Abstract
Background
Nonhuman primates (NHPs) are an important model organism for studies of HIV pathogenesis and pre-clinical evaluation of anti-HIV therapies. The successful translation of NHP-derived data to clinically relevant anti-HIV studies will require better understanding of the viral strains and NHP species used, and their responses to existing antiretroviral therapies (ART).
Methods
Five pigtailed macaques (M. nemestrina) were productively infected with the SIV/HIV chimeric virus SHIV-1157ipd3N4 following intravenous challenge. After 8 or 27 weeks, ART (PMPA, FTC, Raltegravir) was initiated. Viral load, T-Cell counts, and production of SHIV-specific antibodies were monitored throughout the course of infection and ART.
Results
ART led to a rapid and sustained decrease in plasma viral load. Suppression of plasma viremia by ART was independent of the timing of initiation during chronic infection.
Conclusions
We present a new NHP model of HIV infection on antiretroviral therapy, which should prove applicable to multiple clinically relevant anti-HIV approaches.
Keywords: HIV, SHIV, antiretroviral therapy, pigtailed macaque, viral reservoir
INTRODUCTION
Nonhuman primates represent the best available animal model of HIV infection in vivo, and provide important pre-clinical data for various anti-HIV intervention strategies. Over the past decade, the pigtailed macaque (M. nemestrina) model has emerged as a useful system in which to study viral pathogenesis, host immune response, and vaccine efficacy [2,9,10,13,16,19,28,31,34,37,41,53]. Additionally, this species of macaque serves as a well-established large animal model of hematopoietic stem cell transplant gene therapy [3,5,48,52]. However, use of M. nemestrina models for anti-HIV gene therapy and related approaches will require a better understanding of the viral kinetics of various simian immunodeficiency virus (SIV) and simian/human immunodeficiency (SHIV) strains.
Viral replication kinetics for several SIV strains have been previously discussed in the M. nemestrina model [4,11,55]. More recently, several strains of SHIV have also been shown to productively replicate in vivo in the pigtail model [7,17,48,49]. Importantly, relative to the better-established rhesus macaque model, less is known about the response of various SIV and SHIV strains to antiretroviral therapy regimens in pigtails [1,14,22,26,27,35,41,51]. Establishing a model of ART-suppressed HIV infection in pigtail macaques is an essential component in the preclinical evaluation of anti-HIV therapies, namely gene therapy approaches.
SHIVs containing an HIV envelope (env-SHIVs) are a useful challenge virus for macaque models of HIV infection, and avoid complications associated with alternate coreceptor usage by SIV envelopes [25,29,42,45]. Previously, infectivity of the CCR5-tropic env-SHIV virus SHIV-1157ipd3N4 was evaluated in the pigtailed macaque model [18]. Following a single intrarectal inoculation, four animals exhibited peak viral loads nearing 107 RNA copies/mL plasma. One animal was euthanized during acute infection. Of the remaining three animals, two progressed to chronic infection with viral set points in the range of 104–105 copies/mL, while the third animal controlled infection to below the level of quantification. In the chronically infected animals, CD4+ T-cell depletion was most robust in the gut, and the virus was shown to be highly CCR5-tropic.
To examine the response of SHIV-1157ipd3N4-infected pigtailed macaques to 3-drug ART, we administered SHIV to five animals by single intravenous inoculation and initiated ART at either 8 weeks or 27 weeks post-SHIV challenge. Our pre-ART data closely resemble the viral kinetics previously demonstrated for this species and virus following intrarectal challenge. Initiating our 3-drug ART at either 8 or 27 weeks post-infection led to durable suppression of plasma viremia, suggesting that our regimen is able to antagonize viral replication in vivo, independent of the timing of administration during chronic infection. These results demonstrate that the SHIV-1157ipd3N4/M. nemestrina model will be suitable for preclinical studies of anti-HIV therapies for infected patients on ART.
MATERIALS AND METHODS
Animal Welfare Statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committees of the Fred Hutchinson Cancer Research Center and University of Washington.
Virus Stock
SHIV1157-ipd3N4 was kindly provided by Dr. Ruth Ruprecht [46]. The stock used in this study was passaged in rhesus macaque peripheral blood mononuclear cells (PBMC) and its infectivity determined at 1.9×104 TCID50/mL in TZM-bl cells. For SHIV challenge, a single 500μL dose was administered IV to five animals following 6–8 weeks of pre-inoculation baseline sample collections.
Antiretroviral Therapy
Tenofovir (PMPA) and Emtricitabine (FTC) pure compounds were kindly provided by Gilead Sciences [Foster, CA]. Raltegravir pure compound was kindly provided by Merck [Whitehouse Station, NJ]. A dual solution of 40 mg/mL PMPA and 80 mg/mL FTC was prepared in double-distilled water and dissolved in the presence of NaOH. The solution was filter-sterilized and warmed to 37 degrees Celsius prior to subcutaneous administration. Raltegravir pure compound was mixed with food and frozen. Animals were monitored by veterinary staff to confirm complete consumption of Raltegravir dose.
Tissue Collection and Blood Processing
Endoscope-guided pinch biopsies were conducted as previously described [18]. Briefly, twenty-three 1mm pinch biopsies were collected, using 3mm biopsy forceps, into ice-cold RPMI media containing 10% FBS, 25 mM HEPES, 2mM L-glutamine, and 1% pen/strep for isolation and analysis of T lymphocytes (see below).
Peripheral blood was drawn by venipuncture into EDTA tubes (for isolation of plasma and PBMC) or serum separation tubes (SST) (for isolation of serum). Plasma for viral load measurements was obtained from peripheral blood in EDTA by Ficoll centrifugation. Flow cytometry was conducted from whole blood samples as previously described [18].
Immunophenotyping, Plasma Viral Load, and ELISA Measurements from Blood
Cells were stained with CD3-FITC (SP34-2) and CD4-PerCP-Cy5.5 (L200), and fixed with 1% paraformaldehyde prior to analysis on a FACSCalibur flow cytometer (antibodies and instrument from Becton Dickinson and Company). Data acquisition and analysis was conducted as previously described [18]. Viral RNA was isolated from EDTA-plasma, reverse transcribed, and analyzed by real-time PCR as previously described [40]. Whole virus SIVmac and HIV-1SF162 gp120-specific antibody titers were measured by ELISA and analyzed as previously described [20].
Cell Isolation and Immunophenotyping of GI Biopsy Samples
Pinch biopsies in RPMI media were dissociated in the presence of 0.5 mg/mL collagenase and 1 U/mL DNase I, stained with 7-AAD and anti-CD3-PE antibody (SP34-2), and counted on a guava cytometer (EMD Millipore). 2×104 cells were stained with CD3-Ax700 (SP34-2), CD4-PerCP-Cy5.5 (L200) and CD8-APC-Cy7 (SK1) antibodies and AVID Aqua viability dye (Invitrogen) and analyzed on a LSR II flow cytometer (Becton Dickinson and Company). Live cells were gated for CD3+. Data represent the proportion of CD4+ and CD4+CD8+ cells from the CD3+ gate.
RESULTS
Previous reports have demonstrated robust infectivity in multiple macaque species using the Clade C env chimeric virus SHIV-1157ipd3N4 [18,21,46]. To evaluate the response of animals infected with this virus to antiretroviral therapy (ART), we first infected two pigtailed macaques with 9,500 50% tissue culture infectious doses (TCID50) of SHIV1157-ipd3N4 by intravenous (IV) administration. Peak viremia of 1–2×107 viral RNA copies per mL plasma was observed in both animals two weeks after infection (Figure 1). Viral set point was maintained at approximately 1×105 copies/mL, and persisted for 20 weeks in the absence of antiretroviral therapy. To evaluate the response of our SHIV-infected animals to a 3-drug ART regimen, we began daily administration of PMPA (20 mg/kg, subcutaneous injection), FTC (40 mg/kg, subcutaneous injection), and Raltegravir (150 mg, dosed orally with food two times per day) at 27 weeks post-infection. In both animals, a rapid drop in viral load of approximately 4 logs was observed over the course of the first two weeks of ART administration. Subsequently, animal Z09144 maintained suppressed viral loads at or below 100 copies/mL plasma, while viral suppression in animal Z09087 was slightly less robust; however, at eight weeks post-ART, both animals’ plasma viral loads were below 50 copies/mL (Figure 1). These results suggest that IV infection of pigtailed macaques with SHIV1157-ipd3n4 follows similar kinetics to those previously described for intrarectal challenge, and that 3-drug ART leads to a durable and robust suppression of plasma viremia.
Figure 1. SHIV-1157ipd3N4 Infection Kinetics in Pigtailed Macaques and Suppression by 3-Drug ART.
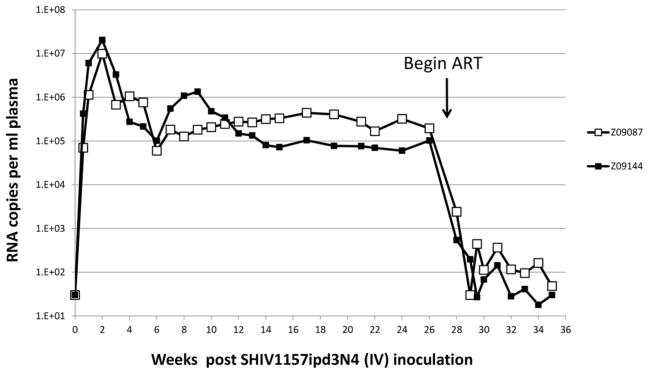
Following intravenous challenge with 9,500 TCID50 of SHIV-1157ipd3N4, two pigtailed macaques (ID’s Z09087 and Z09144) plasma viral loads were monitored by real time RT-PCR at the indicated time points. Arrow denotes time point at which 3-drug ART was initiated.
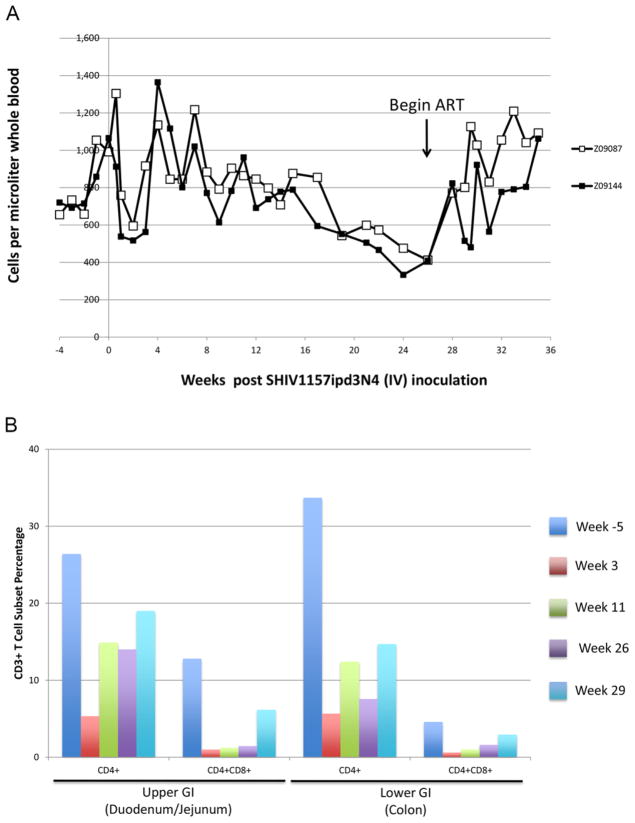
To assess the depletion of circulating helper T-cells after SHIV challenge, we quantified CD3+CD4+ cells in peripheral blood following infection with SHIV1157-ipd3N4. Consistent with past results, decrease in CD4+ T-cell count following infection in these animals was modest (Figure 2A). Whereas counts ranged between 600 and 1000 cells/μL whole blood prior to infection, we observed a nadir count of approximately 500 cells/μL post-infection, corresponding with peak viral load at 2 weeks post-infection (Figure 1). Cell counts rebounded to 1100 (animal Z09087) or 1400 (animal Z09144) cells/μL at 4 weeks post-infection, and gradually declined to 400 cells/μL at week 26 post-infection. These data are consistent with previous findings in SHIV1157-ipd3N4-infected pigtailed macaques [18]. Initiation of ART led to an immediate and sustained increase in CD3+CD4+ cells in peripheral blood. By week 28, levels approximated those found prior to SHIV challenge. These findings demonstrate that our SHIV-macaque model follows the predicted dynamics of T-cell depletion in peripheral blood, and that administration of ART restores CD3+CD4+ counts to pre-infection levels.
Figure 2. Depletion of CD4+T-Cells in Peripheral Blood and GI Tract Following SHIV Challenge and Reconstitution Following ART.
A) Following IV challenge, CD3+CD4+ T-cells from hemolysed whole blood were measured by flow cytometry at the indicated time points. B) Gastrointestinal biopsies were collected from the upper GI tract and lower GI tract of animal Z09087 5 weeks prior to infection (Dark blue) or 3 weeks (red), 11 weeks (green), 26 weeks (purple) or 29 weeks (light blue) post-infection. Collagenase-treated biopsy samples were analyzed for CD3+, CD4+, and CD8+ cells by flow cytometry. Data shown represent the CD4+, and CD4+CD8+ percentages from the CD3+ gate.
In rhesus macaques, the depletion of gut-associated CD4+ T-cells following infection with SHIV1157-ipd3N4 follows a more dramatic course than depletion of T-cells in peripheral blood [18]. Therefore, we examined the percentage of CD4+ T-cells in gut biopsies taken from the duodenum and jejunum (“upper GI”) and colon (“lower GI”) of our pigtailed macaques. As shown in Figure 2B, CD4+ cells made up 26.4% of the total CD3+ cell count in upper GI biopsy samples, and 33.7% in lower GI biopsy samples for animal Z09087; data are not shown for animal Z09144 due to a surgical complication during the week 3 GI biopsy. At week 3 after SHIV challenge in animal Z09087, the proportion of CD4+ T-cells dropped to 5% in both upper and lower GI. We also measured the proportion of CD3+ cells that expressed both CD4 and CD8. These CD4+CD8+ double positive cells in the gut have been previously shown to be highly active, highly CCR5-expressing T-cells that are primary targets for SIV infection in macaques [36,38,50]. Consistent with these findings, double positive T-cells from upper and lower GI biopsies were significantly depleted in animal Z09087. At weeks 11 and 26 post-infection, double positive cells rebounded at a slower rate relative to CD4 single-positive cells (Figure 2). A final GI biopsy was performed at week 29 post-infection, two weeks after initiation of ART therapy. Proportions of CD4 single-positive cells from upper and lower GI biopsies displayed a modest increase following initiation of ART, while double positive cells increased robustly, especially in duodenum/jejunum. Collectively, these results confirm that gut associated helper T-cells, especially double positive cells, are preferential targets for SHIV-dependent depletion, and that ART restores their levels proportionally.
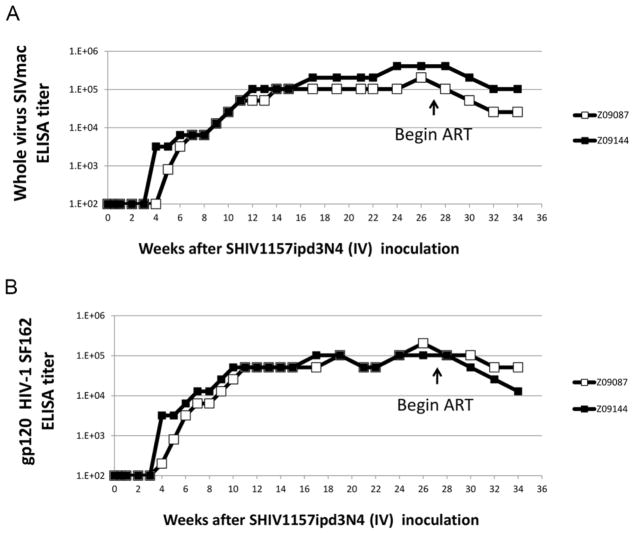
As a final measure of our animals’ response to SHIV1157-ipd3N4 challenge and antiretroviral therapy, we measured anti-SHIV antibodies in serum, using ELISAs directed against SIVmac239, the backbone of SHIV1157-ipd3N4, and against gp120 from HIV-1 SF162 (Figure 3). Anti-SIVmac239 antibodies were first detected in animal Z09144 at 4 weeks post-infection, and in animal Z09087 at 5 weeks post-infection (Figure 3A). Anti-HIV gp120 antibodies were detected in both animals 4 weeks after infection (Figure 3B). Consistent with past results [18], antibody titers increased to approximately 1×105 at 25 weeks post-infection. Following initiation of ART at week 27, these titers dropped by 0.5–1 logs. These data show that humoral immune response to SHIV1157-ipd3N4 arises at the predicted timepoints post-infection, and is reduced following ART-mediated suppression of SHIV viremia.
Figure 3. SHIV-Specific Antibody Responses Before and After ART.
A) Following IV challenge, serum samples were collected at the indicated time points and titered for SIVmac-specific antibodies by whole virus ELISA. B) Same as A), except titer was determined for HIV-1SF162 gp120 env.
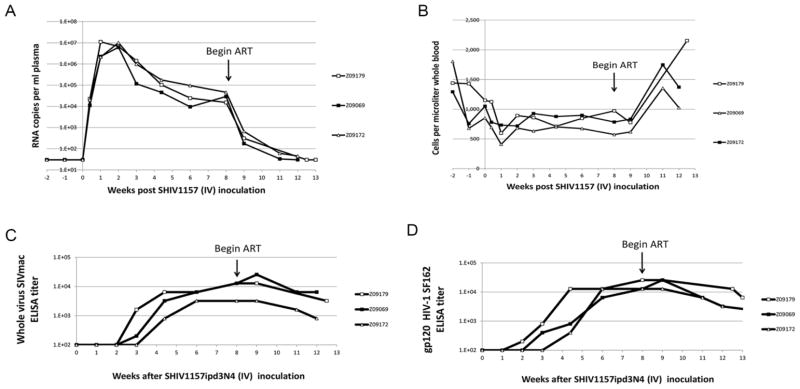
Finally, we investigated whether the time point at which ART was initiated affected the levels of suppressed plasma viremia in our animals. We challenged three animals with the same intravenous dose of SHIV-1157ipd3N4 as Z09087 and Z09144, and then initiated ART at 8 weeks post-infection, rather than 27 weeks post-infection. Figure 4 shows viral load, peripheral blood CD4+ T-cell counts, and antibody response from these three animals, Z09179, Z09069, and Z09172. Peak viral load and viral set point in these animals resembled those recorded for Z09144 and Z09087 (Figure 4A), as did dynamics of CD4+ depletion in peripheral blood (Figure 4B) and anti-SIV and anti-HIV antibody production (Figure 4C and 4D). Importantly, ART mediated similar kinetics of viral suppression, CD4+ rebound, and reduction in anti-SHIV antibody response at 8 weeks (Figure 4) as compared to 27 weeks after infection (Figure 1, 2, 3). One exception was that suppressed viral load took longer to stabilize in the animals receiving ART 27 weeks post-infection relative to the animals receiving ART 8 weeks post-infection. In sum, these results suggest that viral evolution between weeks 8 and 27 post-infection does not significantly impact response to our 3-drug ART regimen.
Figure 4. ART Therapy Similarly Suppresses SHIV Viremia in 8-Week Post-Infection Animals.
A) Plasma viral load was measured as described in Figure 1A, for three animals receiving ART 8 weeks after SHIV challenge. B) CD3+CD4+ T-cell counts from hemolysed whole blood were measured from animals receiving ART 8 weeks after infection. C) SIV-specific and D) HIV-1SF162 gp120 env-specific antibody responses in 8-week post-infection, ART-treated animals.
DISCUSSION
Here, we show the dynamics of SHIV viremia, CD4+ T-cell counts and serological responses in five pigtailed macaques prior to and following the initiation of ART. To our knowledge, this is the first study demonstrating robust response to ART in a pathogenic env-SHIV model: 3-drug ART is capable of suppressing SHIV viremia in our animal model to levels approaching those associated with suppression in human patients. Our findings also suggest that the timing of ART initiation during chronic SHIV infection does not affect the dynamics of drug-mediated viral suppression. Finally, we observe that viral kinetics following IV challenge with SHIV-1157ipd3N4 parallel results observed in intrarectally challenged macaques.
The key finding from these studies is that administration of 3-drug ART consisting of PMPA, FTC, and Raltegravir results in a four-log decrease in plasma viral load in our animals. Most recently, 3-drug ART studies in pigtails have focused on the use of PMPA/FTC/Efavirenz in RT-SHIV infected animals [7,23]. Since our SHIV/macaque model utilized a virus containing SIV RT, we could not use the non-nucleoside reverse transcriptase inhibitor Efavirenz in our experiments [54]. Instead, we added the integrase inhibitor Raltegravir; although this drug has been widely used in rhesus macaques [15,32,44], this is, to our knowledge, the first demonstration of its efficacy in a pigtail model. In combination with PMPA and FTC, Raltegravir showed great success in suppressing viral loads in vivo using the same dosing parameters as rhesus macaques, suggesting that drug metabolism is not adversely affected in pigtails versus rhesus. Notably, PMPA and FTC are much better characterized in the pigtail model than Raltegravir, and thus the success of these drugs in our model is unsurprising [12,43]. In sum, our 3-drug ART regimen was effective in suppressing env-SHIV viremia in M. nemestrina. In future experiments, it will be important to demonstrate that this regimen is equally effective with other SIV and SHIV strains. Recent experiments have also sought to evaluate various intensified ART regimens in rhesus macaque models that suppress viral loads to levels associated with suppression in human patients [44]. It will also be important to evaluate whether intensification with additional ART drugs is capable of driving plasma viremia to lower levels in our SHIV/macaque model.
A related finding in this work was that initiation of 3-drug ART following acute infection (8 weeks post-infection) versus during chronic infection (27 weeks post-infection) did not noticeably affect the extent of ART-mediated viral suppression. A continuing debate within the field centers on the optimal point at which to start ART in nonhuman primate models; while earlier initiation likely results in more robust suppression to levels associated with pharmacological control in human patients, later initiation is more consistent with the time frame at which patients initiate therapy [8,15,30,39]. Our findings suggest that viral replication is comparably susceptible to ART administered either 2 months or 6 months post-infection. Sequencing of the viral population at various time points before and after ART will be important to better understand viral evolution at various time points pre-ART, and the ability of our drug regimen to restrict these populations.
Pre-ART data demonstrated that our intravenous SHIV challenge led to peak viral loads, viral set points, and CD4+ T-cell depletion dynamics that were highly comparable to previous intrarectal challenge experiments [18]. The extent to which viral sequence [24,33] and pathogenesis [6,47] varies between mucosally- and intravenously-inoculated subjects remains unclear. Our results imply that our SHIV-1157ipd3N4 inoculum, which was expanded on rhesus macaque PBMC prior to inoculation, replicates comparably, independent of challenge route. We are currently sequencing our viral stocks, as well as infected animals, to better understand the dynamics of viral evolution and selection in this model following either intrarectal or intravenous challenge.
In conclusion, we have demonstrated effective suppression of SHIV viremia in pigtailed macaques after administration of 3-drug ART. The timing of ART initiation up to 6 months after SHIV challenge had only minor effects on viremic suppression. These findings are directly applicable to multiple anti-HIV intervention models, including anti-HIV gene therapy approaches.
Acknowledgments
Acknowledgements of Funding: The authors would like to acknowledge the impact of NIH-supported Martin Delaney Collaboratory grant U19 AI 096111 to H.-P.K., S.C.D., S.L.H., M.P., A.E.W, and K.R.J. This effort was supported in part by R01 AI80326 and R01 HL84345 (to H.-P.K.). H.-P.K. is a Markey Molecular Medicine Investigator and also supported by the José Carreras/E. Donnall Thomas Endowed Chair for Cancer Research.
We are indebted to Gilead Sciences and Merck for supply of our ART drugs. We thank Dr. Ruth Ruprecht for supplying the SHIV-1157ipd3N4 virus stock, Grace Choi, Helen Crawford and Bonnie Larson for help in preparing this manuscript, Baoping Tian, Ryan Wallerstedt, and Taryn Urion for sample processing and virologic and serologic analyses, Heather Mack for T-cell phenotyping, Leon Flanary and Joel Ahrens for conducting GI biopsy procedures, and Veronica Nelson, Erica Curry, and Kelvin Sze for excellent all-around support in our pigtailed macaque studies.
References
- 1.Ambrose Z, Palmer S, Boltz VF, Kearney M, Larsen K, Polacino P, Flanary L, Oswald K, Piatak M, Jr, Smedley J, Shao W, Bischofberger N, Maldarelli F, Kimata JT, Mellors JW, Hu SL, Coffin JM, Lifson JD, KewalRamani VN. Suppression of Viremia and Evolution of Human Immunodeficiency Virus Type 1 Drug Resistance in a Macaque Model for Antiretroviral Therapy. J Virol. 2007;81:12145–12155. doi: 10.1128/JVI.01301-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baroncelli S, Negri DR, Michelini Z, Cara A. Macaca Mulatta, Fascicularis and Nemestrina in AIDS Vaccine Development (Review) Expert Review of Vaccines. 2008;7:1419–1434. doi: 10.1586/14760584.7.9.1419. [DOI] [PubMed] [Google Scholar]
- 3.Beard BC, Trobridge GD, Ironside C, McCune JS, Adair JE, Kiem H-P. Efficient and Stable MGMT-Mediated Selection of Long-Term Repopulating Stem Cells in Nonhuman Primates. J Clin Invest. 2010;120:2345–2354. doi: 10.1172/JCI40767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belshan M, Kimata JT, Brown C, Cheng X, McCulley A, Larsen A, Thippeshappa R, Hodara V, Giavedoni L, Hirsch V, Ratner L. Vpx Is Critical for SIVmne Infection of Pigtail Macaques. Retrovirology. 2012;9:32. doi: 10.1186/1742-4690-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger C, Berger M, Anderson D, Riddell SR. A Non-Human Primate Model for Analysis of Safety, Persistence, and Function of Adoptively Transferred T Cells. Journal of Medical Primatology. 2011;40:88–103. doi: 10.1111/j.1600-0684.2010.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biesinger T, White R, Yu Kimata MT, Wilson BK, Allan JS, Kimata JT. Relative Replication Capacity of Phenotypic SIV Variants During Primary Infections Differs With Route of Inoculation. Retrovirology. 2010;7:88. doi: 10.1186/1742-4690-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boltz VF, Ambrose Z, Kearney MF, Shao W, KewalRamani VN, Maldarelli F, Mellors JW, Coffin JM. Ultrasensitive Allele-Specific PCR Reveals Rare Preexisting Drug-Resistant Variants and a Large Replicating Virus Population in Macaques Infected With a Simian Immunodeficiency Virus Containing Human Immunodeficiency Virus Reverse Transcriptase. J Virol. 2012;86:12525–12530. doi: 10.1128/JVI.01963-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourry O, Mannioui A, Sellier P, Roucairol C, Durand-Gasselin L, Dereuddre-Bosquet N, Benech H, Roques P, Le GR. Effect of a Short-Term HAART on SIV Load in Macaque Tissues Is Dependent on Time of Initiation and Antiviral Diffusion. Retrovirology. 2010;7:78. doi: 10.1186/1742-4690-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brennan G, Kozyrev Y, Hu SL. TRIMCyp Expression in Old World Primates Macaca Nemestrina and Macaca Fascicularis. PNAS. 2008;105:3569–3574. doi: 10.1073/pnas.0709511105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brennan G, Kozyrev Y, Kodama T, Hu S-L. Novel TRIM5 Isoforms Expressed by Macaca Nemestrina. J Virol. 2007;81:12210–12217. doi: 10.1128/JVI.02499-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen ZW, Shen Y, Davis IC, Shen L, Letvin NL, Fultz PN. Down-Regulation of Macaque Gammadelta + T Cells in Lymphoid Compartments After Rectal Infection With SIVsmmPBj14. Journal of Medical Primatology. 2000;29:143–147. doi: 10.1034/j.1600-0684.2000.290307.x. [DOI] [PubMed] [Google Scholar]
- 12.Clements JE, Li M, Gama L, Bullock B, Carruth LM, Mankowski JL, Zink MC. The Central Nervous System Is a Viral Reservoir in Simian Immunodeficiency Virus--Infected Macaques on Combined Antiretroviral Therapy: a Model for Human Immunodeficiency Virus Patients on Highly Active Antiretroviral Therapy. Journal of Neurovirology. 2005;11:180–189. doi: 10.1080/13550280590922748-1. [DOI] [PubMed] [Google Scholar]
- 13.Ebenezer GJ, McArthur JC, Polydefkis M, Dorsey JL, O’donnell R, Hauer P, Adams RJ, Mankowski JL. SIV-Induced Impairment of Neurovascular Repair: a Potential Role for VEGF. Journal of Neurovirology. 2012;18:222–230. doi: 10.1007/s13365-012-0102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Lerma JG, Heneine W. Animal Models of Antiretroviral Prophylaxis for HIV Prevention. Current Opinion in HIV & AIDS. 2012;7:505–513. doi: 10.1097/COH.0b013e328358e484. [DOI] [PubMed] [Google Scholar]
- 15.Graham DR, Gama L, Queen SE, Li M, Brice AK, Kelly KM, Mankowski JL, Clements JE, Zink MC. Initiation of HAART During Acute Simian Immunodeficiency Virus Infection Rapidly Controls Virus Replication in the CNS by Enhancing Immune Activity and Preserving Protective Immune Responses. Journal of Neurovirology. 2011;17:120–130. doi: 10.1007/s13365-010-0005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatziioannou T, Evans DT. Animal Models for HIV/AIDS Research. Nature Reviews. 2012;10:852–867. doi: 10.1038/nrmicro2911. Microbiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henning T, Fakile Y, Phillips C, Sweeney E, Mitchell J, Patton D, Sturdevant G, Caldwell HD, Secor WE, Papp J, Hendry RM, McNicholl J, Kersh E. Development of a Pigtail Macaque Model of Sexually Transmitted Infection/HIV Coinfection Using Chlamydia Trachomatis, Trichomonas Vaginalis, and SHIV(SF162P3) Journal of Medical Primatology. 2011;40:214–223. doi: 10.1111/j.1600-0684.2011.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho O, Larsen K, Polacino P, Li Y, Anderson D, Song R, Ruprecht RM, Hu SL. Pathogenic Infection of Macaca Nemestrina With a CCR5-Tropic Subtype-C Simian-Human Immunodeficiency Virus. Retrovirology. 2009;6:65. doi: 10.1186/1742-4690-6-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu SL, Abrams K, Barber GN, Moran P, Zarling JM, Langlois AJ, Kuller L, Morton WR, Benveniste RE. Protection of Macaques Against SIV Infection by Subunit Vaccines of SIV Envelope Glycoprotein Gp160. Science. 1992;255:456–459. doi: 10.1126/science.1531159. [DOI] [PubMed] [Google Scholar]
- 20.Hu SL, Zarling JM, Chinn J, Travis BM, Moran PA, Sias J, Kuller L, Morton WR, Heidecker G, Benveniste RE. Protection of Macaques Against Simian AIDS by Immunization With a Recombinant Vaccinia Virus Expressing the Envelope Glycoproteins of Simian Type D Retrovirus. PNAS. 1989;86:7213–7217. doi: 10.1073/pnas.86.18.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Humbert M, Rasmussen RA, Song R, Ong H, Sharma P, Chenine AL, Kramer VG, Siddappa NB, Xu W, Else JG, Novembre FJ, Strobert E, O’Neil SP, Ruprecht RM. SHIV-1157i and Passaged Progeny Viruses Encoding R5 HIV-1 Clade C Env Cause AIDS in Rhesus Monkeys. Retrovirology. 2008;5:94. doi: 10.1186/1742-4690-5-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jasny E, Geer S, Frank I, Vagenas P, Aravantinou M, Salazar AM, Lifson JD, Piatak M, Jr, Gettie A, Blanchard JL, Robbiani M. Characterization of Peripheral and Mucosal Immune Responses in Rhesus Macaques on Long-Term Tenofovir and Emtricitabine Combination Antiretroviral Therapy. J Acquir Immune Defic Syndr. 2012;61:425–435. doi: 10.1097/QAI.0b013e318266be53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kearney JF, Radbruch A, Liesegang B, Rajewsky K. A New Mouse Myeloma Cell Line That Has Lost Immunoglobulin Expression but Permits the Construction of Antibody-Secreting Hybrid Cell Lines. J Immunol. 1979;123:1548–1550. [PubMed] [Google Scholar]
- 24.Keele BF, Li H, Learn GH, Hraber P, Giorgi EE, Grayson T, Sun C, Chen Y, Yeh WW, Letvin NL, Mascola JR, Nabel GJ, Haynes BF, Bhattacharya T, Perelson AS, Korber BT, Hahn BH, Shaw GM. Low-Dose Rectal Inoculation of Rhesus Macaques by SIVsmE660 or SIVmac251 Recapitulates Human Mucosal Infection by HIV-1. J Exp Med. 2009;206:1117–1134. doi: 10.1084/jem.20082831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiene M, Marzi A, Urbanczyk A, Bertram S, Fisch T, Nehlmeier I, Gnirss K, Karsten CB, Palesch D, Munch J, Chiodi F, Pohlmann S, Steffen I. The Role of the Alternative Coreceptor GPR15 in SIV Tropism for Human Cells. Virology. 2012;433:73–84. doi: 10.1016/j.virol.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Kinman L, Bui T, Larsen K, Tsai CC, Anderson D, Morton WR, Hu SL, Ho RJ. Optimization of Lipid-Indinavir Complexes for Localization in Lymphoid Tissues of HIV-Infected Macaques. Journal of Acquired Immune Deficiency Syndromes: JAIDS. 2006;42:155–161. doi: 10.1097/01.qai.0000214822.33905.87. [DOI] [PubMed] [Google Scholar]
- 27.Klatt NR, Canary LA, Sun X, Vinton CL, Funderburg NT, Morcock DR, Quiñones M, Deming CB, Perkins M, Hazuda DJ, Miller MD, Lederman MM, Segre JA, Lifson JD, Haddad EK, Estes JD, Brenchley JM. Probiotic/Prebiotic Supplementation of Antiretrovirals Improves Gastrointestinal Immunity in SIV-Infected Macaques. J Clin Invest. 2013 doi: 10.1172/JCI66227. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klatt NR, Canary LA, Vanderford TH, Vinton CL, Engram JC, Dunham RM, Cronise HE, Swerczek JM, Lafont BA, Picker LJ, Silvestri G, Brenchley JM. Dynamics of Simian Immunodeficiency Virus SIVmac239 Infection in Pigtail Macaques. J Virol. 2012;86:1203–1213. doi: 10.1128/JVI.06033-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lakhashe SK, Wang W, Siddappa NB, Hemashettar G, Polacino P, Hu SL, Villinger F, Else JG, Novembre FJ, Yoon JK, Lee SJ, Montefiori DC, Ruprecht RM, Rasmussen RA. Vaccination Against Heterologous R5 Clade C SHIV: Prevention of Infection and Correlates of Protection. PLoS ONE [Electronic Resource] 2011;6:e22010. doi: 10.1371/journal.pone.0022010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le T, Wright EJ, Smith DM, He W, Catano G, Okulicz JF, Young JA, Clark RA, Richman DD, Little SJ, Ahuja SK. Enhanced CD4+ T-Cell Recovery With Earlier HIV-1 Antiretroviral Therapy. N Engl J Med. 2013;368:218–230. doi: 10.1056/NEJMoa1110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Cleveland B, Klots I, Travis B, Richardson BA, Anderson D, Montefiori D, Polacino P, Hu SL. Removal of a Single N-Linked Glycan in Human Immunodeficiency Virus Type 1 Gp120 Results in an Enhanced Ability to Induce Neutralizing Antibody Responses. J Virol. 2008;82:638–651. doi: 10.1128/JVI.01691-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lugli E, Mueller YM, Lewis MG, Villinger F, Katsikis PD, Roederer M. IL-15 Delays Suppression and Fails to Promote Immune Reconstitution in Virally Suppressed Chronically SIV-Infected Macaques. Blood. 2011;118:2520–2529. doi: 10.1182/blood-2011-05-351155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makedonas G, Bruneau J, Alary M, Tsoukas CM, Lowndes CM, Lamothe F, Bernard NF. Comparison of HIV-Specific CD8 T-Cell Responses Among Uninfected Individuals Exposed to HIV Parenterally and Mucosally. AIDS. 2005;19:251–259. [PubMed] [Google Scholar]
- 34.Maloveste SM, Chen D, Gostick E, Vivian JP, Plishka RJ, Iyengar R, Kruthers RL, Buckler-White A, Brooks AG, Rossjohn J, Price DA, Lafont BA. Degenerate Recognition of MHC Class I Molecules With Bw4 and Bw6 Motifs by a Killer Cell Ig-Like Receptor 3DL Expressed by Macaque NK Cells. J Immunol. 2012;189:4338–4348. doi: 10.4049/jimmunol.1201360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malzahn J, Shen C, Caruso L, Ghosh P, Ramachandra Sankapal S, Barratt-Boves S, Gupta P, Chen Y. Effect of Early Anti-Retroviral Therapy on the Pathogenic Changes in Mucosal Tissues of SIV Infected Rhesus Macaques. Viology Journal. 2013;9:269. doi: 10.1186/1743-422X-9-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsumoto Y, Miura T, Akari H, Goto Y, Haga T. Peripheral Blood CD4 and CD8 Double-Positive T Cells of Rhesus Macaques Become Vulnerable to Simian Immunodeficiency Virus by in Vitro Stimulation Due to the Induction of CCR5. J Vet Med Sci. 2010;72:1057–1061. doi: 10.1292/jvms.09-0588. [DOI] [PubMed] [Google Scholar]
- 37.McClure J, Schmidt AM, Rey-Cuille MA, Bannink J, Misher L, Tsai CC, Anderson DM, Morton WR, Hu SL. Derivation and Characterization of a Highly Pathogenic Isolate of Human Immunodeficiency Virus Type 2 That Causes Rapid CD4+ Cell Depletion in Macaca Nemestrina. Journal of Medical Primatology. 2000;29:114–126. doi: 10.1034/j.1600-0684.2000.290304.x. [DOI] [PubMed] [Google Scholar]
- 38.Pahar B, Lackner AA, Veazey RS. Intestinal Double-Positive CD4+CD8+ T Cells Are Highly Activated Memory Cells With an Increased Capacity to Produce Cytokines. Eur J Immunol. 2006;36:583–592. doi: 10.1002/eji.200535520. [DOI] [PubMed] [Google Scholar]
- 39.Perez-Molina JA, Diaz-Menendez M, Plana MN, Zamora J, Lopez-Velez R, Moreno S. Very Late Initiation of HAART Impairs Treatment Response at 48 and 96 Weeks. Results From a Meta-Analysis of Randomized Clinical Trials (Review) J Antimicrob Chemother. 2012;67:312–321. doi: 10.1093/jac/dkr478. [DOI] [PubMed] [Google Scholar]
- 40.Polacino P, Cleveland B, Zhu Y, Kimata JT, Overbaugh J, Anderson D, Hu SL. Immunogenicity and Protective Efficacy of Gag/Pol/Env Vaccines Derived From Temporal Isolates of SIVmne Against Cognate Virus Challenge. Journal of Medical Primatology. 2007;36:254–265. doi: 10.1111/j.1600-0684.2007.00243.x. [DOI] [PubMed] [Google Scholar]
- 41.Polacino P, Stallard V, Montefiori DC, Brown CR, Richardson BA, Morton WR, Benveniste RE, Hu SL. Protection of Macaques Against Intrarectal Infection by a Combination Immunization Regimen With Recombinant Simian Immunodeficiency Virus SIVmne Gp160 Vaccines. J Virol. 1999;73:3134–3146. doi: 10.1128/jvi.73.4.3134-3146.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ren W, Mumbauer A, Zhuang K, Harbison C, Knight H, Westmoreland S, Gettie A, Blanchard J, Cheng-Mayer C. Mucosal Transmissibility, Disease Induction and Coreceptor Switching of R5 SHIVSF162P3N Molecular Clones in Rhesus Macaques. Retrovirology. 2013;10:9. doi: 10.1186/1742-4690-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen A, Zink MC, Mankowski JL, Chadwick K, Margolick JB, Carruth LM, Li M, Clements JE, Siliciano RF. Resting CD4+ T Lymphocytes but Not Thymocytes Provide a Latent Viral Reservoir in a Simian Immunodeficiency Virus-Macaca Nemestrina Model of Human Immunodeficiency Virus Type 1-Infected Patients on Highly Active Antiretroviral Therapy. J Virol. 2003;77:4938–4949. doi: 10.1128/JVI.77.8.4938-4949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shytaj IL, Norelli S, Chirullo B, Della CA, Collins M, Yalley-Ogunro J, Greenhouse J, Iraci N, Acosta EP, Barreca ML, Lewis MG, Savarino A. A Highly Intensified ART Regimen Induces Long-Term Viral Suppression and Restriction of the Viral Reservoir in a Simian AIDS Model. PLoS Pathogens. 2012;8:e1002774. doi: 10.1371/journal.ppat.1002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sina ST, Ren W, Cheng-Mayer C. Coreceptor Use in Nonhuman Primate Models of HIV Infection (Review) Journal of Translational Medicine. 2011;9 (Suppl 1):S7. doi: 10.1186/1479-5876-9-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song RJ, Chenine AL, Rasmussen RA, Ruprecht CR, Mirshahidi S, Grisson RD, Xu W, Whitney JB, Goins LM, Ong H, Li PL, Shai-Kobiler E, Wang T, McCann CM, Zhang H, Wood C, Kankasa C, Secor WE, McClure HM, Strobert E, Else JG, Ruprecht RM. Molecularly Cloned SHIV-1157ipd3N4: a Highly Replication- Competent, Mucosally Transmissible R5 Simian-Human Immunodeficiency Virus Encoding HIV Clade C Env. J Virol. 2006;80:8729–8738. doi: 10.1128/JVI.00558-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevceva L, Tryniszewska E, Hel Z, Nacsa J, Kelsall B, Washington PR, Franchini G. Differences in Time of Virus Appearance in the Blood and Virus-Specific Immune Responses in Intravenous and Intrarectal Primary SIVmac251 Infection of Rhesus Macaques; a Pilot Study. BMC Infectious Diseases. 2001;1:9. doi: 10.1186/1471-2334-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trobridge GD, Wu RA, Beard BC, Chiu SY, Muñoz NM, von Laer D, Rossi JJ, Kiem H-P. Protection of Stem Cell-Derived Lymphocytes in a Primate AIDS Gene Therapy Model After in Vivo Selection. PLoS ONE. 2009;4:e7693. doi: 10.1371/journal.pone.0007693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tso FY, Tully DC, Gonzalez S, Quince C, Ho O, Polacino P, Ruprecht RM, Hu SL, Wood C. Dynamics of Envelope Evolution in Clade C SHIV-Infected Pig-Tailed Macaques During Disease Progression Analyzed by Ultra-Deep Pyrosequencing. PLoS ONE [Electronic Resource] 2012;7:e32827. doi: 10.1371/journal.pone.0032827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X, Das A, Lackner AA, Veazey RS, Pahar B. Intestinal Double-Positive CD4+CD8+ T Cells of Neonatal Rhesus Macaques Are Proliferating, Activated Memory Cells and Primary Targets for SIVMAC251 Infection. Blood. 2008;112:4981–4990. doi: 10.1182/blood-2008-05-160077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watson A, Ranchalis J, Travis B, McClure J, Sutton W, Johnson PR, Hu SL, Haigwood NL. Plasma Viremia in Macaques Infected With Simian Immunodeficiency Virus: Plasma Viral Load Early in Infection Predicts Survival. J Virol. 1997;71:284–290. doi: 10.1128/jvi.71.1.284-290.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watts KL, Zhang X, Beard BC, Chiu SY, Trobridge GD, Humphries RK, Kiem H-P. Differential Effects of HOXB4 and NUP98-HOXA10hd on Hematopoietic Repopulating Cells in a Nonhuman Primate Model. Hum Gene Ther. 2011;22:1475–1482. doi: 10.1089/hum.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winnall WR, Sexton A, Alcantara S, Roath S, De RR, Kent SJ. Characterisation of Simian Immunodeficiency Virus-Infected Cells in Pigtail Macaques. Virology. 2012;428:11–20. doi: 10.1016/j.virol.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 54.Witvrouw M, Pannecouque C, Switzer WM, Folks TM, De CE, Heneine W. Susceptibility of HIV-2, SIV and SHIV to Various Anti-HIV-1 Compounds: Implications for Treatment and Postexposure Prophylaxis. Antiviral Therapy. 2004;9:57–65. [PubMed] [Google Scholar]
- 55.Xu Y, Weatherall C, Bailey M, Alcantara S, De Rose R, Estaquier J, Wilson K, Suzuki K, Corbeil J, Cooper DA, Kent SJ. SIV Infects Follicular Helper CD4 T Cells in Lymphoid Tissues During Pathogenic Infection of Pigtail Macaques. J Virol. 2013 doi: 10.1128/JVI.02497-12. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]