Abstract
BACKGROUND
The treatment of relapsed chronic lymphocytic leukemia (CLL) has resulted in few durable remissions. Bruton's tyrosine kinase (BTK), an essential component of B-cell–receptor signaling, mediates interactions with the tumor microenvironment and promotes the survival and proliferation of CLL cells.
METHODS
We conducted a phase 1b–2 multicenter study to assess the safety, efficacy, pharmacokinetics, and pharmacodynamics of ibrutinib (PCI-32765), a first-in-class, oral covalent inhibitor of BTK designed for treatment of B-cell cancers, in patients with relapsed or refractory CLL or small lymphocytic lymphoma. A total of 85 patients, the majority of whom were considered to have high-risk disease, received ibrutinib orally once daily; 51 received 420 mg, and 34 received 840 mg.
RESULTS
Toxic effects were predominantly grade 1 or 2 and included transient diarrhea, fatigue, and upper respiratory tract infection; thus, patients could receive extended treatment with minimal hematologic toxic effects. The overall response rate was the same in the group that received 420 mg and the group that received 840 mg (71%), and an additional 20% and 15% of patients in the respective groups had a partial response with lymphocytosis. The response was independent of clinical and genomic risk factors present before treatment, including advanced-stage disease, the number of previous therapies, and the 17p13.1 deletion. At 26 months, the estimated progression-free survival rate was 75% and the rate of overall survival was 83%.
CONCLUSIONS
Ibrutinib was associated with a high frequency of durable remissions in patients with relapsed or refractory CLL and small lymphocytic lymphoma, including patients with high-risk genetic lesions. (Funded by Pharmacyclics and others; ClinicalTrials.gov number, NCT01105247.)
Chronic lymphocytic leukemia (CLL) is the most common leukemia in adults. Therapy for symptomatic CLL has consisted predominantly of chemotherapeutic agents, including chlorambucil, cyclophosphamide, fludarabine, and combinations of these agents that are effective for palliation but do not improve survival.1 The addition of the anti-CD20 antibody rituximab to chemotherapy (fludarabine2 alone or in combination with cyclophosphamide3) results in higher response rates, extended remissions, and improved overall survival.4 However, a subgroup of patients with deletion of 17p13.1 have a poor response to chemoimmunotherapy, and patients with tumors expressing unmutated immunoglobulin variable-region heavy-chain genes have shorter remissions than those with mutated genes.4,5 Furthermore, chemoimmunotherapy is not curative, and treatment options for relapsed disease tend to have increased toxicity and reduced antitumor activity.
Unlike chronic myeloid leukemia,6 CLL lacks a common genetic target. However, B-cell–receptor signaling has emerged as a driving factor for CLL tumor-cell survival.7–17 Downstream of the B-cell receptor and of critical importance to its function is a member of the Tec family of kinases, Bruton's tyrosine kinase (BTK). BTK mutations in humans cause X-linked agammaglobulinemia, which leads to the absence of peripheral-blood B cells, decreased levels of serum immunoglobulin, and increased susceptibility to infections. BTK is essential for activation of several constitutively active pathways of CLL-cell survival, including the Akt,18 extracellular signal–regulated kinase (ERK),19 and nuclear factor kappa light-chain enhancer of activated B cells (NF-κB) pathways.20,21 In addition, BTK is essential to chemokine-mediated homing and adhesion of B cells.22–25 Given the importance of B-cell–receptor signaling in CLL and the central role of BTK in this pathway, an attractive strategy is to target inhibition of this kinase.
Ibrutinib (Pharmacyclics) is an orally bioavailable, potent inhibitor (50% inhibitory concentration, 0.5 nM) that covalently binds to the cysteine-481 amino acid of the BTK enzyme. Preclinical studies have shown that ibrutinib treatment inhibits numerous processes, including ERK signaling, NF-κB DNA binding, cytosine–phosphate–guanine (CpG)–mediated CLL-cell proliferation, and tumor-cell migration.26–28 Ibrutinib does not have toxic effects on normal T cells; this distinguishes it from most regimens used for CLL.27 A phase 1 study of ibrutinib showed mild-to-moderate toxicity and clinical antitumor activity in patients with relapsed or refractory B-cell cancers; 11 of the 16 patients in the study had CLL or small lymphocytic lymphoma.29 These preliminary results prompted the initiation of a phase 1b–2 study of ibrutinib in CLL; this study involved two different therapeutic doses in patients with relapsed or refractory disease.
METHODS
PATIENTS
Eligibility criteria included the following: a diagnosis of relapsed or refractory CLL or small lymphocytic lymphoma, as defined according to the International Workshop on Chronic Lymphocytic Leukemia30 and World Health Organization31 classifications; a need for treatment; adequate renal and hepatic function, defined by a creatinine level of no more than 1.5 times the upper limit of the normal range and an alanine aminotransferase level of no more than 2.5 times the upper limit of the normal range; and an absence of active infection. The first and second cohorts were required to have received at least two previous therapies, including a purine analogue. A third cohort was composed of patients with high-risk disease that did not respond to a chemoimmunotherapy regimen or that progressed within 24 months after completion of the regimen. An absolute neutrophil count of at least 750 cells per cubic millimeter and a platelet count of at least 50,000 per cubic millimeter were required initially. However, an amendment to the protocol allowed enrollment of 22 patients with any degree of cytopenia if it was due to bone marrow involvement. Exclusion criteria were any type of cancer that limited survival to less than 2 years, gastrointestinal disease that might inhibit ibrutinib absorption, and medicines associated with torsades de pointes. Testing to detect interphase cytogenetic abnormalities, immunoglobulin-gene mutational analysis, and measurement of β2-microglobulin levels were performed during the screening period by a central reference laboratory.
STUDY DESIGN AND TREATMENT
This phase 1b–2, open-label, multicenter study was designed to determine the safety, efficacy, pharmacokinetics, and pharmacodynamics of ibrutinib in patients with relapsed or refractory CLL or small lymphocytic lymphoma. In addition, we sought to examine the influence of features of a poor prognosis on the clinical response to ibrutinib. An institutional review board approved the protocol (available with the full text of this article at NEJM.org) at each study site. The study was conducted according to the principles of the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice. All patients provided written informed consent. The study is ongoing.
Two cohorts of patients (27 patients in cohort 1 and 24 patients in cohort 3) were assigned to receive a fixed daily dose of 420 mg of ibrutinib, and one cohort (34 patients in cohort 2) was assigned to receive a daily dose of 840 mg, with both doses administered orally on a continuous schedule until the onset of disease progression or unacceptable toxicity. Enrollment in the three cohorts occurred consecutively from May 2010 through August 2011. On the basis of early data from cohort 1, cohort 3 was added to study the effect of 420 mg in patients with high-risk disease.
ASSESSMENTS
The primary end point was the safety of the two fixed-dose regimens, assessed according to the frequency and severity of adverse events. Safety monitoring, which included a clinical history taking, physical examination, and laboratory tests, was performed weekly for the first month, every other week for the second month, and monthly thereafter. Adverse events were graded with the use of the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 (http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf). Hematologic toxic effects were graded according to the system of the International Workshop on Chronic Lymphocytic Leukemia (2008).30
The secondary end points were the overall response rate, progression-free survival, pharmacodynamics, and pharmacokinetics. A response assessment that included a radiologic examination was performed at the end of cycles 2, 5, 8, 12, 15, and 24. A bone marrow biopsy was performed to confirm a complete response.30 The response in patients with CLL was evaluated according to the criteria of the International Workshop on Chronic Lymphocytic Leukemia,30 with the exception that lymphocytosis was not the sole criterion for disease progression (a summary of the response criteria is available in Table S1 in the Supplementary Appendix, available at NEJM.org).32 For patients with persistent lymphocytosis, a partial response in all other measures was characterized as a partial response with lymphocytosis. The response in patients with small lymphocytic lymphoma was evaluated according to the International Working Group criteria for non-Hodgkin's lymphoma (2007).33 Putative biomarkers of response and overall survival were exploratory end points.
PHARMACOKINETIC AND PHARMACODYNAMIC ANALYSES
We conducted pharmacokinetic studies to determine the exposure to ibrutinib after treatment at both dose levels. The pharmacokinetic measures were derived from the nominal plasma concentration–time curves. Pharmacodynamic testing was performed to assess the level of drug binding to BTK (BTK occupancy) after treatment. A cell-permeable, fluorescently tagged derivative of ibrutinib was used to visualize BTK before treatment and 4 and 24 hours after treatment, as previously described.26
STUDY OVERSIGHT
The study was sponsored by Pharmacyclics and Janssen. All the authors and the sponsors were responsible for designing the study protocol and analysis plan. All the authors and their respective research teams collected all the data, and the sponsors confirmed the accuracy of the data and compiled it for summation and analysis. The authors had full access to the data and analyses for compilation of this report. Manuscript drafts were prepared by the authors with editorial assistance from a professional medical writer paid by Janssen. All the authors vouch for the accuracy and completeness of the data reported and the fidelity of the study to the protocol, and all the authors made the decision to submit the manuscript for publication.
STATISTICAL ANALYSIS
Descriptive statistics, including means, standard deviations, and medians for continuous variables and proportions for discrete variables, were used to summarize the findings in each of the defined cohorts. All analyses included patients who received the study drug. The overall response rate with a 95% confidence interval was calculated. The Kaplan–Meier method was used for time-to-event analysis (curves and corresponding quartiles). Data on progression-free survival were censored when patients received new anticancer therapy or at the last clinical assessment, for patients who were lost to follow-up. No imputation of missing values was performed. In an exploratory analysis, characteristics associated with response and progression-free survival and overall survival were compared by means of Fisher's exact test in each subgroup. Analyses were not adjusted for multiple comparisons.
RESULTS
PATIENTS AND TREATMENT
A total of 85 patients were enrolled at eight sites. The baseline characteristics of the patients are listed in Table 1, and in Table S2 in the Supplementary Appendix. The patients, who were generally considered to have high-risk disease, had received a median of four previous therapies. A total of 65% of the patients had advanced-stage disease, 33% had 17p13.1 deletions, and 36% had 11q22.3 deletions. At a median follow-up of 20.9 months (range, 0.7 to 26.7), 54 patients (64%) were still receiving treatment, and 31 (36%) had discontinued treatment. Reasons for treatment discontinuation (detailed in Table S3 in the Supplementary Appendix) included disease progression in 11 patients (13%); the patient's or investigator's decision in 13 (15%), of whom 5 patients then underwent stem-cell transplantation; and adverse events in 7 patients (8%), including 3 patients who had pneumonia, 2 who had sepsis, 1 who had staphylococcal bacteremia without physiological signs of sepsis, and 1 who had gastrointestinal hemorrhage.
Table 1.
Baseline Demographic and Clinical Characteristics of the Patients.
| Characteristic | Total (N = 85) |
|---|---|
| Age | |
| Median — yr | 66 |
| Range — yr | 37–82 |
| ≥70 yr — no. (%) | 30 (35) |
| Sex — no. (%) | |
| Male | 65 (76) |
| Female | 20 (24) |
| Diagnosis — no. (%) | |
| Chronic lymphocytic leukemia | 82 (96) |
| Small lymphocytic lymphoma | 3 (4) |
| Time since most recent systemic anticancer therapy — mo | |
| Median | 3 |
| Range | 1–98 |
| Rai stage at treatment initiation — no. (%)* | |
| 0, I, or II | 29 (34) |
| III or IV | 55 (65) |
| Data missing | 1 (1) |
| No. of previous therapies | |
| Median | 4 |
| Range | 1–12 |
| Previous therapy — no. (%) | |
| Nucleoside analogue | 81 (95) |
| Rituximab | 83 (98) |
| Alkylator | 76 (89) |
| Alemtuzumab | 18 (21) |
| Bendamustine | 33 (39) |
| Ofatumumab | 22 (26) |
| Bulky nodes — no. (%) | |
| ≥5 cm in diameter | 44 (52) |
| ≥10 cm in diameter | 13 (15) |
| Data missing | 3 (4) |
| Unmutated immunoglobulin variable-region heavy-chain gene — no. (%) | |
| Patients with data that could be evaluated | 69 (81) |
| Data missing | 4 (5) |
| Interphase cytogenetic abnormality — no. (%)† | |
| 17p13.1 deletion | 28 (33) |
| 11q22.3 deletion | 31 (36) |
| β2-microglobulin level — no. (%) | |
| >3 mg/liter | 39 (46) |
| Data missing | 5 (6) |
| Disease resistant to purine analogue — no. (%)‡ | 41 (48) |
Rai stage 0 indicates low-risk, stage I or II intermediate-risk, and stage III or IV high-risk disease.
Cutoff points were defined according to the assay specifications as performed in the central laboratory.
Resistance to a purine analogue was defined as treatment failure (stable disease or progressive disease) or disease progression within 12 months after receipt of a regimen containing a purine analogue.
PHARMACOKINETIC AND PHARMACODYNAMIC MEASUREMENTS
The steady-state concentration–time profiles of ibrutinib after administration of 420 mg or 840 mg per day are shown in Figure S1 in the Supplementary Appendix. The exposure to ibrutinib increased proportionally from a dose of 420 to 840 mg per day. No differences in the time to the peak ibrutinib concentration in the blood (median Tmax, 2 hours [range, 0.5 to 6]) or the terminal half-life (7.8±3.6 hours with 420 mg per day and 8.1±3.4 hours with 840 mg per day) were apparent between doses.
Post-treatment assessments indicated full occupancy of BTK by ibrutinib at both dose levels. The median level of BTK occupancy was 96 to 99% (Fig. S2 in the Supplementary Appendix); it was observed as early as 4 hours after the dose was administered and was maintained 24 hours after the dose was administered, at both dose levels.
SAFETY
Long-term therapy with ibrutinib was associated with modest toxicity; most adverse events were grade 1 or 2 (Table 2). The most common adverse events were diarrhea, fatigue, and upper respiratory tract infection, and most adverse events resolved without the need for a suspension of treatment. Adverse events leading to discontinuation of treatment occurred in 2 patients in the 420-mg cohort (4%) and in 4 patients in the 840-mg cohort (12%). Serious adverse events are listed in Table S4 in the Supplementary Appendix. The most common adverse events of grade 3 or higher were pneumonia (in 10 patients [12%]) and dehydration (in 5 patients [6%]). Infections of grade 3 or higher occurred most frequently early in the course of therapy.
Table 2.
Adverse Events.*
| Adverse Event | Grade 1–2 | Grade 3–4 | Total† |
|---|---|---|---|
| number of patients (percent) | |||
| Diarrhea | 40 (47) | 2 (2) | 42 (49) |
|
| |||
| Upper respiratory tract infection | 28 (33) | 0 | 28 (33) |
|
| |||
| Fatigue | 24 (28) | 3 (4) | 27 (32) |
|
| |||
| Cough | 26 (31) | 0 | 26 (31) |
|
| |||
| Arthralgia | 23 (27) | 0 | 23 (27) |
|
| |||
| Rash | 23 (27) | 0 | 23 (27) |
|
| |||
| Pyrexia | 19 (22) | 4 (5) | 23 (27) |
|
| |||
| Edema, peripheral | 18 (21) | 0 | 18 (21) |
|
| |||
| Muscle spasms | 16 (19) | 1 (1) | 17 (20) |
|
| |||
| Constipation | 14 (16) | 1 (1) | 15 (18) |
|
| |||
| Dizziness | 14 (16) | 1 (1) | 15 (18) |
|
| |||
| Headache | 14 (16) | 1 (1) | 15 (18) |
|
| |||
| Hypertension | 11 (13) | 4 (5) | 15 (18) |
|
| |||
| Nausea | 14 (16) | 1 (1) | 15 (18) |
|
| |||
| Sinusitis | 11 (13) | 4 (5) | 15 (18) |
|
| |||
| Contusion | 14 (16) | 0 | 14 (16) |
|
| |||
| Vomiting | 13 (15) | 1 (1) | 14 (16) |
|
| |||
| Neutropenia‡ | 0 | 13 (15) | 13 (15) |
|
| |||
| Oropharyngeal pain | 13 (15) | 0 | 13 (15) |
Listed are adverse events that were reported in 13 or more of the 85 patients (≥15%), regardless of the cause.
Seven grade 5 adverse events were reported: pneumonia, influenza-like pneumonia, cryptococcal pneumonia, chronic lymphocytic leukemia, Richter's syndrome, sarcoma, and the systemic inflammatory response syndrome. (The event terms are those deemed most appropriate by the investigator.)
Neutropenia did not lead to treatment discontinuation and was managed with the administration of growth factors in 5 of 13 patients.
The average rate of infection was 7.1 per 100 patient-months during the first 6 months and 2.6 per 100 patient-months thereafter (Table S5 in the Supplementary Appendix). The exposure-adjusted rate of infections that were grade 3 or higher was reduced by more than half after 6 months of treatment (Table S5 in the Supplementary Appendix).
IgG and IgM levels remained relatively stable throughout treatment, whereas IgA levels increased at 3, 6, and 12 months (Fig. S3 in the Supplementary Appendix). Grade 3 or 4 hematologic toxic effects were infrequent; 5 patients (6%) had anemia, 13 patients (15%) had neutropenia, and 5 patients (6%) had thrombocytopenia. Bleeding events that were grade 3 or higher in severity occurred in 4 patients. A total of 8 patients died within 30 days after receiving the last dose of ibrutinib: 3 deaths were from pneumonia, 1 was from the systemic inflammatory response syndrome, 1 was from sarcoma, and 3 were related to CLL progression.
EFFICACY
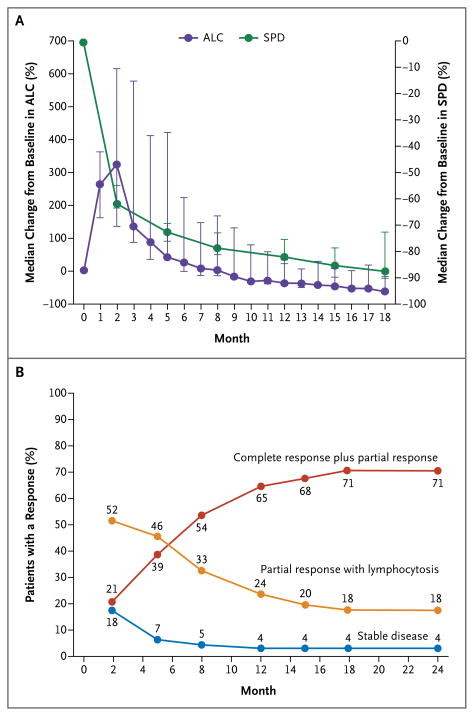
The overall response rate based on the standard criteria30,33 was 71% (2 complete responses and 34 partial responses) in the 420-mg cohort and 71% (24 partial responses) in the 840-mg cohort. In addition, 10 patients in the 420-mg cohort (20%) and 5 patients in the 840-mg cohort (15%) had a partial response with persistent lymphocytosis. Blood lymphocytosis was generally noted by day 7 (in 78% of the patients); it peaked at a median of 4 weeks and then slowly declined. In 50 of 63 patients (79%), the lymphocyte count normalized (absolute lymphocyte count, <4×109 cells per cubic millimeter) or was reduced by 50% from the baseline level. Treatment-related lymphocytosis developed at similar frequencies in patients with unmutated and those with mutated immunoglobulin variable-region heavy-chain genes (77% and 83%, respectively). However, in patients with unmutated immunoglobulin genes, lymphocyte counts normalized more rapidly (median, 6.4 vs. 14.8 months) and more frequently (in 85% vs. 50% of the patients). Lymphocytosis occurred concomitantly with a notable reduction in lymph-node size (Fig. 1A) and spleen size, as well as frequent improvement in cytopenias (Fig. S4 in the Supplementary Appendix). An improved response was time-dependent (Fig. 1B); increasing numbers of partial responses and complete responses occurred during follow-up, whereas the frequency of a partial response with lymphocytosis diminished as the lymphocyte count decreased over time.
Figure 1. Response to Ibrutinib over Time.
Panel A shows the median percent change from baseline in the absolute lymphocyte count (ALC) and the sum of the products of lymph-node diameters (SPD) in all patients. I bars denote distribution-free 95% confidence intervals. Panel B shows the curves for cumulative best response (complete response plus partial response, partial response with lymphocytosis, and stable disease).
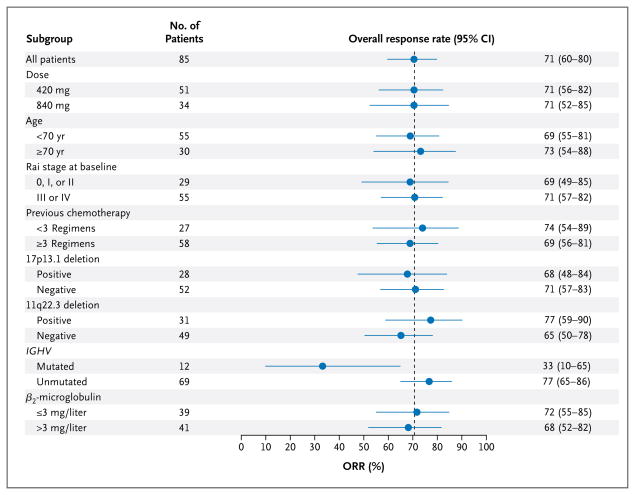
As shown in Figure 2, the response to ibrutinib did not appear to vary according to traditional high-risk prognostic features. The response rate among the patients with a 17p13.1 deletion was 68%, including one complete response, whereas the response rate was 71% among those without this deletion. The only factor associated with a response was the mutation status of the immunoglobulin variable-region heavy-chain gene. Notably, 4 of the 12 patients with a mutated immunoglobulin variable-region heavy-chain gene (33%) had a partial response or complete response and 5 (42%) had a partial response with lymphocytosis. By contrast, 53 of the 69 patients with an unmutated immunoglobulin variable-region heavy-chain gene (77%) had a partial response or complete response and 9 (13%) had a partial response with lymphocytosis. This difference in the overall response rate (partial response plus complete response) was significant (P = 0.005), whereas the combined overall response rate plus the rate of partial response with lymphocytosis did not differ significantly according to mutation status.
Figure 2. Overall Response Rates (ORR) According to Subgroup.
Rai stage 0 indicates low risk, stage I or II intermediate risk, and stage III or IV high risk. The dotted line shows the response rate for all 85 patients. IGHV denotes immunoglobulin variable-region heavy-chain gene.
Sustained improvement in cytopenias, defined as improvement by more than 50% or a hemoglobin level higher than 11 g per deciliter, an absolute neutrophil count higher than 1500 cells per cubic millimeter, or a platelet count higher than 100,000 cells per cubic millimeter (lasting for ≥2 cycles without transfusion or administration of growth factors), was frequently observed during ibrutinib treatment (Fig. S4 in the Supplementary Appendix). Improvement was observed in 32 of 41 patients with baseline thrombocytopenia (78%), 27 of 33 with anemia (82%), and 24 of 31 with neutropenia (77%).
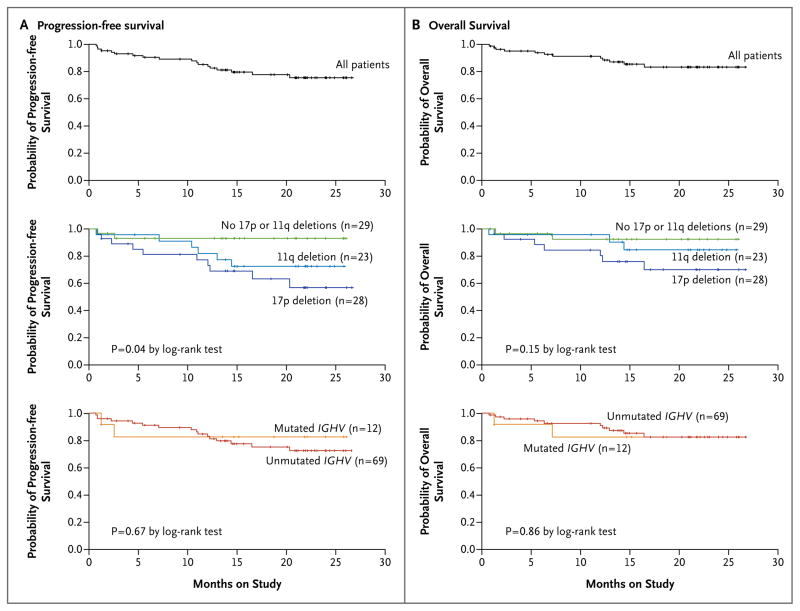
Ibrutinib treatment promoted durable responses, irrespective of the dose. The 26-month estimated rate of progression-free survival (Fig. 3A, top graph) was 75%, and the rate of overall survival (Fig. 3B, top graph) was 83%. Disease progression developed in 11 patients (13%) during follow-up, and 7 of those patients had progression by biologic transformation (Richter's syndrome). The median time from the initial diagnosis of CLL to transformation was 98 months (range, 24 to 143). Among the 11 patients in whom progressive disease developed, 10 patients had a 17p13.1 or a 11q22.3 deletion, and 1 patient did not have high-risk cytogenetic abnormalities (Fig. 3A). Patients had a prolonged time to progression despite high-risk genomic features. Among the 28 patients with a 17p13.1 deletion — a uniformly poor prognostic factor — the 26-month estimated rate of progression-free survival (Fig. 3A) was 57% and the rate of overall survival (Fig. 3B) was 70%. Patients who discontinued ibrutinib for reasons other than progression remained in the study and were followed quarterly until disease progression, the initiation of new anticancer therapy, or death. After disease progression or initiation of subsequent anticancer therapy, follow-up was limited to survival status. Treatment at the time of new treatment initiation was left to the discretion of the treating physician.
Figure 3. Kaplan–Meier Curves for Progression-free Survival and Overall Survival.
Panels A and B show the probability of progression-free survival and overall survival, respectively, for all 85 patients (top graphs) and according to status with respect to the 17p13.1 and 11q22.3 deletions (middle graphs) and IGHV mutation status (bottom graphs). Tick marks indicate censored data.
DISCUSSION
The responses to ibrutinib, the covalent inhibitor of BTK, that we observed in this study were more durable than expected on the basis of previous experience with other single-agent therapies for relapsed CLL. At both doses of ibrutinib that we studied, pharmacokinetic data showed rapid absorption and elimination. Moreover, pharmacodynamic data showed that once-daily ibrutinib provided effective and complete occupancy of BTK, a surrogate of kinase inhibition.
Ibrutinib caused a transient increase in blood lymphocyte levels, which was concurrent with a reduction in lymph-node size, spleen size, or both. Continued treatment with ibrutinib led to resolution of this asymptomatic lymphocytosis, and patients were characterized as having a classic response according to the 2008 criteria of the International Workshop on Chronic Lymphocytic Leukemia, with an observed response rate of 71%. Fifteen additional patients (18%) in this study had a partial response with lymphocytosis. Treatment-related lymphocytosis has been seen with other agents that target B-cell–receptor signaling; these findings have prompted several groups of CLL experts to conclude that such lymphocytosis is not a sign of progressive disease.34,35 Our findings provide support for an ibrutinib-mediated pharmacodynamic effect on CLL by cell mobilization from protected bone marrow, lymphnode, and spleen sites harboring stromal elements that have been shown to promote leukemic-cell proliferation, drug resistance, and survival.25
Despite the immunocompromised condition of the patients, who had received a median of four previous therapies, ibrutinib treatment did not result in an increased incidence of grade 3 or higher infections during the extended therapy period, as compared with the rates reported in several series of patients who received traditional salvage therapies.36–38 The incidence of infection was highest early in the course of ibrutinib treatment and decreased with continued therapy. During treatment, improvement in neutrophil counts occurred in patients with baseline neutropenia (Fig. S4 in the Supplementary Appendix), with significant increases in the level of serum IgA and no detrimental effects on IgG or IgM levels (Fig. S3 in the Supplementary Appendix). The analysis of immunoglobulin levels was limited to patients with relatively preserved immunoglobulin levels, since those who received intravenous immune globulin were excluded. The occurrence of ecchymosis, petechiae, or both was noted on the skin in a subgroup of patients who received ibrutinib, but these conditions were not accompanied by excess bleeding or associated with thrombocytopenia. Randomized trials comparing the safety profile of ibrutinib with that of other therapeutic agents for CLL are ongoing. These trials include A Phase 3 Study of Ibrutinib (PCI-32765) Versus Ofatumumab in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia (RESONATE; ClinicalTrials.gov number, NCT01578707) and A Multicenter, Open-label, Phase 3 Study of the Bruton's Tyrosine Kinase Inhibitor PCI-32765 Versus Chlorambucil in Patients 65 Years or Older with Treatment-naive Chronic Lymphocytic Leukemia or Small Lymphocytic Leukemia (RESONATE-2, NCT01722487).
Several validated high-risk characteristics of CLL, including 17p13.1 deletion, did not influence the objective response to ibrutinib. However, most events associated with disease progression occurred in patients with high-risk cytogenetic lesions (17p13.1 deletion or 11q22.3 deletion), whereas only one patient without these risk factors had an event associated with disease progression. Patients with an unmutated immunoglobulin variable-region heavy-chain gene, perhaps owing to enhanced B-cell–receptor signaling and dependence on this pathway, had earlier resolution of lymphocytosis and were more frequently classified as having a response according to traditional response criteria of the International Workshop on Chronic Lymphocytic Leukemia (P = 0.02); however, survival outcomes were similar to those among patients without this unmutated gene. Greater than 90% occupancy of the pharmacodynamic probe and the similar response in the two dose groups provide support for the use of the 420-mg dose of ibrutinib for relapsed CLL.
Ibrutinib has a favorable therapeutic index, which may facilitate its use in combination with other agents for the treatment of CLL. However, the durable remissions obtained thus far suggest that many patients may be treated successfully with monotherapy. Randomized clinical trials of ibrutinib in patients with CLL or small lymphocytic lymphoma are ongoing.
Supplementary Material
Acknowledgments
Supported by Pharmacyclics, Janssen, and grants from the Leukemia and Lymphoma Society, the D. Warren Brown Foundation, Mr. and Mrs. Michael Thomas, the Harry T. Mangurian, Jr., Foundation, and the National Institutes of Health (P50 CA140158 and P01 CA095426).
We thank the investigators and coordinators at each of the clinical sites; the patients who participated in this trial and their families; the employees of Pharmacyclics who contributed to the design and implementation of the trial; Cathy Zhou, M.S., for assistance with data analysis and interpretation; Raquel Izumi, Ph.D., and Ahmed Hamdy, M.D., for initial work on planning and implementing the trial; Stella Chang, M.S., for assays for pharmacodynamic analysis; and Namit Ghildyal, Ph.D., of Janssen Research and Development for editorial assistance with an earlier version of the manuscript.
Footnotes
Presented in part at the Annual Meeting of the American Society of Hematology, Atlanta, December 8–11, 2012.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Gribben JG, O'Brien S. Update on therapy of chronic lymphocytic leukemia. J Clin Oncol. 2011;29:544–50. doi: 10.1200/JCO.2010.32.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrd JC, Rai K, Peterson BL, et al. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: an updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood. 2005;105:49–53. doi: 10.1182/blood-2004-03-0796. [DOI] [PubMed] [Google Scholar]
- 3.Tam CS, O'Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–80. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–74. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 5.Byrd JC, Gribben JG, Peterson BL, et al. Select high-risk genetic features predict earlier progression following chemoimmunotherapy with fludarabine and rituximab in chronic lymphocytic leukemia: justification for risk-adapted therapy. J Clin Oncol. 2006;24:437–43. doi: 10.1200/JCO.2005.03.1021. [DOI] [PubMed] [Google Scholar]
- 6.O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 7.Ringshausen I, Schneller F, Bogner C, et al. Constitutively activated phosphatidylinositol-3 kinase (PI-3K) is involved in the defect of apoptosis in B-CLL: association with protein kinase Cdelta. Blood. 2002;100:3741–8. doi: 10.1182/blood-2002-02-0539. [DOI] [PubMed] [Google Scholar]
- 8.Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116:2078–88. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernal A, Pastore RD, Asgary Z, et al. Survival of leukemic B cells promoted by engagement of the antigen receptor. Blood. 2001;98:3050–7. doi: 10.1182/blood.v98.10.3050. [DOI] [PubMed] [Google Scholar]
- 10.Lannutti BJ, Meadows SA, Herman SE, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117:591–4. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gobessi S, Laurenti L, Longo PG, Sica S, Leone G, Efremov DG. ZAP-70 enhances B-cell-receptor signaling despite absent or inefficient tyrosine kinase activation in chronic lymphocytic leukemia and lymphoma B cells. Blood. 2007;109:2032–9. doi: 10.1182/blood-2006-03-011759. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Huynh L, Apgar J, et al. ZAP-70 enhances IgM signaling independent of its kinase activity in chronic lymphocytic leukemia. Blood. 2008;111:2685–92. doi: 10.1182/blood-2006-12-062265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Widhopf G, Huynh L, et al. Expression of ZAP-70 is associated with increased B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2002;100:4609–14. doi: 10.1182/blood-2002-06-1683. [DOI] [PubMed] [Google Scholar]
- 14.Richardson SJ, Matthews C, Catherwood MA, et al. ZAP-70 expression is associated with enhanced ability to respond to migratory and survival signals in B-cell chronic lymphocytic leukemia (B-CLL) Blood. 2006;107:3584–92. doi: 10.1182/blood-2005-04-1718. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez-Aguilera A, Rattmann I, Drew DZ, et al. Involvement of RhoH GTPase in the development of B-cell chronic lymphocytic leukemia. Leukemia. 2010;24:97–104. doi: 10.1038/leu.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dal-Bo M, Bertoni F, Forconi F, et al. Intrinsic and extrinsic factors influencing the clinical course of B-cell chronic lymphocytic leukemia: prognostic markers with pathogenetic relevance. J Transl Med. 2009;7:76. doi: 10.1186/1479-5876-7-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–15. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 18.Craxton A, Jiang A, Kurosaki T, Clark EA. Syk and Bruton's tyrosine kinase are required for B cell antigen receptor-mediated activation of the kinase Akt. J Biol Chem. 1999;274:30644–50. doi: 10.1074/jbc.274.43.30644. [DOI] [PubMed] [Google Scholar]
- 19.Tomlinson MG, Woods DB, McMahon M, et al. A conditional form of Bruton's tyrosine kinase is sufficient to activate multiple downstream signaling pathways via PLC Gamma 2 in B cells. BMC Immunol. 2001;2:4. doi: 10.1186/1471-2172-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petro JB, Rahman SM, Ballard DW, Khan WN. Bruton's tyrosine kinase is required for activation of IkappaB kinase and nuclear factor kappaB in response to B cell receptor engagement. J Exp Med. 2000;191:1745–54. doi: 10.1084/jem.191.10.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petro JB, Khan WN. Phospholipase C-gamma 2 couples Bruton's tyrosine kinase to the NF-kappaB signaling pathway in B lymphocytes. J Biol Chem. 2001;276:1715–9. doi: 10.1074/jbc.M009137200. [DOI] [PubMed] [Google Scholar]
- 22.Spaargaren M, Beuling EA, Rurup ML, et al. The B cell antigen receptor controls integrin activity through Btk and PLCgamma2. J Exp Med. 2003;198:1539–50. doi: 10.1084/jem.20011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan WN, Alt FW, Gerstein RM, et al. Defective B cell development and function in Btk-deficient mice. Immunity. 1995;3:283–99. doi: 10.1016/1074-7613(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 24.Rawlings DJ, Saffran DC, Tsukada S, et al. Mutation of unique region of Bruton's tyrosine kinase in immunodeficient XID mice. Science. 1993;261:358–61. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- 25.Burger JA. Nurture versus nature: the microenvironment in chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2011;2011:96–103. doi: 10.1182/asheducation-2011.1.96. [DOI] [PubMed] [Google Scholar]
- 26.Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A. 2010;107:13075–80. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–96. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ponader S, Chen SS, Buggy JJ, et al. Bruton's tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–9. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–56. doi: 10.1182/blood-2007-06-093906. [Erratum, Blood 2008; 112:5259.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaffe ES, Harris NL, Stein H, Isaacson PG. Classification of lymphoid neoplasms: the microscope as a tool for disease discovery. Blood. 2008;112:4384–99. doi: 10.1182/blood-2008-07-077982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hallek M. FCA: forget chemoimmunotherapy with alemtuzumab? Blood. 2012;119:5059–60. doi: 10.1182/blood-2012-04-413096. [DOI] [PubMed] [Google Scholar]
- 33.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 34.Hallek M, Cheson BD, Catovsky D, et al. Response assessment in chronic lymphocytic leukemia treated with novel agents causing an increase of peripheral blood lymphocytes. Blood. 2012 Jun 4; (Epub ahead of print) [Google Scholar]
- 35.Cheson BD, Byrd JC, Rai KR, et al. Novel targeted agents and the need to refine clinical end points in chronic lymphocytic leukemia. J Clin Oncol. 2012;30:2820–2. doi: 10.1200/JCO.2012.43.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perkins JG, Flynn JM, Howard RS, Byrd JC. Frequency and type of serious infections in fludarabine-refractory B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma: implications for clinical trials in this patient population. Cancer. 2002;94:2033–9. [PubMed] [Google Scholar]
- 37.Wierda WG, Kipps TJ, Mayer J, et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1749–55. doi: 10.1200/JCO.2009.25.3187. [Erratum, J Clin Oncol 2010; 28:3670.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keating MJ, Flinn I, Jain V, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99:3554–61. doi: 10.1182/blood.v99.10.3554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.