Abstract
Background
The gram-negative organism, Burkholderia pseudomallei, is responsible for the disease melioidosis. Septic arthritis and osteomyelitis due to B. pseudomallei are rare but recognised presentations of the disease.
Methods
A prospective database of all cases of melioidosis in the Northern Territory of Australia has been kept since October 1989. Entries to April 2009 were reviewed and cases involving bone and/or joint were investigated. We also present in detail the case reports of 3 presentations of bone and joint melioidosis.
Results
There were 536 presentations of melioidosis during the 20-year study period. Amongst these, there were 13 patients with primary septic arthritis and 7 cases of primary osteomyelitis. Septic arthritis and osteomyelitis were secondary to primary melioidosis elsewhere in 14 and 7 patients respectively. Melioidosis patients with bone/joint involvement were more likely to be Indigenous (p = 0.006) and female (p = 0.023) compared to patients with other presentations of disease.
Conclusions
Timely microbiological diagnosis and prompt treatment of melioidosis involving bone and/or joint with appropriate intravenous antibiotics is important, as is adequate surgical drainage and debridement where indicated. A subsequent protracted course of antibiotic eradication therapy is important to avoid relapse of disease.
Keywords: Melioidosis, Septic arthritis, Osteomyelitis, Infection, Bone
1. Background
The gram-negative organism, Burkholderia pseudomallei, is responsible for the disease melioidosis. First described in 1912 by Whitmore and Krishnaswami,1 this infectious disease became a major problem for American troops during conflict in the Vietnam War, many falling victim to the potentially deadly disease. While being endemic to northern Australia and South East Asia,2 melioidosis is considered to be of emerging significance in other tropical locations and in Europe and the USA in those returning or travelling from melioidosis endemic regions.3 How much this reflects improved diagnosis and/or expanding endemic locations remains unclear. We have documented melioidosis to have previously been the commonest cause of fatal community-acquired bacteraemic pneumonia in our region of tropical northern Australia.4 Although it commonly presents as bacteraemic pulmonary disease, melioidosis is notorious for its wide variety of presentations and its relapsing nature.5,6 The major risk factors for the disease are diabetes mellitus, hazardous alcohol use, chronic renal disease and chronic lung disease.7
B. pseudomallei is an environmental bacterium, surviving in soil and surface water. It gains entrance to the body most commonly through percutaneous inoculation, although inhalation and ingestion can also occur.2 The incidence of infection closely parallels rainfall patterns in many endemic locations, and at Royal Darwin Hospital, as in Thailand, the disease is much less common outside of our wet season months (November to May in Darwin).8,9 The vast majority of cases are from recent infection, with most (around 85%) presenting as acute disease following an incubation period of 1–21 days (median 9 days) and a minority (around 10%) having a more chronic illness, defined as being sick for 2 months or longer.6 Under 5% of cases result from reactivation from a latent focus, with the longest documented period between infection and disease being reported from the USA in a WWII veteran 62 years after presumed infection in South East Asia.10
Septic arthritis and osteomyelitis due to B. pseudomallei are rare but recognised presentations of melioidosis.6,11–13 Involvement of bone or joints occurs more often following dissemination of disease from infection elsewhere in the body. Nevertheless, septic arthritis and osteomyelitis can be the primary manifestation of melioidosis. The prospective database in our region has documented all cases of melioidosis over the last 20 years and this enables us to characterize those patients with septic arthritis and/or osteomyelitis as a primary or secondary manifestation of their disease.
2. Methods
The study was conducted at Royal Darwin Hospital, the tertiary referral hospital for the tropical Northern Territory of Australia. A prospective database of all cases of melioidosis in the Northern Territory has been kept since 1989. Entries in this database from October 1989 to April 2009 were retrospectively reviewed. This review was supplemented with data obtained from a review of case notes and laboratory records during the same period. The frequency, nature and presentations of patients with melioidosis septic arthritis and osteomyelitis were reviewed. The features of these presentations and the patient demographics and risk factors were compared to patients without melioidosis involving bone or joint. Patient details were stored in a Microsoft Access (2003) database and analysed using Stata version 10 (Stata Corporation, Texas). Comparisons were made using Fisher's exact test and the Mann Whitney U test for proportions and continuous data respectively; p < 0.05 was considered significant. Multivariate analysis was not performed due to small numbers.
We also present in detail the case reports of 3 cases of bone and joint melioidosis. Each subject was informed that data concerning the case would be submitted for publication. Approval for the study was obtained from the Human Research Ethics Committee of the Northern Territory Department of Health and the Menzies School of Health Research (HREC 02/38). There were no external sources of funding utilised for this study.
3. Results
There were 536 presentations with melioidosis during the 19.4-year study period. Of these, there were 13 patients (2.4%) with primary septic arthritis and 7 patients (1.3%) with primary osteomyelitis. An additional 14 patients (2.6% of all patients) had septic arthritis secondary to primary melioidosis elsewhere. The primary clinical presentations identified for these cases were pneumonia (6 patients), bacteraemia with no focus (4 patients), genitourinary disease (2 patients) and soft tissue abscess (2 patients). 7 further patients had secondary osteomyelitis (1.3%), with the primary diseases being bacteraemia with no focus (3 patients), pneumonia (1 patient), genitourinary disease (1 patient), soft tissue abscess (1 patient) and skin abscess (1 patient). 7 of the 41 patients with primary or secondary bone and joint melioidosis had clinical evidence of both septic arthritis and osteomyelitis.
Table 1 highlights the features of the 20 patients presenting with primary bone and joint melioidosis compared with those presenting with another primary presentation. A smaller proportion of patients with bone/joint melioidosis had occupational exposure or no documented risk factors; patients with bone/joint melioidosis were younger and a smaller proportion had excessive alcohol consumption, but neither was statistically significant.
Table 1.
Features of patients with primary bone and joint melioidosis versus primary melioidosis elsewhere.
| Primary bone/joint melioidosis (n = 20) | Primary melioidosis elsewhere (n = 516) | p | |
|---|---|---|---|
| Age (at 1st diagnosis) | Median 41 (range 1–63) | Median 49 (range 1–91) | >0.05 |
| Indigenous | 14 (70.0%) | 267 (51.8%) | >0.10 |
| Male | 11 (55.0%) | 361 (70.0%) | >0.10 |
| Diabetes mellitus | 10 (50.0%) | 202 (39.2%) | >0.10 |
| Hazardous alcohol use | 4 (20.0%) | 207 (40.2%) | 0.071 |
| Chronic renal impairment | 2 (10.0%) | 64 (12.4%) | >0.05 |
| Other risk factors for melioidosis | 8 (40.0%) | 226 (43.8%) | >0.10 |
| No documented risk factors for melioidosis | 0 | 63 (12.2%) | 0.020 |
| Occupational exposure | 0 (0.0%) | 95 (18.5%) | 0.034 |
| Blood culture positive | 14 (70.0%) | 283 (55.7%) | >0.10 |
| Septic shock | 4 (20.0%) | 113 (21.9%) | >0.10 |
| Required intensive care management | 4 (25.0%) | 118 (22.9%) | >0.10 |
| Mortality | 2 (10.0%) | 71 (13.8%) | >0.10 |
Table 2 highlights the features of the 41 patients with primary or secondary bone and joint melioidosis compared with patients with melioidosis but no bone or joint involvement. Patients with bone or joint involvement were more likely to be Indigenous (p = 0.006). Although melioidosis is a disease more common in the male population, 53.7% of melioidosis patients with bone or joint involvement were male compared to 70.7% in those without (p = 0.023).
Table 2.
Features of patients with primary or secondary bone and joint melioidosis versus melioidosis elsewhere.
| Primary or secondary bone/joint melioidosis (n = 41) | Melioidosis elsewhere (n = 495) | p | |
|---|---|---|---|
| Age (at 1st diagnosis) | Median 47 (range 1–64) | Median 49 (range 1–91) | >0.10 |
| Indigenous | 30 (73.2%) | 251 (50.7%) | 0.006 |
| Male | 22 (53.7%) | 350 (70.7%) | 0.023 |
| Diabetes mellitus | 20 (48.8%) | 192 (38.8%) | >0.10 |
| Hazardous alcohol use | 15 (36.6%) | 196 (39.6%) | >0.10 |
| Chronic renal impairment | 7 (17.1%) | 59 (11.9%) | >0.10 |
| Other risk factors for melioidosis | 17 (41.5%) | 217 (43.8%) | >0.10 |
| No documented risk factors for melioidosis | 8 (19.5%) | 61 (12.3%) | >0.10 |
| Occupational exposure | 2 (4.9%) | 93 (18.8%) | 0.025 |
| Blood culture positive | 29 (70.7%) | 268 (55.0%) | 0.052 |
| Septic shock | 9 (22.0%) | 108 (21.8%) | >0.10 |
| Required intensive care management | 11 (26.8%) | 111 (22.4%) | >0.10 |
| Mortality | 2 (4.9%) | 71 (14.3%) | 0.089 |
Of the 41 presentations (of either primary or secondary bone or joint melioidosis), 37 involved the lower limb, 3 the upper limb and 1 both upper and lower limbs.
4. Case reports
4.1. Case 1
A 34 year-old previously well indigenous man from a remote Aboriginal community was transferred with a swollen painful right knee, hypotension and oliguria. On examination he was unwell, with a blood pressure of 76/32 mmHg, heart rate of 95 bpm, temperature of 36.7° Celsius and a grossly swollen and tender right knee.
Blood tests demonstrated a white cell count of 13.5 × 109/L (normal range 4.0–11.0) with a neutrophilia of 9.9 × 109/L (normal range 2.5–7.5) and a C-reactive protein of 163 mg/L (normal value < 3.0). An X-ray of the right knee suggested a joint effusion but no bony pathology. The arterial blood gas revealed a metabolic acidosis.
An immediate arthrotomy and washout of his right knee was performed and he was subsequently transferred to the Intensive Care Unit for intubation, inotropic support (which he required for one day) and continuous veno-venous haemofiltration.
Blood cultures and right knee aspirates grew B. pseudomallei. He was treated with IV meropenem and commenced on oral trimethoprim/sulfamethoxazole. CT scans of chest, abdomen and pelvis failed to reveal any lung, prostate or abdominal involvement with melioidosis. His condition improved and he was deemed ready for discharge to the ward on day 4 post admission.
On the ward, he had intensive physiotherapy, and continued intravenous antibiotic therapy. Meropenem was changed to ceftazidime on day 4, however the patient's swelling and pain in the right knee persisted. On day 7 he became unwell with temperatures to 40° Celsius and severe pain in the right knee, he was taken to theatre for a second washout. Cultures from the aspirate again grew B. pseudomallei.
Pain and limited range of motion persisted one week following the second washout, and it was decided to perform a further arthrotomy with washout. Cultures again grew B. pseudomallei. This time, however, his condition improved markedly postoperatively, and the swelling and knee pain subsided significantly. He was discharged on day 22 with the plan to provide ongoing intravenous ceftazidime and oral trimethoprim/sulfamethoxazole through the Hospital In The Home (HITH) programme, to complete a total of four weeks IV ceftazidime therapy from the time of the last washout. Ceftazidime can be given as a constant intravenous infusion through a peripherally inserted central catheter (PICC), enabling outpatient therapy as “Hospital in the Home” (HITH).
A follow up CT scan in clinic at 3 weeks post discharge revealed an ongoing right knee joint effusion and distal femoral osteomyelitis (see Fig. 1). As such he was readmitted for further investigation. The decision was made to provide conservative management without more surgical intervention. Intravenous ceftazidime was continued for a further 6 weeks, together with oral trimethoprim/sulfamethoxazole, which was then continued for a subsequent further 3 months as standard melioidosis eradication therapy. Follow up CT scans at 3 and 6 months demonstrated resolution of the osteomyelitis and he was considered cured of his infection.
Fig. 1.
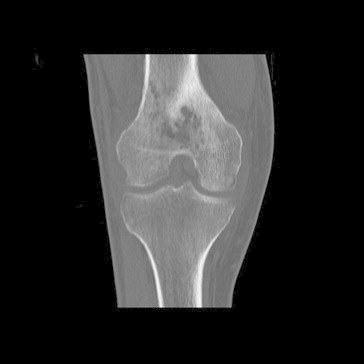
CT images 3 weeks post discharge, demonstrating ongoing distal femoral osteomyelitis (Case 1).
4.2. Case 2
A 63-year-old non-Indigenous man with type 2 diabetes mellitus was referred from his primary care practitioner with a 2-week history of weight loss, night sweats and lethargy. He also reported a 4-week history of right leg pain which he described as a constant deep ache in the right thigh and knee region. This was progressively worsening such that weight-bearing was now difficult. His doctor reported an ESR of 95 mm/h (normal value < 20) and recent positive melioidosis serology by indirect haemagglutination test.
On examination he had a temperature of 38.5° Celsius, a BP of 183/85 mmHg and heart rate of 118 bpm. There was full and pain free range of motion of the right knee. The right hip demonstrated a full range of motion, but with pain throughout all movements. There was no localised tenderness. Blood tests demonstrated a normal white cell count and a C-reactive protein of 64 mg/L (normal value <3.0).
Blood cultures grew B. pseudomallei. A bone scan showed increased uptake in the distal femur and CT and MRI imaging were suggestive of distal femoral osteomyelitis and right hip joint effusion (see Fig. 2). The patient was started on IV ceftazidime and oral trimethoprim/sulfamethoxazole.
Fig. 2.
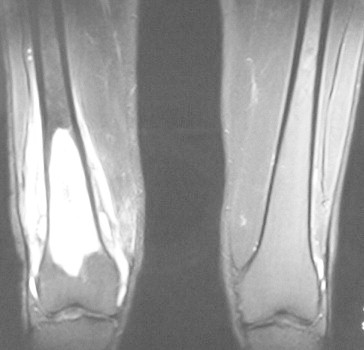
MR images showing an intramedullary sclerotic rim in the distal shaft and metaphysis of the right femur with surrounding periosteal region fluid, consistent with osteomyelitis (Case 2).
An ultrasound guided right hip joint aspiration was performed on day 5, the cultures of which grew B. pseudomallei. A right hip joint washout via a posterior approach was performed on day 8. A repeat MRI on day 12 demonstrated no significant serial changes. He made a gradual clinical improvement over some weeks and received a total of 4 weeks IV ceftazidime and oral trimethoprim/sulfamethoxazole following the washout, followed by a further 3 months of oral trimethoprim/sulfamethoxazole and doxycycline eradication therapy.
Six months following the initial admission, the patient reported a one-month history of intermittent fevers and progressive swelling around the distal thigh. An ultrasound guided aspirate was repeated which again grew B. pseudomallei from the right hip joint. CT and MRI imaging demonstrated bony destruction and fluid collection in the distal femoral shaft and new sequestrum formation within the distal medullary cavity.
The patient was again started on IV ceftazidime and taken to theatre for washout of his left hip joint and exploration, corticotomy and curettage of the right distal femoral medullary cavity. He had insertion of bone cement impregnated with meropenem to the right distal femur. Tissue culture grew B. pseudomallei. The patient remained resting in bed for 2 weeks postoperatively. Thereafter he was discharged to complete a further 6 weeks of IV ceftazidime therapy plus oral trimethoprim/sulfamethoxazole, with a subsequent further 3 months of eradication therapy with oral trimethoprim/sulfamethoxazole.
4.3. Case 3
A 60-year-old man with type 2 diabetes mellitus was referred by his primary care practitioner with 4 weeks of fever and weight loss (approximately 10 kg over 1 month). On admission he also reported poor appetite and recent nausea with vomiting. He described a 4-day history of severe burning pain in his right calf.
On examination the right calf was tender and warm on palpation with slight erythema. He was afebrile with stable observations and the rest of the clinical examination was normal. His white cell count was 8.4 × 109/L (normal range 4.0–11.0) and C-Reactive protein 130 mg/L (normal value <3.0) on admission.
An X-ray of the right leg was reported as normal, however on a subsequent bone scan there was evidence of increased bony reactivity involving the right tibia at the mid and distal shaft along with the tibial tuberosity. These findings were in keeping with right tibial osteomyelitis, which was confirmed by MRI scan (see Fig. 3).
Fig. 3.
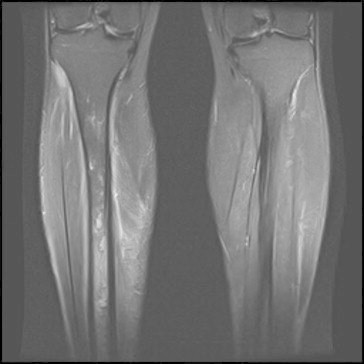
MR images showing marrow oedema from the upper third of the diaphysis to the distal metaphysis of the right tibia. There are also some cortical breaches and localised signal abnormalities consistent with intramedullary abscess (Case 3).
Blood cultures grew B. pseudomallei and the patient was started on IV meropenem and later changed to IV ceftazidime and oral trimethoprim/sulfamethoxazole. The patient received a total of 6 weeks of IV ceftazidime and trimethoprim/sulfamethoxazole, with a subsequent further 3 months of oral trimethoprim/sulfamethoxazole. At follow up the patient's symptoms had resolved with resolution of osteomyelitis on imaging.
5. Discussion
Bone and joint involvement in melioidosis was identified in this study as occurring in 41/536 (7.6%) of melioidosis patients. This proportion is much lower than the 14–27% reported in previous series of patients from northeast Thailand.14,15 This may possibly be explained by differences between the centres in the average time to receive appropriate treatment for melioidosis, with delays greater than 2 weeks thought to be a risk factor for bone and joint involvement.14 Diabetes mellitus has been identified as being an independent risk factor for bone and joint involvement in melioidosis14,15 however this was not evident in our series. Although melioidosis is more common overall in males, females were over-represented in the bone and joint cohort. Joint involvement was more often in the lower extremity, particularly knee and ankle, contrary to the findings of the Thai study.15
Melioidosis is an important infectious disease endemic throughout various tropical regions of the world. The potential for primary osteomyelitis and septic arthritis as a result of this disease exists, and an appropriate degree of suspicion is warranted for an accurate diagnosis. In addition, all patients presenting with another primary manifestation of melioidosis, such as pneumonia, require careful observation over the time course of therapy for the development of secondary osteomyelitis or septic arthritis. These considerations are particularly important for clinicians working in endemic areas. Elsewhere, melioidosis should be on the differential diagnosis list in travellers arriving or returning from these areas, especially if they are at risk for the illness (diabetes mellitus, hazardous alcohol use, chronic renal disease or chronic lung disease).
The presentation of osteomyelitis due to B. pseudomallei can range from acute sepsis to chronic disease. Often the metaphyseal regions of long bones are the sites of osseous lesions, along with the vertebral bodies. The radiographic appearances may demonstrate erosion of cortical bone or cystic change. Prompt microbiological diagnosis is important to differentiate from neoplasm, sarcoidosis, tuberculosis and other granulomatous disease.11
A high degree of awareness and active radiological screening for melioidosis are important for institutions within endemic areas. Appropriate antibiotic therapy, vigorous and sometimes repeated joint washouts and extensive debridement of infected bone, where indicated, are essential for successful treatment.11,13 We have used antibiotic impregnated bone cement for extensive or recurrent osteomyelitis but it is important to note however that B. pseudomallei is resistant to gentamicin. In our institution, the antibiotic treatment protocol is initial intensive therapy with high dose intravenous ceftazidime or meropenem, together with high dose oral trimethoprim/sulfamethoxazole and folic acid.2,6 This is followed by eradication therapy for at least 3 months with oral high dose trimethoprim/sulfamethoxazole plus folic acid. Doxycycline is an alternative oral therapy for the eradication phase in those intolerant of trimethoprim/sulfamethoxazole because of rash or bone marrow suppression.
Completion of therapy is important as relapse with inadequate therapy is well documented.7 The duration of intensive and eradication therapy may need to be prolonged in more extensive pneumonia and deep-seated bone, joint and neurological infections. For osteomyelitis, prolonged intravenous therapy (4–8 weeks) is recommended.
6. Conclusion
Due to increasing numbers of people visiting and living in endemic areas, it is likely that melioidosis will become more common as a cause of septic arthritis and osteomyelitis.13 The possibility of chronic, mild disease and the long potential latency of this condition before reactivation mean that presentation may occasionally be months to years after infection. Given the high mortality associated with this serious infection, it is important that due suspicion translates into prompt diagnosis and appropriate treatment.
Ethical approval
Approval for the study was obtained from the Human Research Ethics Committee of the Northern Territory Department of Health and the Menzies School of Health Research (HREC 02/38).
Conflicts of interest
All authors have none to declare.
Acknowledgements
We thank our clinical colleagues at Royal Darwin Hospital for support in patient diagnosis and therapy and the Microbiology Department for expertise in bacterial isolate identification.
References
- 1.Whitmore A., Krishnaswami C.S. An account of the discovery of a hitherto undescribed infective disease occurring among the population of Rangoon. Indian Med Gaz. 1912;47:262–267. [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng A.C., Currie B.J. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dance D.A. Melioidosis as an emerging global problem. Acta Trop. 2000;74:115–119. doi: 10.1016/s0001-706x(99)00059-5. [DOI] [PubMed] [Google Scholar]
- 4.Meumann E.M., Cheng A.C., Ward L., Currie B.J. Clinical features and epidemiology of melioidosis pneumonia: results from a 21-year study and review of the literature. Clin Infect Dis. 2012;54:362–369. doi: 10.1093/cid/cir808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White N.J. Melioidosis. Lancet. 2003;361:1715–1722. doi: 10.1016/s0140-6736(03)13374-0. [DOI] [PubMed] [Google Scholar]
- 6.Currie B.J., Ward L., Cheng A.C. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis. 2010;4:e900. doi: 10.1371/journal.pntd.0000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suputtamongkol Y., Chaowagul W., Chetchotisakd P. Risk factors for melioidosis and bacteremic melioidosis. Clin Infect Dis. 1999;29:408–413. doi: 10.1086/520223. [DOI] [PubMed] [Google Scholar]
- 8.Currie B.J., Fisher D.A., Howard D.M. The epidemiology of melioidosis in Australia and Papua New Guinea. Acta Trop. 2000;74:121–127. doi: 10.1016/s0001-706x(99)00060-1. [DOI] [PubMed] [Google Scholar]
- 9.Suputtamongkol Y., Hall A.J., Dance D.A. The epidemiology of melioidosis in Ubon Ratchatani, northeast Thailand. Int J Epidemiol. 1994;23:1082–1090. doi: 10.1093/ije/23.5.1082. [DOI] [PubMed] [Google Scholar]
- 10.Ngauy V., Lemeshev Y., Sadkowski L., Crawford G. Cutaneous melioidosis in a man Who was taken as a Prisoner of War by the Japanese during World War II. J Clin Microbiol. 2005;43:970–972. doi: 10.1128/JCM.43.2.970-972.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subhadrabandhu T., Prichasuk S., Sathapatayavongs B. Localised melioidotic osteomyelitis. J Bone Jt Surg [Br] 1995;77:445–449. [PubMed] [Google Scholar]
- 12.Popoff I., Nagamori J., Currie B. Melioidotic osteomyelitis in northern Australia. Aust N Z J Surg. 1997;67:692–695. doi: 10.1111/j.1445-2197.1997.tb07111.x. [DOI] [PubMed] [Google Scholar]
- 13.Kosuwon W., Saengnipanthkul S., Mahaisavariya B., Laupattarakasem W. Musculoskeletal melioidosis. J Bone Jt Surg [Am] 1993;75:1811–1815. doi: 10.2106/00004623-199312000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Teparrakkul P., Tsai J.J., Chierakul W. Rheumatological manifestations in patients with melioidosis. Southeast Asian J Trop Med Public Health. 2008;39:649–655. [PubMed] [Google Scholar]
- 15.Kosuwon W., Taimglang T., Sirichativapee W., Jeeravipoolvarn P. Melioidotic septic arthritis and its risk factors. J Bone Jt Surg [Am] 2003;85-A:1058–1061. doi: 10.2106/00004623-200306000-00011. [DOI] [PubMed] [Google Scholar]


