Abstract
Live High:Train Low (LHTL) altitude training is a popular ergogenic aid amongst athletes. An alternative hypoxia protocol, acute (60-90 min daily) Intermittent Hypoxic Exposure (IHE), has shown potential for improving athletic performance. The aim of this study was to compare directly the effects of LHTL and IHE on the running and blood characteristics of elite triathletes. Changes in total haemoglobin mass (Hbmass), maximal oxygen consumption (VO2max), velocity at VO2max (vVO2max), time to exhaustion (TTE), running economy, maximal blood lactate concentration ([La]) and 3 mM [La] running speed were compared following 17 days of LHTL (240 h of hypoxia), IHE (10.2 h of hypoxia) or Placebo treatment in 24 Australian National Team triathletes (7 female, 17 male). There was a clear 3.2 ± 4.8% (mean ± 90% confidence limits) increase in Hbmass following LHTL compared with Placebo, whereas the corresponding change of -1.4 ± 4.5% in IHE was unclear. Following LHTL, running economy was 2.8 ± 4.4% improved compared to IHE and 3mM [La] running speed was 4.4 ± 4.5% improved compared to Placebo. After IHE, there were no beneficial changes in running economy or 3mM [La] running speed compared to Placebo. There were no clear changes in VO2max, vVO2max and TTE following either method of hypoxia. The clear difference in Hbmass response between LHTL and IHE indicated that the dose of hypoxia in IHE was insufficient to induce accelerated erythropoiesis. Improved running economy and 3mM [La] running speed following LHTL suggested that this method of hypoxic exposure may enhance performance at submaximal running speeds. Overall, there was no evidence to support the use of IHE in elite triathletes.
Key Points.
Despite a clear 3.2% increase in haemoglobin mass following 17 days of Live High: Train Low altitude training, no change in maximal aerobic capacity was observed.
There were positive changes in running economy and the lactate-speed relationship at submaximal running speeds following Live High: Train Low altitude training.
There was no evidence to support the use of daily 60-90 minute Intermittent Hypoxic Exposure in elite triathletes.
Key words: Red cell mass, HiLo altitude, blood volume
Introduction
Altitude training first became popular with athletes as part of their physical preparation for competition nearly fifty years ago, and over the intervening period many different altitude training protocols have evolved (Millet et al., 2010; Wilber, 2007). In the past 15 years, numerous investigations have been conducted to examine the effects of Live High:Train Low (LHTL) altitude training, where athletes live at moderate altitude (2000-3000 m) but train near sea-level, on subsequent sports performance [(Levine and Stray-Gundersen, 1997) for instance]. Several researchers have concluded that, provided athletes are exposed to an adequate 'dose' of altitude (a combination of the duration and severity of hypoxic exposure), LHTL can lead to worthwhile performance improvements of 1-2% (Bonetti and Hopkins, 2009; Levine and Stray-Gundersen, 1997; Robertson et al., 2010a). An hypoxic dose of > 12 hours per day for at least 3 weeks at an elevation of 2100 m to 2500 m has been suggested as sufficient for athletes to benefit from the exposure (Rusko et al., 2004; Wilber, 2007). Despite other researchers finding no performance benefit from LHTL, including one recent placebo- controlled double-blind study (Siebenmann et al., 2012), this form of altitude training remains popular amongst elite athletes. The specific facilities required for LHTL altitude protocols can be logistically and financially inaccessible to many athletes. Either a location with rapid travel options between a low altitude training venue and a moderate altitude residential facility, or a special purpose 'altitude house' is required where the hypoxic environment can be simulated by reducing the oxygen content of the ambient air.
One alternative, acute (60-90 min daily) Intermittent Hypoxic Exposure (IHE), was highlighted by a recent meta-analysis (Bonetti and Hopkins, 2009) as one of the most beneficial forms of altitude training in sub-elite athletes. However, the authors of two recent reviews of the literature (Lundby et al., 2012; Millet et al., 2010) held an opposing view: that more studies suggest unchanged or impaired performance resulting from IHE (Hamlin et al., 2010; Julian et al., 2004) than improved performance (Katayama et al., 2003; Wood, 2006). Although the effectiveness of IHE is highly debated, this method offers major practical advantages over LHTL in terms of cost-effectiveness, time-efficiency, accessibility and portability. The combination of these practical advantages and the 1-5% performance improvements that have been reported by some researchers (Katayama et al., 2003, Wood, 2006) ensure that IHE remains a method of interest to elite athletes and coaches. However, there have been no previous studies that have directly compared these two methods in the same population and very few studies have used elite athletes.
In light of the debate surrounding the purported effects of LHTL and IHE on sports performance, insight might be gained by examining the underlying physiological effects of these protocols. The erythropoietic effect of prolonged hypoxic exposure has been studied in detail due to the positive relationship between haemoglobin mass (Hbmass) and maximal oxygen consumption (VO2max) (Schmidt and Prommer, 2010). Haemoglobin mass increases of 1-4% following an adequate dose of LHTL have been demonstrated by several researchers (Robach et al., 2006; Robertson et al., 2010a; Wehrlin et al., 2006), although it has been suggested recently that athletes with already high Hbmass may benefit less (Robach and Lundby, 2012). The effect of IHE on Hbmass has not been examined, although, evidence of increased haemoglobin concentration ([Hb]) (Bonetti et al., 2006; Hellemans, 1999) and increased serum erythropoietin concentration (Rodriguez et al., 2000) following IHE are suggestive of a positive haematological adaptation. However, due to the confounding influence of plasma volume fluctuations on [Hb], measurement of Hbmass is a more relevant method for the true effect of IHE on athletes' blood to be determined.
Considerations of non-haematological adaptations to hypoxia reveal a potentially common mechanism of physiological adaptation following IHE and LHTL; there is evidence of similar improvements in sub-maximal exercise efficiency in runners from two separate studies, following IHE (Katayama et al., 2003) and LHTL (Saunders et al., 2009).
The aim of this study was to compare directly the effects of two different forms of hypoxic exposure, LHTL and IHE, on the running and blood characteristics of elite athletes.
Methods
Study design
Twenty-four Australian National Team triathletes (7 female, 17 male) took part in this randomised placebo-controlled study (Table 1). This study was approved by the ethics committees of the Australian Institute of Sport and the University of Canberra and all athletes provided their written informed consent to participate. The athletes attended a 21-day running-focused training camp during the domestic competition season in Canberra, Australia (600 m) in which they were randomly assigned to one of three groups: LHTL, IHE or Placebo. The groups were evenly matched for VO2max, as measured during the incremental treadmill test at the start of the camp. All athletes trained in the normal Canberra environment (minimum temp 16.5 ± 3.5°C, maximum temp 31.6 ± 5.1°C; mean ± SD), completing ~30km swimming, ~400km cycling, ~85km running, and one strength session in the gym per week. During the camp, all athletes followed the same training plan, modified slightly to take into account differences in physical capability between athletes. As a result, the athletes trained together for most sessions during the camp. The athletes recorded the duration (min), distance (km) and intensity (1-10 rating of perceived exertion scale) of all training completed one month prior to, and during the camp. The training impulse (TRIMP) for each session, which can be interpreted as the integrated training load (Banister et al., 1975), was calculated by multiplying duration and intensity of the session. Total body Hbmass, venepuncture for serum ferritin ([Ferr]) and soluble transferrin receptor ([sTfR]) concentrations, and various physiological variables associated with running were measured before and after the intervention (Figure 1).
Table 1.
Physical characteristics of participants at baseline.
| LHTL | IHE | Placebo | ||||
|---|---|---|---|---|---|---|
| Sex | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ |
| N | 5 | 2 | 5 | 2 | 6 | 3 |
| Age (yr) | 21.2 (1.6) | 20.9 (3.5) | 20.0 (2.6) | 23.4 (6.4) | 21.2 (1.6) | 20.4 (3.9) |
| Height (m) | 1.80 (.08) | 1.65 (.04) | 1.78 (.04) | 1.70 (.01) | 1.79 (.04) | 1.62 (.04) |
| Mass (kg) | 70.5 (5.9) | 53.7 (1.2) | 68.9 (3.3) | 53.6 (1.2) | 66.2 (6.1) | 51.7 (0.8) |
| Haemoglobin mass (g·kg-1) | 13.6 (.3) | 11.7 (.3) | 13.9 (.6) | 11.5 (.0) | 13.5 (.7) | 10.9 (.8) |
Data presented are the raw values, expressed as group means (± standard deviation).
Figure 1.
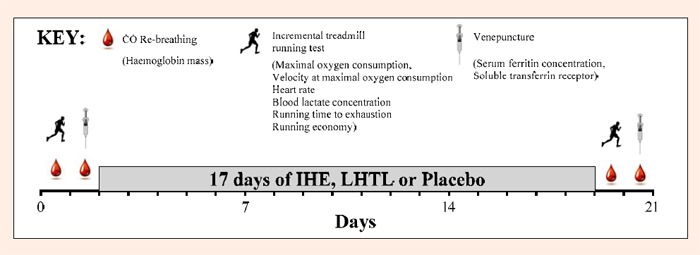
Outline of the study design illustrating the sequence of carbon monoxide (CO) re-breathing tests, incremental treadmill tests and venepuncture blood sampling.
Hypoxic exposure
The LHTL group spent 14.1 ± 0.1 h·day-1 for 17 days (240 h) in normobaric hypoxia equivalent to an altitude of3000m. The normobaric hypoxic environment was generated within a purpose-built 'altitude house' facility using oxygen filtration technology (Kinetic Performance Technology, Canberra, Australia). For the IHE group, hypoxia was produced using a commercially available re-breathing device (AltO2lab, Pharma Pacific, Phoenix, Arizona, USA), which uses spacers to increase respiratory deadspace and thereby reduce the partial pressure of oxygen in inspired air at the lungs. Expired air was passed through a soda-lime absorbent to reduce carbon dioxide (CO2) in the AltO2lab. Over a 17 day period, the IHE group completed one IHE session per day, during which they alternated breathing through the device for 6 min and normal room air for 4 min; this cycle was repeated six times, totalling 60 min. Peripheral oxygen saturation (SpO2) was monitored continuously by experimenters via pulse oximetry (Avant 4000, Nonin Medical Inc., Plymouth, Minnesota, USA) and was progressively reduced from 90% on day 1, to 76% on days 14 to 17 by the addition of more spacers. The hypoxic stimulus was equivalent to that of 3500-6000 m altitude (http://www.high-altitude-medicine.com/SaO2-table.html (Hackett and Roach, 1995)). Over the course of 17 days, the total duration of hypoxic exposure for the IHE group was 10.2 h. The Placebo group completed identical duration 'IHE' sessions daily for 17 days, but their re-breathing devices had been modified by removing the soda lime absorbent. This method of creating a Placebo IHE condition has been published (Wood, 2006). In the current study, the placebo configuration of the re-breathing device had the effect of generating only mild hypoxia (SpO2 96 ± 0.5%, mean ± SD) and mild hypercapnia during the 6 min intervals from days 1 to 17. The magnitude of hypercapnia during IHE and Placebo sessions was explored in a pilot study (n=4); end-expiratory CO2 concentrations of 4.7 ± 0.3% and 5.7 ± 0.4% were recorded for IHE and Placebo, respectively, and the corresponding end-inspiratory CO2 concentrations were 1.2 ± 0.3% and 2.9 ± 0.4%. By using telemetered pulse oximeters, athletes in both the IHE and Placebo groups were blinded to their SpO2 throughout the intervention period.
Blood parameters
Changes in Hbmass from the start to the end of the training camp were assessed using the optimised carbon monoxide (CO) re-breathing technique as published by our group previously (Gough et al., 2011). Duplicate measures of Hbmass were made both pre- and post-intervention and averaged to a single value at each time point for analysis. The typical error of Hbmass (with 90% confidence limits) was 2.4 (2.1-2.9)% from the pooled duplicate data of all three groups. Using the mean of the duplicate pairs reduced error by a factor of √2, when compared with singleton measures (Alexander et al., 2011).
A venous blood sample (4mL) was collected from the athletes at the start and the end of the camp and analysed for [Ferr] and [sTfR] using immunoturbidimetric assay on a Hitachii 911 automatic analyser (Boehringer Mannheim, Germany). Iron supplementation for all three groups (Ferrograd C, Ferrogradumet; Abbott Australia, Botany, Australia equivalent to 105 mg elemental iron per day) started two weeks prior to the first day of the camp and supplementation continued for the duration of the training camp.
Incremental treadmill test
Following a 5 min warm-up at 14 km·h-1 (12 km·h-1 for females), athletes ran at 14, 15, 16 and 17 km.h-1 (12, 13, 14 and 15 km·h-1 for females) for four mins at each speed, separated by 1-min rest periods. Heart rate (HR) was continuously recorded using short-range telemetry (Polar Vantage NV, Kempele, Finland). After each 4-min stage, a capillary blood sample was taken from a finger and measured for blood lactate concentration ([La]) using a portable analyser (Lactate Pro, Arkray, KDK Corporation, Kyoto, Japan). During pre-camp testing, the [La] of most athletes was >4 mM by the end of the fourth submaximal stage, but athletes for whom this was not the case (n = 6) completed a fifth submaximal stage at 18 km·h-1 (16 km·h-1 for females). During post-camp testing, regardless of [La], athletes completed the same number of submaximal stages as they had done pre-camp, which resulted in some athletes' [La] being <4 mM post-camp. Consequently, in order to assess changes in the submaximal [La] profile from pre- to post- intervention, the running speed corresponding to 3 mM [La], rather than the traditional 4 mM (Heck et al., 1985), was calculated using an integrative technique of plotting speed versus [La] using an exponential fit. Three mM [La] thus provided a consistent value that could be compared between pre- and post- testing for all subjects.
An in-house automated metabolic system, which has been described previously (Saunders et al., 2004), was used for measurement of oxygen consumption (VO2) throughout the protocol. The VO2 values for the final minute of the first two submaximal stages were pooled and averaged to give a measure of running economy. Upon completion of the final submaximal stage, participants rested for 5 min before completing an incremental run to maximal volitional fatigue, beginning at 16 km·h-1 (14 km·h-1 for females). The speed was increased by 1 km.h-1 each minute until 20 km·h-1 (18 km·h-1 for females), then the gradient was increased by 0.5% per minute until volitional exhaustion. Every athlete was familiar with this test format from previous periodic testing. Time to exhaustion (TTE) during the maximal test and VO2max, taken as the highest two consecutive 30 sec VO2 values, were recorded and the velocity at VO2max (vVO2max) was calculated using an integrative technique of plotting speed versus VO2 for the 4 submaximal stages and forming a regression equation that was solved for VO2max (Billat and Koralsztein, 1996). For each athlete, the pre- and post-camp treadmill tests were completed at the same time of day, and the nutritional intake for 24 hours prior to the first test was recorded and athletes were asked to replicate that same diet prior to their second test.
Participation variations
Three athletes did not participate in the post-camp incremental treadmill tests due to injuries (ankle sprain, calf strain and shin soreness, respectively) sustained in the latter stages of the training camp. One athlete completed the submaximal but not the maximal steps of the post-camp treadmill test due to a hip injury that limited top-speed running only. In addition, the treadmill test results of a further two athletes were excluded because their data indicated a leak in the gas analysis system during one of their tests; the likely source was air leakage around the mouthpiece. Due to time limitations it was not possible for these tests to be repeated. All 24 athletes completed tests for blood parameters including Hbmass. After exclusions, there were 6, 8 and 6 athletes in the LHTL, IHE and Placebo groups, respectively, for submaximal running variables; and the corresponding numbers for the maximal running variables were 5, 8 and 6 athletes.
Statistical analysis
Data were analysed using a contemporary analytical approach involving magnitude-based inferences (Hopkins et al., 2009), which enables small effects that are of practical importance in an elite athlete population to be detected. In order to reduce any effects of non-uniformity of error all measures were log-transformed before analysis. Preliminary analyses revealed large between-group differences in pre-camp training load (4922 ± 21.1%, 5246 ± 23.3% and 3618 ± 19.7% arbitrary TRIMP units for LHTL, IHE and Placebo, respectively) but only a small between-group difference in training load during the camp (5627 ± 36.8%, 6691 ± 28.4% and 6397 ± 6.4% arbitrary TRIMP units for LHTL, IHE and Placebo, respectively). To reduce the likelihood of training-induced changes impacting the findings of the study, the percent change in weekly training load from pre- to during-camp for each individual athlete (range 0.3% to 140%) was incorporated as a covariate in the analysis of the blood and running variables. The mean percent change in each of the blood and running variables from pre- to post-intervention was calculated and the differences in the response of each of the hypoxic groups were compared to changes in the Placebo group (± 90% confidence limits (CL)) using independent t-tests (Hopkins, 2006). The magnitude of differences were assessed in relation to the smallest worthwhile change (SWC) which, for each variable, was calculated as one fifth of the between-subject standard deviation of athletes' baseline data. SWCs were calculated separately for male and female athletes, and the mean value taken as the final SWC because a mixed-sex cohort led to large between-subject standard deviations in body mass-related variables such as VO2max, Hbmass and running economy.
Effects were termed positive, trivial or negative depending on the magnitude of the change relative to the SWC and the spreads of the 90% CL were used to ascertain the certainty with which the effects could be classified: 50-74%, possibly; 75-95%, likely; 95-99%, very likely; and >99%, almost certainly. The effect was deemed “unclear” if its confidence interval overlapped the SWC thresholds for both positive and negative change.
Results
Blood parameters
There were small and variable changes in [Ferr] in all groups, resulting in group mean (± standard deviation expressed as % coefficient of variation) post-camp values of 68 ng·mL-1 ± 43%, 51 ng·mL-1 ± 72% and 56 ng·mL-1 ± 131% for LHTL, IHE and Placebo, respectively (Table 2).
Table 2.
Blood and running parameters pre and post Live High:Train Low (LHTL) altitude training, Intermittent Hypoxic Exposure (IHE), or Placebo.
| LHTL | IHE | Placebo | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Blood measures | ||||||
| Haemoglobin mass (g) | 869 (182) | 900 (193) | 847 (189) | 850 (184) | 775 (191) | 789 (195) |
| Serum ferritin (ng·mL-1) | 62.8 (33.4) | 68.0(27.3) | 54.2(27.5) | 51.0(23.4) | 71.6(46.4) | 56.4(36.2) |
| Soluble transferrin receptor (mg·L-1) | 2.5 (.3) | 2.9 (.6) | 2.7 (.6) | 3.2 (.7) | 2.4 (.4) | 2.8 (.6) |
| Submaximal running | ||||||
| Running economy (mL·kg-1·min-1) | 3.72 (.58) | 3.55 (.62) | 3.39 (.63) | 3.35 (.63) | 3.29 (.66) | 3.35 (.59) |
| 3mM [La] running speed (km·h-1) | 16.8 (.4) | 17.1 (.7) | 17.0 (1.0) | 17.4 (.8) | 16.5 (.7) | 16.8 (.8) |
| Maximal running | ||||||
| VO2max (L·min-1) | 4.82 (1.1) | 4.84 (.89) | 4.56 (.87) | 4.63 (.79) | 4.47 (.65) | 4.60 (.72) |
| vVO2max (km·h-1) | 19.3 (.6) | 19.6 (.4) | 18.9 (1.4) | 19.1 (1.3) | 19.6 (.9) | 19.6 (1.1) |
| TTE (s) | 528 (46) | 540 (37) | 518 (73) | 521 (36) | 540 (47) | 570 (43) |
| Maximal heart rate (beats·min-1) | 192 (12) | 185 (16) | 200 (6) | 196 (5) | 195 (9) | 195 (9) |
| Maximal [La] (mM·L-1) | 9.7 (2.0) | 8.3 (1.7) | 8.8 (1.6) | 7.4 (.9) | 10.0 (2.4) | 10.4 (2.0) |
Data presented are the raw values, expressed as group means (± standard deviation). Blood lactate concentration: [La]. Maximal oxygen consumption: VO2max. Running velocity at VO2max: vVO2max. Running time to exhaustion: TTE
After 17 days of LHTL, the change in Hbmass was possibly higher than Placebo (3.2 ± 4.8%; mean percent difference ± 90% CL; Table 3) and likely higher than IHE (4.7 ± 3.5%). There was no clear difference in Hbmass response between IHE and Placebo conditions. Similarly, the change in [sTfR] over the period of training was likely higher in LHTL than in both IHE and Placebo (Table 2 and 3).
Table 3.
Percent difference in the changes in blood and running parameters after either Live High:Train Low (LHTL) altitude training or Intermittent Hypoxic Exposure (IHE) compared with Placebo.
| LHTL | IHE | Placebo | |||||
|---|---|---|---|---|---|---|---|
| SWC (%) | Diff. from Placebo (± 90% CL) | Qualitative Inference | Diff. from Placebo (± 90% CL) | Qualitative Inference | Diff. from Placebo (± 90% CL) | Qualitative Inference | |
| Blood measures | |||||||
| Haemoglobin mass (g) | 1.6 | 3.2 (4.8) | Possibly higher | −1.4 (4.5) | Unclear | 4.7 (3.5) | Likely higher |
| Serum ferritin (ng·mL-1) | 8.2 | 17.5 (46.6) | Unclear | 31.0 (40.9) | Likely higher | −10.3 (43.3) | Unclear |
| Soluble transferrin receptor (mg·L-1) | 3.0 | 14.3 (16.9) | Likely higher | 3.4 (14.1) | Unclear | 10.5 (12.8) | Likely higher |
| Submaximal running | |||||||
| Running economy (mL·kg-1·min-1) | 1.9 | −1.1 (4.2) | Unclear | 1.7 (3.4) | Possibly trivial | −2.8 (4.4) | Possibly lower |
| 3mM [La] running speed (km·h-1) | .8 | 4.4 (4.5) | Likely higher | 2.7 (3.7) | Unclear | 1.7 (4.6) | Unclear |
| Maximal running | |||||||
| VO2max (L·min-1) | 1.5 | 1.7 (9.0) | Unclear | 3.9 (7.1) | Unclear | −2.1 (8.5) | Unclear |
| vVO2max (km·h-1) | .9 | 1.7 (9.7) | Unclear | 1.3 (8.3) | Unclear | .4 (9.5) | Unclear |
| TTE (s) | 3.0 | −.1 (20.0) | Unclear | −4.7 (12.2) | Unclear | 4.8 (20.0) | Unclear |
| Maximal heart rate (beats·min-1) | .8 | −5.7 (3.4) | Very likely lower | −3.0 (2.8) | Likely lower | −2.8 (3.5) | Likely lower |
| Maximal [La] (mM·L-1) | 3.5 | −32.4 (27.2) | Very likely lower | −20.7 (26.8) | Likely lower | −14.7 (32.4) | Unclear |
Values are the net difference between groups of the percent change in variables from pre-camp to post-camp with 90% confidence limits (± 90% CL). Smallest Worthwhile Change in the variable, expressed as a percentage: SWC. Differences: Diff. Blood lactate concentration: [La]. Maximal oxygen consumption: VO2max. Running velocity at VO2max: vVO2max. Running time to exhaustion: TTE.
Running parameters
Athletes in the LHTL group experienced a possible improvement in running economy compared to the IHE group (-2.8 ± 4.4%; Table 3), although the difference from Placebo was not clear (-1.1 ± 4.2%). The difference between the running economy changes of the IHE and Placebo groups was trivial. The only clear between-group difference in 3mM [La] running speed was an improvement in LHTL by 4.4 ± 4.5% compared with Placebo. Both LHTL and IHE demonstrated substantial decreases in maximal [La] and HR, compared with Placebo. There were no clear between-group differences in VO2max, vVO2max, or TTE.
Discussion
The clear increases in Hbmass and [sTfR] following LHTL demonstrate an erythropoietic response to this form of hypoxic exposure, which was absent following IHE. The trivial change in [Ferr] in the IHE group during the training camp confirms that iron availability was not a limiting factor for Hbmass increases. A more likely reason for the null haematological response is the difference in hypoxic dose between LHTL and IHE. It has been suggested previously that the minimum hypoxic dose needed to stimulate haematological adaptation is >12 h·d-1 for at least 3 wk at an altitude or simulated altitude of 2100-2500 m (Rusko et al., 2004). The mean 3.2% increase in Hbmass measured here after 17d of LHTL, at 14 h·d-1 and a simulated altitude of 3000 m, demonstrates that haematological adaptation can be achieved within fewer days if the severity of altitude and duration of exposure per day are increased sufficiently. The magnitude of the Hbmass change observed following LHTL in the present study (3.2%) fits well with the prediction of a 4% change in this population, originating from a recently published model relating initial Hbmass of athletes to Hbmass response following LHTL (Robach and Lundby, 2012). Although the hypoxia to which the athletes in the IHE group were exposed was more severe again (equivalent to 3500-6000 m), the findings of the present study confirm that this dose is insufficient to stimulate erythropoeisis since there was no increase in Hbmass. This is the first time that changes in Hbmass in response to IHE sessions lasting <3h·d-1 have been examined. The lack of haematological response to IHE is unsurprising. It has previously been demonstrated that Hbmass was unchanged after 4 weeks of 3h·d-1 at 4000-5500 m (Gore et al., 2006), which is likely to be a larger cumulative dose of hypoxia compared to IHE. These findings refute the suggestions of other researchers who, based on measured increases in Hb concentration and haematocrit coupled with decreased [Ferr] following 60-90 min IHE, concluded that Hbmass may have increased (Bonetti et al., 2006; Hellemans, 1999).
At maximal running speeds, there was no evidence of improvements in either the LHTL or IHE groups. It was somewhat surprising that there was no clear change in VO2max after LHTL given the substantial increase in Hbmass that should theoretically transfer to a worthwhile improvement in VO2max of ~2% (Schmidt and Prommer, 2010). Whilst there was an unclear 1.7% improvement in VO2max following LHTL compared with Placebo, the majority of this difference was due to a 1.6% decrease in VO2max in the Placebo group, not an increase in the LHTL group. One possible explanation for the incongruence between Hbmass and VO2max in the LHTL group is that the decrease in maximal HR recorded after LHTL could have counteracted the positive effect of improved oxygen carrying capacity and resulted in no change to VO2max. A decreased maximal HR has previously been reported after LHTL at moderate altitude (Saunders et al., 2009; Wehrlin et al., 2006) and similar changes after acclimatisation to severe and chronic altitude exposure have been attributed to changes in myocardial B-adrenergic and myocardial receptor density (Favret et al., 2001). Interestingly, the IHE group also experienced a similar decrease in maximal HR.
Running economy is one factor that, together with changes in VO2max and lactate threshold, can account for 70% of the variance in endurance running performance (Di Prampero et al., 1986).Various non-haematological changes in athletes' physiology have been measured in response to hypoxia (Gore et al., 2007) and may contribute to improved performance in the absence of increased Hbmass. Improvements to the efficiency of oxygen usage during submaximal exercise (running economy) is one such non-haematological change that has previously been demonstrated after LHTL (Gore et al., 2001; Saunders et al., 2009) and IHE (Katayama et al., 2003). On the other hand, a recent double-blind placebo study of LHTL concluded that there was no statistically significant change in running economy after 4 weeks of LHTL (Siebenmann et al., 2012). In the present study, there was a 2.8% improvement in running economy in the LHTL group when compared to IHE. Together with the likely higher 3mM [La] running speed following LHTL, the small improvement in running economy suggests that LHTL altitude training was advantageous for submaximal running. In contrast, there was no evidence of IHE leading to benefitcial changes to running at submaximal speeds.
A rightward shift in the lactate-power profile indicates that an athlete is able to run at a higher speed for the same or reduced lactate accumulation, and typically leads to improved performance (Amann et al., 2006). The 4.4% increase in 3mM [La] running speed and decreased maximal [La] following LHTL indicates a positive shift in the lactate profile, and although there were no clear changes in 3 mM [La] running speed following IHE, there was decreased maximal [La] of a similar magnitude in both hypoxic exposure methods. However, these changes do not appear to be consistent, with other researchers having reported no changes in the lactate profile following both LHTL (Gore et al., 2001; Robertson et al., 2010b) and IHE (Bonetti et al., 2006; Tadibi et al., 2007). In fact, the inconsistent nature of this adaptation has been demonstrated for both methods of hypoxia; the running speed corresponding to 4mM [La] was improved in one bout but not in a subsequent identical bout of LHTL completed five weeks later (Robertson et al., 2010a). Furthermore, despite using almost-identical IHE protocols, one research group recorded substantial changes in lactate profile (Wood, 2006) whilst another found no such changes (Tadibi et al., 2007).
It is possible that the decreases in maximal HR and [La] following LHTL and IHE were transient changes indicative of increased fatigue resulting from over-reaching (Meeusen et al., 2006) that have been observed a number of times following periods of intense training and could be reversed with a few days of sufficient recovery (Faude et al., 2009). Hypoxia induces an additional physiological stress and can increase the occurrence of overtraining (Rusko et al., 2004). A high training load, and specifically an associated plasma volume expansion (Fellmann, 1992), could be responsible for the clear decreases in maximal HR in both LHTL and IHE. However, in this instance, if any groups were to suffer from undue fatigue or training effects, it is more likely that it would have been the Placebo group rather than the LHTL or IHE groups since the training load during the camp represented a much greater relative increase from their normal training. Unfortunately, due to the athletes' competition schedule it was not possible to delay the post-intervention treadmill tests until a few days after the end of the camp, although this would have allowed a short period of recovery and thereby minimised the possible influences of fatigue or plasma volume expansion on the results.
It has been demonstrated previously that one additional parameter, vVO2max, can alone predict up to 94% of the total variance in 16-km running performance (McLaughlin et al., 2010) and, as such, is a good indicator because it integrates both the maximal aerobic power and running economy (Billat and Koralsztein, 1996). Again, there were no clear changes to this parameter relating to either method of hypoxic exposure in the current study. Of the four factors discussed here that have been shown to account for variance in running performance, positive changes in 3mM [La] running speed and running economy in LHTL alone suggest any likely benefit.
Limitations
The groups differed in the amount of training they had completed in the lead-up to the study, and consequently the training load of the camp would have served as a greater stimulus for some athletes than others. In order to neutralise the potential inequality of the training effect, the change in training load from pre-camp to during-camp was incorporated into the analyses as a covariate; however, the possibility of the results being affected by these training differences cannot be discounted.
The number of participants for whom there are running data is less than those for blood parameters due to athlete injury drop-outs. Therefore, interpretations of these data are more difficult due to the effects being relatively small in magnitude with moderate variability between subjects.
Conclusion
The clear difference in Hbmass response between LHTL and IHE indicated that the dose of hypoxia in daily 60-90 min IHE is insufficient to induce accelerated erythropoiesis. Improved running economy and 3mM [La] running speed following LHTL suggested that this method of hypoxic exposure may enhance performance at submaximal running speeds. Overall, there was no evidence to support the use of IHE in elite triathletes.
Acknowledgements
The authors wish to thank all the triathletes and coaches who took part in this project. This project was jointly-funded by Triathlon Australia, the Australian Institute of Sport and the University of Canberra. Procedures followed in this research are in accordance with Australian law.
Biographies
Clare E. Humberstone-Gough
Employment
Senior Physiologist, Australian Institute of Sport
Degree
BSc. Sports Science and Physiology, PhD
Research interests
Anti-doping, combat sports, talent development
E-mail: clare.humberstone@ausport.gov.au
Philo U. Saunders
Employment
Senior Physiologist, Australian Institute of Sport
Degrees
Bachelor Applied Science, Bachelor of Science Honours, PhD
Research interests
Altitude training, running physiology, heat training, plyometric training, running mechanics, swimming physiology.
Darrell L. Bonetti
Employment
Aviation Physiologist, Royal New Zealand Air Force
Degree
PhD
Shaun Stephens
Employment
Performance Coach, Team Sky Pro Cycling
Degrees
BHMS, Exercise management (Hons 1)
Nicola Bullock
Employment
Senior Physiologist, Australian Institute of Sport and Australian Canoeing
Degree
PhD
Research interests
Kayaking, training monitoring
Christopher J. Gore
Employment
Head of Physiology, Australian Institute of Sport
Degrees
DipT, BEd (Hons), PhD
Research interests
Altitude training, anti-doping
References
- Alexander A., Garvican L.A., Burge C.M., Clark S.A., Plowman J.S., Gore C.J. (2011) Standardising analysis of carbon monoxide rebreathing for application in anti-doping. Journal of Science and Medicine in Sport 14, 100-105 [DOI] [PubMed] [Google Scholar]
- Amann M., Subudhi A. W., Foster C. (2006) Predictive validity of ventilatory and lactate thresholds for cycling time trial performance. Scandinavian Journal of Medicine and Science in Sports 16, 27-34 [DOI] [PubMed] [Google Scholar]
- Banister E.W., Calvert T.W., Savage M.V., Bach T.M. (1975) A systems model of training for athletic performance. Australian Journal of Sports Medicine 7, 57-61 [Google Scholar]
- Billat L.V., Koralsztein J.P. (1996). Significance of the velocity at VO2max and time to exhaustion at this velocity. Sports Medicine 22, 90-108 [DOI] [PubMed] [Google Scholar]
- Bonetti D.L., Hopkins W.G. (2009) Sea-level exercise performance following adaptation to hypoxia: a meta-analysis. Sports Medicine 39, 107-127 [DOI] [PubMed] [Google Scholar]
- Bonetti D.L., Hopkins W.G., Kilding A.E. (2006) High-intensity kayak performance after adaptation to intermittent hypoxia. International Journal of Sports Physiology and Performance 1, 246-260 [DOI] [PubMed] [Google Scholar]
- Di Prampero P.E., Atchou G., Bruckner J.C., Moia C. (1986) The energetics of endurance running. European Journal of Applied Physiology and Occupational Physiology 55, 259-266 [DOI] [PubMed] [Google Scholar]
- Faude O., Meyer T., Urhausen A., Kindermann W. (2009) Recovery training in cyclists: ergometric, hormonal and psychometric findings. Scandinavian Journal of Medicine and Science in Sports 19, 433-441 [DOI] [PubMed] [Google Scholar]
- Favret F., Richalet J.P., Henderson K.K., Germack R., Gonzalez N.C. (2001) Myocardial adrenergic and cholinergic receptor function in hypoxia: correlation with O(2) transport in exercise. American Journal of Physiology Regulatory Integrative and Comparative Physiology 280, R730-738 [DOI] [PubMed] [Google Scholar]
- Fellmann N. (1992) Hormonal and plasma volume alterations following endurance exercise. A brief review. Sports Medicine 13, 37-49 [DOI] [PubMed] [Google Scholar]
- Gore C.J., Clark S.A., Saunders P.U. (2007) Nonhematological mechanisms of improved sea-level performance after hypoxic exposure. Medicine and Science in Sports and Exercise 39, 1600-1609 [DOI] [PubMed] [Google Scholar]
- Gore C.J., Hahn A.G., Aughey R.J., Martin D.T., Ashenden M.J., Clark S.A., Garnham A.P., Roberts A.D., Slater G.J., McKenna M.J. (2001) Live high:train low increases muscle buffer capacity and submaximal cycling efficiency. Acta Physiologica Scandinavica 173, 275-286 [DOI] [PubMed] [Google Scholar]
- Gore C.J., Rodriguez F.A., Truijens M.J., Townsend N.E., Stray-Gundersen J., Levine B.D. (2006) Increased serum erythropoietin but not red cell production after 4 wk of intermittent hypobaric hypoxia (4,000-5,500 m). Journal of Applied Physiology 101, 1386-1393 [DOI] [PubMed] [Google Scholar]
- Gough C., Sharpe K., Ashenden M., Anson J.M., Saunders P.U., Garvican L.A., Bonetti D.L., Gore C.J., Prommer N. (2011) Quality control technique to reduce the variability of longitudinal measurement of hemoglobin mass. Scandinavian Journal of Medicine and Science in Sports 21, e365-371 [DOI] [PubMed] [Google Scholar]
- Hackett P.H., Roach R. (1995) High-altitude medicine. St Louis, MO, Mosby [Google Scholar]
- Hamlin M.J., Marshall H.C., Hellemans J., Ainslie P.N. (2010) Effect of intermittent hypoxia on muscle and cerebral oxygenation during a 20-km time trial in elite athletes: a preliminary report. Applied Physiology Nutrition and Metabolism 35, 548-559 [DOI] [PubMed] [Google Scholar]
- Heck H., Mader A., Hess G., Mucke S., Muller R., Hollmann W. (1985) Justification of the 4-mmol/l lactate threshold. International Journal of Sports Medicine 6, 117-130 [DOI] [PubMed] [Google Scholar]
- Hellemans J. (1999) Intermittent hypoxic training: a pilot study. In:Proceedings of the Second Annual International Altitude Training Symposium, Flagstaff. 145-154 [Google Scholar]
- Hopkins W.G. (2006) Spreadsheets for analysis of controlled trials, with adjustment for a subject characteristic. Sportscience 10, 46-50 [Google Scholar]
- Hopkins W.G., Marshall S.W., Batterham A.M., Hanin J. (2009) Progressive statistics for studies in sports medicine and exercise science. Medicine and Science in Sports and Exercise 41, 3-13 [DOI] [PubMed] [Google Scholar]
- Julian C.G., Gore C.J., Wilber R.L., Daniels J.T., Fredericson M., Stray-Gundersen J., Hahn A.G., Parisotto R., Levine B.D. (2004) Intermittent normobaric hypoxia does not alter performance or erythropoietic markers in highly trained distance runners. Journal of Applied Physiology 96, 1800-1807 [DOI] [PubMed] [Google Scholar]
- Katayama K., Matsuo H., Ishida K., Mori S., Miyamura M. (2003) Intermittent hypoxia improves endurance performance and submaximal exercise efficiency. High Altitude Medicine and Biology 4, 291-304 [DOI] [PubMed] [Google Scholar]
- Levine B.D., Stray-Gundersen J. (1997) "Living high-training low": effect of moderate-altitude acclimatization with low-altitude training on performance. Journal of Applied Physiology 83, 102-112 [DOI] [PubMed] [Google Scholar]
- Lundby C., Millet G.P., Calbet J.A., Bartsch P., Subudhi A.W. (2012) Does 'altitude training' increase exercise performance in elite athletes?. British Journal of Sports Medicine 46, 792-795 [DOI] [PubMed] [Google Scholar]
- McLaughlin J.E., Howley E.T., Bassett D.R., JR., Thompson D.L., Fitzhugh E.C. (2010) Test of the classic model for predicting endurance running performance. Medicine and Science in Sports and Exercise 42, 991-997 [DOI] [PubMed] [Google Scholar]
- Meeusen R., Duclos M., Gleeson M., Rietjens G., Steinacker J.M., Urhausen A. (2006) Prevention, diagnosis and treatment of the Overtraining Syndrome: ECSS Position Statement 'Task Force'. European Journal of Sport Science 6, 1-14 [DOI] [PubMed] [Google Scholar]
- Millet G. P., Roels B., Schmitt L., Woorons X., Richalet J.P. (2010) Combining hypoxic methods for peak performance. Sports Medicine 40, 1-25 [DOI] [PubMed] [Google Scholar]
- Robach P., Lundby C. (2012) Is live high-train low altitude training relevant for elite athletes with already high total hemoglobin mass?. Scandinavian Journal of Medicine and Science in Sports 22, 303-305 [DOI] [PubMed] [Google Scholar]
- Robach P., Schmitt L., Brugniaux J.V., Roels B., Millet G., Hellard P., Nicolet G., Duvallet A., Fouillot J.P., Moutereau S., Lasne F., Pialoux V., Olsen N.V., Richalet J.P. (2006) Living high-training low: effect on erythropoiesis and aerobic performance in highly-trained swimmers. European Journal of Applied Physiology 96, 423-433 [DOI] [PubMed] [Google Scholar]
- Robertson E.Y., Saunders P.U., Pyne D.B., Aughey R.J., Anson J.M., Gore C J. (2010a) Reproducibility of performance changes to simulated live high/train low altitude. Medicine and Science in Sports and Exercise 42, 394-401 [DOI] [PubMed] [Google Scholar]
- Robertson E.Y., Saunders P.U., Pyne D.B., Gore C.J., Anson J.M. (2010b) Effectiveness of intermittent training in hypoxia combined with live high/train low. European Journal of Applied Physiology 110, 379-387 [DOI] [PubMed] [Google Scholar]
- Rodriguez F.A., Ventura J.L., Casas M., Casas H., Pages T., Rama R., Ricart A., Palacios L., Viscor G. (2000) Erythropoietin acute reaction and haematological adaptations to short, intermittent hypobaric hypoxia. European Journal of Applied Physiology 82, 170-177 [DOI] [PubMed] [Google Scholar]
- Rusko H.K., Tikkanen H.O., Peltonen J.E. (2004) Altitude and endurance training. Journal of Sports Science 22, 928-945 [DOI] [PubMed] [Google Scholar]
- Saunders P.U., Pyne D.B., Telford R.D., Hawley J.A. (2004) Reliability and variability of running economy in elite distance runners. Medicine and Science in Sports and Exercise 36, 1972-1976 [DOI] [PubMed] [Google Scholar]
- Saunders P.U., Telford R.D., Pyne D.B., Hahn A.G., Gore C.J. (2009) Improved running economy and increased hemoglobin mass in elite runners after extended moderate altitude exposure. Journal of Science and Medicine in Sport 12, 67-72 [DOI] [PubMed] [Google Scholar]
- Schmidt W., Prommer N. (2010) Impact of alterations in total hemoglobin mass on VO2max. Exercise and Sports Science Reviews 38, 68-75 [DOI] [PubMed] [Google Scholar]
- Siebenmann C., Robach P., Jacobs R.A., Rasmussen P., Nordsborg N., Diaz V., Christ A., Olsen N. ., Maggiorini M., Lundby C. (2012) Live high-train low" using normobaric hypoxia: a double-blinded, placebo-controlled study. Journal of Applied Physiology 112, 106-17 [DOI] [PubMed] [Google Scholar]
- Tadibi V., Dehnert C., Menold E., Bartsch P. (2007) Unchanged anaerobic and aerobic performance after short-term intermittent hypoxia. Medicine and Science in Sportsand Exercise 39, 858-864 [DOI] [PubMed] [Google Scholar]
- Wehrlin J.P., Zuest P., Hallen J., Marti B. (2006) Live high-train low for 24 days increases hemoglobin mass and red cell volume in elite endurance athletes. Journal of Applied Physiology 100, 1938-1945 [DOI] [PubMed] [Google Scholar]
- Wilber R.L. (2007) Application of altitude/hypoxic training by elite athletes. Medicine and Science in Sports and Exercise 39, 1610-1624 [DOI] [PubMed] [Google Scholar]
- Wood M.R., Dowson M.N., Hopkins W.G. (2006) Running performance after adaptation to acutely intermittent hypoxia. European Journal of Sports Science 6, 163-172 [Google Scholar]


