Abstract
Background
Rotator cuff repair re-tear rates range from 25-90% necessitating methods to improve repair strength. While numerous laboratory studies have compared single to double row fixation properties, little is known regarding regional (i.e., medial versus lateral) suture retention properties in both intact and torn tendons.
Hypothesis
A torn supraspinatus tendon will have reduced suture retention properties on the lateral aspect of the tendon compared to the more medial musculotendinous junction.
Study Design
Controlled Laboratory Study
Methods
Human supraspinatus tendons (torn and intact) were randomly assigned for suture retention mechanical testing, ultrastructural collagen fibril analysis, or histology following suture pullout testing. For biomechanical evaluation, sutures were placed either at the musculotendinous junction (medial) or 10 mm from the free margin (lateral), and tendons were elongated to failure. Collagen fibril assessments were performed using transmission electron microscopy (TEM).
Results
Intact tendons showed no regional differences with respect to suture retention properties. In contrast, among torn tendons the medial region exhibited significantly higher stiffness and work values relative to the lateral region. For the lateral region, work to 10 mm displacement (1592±261 N-mm) and maximum load (265±44 N) for intact tendons were significantly higher (p<0.05) than those of torn tendons (1086±388 N-mm and 177±71 N, respectively). For medial suture placement, maximum load, stiffness and work of intact and torn tendons were similar (p>0.05). Regression analyses for the intact and torn groups revealed generally low correlations between donor age and the three biomechanical indices. For both intact and torn tendons, the mean fibril diameter and area density were greater in the medial region relative to the lateral (p≤0.05). In the lateral tendon, but not the medial region, torn specimens showed a significantly lower fibril area fraction (48.3±3.8%) than intact specimens (56.7±3.6%, p<0.05).
Conclusions
Superior pullout resistance of medial placed sutures may provide a strain shielding effect for the lateral row following double row repair. Larger diameter collagen fibrils as well as greater fibril area fraction in the medial supraspinatus tendon may provide greater resistance to suture migration.
Clinical Relevance
While clinical factors such as musculotendinous integrity warrant strong consideration for surgical decision making, the present ultrastructural and biomechanical results appear to provide a scientific rationale for double-row rotator cuff repair where sutures are placed more medial at the muscle tendon junction.
Keywords: Supraspinatus, Biomechanics, Rotator Cuff Repair, Collagen
INTRODUCTION
Rotator cuff tears have an estimated incidence of 3.7 per 100,000 individuals per year, with peak occurrence during the 5th decade in men and 6th decade for women.2 Greater than 300,000 repairs are performed each year, with observed retear rates ranging from 25% to 90%.16 Successful surgical outcomes are dependent upon both intrinsic musculotendinous properties (e.g., architectural, biomechanical, and healing capacity) as well as the surgical repair technique. In chronic tendon ruptures, repair becomes more technically difficult due to irreversible architectural changes in the musculotendinous unit, including fatty infiltration, muscle fiber shortening and increased muscle pennation angle.6
Prior laboratory studies have suggested that superior initial fixation strength, tendon to bone footprint contact and stability under cyclic loading may lead to improved repair outcomes.3 Despite some conflicting reports, biomechanical results generally indicate that multi-row and transosseous-equivalent configurations confer improved fixation relative to single-row techniques.11, 18, 19, 24 Technical developments such as double-row and transosseous-equivalent repairs increase pressurized contact at the insertion footprint18, 19 but necessitate additional anchors and sutures. Furthermore, the latter findings are derived from cadaveric studies utilizing tendon detachment, immediate repair, and mechanical testing of tendon–suture–anchor–bone constructs. In contrast, little attention has focused on suture retention properties of the medial and lateral regions of the tendon (independent of fixation technique) in both intact and torn states. An improved understanding of supraspinatus tendon regional collagen fiber functional and architectural properties may provide additional guidance with regard to intraoperative suture placement during rotator cuff repair. Therefore, the present investigation evaluated the mechanical capacity of intact and torn human supraspinatus tendons to retain a suture with both lateral and medial placements. As tears typically propagate from the insertion site, we hypothesized that torn tendons and specifically the lateral region would exhibit inferior suture retention and ultrastructural properties compared to intact specimens. In addition, collagen fibril and immunohistochemical characteristics were examined to provide additional insight into structure-function relationships.
MATERIALS AND METHODS
Specimen Preparation
Twenty-seven fresh frozen human cadaveric shoulders were obtained from LifeLegacy Foundation (Tucson, AZ, USA) and stored at −20°C until use. Among these specimens, twenty (10 torn and 10 intact supraspinatus tendons, mean age 73 ± 12 years, range 52 to 93) were utilized for suture retention testing while seven (3 torn and 4 intact supraspinatus tendons, mean age 66 ± 19, range 48-92) were used for ultrastructural analyses. Of those specimens used for mechanical testing, six intact and torn tendons each (as classified prior to testing) were subsequently analyzed using (immuno)histologic methods. Donor gender and age characteristics for all assays are provided in Table 1. After thawing, the supraspinatus muscle was harvested from its fossa and the tendon sharply detached from its footprint on the greater tuberosity. Tendons were divided into two experimental groups (intact and torn) based on gross evaluation during dissection. For suture retention testing, specimens were assigned to one of four groups (n=5 per group): (1) intact tendon with medial suture placement, (2) intact tendon with lateral suture placement, (3) torn tendon with medial suture placement and (4) torn tendon with lateral suture placement. Specifically, intact tendons were randomly selected for either medial or lateral suture placement. For torn tendons, suture placement was selected on the basis of tear size (i.e., massive tears only permitted medial sutures while either medial or lateral placement was possible for sufficiently small tears).
Table 1. Donor age (mean ± SD) and gender characteristics of specimens utilized for each laboratory assay.
| Mechanical Testing | ||
|---|---|---|
|
|
||
|
Age
Gender |
Intact (n=10) | Torn (n=10) |
| 69 ± 12 | 76 ± 11 | |
| 7 M / 3 F | 9 M / 1 F | |
| TEM | ||
|---|---|---|
|
|
||
|
Age
Gender |
Intact (n=4) | Torn (n=3) |
| 53 ± 4 | 85 ± 13 | |
| 4 M | 3 F | |
| Histology | ||
|---|---|---|
|
|
||
|
Age
Gender |
Intact (n=6) | Torn (n=6) |
| 68 ± 14 | 76 ± 11 | |
| 2 M / 4 F | 6 M | |
Biomechanical Testing
Biomechanical testing was performed using an electromechanical materials testing system (MTS Insight 5, Eden Prairie, MN, USA). A custom made cryoclamp, positioned such that its base was 4-5 millimeters proximal to the musculotendinous junction (Figure 1), was used to secure the muscle belly and freeze it to 0°C using dry ice. During specimen preparation and testing, tissue temperatures were monitored using an infrared thermometer (Extech Instruments, Waltham, MA, USA). The tendon temperature was maintained above 15°C and within a maximum gradient of 5°C along its length throughout the duration of each experiment by irrigating the tendon with warmed saline. Maintenance of the tissue temperature in this range ensured that thermal gradients would not influence the elastic or viscoelastic properties of the supraspinatus tendon.7
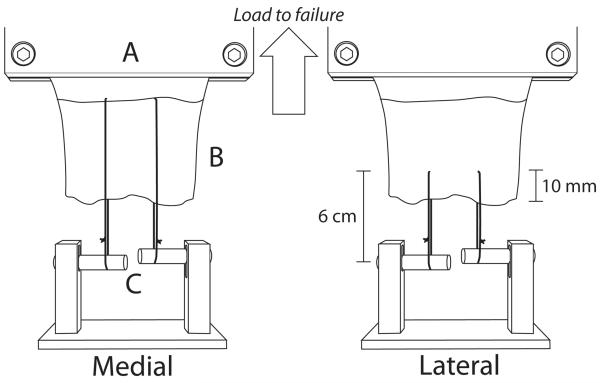
Figure 1.
Schematic diagram illustrating suture placement for tendon suture retention testing. Sutures trisected the tendon width and were placed at either the musculotendinous junction for medial tendon testing (left picture) or 10mm from the free tendon margin for lateral region testing (right picture). The cryoclamp was attached in series to a 1000N load cell and crosshead of the materials testing system (not shown). A: base of cryoclamp, B: tendon, C: adjustable bars for each suture
Two #2 FiberWire (Arthrex, Naples, FL, USA) sutures, trisecting the width of the tendon, were placed at either 10mm from the free margin (lateral) or at the musculotendinous junction (medial) (Figure 1). Since the placement of the sutures within the tendon was standardized for all specimens, it was deemed unnecessary to determine an initial length. Suture length was standardized to 6 cm, with each of the two sutures per tendon looped over an adjustable fixture (i.e., one movable bar for each suture) attached to the testing platform; this experimental setup facilitated the establishment of approximately equal initial tension in the two sutures. For consistency, the same experimentalist performed the suture placement and bar adjustment (i.e., suture tensioning) for all specimens tested. As the bar fixture remained stationary throughout testing, upward displacement of the MTS crosshead (Figure 1) resulted in tensile loading of the tendon, thereby pulling the sutures through the tendon. Tensile loads were measured using a 1000N load cell attached between the cryoclamp and machine crosshead. Prior to initiating mechanical loading, care was taken to avoid direct contact between the suture knot and either the tendon or test fixture. Each suture-tendon construct was preloaded to 5N for 1 minute, followed by cyclic preconditioning between 5N and 30N at 1 mm/sec for 50 cycles. Finally, a pull to failure test was applied at a rate of 1 mm/s. Since the potential suture migration distance during failure testing was greater for medial than lateral placements (Figure 1), for objective comparison the following mechanical parameters were quantified from the first 10mm of the force-displacement curve for each failure test: load at 10mm, linear stiffness (steepest slope spanning 30% of data points26 from the initial force to load at 10mm), and work to 10mm displacement (area under curve).
Transmission Electron Microscopy (TEM)
From the prominent, “tubular” anterior tendon region5, 22 of three torn and four intact specimens, medial and lateral specimen cubes (approximately 3 mm × 3 mm × 3 mm) were harvested and fixed in 4% paraformaldehyde and 2.5% glutaraldehyde EM grade in 1M Sodium Cacodylate Buffer (Electron Microscopy Sciences, Hatfield, PA) for 48 hours. Samples were post-fixed in 1% osmium tetroxide, dehydrated in a series of alcohols and embedded in epoxy resin. Tendon specimens were sectioned transversely at 80-90nm thickness on a Leica UltraCut UCT ultramicrotome (Leica Microsystems, Wetzlar, Germany), placed on 3mm 200 mesh copper grids and stained with uranyl acetate and lead citrate. Digital images of the sections were captured at a magnification of 150,000x using a transmission electron microscope (JEM-1220, Jeol, Boston, MA, USA). Collagen fibril diameters and area fraction (i.e., percentage of a microscopic field occupied by collagen fibrils) were quantified using image analysis software (Image J, National Institutes of Health, USA) from 960 nm × 1280 nm microscopic imaging fields. Individual collagen fibrils were manually traced for area determination. Collagen fibril diameter was calculated as 2*√ (fibril cross sectional area / π) and area fraction (%) as (Σ collagen fibril areas) / area of microscopic image field.21 Fibril diameter frequency distribution was evaluated per image field at 10nm intervals.
Histology and Immunohistochemistry
Medial and lateral portions of the biomechanically tested tendons were fixed in 10% neutral buffered formalin, paraffin embedded and sectioned at 8μm. Slides were stained with hematoxylin and eosin (H&E) and Safranin-O/Fast Green. For immunohistochemical analysis, sections were deparaffinized and rinsed in distilled water and phosphate buffered saline. Endogenous peroxidase activity was blocked with 2% hydrogen peroxide and non-specific antibody binding blocked with 10% normal goat serum. Primary antibodies were rabbit polyclonal anti-Collagen I (Abcam, Cambridge, MA; dilution 1:50 in 1% BSA/PBS) and mouse monoclonal anti-Collagen III (Sigma-Aldrich, St Louis, MO;, dilution 1:500 in 1% BSA/PBS). Peroxidase conjugated fragment goat anti-rabbit antibody (Accurate Chemical & Scientific Inc., Westbury, NY; dilution 1:100 in 1% BSA/PBS) and peroxidase conjugated rabbit anti-mouse antibody (Sigma-Aldrich, dilution 1:100 in 1% BSA/PBS) respectively were used as secondary antibodies. Immunohistochemistry was performed in parallel with positive and negative (no primary antibody) controls.
Statistical Analysis
Statistical analyses were performed using SPSS 15.0 (SPSS Science Inc, Chicago, IL, USA). Biomechanical parameters were compared using a two-way ANOVA followed by pair wise post-hoc testing to determine differences attributable to tendon integrity (intact or torn) and anatomic region (medial or lateral). Due to the relatively small sample sizes for TEM measurements, unpaired t-tests were used to examine differences in mean collagen fibril diameter and area fraction of medial and lateral specimens from both intact and torn groups. A Kolmogorov-Smirnov test was used to compare the collagen fibril diameter distributions between groups. Linear regression analysis was performed to determine potential correlations between suture retention properties and donor age. Results were considered statistically significant for p-values less than or equal to 0.05.
RESULTS
Suture Retention
Biomechanical failure occurred via suture pull-through of the tendon in all specimens. Typically, failure initiated at the posterior aspect of the tendon prior to the anterior aspect. Muscle slippage from the cryoclamp, knot slippage or suture breakage was not observed as a mode of failure.
No differences in suture retention properties were observed between the medial and lateral regions of the intact tendons (Table 2). In contrast, among torn tendons, work to 10mm (1086 ± 388N-mm) and stiffness (36 ± 8 N/mm) for the lateral region were significantly lower than those of the medial (1568 ± 283N-mm and 50 ± 12 N/mm, respectively).
Table 2. Suture Retention Properties.
| Medial | Lateral | p | ||
|---|---|---|---|---|
|
| ||||
| Work (N-mm) | Intact | 1697 ± 139 | 1592 ± 261 | n.s. |
| Torn | 1568 ± 283 | 1086 ± 388 | 0.03 | |
|
| ||||
| P | n.s. | 0.02 | ||
|
| ||||
| Load at 10mm (N) |
Intact | 280 ± 46 | 265 ± 44 | n.s. |
| Torn | 262 ± 47 | 177 ± 71 | n.s. | |
|
| ||||
| P | n.s. | 0.05 | ||
|
| ||||
| Stiffness (N/mm) |
Intact | 47 ± 6 | 47 ± 11 | n.s |
| Torn | 50 ± 12 | 36 ± 8 | 0.05 | |
|
| ||||
| P | n.s. | n.s. | ||
No differences were observed in the work to 10 mm (p=0.49), load at 10mm (p=0.63) or stiffness (p=0.62) of torn compared to intact tendons with medial suture placement. However, for the lateral region, work to 10 mm displacement (1592 ± 261 N-mm, p=0.02) and load at 10mm (265 ± 44 N, p=0.05) for intact tendons were significantly higher than those of torn tendons (1086 ± 388 N-mm and 177 ± 71 N, respectively).
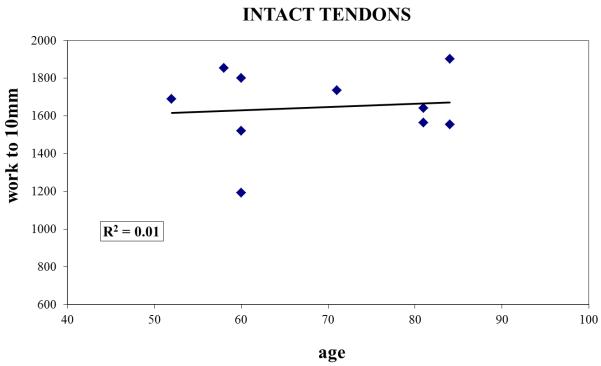
Regression analyses for the intact and torn groups revealed generally low correlations between donor age and the three biomechanical indices. Specifically, for separate analyses of intact and torn groups (n=10 samples each), R2 values (where R represents the correlation coefficient) were less than or equal to 0.23 (Figure 2) for each mechanical outcome measure.
Figure 2.
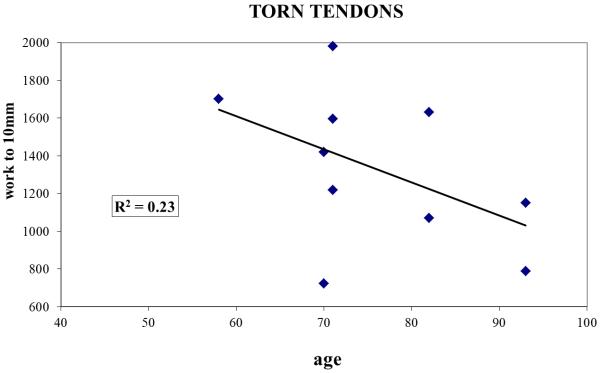
Work to 10mm plotted as a function of specimen age for (A) intact and (B) torn tendons, demonstrating that age was not a confounding factor for the differences in suture retention properties between the two groups.
Collagen Fibril TEM
For both intact and torn tendons, the mean fibril diameter and area density were greater in the medial region relative to the lateral (p<0.05, Table 3). Ultrastructural analysis revealed that the mean intact tendon collagen fibril diameter was larger in medial (98 ± 14 nm) compared with lateral tendon region (57 ± 3 nm; p<0.05, Figure 3). Similarly, among torn tendons (Figure 4), medial regions (125 ± 15 nm) exhibited a larger fibril diameter than the lateral (51 ± 3 nm).
Table 3. Collagen Fibril Analyses.
| Medial | Lateral | p | ||
|---|---|---|---|---|
|
| ||||
| Fibril Diameter (nm) |
Intact | 98 ± 14 | 57 ± 3 | 0.04 |
| Torn | 125 ± 15 | 51 ± 3 | 0.05 | |
|
| ||||
| P | n.s. | n.s. | ||
|
| ||||
| Area Fraction (%) |
Intact | 66.7 ± 4.5 | 56.7 ± 3.6 | 0.01 |
| Torn | 62.8 ± 6.9 | 48.3 ± 3.8 | 0.05 | |
|
| ||||
| p | n.s. | 0.03 | ||
Figure 3.
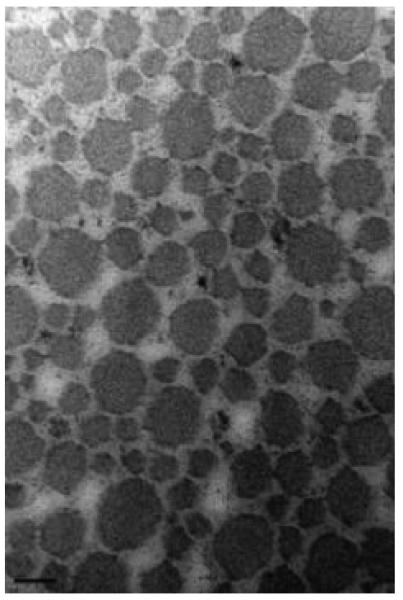
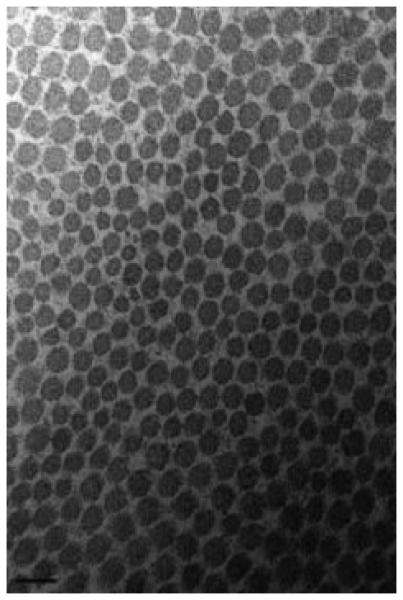
Collagen fibrils of the medial (A) and lateral (B) intact supraspinatus (magnification: 150,000). A bimodal fibril diameter distribution is apparent in the medial region. Scalebar = 100nm
Figure 4.
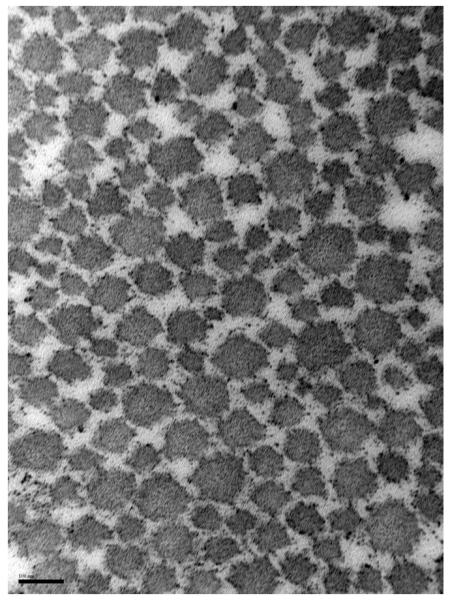
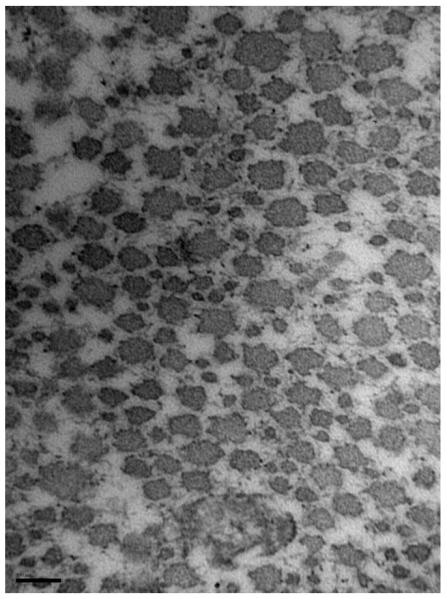
Collagen fibrils of the medial (A) and lateral (B) regions of torn supraspinatus (magnification: 150,000). Scalebar = 100nm
For medial region tendon samples, the mean fibril diameter and area fraction were similar between intact and torn tendons. The lateral region of torn specimens exhibited a decreased collagen fibril area fraction (48.3 ± 3.8%) compared to intact specimens (56.7 ± 3.6%, p<0.05, Table 3); however, the mean fibril diameter was not different.
Torn and intact tendons exhibited significantly different fibril diameter distributions for both medial and lateral locations. The medial region of intact specimens showed a nearly bimodal distribution with peak frequencies at approximately 70-90 nm and 170-190 nm; in contrast, the medial region of torn tendons showed a peak frequency in the 150-170 nm range (Figure 5a). In the lateral region, intact and torn tendons both exhibited a more symmetric distribution curve relative to the medial region. Among lateral region tendons, torn specimens showed a wider range of fibril diameters while for intact tissues the distribution plot demonstrated a trend towards larger fibrils (Figure 5b).
Figure 5.
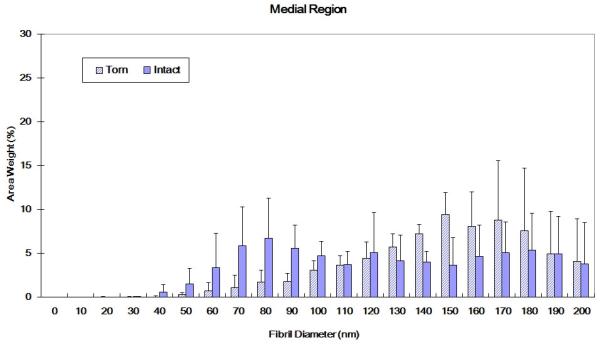
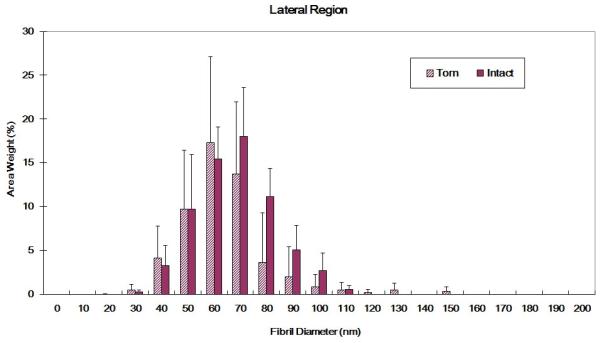
(A) Collagen fibril diameter distributions for intact and torn medial tendon regions (medial region of intact tendon shows a bimodal distribution; torn tendons exhibited more large diameter collagen fibrils. K-S test: p<0.01) (B) Collagen fibril diameter distributions for intact and torn lateral tendon regions (torn tendon shows trend towards smaller fibrils and wider distribution than intact tendon, K-S test: p<0.01)
Histology and Immunohistochemistry
Intact tendons exhibited closely arranged, parallel fibers and low cellularity (Figure 6A). In contrast, torn tendons revealed longitudinal splitting and thin fibers in a disorganized pattern. Increased cellularity and vascularity were noted in many of the specimens. No apparent cell or matrix differences were observed between the medial and lateral regions of torn specimens (Figure 6B and 6C). Furthermore, immunohistochemical staining for types I and III collagen did not appear to differ between the medial and lateral regions of torn tendons (Figure 6D and 6E).
Figure 6.
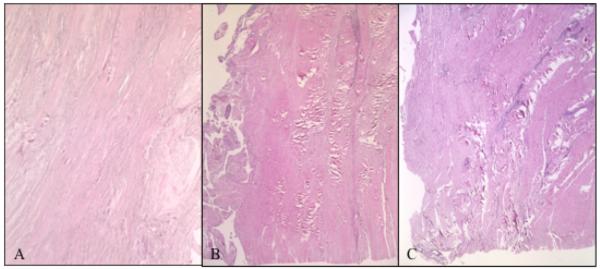
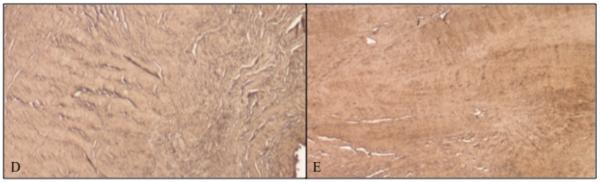
Tendon Histology. H+E staining of (A) Intact tendon from anterior region; (B) Torn tendon, anteromedial region; and (C) Torn tendon, anterolateral region. Immunohistochemical histology, stained for type III collagen of (D) Anteromedial supraspinatus tendon; and (E) Anterolateral supraspinatus tendon. No differences were noted between medial and lateral tendon.
DISCUSSION
The primary objective of the present study was to evaluate regional suture retention properties in the human supraspinatus tendon. Our results demonstrate that the medial and lateral regions of the intact supraspinatus tendon exhibit similar biomechanical properties with respect to suture retention. Interestingly, the presence of a tear altered the suture retention properties of the lateral, but not the medial supraspinatus tendon. Among torn tendons, the mechanical parameters pullout stiffness and work were significantly lower when sutures were placed in the lateral region, relative to a medial position (Table 2). These findings confirm our hypothesis that suture retention by the lateral supraspinatus tendon is impaired in a torn state. The impairment of such properties may in part help to explain higher re-tear or failure-to-heal rates following primary rotator cuff repair. More specifically, single-row techniques that utilize sutures placed more laterally in the diseased tendon may experience a higher failure-to-heal rate than otherwise might be anticipated with healthier tissue or more medially placed sutures.
TEM analyses revealed that both the mean collagen fibril diameter as well as the area fraction were significantly lower for the lateral tendon, irrespective of whether the tendon was intact or torn (Table 3). The similar fibril characteristics of the medial region of torn and intact tendons parallels the findings of Magnusson et al., who observed that fibril diameters were similar between intact and ruptured Achilles tendons, but that the tendon rupture site showed a focal loss of larger collagen fibrils.15 For the lateral supraspinatus, area fraction - but not mean fibril diameter - was noted to be reduced in torn relative to intact tissues. We speculate that the larger diameter collagen fibrils as well as altered fibril diameter distribution of the medial region of torn tendons (Figures 3-5) may reflect adaptation to increasing functional demands in non-injured areas of tissue. Taken together, the aforementioned TEM findings appear to provide a structural basis for the superior suture retention properties of the medial aspect of torn supraspinatus tendons; namely, the larger collagen fibrils as well as greater fibril density of the medial relative to the lateral tendon may provide a more robust matrix for resisting suture migration.
Prior studies have described the complex mechanical behavior of the human supraspinatus tendon in its intact and repaired states, with the vast majority of investigators either preserving the tendon’s native insertion site on the greater tuberosity of the humeral head or following fixation via sutures to bone, respectively. Differential intratendinous tendon strain exists from the bursal to the articular surface, while surface strain near the insertion site exceeds that of the more proximal tendon regions1, 8; in both of these studies, strain magnitudes varied significantly with glenohumeral abduction. Recently, Lake et al.13 compared the material properties of specific tendon regions and reported that samples harvested from the medial supraspinatus were stiffer than those from the lateral region. Presently, it is unclear whether the latter results support the suture retention and ultrastructural findings of the current study, as the nature of testing in these two studies differs (tensile loading of tendon strips versus sutures pulled through an entire tendon).
More recently developed rotator cuff tendon fixation techniques such as double-row and transosseous equivalent repairs have been noted to promote significantly higher ultimate load, increased stability under cyclic loading, and decreased gap formation.11, 14, 19, 24, 28 While these experiments convey clinically important information, their results are a manifestation of multiple factors including bone quality, suture-tendon and anchor-bone interaction. By contrast, the present study sought to determine whether regional properties of the supraspinatus tendon’s collagenous matrix might provide an additional rationale for surgical repair technique. In light of the perceived improvement in structural properties resulting from a second row of sutures,11, 14, 24, 28 we postulate that the superior pullout resistance of medial row sutures may provide a strain shielding effect for the lateral row following double-row repair.
Our histolopathologic findings of collagen fiber splitting and disorganization as well as increased cellularity and vascularity in torn supraspinatus tendons are typical of light microscopic observations of ruptured tendons.10 However, no apparent differences in immunohistochemical staining for types I and III collagen were noted between medial and lateral regions of the torn tendons examined (Figure 6D-E). These observations contrast those of previous studies4, 17 which show an increase in type III collagen (with no increase in type I) at the rupture site of human Achilles tendons. A potential explanation for this discrepancy is that the tissues analyzed may represent different stages of repair, as collagen III production peaks in the early remodeling phase and is gradually replaced by collagen I23; furthermore, in the Achilles tendon studies samples were collected intraoperatively, within 48 hours after rupture, while the chronicity of the supraspinatus tears was unknown. Future studies examining biochemical properties such as collagen cross-linking or proteoglycan deposition may provide additional insight into the nature of the matrix-level biologic changes.
The mean donor ages of intact and torn specimens used for mechanical testing were similar (Table 1). Statistical correlation analyses revealed that donor age was a poor predictor of suture retention properties of both intact and torn samples (Figure 2); hence, we conclude that age was not a confounding variable for detecting differences between intact and torn samples. For TEM analyses, we elected to use a separate set of specimens due to concerns that the suture retention testing would damage the microstructure of the collagenous matrix. Our consistent observation during failure testing (in both pilot experiments as well as those of the formal study) was that tissue regions throughout the tendon underwent considerable elongation. Prior studies have reported that the threshold for fibril damage occurs at low levels of subfailure strain (i.e., 5.8% strain for rat MCL and 10% strain for rabbit MCL).20, 29 For TEM measurements, the mean donor age of the torn tendon specimens was noticeably higher than that of the intact group (Table 1), which constitutes a study limitation. However, based on our limited sample size, anatomic region appeared to have a greater influence on ultrastructural properties than the donor age (Table 3). It is possible that the TEM results reported herein reflect the combined effects of age and variability across human subjects.
Additional limitations of this study include the fact that, due to the destructive nature of suture testing, results from medial and lateral tendon regions were obtained from different tissue specimens. The use of cadaveric material precluded the comparative study of tissues pre- and post-tearing. In addition, all TEM specimens were harvested from the anterior aspect of the supraspinatus tendon. Anatomical studies have described the anterior tendon as a cord-like bundle that is longer and more narrow than the posterior tendon.12, 22, 27 While we are unaware of potential differences in ultrastructural characteristics between anterior and posterior tendon regions, it is likely that the anterior portion plays a more prominent functional role in vivo due to its superior tensile properties (when compared to middle and posterior thirds)9 and smaller amount of gapping relative to the posterior tendon following repair.14, 25 Indeed, during biomechanical testing, the posterior suture consistently pulled completely through the tendon prior to the anterior suture. Regarding our experimental setup, though a combination of simple and/or mattress suture configurations would likely be performed in clinical practice, all specimens were tested with only two simple sutures. Lastly, the current investigation did not examine responses to cyclic loading.
In summary, the present biomechanical data indicate that the medial portion of the torn supraspinatus tendon confers greater suture retention properties than the lateral region. The inferior resistance to suture migration might be attributable to reduced collagen fibril area fraction and/or generalized matrix degeneration. Importantly, the suture retention capacity of the medial supraspinatus appears to be preserved following a tendon tear. Collectively, these results may explain why double-row and transosseous equivalent rotator cuff repair techniques, which engage more medial tendon regions, confer greater fixation stability than that of a more traditional single-row approach.11, 14, 19, 24, 28 While clinical factors such as musculotendinous integrity (e.g., retraction and atrophy) warrant strong consideration for surgical decision making, our ultrastructural and biomechanical results appear to provide a scientific rationale for double-row rotator cuff repair.
Acknowledgements
Funding provided by Arthrex, Inc (BJC); Alpha Omega Alpha (ABY); and NIH/NIAMS T32 AR052272
REFERENCES
- 1.Bey MJ, Song HK, Wehrli FW, Soslowsky LJ. Intratendinous strain fields of the intact supraspinatus tendon: the effect of glenohumeral joint position and tendon region. J Orthop Res. 2002;20:869–74. doi: 10.1016/S0736-0266(01)00177-2. [DOI] [PubMed] [Google Scholar]
- 2.Clayton RA, Court-Brown CM. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury. 2008;39:1338–44. doi: 10.1016/j.injury.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Cole BJ, ElAttrache NS, Anbari A. Arthroscopic rotator cuff repairs: an anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy. 2007;23:662–9. doi: 10.1016/j.arthro.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 4.Eriksen HA, Pajala A, Leppilahti J, Risteli J. Increased content of type III collagen at the rupture site of human Achilles tendon. J Orthop Res. 2002;20:1352–7. doi: 10.1016/S0736-0266(02)00064-5. [DOI] [PubMed] [Google Scholar]
- 5.Fallon J, Blevins FT, Vogel K, Trotter J. Functional morphology of the supraspinatus tendon. J Orthop Res. 2002;20:920–6. doi: 10.1016/S0736-0266(02)00023-2. [DOI] [PubMed] [Google Scholar]
- 6.Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 2004;86:1973–82. doi: 10.2106/00004623-200409000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Huang CY, Wang VM, Flatow EL, Mow VC. Temperature-dependent viscoelastic properties of the human supraspinatus tendon. J Biomech. 2009;42:546–9. doi: 10.1016/j.jbiomech.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang CY, Wang VM, Pawluk RJ, Bucchieri JS, Levine WN, Bigliani LU, et al. Inhomogeneous mechanical behavior of the human supraspinatus tendon under uniaxial loading. J Orthop Res. 2005;23:924–30. doi: 10.1016/j.orthres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Itoi E, Berglund LJ, Grabowski JJ, Schultz FM, Growney ES, Morrey BF, et al. Tensile properties of the supraspinatus tendon. J Orthop Res. 1995;13:578–84. doi: 10.1002/jor.1100130413. [DOI] [PubMed] [Google Scholar]
- 10.Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507–25. [PubMed] [Google Scholar]
- 11.Kim DH, ElAttrache NS, Tibone JE, Jun BJ, DeLaMora SN, Kvitne RS, et al. Biomechanical comparison of a single-row versus double-row suture anchor technique for rotator cuff repair. Am J Sports Med. 2006;34:407–14. doi: 10.1177/0363546505281238. [DOI] [PubMed] [Google Scholar]
- 12.Kim SY, Boynton EL, Ravichandiran K, Fung LY, Bleakney R, Agur AM. Three-dimensional study of the musculotendinous architecture of supraspinatus and its functional correlations. Clin Anat. 2007;20:648–55. doi: 10.1002/ca.20469. [DOI] [PubMed] [Google Scholar]
- 13.Lake SP, Miller KS, Elliott DM, Soslowsky LJ. Effect of fiber distribution and realignment on the nonlinear and inhomogeneous mechanical properties of human supraspinatus tendon under longitudinal tensile loading. J Orthop Res. 2009;27:1596–602. doi: 10.1002/jor.20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma CB, Comerford L, Wilson J, Puttlitz CM. Biomechanical evaluation of arthroscopic rotator cuff repairs: double-row compared with single-row fixation. J Bone Joint Surg Am. 2006;88:403–10. doi: 10.2106/JBJS.D.02887. [DOI] [PubMed] [Google Scholar]
- 15.Magnusson SP, Qvortrup K, Larsen JO, Rosager S, Hanson P, Aagaard P, et al. Collagen fibril size and crimp morphology in ruptured and intact Achilles tendons. Matrix Biol. 2002;21:369–77. doi: 10.1016/s0945-053x(02)00011-2. [DOI] [PubMed] [Google Scholar]
- 16.Nho SJ, Shindle MK, Sherman SL, Freedman KB, Lyman S, MacGillivray JD. Systematic review of arthroscopic rotator cuff repair and mini-open rotator cuff repair. J Bone Joint Surg Am. 2007;89(Suppl 3):127–36. doi: 10.2106/JBJS.G.00583. [DOI] [PubMed] [Google Scholar]
- 17.Pajala A, Melkko J, Leppilahti J, Ohtonen P, Soini Y, Risteli J. Tenascin-C and type I and III collagen expression in total Achilles tendon rupture. An immunohistochemical study. Histol Histopathol. 2009;24:1207–11. doi: 10.14670/HH-24.1207. [DOI] [PubMed] [Google Scholar]
- 18.Park MC, ElAttrache NS, Tibone JE, Ahmad CS, Jun BJ, Lee TQ. Part I: Footprint contact characteristics for a transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16:461–8. doi: 10.1016/j.jse.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Park MC, Tibone JE, ElAttrache NS, Ahmad CS, Jun BJ, Lee TQ. Part II: Biomechanical assessment for a footprint-restoring transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16:469–76. doi: 10.1016/j.jse.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Provenzano PP, Heisey D, Hayashi K, Lakes R, Vanderby R., Jr. Subfailure damage in ligament: a structural and cellular evaluation. J Appl Physiol. 2002;92:362–71. doi: 10.1152/jappl.2002.92.1.362. [DOI] [PubMed] [Google Scholar]
- 21.Robinson PS, Lin TW, Jawad AF, Iozzo RV, Soslowsky LJ. Investigating tendon fascicle structure-function relationships in a transgenic-age mouse model using multiple regression models. Ann Biomed Eng. 2004;32:924–31. doi: 10.1023/b:abme.0000032455.78459.56. [DOI] [PubMed] [Google Scholar]
- 22.Roh MS, Wang VM, April EW, Pollock RG, Bigliani LU, Flatow EL. Anterior and posterior musculotendinous anatomy of the supraspinatus. J Shoulder Elbow Surg. 2000;9:436–40. doi: 10.1067/mse.2000.108387. [DOI] [PubMed] [Google Scholar]
- 23.Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87:187–202. doi: 10.2106/JBJS.D.01850. [DOI] [PubMed] [Google Scholar]
- 24.Smith CD, Alexander S, Hill AM, Huijsmans PE, Bull AM, Amis AA, et al. A biomechanical comparison of single and double-row fixation in arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2006;88:2425–31. doi: 10.2106/JBJS.E.00697. [DOI] [PubMed] [Google Scholar]
- 25.Tashjian RZ, Levanthal E, Spenciner DB, Green A, Fleming BC. Initial fixation strength of massive rotator cuff tears: in vitro comparison of single-row suture anchor and transosseous tunnel constructs. Arthroscopy. 2007;23:710–6. doi: 10.1016/j.arthro.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 26.Van Thiel GS, Wang VM, Wang FC, Nho SJ, Piasecki DP, Bach BR, Jr, et al. Biomechanical similarities among subscapularis repairs after shoulder arthroplasty. J.Shoulder Elbow Surg. 2010 doi: 10.1016/j.jse.2010.01.014. in press. [DOI] [PubMed] [Google Scholar]
- 27.Volk AG, Vangsness CT., Jr. An anatomic study of the supraspinatus muscle and tendon. Clin Orthop Relat Res. 2001;(384):280–5. doi: 10.1097/00003086-200103000-00032. [DOI] [PubMed] [Google Scholar]
- 28.Waltrip RL, Zheng N, Dugas JR, Andrews JR. Rotator cuff repair. A biomechanical comparison of three techniques. Am J Sports Med. 2003;31:493–7. doi: 10.1177/03635465030310040301. [DOI] [PubMed] [Google Scholar]
- 29.Yahia L, Brunet J, Labelle S, Rivard CH. A scanning electron microscopic study of rabbit ligaments under strain. Matrix. 1990;10:58–64. doi: 10.1016/s0934-8832(11)80138-1. [DOI] [PubMed] [Google Scholar]