Abstract
The transfer of acetyl groups from acetyl coenzyme A to the ε amino group of internal lysine residues is catalyzed by Tip60, which is in the MYST family of nuclear histone acetyltransferases (HATs). The tyrosine phosphorylation of Tip60 seems to be a unique modification. We present evidence that Tip60 is modified on tyrosine 327 by Abl kinase. We show that this causes functional changes in HAT activity and the subcellular localization of TIP60, which forms a complex with Abl kinase. The Tip60 mutation Y327F abolished tyrosine phosphorylation, reduced the inhibition of Tip60 HAT activity, and caused G0-G1 arrest and association with FE65. Thus, our findings for the first time suggested a novel regulation mechanism of Tip60. Regulation was through phosphorylation of tyrosine 327 by Abl tyrosine kinase and depended on environmental conditions, suggesting that the tyrosine residue of Tip60 is important for the activation process.
Keywords: TIP60, Fe65, Abl kinase, HAT, phosphorylation.
INTRODUCTION
Tip60 was characterized as a partner of the human immunodeficiency virus, type 1-encoded transactivator protein Tat [1, 2]. Tip60 was subsequently identified as a member of the MYST family of nuclear histone acetyltransferases (HATs). While attempting to elucidate its function and identify proteins associated with ectopically expressed Tip60, the presence of associated ATPase and DNA helicase activities was demonstrated [3, 4]. Functional tests demonstrated that Tip60 and its associated proteins may perform essential functions in DNA repair and apoptosis [3, 4]. Moreover, Tip60 appears to be involved in a broad variety of cellular functions [5, 6]. The acetylation is a modification by the acetyltransferases that catalyze the transfer of acetyl groups from acetyl coenzyme A to either the amino group of N-terminal amino acids or the έ-amino group of internal lysine residues [1, 2].
Tyrosine phosphorylation is an important biological messenger that plays a role in physiological and pathophysiological, as a signal mediator showing numerous important biological effects [7, 8]. Tyrosine phosphorylation has been shown in many instances to exhibit its action via the protein phosphorylation regulate protein function. The direct link between protein tyrosine phosphorylation and functional modulation, however, has been demonstrated only in limited examples [9, 10].
The c-Abl proto-oncogene encodes a unique protein-tyrosine kinase (Abl) which is distinct from other cytoplasmic tyrosine kinases [11, 12]. In normal cells, Abl functions in cellular responses to genotoxic stress as well as regulation of the actin cytoskeleton [13]. Abl is also well known in the context of Bcr-Abl, the oncogenic fusion protein characteristic of chronic myelogenous leukemia (CML) [14, 15].
FE65 which was originally identified as an EST corresponding to an mRNA expressed at high levels in the rat brain [16] has the characteristics of an adaptor protein [17,18]. FE65 which contains one WW domain and two phosphotyrosine interaction/phosphotyrosine binding domains (PID1/PID2) as an adaptor protein is able to interact with β-amyloid precursor protein (APP) through the PID2 of FE65, as well as with other members of APP protein family [19,20]. PID2 domain of FE65 binds the YENPTY sequence in the cytoplasmic domain of APP which acts as a cytoplasmic docking site for FE65 [18, 19].
In this study, we present evidence that Tip60 is modified through tyrosine phosphorylation of tyrosine 327 by Abl tyrosine kinase. We found that this induced functional changes in Tip60 HAT activity, subcellular localization, and FE65 binding affinity. Mutational analysis of Tip60 (Y327F) further showed that tyrosine 327 is important in the phosphorylation process. Mutation of the tyrosine residue completely ablated tyrosine phosphorylation of Tip60, but enhanced its HAT activity. Thus, we report for the first time a novel regulatory mechanism of Tip60 by phosphorylation of the 327 tyrosine by Abl tyrosine kinase, resulting in the modulation of the interaction of Tip60 with FE65.
MATERIALS AND METHODOLOGY
Materials
Abl (80 units/mg) was bought from Merck Millipore (Darmstadt, Germany). Other chemicals were of the highest grade of purity from Sigma. Monoclonal or polyclonal antibodies against, Actin, Tip60, and phosphor 327 Tyr Tip60 was purchased from Santa Cruz Biotech Inc (Santa Cruz Biotech. USA) or from Cell Signaling (Boston Ma, USA). All chemicals were purchased from Sigma–Aldrich unless otherwise stated.
Cell Culture and Transfection
HEK293 cells were maintained in DMEM (Gibco) supplemented with 10% FBS and 1% penicillin/streptomycin (Gibco) at 37 °C with 5% CO2. Transfection was performed with Lipofectamine and Plus Reagent (Invitrogen) according to the manufacturer's instruction.
DNA Constructions
Tip60 (WT), Tip60 (Y327F, Y345F Y409F) were cloned from Human Tip60 (isoform1; NP-874369). Full-length Tip60 was cloned into pCMV-AC-GFP vector for cell culture study and into pGE5X-1 vector for recombinant protein production. Sequence integrity of all constructs was verified by sequencing.
Overexpression and Purification of Recombinant Proteins
Recombinant GST, GST-Tip60, and GST-Tip60 Y327F were expressed in in Escherichia coli BL21. Overexpression of bacterial culture in linear growing phase (0.6 OD) were induced by 0.2 mM IPTG at 18 °C overnight, and the recombinant proteins were then purified by GST agarose beads (Amersham Biosciences Co). Concentrations of the recombinants protein were quantified by SDS/PAGE with the use of BSA as standard.
Immunprecipitation of Tip60
Total cell lysate of HEK293 cell was denatured by incubation with 0.5% SDS, 50 mM sodium phosphate, pH 8.0, and 2 mM EDTA at 90 °C, to allow immunoprecipitation. The samples were supplemented to obtain a composition of 50 mM sodium phosphate, pH 7.2, 1% sodium deoxycholate, 1% Triton X-100, 0.5% SDS, 150 mM NaCl, 2 mM EDTA, 5 mM NaF, 2 mM Na4P2O7, 2 mM Na3VO4, 1% aprotinin, and 200 µg/ml leupeptin at 4 °C. Mouse anti-Tip60 monoclonal antibody (usually at a 1:100 dilution) was added, followed 18 h later by protein A-Sepharose. Washed immunoprecipitates were analyzed by the specific antibody after separation in a 11% sodium dodecyl sulfate-polyacrylamide gel.
Cell Cycle Analysis
HEK293 cells were transfected with 0.25 μg of GFP- Tip60 (WT), Tip60 (Y327F, Y345F Y409F) vector was transfected and the rate of apoptosis measured by Annexin V-PE apoptosis detection kit I (BD Biosciences, CA, USA), according to the manufacturer’s instructions. The cells were vortexed gently and incubated for 15 min at 25 °C in the dark. 400 μl of binding buffer was added to each tube. Within 1 hr, FACS was performed on a Coulter Epics Elite equipped with a gated amplifier and upgraded with enhanced system performance in The Core Facility of Chungbuk National University [21].
Protein Tyrosine Phosphorylation Assay
The assay kit and active Abl kinase were purchased from Merck Millipore. After Abl kinase reaction in the reaction buffer (20 mM MOPS, 1 mM EDTA, 0.01% Brij-35, 5% Glycerol, 0.1% β-mercaptoethanol, 1 mg/mL BSA, 100uM ATP) with GST- Tip 60 recombinant protein (1ug/10ul) , the reactant was western blotted with an anti- phosphor Tyr antibody of Cell Signaling Technolgy Inc (Boston MA, USA).
ELISA for Histone Acetyltransferase (HAT)
Extracts were immunoprecipitated as above, except that the high salt wash was omitted. The immunoprecipitates were washed twice in HAT assay buffer (50 mM Tris, pH 8/10% glycerol/0.1 mM EDTA/1 mM DTT), and incubated in 60 μl of HAT assay buffer containing acetyl-CoA (100 μM) and biotinylated histone H4 peptide (0.5 μg) for 30 min at 30°C. An aliquot of the reaction was immobilized onto streptavidin plates and tyrosine phosphorylation was detected via HAT ELISA according to the manufacturer's instructions (biovision). In some assays, HAT activity was analyzed with Tip60 immobilized on Glutathione-sepharose beads. Its HAT activity was monitored by recording the fluorescence emission above 430 nm after excitation at 313 and 366 nm, respectively. Sample without ATP was served as the negative control[22].
RESULTS
Phosphorylation of Tyrosine 327 on TIP60 by Abl Kinase
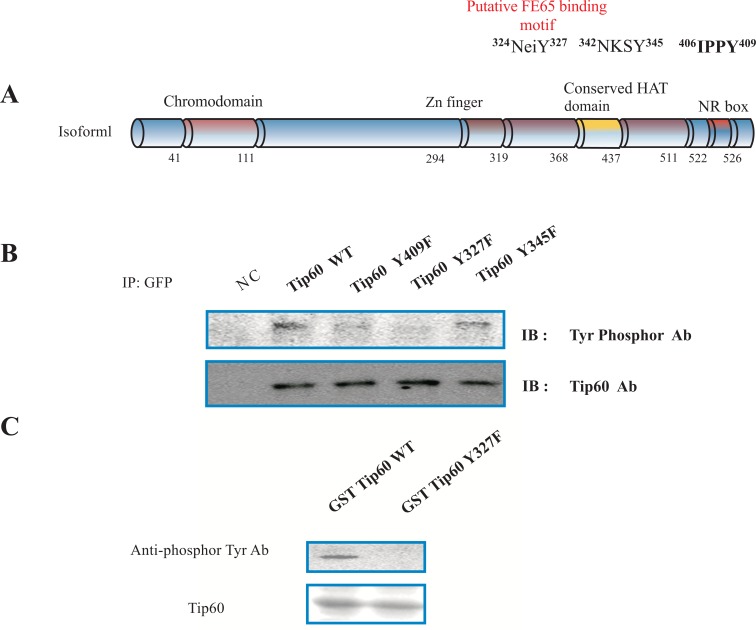
Based on the identification of the tyrosine phosphorylation site in TIP60 by consensus sequences analysis (Fig. 1A) and the information on the protein-protein interaction between TIP60 and Abl kinase (unpublished data, [18, 19, 22]), we examined whether TIP60 is phosphorylated by Abl kinase in HEK293 cells. To detect the tyrosine phosphor modifications of the Tip60, we used anti phosphor tyrosine rabbit antibody which recognized specifically the phosphorylated tyrosine (Fig. 1A). Human Tip60 (isoform1; NP-874369) has 546aa amino acids, and functional domains and several key tyrosine phosphorylation acids (Y327 or Y345) [5, 18]. The putative FE65 binding motif (324NeiY327) of Tip60 binds to PID1 of FE65, depending on its 327 tyrosine residue phosphorylation by Abl kinase [5, 6]. Further because it has been demonstrated that FE65 interacts with Tip60 through its PID1 domain [18, 19], we attempted to test whether the putative tyrosine phosphorylation site in human Tip60, each tyrosine residue was replaced with the phenylalanine (Y327F, Y345F). The functional domains (chromodomain, Zn finger, the conserved HAT domain, and NR box) in Tip60 are indicated with the amino acid number (Fig. 1A). In order to know whether Tip60 is a tyrosine phosphorylated protein or not, we performed the experiment with the site directed mutant (Y327 or Y345). The panel is the representative data for immunoblotting with an anti-Phosphor Tyr specific antibody and Tip60 antibody (Fig. 1B). After transfected HEK293 cell with each EGFP DNA constructs which are indicated above, each cell lysate was immunoprecipitated with an anti-GFP antibody. Each immunoprecipitant was immunoblotted with an anti-Phosphor specific antibody or Tip60 antibody. Because only EGFP-Tip60 Y327F was not recognized by anti-Phosphor specific antibody, the 327 tyrosine residue is the phosphorylation site by Abl kinase (above panel in Fig. 1B). The expression of EGFP Tip60 protein in each construct was monitored with the western blot (bottom panel in Fig. 1B). The negative control (NC) indicates the untransfected HEK293 cell lysate. To monitor the point mutation effect on Tip60 binding FE65, EGFP Tip60 Y409F mutant was constructed (Fig. 1A). The mutant Tip60 (Y409F) did not ablate the interaction with FE65 (above panel in Fig. 1B).
Fig. (1).
Phosphorylation on tyrosine 327 of Tip60. (A) Rat Tip60 (isoform 1) has 546 amino acids, functional domains, several key tyrosine phosphorylation targets (Y327, Y345, and Y409), and a putative PID1 binding motif of FE65. The putative tyrosine phosphorylation site in human Tip60 was replaced with phenylalanine (Y327F, Y345F, and Y409F). Functional domains (chromodomain, Zn finger, the conserved HAT domain, and NR box) in Tip60 are indicated with amino acid number. (B) Representative immunoblot data using antibodies against phosphorylated tyrosine and Tip60. HEK293 cells were transfected with EGFP DNA constructs as indicated for immunoprecipitation with anti-GFP and immunoblotting. Expression of EGFP Tip60 protein was monitored by Western blot. Negative control (NC) was untransfected HEK293 cells. (C) GST fusion proteins of Tip60 WT or Y327F from E. coli were subjected to in vitro tyrosine kinase assay. GST fusion proteins with Tip60 WT or Y327F were monitored with Coomassie blue staining (bottom). Experiments were repeated at least three times.
In order to further demonstrate our observation, GST fusion protein with Tip60 containing WT or Y327F was prepared from E. coli, and subjected to a tyrosine kinase assay in vitro (as shown in Fig. 1C). The reactant was immunoblotted with an anti-Phosphor specific antibody. The reactant of Tip60 WT was recognized by an anti-Phosphor specific antibody, while Tip60 Y327F was not (above panel in Fig, 1C). The protein amount of GST fusion protein with Tip60 containing WT or Y327F was monitored with the Coomassie Blue staining (bottom panel in Fig. 1C). It has been reported that Abl kinase forms a protein complex with Tip60 in the cell. We also observed the protein-protein interaction between Tip60 and Abl kinase (data not shown). Thus, this result also suggests that Tip60 is a tyrosine phosphorylation protein which is modified by Abl kinase.
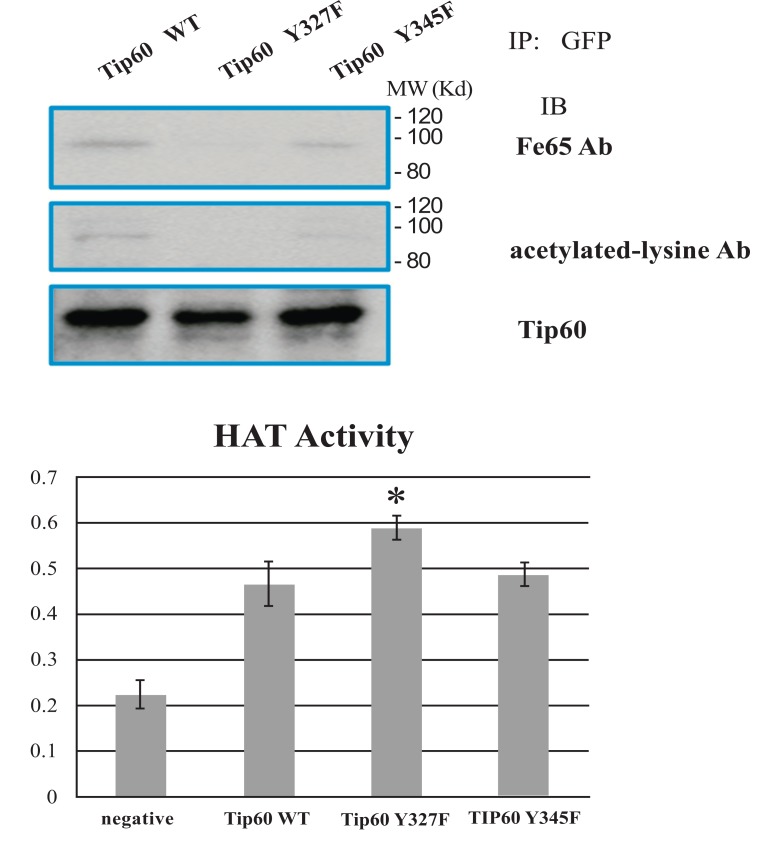
Phosphorylation on Tyrosine 327 Enhances Interaction between Tip60 and FE65 but Not HAT Activity
Because a number of physiological functions of Tip60 are associated with several proteins, including FE65, we suspected that the tyrosine phosphorylation of Tip60 could effect on its Fe65 binding activity in cells exposed to various cell death stimuli [5, 6, 13, 18, 22]. Further, because it has been reported that the tyrosine residue of Tip60 binds to PID1 of FE65 and inhibits its HAT activity, we investigated which position tyrosine phosphorylation could affect its Tip60 inhibition activity. To compare the tyrosine phosphorylation effect on Tip60, we used Tip60 Y327F mutant in parallel with both WT and Y345F. As shown Fig. (2A), theinteraction between Tip60 Y327F mutant and FE65 was ablated, compared with that of either WT or Y345F. The result is contradicted with other’s report, suggesting that Tip60 Y345 phosphorylation was responsible to interact with PID1 of FE65. Thus, our results suggested that the interaction between Tip60 and FE65 is dependent on the Y327 residue, but not Y345 residue where can be phosphorylated by Abl kinase.
Fig. (2).
Tyrosine 327 phosphorylation of Tip60 promoted binding with FE65 and inhibited HAT activity. (A) Effect of tyrosine phosphorylation on the protein interaction with FE65. EGFP TIP60 WT, Y327F, and Y345F in HEK293 lysates were immunoprecipitated with anti-GFP and immunoblotted with FE65 antibody (above), anti-acetylated lysine (middle), or anti-TIP60 (bottom). (B) Quantification of HAT activity of EGFP Tip60 WT, Y327F, or Y345F by ELISA (the number is an artificial optical density. * means the statistical significance.
To investigate further the effect of Y327 residue phosphorylation, we monitored Tip60 activity by ELISA measurement. Surprisingly, the HAT activity of Tip60 Y327F mutant is higher than that WT or Y345F mutant (Fig. 2B). The results suggested that the interaction between Tip60 and FE65, together with the auto-acetylation (Fig. 2B), seem to be the inhibitory effect to the HAT activity of Tip60 (Fig. 2B). Thus, these observations suggested that the tyrosine 327 phosphorylation of Tip60 influences on not only its association with FE65 but also its auto acetylation and HAT activity. Further the results also suggested that the FE65's anti Tip60 activity depends on the direct physical interaction of FE65 with Tip60 (data not shown). Therefore, Tip60 tyrosine phosphorylation may induce to increase FE65's anti Tip60 role by enhancing with the direct interaction between FE65and Tip60.
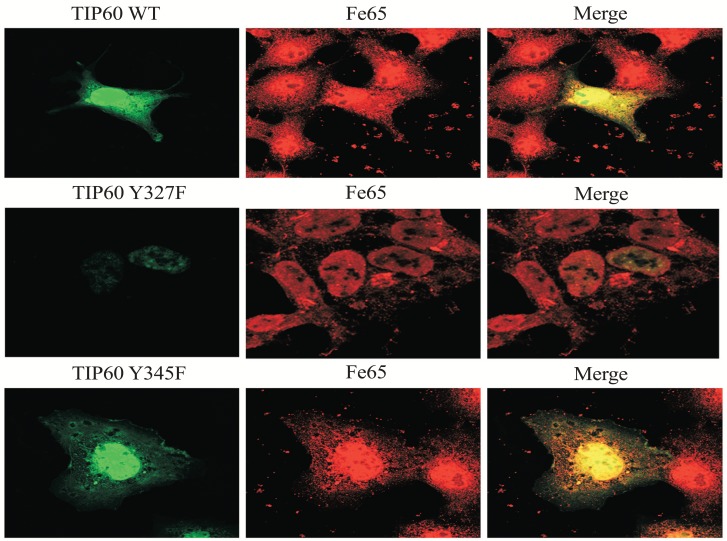
Tyrosine 327 Phosphorylation of Tip60 Affects Subcellular Localization
Through its HAT activity, Tip60 targets a number of substrates for acetylation. Tip60 also targets itself for auto acetylation, which can be used as an indicator for its activity. To determine whether the tyrosine phosphrylation could affect Tip60's activity, HEK293 cells transfected with GFP-Tip60 were treated with the tyrosine phosphorylation, which indicated that the HAT activity was affected by FE65 binding (Fig. 3). We observed that the localization of Tip60 Y327F was mainly in the nuclear, compared with Tip60 WT and Y345F (which were found in both nuclear and cytoplasm). Therefore it seems to be that the depletion of the tyrosine phoshorylation on 327 residue promotes Tip60 nuclear localization (Fig. 3 middle).
Fig. (3).
Subcellular localization of Tip60 WT, Y327, or Y345F. HEK293 cells were transfected with EGFP-Tip60 WT, Y327F, or Y345F. Endogenous FE65 was stained with specific antibody. Confocal microscopy: EGFP-Tip60 WT (upper), Y345F (bottom), Y327F (middle). Experiments were repeated at least three times.
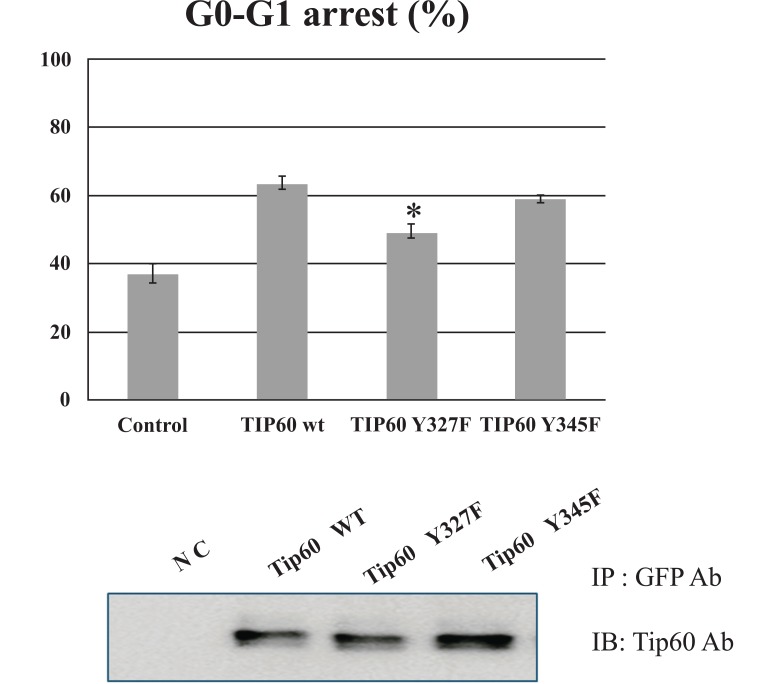
Tyrosine 327 Phosphorylation of Tip60 Promotes G0-G1
To determine further the functional role of tyrosine 327 phosphorylation of Tip 60, we compared the cell cycle regularity of Tip60 Y327F mutant in parallel with both WT and Y345F. After transfected at day 3 with each DNA construct (EGFP-Tip60 WT, Y327F, or Y345F), cells were harvested at different days post-transfection as indicated. HEK293 cells transfected with the control vector or Tip60 constructs (WT, Y327F or Y345F) were harvested and subjected to FACS analysis. As shown in Fig. (4A), Tip60 Y327F construct significantly reduced G0-G1 arrest, compared with the control vector or WT or Y345F, even though each protein expression level was similar (Fig. 4B). Thus, these results suggest that tyrosine 327 phosphorylation on Tip60 by Abl kinase is required to promote G0-G1 stage.
Fig. (4).
Tip60 Y327F reduces G0-G1 arrest. HEK293 cells with EGFP-Tip60 WT, Y327F, or Y345F were harvested as indicated and subjected to FACS analysis (A). The expression level of each protein was monitored in the western blot (B). * means the statistical significance.
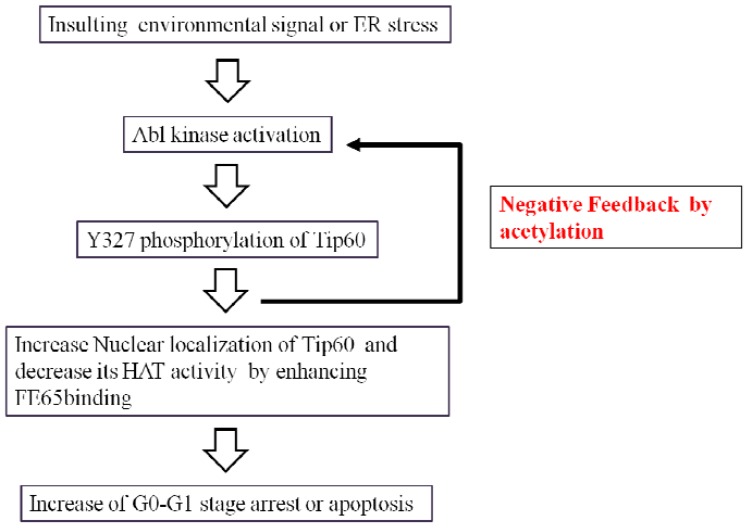
Hypothetical Signal Transduction to Tip60 by Abl Tyrosine Kinase Phosphorylation
We describe here for the first time an interaction between FE65 and TIP60 that was enhanced by posttranslational modification of tyrosine 327 on Tip60. This interaction resulted in cell death and the inhibition of Tip60 HAT activity. Furthermore, we observed that phosphorylation on tyrosine 327 of Tip60 by Abl kinase inhibited HAT activity, with FE65 being part of a feedback regulatory mechanism. Thus, our results revealed a new regulatory mechanism controlling the apoptotic activity of Abl kinase during environmental or ER stress [18, 22]. When a stress signal from the environment reaches the cell membrane, Abl tyrosine kinase is activated through a signaling pathway. Tip60, as an Abl substrate, is phosphorylated on tyrosine 327. Activated Abl kinase phosphorylates tyrosine 376 on Tip60, resulting in increased FE65 binding. Ultimately, the cell enters G0 arrest or apoptosis. Activated Tip60 acetylates Abl kinase as a feedback mechanism (Fig. 5).
Fig. (5).
Hypothetical signal transduction to Tip60 by Abl tyrosine kinase phosphorylation.
When an environmental signal reaches the cell membrane, Abl tyrosine kinase is activated through a signal pathway and Tip60 is phosphorylated on tyrosine 327. Activated Abl kinase phosphorylates Tip60 tyrosine 376, resulting in increased FE65 binding and the cell enters G0-G1 arrest or apoptosis. Activated Tip60 acetylates Abl kinase, as a feedback mechanism.
DISCUSSION
In this study, we demonstrated that Tip60 can be phosphorylated on tyrosine 327 by Abl kinase, which forms a complex with Tip60. Tyrosine phosphorylation of Tip60 compromises its Tip60 HAT activity through binding with the scaffolding protein FE65 (Fig. 1).
The mechanism by which the tyrosine phosphorylation of Tip60 impairs its HAT activity appears to occur through a feedback inhibitory mechanism. This idea contrasts with the mechanism of impairment of FE65 to Tip60 binding function by the tyrosine phosphorylation. FE65 binding function of Tip60 is induced by the tyrosine phosphorylation on 327 tyrosine residue of Tip60 (Fig. 5).
Apart from the regulatory circuit, the tyrosine phosphorylation is also involved in mediating cell death through Tip60 inhibition (Fig. 4). Our studies showed that the tyrosine phosphorylation of Ti60 can mediate the translocation of FE65–Tip60 protein complex to the nucleus and initiates cell cycle G0-G1 arrest [6, 18]. These findings that the tyrosine phosphorylation of Tip60 can affect its FE65 binding function further suggest that other environmental cell stress can affect the survival of cell through targeting a number of pathways. It has been already demonstrated that Tip60 physically associates with Abl kinase, and Abl is acetylated by Tip60 [6, 18].
It has been characterized that Tip60 is essential for K921 Abl acetylation through protein-protein interaction [6, 18]. However, several recent studies do not preclude hMOF acetylase in mediating the modification since hMOF-suppressed cells showed partially abrogated acetylation. Further the cross-talk between Tip60 and human MOF has been reported. Moreover, hMOF has been reported to influence Abl activation in response to DNA damage as depletion of hMOF prevents Abl-mediated phosphorylation of p53 and Chk2 [5, 14, 15]. These findings suggested that the acetylation of Abl is also performed by hMOF (a histone H4K16-specific HAT) in response to DNA damage, in addition to Tip60. It is interesting to note that pro-apoptotic proteins such as p53 and E2F-1 are initially modified by phosphorylation, and at latter time-point, by acetylation. Similarly, Tip60 seems to be auto acetylated after Y327 phosphorylation by Abl kinase (Fig. 5). Whereas phosphorylation of p53 leads to stabilization, enhancement of promoter DNA binding and activation of its cell-cycle arrest function, acetylation is shown to positively modulate its apoptotic activity [5, 6]. Clearly, a parallel can be drawn between Abl and Tip60 proteins in terms of regulation of their activity by coupled process of phosphorylation/acetylation [14, 15]. However, in present time we do not sure whether the process of phosphorylation/acetylation of Tip60 is coupled in the cell. Specifically, when taken with the previous findings that observation that Abl contributes to growth arrest response, and is activated by Abl-mediated phosphorylation upon environmental or ER stress lead us to speculate that combined modification by phosphorylation and acetylation modulates the cell growth and apoptotic activity of Abl, following DNA damage [5, 14, 15]. In present time, we do not know what kinds of the environmental (or ER) stress signal to Abl kinase activation.
In conclusion, we describe here a FE65 and Tip60 interaction that is enhanced by posttranslational phosphorylation on tyrosine 327 Tip60. This interaction resulted in the inhibition of Tip60 HAT activity and cell cycle arrest (Fig. 5). Understanding of the interaction between FE65 and Tip60 would contribute to understanding the functions of both FE65 and Tip60 in neurodegenerative disorders and would help establish therapeutic strategies based on tyrosine phosphorylation by Abl kinase.
ACKNOWLEDGEMENTS
This work was supported by National Research Foundation of Korea (NRF) grants (2009-0076024 and 2009-0069007) funded by the Korea Government (MEST) to S S Kang. We also appreciated The Core Facility of Chungbuk National University for their excellent skills.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Kamine J, Elangovan B, Subramanian T, Coleman D, Chinnadurai G. Identification of a cellular protein that specifically interacts with the essential cysteine region of the HIV-1 Tat transactivator. Virology. 1996;216:357–366. doi: 10.1006/viro.1996.0071. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto T, Horikoshi M. Novel substrate specificity of the histone acetyltransferase activity of HIV-1-Tat interactive protein Tip60. J. Biol. Chem. 1997;272:30595–30598. doi: 10.1074/jbc.272.49.30595. [DOI] [PubMed] [Google Scholar]
- 3.Kimura A, Horikoshi M. Tip60 acetylates six lysines of a specific class in core histones in vitro. Genes Cells. 1998;3:789–800. doi: 10.1046/j.1365-2443.1998.00229.x. [DOI] [PubMed] [Google Scholar]
- 4.Sterner DE, Berger SL. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 2000;64:435–459. doi: 10.1128/mmbr.64.2.435-459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sapountzi V, Logan IR, Robson CN. Cellular functions of TIP60. Int. J. Biochem. Cell. Biol. 2006;38:1496–1509. doi: 10.1016/j.biocel.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Szumiel I, Foray N. Chromatin acetylation, beta-amyloid precursor protein and its binding partner FE65 in DNA double strand break repair. Acta. Biochim. Pol. 2011;58:11–18. [PubMed] [Google Scholar]
- 7.Krebs EG, Beavo JA. Phosphorylation-dephosphorylation of enzymes. Annu. Rev. Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- 8.Hunter T, Cooper JA. Tyrosine protein kinases and their substrates: an overview. Adv. Cyclic. Nucleotide. Protein. Phosphorylation Res. 1984;17:443–455. [PubMed] [Google Scholar]
- 9.Kim SJ, Ryu SE. Structure and catalytic mechanism of human protein tyrosine phosphatome. BMB Rep. 2012;45:693–699. doi: 10.5483/BMBRep.2012.45.12.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Locascio LE, Donoghue DJ. KIDs rule: regulatory phosphorylation of RTKs. Trends Biochem Sci. 2013;38:75–84. doi: 10.1016/j.tibs.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Lambrechts A, Kwiatkowski AV, Lanier LM, Bear JE, Vandekerckhove J, Ampe C, Gertler FB. cAMP-dependent protein kinase phosphorylation of EVL, a Mena/VASP relative, regulates its interaction with actin and SH3 domains. J. Biol. Chem. 2000;275:36143–36151. doi: 10.1074/jbc.M006274200. [DOI] [PubMed] [Google Scholar]
- 12.Panjarian S, Iacob RE, Chen S, Engen JR, Smithgall TE. Structure and Dynamic Regulation of Abl Kinases. J. Biol. Chem. 2013;288:5443–5450. doi: 10.1074/jbc.R112.438382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang Z, Kamath R, Jin S, Balasubramani M, Pandita TK, Rajasekaran B. Tip60-mediated acetylation activates transcription independent apoptotic activity of Abl. Mol. Cancer. 2011;10:88. doi: 10.1186/1476-4598-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kusio-Kobialka M, Wolanin K, Podszywalow-Bartnicka P, Sikora E, Skowronek K, McKenna SL, Ghizzoni M, Dekker FJ, Piwocka K. Increased acetylation of lysine 317/320 of p53 caused by BCR-ABL protects from cytoplasmic translocation of p53 and mitochondria-dependent apoptosis in response to DNA damage. Apoptosis. 2012;17:950–963. doi: 10.1007/s10495-012-0739-9. [DOI] [PubMed] [Google Scholar]
- 15.Griaud F, Williamson AJ, Taylor S, Potier DN, Spooncer E, Pierce A, Whetton AD. BCR/ABL modulates protein phosphorylation associated with the etoposide-induced DNA damage response. J Proteomics. 2012;77:14–26. doi: 10.1016/j.jprot.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Duilio A, Zambrano N, Mogavero AR, Ammendola R, Cimino F, Russo T. A rat brain mRNA encoding a transcriptional activator homologous to the DNA binding domain of retroviral integrases. Nucleic Acids Res. 1991;19:5269–5274. doi: 10.1093/nar/19.19.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russo T, Faraonio R, Minopoli G, De Candia P, De Renzis S, Zambrano N. Fe65 and the protein network centered around the cytosolic domain of the Alzheimer's beta-amyloid precursor protein. FEBS Lett. 1998;434:1–7. doi: 10.1016/s0014-5793(98)00941-7. [DOI] [PubMed] [Google Scholar]
- 18.Cao X, Sudhof TC. A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science. 2001;293:115–120. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- 19.McLoughlin DM, Miller CC. The FE65 proteins and Alzheimer's disease. J Neurosci Res. 2008;86:744–754. doi: 10.1002/jnr.21532. [DOI] [PubMed] [Google Scholar]
- 20.Schettini G, Govoni S, Racchi M, Rodriguez G. Phosphorylation of APP-CTF-AICD domains and interaction with adaptor proteins: signal transduction and/or transcriptional role--relevance for Alzheimer pathology. J. Neurochem. 2010;115:1299–1308. doi: 10.1111/j.1471-4159.2010.07044.x. [DOI] [PubMed] [Google Scholar]
- 21.Shin SH, Lee EJ, Chun J, Hyun S, Kim YI, Kang SS. The nuclear localization of glycogen synthase kinase 3beta is required its putative PY-nuclear localization sequences/ Mol. Cells. 2012;34:375–382. doi: 10.1007/s10059-012-0167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee EJ, Shin SH, Hyun S, Chun J, Kang SS. Endoplasmic Reticulum (ER) Stress Enhances Tip60 (A Histone Acetyltransferase) Binding to the Concanavalin A. Open Biochem. J. 2012;6:1–10. doi: 10.2174/1874091X01206010001. [DOI] [PMC free article] [PubMed] [Google Scholar]