To the Editor:
Epidemiologic studies have shown that low serum 25-hydroxyvitamin D (25[OH]D) levels are associated with increased risk of lower respiratory tract infections in young children.1, 2 Moreover, both low maternal intake of vitamin D during pregnancy and low umbilical cord blood 25(OH)D levels have been associated with increased risk of childhood wheezing.2, 3 These associations are supported by intervention trials demonstrating decreased respiratory tract infections in children receiving vitamin D supplementation.4, 5 To date, only 1 virus-specific study is available, a randomized placebo-controlled trial demonstrating that a group of school-aged children receiving a 1,200 IU vitamin D3 supplement had fewer influenza A viral infections.5 In Finland national recommendations are that all young children receive vitamin D supplementation because of Finland’s northern latitude, but parental compliance is generally low.6 We hypothesized that 25(OH)D levels would be inversely associated with the risk of rhinovirus (the main viral trigger of wheezing), more than 1 concurrent viral infection (ie, viral coinfections), or both.7
In the VINKU study cohort of 284 hospitalized wheezing children, nasopharyngeal aspirate samples were collected and blood was drawn during the acute illness (see Fig E1 in this article’s Online Repository at www.jacionline.org).7 In this 2-year study (September 2000 to May 2002, excluding June and July 2001), 75% of the children were recruited during September to February. We tested the nasopharyngeal aspirate samples for 18 different viruses using all available conventional and molecular methods. Specifically, we performed PCR, culture, antigen detection, and serology, if available, for rhinovirus; respiratory syncytial virus (RSV); enteroviruses; coronaviruses 229E, OC43, NL63, and HKU1; metapneumovirus, human bocavirus; influenza A and B viruses; adenovirus; parainfluenza virus types 1 to 4; and WU and KI polyomaviruses.
Serum 25(OH)D measurements were done by means of liquid chromatography–tandem mass spectrometry at Massachusetts General Hospital. Specific IgE levels for common allergens were measured from serum by using a fluoroenzyme immunoassay (cutoff for specific allergens 0.35 kU/L; CAP FEIA, Phadiatop Combi; Phadia, Uppsala, Sweden). These data allowed us to perform a cross-sectional analysis of the association between serum 25(OH)D levels and the viral cause of acute wheezing necessitating hospitalization. The methods are described in more detail in the Methods section of this article’s Online Repository at www.jacionline.org.
Statistical analyses included descriptive statistics, multivariable logistic regression models, and unadjusted Lowess plots to visually present nonlinear associations, if present, between 25(OH)D level and outcomes (Stata 10.0 software; StataCorp, College Station, Tex).
The median age of the children was 1.6 years (interquartile range, 1.0–2.8 years), and 189 (67%) were boys (see Table E1 in this article’s Online Repository at www.jacionline.org). The mean 25(OH)D concentration was 68 nmol/L. Eighty-eight (31%) children had a serum 25(OH)D level of less than 50 nmol/L, and 16 (6%) had a level of less than 25 nmol/L. A viral infection was identified in 271 (95%) children, most commonly rhinovirus (40%), RSV (27%), enterovirus (26%), and bocavirus (19%). More than 1 virus was found in 112 (39%) children. Of the viral coinfections, 51% involved rhinovirus, 35% involved RSV, 33% involved enteroviruses, and 35% involved bocavirus. More than one third of the children (102/277 [37%]) had a specific allergic sensitization.
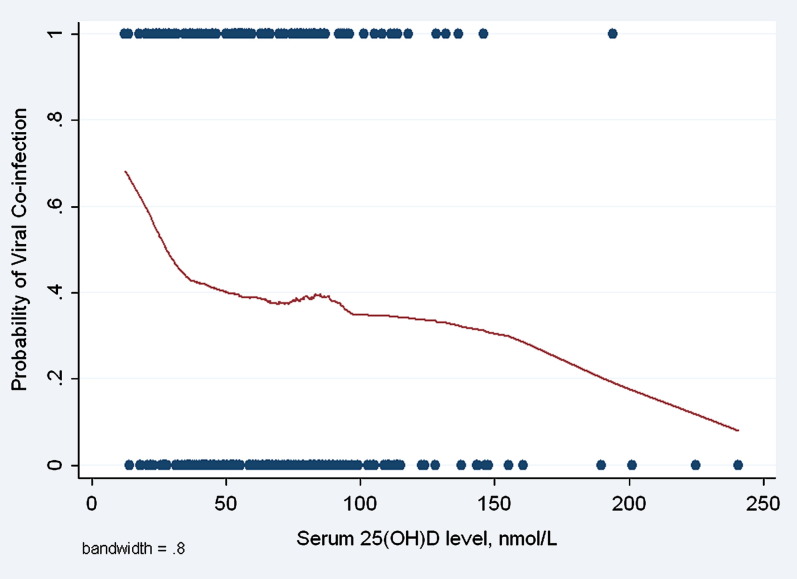
In both unadjusted and adjusted analyses (with the latter adjusting for age, sex, sensitization status, and fall-winter hospitalization), we found that the child’s serum 25(OH)D level was inversely associated with RSV (odds ratio [OR] per 10 nmol/L increase, 0.91; 95% CI, 0.83-0.99), rhinovirus (OR, 0.92; 95% CI, 0.85-0.99), and multiple viral cause (OR, 0.91; 95% CI, 0.84-0.99; see Table E2 in this article’s Online Repository at www.jacionline.org). By contrast, no association was found with other viral infections. In these same multivariable models we found a positive association between atopy status and rhinovirus (OR, 2.71; 95% CI, 1.54-4.75), a negative association between atopy status and RSV (OR, 0.43; 95% CI, 0.19-0.93), and no significant association between atopy status and viral coinfection (OR, 0.82; 95% CI, 0.47-1.45) or other viral infections. Further control for parental smoking, number of siblings, and day care attendance did not materially change these results. Fig 1 shows the unadjusted association between serum 25(OH)D levels and the risk of viral coinfections.
Fig 1.
Unadjusted association of serum 25(OH)D levels with the probability of viral coinfection in hospitalized wheezing children. The graph was smoothed by carrying out weighted regression of outcomes on vitamin D (Lowess plot). Viral coinfection was a dichotomous outcome. Dots represent subjects.
Thus our study has 2 main findings. First, we provide the first link between a low serum 25(OH)D level and risk of viral coinfection, specifically the risk of RSV, rhinovirus, or both infections, which were involved in most of the coinfections. Interestingly, the association was demonstrated in the most severe spectrum of viral illness in children with acute wheezing necessitating hospitalization. Moreover, RSV is the dominant cause of bronchiolitis, whereas rhinovirus is the main trigger of wheezing in slightly older children and an important risk factor for asthma.8 Our results are consistent with multiple epidemiologic studies examining the association between vitamin D intake/level and risk of respiratory tract illness and one randomized controlled trial examining influenza.1, 2, 3, 4, 5 Together these findings suggest that vitamin D might play a role in antiviral defense and might be particularly important in children at increased risk of moderate-to-severe viral infections, such as young infants exposed to RSV and high asthma risk children exposed to rhinovirus. The potential beneficial effects of vitamin D in the defense against respiratory tract infections might involve changes in both the innate and adaptive immune systems.9
Second, one third of the children had low serum 25(OH)D levels. During the study period, the Finnish national recommendation (by the Ministry of Social Affairs and Health) for vitamin D supplementation for children less than 12 months of age was 10 μg (1000 IU) per day for 1 year. For children aged 1 to 15 years, the recommendation was 5 to 10 μg/d during the winter months depending on the use of other vitamin D–containing products. Unfortunately, most 2- to 3-year-olds in Finland do not take vitamin supplements,7 a finding that helps explain the children’s low vitamin D status.
The study has several strengths. Serum 25(OH)D concentrations were measured during the child’s acute wheezing illness. Comprehensive viral diagnostics were used (ie, all available conventional and molecular methods were applied). Statistical analyses were carefully adjusted for important covariates. Nevertheless, causality cannot be assessed by this cross-sectional analysis.
In summary, our study provides additional evidence supporting a role of vitamin D in antiviral defense and suggests that it might be particularly important in wheezing children. The inverse associations between 25(OH)D levels and specific types of viral infection raise the question of whether vitamin D could moderate the frequency or severity of acute respiratory tract infections. Randomized controlled trials of vitamin D supplementation are warranted to determine whether our findings are truly causal and reversible. One third of the study children had low 25(OH)D levels. Intensified counseling of the parents is clearly needed to prevent these low levels and their likely untoward effects on general health.
Footnotes
Supported by the Academy of Finland (grant nos. 114034 and 132595) and the Foundation for Pediatric Research, both in Helsinki, Finland; the National Institutes of Health (K23 AI-77801); and the Massachusetts General Hospital Center for D-receptor Activation Research in Boston, Mass.
Disclosure of potential conflict of interest: O. Ruuskanen has served as a consultant to Novartis Vaccines and Abbott. The other authors have declared that they have no conflict of interest.
Methods
Subjects
The current analysis is a substudy of the VINKU project, which took place in the Department of Pediatrics of Turku University Hospital (September 2000-May 2001, excluding Christmas week, and August 2001-May 2002). Its original aim was to study the efficacy of oral prednisolone in hospitalized wheezing children in relation to viral cause; that is, half of the patients were randomized to receive oral prednisolone for 3 days, and the other half were randomized to receive placebo in a double-blind design.E1, E2, E3, E4 The present analysis included all 284 children of the VINKU study who had serum samples available for 25(OH)D analyses at study entry (Fig E1). Predefined inclusion criteria were age of 3 months or greater and less than 16 years and hospitalization for acute wheezing. Predefined exclusion criteria were chronic disease (other than asthma or allergy), systemic glucocorticoid treatment within 4 weeks before the study, severe wheezing necessitating intensive care unit treatment, and previous participation in this study. The initial decisions of hospitalizations were done by on-duty physicians not participating in the study. The presence of wheezing was confirmed by the study physician at enrollment. The study protocol was approved by the Ethics Committee of Turku University Hospital, and informed consent was obtained from the guardian before commencing the study.
Sample collection and analysis
Blood was drawn at the acute and convalescence phases (2 weeks later), and sera were stored in small aliquots at −70°C before processing. On admission, a nasopharyngeal aspirate sample was also obtained by using a standardized procedure, as described elsewhere.E1
Serum aliquots for each enrolled subject were shipped on dry ice to Massachusetts General Hospital for serum 25(OH)D measurement by means of liquid chromatography–tandem mass spectrometry, which is replacing DiaSorin radioimmunoassay as the criterion standard.E5 The method used was an isotope dilution, liquid chromatography–tandem mass spectrometry assay optimized based on published procedures.E6 The limit of detection is 5 nmol/L for vitamin D2 and 7.5 nmol/L for vitamin D3. The between-run coefficient of variation for a quality control serum containing a total vitamin D concentration of 57 nmol/L is 7.5%.
Viral antigen detection and viral culture were carried out immediately, and the samples obtained for PCR assays were stored in small aliquots at −70°C before processing. The diagnostic evaluation of nasopharyngeal aspirate specimens included the following: single PCR testing for 18 viruses (enteroviruses; rhinoviruses; RSV; coronaviruses 229E, OC43, NL63, and HKU1; metapneumovirus; human bocavirus; influenza A and B viruses; adenovirus; parainfluenza virus [PIV] types 1-4; and WU and KI polyomaviruses), viral culture for 10 viruses (adenovirus, influenza A and B viruses, PIV types 1-3, RSV, enteroviruses, rhinovirus, and metapneumovirus), antigen detection for 7 viruses (adenovirus, influenza A and B viruses, PIV types 1-3, and RSV), IgG serologic testing of acute and convalescent phase serum samples for 7 viruses (adenovirus, enteroviruses, influenza A and B viruses, PIV type 1/type 3, and RSV), and IgM serology for enteroviruses.E1, E7, E8, E9, E10 Two hundred forty-seven (87%) of 284 study subjects had samples available for complete viral testing. Thirteen percent missed bocavirus and polyomavirus testing. All rhino-enterovirus PCR-positive samples could not be typed by means of hybridization. Twelve such samples, which were available for sequence analysis, showed rhinoviruses. On the basis of this finding, all 35 nontypable rhino-enteroviruses were classified as rhinoviruses.
Specific IgE levels for common allergens were measured from serum with a fluoroenzyme immunoassay (cutoff for specific allergens, 0.35 kU/L; CAP FEIA, Phadiatop Combi; Phadia, Uppsala, Sweden). The tested allergens included codfish, cow’s milk, egg, peanut, soybean, wheat, cat, dog, horse, birch, mugwort, timothy, Cladosporium herbarum, and Dermatophagoides pteronyssinus.
Fig E1.
Study flow chart.
Table E1.
Patients’ characteristics
| Characteristic | n = 284 |
|---|---|
| Age (y), median (interquartile range) | 1.6 (1.0-2.8) |
| 25(OH)D (nmol/L), mean (SD) | 68 (35) |
| <25 nmol/L | 16 (6%) |
| 25-49 nmol/L | 72 (25%) |
| 50-74 nmol/L | 92 (32%) |
| 75-99 nmol/L | 62 (22%) |
| ≥100 nmol/L | 42 (15%) |
| Viral infection | |
| Any virus | 271 (95%) |
| Rhinovirus | 115 (40%) |
| RSV | 77 (27%) |
| Enterovirus | 70 (26%) |
| Bocavirus | 51 (19%) |
| Other virus | 65 (23%) |
| Multiple viruses | 112 (39%) |
| Male sex | 189 (67%) |
| Parental smoking | 119 (43%) |
| No. of siblings at home, median (interquartile range) | 2 (1-3) |
| Day care attendance | 122 (45%) |
| Sensitized∗ | 102 (37%) |
| First episode of wheezing | 153 (56%) |
| History of asthma | 42 (15%) |
| Inhaled corticosteroid at study entry | 28 (10%) |
| Season of hospitalization | |
| Spring (March-May) | 64 (23%) |
| Summer (June-August) | 8 (3%) |
| Fall (September-November) | 123 (43%) |
| Winter (December-February) | 89 (31%) |
Specific IgE level for any of the common allergens greater than 0.35 kU/L.
Table E2.
Association between serum 25(OH)D level (per 10 nmol/L increase) and viral cause of wheezing
| OR (95% CI) |
||
|---|---|---|
| Outcome | Univariable | Multivariable∗ |
| Rhinovirus | 0.92 (0.86-0.99) | 0.92 (0.85-0.99) |
| RSV | 0.98 (0.91-1.06) | 0.91 (0.83-0.99) |
| Enterovirus | 1.04 (0.96-1.12) | 1.05 (0.97-1.14) |
| Bocavirus | 0.97 (0.89-1.06) | 0.97 (0.88-1.07) |
| Multiple viruses | 0.93 (0.87-1.00) | 0.91 (0.84-0.99) |
Adjusted for age, sex, atopy, and fall/winter hospitalization.
References
- 1.Wayse V., Yousafzai A., Mogale K., Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 years. Eur J Clin Nutr. 2004;58:563–567. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- 2.Camargo C.A., Jr., Ingham T., Wickens K., Thadhani R.I., Silvers K.M., Epton M.J. Cord blood 25-hydroxyvitamin D levels and risk of respiratory infection, childhood wheezing, and asthma. Pediatrics. 2010 doi: 10.1542/peds.2010-0442. (In press) [DOI] [PubMed] [Google Scholar]
- 3.Camargo C.A., Jr., Rifas-Shiman S.L., Litonjua A.A., Rich-Edwards J.W., Weiss S.T., Gold D.R. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 years of age. Am J Clin Nutr. 2007;85:788–795. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linday L.A., Shindledecker R.D., Tapia-Mendoza J., Dolitsky J.N. Effect of daily cod liver oil and a multivitamin-mineral supplement with selenium on upper respiratory tract pediatric visits by young, inner-city, Latino children: randomized pediatric sites. Ann Otol Rhinol Laryngol. 2004;113:891–901. doi: 10.1177/000348940411301108. [DOI] [PubMed] [Google Scholar]
- 5.Urashima M., Segawa T., Okazaki M., Kurihara M., Wada Y., Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91:1255–1260. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 6.Marjamäki L., Räsänen M., Uusitalo L., Ahonen S., Veijola R., Knip M. Use of vitamin D and other dietary supplements by Finnish children at the age of 2 and 3 years. Int J Vitam Nutr Res. 2004;74:27–34. doi: 10.1024/0300-9831.74.1.27. [DOI] [PubMed] [Google Scholar]
- 7.Jartti T., Lehtinen P., Vuorinen T., Osterback R., van den Hoogen B., Osterhaus A.D. Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerg Infect Dis. 2004;10:1095–1101. doi: 10.3201/eid1006.030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson D.J., Gangnon R.E., Evans M.D., Roberg K.A., Anderson E.L., Pappas T.E. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mora J.R., Iwata M., von Andrian U.H. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- Jartti T., Lehtinen P., Vuorinen T., Osterback R., van den Hoogen B., Osterhaus A.D. Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerg Infect Dis. 2004;10:1095–1101. doi: 10.3201/eid1006.030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jartti T., Lehtinen P., Vanto T., Hartiala J., Vuorinen T., Mäkelä M.J. Evaluation of the efficacy of prednisolone in early wheezing induced by rhinovirus or respiratory syncytial virus. Pediatr Infect Dis J. 2006;25:482–488. doi: 10.1097/01.inf.0000215226.69696.0c. [DOI] [PubMed] [Google Scholar]
- Jartti T., Lehtinen P., Vanto T., Vuorinen T., Hartiala J., Hiekkanen H. Efficacy of prednisolone in children hospitalized for recurrent wheezing. Pediatr Allergy Immunol. 2007;18:326–334. doi: 10.1111/j.1399-3038.2007.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtinen P., Ruohola A., Vanto T., Vuorinen T., Ruuskanen O., Jartti T. Prednisolone reduces recurrent wheezing after a first wheezing episode associated with rhinovirus infection or eczema. J Allergy Clin Immunol. 2007;119:570–575. doi: 10.1016/j.jaci.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth H.J., Schmidt-Gayk H., Weber H., Niederau C. Accuracy and clinical implications of seven 25-hydroxyvitamin D methods compared with liquid chromatography-tandem mass spectrometry as a reference. Ann Clin Biochem. 2008;45:153–159. doi: 10.1258/acb.2007.007091. [DOI] [PubMed] [Google Scholar]
- Singh R.J., Taylor R.L., Reddy G.S., Grebe S.K.G. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab. 2006;91:3055–3061. doi: 10.1210/jc.2006-0710. [DOI] [PubMed] [Google Scholar]
- Allander T., Jartti T., Gupta S., Niesters H.G., Lehtinen P., Osterback R. Human bocavirus and acute wheezing in children. Clin Infect Dis. 2007;44:904–910. doi: 10.1086/512196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantola K., Hedman L., Allander T., Jartti T., Lehtinen P., Ruuskanen O. Serodiagnosis of human bocavirus infection. Clin Infect Dis. 2008;46:540–546. doi: 10.1086/526532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderlund-Venermo M., Lahtinen A., Jartti T., Hedman L., Kemppainen K., Lehtinen P. Clinical assessment and improved diagnosis of bocavirus-induced wheezing in children, Finland. Emerg Infect Dis. 2009;15:1423–1430. doi: 10.3201/eid1509.090204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantola K., Sadeghi M., Lahtinen A., Koskenvuo M., Aaltonen L.M., Möttönen M. Merkel cell polyomavirus DNA in tumor-free tonsillar tissues and upper respiratory tract samples: implications for respiratory transmission and latency. J Clin Virol. 2009;45:292–295. doi: 10.1016/j.jcv.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]