Abstract
Increased proportions of CD8 T lymphocytes lacking expression of the CD28 costimulatory receptor have been documented during both aging and chronic infection with HIV-1, and their abundance correlates with numerous deleterious clinical outcomes. CD28-negative cells also arise in cell cultures of CD8+CD28+ following multiple rounds of Ag-driven proliferation, reaching the end stage of replicative senescence. The present study investigates the role of a second T cell costimulatory receptor component, adenosine deaminase (ADA), on the process of replicative senescence. We had previously reported that CD28 signaling is required for optimal telomerase upregulation. In this study, we show that the CD8+CD28+ T lymphocytes that are ADA+ have significantly greater telomerase activity than those that do not express ADA and that ADA is progressively lost as cultures progress to senescence. Because ADA converts adenosine to inosine, cells lacking this enzyme might be subject to prolonged exposure to adenosine, which has immunosuppressive effects. Indeed, we show that chronic exposure of CD8 T lymphocytes to exogenous adenosine accelerates the process of replicative senescence, causing a reduction in overall proliferative potential, reduced telomerase activity, and blunted IL-2 gene transcription. The loss of CD28 expression was accelerated, in part due to adenosine-induced increases in constitutive caspase-3, known to act on the CD28 promoter. These findings provide the first evidence for a role of ADA in modulating the process of replicative senescence and suggest that strategies to enhance this enzyme may lead to novel therapeutic approaches for pathologies associated with increases in senescent CD8 T lymphocytes.
The CD28 costimulatory receptor, a membrane glycoprotein, provides the requisite second signal for initiating immune responses by T lymphocytes (1). Binding of CD28 to the CD80 ligand on APCs transduces survival and proliferation signals, including induction of IL-2, telomerase activation, and stabilization of mRNA for several cytokines (2–4). During aging and in younger persons infected with HIV-1, high proportions of T cells lacking CD28 expression have been observed, particularly within the CD8 T cell subset (5, 6). The abundance of CD8+CD28− T lymphocytes correlates with a variety of negative outcomes, such as decreased vaccine responsiveness in the elderly, reduction in overall TCR repertoire, and more rapid progression to AIDS (7–10). The irreversible loss of CD28 is also observed in vitro as human T lymphocytes undergo repeated rounds of Ag-driven proliferation in culture, reaching the end stage of replicative senescence (11).
Adenosine deaminase (ADA) is best known for its critical role in lymphoid development, where its absence results in SCID (12). However, ADA, present both intracellularly and on the surface as ecto-ADA that is bound to CD26 (13), is also essential for optimal function of mature T lymphocytes. Indeed, the ADA/CD26 complex, which, like CD28, is a component of the immunological synapse, delivers a costimulatory signal upon T cell stimulation (13–17). Although CD26 has been reported to show an age-related reduced expression on CD8 T lymphocytes, the effect of aging on ADA has not been addressed. Interestingly, in persons with HIV/AIDS, which is associated with premature aging of the immune system (18, 19), ADA binding to CD26 on the cell surface is inhibited by HIV glycoproteins and viral particles (20, 21). Alterations in ADA have also been documented during the progression of human fibroblasts to replicative senescence, where intracellular ADA activity declines progressively with increasing population doublings (PDs) (22).
Adenosine, a potent extracellular messenger, is continuously produced, and, under normal conditions, its level in the blood is maintained at concentrations in the 100–300 nM range (12). However, under metabolically unfavorable conditions, such as tissue hypoxia and inflammation, a variety of cell types, such as regulatory T cells (Tregs), mast cells, and neural cells, secrete adenosine, which can have severe clinical implications (23). Adenosine is also present in the microenvironment of tumor cells, possibly contributing to reduced cancer immunosurveillance (24). ADA is the enzyme that converts adenosine to inosine, thereby playing a key role in normal immune function (16, 23). Indeed, a variety of studies have documented some of the negative effects of adenosine on T lymphoctyes, such as suppression of IL-2 production (25), and, at high levels, induction of apoptosis (26). Short-term (∼36 h) exposure of murine T lymphocytes to adenosine alters multiple effector functions (27) and also results in a loss of CD28 expression (28), but the role of ADA/CD26 in the downregulation of CD28 in chronically stimulated human T lymphocytes has not been addressed.
The goal of the current study, therefore, was to analyze the dynamics of ADA expression as human T lymphocytes progress to replicative senescence. We show that ADA expression is key to upregulation of telomerase activity and that both intracellular and ecto-ADA decrease with increasing PDs. Exposure to exogenous adenosine, to mimic the in vivo environment, was shown to accelerate the progression of CD8 T cells to replicative senescence, causing more rapid loss of CD28 expression and telomerase activity and reduced proliferative potential.These results demonstrate a novel role for ADA in T cell biology that is highly relevant to aging, HIV disease, and other clinical situations involving chronic stimulation of T lymphocytes.
Materials and Methods
Cell cultures
Human peripheral blood samples were acquired by venipuncture after informed consent and in accordance with the University of California, Los Angeles, Institutional Review Board. Postcentrifugation, the layer of PBMCs was carefully removed and washed twice in complete RPMI 1640 (10% FBS, 10 mM HEPES, 2 mM glutamine, 50 IU/ml penicillin/streptomycin). The PBMCs were then used to initiate long-term T cell cultures, as described previously (29). Briefly, PBMCs were exposed to T cell activation microbeads (anti-CD2/3/28; Miltenyi Biotec, Auburn, CA) with 10 µl microbead mixture added for every 1 × 106 cells and stimulation repeated every 14 d. Prior to the second stimulation, CD8+ T cells were isolated by CD8 negative selection using the EasySep CD8 enrichment kit (Stemcell Technologies, Vancouver, British Columbia, Canada). Purity of the CD8+ T cells was verified by flow cytometry. In some experiments, the cultures were initiated by combining PBMCs with irradiated (8000 rad) allogeneic EBV-transformed B lymphoblastoid cells, which serve as APCs in complete RPMI 1640 supplemented with recombinant IL-2 (20 U/ml). For all cultures, every 3 to 4 d, viable cell concentration was determined by trypan blue exclusion, and when the concentration reached ≥8 × 105/ml, cells were sub-cultivated to a density of 5 ×105 cells/ml. PDs were determined according to the formula: PD = log2 (final cell concentration/initial cell concentration).
Adenosine treatment
Cultures, established as described above using T cell activation microbeads, were exposed to erythro-9-(2-hydroxy-3-nonyl)adenine hydrochloride (EHNA,an ADA inhibitor; Sigma-Aldrich, St. Louis, MO),a combination of adenosine/ADA inhibitor, or a combination of adenosine/ADA inhibitor/ caspase-3 inhibitor (Sigma-Aldrich) at day 0 and every 3 to 4 d throughout the culture period. Control cultures were exposed to the diluent DMSO. The concentration of 10 µM, for both adenosine and EHNA, was used in these experiments, based on initial titration studies and on previous results describing the concentration of adenosine in ADA-SCID patients (12).
Flow cytometry
Surface expression of CD4, CD28, CD8, ADA, and CD3 was examined by immunostaining and flow cytometry. Cells were incubated with FITC-conjugated anti-CD4, PE-conjugated anti-CD28, APC-conjugated anti-CD3, and PerCp-conjugated anti-CD8 (BD Biosciences, San Jose, CA) or anti-ADA biotinylated (Abcam, Cambridge, MA) with strepavidin-PerCP (BD Biosciences) mAbs at 4˚C for 20 min, washed, and fixed in PBS containing 1% paraformaldehyde. Parallel samples were incubated with Ig isotype control Ab or secondary Abs (BD Biosciences). All samples were analyzed on an FACS Calibur flow cytometer (BD Biosciences). Fluorescence data from at least 25,000 cells were acquired. Analysis of data was performed using CellQuest Pro (BDBiosciences).
Intracellular ADA activity quantification
Intracellular ADA activity was determined using the colorimetric assay described by Vielh and Castellazzi (30). Briefly, 3 × 106 cells were removed 4 d postrestimulation, centrifuged, and 1 × 106 cells were lysed by sonication in PBS. Total protein was normalized using the Bradford assay, and cell lysates were measured for ADA activity in triplicate wells and compared with a standard solution of ammonium sulfate.
Real-time quantitative PCR
RNAwas isolated using the Qiagen RNAeasy kit (Qiagen, Valencia, CA) and quantified using the Quant-iT Ribogreen RNA Assay Kit (Molecular Probes, Eugene, OR). cDNAs were synthesized with the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) using 500 ng RNA. Real-time quantitative PCR assays were performed using the iQ SYBER Green SuperMix and IQCycler (Bio-Rad). GAPDH was used as an internal control. The sequences were designed with the aid of Beacon Designer software (www.premierbiosoft.com/ molecular_beacons/index.html) and synthesized at Integrated DNA Technologies, Coralville, IA. CD28: sense TCCTTACCTAGACAATGAGAAGAGC-AATC, antisense CCACCAACCACCACCAGCAC; ADA: sense CAGGCT-AACTACTCGCTCAACACAG; antisense TGTCCCGTTTGGTCATCTGG-TAATC; IL-2: sense TCACCAGGATGCTCACATTTAAGTTTTAC; anti-sense TTCCTCCAGAGGTTTGAGTTCTTCTTC; and GAPDH: sense GGT-CATGAGTCCTTCCACGATACCA; antisense CCTCAAGATCATCAGCA-ATGCCTCCT.
Samples were run in triplicate in a 96-well plate at the following settings: 95˚C for 15 s, 61˚C for 30 s, and 72˚C for 30 s using single fluorescence measurement.
Separation of cell subpopulations
Purified CD3+ or CD8+ T cells were obtained by negative selection using the EasySep cell isolation kit (Stemcell Technologies). In some experiments, CD8+ T cells were further divided into either ADA+ or ADA− subsets, using an ADA-biotin mAb (Abcam) conjugated to antibiotin magnetic beads (Miltenyi Biotec). Purity of the various cell populations was routinely >95% as verified by flow cytometry.
Caspase-3 assay
The ApoAlert assay kit (Calbiochem, San Diego, CA) was used to detect caspase-3 activity. Briefly,14 d poststimulation, when cells were quiescent, 2 × 106 purified CD8 T cells were centrifuged, and the pellet was lysed using the lysis buffer provided in the kit. Each lysate was incubated with 2× reaction buffer containing 10 mM DTTand incubated for 1 h at 37˚C. Caspase-3 protease activity was detected by spectrophotometry (405 nm).
Telomerase activity measurements
Telomerase activity was determined using the telomere repeat amplification protocol (TRAP), as previously described in Saldanha et al. (31), with minor modifications.
Statistical analysis
Mean values and SDs were calculated for each time point. Significance was established by using a two-tail Student t test, and a p value of <0.05 is considered significant.
Results
Higher telomerase activity in CD8+CD28+ADA+ versus CD8+ CD28+ADA− T lymphocytes
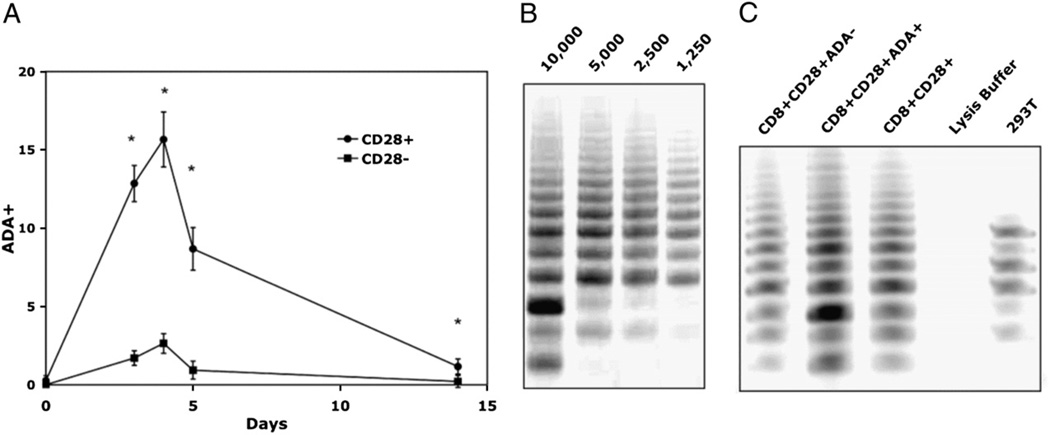
In earlier studies, we showed that CD28 costimulation is required for optimal telomerase upregulation and that the loss of telomerase activity parallels that of CD28 in repeatedly stimulated CD8 T lymphocytes followed in long-term cultures (2). Ecto-ADA (the surface form of ADA), bound to CD26 on lymphocytes and part of the immunological synapse, also participates in costimulation upon complexing withADA-anchoring proteins on APCs(14,17).Wetherefore tested whether ADA might also enhance telomerase activity. We first verified that in the absence of CD26, no ecto-ADA is present, indicating that CD26 was required for ADA to bind to the cell surface (data not shown). Next,wetested the kinetics of ecto-ADA expression during the early period following T cell activation. Flow cytometric analysis indicated that ecto-ADA expression peaked on CD8 T cell sat ∼4 d poststimulation and gradually decreased as the cells reach quiescence (day 14). The expression of ecto-ADA was more pronounced in the CD8+CD28+ subset across all time points, with the CD8+ CD28− subset showing only a minimal increase in ecto-ADA expression following stimulation (Fig. 1A). The fact that ecto-ADAwas only expressed on a subset (∼16%) of the CD28+ T cells allowed us to compare telomerase activity in two phenotypically distinct sub-populations, namely the CD8+CD28+ADA+ versus CD8+CD28+ ADA− T lymphocyte subsets. For these experiments, we stimulated PBMC with an allogeneic B cell line, as described in our earlier telomerase studies (2), and on day 4, purified the total CD3+CD8+ population, which we verified was. >95% CD28+ by flow cytometry. We then further separated these CD8+CD28+ cells into ADA+ and ADA− for the telomerase activity assay. An initial titration was performed to ensure that the samples had telomerase activity in the linear range (Fig. 1B). Comparing lysates from 2500 cell equivalents of each cell population, we observed that the telomerase activity of the CD8+ CD28+ADA+ subset was higher than that of the CD8+CD28+ADA− subset (Fig. 1C) and was, in fact, even higher than that of the total CD28+ population. The results comparing T cell cultures derived from five different individuals show a highly significant (p = .000057) fold increase (1.83) in telomerase activity between CD8+CD28+ ADA+ and CD8+CD28+ADA− (Table I).
FIGURE 1.
Higher telomerase activity in CD8+CD28+ T cells that express ADA. A, Kinetics of ADA cell surface expression following stimulation with Ab-coated microbeads. Gated populations of CD3+CD8+CD28+ versus CD3+CD8+CD28− T lymphocytes were evaluated from four individual cultures. B, Representative gel showing that four different cell equivalents of CD8+ T cells (10,000, 5,000, 2,500, 1,250 cell equivalents) fall in the linear range of telomerase activity. Cultures were stimulated with irradiated allogeneic B lymphoblastoid cells, and, on day 4, cell lysates were prepared, and telomerase activity was analyzed using the modified TRAP assay as described in Materials and Methods. C, TRAP assay gel from a representative culture after separation of CD8+CD28+ subset into ADA+ and ADA− using 2500 cell equivalents per lane.
Table I.
Higher telomerase activity in CD8+CD28+ADA+ versus CD8+ CD28+ADA− T lymphocytes
| CD8+CD28+ADA+/CD8+CD28+ADA- | |
|---|---|
| Donor | |
| 1 | 1.81 |
| 2 | 1.88 |
| 3 | 1.77 |
| 4 | 2.18 |
| 5 | 1.51 |
| Mean ± SD | 1.83 ± 0.241 |
| p value | 0.000057 |
PBMCs were stimulated with an irradiated allogeneic EBV-transformed B cell line as described in Materials and Methods. On day 4, purified CD8+ T cells, which were 97% (± 2%) CD28+, were separated into ADA+ and ADA− and purity verified by flow cytometry. The TRAP assay was performed on lysates from the equivalent of 2500 cells per lane. Data are expressed as the fold increase in telomerase activity of CD8+CD28+ADA+ over CD8+CD28+ADA− for each donor.
Decrease in ADA intracellular activity and surface expression with culture age
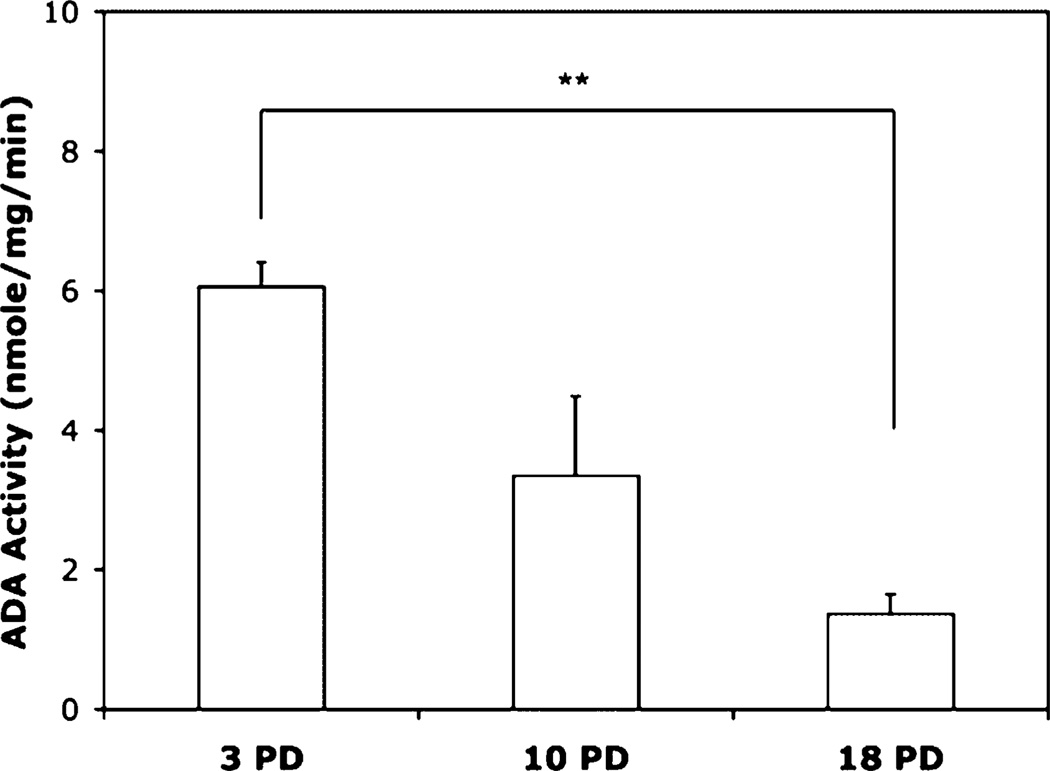
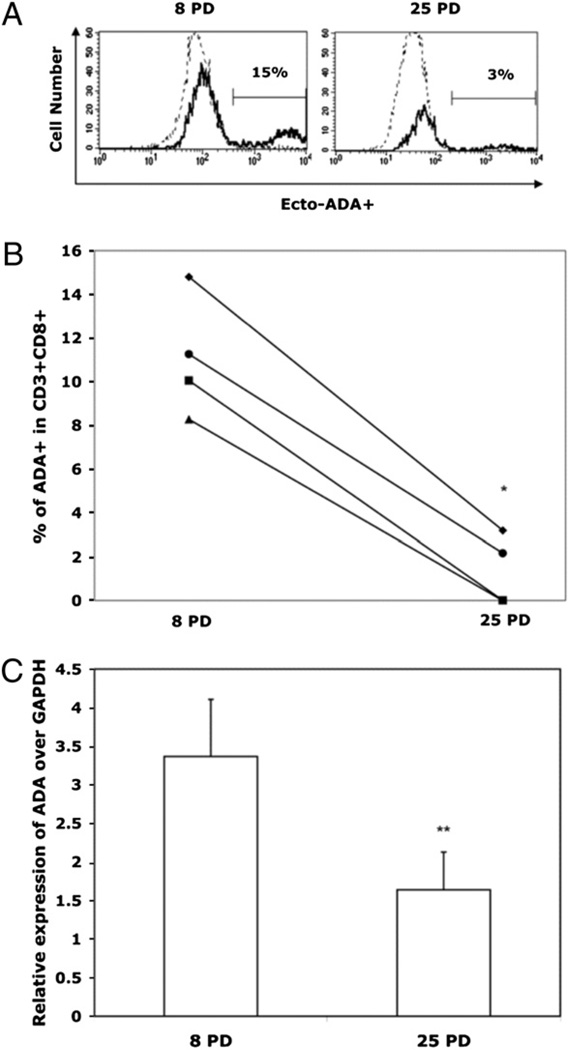
Replicative senescence has been documented in a variety of cell types, with the most extensive studies done on fibroblasts. Interestingly, in that cell type, the activity of intracellular ADA was shown to progressively decline with increasing numbers of PDs (22). Based on numerous similarities in senescence-associated patterns of fibroblasts and lymphocytes (11), we tested whether ADA activity also changed during the in vitro life span of CD8 T lymphocytes. Our data show a progressive decline in intracellular ADA activity as the cells approached replicative senescence. This pattern was true both soon after each stimulation, when the cells were actively dividing, and where the difference between ADA activity at early (3 PDs) and late (18 PDs) was highly significant (p < 0.01; Fig. 2), as well as on day 14 post-stimulation, when the cells were quiescent (data not shown).Asimilar pattern was observed in the expression of ecto-ADA, which is biologically identical to the intracellular enzyme. A representative flow cytometry histogram (Fig. 3A) and the composite results from four individual cultures (Fig. 3B) show that on day 4 poststimulation, surface expression of ecto-ADA declined with culture age for all donors tested. This decrease was associated with reduced ADA gene transcription (Fig. 3C).
FIGURE 2.
Intracellular ADA activity decreases with culture age. Long-term T cultures were established by activation with Ab-coated microbeads. CD8+ T lymphocytes were purified 4 d postsimulation, and cell lysates were assessed for ADA activity using the colorimetric assay described by Vielh and Castellazzi (30). Intracellular ADA activity for five individual cultures, all at equivalent PDs, is presented. **p , 0.01.
FIGURE 3.
Surface ADA expression is reduced as human CD8 T cells approach replicative senescence. Cultures were established by activation with Ab-coated microbeads, and ADA expression was determined by flow cytometry 4 d after each stimulation (i.e., every ∼14 d). A, Histogram showing expression of ecto-ADA (determined by flow cytometry) on CD3+CD8+ cells from a representative culture at 8 PDs and 25 PDs. B, Mean (±SD) percentage of the CD8+ T cells that express ecto-ADA at early (8 PDs) and late (25 PDs) time points; the lines connect data from four individual cultures. *p < 0.05. C, ADA tran-scriptional expression (n = 4) normalized against GAPDH. **p < 0.01.
Exposure to adenosine leads to CD28 downregulation
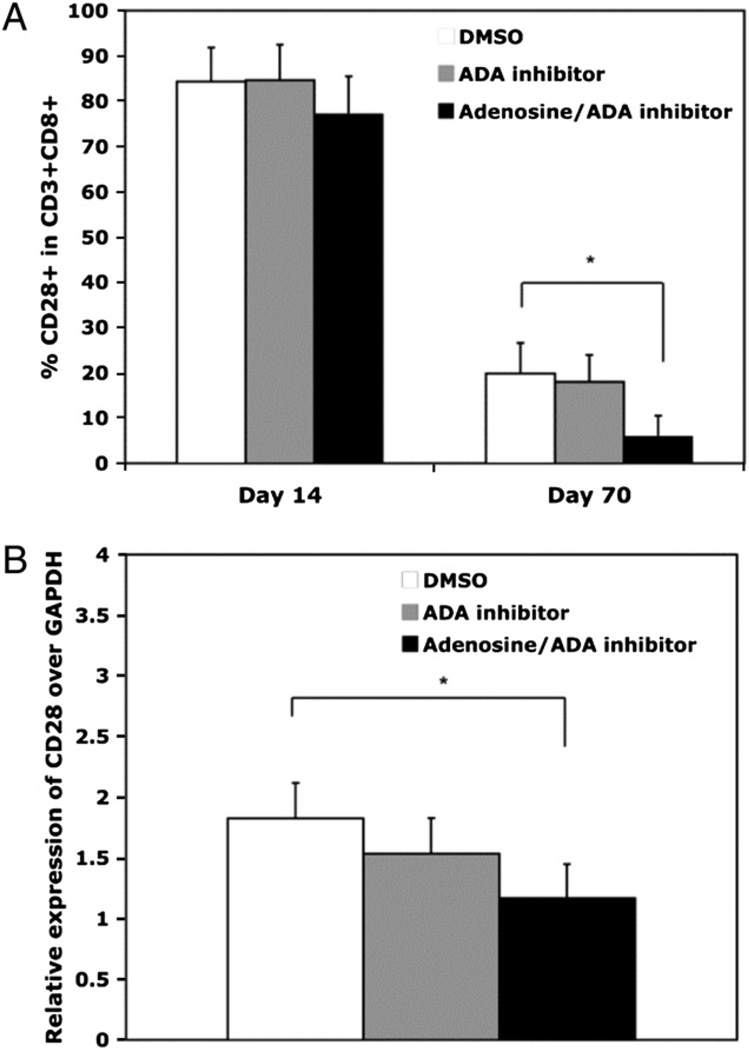
The loss of ADA activity and expression with culture age led us to investigate potential effects that chronic exposure to adenosine might exert on CD8 T lymphocytes as they progress to replicative senescence. Although there is no evidence of adenosine production by CD8 T cells, within the invivo environment they are exposed to a variety of cell types, such as Tregs, that secrete adenosine (32–35). We focused on CD28 expression, because in mouse T cells, a short-termexposure to adenosine was shown to result in downregulation of CD28 (28). Initial titration experiments showed treatment of CD8 T lymphocytes with exogenous adenosine every 3 to 4 d resulted in a dose-dependent decrease in CD28 surface expression (data not shown). We chose to use the 10 µM adenosine concentration based on its maximal downregulation of CD28 and its documented physiological effect on immune function (12). To control for any residual ADA in the cultures, which could confound interpretation of the results, an ADA inhibitor (EHNA, see Materials and Methods) was included in the adenosine-treated cultures at a concentration of 10 µM; this inhibitor alone, in the absence of adenosine, had no effect on the process of replicative senescence. Our data show that chronic exposure to adenosine caused a significant downregulation of CD28 expression, especially at 70 d, both on the cell surface (Fig. 4A) and at the transcriptional level (Fig. 4B).
FIGURE 4.
CD8 T lymphocytes exposed to adenosine have lower CD28 expression. Long-term cultures were established by stimulation with Ab-coated microbeads. DMSO, the ADA inhibitor (10 µM),oradenosine (10µM) plus ADA inhibitor (10 µM) were added on day0 and every 3 to 4 d thereafter. A, CD28 expression on CD3+CD8+ T cells in a long-term culture exposure to DMSO, ADA inhibitor (10 µM), or adenosine (10 µM) with ADA inhibitor (10µM).B,CD28messagewascomparedwithGAPDHbyQT-PCRatday70. n = 4. *p < 0.05.
Caspase-3 activity increases in CD8 T lymphocytes chronically exposed to adenosine
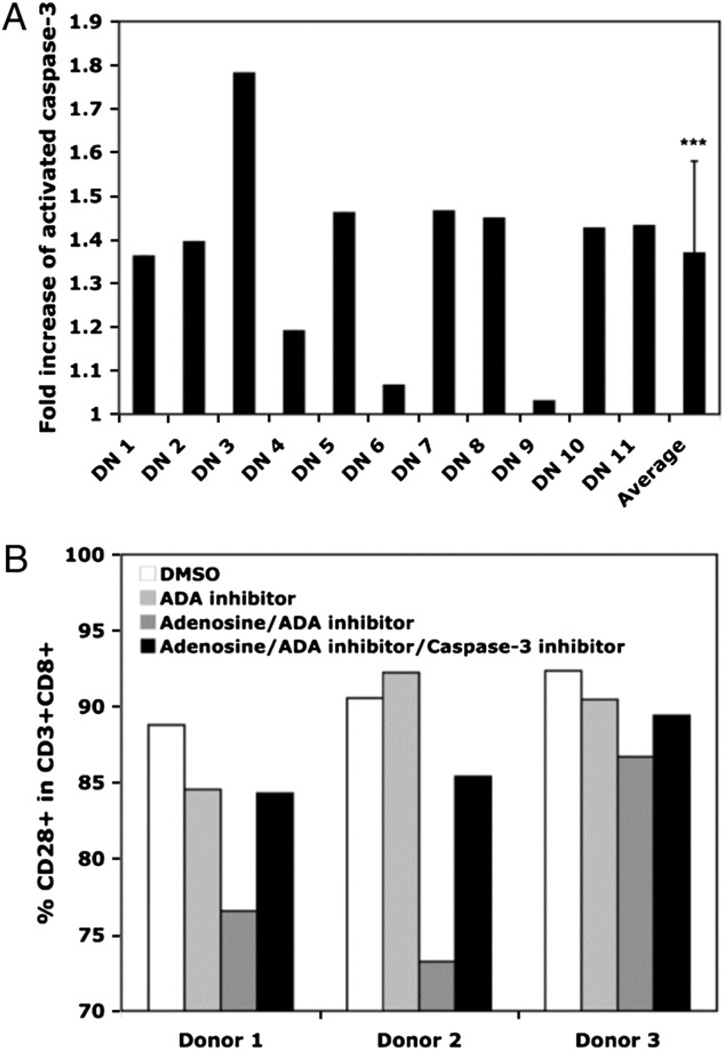
Previous studies have shown that in the Jurkat CD4 T cell tumor line, gene transcription of CD28 was reduced in the presence of constitutive caspase-3 levels that were below the threshold required for apoptosis; this effect was totally abrogated in the presence of a caspase-3 inhibitor (36, 37). We recently extended those findings to normal T lymphocytes, showing that constitutive caspase-3 activity is higher in CD8 T cells that lack CD28 expression (i.e., the senescent phenotype) than in those that are CD28+(38). To determine whether the downregulation of CD28 by adenosine also involved the caspase-3 pathway, we evaluated caspase-3 activity in the adenosine-treated cultures. For these experiments, we selected 11 adenosine-treated cultures in which the levels of CD28 expression were substantially reduced by day 14 and compared the caspase-3 activity to parallel control (DMSO)-treated cultures at the same time point. The fold increase in constitutive caspase-3 activity was significantly greater in the adenosine-treated cultures (p < 0.05) compared with the DMSO control cultures (Fig. 5A). Because the inclusion of a caspase-3 inhibitor only partially reversed the loss of CD28 expression induced by adenosine treatment (Fig. 5B), our data suggest that the adenosine-mediated downregulation of CD28 expression in CD8 T lymphocytes operates through both caspase-3– dependent and –independent pathways.
FIGURE 5.
Adenosine downregulation of CD28 involves activated caspase-3. T lymphocytes were stimulated with Ab-coated microbeads. DMSO, the ADA inhibitor (10 µM), or adenosine (10 µM) plus ADA inhibitor (10 ) were added at day 0 and every 3 to 4 d thereafter. Constitutive caspase-3 levels in CD8+ T lymphocytes that were purified on day 14 were determined using Apo-Alert commercial kit (Calbiochem). A, Fold increase in caspase-3 level for 11 individual cultures treated with adenosine/ADA inhibitor versus DMSO and mean fold increase for all cultures. B, CD28 surface expression at day 14 in CD3+CD8+ T cell cultures treated with DMSO, ADA inhibitor (10 ), adenosine (10 )/ ADA inhibitor (10 ), or adenosine/ADA inhibitor/caspase-3 inhibitor. ***p < 0.001.
Chronic exposure to adenosine accelerates replicative senescence in CD8 T lymphocytes
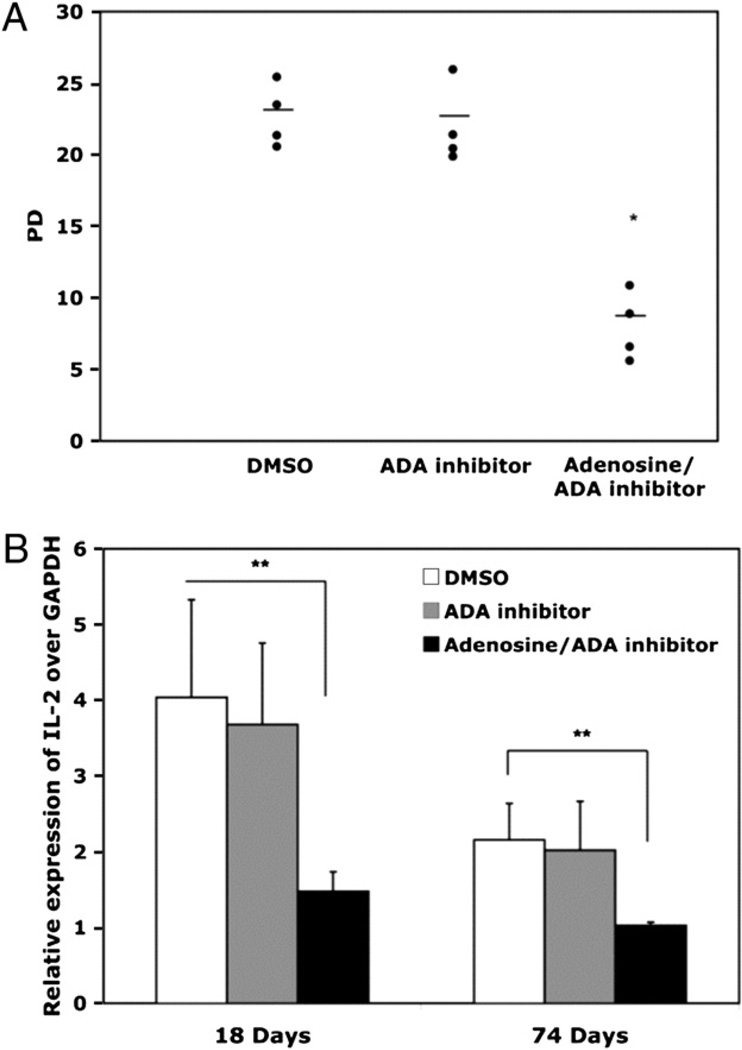
In addition to the loss of CD28, replicative senescence is associated with a variety of functional alterations, including the inability to upregulate telomerase activity (2), altered cytokine profiles, and irreversible cell cycle arrest (39, 40). Chronic exposure to adenosine throughout the long-term culture period significantly accelerated this process. We observed that the adenosine-treated cultures had a reduced rate of proliferation, as well as reduced total proliferative potential compared with control cultures from the same donor. Because adenosine exposure was associated with a reduced rate of proliferation, these cultures acquired the senescent phenotype after the same number of days as the controls, but had undergone fewer PDs. Indeed, whereas the control cultures (exposed to DMSO or to the ADA inhibitor alone) underwent an average of 23 PDs prior to reaching replicative senescence, the cultures exposed to adenosine plus the ADA inhibitor reached senescence after only 8 PDs (Fig. 6A) and were not able to proliferate further even when the adenosine/ADA inhibitor was removed by extensive washing (data not shown).
FIGURE 6.
Adenosine accelerates replicative senescence in human CD8 T lymphocytes. A, Total PD for DMSO, EHNA, and adenosine/EHNA-treated cultures when replicative senescence was reached; n = 4, mean value shown with horizontal line. B, IL-2 mRNA was analyzed by QT-PCR and results expressed relative to GAPDH. *p < 0.05; **p < 0.01.
Because CD28 signaling is essential for optimal telomerase upregulation (2), and adenosine exposure affects CD28 expression, we also evaluated the effect of adenosine on telomerase activity. CD8 T lymphocytes were isolated from adenosine-treated and control cultures after the second stimulation, and equivalent numbers of cells were evaluated in the TRAP assay. Our data show that the adenosine-mediated reduction in CD28 expression is associated with a significantly lower level of telomerase activity. The fold decrease in telomerase activity for four individual cultures is summarized in Table II. CD28 signaling is also central to the upregulation of both IL-2 and its receptor (4, 41, 42). We observed that chronic exposure to adenosine led to a significant (p < 0.01) decrease in IL-2 message both at early and late time points in the culture (Fig. 6B). Replicative senescence of CD8 T lymphocytes is also associated with increased production of TNF-a and IL-6 (40). We noted, however, that this aspect of senescence was not accelerated by adenosine (data not shown), possibly due to the documented anti-inflammatory properties of adenosine (23). In sum, our data show that chronic exposure of CD8 T lymphocytes to adenosine accelerates multiple facets of the replicative senescence program.
Table II.
Adenosine downregulates telomerase activity in CD8 T lymphocytes
| ADA Inhibitor | Adenosine/ADA Inhibitor | |
| Donor | ||
| 1 | 0.888 | 0.520 |
| 2 | 0.936 | 0.374 |
| 3 | 0.926 | 0.687 |
| 4 | 1.036 | 0.519 |
| Mean ± SD | 0.946 ± 0.0632 | 0.493 ± 0.0826 |
| value | 0.14 | 0.00031 |
Purified CD8+ T cells from cultures established from four individual donors were isolated after the second stimulation (with Ab-coated microbeads). The TRAP assay was performed on 2500 cell equivalents. For each donor, the fold change in telomer-ase activity of cultures containing either the ADA inhibitor alone or adenosine plus the ADA inhibitor as compared with the DMSO control is shown.
Discussion
The major finding of the current study is that ADA, which when complexed to CD26 constitutes a key costimulatory component of the immunological synapse, undergoes significant changes as human CD8 T cells progress to replicative senescence. We also document that ADA is essential for optimal telomerase activity in CD8 T lymphocytes. Because ADA is required for the conversion of adenosine to inosine, the reduction of ADA activity and surface expression during progressive rounds of cell division might result in increased exposure of senescent CD8 T lymphocytes present in vivo to adenosine. Our data demonstrate that chronic exposure to adenosine significantly accelerated the process of replicative senescence in CD8 T lymphocytes, causing reduced proliferative potential, early loss of CD28 expression and telomerase activity, and reduced IL-2 message. Because increased proportions of senescent CD8 T lymphocytes are associated with a variety of deleterious clinical outcomes, including more rapid progression to AIDS (43), reduced vaccine responsiveness (7, 9), increased osteoporotic fractures (44), and early mortality in the elderly (8), our results may lead to novel approaches for manipulating the process of replicative senescence via ADA, thereby leading to improved health span.
It is well established that CD28, a key costimulatory molecule required for proper cellular immune responses, is downregulated in CD8 T lymphocytes that undergo extensive Ag-driven proliferation in cell culture to reach the end stage of replicative senescence (40). The in vivo relevance of this process is underscored by abundance of CD8+CD28− T lymphocytes in a variety of clinical situations involving chronic immune activation, including aging, HIV/AIDS, rheumatoid arthritis, latent infections, and several types of cancer (7, 8, 45–48). The present study expands upon these earlier findings by showing that ecto-ADA, known primarily for its role in the removal of extracellular adenosine (which can downregulate CD28 expression), is also an integral component of the telomerase activation pathway. We show that the modulation of CD28 involves both caspase-3–dependent and –independent pathways. This observation contrasts with our recent report showing that the modulation of CD28 expression by TNF-α is more highly dependent on caspase-3, although those studies did not include confirmatory experiments using a caspase inhibitor (38).
Our studies also help elucidate the role of ADA in optimizing CD8 T lymphocyte telomerase upregulation during activation. Previous reports have shown that T cell activation is more robust when the surface CD26 is complexed with ADA as compared with CD26 in the absence of ADA (14, 17).We expanded on those studies to show that the CD8+CD28+ADA+ subset has higher telomerase activity than the CD8+CD28+ADA− subset (Fig. 1), reinforcing the importance of the costimulatory contribution of the ADA− CD26 signaling pathway in maximizing CD8 T cell activation. This observation is highly relevant to chronic HIV-1 infection, where gp120 inhibition of ADA binding to CD26 may contribute to the well-documented telomere shortening (6) due to loss of telomerase activity in CD8 T lymphocytes (21). We also found that the reduced expression of ecto-ADA with culture age was not due to changes in surface expression of CD26 over the culture life span (data not shown). This observation, which is based on the longitudinal analysis of the same CD8 T cell population over time, contrasts with the cross-sectional analysis that showed lower expression CD26 on CD8 Tlymphocytes of older versus younger individuals (49).
The decrease in ADA with progressive cell divisions occurs not only at the cell surface, but also with respect to the actual activity of ADAwithin the cell. Although we documented one possible cause, namely the reduced ADA gene transcription, it remains to be determined if there is also a decrease in the transport of ADA to the surface for binding to CD26. The loss of ADA in human CD8 T cells expands upon studies on other cell types that showed a progressive reduction of ADA as cells transition to the end stage of replicative senescence (22). Because the ADA reduction pattern occurs in both immune and nonimmune cell types, the change is not likely specifically due to prolonged exposure to Ag, but rather relates to extensive rounds of proliferation. For T lymphocytes present in vivo, replicative senescence can be due to both chronic antigenic exposure and altered cytokine milieu or a combination of both. Irrespective of the precise mechanisms involved in the reduced surface ADA expression, cells that are ecto-ADA negative are at increased risk for the immunosuppressive effects of adenosine, which is secreted by Tregs and is also found in the microenvironment of tumor cells in vivo (24, 32, 34, 50, 51).
To isolate the specific effect of adenosine on the process of replicative senescence, our experiments included an ADA inhibitor (EHNA) in combination with the exogenously added adenosine. The presence of an ADA inhibitor in the cultures was intended to mimic in vivo conditions, where CD8 T cells lacking ADA might be exposed to adenosine, which can be secreted by multiple cell types in their extracellular environment. Our results showed that in the absence of ADA, adenosine had multiple effects on the process of replicative senescence. Indeed, chronic exposure of CD8 T lymphocytes to adenosine resulted in the early acquisition of the senescent phenotype, which comprises numerous genetic and functional alterations, including transcriptional repression of the CD28 gene, reduced telomerase activity (2, 52), and altered cytokine profiles (1, 3, 4, 40, 41). Adenosine exposure accelerated this process, resulting in markedly reduced overall total PDs before senescence was reached. These effects can all be attributed to adenosine, because the presence of the ADA inhibitor alone had no effect on replicative senescence, presumably because CD8 T cells do not produce adenosine. Exposure to adenosine was associated with a >50% reduction in telomerase activity and a significantly lower level of IL-2 gene transcription at all time points. Because telomerase activity and IL-2 production are directly linked with CD28 gene expression, these observations are consistent with previous findings regarding the multiple roles of CD28 signaling in T lymphocytes (2, 41, 42). The decrease in telomerase activity could explain the reduction in overall proliferative potential, because telomerase is needed to maintain CD8 T cell proliferation (53). Furthermore, the presence of IL-2 has been previously shown to be key in the upregulation of ADA and CD26 on the cell surface (54). Therefore, the gradual loss of IL-2 in vivo during aging could contribute to the loss of ADA on the cell surface, thereby synergizing with the loss of CD28.
Both TNF-α and adenosine have now been shown to be key modulators of replicative senescence, raising the question of their potential association. Although our own studies show that both can affect CD28 expression, reports in the literature suggest that the relationship between adenosine and TNF-α may be highly complex. Although in some cases adenosine has been shown to have anti-inflammatory effects, causing reduced TNF-α production (55), other reports have shown opposite results (56). Furthermore, there is evidence that TNF-α can upregulate specific adenosine receptors, thereby increasing a cell’s responsiveness to adenosine (57–59). However, our own observation on long-term cultures was that, irrespective of the presence of absence of exogenous adenosine, the level of TNF-α increased with culture age (data not shown). Future studies will explore the kinetics of the various adenosine receptors in the presence and absence of TNF-α to determine how these kinetics relate to replicative senescence. Our current hypothesis is that that adenosine may be a second pathway, in addition to that mediated by TNF-α, involved in CD28 expression regulation.
In summary, our study further elucidates the dynamics of CD28 regulation in human CD8 T cells as they undergo progressive differentiation to the end stage of replicative senescence. Our data show that ADA is instrumental in telomer aseactivation when complexed to CD26 on the cell surface. Furthermore, the loss of ADA reduces the ability of T lymphocytes to regulate adenosine levels, which when present in the extracellular space leads to CD28 downregulation through both caspase-dependent and -independent pathways, ultimately leading to premature replicative senescence. Our findings highlight novel aspects of ADA and adenosine in memory T lymphocyte biology and suggest possible new therapeutic approaches to prevent the generation of senescent CD8 T cells, which hare associated with and involved in multiple clinical pathologies.
Acknowledgments
This work was supported by National Institutes of Health Grants AG023720 and AI060362 (to R.B.E).
Abbreviations used in this paper
- ADA
adenosine deaminase
- EHNA
erythro-9-(2-hydroxy-3-nonyl)adenine
- PD
population doubling
- TRAP
telomere repeat amplification protocol
- Treg
regulatory T cell
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 2.Valenzuela HF, Effros RB. Divergent telomerase and CD28 expression patterns in human CD4 and CD8 T cells following repeated encounters with the same antigenic stimulus. Clin. Immunol. 2002;105:117–125. doi: 10.1006/clim.2002.5271. [DOI] [PubMed] [Google Scholar]
- 3.Lindstein T, June CH, Ledbetter JA, Stella G, Thompson CB. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989;244:339–43. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- 4.Powell JD, Ragheb JA, Kitagawa-Sakakida S, Schwartz RH. Molecular regulation of interleukin-2 expression by CD28 co-stimulation and anergy. Immunol. Rev. 1998;165:287–300. doi: 10.1111/j.1600-065x.1998.tb01246.x. [DOI] [PubMed] [Google Scholar]
- 5.Posnett DN, Sinha R, Kabak S, Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to “benign monoclonal gammapathy”. J. Exp. Med. 1994;179:609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Effros RB, Allsopp R, Chiu CP, Hausner MA, Hirji K, Wang L, Harley CB, Villeponteau B, West MD, Giorgi JV. Shortened telomeres in the expanded CD28-CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS. 1996;10:F17–F22. doi: 10.1097/00002030-199607000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O’Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J. Virol. 2001;75:12182–12187. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hadrup SR, Strindhall J, Køllgaard T, Seremet T, Johansson B, Pawelec G, thor Straten P, Wikby A. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J. Immunol. 2006;176:2645–2653. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- 9.Saurwein-Teissl M, Lung TL, Marx F, Gschösser C, Asch E, Blasko I, Parson W, Böck G, Schönitzer D, Trannoy E, Grubeck-Loebenstein B. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J. Immunol. 2002;168:5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- 10.Cao W, Jamieson BD, Hultin LE, Hultin PM, Effros RB, Detels R. Premature aging of T cells is associated with faster HIV-1 disease progression. J. Acquir. Immune Defic. Syndr. 2009;50:137–147. doi: 10.1097/QAI.0b013e3181926c28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Effros RB. From Hayflick to Walford: the role of T cell replicative senescence in human aging. Exp. Gerontol. 2004;39:885–890. doi: 10.1016/j.exger.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Hershfield MS. New insights into adenosine-receptor-mediated immu-nosuppression and the role of adenosine in causing the immunodeficiency associated with adenosine deaminase deficiency. Eur. J. Immunol. 2005;35:25–30. doi: 10.1002/eji.200425738. [DOI] [PubMed] [Google Scholar]
- 13.Kameoka J, Tanaka T, Nojima Y, Schlossman SF, Morimoto C. Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science. 1993;261:466–469. doi: 10.1126/science.8101391. [DOI] [PubMed] [Google Scholar]
- 14.Martín M, Huguet J, Centelles JJ, Franco R. Expression of ecto-adenosine deaminase and CD26 in human T cells triggered by the TCR-CD3 complex. Possible role of adenosine deaminase as costimulatory molecule. J. Immunol. 1995;155:4630–4643. [PubMed] [Google Scholar]
- 15.Aran JM, Colomer D, Matutes E, Vives-Corrons JL, Franco R. Presence of adenosine deaminase on the surface of mononuclear blood cells: immunochemical localization using light and electron microscopy. J. Histochem. Cytochem. 1991;39:1001–1008. doi: 10.1177/39.8.1856451. [DOI] [PubMed] [Google Scholar]
- 16.Hovi T, Smyth JF, Allison AC, Williams SC. Role of adenosine deaminase in lymphocyte proliferation. Clin. Exp. Immunol. 1976;23:395–403. [PMC free article] [PubMed] [Google Scholar]
- 17.Pacheco R, Martinez-Navio JM, Lejeune M, Climent N, Oliva H, Gatell JM, Gallart T, Mallol J, Lluis C, Franco R. CD26, adenosine deaminase, and adenosine receptors mediate costimulatory signals in the immunological synapse. Proc. Natl. Acad. Sci. USA. 2005;102:9583–9588. doi: 10.1073/pnas.0501050102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis DE, Yang L, Luo W, Wang X, Rodgers JR. HIV-specific cytotoxic T lymphocyte precursors exist in a CD28-CD8+ T cell subset and increase with loss of CD4 T cells. AIDS. 1999;13:1029–133. doi: 10.1097/00002030-199906180-00005. [DOI] [PubMed] [Google Scholar]
- 19.Appay V, Almeida JR, Sauce D, Autran B, Papagno L. Accelerated immune senescence and HIV-1 infection. Exp. Gerontol. 2007;42:432–437. doi: 10.1016/j.exger.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Valenzuela A, Blanco J, Callebaut C, Jacotot E, Lluis C, Hovanessian AG, Franco R. Adenosine deaminase binding to human CD26 is inhibited by HIV-1 envelope glycoprotein gp120 and viral particles. J. Immunol. 1997;158:3721–729. [PubMed] [Google Scholar]
- 21.Valenzuela A, Blanco J, Callebaut C, Jacotot E, Lluis C, Hovanessian AG, Franco R. HIV-1 envelope gp120 and viral particles block adenosine deaminase binding to human CD26. Adv. Exp. Med. Biol. 1997;421:185–192. doi: 10.1007/978-1-4757-9613-1_24. [DOI] [PubMed] [Google Scholar]
- 22.Ghneim HK, Al-Saleh SS, Al-Shammary FJ, Kordee ZS. Changes in adenosine deaminase activity in ageing cultured human cells and the role of zinc. Cell Biochem. Funct. 2003;21:275–282. doi: 10.1002/cbf.1023. [DOI] [PubMed] [Google Scholar]
- 23.Gessi S, Varani K, Merighi S, Fogli E, Sacchetto V, Benini A, Leung E, Mac-Lennan S, Borea PA. Adenosine and lymphocyte regulation. Purinergic Signal. 2007;3:109–116. doi: 10.1007/s11302-006-9042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacKenzie WM, Hoskin DW, Blay J. Adenosine inhibits the adhesion of anti-CD3-activated killer lymphocytes to adenocarcinoma cells through an A3 receptor. Cancer Res. 1994;54:3521–3526. [PubMed] [Google Scholar]
- 25.DosReis GA, Nóbrega AF, de Carvalho RP. Purinergic modulation of T-lymphocyte activation: differential susceptibility of distinct activation steps and correlation with intracellular 3′,5′-cyclic adenosine monophosphate accumulation. Cell. Immunol. 1986;101:213–21. doi: 10.1016/0008-8749(86)90199-1. [DOI] [PubMed] [Google Scholar]
- 26.Szondy Z. Adenosine stimulates DNA fragmentation in human thymocytes by Ca(2+)-mediated mechanisms. Biochem. J. 1994;304:877–885. doi: 10.1042/bj3040877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohta A, Ohta A, Madasu M, Kini R, Subramanian M, Goel N, Sitkovsky M. A2A adenosine receptor may allow expansion of T cells lacking effector functions in extracellular adenosine-rich microenvironments. J. Immunol. 2009;183:5487–5493. doi: 10.4049/jimmunol.0901247. [DOI] [PubMed] [Google Scholar]
- 28.Butler JJ, Mader JS, Watson CL, Zhang H, Blay J, Hoskin DW. Adenosine inhibits activation-induced T cell expression of CD2 and CD28 co-stimulatory molecules: role of interleukin-2 and cyclic AMP signaling pathways. J. Cell. Biochem. 2003;89:975–991. doi: 10.1002/jcb.10562. [DOI] [PubMed] [Google Scholar]
- 29.Perillo NL, Walford RL, Newman MA, Effros RB. Human T lymphocytes possess a limited in vitro life span. Exp. Gerontol. 1989;24:177–187. doi: 10.1016/0531-5565(89)90009-0. [DOI] [PubMed] [Google Scholar]
- 30.Vielh P, Castellazzi M. A colorimetric assay for serial determination of adenosine deaminase activity in small lymphocyte populations. J. Immunol. Methods. 1984;73:313–320. doi: 10.1016/0022-1759(84)90406-x. [DOI] [PubMed] [Google Scholar]
- 31.Saldanha SN, Andrews LG, Tollefsbol TO. Analysis of telomerase activity and detection of its catalytic subunit, hTERT. Anal. Biochem. 2003;315:1–21. doi: 10.1016/s0003-2697(02)00663-2. [DOI] [PubMed] [Google Scholar]
- 32.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marquardt DL, Gruber HE, Wasserman SI. Adenosine release from stimulated mast cells. Proc. Natl. Acad. Sci. USA. 1984;81:6192–6196. doi: 10.1073/pnas.81.19.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bynoe MS, Viret C. Foxp3+CD4+ T cell-mediated immunosuppression involves extracellular nucleotide catabolism. Trends Immunol. 2008;29:99–102. doi: 10.1016/j.it.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Mandapathil M, Hilldorfer B, Szczepanski MJ, Czystowska M, Szajnik M, Ren J, Lang S, Jackson EK, Gorelik E, Whiteside TL. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25high-FOXP3+ regulatory T cells (TREG) J. Biol. Chem. 2009 doi: 10.1074/jbc.M109.047423. doi:10.1074/jbc.M109.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma S, Ochi H, Cui L, He W. FasL-induced downregulation of CD28 expression on jurkat cells in vitro is associated with activation of caspases. Cell Biol. Int. 2003;27:959–964. doi: 10.1016/s1065-6995(03)00170-7. [DOI] [PubMed] [Google Scholar]
- 37.Ma S, Ochi H, Cui L, Zhang J, He W. Hydrogen peroxide induced down-regulation of CD28 expression of Jurkat cells is associated with a change of site alpha-specific nuclear factor binding activity and the activation of caspase-3. Exp. Gerontol. 2003;38:1109–1118. doi: 10.1016/s0531-5565(03)00166-9. [DOI] [PubMed] [Google Scholar]
- 38.Parish ST, Wu JE, Effros RB. Modulation of T lymphocyte replicative senescence via TNF-alpha inhibition: role of caspase-3. J. Immunol. 2009;182:4237–4243. doi: 10.4049/jimmunol.0803449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tarazona R, DelaRosa O, Alonso C, Ostos B, Espejo J, Peña J, Solana R. Increased expression of NK cell markers on T lymphocytes in aging and chronic activation of the immune system reflects the accumulation of effector/ senescent T cells. Mech. Ageing Dev. 2000;121:77–88. doi: 10.1016/s0047-6374(00)00199-8. [DOI] [PubMed] [Google Scholar]
- 40.Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8+ T-cell replicative senescence in human aging. Immunol. Rev. 2005;205:147–157. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 41.Verweij CL, Geerts M, Aarden LA. Activation of interleukin-2 gene transcription via the T-cell surface molecule CD28 is mediated through an NF-kB-like response element. J. Biol. Chem. 1991;266:14179–14182. [PubMed] [Google Scholar]
- 42.Fraser JD, Irving BA, Crabtree GR, Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991;251:313–316. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- 43.Cao W, Jamieson BD, Hultin LE, Hultin PM, Detels R. Regulatory T cell expansion and immune activation during untreated HIV type 1 infection are associated with disease progression. AIDS Res. Hum. Retroviruses. 2009;25:183–191. doi: 10.1089/aid.2008.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pietschmann P, Grisar J, Thien R, Willheim M, Kerschan-Schindl K, Preisinger E, Peterlik M. Immune phenotype and intracellular cytokine production of peripheral blood mononuclear cells from postmenopausal patients with osteoporotic fractures. Exp. Gerontol. 2001;36:1749–1759. doi: 10.1016/s0531-5565(01)00125-5. [DOI] [PubMed] [Google Scholar]
- 45.Sze DM, Giesajtis G, Brown RD, Raitakari M, Gibson J, Ho J, Baxter AG, Fazekas de St Groth B, Basten A, Joshua DE. Clonal cytotoxic T cells are expanded in myeloma and reside in the CD8(+)CD57(+)CD28(−) compartment. Blood. 2001;98:2817–2827. doi: 10.1182/blood.v98.9.2817. [DOI] [PubMed] [Google Scholar]
- 46.Vingerhoets JH, Vanham GL, Kestens LL, Penne GG, Colebunders RL, Vandenbruaene MJ, Goeman J, Gigase PL, De Boer M, Ceuppens JL. Increased cytolytic T lymphocyte activity and decreased B7 responsiveness are associated with CD28 down-regulation on CD8+ T cells from HIV-infected subjects. Clin. Exp. Immunol. 1995;100:425–433. doi: 10.1111/j.1365-2249.1995.tb03717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsukishiro T, Donnenberg AD, Whiteside TL. Rapid turnover of the CD8(+)CD28(−) T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol. Immunother. 2003;52:599–607. doi: 10.1007/s00262-003-0395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Håkansson A, Håkansson L, Gustafsson B, Krysander L, Rettrup B, Ruiter D, Bernsen MR. Biochemotherapy of metastatic malignant melanoma. On down-regulation of CD28. Cancer Immunol. Immunother. 2002;51:499–504. doi: 10.1007/s00262-002-0304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Czesnikiewicz-Guzik M, Lee WW, Cui D, Hiruma Y, Lamar DL, Yang ZZ, Ouslander JG, Weyand CM, Goronzy JJ. T cell subset-specific susceptibility to aging. Clin. Immunol. 2008;127:107–118. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burnstock G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology. 1997;36:1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- 51.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc. Natl. Acad. Sci. USA. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Effros RB. Telomerase induction in T cells: a cure for aging and disease? Exp. Gerontol. 2007;42:416–420. doi: 10.1016/j.exger.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Plunkett FJ, Franzese O, Finney HM, Fletcher JM, Belaramani LL, Salmon M, Dokal I, Webster D, Lawson AD, Akbar AN. The loss of telomerase activity in highly differentiated CD8+CD28-CD27- T cells is associated with decreased Akt (Ser473) phosphorylation. J. Immunol. 2007;178:7710–7719. doi: 10.4049/jimmunol.178.12.7710. [DOI] [PubMed] [Google Scholar]
- 54.Cordero OJ, Salgado FJ, Fernández-Alonso CM, Herrera C, Lluis C, Franco R, Nogueira M. Cytokines regulate membrane adenosine deaminase on human activated lymphocytes. J. Leukoc. Biol. 2001;70:920–930. [PubMed] [Google Scholar]
- 55.Perni U, Sezen D, Bongiovanni AM, Linhares IM, Skupski D, Witkin SS. Endogenous adenosine down-modulates mid-trimester intraamniotic tumor necrosis factor-alpha production. Am. J. Reprod. Immunol. 2009;62:232–237. doi: 10.1111/j.1600-0897.2009.00730.x. [DOI] [PubMed] [Google Scholar]
- 56.Nakav S, Naamani O, Chaimovitz C, Shaked G, Czeiger D, Zlotnik M, Douvdevani A. Regulation of adenosine system at the onset of peritonitis. Nephrol Dial Transplant. 2009 doi: 10.1093/ndt/gfp542. doi:10.1093/ndt/gfp542. [DOI] [PubMed] [Google Scholar]
- 57.Varani K, Vincenzi F, Tosi A, Gessi S, Casetta I, Granieri G, Fazio P, Leung E, Maclennan S, Granieri E, Borea PA. A2A adenosine receptor overexpression and functionality, as well as TNF-{alpha} levels, correlate with motor symptoms in Parkinson’s disease. FASEB J. 2010;24:587–598. doi: 10.1096/fj.09-141044. [DOI] [PubMed] [Google Scholar]
- 58.St Hilaire C, Koupenova M, Carroll SH, Smith BD, Ravid K. TNF-alpha upregulates the A2B adenosine receptor gene: The role of NAD(P)H oxidase 4. Biochem. Biophys. Res. Commun. 2008;375:292–296. doi: 10.1016/j.bbrc.2008.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Capecchi PL, Camurri A, Pompella G, Mazzola A, Maccherini M, Diciolla F, Lazzerini PE, Abbracchio MP, Laghi-Pasini F. Up-regulation of A2A adenosine receptor expression by TNF-alpha in PBMC of patients with CHF: a regulatory mechanism of inflammation. J. Card. Fail. 2005;11:67–73. doi: 10.1016/j.cardfail.2004.04.005. [DOI] [PubMed] [Google Scholar]