doi: 10.1371/journal.pbio.1000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welburn SC, et al. Controlling sleeping sickness - a review. Parasitology. 2009;136:1943–1949. doi: 10.1017/S0031182009006416. [DOI] [PubMed] [Google Scholar]
- Werren JH. Biology of Wolbachia. Annual Review of Entomology. 1997;42:587–609. doi: 10.1146/annurev.ento.42.1.587. [DOI] [PubMed] [Google Scholar]
- Werren JH, et al. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- Wiwatanaratanabutr I, Kittayapong P. Effects of crowding and temperature on Wolbachia infection density among life cycle stages of Aedes albopictus. J Invertebr Pathol. 2009;102:220–224. doi: 10.1016/j.jip.2009.08.009. [DOI] [PubMed] [Google Scholar]