Abstract
The intention of this study was to systematically analyze the impact of biomechanical variables in terms of different vibration frequencies, amplitudes and knee angles on quadriceps femoris and hamstring activity during exposure to whole-body vibration (WBV). 51 healthy men and women (age 55 ± 8 years) voluntary participated in the study and were randomly allocated to five different vibration-frequency groups. Each subject performed 9 static squat positions (3 amplitudes x 3 knee angles) on a side alternating vibration platform. Surface electromyography (EMG) was used to record the neuromuscular activity of the quadriceps femoris and hamstring muscles. Maximal voluntary contractions (MVCs) were performed prior to the measurements to normalize the EMG signals. A three-way mixed ANOVA was performed to analyze the different effects of the biomechanical variables on muscle activity. Depending on the biomechanical variables, EMG muscle activity ranged between 18.2 and 74.1 % MVC in the quadriceps femoris and between 5.2 and 27. 3 % MVC in the hamstrings during WBV. The highest levels of muscle activation were found at high frequencies and large amplitudes. Especially in the quadriceps femoris muscle, a WBV frequency of 30 Hz led to a significant increase in muscle activity compared to the other tested frequencies. However, it seems that knee angle is only relevant for the quadriceps femoris muscle. The results of this study should give more information for developing individual training protocols for WBV treatment in different practical applications.
Key Points.
WBV leads to a higher muscle activity of the quadriceps femoris than of the hamstrings.
The maximum levels of muscle activity were significantly reached at high amplitude and high frequency.
The knee angle only significantly affects the quadriceps femoris.
Certain combinations of the biomechanical variables have similar effects on the level of muscle activity.
Key words: Vibration training, surface electromyography, muscle strength, muscle tuning
Introduction
In the last ten years, training on vibration plates has become a steadily increasing field of interest in sports science and research. The mechanical vibration stimulus applied to the muscles and tendons during vibration training leads to a neuromuscular response. This physiological mechanism has been named “tonic vibration reflex” (TVR), where muscle spindle reflexes facilitate the activation of Ia-motoneurons, leading to muscle contractions (Hagbart and Eklund, 1966; Lance et al., 1973). Monosynaptic and polysynaptic pathways have been shown to mediate TVR (Desmedt and Godeaux, 1980; Luo et al., 2005).
In fact, previous studies have shown that whole-body vibration (WBV) exercises have positive effects in increasing muscle strength and performance (Bosco et al., 1999b; Cardinale and Wakeling, 2005; Delecluse et al., 2003; Luo et al., 2005, Ruiter et al., 2003), as well as improvements in jumping ability (Lamont et al., 2009). Other studies reported metabolic and hormonal responses in men (Bosco et al., 2000; Kerschan-Schindl et al., 2001; Kvorning et al., 2006; Rittweger et al., 2000 and 2002). However, there are some studies reporting no or less effects of WBV intervention (Cochrane et al., 2004; Colson et al., 2009; Schlumberger et al., 2001; Torvinen et al., 2002a; 2002b). Although it has been shown that WBV has the most effective impact on elderly people or individuals with clinical complaints (Bogaerts et al., 2009; Johnson et al., 2010; Rapp et al., 2009a; 2009b; Rittweger et al., 2006; Schuhfried and Mittermaier, 2005), there is still a lack of knowledge about effective training protocols for WBV exercise (Cardinale and Lim, 2003). Limitations of previous studies are different training procedures with regard to the exercise parameters (i.g. side alternating or synchronous vibration platform type, duration of WBV, volume of WBV exercises) and the biomechanical variables (vibration frequency, vibration amplitude, and joint angle) determining the vibration load. Especially the possible combinations of these biomechanical variables are too multifarious and could explain the different results of muscle strength and performance in previous studies. Therefore the previous study is focusing on the three biomechanical variables affecting the intensity of neuromuscular activation.
In this context, several studies have investigated the responsiveness of different muscles in different exercises during vibration stimulus using electromyography (EMG). It has been reported that a vibration stimulus applied to the arm increased the neuromuscular activity during elbow flexion compared to the same exercise without vibration (Bosco et al., 1999a). Furthermore, Roelants et al., 2006 showed that WBV increased leg muscle activity in three different squat exercises. Thereby, the muscle activity of the leg muscles varied between 12.6 and 82.4 % of maximal voluntary contraction (MVC) values. Another study investigated the neuromuscular activity of the vastus lateralis muscle during WBV and reported that different frequencies led to different activation levels (Cardinale and Lim, 2003). Variations in neuromuscular responses comparing different vibration platforms, contraction, and squatting types have also been reported (Abercromby et al., 2007). In a recent study, Krol et al., 2011 investigated the interaction of different vibration frequencies and amplitudes on the activity of the vastus medialis and lateralis muscles. They showed that the muscle activity increased both with increased frequency and amplitude. However, it should be pointed out that, to date, no study has examined the effectiveness of combinations of all three biomechanical variables (different vibration frequencies, amplitudes, and knee angles) on quadriceps femoris and hamstrings.
Therefore, the aim of this study was to investigate the optimal frequency, amplitude, and knee angle to achieve the highest level of activity of the quadriceps and hamstring muscles. We hypothesized that the biomechanical variables, which determine the vibration stimulus, affect the level of neuromuscular activity in different dimensions. We first examined to what extent quadriceps femoris and hamstring muscles were active during WBV. In a second step we investigated how the level of activation was affected by the biomechanical variables. Both steps were important for determining the optimal vibration stimulus for effective exercise protocols for WBV intervention and training of different thigh muscles.
Methods
Experimental approach to the problem
To test the hypothesis put forward in the introduction, measurements of a randomised controlled trial were done to analyze the neuromuscular activity of the quadriceps femoris and hamstrings during WBV exposure. Surface EMG was used to record the signals from the rectus femoris, vastus medialis, vastus lateralis muscles, semitendinousus and biceps femoris muscles in different conditions of WBV. The neuromuscular activation levels of the quadriceps femoris and hamstrings were the dependent variables. The independent variables were vibration frequency (6 Hz, 12 Hz, 18 Hz, 24, Hz and 30 Hz), three vibration peak amplitudes (1.3, 2.6 and 3.9 mm) and knee angles at 30°, 45° and 60°. To normalize EMG, muscle activation was recorded during isolated and isometric maximal voluntary contractions (MVC). EMG treatment procedure and data analysis were performed according to Merletti, 1999.
Subjects
Fifty-one healthy and physically active men (n = 17) and women (n = 34) (age 55 ± 8 years, height 1.69. ± 0.08 m, weight 69.4 ± 11.7 kg, BMI 23.7 ± 2.7 kg/m2) volunteered to participate in the present study. All subjects gave written informed consent to participate in the experiment. Reasons for exclusion were any history of recent injuries or fractures of the lower extremities, total knee or hip arthroplasty, or diseases related to gallstones and kidney stones. The study was approved by the Human Ethics Committee of the university according to the declaration of Helsinki.
EMG analyses
The surface EMG (Noraxon Telemyo 2400T V2, Scottsdale, AZ) was recorded from the rectus femoris, vastus medialis, vastus lateralis, semitendinousus and biceps femoris muscles of the dominant leg. Bipolar surface electrodes (Ag/AgCL, 3M Health Care, St. Paul, MN) were applied over the muscle belly (interelectrode distance 25 mm) in accordance with SENIAM recommendations (Hermens et al., 1999). A ground electrode was placed over the epicondyle of the tibia. The preamplified EMG signals were amplified (x1000), bandpass filtered at 10-500 Hz ± 2% cut-off (Butterworth/Bessels), and sampled at 1,500 Hz. MyoResearch XP software (Noraxon, Scottsdale, AZ) was used to collect and store the data for subsequent analysis. EMG cables were properly fixed to the skin with tape to prevent the cables from swinging and to avoid movement artifacts.
Treatment protocol
The participants were randomized into five groups with different vibration frequencies (6 Hz, 12 Hz, 18 Hz, 24, Hz and 30 Hz), so that each subject was only exposed to a single frequency. After attaching the electrodes, an impedance test was performed. Maximal skin impedance from 1 to 5 kΩ was accepted for the experimental session. If the impedance level was verified, the test protocol started by performing isometric MVCs to record muscle activity (Burden, 2010). The MVCs were performed on the IsoMed 2000 (D&R Ferstl GmbH, Hernau) with isolated leg extension and flexion at knee angles of 30°, 45° and 60°.
The participants were exposed to WBV using a side alternating vibration platform (Galileo Fitness, Novotec Medical GmbH, Pforzheim). Such a vibration device reciprocates vertical displacements on the left and right side of a fulcrum. So the amplitudes on a side alternating platform depend on the width of the foot position (Rittweger, 2010). Therefore muscle activity was recorded during 9 different static squat positions: three different foot positions each performed at knee angles of 30°, 45° and 60°. Foot positions, which determine the amplitudes, were marked on the vibration platform: FP1 (vibration peak amplitude of 1.3 mm), FP2 (vibration peak amplitude of 2.6 mm) and FP3 (vibration peak amplitude of 3.9 mm). Subjects were asked to stand barefoot in the relevant squat position with the centre of the heel on the marker for the particular foot position while their hands were kept on their waist. Knee angles were simultaneous monitored and recorded with EMG signals using a flexible axis goniometer (Noraxon 2D- Goniometer, Scottsdale, AZ) connected to the EMG device. The goniometer was properly fixed to the skin with tape on the lateral line of the tibiofemural joint of the dominant leg with its center placed over the joint space. Zero offset calibration was performed at a knee angle of 0° additionally controlled with a manual orthopedic goniometer. During all test positions, a straight back and an upright body were required. Posture was permanently monitored visually during all conditions. Before starting the measurements, participants were able to familiarize themselves with the selected vibration stimulus during a warm-up period.
The EMG signals were recorded once the subjects had taken up the correct position on the vibration platform. In the first 6-8 seconds, muscle activity was recorded without WBV stimulus. Then WBV was applied at the frequency the participant was randomized to and EMG was recorded for the next 15 seconds. Each of the nine test positions were measured once only in a single session at the frequency the participant was randomized. To unload the muscles, subjects paused for 60 seconds and sat on a chair before the next measurement started.
Data analyses
The EMG raw signals were rectified and smoothed. Root mean square calculation (rms) was applied as a digital smoothing algorithm in a defined time window (300 ms). Then the EMGrms signals were MVC-normalized according to the isolated MVCs done prior to the WBV trials. Subsequently, muscle activity during WBV exposure was expressed as a percentage of the muscle activity during the measured MVC (100 %). Muscle activity of the rectus femoris, vastus lateralis, and medialis muscles was expressed relative to muscle activity during maximal isometric leg extension. Muscle activity of the biceps femoris and semitendinousus muscles was expressed relative to muscle activity during maximal isometric leg flexion. In each case, maximal leg extension and flexion were performed at knee angles of 30, 45 and 90° which correspond to the WBV squat conditions. The mean EMG%MVC value was calculated over a period of 5 seconds. Activity of the quadriceps femoris was calculated as the average responses of the rectus femoris, vastus lateralis, and vastus medialis muscles, whereas the activity of the hamstrings was calculated as the average of the semitendinousus and biceps femoris muscles. Data were reported as mean ± SE.
Statistical analysis
Statistical analyses were performed using the statistical software package SPSS, Version 20 (IBM, Armonk, NY). To analyze differences in EMG%MVC, a three-way mixed ANOVA with repeated measures independent variables (3 [amplitudes] × 3 [knee angle]) and between-group independent variables (5 [frequency group]) was performed (Field, 2009). After significant F values were found, a Tukey-Kramer HSD post hoc test for between-group comparison followed and contrast analyses were used for further comparisons within frequency groups. A Bonferroni correction was used to adjust the p value related to the number of conditions that were performed. Significance level was set at p ≤ 0.05.
Results
Quadriceps femoris
Addressing our first research question,the muscle activity of the quadriceps femoris varied between 18.2 and 74.1% MVC, whereby the highest level was found at a frequency of 30 Hz combined with vibration peak amplitude of 3.9 mm and a knee angle of 60°. Regarding our second research question, we found that the biomechanical variables had different effects on the muscle activity of the quadriceps femoris (see Figure 1 and Table 1). Our results show there was a significant main effect of ‘frequency group’ (F (4, 46) = 3.75, p < 0.01). The magnitude of EMG%MVC was consistently higher at a frequency of 30 Hz compared to the lower frequencies tested. Post hoc tests clarified statistically significant differences between the group at 30 Hz and that at 6 Hz (p < 0.01) and between 30 Hz and 12 Hz (p < 0.05). No significant differences (p > 0.05) were found between any of the other frequency groups.
Figure 1.
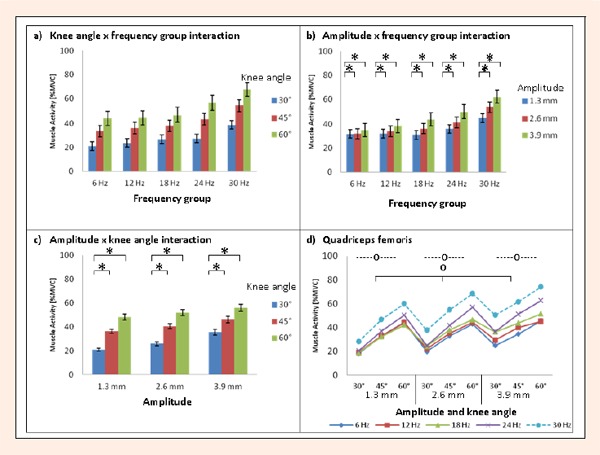
Mean ± SE EMG% MVC values of the quadriceps femoris for ‘knee angle x frequency group’ interaction effect (a), ‘amplitude x frequency group’ interaction effect (b), ‘amplitude x knee angle’ interaction effect (c) and mean EMG% MVC values regarding all biomechanical variables (d). * Significant differences in muscle activity (p < 0.05). -O- Significant differences in main effect of ‘knee angle’ (p < 0.05). O Significant differences in main effect of ‘amplitude (p < 0.05).
Table 1.
Muscle activity of the quadriceps femoris (mean value of rectus femoris, vastus lateralis, and medialis) and hamstrings (mean value of biceps femoris and semitendinosus) with vibration peak amplitude of 1.3 mm, 2.6 mm and 3.9 mm at different knee angles (30°, 45° and 60°) according to different frequency groups. Values are presented as a percentage of muscle activity during an isolated MVC (100%). Values are mean (± SE).
| 30° | 1.3 mm 45° |
60° | 30° | 2.6 mm 45° |
60° | 30° | 3.9 mm 45° |
60° | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 Hz | n = 10 | Quadriceps | 18.2 (2.1) | 33.0 (4.7) | 44.4 (6.5) | 19.9 (3.3) | 32.9 (5.2) | 42.9 (6.7) | 24.8 (4.3) | 34.5 (4.5) | 45.5 (6.5) |
| (4 ♂, 6 ♀) | Hamstring | 7.9 (2.3) | 5.2 (.7) | 6.8 (1.0) | 10.3 (2.3) | 7.4 (1.3) | 7.2 (1.1) | 11.3 (2.1) | 9.4 (1.8) | 8.4 (1.6) | |
| 12 Hz | n = 10 | Quadriceps | 19.2 (2.7) | 33.1 (4.0) | 44.0 (4.5) | 22.4 (2.7) | 35.2 (4.4) | 44.3 (4.0) | 29.5 (4.0) | 40.2 (5.6) | 45.2 (4.0) |
| (3 ♂, 7 ♀) | Hamstring | 5.9 (1.2) | 6.3 (1.3) | 8.6 (1.6) | 9.0 (1.5) | 8.0 (1.5) | 15.9 (6.5) | 15.6 (2.3) | 13.2 (2.6) | 11.4 (1.9) | |
| 18 Hz | n = 10 | Quadriceps | 19.0 (2.8) | 32.2 (4.2) | 42.2 (5.3) | 24.3 (3.8) | 38.0 (5.5) | 46.5 (5.7) | 36.5 (6.0) | 43.7 (5.3) | 51.6 (6.2) |
| (3 ♂, 7 ♀) | Hamstring | 5.8 (.9) | 6.9 (1.3) | 8.9 (1.3) | 8.5 (2.1) | 8.3 (1.5) | 9.9 (1.6) | 18.9 (5.1) | 10.5 (1.6) | 12.4 (2.1) | |
| 24 Hz | n = 10 | Quadriceps | 20.4 (2.1) | 37.1 (3.3) | 50.4 (4.6) | 25.0 (3.7) | 42.0 (4.5) | 57.1 (6.2) | 36.6 (5.5) | 51.1 (6.7) | 62.6 (6.9) |
| (3 ♂, 7 ♀) | Hamstring | 7.9 (1.7) | 8.8 (1.4) | 11.2 (2.0) | 13.1 (2.1) | 11.0 (1.4) | 13.1 (1.7) | 18.2 (3.0) | 17.2 (2.2) | 18.3 (3.1) | |
| 30 Hz | n = 11 | Quadriceps | 28.2 (1.9) | 46.7 (3.4) | 60.2 (6.5) | 37.9 (4.1) | 55.0 (4.8) | 68.5 (7.1) | 50.3 (6.7) | 61.6 (7.5) | 74.1 (8.0) |
| (4 ♂, 7 ♀) | Hamstring | 12.1 (2.3) | 11.8 (3.1) | 12.8 (3.3) | 20.6 (3.3) | 17.2 (4.0) | 17.3 (5.2) | 27.3 (5.1) | 19.3 (5.0) | 19.0 (6.1) |
We also found a significant main effect of the amplitudes tested (F (1. 3, 60.9) = 59.7, p < 0.01). Contrast analyses showed significant (p < 0.001) differences in EMG%MVC level between each amplitude tested. In addition, the main effect of ‘knee angle’ (F (1.6, 71.6) = 120.1, p < 0.001) was also significant. Here, the differences in muscle activity level were significant between all three knee angles tested. Differences in the effect of amplitude among frequency groups were found as the ‘amplitude x frequency group’ interaction effect, which was found to be significant (F (15.3, 60.9) = 3.6, p < 0.01). The amplitude effect between 1.3 mm and 2.6 mm and between 1.3 mm and 3.9 mm was significantly different (p < 0.001) , but no significant difference (p > 0.05) was found between 2.6 mm and 3.9 mm. There was also a significant interaction effect between amplitudes and knee angles (F (2.8, 130.9) = 7.5, p < 0. 001). Contrast analyses showed significant differences in EMG%MVC level between knee angles of 30° and 60° (p < 0.001) and between knee angles of 30° and 45° (p < 0.05) for each amplitude (see Figure 1c). However, neither a significant ‘knee angle x frequency group’ interaction effect (F (6.6, 71.6) = 0.7, p > 0.05), nor a significant interaction between knee angles, amplitudes, and frequency groups (F (11.4, 130.9) = 0. 4, p > 0.05) was found. This indicates that some combinations of the biomechanical variables have similar effects on muscle activity (see Figure 1a and d).
There were no statistical gender-specific differences concerning the main effects and the interaction effects.
Hamstrings
Addressing our first research question, normalized EMG activity of the hamstrings ranged between 5.2 and 27.3 % MVC. The highest activation level was detected in the 30 Hz-group with vibration peak amplitude of 3.9 mm and a knee angle of 30° (see Figure 2 and Table 1). Regarding our second research question, we found that the biomechanical variables had different effects on the muscle activity of the hamstrings. Statistical analyses confirmed these observations as follows: a significant ‘frequency group’ effect was found (F (4, 46) = 2.64, p < 0.05).The magnitude of EMG%MVC was consistently higher at a frequency of 30 Hz compared to the lower frequencies tested. Post hoc tests showed a statistically significant difference between the 30 Hz and 6 Hz groups (p < 0.05). No significant differences (p > 0. 05) were found between any of the other frequency groups. We can also report a significant main effect of ‘amplitude’ (F (2, 92) = 42.2, p < 0.001). Contrast analyses showed significant (p < 0.001) differences in EMG%MVC levels between all three amplitudes tested. However, the muscle activity level remained similar when the knee angles changed and there was no statistically significant effect of the knee angles tested (F (1.4, 62.4) = 3.5, p > 0.05). Furthermore, the muscle activity level within the frequency groups was not affected differently by the amplitudes and no significant ‘amplitude x frequency group’ interaction was found (F (8, 92) = 1.5, p > 0. 05). However, differences in the effect of knee angle among the amplitudes were found as the ‘amplitude x knee angle’ interaction effect and was significant (F (2.6, 118.2) = 5.7, p < 0.05). Contrast analyses showed significant differences in EMG%MVC levels between all knee angles tested within each amplitude (p < 0.01). In addition, there was no significant interaction between knee angles and frequency groups (F (5.4, 62.4) = 0.8, p > 0.05) or any significant interaction of ‘knee angle x amplitude x frequency group’ (F (10.3, 118.2) = 1.3, p > 0.05). This indicates that some combinations of the biomechanical variables had similar effects on muscle activity (see Figure 2a and d).
Figure 2.
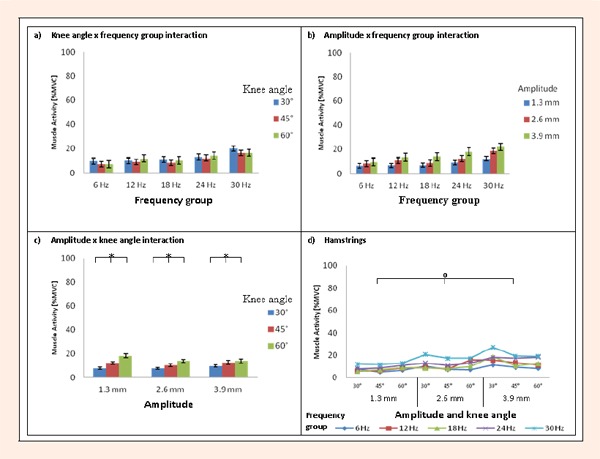
Mean ± SE EMG% MVC values of the hamstrings for ‘knee angle x frequency group’ interaction effect (a), ‘amplitude x frequency group’ interaction effect (b), ‘amplitude x knee angle’ interaction effect (c) and mean EMG% MVC values regarding all biomechanical variables (d). * Significant differences in muscle activity (p < 0.05). O Significant differences in main effect of ‘amplitude (p < 0.05).
There were no statistical gender- specific differences concerning the main effects and the interaction effects.
Discussion
This is the first study examining how different combinations of biomechanical variables (vibration frequencies, vibration amplitudes, and knee angles) impact quadriceps femoris and hamstring muscle activity during WBV exposure. The results of the present study show that these variables affect these muscles in different ways when exposed to vibration stimulus from a side alternating platform, thus confirming our hypothesis. In answer to our first question, we found that the muscle activity of the quadriceps femoris varied between 18.2 ± 2.1 and 74.1 ± 8.0 % MVC and was higher than the variation in the hamstrings, which was between 5.2 ± 0.7 and 27.3 ± 5.1 % MVC.
Vibration frequency is an important factor influ-encing muscle activity during vibration stimulus (Martin and Park, 1997). It is assumed that vibration leads to a higher stimulation of the mechanoreceptors of the relevant muscle, which in turn leads to increased tonicity. Subsequently, synchronized discharges of motor units lead to higher contraction forces in the muscle (Weber, 1997). In this context, Nigg, 2010 reported a muscle tuning hypothesis in runners, in which impact forces are an input signal characterized by frequency and amplitude. This paradigm may also be transferred to WBV: vibration signals are sensed and the central nervous system responds by tuning the activation of the corresponding muscles to minimize soft tissue vibration and injuries. The greatest effects of muscle tuning are achieved when the input frequency and the natural frequency of the soft tissue are similar. The results of the present study support the results of an earlier study (Cardinale and Lim, 2003) and suggest that a frequency of 30 Hz in specified squat positions results in the highest EMG responses, especially in leg extensor muscles. Frequency ranges above or below 30 Hz showed less EMG activity values in both studies. Nigg, 2010 also reported that the effects of muscle tuning are subject-specific depending on the characteristics of every single soft tissue compartment. This could also explain the SE values in the present study, even though movement artifacts in the EMG signal caused by vibration frequency could be removed (Ritzmann et al., 2010).
Another factor clarified in the present study is the impact of vibration amplitude on neuromuscular activity. Although this factor hardly plays a role in hamstring muscle activity, the vibration amplitude has an important effect on the quadriceps femoris. Here, the EMG%MVC increased with higher amplitudes. When vibration amplitude was increased by changing the foot position, a statistically equivalent EMG%MVC value was measured at a decreased knee angle. Both findings are in accordance with the results of Krol (2011), who also reported similar effects of vibration amplitude on muscle activity.
The results of the present study support findings on knee angles reported in previous studies. It has been reported that muscle length and preactivation are two main factors mediating WBV effect (Hopkins et al., 2009; Mester et al., 1999). Reducing muscle length or increasing preactivation led to better effects due to vibration exposure (Rohmert et al., 1989). The significant increase in quadriceps femoris activity by increasing the knee angle in the present study may be explained by these previous findings. On the other hand, we were able to show a lower hamstring muscle EMG%MVC, which was possibly caused by a lower preactivation level of these muscles. Noticeably, another aspect of lower hamstring muscle activity was the distance between the muscle and the vibration platform. Muscles closer to the vibration inducing system were more receptive to vibration stimulus (Nigg, 2010; Roelants et al., 2006).
Roelants et al., 2006 investigated leg muscle activity during different squat exercises with subjects standing on a synchronous vibrating platform at 35 Hz and a peak amplitude of 2.5 mm. They reported EMG%MVC activity between 33.0 ± 3.9 and 58.8 ± 7.9 % MVC for rectus femoris, vastus lateralis, and medialis muscles for low squats, which was higher than for high squats. These activation levels are clearly different compared to the muscle activity of the quadriceps femoris of 68.5 ± 7.1 % MVC in nearly the same conditions in the present study. This difference may be caused by small differences in the study design with regard to vibration frequency (35 Hz vs. 30 Hz), vibration peak amplitude (2.5 mm vs. 2.6 mm), knee angle (55° vs. 60°), and vibration platform type (synchronous vs. side alternating). Nevertheless, both studies showed an increase in muscle activity (EMG%MVC) during WBV while increasing the knee angle. Another EMG study (Cardinale and Lim, 2003) reported an increase of +34% (EMGrms) in vastus lateralis activity on a synchrounus platform at a frequency of 30 Hz with peak amplitude of 5 mm. The present study also confirms the effect of this frequency range combined with large vibration amplitude of 3.9 mm.
Several studies using similar vibration settings in their WBV treatment protocols as in the present study reported improvement in neuromuscular performance and muscle strength (side alternating platforms: Bosco et al., 1999b; Ruiter et al., 2003; synchronous platforms: Delecluse et al., 2003; Luo et al., 2005). Each study implemented a study design with vibration frequencies of 25 Hz and beyond, as well as vibration peak amplitudes between 4 mm and 5 mm. Similar treatment protocols have also been used in WBV studies on injured subjects or individuals with clinical complaints (Issurin and Tenenbaum, 1999; Rapp et al., 2009a). The vibration frequencies also ranged between 24 Hz and 35 Hz, the peak amplitude between 2 mm and 5 mm, and knee angles from 45° to 60°. This led to improvements in muscle strength or performance and stability in the knee joint.
To date, the mentioned frequency levels have been tolerated in elderly people as well as injured subjects (Bogaerts, 2009). Individuals with total knee arthroplasty using WBV during physical therapy showed an outstanding tolerance with respect to a vibration frequency of 35 Hz (Johnson et al., 2010). Rapp has also reported that subjects with gonarthrosis were able to achieve equal improvements in muscle strength with WBV training sessions with 30 Hz over a shorter period of time compared to traditional resistance training sessions (Rapp et al., 2009b).
In conclusion, this study investigated the interaction of the WBV determining biomechanical variables with regard to levels of quadriceps femoris and hamstring activation. First, we found that combining vibration frequency, amplitude, and knee angle in different ways led to a different range of EMG%MVC. A frequency of 30 Hz proved to be the most effective vibration stimulus, whereas the quadriceps femoris was particularly stimulated in combination with increased vibration amplitudes and knee angles. This study also indicates equal EMG%MVC values for this muscle group within the same frequency range when vibration amplitude is increased and knee angle decreased, concurrently. Second, EMG%MVC in hamstring muscles were much lower than in the quadriceps femoris muscle. The reported data is relevant to determine the load that vibration imposes on the neuromuscular system. However, further studies are needed to clarify the impact of the biomechanical variables on other muscle groups (i.g. lower leg and foot muscles or trunk and lower back muscles).
Conclusion
WBV exercises have been shown to have a positive influence on gaining muscle strength and performance (Lamont et al., 2009). Nevertheless, there is still a lack of knowledge about effective training protocols and procedures to describe an evidence-based vibration exercise program. The current study is the first step toward detecting the best parameters for combining biomechanical variables to produce the ideal vibration stimulus on a side alternating platform. Currently, a maximized vibration load can be achieved at a frequency of 30 Hz with amplitude of ca. 4 mm during a knee angle of 60°.
The current study has given us a better understanding of the effectiveness of different vibration loads for WBV. So the results will help physical therapists, coaches, and athletes in diverse sectors to activate the quadriceps femoris and hamstrings most effectively during a WBV session. Yet the effect of WBV on strength and power development depends on both the biomechanical variables (determining the vibration load) and the exercise protocols. Therefore, based on our findings, further studies are still needed to create and evaluate effective vibration training procedures and protocols for the lower extremities. Furthermore, additional studies are still needed to get better insight into the potential of biomechanical variables on other muscle groups and/or training goals.
Biographies
Dennis Perchthaler
Employment
PhD student, Department of Sports Medicine, Medical Clinic, University of Tuebingen, Germany
Degree
MSc
Research interests
Influence of Whole-Body Vibration in men, clinical biomechanics, physical therapy
E-mail: dennis.perchthaler@med.uni-tuebingen.de
Thomas Horstmann
Employment
Prof., Medical Park Bad Wiessee St. Hubertus, Bad Wiessee, Germany. Faculty for Sport and Health Sciences, Technische Universität München, Germany
Degree
MD
Research interests
Conservative and rehabilitative orthopedics, physical therapy
E-mail: t.horstmann@medicalpark.de
Stefan Grau
Employment
Prof., Department of Sports Medicine, Medical Clinic, University of Tuebingen, Germany
Degree
PhD
Research interests
Clinical biomechanics, overused injuries, footwear biomechanics.
E-mail: stefan.grau@med.uni-tuebingen.de
References
- Abercromby A.F., Amonette W.E., Layne C.S., McFarlin B.K., Hinman M.R., Paloski W.H. (2007) Variation in neuromuscular responses during acute whole-body vibration exercise. Medicine and Science in Sports and Exercise 39, 1642-1650 [DOI] [PubMed] [Google Scholar]
- Bogaerts A., Delecluse C., Claessens A., Troosters T., Boonen S., Verschueren S. (2009) Effects of whole body vibration training on cardiorespiratory fitness and muscle strength in older individuals (a 1-year randomised controlled trial). Age Ageing 38, 448-454 [DOI] [PubMed] [Google Scholar]
- Bosco C., Cardinale M., Tsarpela O. (1999a) Influence of vibration on mechanical power and electromyogram activity in human arm flexor muscles. European Journal of Applied Physiology and Occupational Physiology 79, 306-311 [DOI] [PubMed] [Google Scholar]
- Bosco C., Colli R., Introini E., Cardinale M., Tsarpela O., Madella A., Tihanyi J., Viru A. (1999b) Adaptive responses of human skeletal muscle to vibration exposure. Clinical Physiology 19, 183-187 [DOI] [PubMed] [Google Scholar]
- Bosco C., Iacovelli M., Tsarpela O., Cardinale M., Bonifazi M., Tihanyi J., Viru M., De L. A., Viru A. (2000) Hormonal responses to whole-body vibration in men. European Journal of Applied Physiology 81, 449-454 [DOI] [PubMed] [Google Scholar]
- Burden A. (2010) How should we normalize electromyograms obtained from healthy participants? What we have learned from over 25 years of research. Journal of Electromyography and Kinesiology 20, 1023-1035 [DOI] [PubMed] [Google Scholar]
- Cardinale M., Lim J. (2003) Electromyography activity of vastus lateralis muscle during whole-body vibrations of different frequencies. Journal of Strength and Conditioning Research 17, 621-624 [DOI] [PubMed] [Google Scholar]
- Cardinale M., Wakeling J. (2005) Whole body vibration exercise: are vibrations good for you? British Journal of Sports Medicine 39, 585-589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane D.J., Legg S.J., Hooker M.J. (2004) The short-term effect of whole-body vibration training on vertical jump, sprint, and agility performance. Journal of Strength and Conditioning Research 18, 828-832 [DOI] [PubMed] [Google Scholar]
- Colson S.S., Petit P.D., Hebreard L., Tessaro J., Pensini M. (2009) Whole body vibration does not enhance muscle activation. International Journal of Sports Medicine 30, 841-844 [DOI] [PubMed] [Google Scholar]
- De Ruiter C.J., Van Raak S.M., Schilperoort J.V., Hollander A.P., de H.A. (2003) The effects of 11 weeks whole body vibration training on jump height, contractile properties and activation of human knee extensors. European Journal of Applied Physiology 90, 595-600 [DOI] [PubMed] [Google Scholar]
- Delecluse C., Roelants M., Verschueren S. (2003) Strength increase after whole-body vibration compared with resistance training. Medicine and Science in Sports and Exercise 35, 1033-1041 [DOI] [PubMed] [Google Scholar]
- Desmedt J.E., Godeaux E. (1980) The tonic vibration reflex and the paradox in limb and jaw muscle in man. Spinal and Supraspinal Mechanisms of Voluntary Motor Control and Locomotion. Desmedt J.E.Basel: Karger; 215-242 [Google Scholar]
- Field A.P. (2009) Discovering statistics using SPSS: and sex and drugs and rock 'n' roll. 3rd edition Sage: London [Google Scholar]
- Hagbart K.E., Eklund G. (1966) Tonic vibrations reflexes (TVR) in spasticity. Brain Research 2, 201-203 [DOI] [PubMed] [Google Scholar]
- Hermens H.J., Freriks B., Merletti R., Stegeman D., Blok J., Rau G., Disselhorst-Klug C., Hägg G. (1999) European recommendations for surface electromyography, results of SENIAM project. 8th edition Enschede: Roessingh Research and Development [Google Scholar]
- Hopkins J.T., Fredericks D., Guyon P.W., Parker S., Gage M., Feland J.B., Hunter I. (2009) Whole body vibration does not potentiate the stretch reflex. International Journal of Sports Medicine 30, 124-129 [DOI] [PubMed] [Google Scholar]
- Issurin V.B., Tenenbaum G. (1999) Acute and residual effects of vibratory stimulation on explosive strength in elite and amateur athletes. Journal of Sports Sciences 17, 177-182 [DOI] [PubMed] [Google Scholar]
- Johnson A.W., Myrer J.W., Hunter I., Feland J.B., Hopkins J.T., Draper D.O., Eggett D. (2010) Whole-body vibration strengthening compared to traditional strengthening during physical therapy in individuals with total knee arthroplasty. Physiotherapy: Theory and Practice 26, 215-225 [DOI] [PubMed] [Google Scholar]
- Kerschan-Schindl K., Grampp S., Henk C., Resch H., Preisinger E., Fialka-Moser V., Imhof H. (2001) Whole-body vibration exercise leads to alterations in muscle blood volume. Clinical Physiology 21, 377-382 [DOI] [PubMed] [Google Scholar]
- Krol P., Piecha M., Slomka K., Sobota G., Polak A., Juras G. (2011) The effect of whole-body vibration frequency and amplitude on the myoelectric activity of vastus medialis and vastus lateralis. Journal of Sports Science and Medicine 10, 169-174 [PMC free article] [PubMed] [Google Scholar]
- Kvorning T., Bagger M., Caserotti P., Madsen K. (2006) Effects of vibration and resistance training on neuromuscular and hormonal measures. European Journal of Applied Physiology 96, 615-625 [DOI] [PubMed] [Google Scholar]
- Lamont H.S., Cramer J.T., Bemben D.A., Shehab R.L., Anderson M. A., Bemben M.G. (2009) Effects of a 6-week periodized squat training program with or without whole-body vibration on jump height and power output following acute vibration exposure. Journal of Strength and Conditioning Research 23, 2317-2325 [DOI] [PubMed] [Google Scholar]
- Lance J.W, Burke D., Andrews C.J. (1973) The reflex effects of muscle vibrations. New Developments in Electromyography and Clinical Neurophysiology. Desmedt J.E.Basel: Karger; 444-462 [Google Scholar]
- Luo J., McNamara B., Moran K. (2005) The use of vibration training to enhance muscle strength and power. Sports Medicine 35, 23-41 [DOI] [PubMed] [Google Scholar]
- Martin B.J., Park H.S. (1997) Analysis of the tonic vibration reflex: influence of vibration variables on motor unit synchronization and fatigue. European Journal of Applied Physiology and Occupational Physiology 75, 504-511 [DOI] [PubMed] [Google Scholar]
- Merletti R. (1999) Standards for Reporting EMG Data. Journal of Electromyography and Kinesiology 9, 3-5 [Google Scholar]
- Mester J., Spitzenfeil P., Schwarzer J., Seifriz F. (1999) Biological reaction to vibration—implications for sport. Journal of Science and Medicine in Sport 2, 211-226 [DOI] [PubMed] [Google Scholar]
- Nigg B.M. (2010) Biomechanics in Sport Shoes. Topline Printing Inc; Calgary [Google Scholar]
- Rapp W., Boeer J., Albrich C., Heitkamp H. (2009a) Efficiency of vibration or strength training for knee stability in osteoarthritis of the knee. Aktuelle Rheumatologie 34, 240-245 (In German: English abstract) [Google Scholar]
- Rapp W., Feil P., Grau S., Heitkamp H.C. (2009b) Vibration training compared with resistance training in patients with coxarthrosis. German Journal of Sports Medicine 60, 174 (In German) [Google Scholar]
- Rittweger J., Beller G., Felsenberg D. (2000) Acute physiological effects of exhaustive whole-body vibration exercise in man. Clinical Physiology 20, 134-142 [DOI] [PubMed] [Google Scholar]
- Rittweger J., Ehrig J., Just K., Mutschelknauss M., Kirsch K. A., Felsenberg D. (2002) Oxygen uptake in whole-body vibration exercise: influence of vibration frequency, amplitude, and external load. International Journal of Sports Medicine 23, 428-432 [DOI] [PubMed] [Google Scholar]
- Rittweger J., Belavy D., Hunek P., Gast U., Boerst H., Feilcke B., Armbrecht G., Mulder E., Schubert H., Richardson C., de H. A., Stegeman D. F., Schiessl H., Felsenberg D. (2006) Highly demanding resistive vibration exercise program is tolerated during 56 days of strict bed-rest. International Journal of Sports Medicine 27, 553-559 [DOI] [PubMed] [Google Scholar]
- Rittweger J. (2010) Vibration as an exercise modality: how it may work, and what its potential might be. European Journal of Applied Physiology 108, 877-904 [DOI] [PubMed] [Google Scholar]
- Ritzmann R., Kramer A., Gruber M., Gollhofer A., Taube W. (2010) EMG activity during whole body vibration: motion artifacts or stretch reflexes? European Journal of Applied Physiology 110, 143-151 [DOI] [PubMed] [Google Scholar]
- Roelants M., Verschueren S.M., Delecluse C., Levin O., Stijnen V. (2006) Whole-body-vibration-induced increase in leg muscle activity during different squat exercises. Journal of Strength and Conditioning Research 20, 124-129 [DOI] [PubMed] [Google Scholar]
- Rohmert W., Wos H., Norlander S., Helbig R. (1989) Effects of vibration on arm and shoulder muscles in three body postures. European Journal of Applied Physiology and Occupational Physiology 59, 243-248 [DOI] [PubMed] [Google Scholar]
- Schlumberger A, Salin D, Schmidtbleicher D. (2001) Strength training with superimposed vibrations. Sportverletzung Sportschaden 15, 1-7 (In German: English abstract) [DOI] [PubMed] [Google Scholar]
- Schuhfried O., Mittermaier C., Jovanovic T., Pieber K., Paternostro-Sluga T. (2005) Effects of whole-body vibration in patients with multiple sclerosis: a pilot study. Clinical Rehabilitation 19, 834-842 [DOI] [PubMed] [Google Scholar]
- Torvinen S., Kannus P., Sievanen H., Jarvinen T. A., Pasanen M., Kontulainen S., Jarvinen T. L., Jarvinen M., Oja P., Vuori I. (2002a) Effect of four-month vertical whole body vibration on performance and balance. Medicine and Science in Sports and Exercise 34, 1523-1528 [DOI] [PubMed] [Google Scholar]
- Torvinen S., Sievanen H., Jarvinen T. A., Pasanen M., Kontulainen S., Kannus P. (2002b) Effect of 4-min vertical whole body vibration on muscle performance and body balance: a randomized cross-over study. International Journal of Sports Medicine 23, 374-379 [DOI] [PubMed] [Google Scholar]
- Weber R. (1997) Muskelstimulation durch Vibration. Leistungssport 27, 53-57, 1997. (In German) [Google Scholar]


