Abstract
The purpose of this study was to investigate in vivo three- dimensional tibiofemoral kinematics and femoral condylar motion in knees with anterior cruciate ligament (ACL) deficiency during a knee bend activity. Ten patients with unilateral ACL rupture were enrolled. Both the injured and contralateral normal knees were imaged using biplane radiography at extension and at 15°, 30°, 60°, 90°, and 120° of flexion. Bilateral knees were next scanned by computed tomography, from which bilateral three-dimensional knee models were created. The in vivo tibiofemoral motion at each flexion position was reproduced through image registration using the knee models and biplane radiographs. A joint coordinate system containing the geometric center axis of the femur was used to measure the tibiofemoral motion. In ACL deficiency, the lateral femoral condyle was located significantly more posteriorly at extension and at 15° (p < 0.05), whereas the medial condylar position was changed only slightly. This constituted greater posterior translation and external rotation of the femur relative to the tibia at extension and at 15° (p < 0.05). Furthermore, ACL deficiency led to a significantly reduced extent of posterior movement of the lateral condyle during flexion from 15° to 60° (p < 0.05). Coupled with an insignificant change in the motion of the medial condyle, the femur moved less posteriorly with reduced extent of external rotation during flexion from 15° to 60° in ACL deficiency (p < 0.05). The medial- lateral and proximal-distal translations of the medial and lateral condyles and the femoral adduction-abduction rotation were insignificantly changed after ACL deficiency. The results demonstrated that ACL deficiency primarily changed the anterior-posterior motion of the lateral condyle, producing not only posterior subluxation at low flexion positions but also reduced extent of posterior movement during flexion from 15° to 60°.
Key Points.
Three-dimensional tibiofemoral kinematics and femoral condylar motion in ACL-deficient knees during upright weight-bearing flexion were measured using biplane radiography with the geometric center axis.
ACL deficiency caused posterior subluxation of the lateral condyle with excess external femoral rotation at early flexion positions.
On flexion from 15° to 60°, the lateral condyle moved slightly posteriorly in ACL deficiency leading to reduced extent of external femoral rotation.
Key words: anterior cruciate ligament, injury, kinematics, tibiofemoral, femoral condyle, radiography
Introduction
Anterior cruciate ligament (ACL) rupture is a common sport injury in the young and active population. The ACL rupture leads to abnormal tibiofemoral kinematics, producing secondary injury to the menisci and articular cartilage and subsequent osteoarthritic changes over time. A reconstructive operation is often recommended to repair the abnormal kinematics following ACL injury. However, current reconstructive techniques are not able to completely repair this kinematic abnormality (Logan et al., 2004b). This may be due, in part, to the fact that the abnormal kinematics caused by ACL deficiency is inadequately understood.
As the core ligament for normal knee motion, the ACL mainly restrains the anterior translation and internal rotation of the tibia. In ACL deficiency, anterior and medial translations and internal rotation of the tibia increase abnormally during weight-bearing flexion (Defrate et al., 2006; Georgoulis et al., 2003; Li et al., 2007; Yoo et al., 2005). However, tibiofemoral motion is relatively complex and includes components of three- dimensional (3D) kinematics and femoral condylar motion. Many studies on tibiofemoral motion after ACL deficiency regarded femoral motion as one movement, neglecting the differential motion between the medial and lateral condyles (Defrate et al., 2006; Georgoulis et al., 2003; Yamaguchi et al., 2009). Therefore, the effect of ACL deficiency on condylar motion remains poorly understood. In addition, few studies have thoroughly measured the 3D tibiofemoral kinematics and condylar motion in ACL deficiency (Brandsson et al., 2001; Shefelbine et al., 2006). A thorough knowledge of tibiofemoral motion in ACL-deficient knees might contribute to improving reconstructive surgical techniques and postoperative rehabilitation programs. Moreover, measuring medial and lateral condylar motions individually rather than studying femoral motion as a whole might contribute to clarifying the mechanism of chondral lesions in medial and lateral compartments after ACL injury.
Methods have been developed to thoroughly measure in vivo tibiofemoral motion during weight-bearing flexion/extension. Bilateral radiostereometry was used to measure tibiofemoral motion when the subject ascended a platform (Brandsson et al., 2001). This technique is plagued by problems such as the cross-talk phenomenon and the need for an invasive operation to implant tantalum beads in the knees. High-field supine magnetic resonance imaging (MRI) with a leg press device is another method (Shefelbine et al., 2006). The leg press device only provides a small load to the knee, which is much less than upright body weight. Upright weight bearing is vitally important to pathological knee kinematic assessment (Nicholson et al., 2012). The relatively small load to the knee utilized in supine MRI may be responsible for the insignificant difference in kinematics between ACL-deficient and normal knees previously described (Scarvell et al., 2005). Biplane radiography with image registration is a useful noninvasive technique for thoroughly evaluating tibiofemoral motion during upright weight-bearing flexion. When this method is used, the geometric center axis passing through the centers of the medial and lateral condyles can be used together to measure condylar motion (Asano et al., 2001; Kozanek et al., 2009; Shefelbine et al., 2006).
The present study used biplane radiography with image registration and the geometric center axis to measure the 3D tibiofemoral kinematics and condylar motion in ACL-deficient and normal knees during a knee bend activity. Tibiofemoral motion was then compared between the two knees to investigate the adverse effect of ACL deficiency.
Methods
Patients
Ten patients with unilateral ACL rupture (age: 19-40 years; 8 males, 2 females) participated in the study, which was approved by our ethics committee and followed the Declaration of Helsinki for ethical principles. All patients signed an informed consent form. The injured knees were unstable on pivot shift and Lachman tests, but had no other ligament injuries or osteoarthritic changes on radiographs. The ACL rupture was also diagnosed by MRI and confirmed during reconstructive surgery. The contralateral knees had no history of pain, injury, or surgery and were regarded as the normal controls (Kozanek et al., 2008).
Image collection and registration
Biplane radiographs of the knee were captured using two X-ray machines placed at a 3-m focus-film distance (Figure 1). The machines consisted of ceiling-mounted (AXIOM Aristos VX plus, Siemens, Germany) and mobile systems (Polymobil plus, Siemens, Germany) with 2,812×2,812 pixels per image (0.50 mm/pixel in-plane resolution) in a radiographic mode at 73 KV, 9 mAs and 75 KV, 10 mAs, respectively. Two X-ray beams were projected orthogonally to their corresponding film exchangers, which were angled 90° in relation to each other. After attaching a spherical marker (steel, 30 mm in diameter) to the middle part of the thigh, proximal to the patella, the patients performed a knee bend activity between the two film exchangers. The knee was maintained in a neutral status during bending from extension to 15°, 30°, 60°, 90°, and 120°, as measured using a hand goniometer. Each position was held for approximately 5 s, and two X-ray tubes simultaneously captured posteromedial and posterolateral radiographs of the knee. The ACL-deficient knee was imaged before the normal knee.
Figure 1.
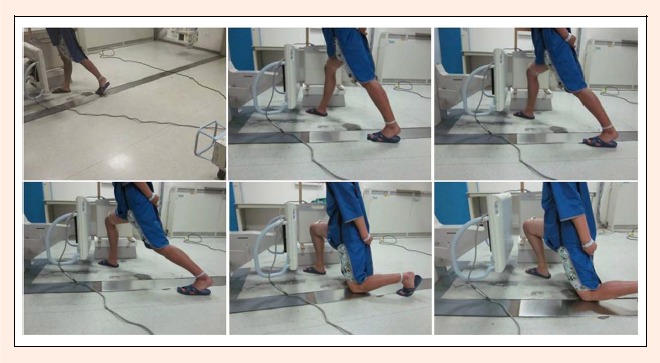
The knee was imaged by two X-ray machines from orthogonal directions during flexion from extension to 15°, 30°, 60°, 90°, and 120°.
Bilateral knees were then scanned by computed tomography (CT, SOMATOM Emotion 16, Siemens, Germany) with 28 cm field of view using 110 KV and 230 mAs, 512×512 pixels per image (0. 536 mm/pixel in-plane resolution), and a 1-mm thickness for 3D reconstruction. These CT scans were imported into Mimics 15.0 (Materialise, Leuven, Belgium), and bilateral 3D bone models of the tibiofemoral joints were created. The tibiofemoral model and its corresponding biplane radiographs were then imported into UG NX 7.0 (Siemens PLM Software, Plano, Texas, USA) for 3D-to-2D image registration. The biplane radiographs were placed perpendicularly to each other, simulating the disposition of the two X-ray film exchangers to create a virtual radiographic system. The biplane radiographs were then enlarged based on the magnification of the spherical marker such that the size of the tibiofemoral image on the radiographs was identical to that of the tibiofemoral model. The two radiographs were then adjusted vertically to the same level relative to the center of the sphere. The tibiofemoral model was moved into the virtual radiographic system for image registration. The tibial and femoral models were respectively translated and rotated in six degrees of freedom until they were matched well to their images on biplane radiographs (Figure 2). As the 3D-to-2D image registration for each knee position was achieved, the tibiofemoral motion of the 3D model reproduced that of the patient's knee during the knee bend activity.
Figure 2.
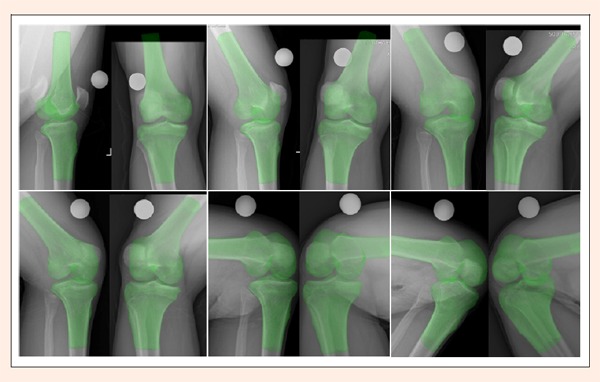
The 3D tibiofemoral model was simultaneously matched to its corresponding two 2D images on biplane radiographs at extension, 15°, 30°, 60°, 90°, and 120°. The spherical marker was indicated in white
Kinematics measurement
Using a joint coordinate system established on the tibiofemoral model (Figure 3), the 3D tibiofemoral kinematics and condylar motion were measured. The coordinate system was first established on the left tibiofemoral model of each patient and was then mirrored to the right model, so that the kinematics of both knees were measured using the same coordinate system. Two axes were created on the femur: the long axis and the geometric center axis. The former was defined as the anatomic axis of the distal femoral shaft. The latter was created as follows: The medial and lateral posterior condyles were best fitted by two spheres whose radii were determined from the profiles of posterior condyles (Leszko et al., 2011); the line connecting the centers of the two spheres was defined as the geometric center axis (Shefelbine et al., 2006). The midpoint between the two centers was defined as the femoral center. The tibial coordinate system was established as a Cartesian coordinate system, in which the medial-lateral axis bisected the anterior-posterior halves of the tibial plateau, perpendicular to the long axis of the tibia (Yamaguchi et al., 2009). The midpoint of the medial-lateral axis was defined as the tibial center. The proximal-distal axis passed through the tibial center and was parallel to the long axis. The anterior-posterior axis was the cross product of the proximal-distal and the medial-lateral axes.
Figure 3.
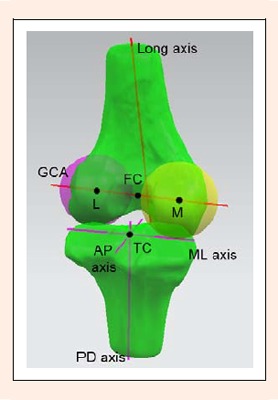
A joint coordinate system was used to measure the tibiofemoral kinematics and condylar motion. The geometric center axis (GCA) of the femur passed the centers of the medial (M) and lateral (L) posterior condyles. AP: anterior-posterior, ML: medial-lateral, PD: proximal-distal, FC: femoral center, TC: tibial center.
Tibiofemoral motion was measured using a method similar to that proposed by Grood and Suntay, 1983. Femoral translation was defined as the motion of the femoral center relative to the tibial coordinate system. Condylar translation was similarly defined as the motions of the posterior condylar centers relative to the tibia. Femoral flexion-extension was defined as the angle between the femoral long axis and the tibial proximal-distal axis on the tibial sagittal plane. Internal-external rotation was defined as the angle between the geometric center axis and the tibial medial-lateral axis on the tibial horizontal plane; adduction-abduction rotation was defined as the angle between these two axes on the tibial coronal plane. In addition, the motional extent was defined as the difference between the translations or rotations at two knee flexion angles.
Evaluation of biplane radiography accuracy
The accuracy of biplane radiography was evaluated by comparing the kinematics based on the radiographs with that based on CT data (as a reference standard) using three cadaver knees (McPherson et al., 2005). The cadaver knees were left knees donated by male citizens of Chongqing. The specimens were intact based on naked eye and X-ray examinations. Each cadaver knee was fixed on a wooden board, medial side down, using two iron pegs through each of the tibia and the femur. The tibia was immobilized, and the femur was moved in sequence from extension to 15°, 30°, 60°, 90°, and 120° of flexion using manual force and was fixed at the position each time. With the knee fixed at each position, the board was first placed between the X-ray film exchangers to obtain biplane radiographs; then, the board was placed into the CT and scanned.
Tibiofemoral models at six positions were then created using CT scans (CT models). The tibiofemoral model at extension was used to best fit with the tibiofemoral models at other five positions using Geomagic Studio 12.0 (Raindrop Geomagic, Morrisville, NC, USA), so that the same joint coordinate system could be used for all six CT models. On the other hand, the tibiofemoral model at extension was matched to the tibiofemoral images on biplane radiographs at six positions, resulting in six models based on biplane radiography (BR models). A joint coordinate system was then established on the tibiofemoral model at extension in the manner described above. The 3D tibiofemoral kinematics of the CT and BR models were measured individually and compared to each other using this coordinate system.
Statistical analysis
The statistical data were analyzed using SPSS 10.0 (SPSS Inc., Chicago, IL, USA). Comparisons of translation and rotation were performed between ACL-deficient and normal knees using a repeated-measures analysis of variance. A paired Student's t-test was used to compare the motional extents from extension to 120° between the two sides. All comparisons with p < 0.05 were considered significant.
Results
Biplane radiography accuracy
The differences in kinematics between the CT and BR models were -0.52 ± 0.88 mm, 0. 26 ± 0.65 mm, and 0.19 ± 0.61 mm in anterior-posterior, medial-lateral, and proximal-distal translation, respectively; and -0.50° ± 1.03°, -0.86° ± 1.09°, and 0.14° ± 0.76° in flexion- extension, internal-external, and adduction-abduction rotation, respectively. The accuracy of our biplane radiography was considered acceptable for clinical applications.
Translations of the femur and the femoral condyles
Anterior-posterior translation: From extension to 120°, the femur was located posterior to the tibial center in both ACL-deficient and normal knees (Table 1). At extension and 15°, significantly increased posterior translation of the femur was observed in ACL-deficient knees when compared to normal knees. There was no significant difference in anterior-posterior femoral translation beyond 15°.
Table 1.
Comparison of translations of the femur and the femoral condyles between ACL-deficient and normal knees at 6 flexion positions. Data are means (±SD).
| Translation | Extension | 15° | 30° | 60° | 90° | 120° | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mm) | ACLD | normal | ACLD | normal | ACLD | normal | ACLD | normal | ACLD | normal | ACLD | normal | |
| AP | Femur | 4.2(1.1) | 2.1(1.5)* | 7.2(2.7) | 4.6(2.2)* | 6.4(1.8) | 5.8(1.5) | 6.7(1.9) | 7.0(1.9) | 8.0(1.6) | 7.2(1.0) | 9.6(1.6) | 9.5(1.2) |
| MC | 4.9(.7) | 5.3(1.4) | 4.1(1.1) | 3.7(1.6) | 2.5(1.3) | 2.5(1.2) | 2.5(0.9) | 2.8(1.7) | 2.6(1.1) | 2.2(1.0) | 4.1(1.3) | 4.4(0.6) | |
| LC | 3.4(2.3) | -1.0(2.5)* | 10.7(4.7) | 5.6(3.2)* | 10.2(2.7) | 9.1(2.3) | 11.0(3.4) | 11.2(2.3) | 13.4(2.6) | 12.2(1.4) | 15.1(2.8) | 14.7(2.5) | |
| ML | Femur | 5.7(1.1) | 5.1(1.1) | 4.6(1.0) | 3.8(1.1) | 3.5(1.0) | 3.7(0.5) | 2.8(0.8) | 3.2(1.2) | 2.6(1.2) | 3.2(.7) | 2.7(1.0) | 2.9(1.2) |
| MC | 30.0(2.1) | 29.2(2.7) | 28.5(2.2) | 28.2(2.1) | 27.6(1.6) | 27.6(2.0) | 26.7(1.9) | 27.0(1.8) | 26.0(1.8) | 26.6(2.1) | 26.8(2.2) | 26.7(2.0) | |
| LC | -18.5(2.9) | -19.0(2.2) | -19.6(2.8) | -20.3(2.8) | -20.5(3.0) | -20.1(2.2) | -21.1(2.7) | -20.5(3.1) | -20.8(3.4) | -20.3(2.9) | -21.4(2.5) | -20.9(3.2) | |
| PD | Femur | 24.7(1.6) | 24.8(2.0) | 22.2(1.7) | 22.6(1.5) | 22.3(1.8) | 22.0(1.6) | 22.0(1.4) | 22.0(1.8) | 21.3(1.6) | 22.1(2.2) | 20.8(1.8) | 21.3(1.6) |
| MC | 22.7(2.2) | 23.2(2.2) | 20.1(2.2) | 20.6(1.5) | 20.1(2.4) | 20.1(1.6) | 19.9(1.9) | 20.1(2.0) | 19.7(2.0) | 20.4(1.9) | 19.3(2.4) | 19.6(1.2) | |
| LC | 26.7(1.2) | 26.4(2.0) | 24.3(1.3) | 24.6(1.7) | 24.5(1.4) | 23.9(1.8) | 24.2(1.0) | 23.9(1.9) | 23.0(1.3) | 23.9(2.7) | 22.3(1.5) | 23.0(2.3) | |
* Significant change between ACL-deficient and normal knees (p < 0.05). Posterior, medial and proximal translations are positive. Anterior, lateral and distal translations are negative. ACLD: ACL-deficient. AP: anterior-posterior, ML: medial-lateral, PD: proximal-distal. MC: medial condyle, LC: lateral condyle.
In ACL- deficient and normal knees, the medial condyle was positioned posterior to the tibial center throughout flexion arc (Table 1). At extension, the posterior translation of the medial condyle was adjusted slightly according to the movement of the medial flexion facet (Freeman and Pinskerova, 2005). There was no significant change in the anterior-posterior translation of the medial condyle in ACL deficiency.
In ACL-deficient knees, the lateral condyle was positioned posteriorly from extension to 120°. In normal knees, it was positioned anteriorly at extension but then posteriorly until 120° of flexion (Table 1). At extension and 15°, the lateral condyle was located in a more posterior position in ACL-deficient knees than in normal knees. Beyond 15°, the anterior-posterior translation of the lateral condyle was comparable between the two sides.
Medial-lateral translation: Both ACL-deficient and normal femurs were located medial to the tibial center from extension to 120° (Table 1). No significant difference was found in medial-lateral femoral translation between the two sides. The medial and lateral condyles were located medially and laterally, respectively, relative to the tibial center throughout the flexion arc (Table 1). Medial-lateral translations of both the medial condyle and lateral condyle were comparable in ACL- deficient and normal knees.
Proximal-distal translation: The femur was located proximal to the tibial center from extension to 120° of flexion (Table 1). There was no significant difference in proximal-distal femoral translation between ACL-deficient and normal knees. Proximal-distal translations of both the medial condyle and lateral condyle were not significantly different in ACL-deficient knees compared to normal knees.
Rotation of the femur
In both ACL-deficient and normal knees, the femur was located in internal rotation at extension but then external rotation until 120° of flexion relative to the tibia (Table 2). At extension and 15°, the femur demonstrated more external rotation in ACL-deficient knees than in normal knees. Beyond 15°, internal-external femoral rotation was similar between the two sides.
Table 2.
Comparison of femoral rotation between ACL-deficient and normal knees at 6 flexion positions. Data are means (±SD).
| Rotation | Extension | 15° | 30° | 60° | 90° | 120° | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (°) | ACLD | normal | ACLD | normal | ACLD | normal | ACLD | normal | ACLD | normal | ACLD | normal |
| IE | -1.8(2.8) | -7.2(3.2)* | 7.9(4.8) | 2.3(3.1)* | 9.2(2.9) | 7.7(2.6) | 10.0(3.7) | 9.8(1.5) | 12.8(2.8) | 11.9(1.6) | 13.0(3.4) | 12.0(3.3) |
| AA | 4.5(1.8) | 3.7(1.2) | 5.1(1.7) | 4.8(0.9) | 5.3(2.0) | 4.3(0.8) | 5.1(1.5) | 4.3(1.0) | 3.9(1.7) | 4.1(1.2) | 3.5(1.8) | 3.9(0.9) |
*Significant change between ACL-deficient and normal knees (p < 0.05). External and adduction rotations are positive, internal and abduction rotations are negative. ACLD: ACL-deficient.IE: internal-external, AA: adduction-abduction.
In adduction- abduction rotation, both ACL-deficient and normal femurs maintained adduction relative to the tibia from extension to 120° (Table 2). No significant difference between the adduction-abduction rotation of ACL-deficient and normal knees was observed at any flexion angle.
Motional extents of the femur and the femoral condyles
Extent of anterior-posterior movement: According to the comparison results between the extents of femoral movement of ACL-deficient and normal knees, the whole flexion arc was divided into three phases: extension to 15° (first), 15° to 60° (second), and 60° to 120° (third) (Table 3). From extension to 15°, the femur moved posteriorly in ACL-deficient and normal knees, and the extent of posterior movement was similar between the two sides. From 15° to 60°, the normal femur still moved posteriorly, but the ACL-deficient femur moved slightly anteriorly, and the extent of movement was significantly different between the two sides. From 60° to 120°, both femurs moved posteriorly, and the extent of posterior movement was comparable between the two sides.
Table 3.
Comparison of extents of movement and rotation between ACL-deficient and normal knees from extension to 120°. Data are means (±SD).
| Extent | Extension-15° | 15°-60° | 60°-120° | |||
|---|---|---|---|---|---|---|
| ACLD | normal | ACLD | normal | ACLD | normal | |
| AP movement of femur (mm) | 3.0(1.9) | 2.5(1.7) | -.4(1.4) | 2.4(2.1)* | 2.9(2.1) | 2.5(1.6) |
| AP movement of lateral condyle (mm) | 7.3(3.7) | 6.6(3.1) | .3(2.3) | 5.6(3.6)* | 4.1(3.3) | 3.4(2.6) |
| IE rotation of femur (°) | 9.7(4.2) | 9.5(3.5) | 2.2(2.5) | 7.5(4.1)* | 3.0(3.4) | 2.2(3.1) |
* Significant change between ACL-deficient and normal knees (p < 0.05). Posterior movement and external rotation are positive, anterior movement and internal rotation are negative. ACLD: ACL-deficient. AP: anterior-posterior, IE: internal-external.
The medial condyle moved anteriorly from extension to 90° and posteriorly from 90° to 120° in both ACL-deficient and normal knees (Figure 4).The extent of anterior movement from extension to 90° was comparable between ACL-deficient and normal knees (2.4 ± 1.2 mm vs. 3.0 ± 1.2 mm), as was the extent of posterior movement from 90° to 120° between ACL-deficient and normal knees (1.6 ± 1.1 mm vs. 2.1 ± 0.9 mm).
Figure 4.
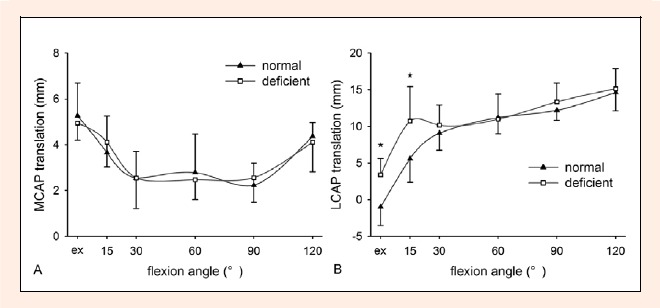
Anterior-posterior (AP) translations of the femoral condyles relative to the tibia (* p < 0.05). A Medial condylar (MC) translations. B Lateral condylar (LC) translations. Posterior translation is positive and anterior translation is negative. Ex: extension
The lateral condyle moved posteriorly from extension to 120° in ACL-deficient and normal knees (Table 3, Figure 4). Like the femoral movement, the extent of posterior movement of the lateral condyle was also compared between the two sides on the three flexion phases. Compared to normal knees, the extent of posterior movement was significantly reduced in ACL-deficient knees from 15° to 60°, but was similar between the two sides from extension to 15° and from 60° to 120°.
Extent of medial-lateral movement: As the knee flexed from extension to 120°, the femur moved laterally in ACL-deficient and normal knees. The extent of lateral femoral movement was 3.0 ± 1.2 mm in ACL-deficient knees from extension to 120°, which was similar to 2.2 ± 1.5 mm in normal knees. The extent of lateral movement of the medial condyle was also comparable between ACL-deficient (3.1 ± 1.7 mm) and normal knees (2.5 ± 1.9 mm), as was the lateral movement of the lateral condyle between ACL-deficient (2.8 ± 1.7 mm) and normal knees (1.9 ± 1.2 mm).
Extent of proximal-distal movement: Both the ACL-deficient and normal femurs moved distally throughout the flexion arc. From extension to 120°, the extent of distal femoral movement was 3.9 ± 0.9 mm in ACL-deficient knees, similar to 3.5 ± 0.8 mm in normal knees. The extent of distal movement of the medial condyle in ACL-deficient knees (3.4 ± 0.4 mm) was nearly the same as that in normal knees (3.5 ± 1.3 mm). The lateral condyles of ACL- deficient and normal knees were comparable in distal movement (4.3 ± 1.6 mm vs. 3.4 ± 1.3 mm).
Extent of femoral rotation: During the flexion arc from extension to 120°, the femur rotated externally in ACL-deficient and normal knees (Table 3). Like anterior-posterior femoral movement, the extent of external femoral rotation was also compared between the two sides on the three flexion phases. The extent of external femoral rotation was comparable between the two sides from extension to 15° and from 60° to 120°, but was significantly less from 15° to 60° in ACL-deficient knees than in normal knees.
Both ACL-deficient and normal femurs showed adduction rotation from extension to 30° and abduction rotation from 30° to 120°. From extension to 30°, the adduction extent of ACL-deficient knees (0.8° ± 0.9°) was similar to that of normal knees (0.6° ± 1.7°). The abduction extent from 30° to 120° was also comparable between ACL-deficient (1.8° ± 2.1°) and normal knees (0.5° ± 0.9°).
Discussion
We used biplane radiography with image registration and the geometric center axis to thoroughly measure the in vivo tibiofemoral motion of ACL-deficient knees during upright weight- bearing flexion from extension to 120°, particularly focusing on the motions of the medial and lateral condyles in three dimensions. Previous studies on knee kinematics after ACL deficiency either regarded femoral motion as one movement (Defrate et al., 2006; Georgoulis et al., 2003; Yamaguchi et al., 2009) or measured condylar motion only in anterior-posterior orientation (Brandsson et al., 2001; Logan et al., 2004a; Nicholson et al., 2012). By comparing the tibiofemoral motion between ACL-deficient and contralateral normal knees, we found increased posterior translation of the lateral condyle with excess external femoral rotation at extension and 15° of flexion in ACL deficiency. Furthermore, the lateral condyle moved slightly posteriorly in ACL-deficient knees during flexion from 15° to 60°, which was accompanied by reduced extent of external femoral rotation.
Previous studies have reported inconsistent results regarding the extents of tibiofemoral anterior-posterior movement and internal-external rotation in ACL deficient knees during flexion. Dennis et al., 2005 observed that in ACL deficiency, the lateral condyle moved less posteriorly with reduced extent of external rotation during a knee bend activity from extension to 120°. Logan et al., 2004a, however, observed that the extent of posterior movement of the lateral condyle in ACL-deficient knees did not differ from that in normal knees during squatting from extension to 90°. Yamaguchi et al., 2009 also observed that the extents of anterior tibial movement and internal rotation were comparable between ACL-deficient and normal knees during squatting from extension to 110°. These previous authors investigated the total extents of tibiofemoral movement and rotation throughout the flexion path in ACL-deficient knees. In the present study, we divided the whole flexion arc into three phases and examined the respective extents of movement and rotation to better understand the effect of ACL deficiency. Reduced extents of posterior movement of the lateral condyle and of external femoral rotation were noted in the second phase after ACL deficiency but were not observed in the first and third phases.
Our finding that ACL deficiency resulted in increased posterior translation of the lateral condyle with external rotation at low flexion angles is similar to findings seen in previous studies. Defrate et al., 2006 observed that ACL deficiency led to an increased anterior shift and internal rotation of the tibia at low flexion angles during a quasi-static lunge. Nicholson et al., 2012 also found that the lateral condyle was more posteriorly displaced with increased external femoral rotation in ACL-deficient knees at early angles of flexion during squatting. However, another study showed that the lateral condyle was more posteriorly displaced in ACL-deficient knees at all angles of flexion during squatting from extension to 90° (Logan et al., 2004a). When the ACL is intact, it carries a peak force within 30° of flexion under various loads; however, the force in the ACL decreases significantly at 60° (Li et al., 2004; Sakane et al., 1997), suggesting that the intact ACL exerts its restraining function primarily within early flexion. This correlates well with our observation that ACL deficiency caused posterior subluxation of the lateral condyle at early flexion angles. This abnormal posterior subluxation of the lateral condyle might produce an additional shear force on the medial tibial plateau, thereby increasing the risk of meniscal tears and chondral lesions in the medial compartment.
During the second flexion phase, the extent of posterior movement of the lateral condyle was significantly reduced after ACL deficiency, which might increase the risk of secondary chondral lesions in the lateral compartment. After ACL rupture, the lateral condyle appeared posteriorly subluxed and was located in a significantly posterior position at 15° of flexion, approximating its position at 60°, so that the lateral condyle moved slightly posteriorly from 15° to 60° (Figure 4). The extent of its posterior movement in the second phase was quite smaller than that in the first and third phases. Thus, during flexion from extension to 120° in ACL-deficient knees, the lateral condyle moved posteriorly with a sharp deceleration in the middle phase. This deceleration might lead to a large increase in contact stress between tibial and femoral cartilages in the lateral compartment. Increased contact stress might in turn predispose the cartilage to damage and degeneration. This potential injury mechanism might correlate with common chondral lesions in the lateral compartment of patients with ACL rupture.
Our study also showed that ACL deficiency affected the motion of the lateral condyle but not the medial condyle during weight- bearing flexion. This result is inconsistent with the findings of some previous MRI studies. In an open MRI study (von Eisenhart-Rothe et al., 2004), the condylar motion of ACL-deficient knees was examined as the subject lay on his side with flexion/extension torques applied to the knee. The results showed that the extent of posterior movement of the medial condyle from 30° to 90° was significantly greater in ACL-deficient knees than in normal knees. In another closed MRI study (Shefelbine et al., 2006), the subjects were supine with an axial load to the foot. The results showed that the medial condyle moved more anteriorly in ACL-deficient knees from extension to 45° when compared to normal knees. In our study, as in other previous studies on the knee kinematics, subjects performed the knee flexion activity while standing and weight bearing, unlike the above MRI studies, in which subjects were lying down while weight bearing. The influence of ACL deficiency on the movement of the medial condyle differed by position (lying vs. standing). In the lying position, injured knees might exhibit a different biomechanical response to load from that in the standing position. This indicates that it is necessary to examine not only the motion of the femur as a whole but also the respective motion of the medial and lateral condyles in various weight-bearing positions to better understand the effect of ACL deficiency on knee kinematics.
In the present study, ACL rupture was accompanied by small medial meniscus tears in injured knees, which may have an additional effect on knee kinematics. This effect might be small, considering that knee kinematics would not significantly change unless the posterior meniscal horn suffers serious injury or excision, or the entire medial meniscus is resected (Ahn et al., 2011; Allen et al., 2000). Another limitation is that we used a goniometer to determine knee flexion angles, as did previous authors (Defrate et al., 2006; Logan et al., 2004a; Nicholson et al., 2012). Although it was difficult to ensure the same angle of flexion in injured and contralateral normal knees, the statistical data showed a consistent trend of altered kinematics after ACL rupture in all patients. Thus, the deviation produced by the goniometer might have a small effect on the comparison of kinematics between the injured and normal knees. In addition, the difference in kinematics between injured and normal knees was acquired from static poses, which might limit our ability to apply our results to dynamic activities such as walking and running. In future studies on the dynamic activities of ACL-deficient knees, the anterior-posterior motion of the medial and lateral condyles might become a point of interest. Future studies also could apply finite element method to investigate the effect of altered kinematics after ACL deficiency on the biomechanics of meniscus and cartilage.
Conclusion
ACL deficiency caused posterior subluxation of the lateral condyle with excess external femoral rotation at early flexion angles. This in turn led to reduced extent of posterior movement of the lateral condyle during the middle flexion phase from 15° to 60°, which might increase cartilage-to-cartilage contact stress in the lateral compartment.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 31070837) and the Natural Science Foundation Project of CQ CSTC (No. 2011BB5042). The authors are grateful to Mr. Peng He for his technical assistance. The authors declare that they have no conflict of interest.
Biographies

Kaining Chen
Employment
Center for Joint Surgery in Southwest Hospital, the Third Military Medical University, China
Degree
MSc
Research interests
Sports medicine, biomechanics.
E-mail: ckn1979@gmail.com

Li Yin
Employment
Center for Joint Surgery in Southwest Hospital, the Third Military Medical University, China
Degree
MSc
Research interests
Sports biomechanics.
E-mail: erie.yin@hotmail.com

Liangjun Cheng
Employment
Depart. of Radiology in Southwest Hospital, the Third Military Medical University, China
Degree
MSc
Research interests
Diagnostic radiology, sports traumatology.
E-mail: 491490691@qq.com

Chuan Li
Employment
Department of Radiology in Southwest Hospital, the Third Military Medical University, China
Degree
PhD
Research interests
Diagnostic radiology, sports traumatology.
E-mail: jessica0023@sina.com

Cheng Chen
Employment
Center for Joint Surgery in Southwest Hospital, the Third Military Medical University, China
Degree
PhD
Research interests
Sports biomechanics.
E-mail: cchen@hsph.harvard.edu

Liu Yang
Employment
Center for Joint Surgery in Southwest Hospital, the Third Military Medical University, China
Degree
PhD
Research interests
Sports medicine, biomechanics.
E-mail: greennature@126.com
References
- Ahn J.H., Bae T.S., Kang K.S., Kang S.Y., Lee S.H. (2011) Longitudinal tear of the medial meniscus posterior horn in the anterior cruciate ligament-deficient knee significantly influences anterior stability. American Journal of Sports Medicine 39(10), 2187-2193 [DOI] [PubMed] [Google Scholar]
- Allen C.R., Wong E.K., Livesay G.A., Sakane M., Fu F.H., Woo S.L. (2000) Importance of the medial meniscus in the anterior cruciate ligament-deficient knee. Journal of Orthopaedic Research 18(1), 109-115 [DOI] [PubMed] [Google Scholar]
- Asano T., Akagi M., Tanaka K., Tamura J., Nakamura T. (2001) In vivo three-dimensional knee kinematics using a biplanar image-matching technique. Clinical Orthopaedics and Related Research 388, 157-166 [DOI] [PubMed] [Google Scholar]
- Brandsson S., Karlsson J., Eriksson B.I., Karrholm J. (2001) Kinematics after tear in the anterior cruciate ligament: dynamic bilateral radiostereometric studies in 11 patients. Acta Orthopaedica Scandinavica 72(4), 372-378 [DOI] [PubMed] [Google Scholar]
- Defrate L.E., Papannagari R., Gill T.J., Moses J.M., Pathare N.P., Li G. (2006) The 6 degrees of freedom kinematics of the knee after anterior cruciate ligament deficiency: an in vivo imaging analysis. American Journal of Sports Medicine 34(8), 1240-1246 [DOI] [PubMed] [Google Scholar]
- Dennis D.A., Mahfouz M.R., Komistek R.D., Hoff W. (2005) In vivo determination of normal and anterior cruciate ligament-deficient knee kinematics. Journal of Biomechanics 38(2), 241-253 [DOI] [PubMed] [Google Scholar]
- Freeman M.A., Pinskerova V. (2005) The movement of the normal tibio-femoral joint. Journal of Biomechanics 38(2), 197-208 [DOI] [PubMed] [Google Scholar]
- Georgoulis A.D., Papadonikolakis A., Papageorgiou C.D., Mitsou A., Stergiou N. (2003) Three-dimensional tibiofemoral kinematics of the anterior cruciate ligament-deficient and reconstructed knee during walking. American Journal of Sports Medicine 31(1), 75-79 [DOI] [PubMed] [Google Scholar]
- Grood E.S., Suntay W.J. (1983) A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. Journal of Biomechanical Engineering 105(2), 136-144 [DOI] [PubMed] [Google Scholar]
- Kozanek M., Van de Velde S.K., Gill T.J., Li G. (2008) The contralateral knee joint in cruciate ligament deficiency. American Journal of Sports Medicine 36(11), 2151-2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozanek M., Hosseini A., Liu F., Van de Velde S.K., Gill T.J., Rubash H.E., Li G. (2009) Tibiofemoral kinematics and condylar motion during the stance phase of gait. Journal of Biomechanics 42(12), 1877-1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leszko F., Hovinga K.R., Lerner A.L., Komistek R.D., Mahfouz M.R. (2011) In vivo normal knee kinematics: is ethnicity or gender an influencing factor? Clinical Orthopaedics and Related Research 469(1), 95-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Zayontz S., Most E., DeFrate L.E., Suggs J.F., Rubash H.E. (2004) In situ forces of the anterior and posterior cruciate ligaments in high knee flexion: an in vitro investigation. Journal of Orthopaedic Research 22(2), 293-297 [DOI] [PubMed] [Google Scholar]
- Li G., Papannagari R., DeFrate L.E., Yoo J.D., Park S.E., Gill T.J. (2007) The effects of ACL deficiency on mediolateral translation and varus-valgus rotation. Acta Orthopaedica 78(3), 355-360 [DOI] [PubMed] [Google Scholar]
- Logan M., Dunstan E., Robinson J., Williams A., Gedroyc W., Freeman M. (2004a) Tibiofemoral kinematics of the anterior cruciate ligament (ACL)-deficient weightbearing, living knee employing vertical access open "interventional" multiple resonance imaging. American Journal of Sports Medicine 32(3), 720-726 [DOI] [PubMed] [Google Scholar]
- Logan M.C., Williams A., Lavelle J., Gedroyc W., Freeman M. (2004b) Tibiofemoral kinematics following successful anterior cruciate ligament reconstruction using dynamic multiple resonance imaging. American Journal of Sports Medicine 32(4), 984-992 [DOI] [PubMed] [Google Scholar]
- McPherson A., Karrholm J., Pinskerova V., Sosna A., Martelli S. (2005) Imaging knee position using MRI, RSA/CT and 3D digitisation. Journal of Biomechanics 38(2), 263-268 [DOI] [PubMed] [Google Scholar]
- Nicholson J.A., Sutherland A.G., Smith F.W., Kawasaki T. (2012) Upright MRI in kinematic assessment of the ACL-deficient knee. Knee 19(1), 41-48 [DOI] [PubMed] [Google Scholar]
- Sakane M., Fox R.J., Woo S.L., Livesay G.A., Li G., Fu F.H. (1997) In situ forces in the anterior cruciate ligament and its bundles in response to anterior tibial loads. Journal of Orthopaedic Research 15(2), 285-293 [DOI] [PubMed] [Google Scholar]
- Scarvell J.M., Smith P.N., Refshauge K.M., Galloway H., Woods K. (2005) Comparison of kinematics in the healthy and ACL injured knee using MRI. Journal of Biomechanics 38(2), 255-262 [DOI] [PubMed] [Google Scholar]
- Shefelbine S.J., Ma C.B., Lee K.Y., Schrumpf M.A., Patel P., Safran M.R., Slavinsky J.P., Majumdar S. (2006) MRI analysis of in vivo meniscal and tibiofemoral kinematics in ACL-deficient and normal knees. Journal of Orthopaedic Research 24(6), 1208-1217 [DOI] [PubMed] [Google Scholar]
- von Eisenhart-Rothe R., Bringmann C., Siebert M., Reiser M., Englmeier K.H., Eckstein F., Graichen H. (2004) Femoro-tibial and menisco-tibial translation patterns in patients with unilateral anterior cruciate ligament deficiency—a potential cause of secondary meniscal tears. Journal of Orthopaedic Research 22(2), 275-282 [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., Gamada K., Sasho T., Kato H., Sonoda M., Banks S.A. (2009) In vivo kinematics of anterior cruciate ligament deficient knees during pivot and squat activities. Clinical Biomechanics 24(1), 71-76 [DOI] [PubMed] [Google Scholar]
- Yoo JD, Papannagari R, Park SE, DeFrate LE, Gill TJ, Li G. (2005) The effect of anterior cruciate ligament reconstruction on knee joint kinematics under simulated muscle loads. American Journal of Sports Medicine 33 (2), 240-246 [DOI] [PubMed] [Google Scholar]


