Abstract
Context
Preclinical prediction of Alzheimer’s disease is important, critical to effective intervention. Plasma levels of amyloid β-peptides have been a principal focus of the growing literature on blood-based biomarkers, but studies to date have varied in design, assay methods and sample size, making it difficult to readily interpret the overall data.
Objective
To conduct a systematic review and meta-analysis of relevant prospective studies in order to determine if plasma amyloid β levels may predict development of dementia, Alzheimer’s disease, and cognitive decline.
Data Sources
Prospective studies published between 1995 and 2011 indexed in the PubMed, EMBASE, and PsycInfo databases were searched.
Study Selection
Selected studies included those measuring at least one relevant plasma amyloid β species (Aβ40, Aβ42, Aβ42:Aβ40 ratio) and reporting an effect estimate for dementia, Alzheimer’s disease, or cognitive change.
Data Extraction
Using a standardized extraction form, appropriate study parameters on subject information, exposure, and outcome were extracted. Random effects models were utilized to generate summary risk ratios and 95% confidence intervals, comparing the bottom versus top quantile for each plasma measure.
Results
Thirteen studies with a total of 10,303 subjects met inclusion criteria for meta-analysis. Lower Aβ42:Aβ40 ratios were significantly associated with development of Alzheimer’s disease (summary RR=1.60, 95% CI=1.04,2.46; p=0.03) and dementia (RR=1.67 95% CI=1.02,2.75; p=0.04). Significant heterogeneity was found for both summary estimates, which could not be explained by participants’ age, sex distribution, the study’s follow-up time, or year of publication. Plasma levels of Aβ40 and Aβ42 alone were not significantly associated with either outcome.
Conclusions
Overall, the literature indicates that plasma Aβ42:Aβ40 ratios predict development of Alzheimer’s disease and dementia. However, significant heterogeneity in the meta-analysis underlines the need for substantial further investigation of plasma amyloid β levels as a preclinical biomarker.
Introduction
An enormous public health burden is caused by senile dementia, with Alzheimer’s disease (AD) alone being the seventh leading cause of death in the United States and costing an estimated $172 billion annually1. Current therapies to treat AD are minimally effective and do not alter the disease process. It is widely believed that novel therapeutic agents expected to be developed in the coming years will be optimally administered preclinically, before patients develop full dementia. Thus, preclinical prediction of dementia through biomarkers is an important field, critical to effective intervention and disease modification2. Although the Alzheimer’s Association and the National Institute on Aging recently established research guidelines for identifying preclinical dementia using neuroimaging and cerebrospinal fluid (CSF) proteins3, a blood-based biomarker would be less invasive and more cost-effective than CSF or imaging-based methods. Moreover, a blood-based biomarker might also be used in a complementary role to CSF and imaging, as a first-step screen for high-risk individuals who would maximally benefit from these more invasive and expensive modalities.
Plasma levels of amyloid β-peptides have been a focus of the growing literature on blood-based biomarkers for dementia4–17, but studies to date have varied substantially in their design, assay methods and sample size - making it difficult to interpret the overall data. Therefore, we performed a systematic review and meta-analysis to evaluate the scientific literature, asking whether plasma Aβ levels predict development of dementia, including AD, and cognitive decline.
Methods
Search Strategy
Following a pre-established protocol, a systematic review was conducted by two investigators with methodological expertise (A.K. and F.G.) using a Boolean search strategy on the electronic databases MEDLINE, EMBASE, and PsycInfo. Keywords shown in eFigure 1 were used to search for the exposure and outcomes of interest, as well as to confine our search to epidemiological studies. Studies were limited to those published after 1995, due to the lack of well-developed Aβ assays before this time. The bibliographies of all relevant articles and review papers were also hand-searched; abstracts from major scientific meetings were also examined by the authors, and experts in the field consulted for any further studies.
Inclusion Criteria
Study selection was carried out in two stages, using the same inclusion criteria. The first stage involved reviewing only the title and abstract of each article, and the second stage involved reviewing the full text. For an article to be included in either stage, it had to fulfill four criteria for study quality: a prospective cohort (including case-cohort or nested case-control designs); measurement of the relevant plasma amyloid β species (Aβ40, Aβ42, and/or Aβ42:Aβ40 ratio); report of the relative risk or equivalent effect estimates for incident AD, total dementia, and/or mean differences in cognitive decline for studies of that outcome; be adjusted for age at a minimum. All languages were included in the searches.
Data Extraction
Data extraction was performed using a standardized extraction form. We extracted the following variables from each study: year of publication; study design; country of study population; name of cohort, exposures measured and variable coding method; outcomes measured and standard for diagnosis; length of followup; sample size; demographics (mean age at baseline, gender, ethnicity); effect measures, respective p values and confidence intervals and/or standard errors; number of cases in each group; covariates used in modeling.
Data Synthesis
For the analyses, odds ratios, incidence rate ratios, hazard ratios, or risk ratios for dichotomous outcomes were considered as equivalent effect measures18. For the sake of simplicity, these effect measures will hereafter be referred to as risk ratios (RR). We focused on data regarding Aβ42 and Aβ42:Aβ40, since these likely provide the most relevant information for risk prediction based on the existing literature. In addition, there is less biological rationale supporting the measurement of Aβ40 alone as a predictor of dementia; therefore, we evaluated those studies secondarily. In studies reporting plasma amyloid β-protein as a categorical variable, we considered the highest quantile as the reference group for our meta-analysis and generated a summary effect estimate for the comparison of the bottom versus the top quantile. These categorical analyses were considered, a priori, as our primary analyses for several reasons. First, because absolute measures of Aβ can differ widely between current plasma Aβ assays19, the categorical classification of Aβ is subject to less misclassification than a continuous variable. That is, while a continuous measure requires that each unit is appropriately estimated, an ordered categorical variable only requires that subjects are generally ranked correctly across three or four categories and thus yields less misclassification. Additionally, ordered categories are less susceptible to outliers of high levels of Aβ as well as very low levels that approach the detection limit of the assay, again resulting in less misclassification when using quantiles. Most importantly, in eventual clinical practice, it is most likely that Aβ will not be utilized as a continuous measure, but rather that threshold categories will be defined for different risk states. Finally, the majority of studies presented analyses of Aβ as a categorical variable. However, secondary analyses were also performed to derive a summary effect estimate from the incremental dose-response RR for each study, when available. The four studies reporting cognitive decline as an outcome4, 10 were not included in the meta-analysis due to large variations in the methods by which cognition was assessed, but are reviewed here qualitatively.
For the dementia outcomes (total dementia and incident AD), both fixed and random effects models were used to generate summary risk ratios across relevant studies. As results were similar using both models, only DerSimonian and Laird random effects estimates are presented20. Heterogeneity was assessed using the I2 statistic, and, if heterogeneity was found, we explored possible explanations using meta-regression models;21 we tested mean age, gender percentage, year of publication and follow-up time in the meta-regression models. We also conducted meta-analyses excluding certain studies with which were meaningfully different from other investigations in terms of sex. We could not conduct stratified analyses according to follow-up time, since this would have yielded strata with an insufficient number of studies to provide meaningful information in a summary estimate. To assess study quality, since many studies reported results from multiple regression models with minimal and maximal control for potential confounding factors, we conducted two separate meta-analyses of the least and most adjusted risk ratios, and the pooled estimates for each were compared for significant differences. Besides evaluating maximal control of confounding factors, we did not conduct additional analyses examining study quality, since our inclusion criteria (see above) already addressed many primary issues of study quality; that is, given our assessment of study quality as part of inclusion criteria, attempts to further stratify studies by quality within the meta-analysis would have resulted in strata with insufficient studies to yield meaningful summary estimates. Publication bias was assessed by means of the Egger test22, and was found to be nonsignificant for our primary meta-analyses of Aβ42 and Aβ42:Aβ40. All calculations were performed using STATA version 11 (StataCorp 2007, College Station, TX).
Results
Study Selection
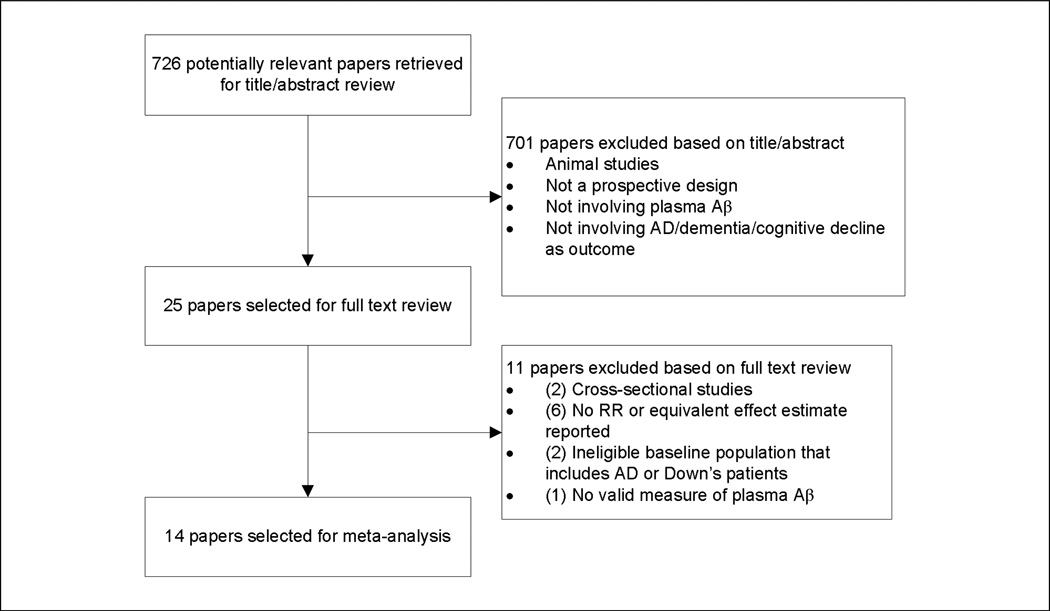
After the initial keyword search, there were 424 results from Pubmed, 82 from Embase, and 252 from PSYCInfo, for a total of 758 studies (Figure 1). After compilation of all studies into Endnote version X3 (Thomson Reuters, 2009, New York, NY), removal of duplicates resulted in 726 distinct studies. Two investigators (A.K. and F.G.) independently reviewed the remaining articles and after the first stage of study selection, 25 studies were identified for further consideration. After reviewing the full text of these articles, 14 publications remained which met all of our inclusion criteria.
Figure 1.
Study Selection
Description of Studies
Fourteen publications met inclusion criteria for meta-analysis. Of these, two publications were each included as two distinct studies in the meta-analysis rather than as one, because results in each of these publications were presented for two separate subcohorts9, 23. In addition, two publications utilized the same cohort, and only the more recent was included as it contained a larger sample14, 15. All studies in the meta-analysis were published in 2003 or after. All studies were prospective cohorts, although two were case-cohort studies. The total subject pool was largely female (48–69% across the studies), with one study comprised exclusively of females4 and another of males9.
Plasma Amyloid β-Protein and Dementia
1. Plasma Aβ42 and Ratio of Aβ42:Aβ40
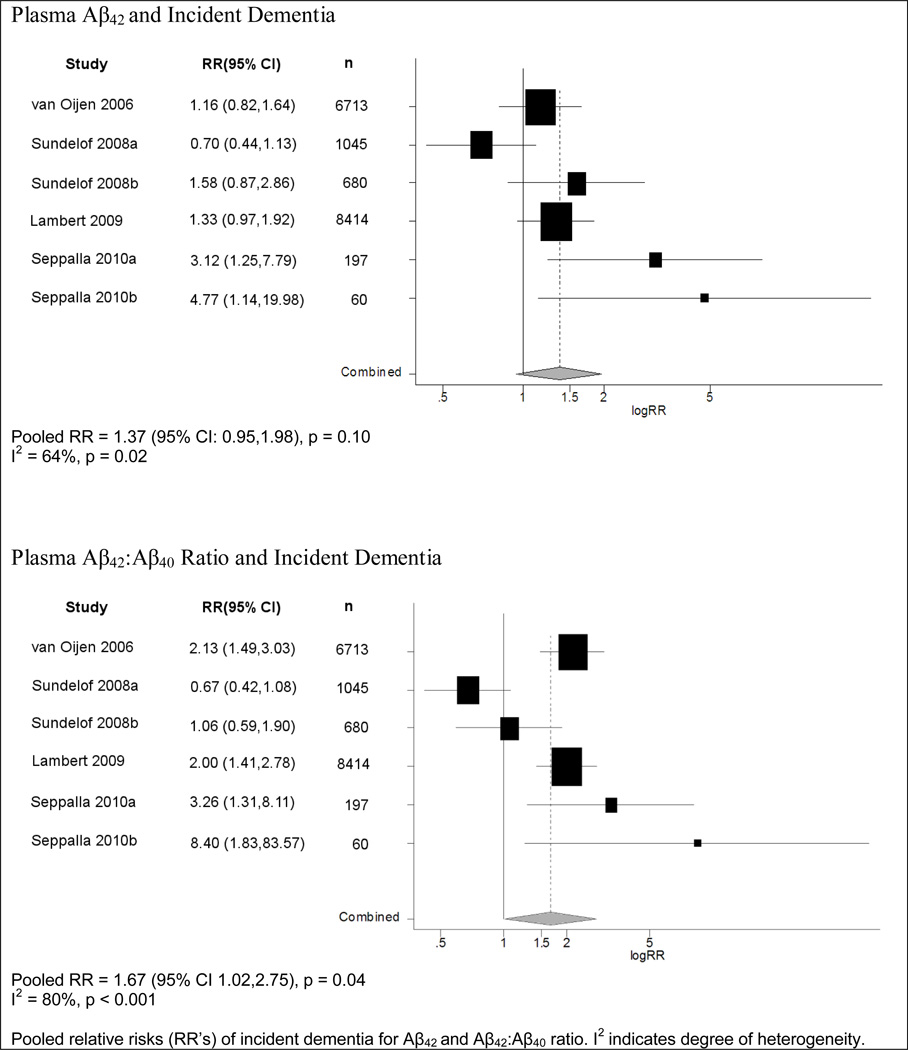
Six studies reported risk ratios for the association between Aβ42 levels and risk of dementia, and all used ordered categories of Aβ42 levels (Table 1). Of these, five reported increased risks of developing dementia for lower levels of Aβ42 in the least adjusted models, although only two were statistically significant23. The pooled risk ratio estimate across the studies was modest, and not statistically significant (summary RR=1.37; 95% CI=0.95,1.98; p=0.10) (Figure 2).
Table 1.
Baseline characteristics of studies
| Study | Design | Outcomes | No. at risk |
No. of Events |
Mean Age |
Sex (female) |
Follow- up time (yrs) |
Adjusted For | Dementia | AD | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dem. | AD | RR (Aβ42) |
RR (Aβ42:Aβ40) |
RR (Aβ42) |
RR (Aβ42:Aβ40) |
||||||||
| Abdullah 2009 | cohort | AD | 203 | -- | 14 | 76.8 | 48.3% | 2.1 (median) | age, sex, education | -- | -- | 4.47 (1.19,9.85) | 3.91 (1.36,11.24) |
| Graff-Radford 2007 | cohort | AD, cognitive change | 563 | -- | 17 | 78.0 (median) | 62.0% | 3.7 (median) | age, APOE | -- | -- | 1.18 (0.50,2.75) | 3.08 (1.12,8.3) |
| Lambert 2009 | case-cohort | AD, dementia | 8414 | 233 | 154 | 74.6 | 60.4% | 4 | age, gender, education, site | 1.33 (0.97,1.92) | 2.00 (1.41,2.78) | -- | 2.08 (1.30,3.23) |
| Mayeux 2003 | cohort | AD | 451 | -- | 86 | 76.2 | 68.9% | 5 | age, education, APOE, Aβ40 level, BMI | -- | -- | 0.4 (0.21,0.77) | -- |
| Schupf 2008 | cohort | AD | 1125 | -- | 104 | 76.9 | 68.3% | 4.6 (mean) | age, sex, APOE, education, ethnicity, BMI, cohort membership | -- | -- | 0.29 (0.12,0.71) | 1.11 (0.59,2.00) |
| Seppalla 2010a | cohort | Dementia | 197 | 9c | -- | 70.0a | 55.0%a | 3 | unknown | 3.12 (1.25,7.79) | 3.26 (1.31,8.11) | -- | -- |
| Seppalla 2010b | cohort | Dementia | 60 | 7c | -- | 70.0a | 55.0%a | 6 | unknown | 4.77 (1.14,19.98) | 8.4 (1.83,83.57) | -- | -- |
| Sundelof 2008a | cohort | AD, dementia | 1045 | 146 | 82 | 71.0 | 0.0% | 11.2 (median) | age, APOE | 0.7 (0.44,1.13) | 0.67 (0.42,1.08) | 0.85 (0.48,1.50) | 0.89 (0.54,1.48) |
| Sundelof 2008b | cohort | AD, dementia | 680 | 74 | 46 | 77.6 | 0.0% | 5.3 (median) | AD: age dementia: age, APOE, diabetes | 1.58 (0.87,2.86) | 1.06 (0.59,1.90) | 2.5 (1.04,6.03) | 1.27 (0.61,2.66) |
| van Oijen 2006b | case-cohort | AD, dementia | 6713 | 392 | 289 | 68.6 | 61.0% | 8.6 (mean) | age, sex | 1.16 (0.82,1.64) | 2.13 (1.49,3.03) | -- | -- |
Abbreviations: AD = Alzheimer’s disease; Aβ = Amyloid beta; APOE = Apolipoprotein E; BMI = Body Mass Index; Dem = Dementia
study population data is for entire cohort
only continuous RR reported for AD outcome
number of events estimated from published data
Note: confidence intervals reflect published results, before random-effects weighting
Figure 2.
Meta-Analysis of Plasma Aβ and Incident Dementia
Among the six studies investigating Aβ42:Aβ40 ratio, five reported an increased risk of developing dementia for the lowest Aβ42:Aβ40 ratios5, 12, 23 compared to the highest quantile; four of these found statistically significant increased relative risks. All studies reported the Aβ42:Aβ40 ratio in quantiles. A pooled analysis yielded a statistically significant RR of 1.67 (95% CI=1.02,2.75; p=0.04).
In all these meta-analyses of plasma Aβ and dementia, the pooled estimate did not change significantly when using results from the most adjusted models, or when excluding the study which only included men. Significant heterogeneity was found in the each of the above meta-analyses, which was not explained by age or gender distribution of the populations studied, or by followup time or year of publication. In a secondary analysis of Aβ42 as a continuous measure, results were consistent with those reported here for the quantile comparisons, although as expected, with increased misclassification of Aβ level in the continuous variable, the summary RRs were generally weaker.
2. Plasma Aβ40
Four studies reported effect estimates for the association between Aβ40 levels and risk of dementia, and all used categorical exposures5, 9, 12. A pooled analysis indicated no relation between Aβ40 and dementia development (RR=1.01; 95% CI: 0.60,1.71; p=0.97).
Plasma Amyloid β-Protein and Cognitive Decline
Four studies reported effect estimates for the association between plasma Aβ levels and cognitive decline4, 10, 24, 25. No association between Aβ42 levels and cognitive decline was found in two4, 10 of the studies. One study reported a statistically significant association between decreased baseline Aβ42 levels and subsequent cognitive decline25, while the remaining study also reported a significant association but for increased baseline Aβ42 levels24. Thus, findings for plasma Aβ42 as a predictor of cognitive decline were inconsistent. However, similar to the meta-analysis of dementia, three of four studies reported that lower Aβ42:Aβ40 ratio at baseline significantly predicted greater cognitive decline10. In addition, one of these studies further measured change over ten years of the Aβ42:Aβ40 ratio, reporting that a decrease in this ratio over time predicted greater subsequent cognitive decline4. The most recent study25 reported an interaction between plasma Aβ42:Aβ40 and cognitive reserve, such that the relation between Aβ42:Aβ40 and cognition was strongest in those with the least education. No other studies have examined such an interaction, although the Nurses’ Health Study4 found that Aβ42:Aβ40 predicted cognitive decline in a well-educated population of women.
Plasma Amyloid β-Protein and Alzheimer’s Disease
1. Plasma Aβ42 and Ratio of Aβ42:Aβ40
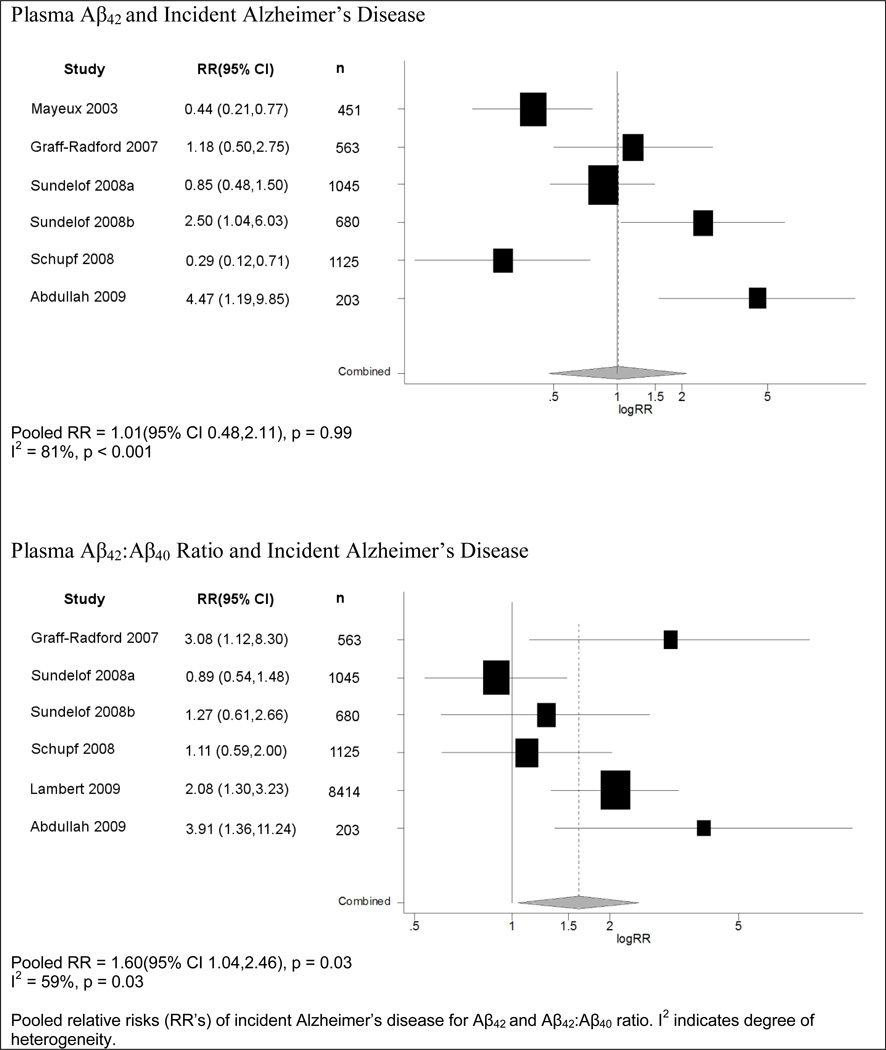
Nine studies reported risk ratios for plasma Aβ42 as a predictor of the development of clinically diagnosed AD. Six of these studies employed categorical exposures, and most of the studies were smaller in size than those of total dementia (Table 1). Results for Aβ42 were inconsistent: three studies reported risk ratios above 1.0 for lower baseline levels of Aβ42 (with 2 achieving statistical significance), while three reported risk ratios below 1.0 (with 2 achieving statistical significance). Reflecting these results, a pooled analysis (Figure 3) showed no relation between levels of Aβ42 and risk of developing AD (RR=1.01, 95% CI=0.48,2.11; p=0.99). Of the three studies reporting continuous levels of Aβ42, which were thus not included in the meta-analysis, one benefited from a fairly large sample (n=1756; 289 cases), but still observed a null result12. The two remaining studies showed a statistically significant decreased risk for lower levels of Aβ4226, although one was no longer significant in the maximally adjusted model8.
Figure 3.
Meta-Analysis of Plasma Aβ and Incident Alzheimer’s Disease
Among eight studies that reported risk ratios for plasma Aβ42:Aβ40 ratio and risk of developing AD, six employed ordered categories of Aβ42 (Table 1). These data were more consistent than for Aβ42 alone. Five of the six studies reported an increase in the likelihood of developing AD for the lowest compared to highest quantile of Aβ42:Aβ40 ratio, and three were statistically significant5, 6, 10. In our pooled analysis, there was a significant increase in risk of AD comparing the bottom versus top quantiles of Aβ42:Aβ40 ratios (RR=1.60, 95% CI=1.04,2.46; p=0.03) (Figure 3). Of the two studies not included in the meta-analysis due to the absence of categorical data on Aβ, one published null results8 while the other study reported that a lower ratio of Aβ42:Aβ40 was a significant predictor of a higher rate of AD development12, consistent with the findings of our meta-analysis.
In all meta-analyses of plasma Aβ species and AD, the pooled estimate did not significantly change when using results from the most adjusted models or when excluding the all-male cohort. Heterogeneity was found in the above meta-analyses with an I2 statistic of 64% for Aβ42 and 80% for Aβ42:Aβ40 ratio; thus, the summary risk ratios must be interpreted cautiously.
2. Plasma Aβ40
Seven studies reported effect estimates for plasma Aβ40 as a predictor of AD, of which four presented Aβ levels in ordered categories. Our meta-analysis found an elevated risk of AD with lower Aβ40, but the confidence interval was fairly wide and the summary estimate was not statistically significant (RR=1.66; 95% CI=0.98,2.83; p=0.06). Of the three studies not included in the meta-analysis, two reported null results6, 8, and one showed a statistically significant decreased risk of AD with lower levels of Aβ4012. Heterogeneity was not significant with an I2 statistic of 45% for Aβ40. Results remained consistent when Aβ40 was analyzed as a continuous exposure.
Discussion
In this systematic review, we examined the literature regarding plasma Aβ42, as well as the ratio of Aβ42:Aβ40, as predictors of dementia and AD. We found that plasma levels of Aβ42 alone were not strong predictors of dementia or AD risk, with non-significant risk ratios across studies of both these outcomes. In contrast, the data across studies of Aβ42:Aβ40 were more promising; we found a significant elevated risk for developing dementia or AD in subjects with lower Aβ42:Aβ40 ratios, with most studies reporting fairly similar findings. These results were robust to sensitivity analyses when using data from the most adjusted models reported, when analyzed as continuous levels rather than as ordered categories, and when the single study comprised of an all male cohort was excluded. No evidence for publication bias was found. Moreover, four studies reported results for cognitive decline as the outcome, and results from three of these were consistent with our meta-analysis in observing that a lower ratio of Aβ42:Aβ40 predicted worse cognitive decline. Collectively, the existing research offers cautious support of the hypothesis that lower levels of the plasma Aβ42:Aβ40 ratio reflect a process of selective deposition of Aβ42 in the brain as insoluble amyloid plaques, thus predictive of dementia development.
While we calculated summary risk ratios across studies to produce a quantitative estimate of effect from the existing data, a key limitation of our findings was the significant heterogeneity in each pooled estimate, necessitating caution in our interpretation of findings. We could not formally identify the source of heterogeneity, however we can hypothesize as to its causes. First, we suspect that much of the heterogeneity was likely the consequence of known measurement issues for plasma Aβ. The studies used in this meta-analysis employed varying ELISA assays and multiplex platforms, resulting in a wide distribution of median Aβ levels between studies. The development of a standardized assay is therefore highly important to achieve more comparable results in further research on plasma Aβ. Second, Aβ levels likely have differing implications at different stages in the pathogenesis of dementia, and the follow-up time varied considerably among studies; although meta-regression did not show this to significantly contribute to heterogeneity. Yet, variation of baseline Aβ levels and the subjects’ degree of underlying, preclinical dementia at baseline may have been important contributors to heterogeneity, regardless of follow-up time. Most studies in the meta-analysis did not assess baseline levels of mild cognitive impairment in participants, and criteria for pre-clinical AD have only recently been promoted3. Thus there was no clear means of evaluating any influence of varying levels of cognitive health at baseline. In two small studies which reported MCI prevalence, estimates ranged from 9.6%14 to 19.3%23, indicating there is likely wide variation in the level of early or underlying dementia across studies. Future research will be informed by more standard assessment of preclinical dementia both at baseline and during follow-up These issues can be addressed, in part, by measuring the relative change of Aβ levels at multiple time points, as opposed to a single baseline measurement. Some studies have already employed this temporal design and have been relatively consistent in showing significant associations between decreasing levels of Aβ42 or Aβ42:Aβ40 ratio over time and cognitive status4, 13, 14, 23, 24. Overall, our results emphasize the need for further research to better understand all of the issues pertaining to heterogeneity before plasma Aβ can be of broader predictive utility as a biomarker of impending dementia.
Other limitations of our meta-analysis should be considered. Results from individual studies are subject to potential unmeasured confounding and bias, and a meta-analysis cannot eliminate these issues (although we found similar results when using both minimally and maximally adjusted RR’s). Missing data could introduce bias if missingness is related to both the exposure and outcome, which is often likely, although the majority of studies reported reasonable follow-up rates. Additionally, some studies may be inappropriate to pool together. For example, one study9 included an all male cohort, potentially limiting the generalizability of this study, as women formed the majority of the other cohorts. However, excluding this study did not alter findings of our meta-analysis. Lastly, data extraction was not blinded, which may also be a source of bias, although this issue is debatable27.
There are numerous reasons why plasma Aβ is a particularly appealing biomarker: (1) most interventions currently under investigation for AD focus on manipulating Aβ levels, and thus an Aβ-based biomarker may be especially relevant for identifying those who will benefit if such treatments become available; (2) Aβ accumulation appears to be the initial step in AD pathogenesis28 and thus an Aβ-based biomarker should be especially suitable for identifying patients at the earliest stages of the disease process, when intervention will likely be most effective; and (3) a plasma-based biomarker is simple, inexpensive and non-invasive, all of which are important qualities for population-based screening tools.
In conclusion, despite the limitations of existing research and heterogeneity across the studies considered, this systematic review and meta-analysis suggests that the ratio of plasma Aβ42:Aβ40 may have value in predicting the risk of later development of dementia or AD and merits further investigation.
Supplementary Material
Table 2.
Baseline characteristics of studies (cont’d)
| Study | Design | Outcomes | No. at risk |
No. of Events |
Mean Age |
Sex (female) |
Follow- up time (yrs) |
Adjusted For | Reason not included | |
|---|---|---|---|---|---|---|---|---|---|---|
| Dem. | AD | |||||||||
| Blasko 2010 | cohort | AD | 406 | -- | 33 | 75.8 | 56.5% | 5 | sex, education, creatinine, smoking, stroke/infarction in MRI, sGDS score, interaction between Aβ42 and APOE | only continuous RR reported |
| Cosentino 2010 | cohort | cognitive change | 880 | -- | -- | 76.1 | 68.0% | 4.5 | age, sex, race, BMI, APOE, recruitment wave | cognitive change as outcome |
| Lopez 2008 | cohort | AD | 274 | -- | 88 | 79.3 | 60.9% | 4.5 (mean) | age | only continuous RR reported |
| Okereke 2009 | cohort | cognitive change | 481 | -- | -- | 63.6 | 100.0% | 10 | age, education, BMI, hypertension, dyslipidemia, heart disease, smoking, HT, physical activity, alcohol, depression | cognitive change as outcome |
| Yaffe 2011 | cohort | cognitive change | 997 | 72 | -- | 74.0 | 55.1% | 10 | age, race, education, diabetes, smoking, APOE | cognitive change as outcome |
Abbreviations: APOE = Apolipoprotein E; BMI = Body Mass Index; HT = postmenopausal hormone therapy; MRI = Magnetic Resonance Imaging; sGDS = short form of the Geriatric Depression Scale
Note: confidence intervals reflect published results, before random-effects weighting
Acknowledgement
This research was funded by National Institutes of Health Grant AG24215. The corresponding author had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis
References
- 1.2010 Alzheimer's disease facts and figures. Alzheimers Dement. 2010 Mar;6(2):158–194. doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Eschweiler GW, Leyhe T, Kloppel S, Hull M. New developments in the diagnosis of dementia. Dtsch Arztebl Int. 2010 Oct;107(39):677–683. doi: 10.3238/arztebl.2010.0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alzheimer's Association NIoA. Recommendations to Update Diagnostic Criteria. http://www.alz.org/research/diagnostic_criteria/. [Google Scholar]
- 4.Okereke OI, Xia W, Selkoe DJ, Grodstein F. Ten-year change in plasma amyloid beta levels and late-life cognitive decline. Arch Neurol. 2009 Oct;66(10):1247–1253. doi: 10.1001/archneurol.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambert JC, Schraen-Maschke S, Richard F, et al. Association of plasma amyloid beta with risk of dementia: the prospective Three-City Study. Neurology. 2009 Sep 15;73(11):847–853. doi: 10.1212/WNL.0b013e3181b78448. [DOI] [PubMed] [Google Scholar]
- 6.Abdullah L, Luis C, Paris D, et al. Serum Abeta levels as predictors of conversion to mild cognitive impairment/Alzheimer disease in an ADAPT subcohort. Mol Med. 2009 Nov-Dec;15(11–12):432–437. doi: 10.2119/molmed.2009.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schupf N, Tang MX, Fukuyama H, et al. Peripheral Abeta subspecies as risk biomarkers of Alzheimer's disease. Proc Natl Acad Sci U S A. 2008 Sep 16;105(37):14052–14057. doi: 10.1073/pnas.0805902105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez OL, Kuller LH, Mehta PD, et al. Plasma amyloid levels and the risk of AD in normal subjects in the Cardiovascular Health Study. Neurology. 2008 May 6;70(19):1664–1671. doi: 10.1212/01.wnl.0000306696.82017.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sundelof J, Giedraitis V, Irizarry MC, et al. Plasma beta amyloid and the risk of Alzheimer disease and dementia in elderly men: a prospective, population-based cohort study. Arch Neurol. 2008 Feb;65(2):256–263. doi: 10.1001/archneurol.2007.57. [DOI] [PubMed] [Google Scholar]
- 10.Graff-Radford NR, Crook JE, Lucas J, et al. Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol. 2007 Mar;64(3):354–362. doi: 10.1001/archneur.64.3.354. [DOI] [PubMed] [Google Scholar]
- 11.Blasko I, Jellinger K, Kemmler G, et al. Conversion from cognitive health to mild cognitive impairment and Alzheimer's disease: prediction by plasma amyloid beta 42, medial temporal lobe atrophy and homocysteine. Neurobiol Aging. 2008 Jan;29(1):1–11. doi: 10.1016/j.neurobiolaging.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 12.vanOijen M, Hofman A, Soares HD, Koudstaal PJ, Breteler MM. Plasma Abeta(1–40) and Abeta(1–42) and the risk of dementia: a prospective case-cohort study. Lancet Neurol. 2006 Aug;5(8):655–660. doi: 10.1016/S1474-4422(06)70501-4. [DOI] [PubMed] [Google Scholar]
- 13.Pomara N, Willoughby LM, Sidtis JJ, Mehta PD. Selective reductions in plasma Abeta 1–42 in healthy elderly subjects during longitudinal follow-up: a preliminary report. Am J Geriatr Psychiatry. 2005 Oct;13(10):914–917. doi: 10.1176/appi.ajgp.13.10.914. [DOI] [PubMed] [Google Scholar]
- 14.Mayeux R, Honig LS, Tang MX, et al. Plasma A[beta]40 and A[beta]42 and Alzheimer's disease: relation to age mortality, and risk. Neurology. 2003 Nov 11;61(9):1185–1190. doi: 10.1212/01.wnl.0000091890.32140.8f. [DOI] [PubMed] [Google Scholar]
- 15.Mayeux R, Tang MX, Jacobs DM, et al. Plasma amyloid beta-peptide 1–42 and incipient Alzheimer's disease. Ann Neurol. 1999 Sep;46(3):412–416. doi: 10.1002/1531-8249(199909)46:3<412::aid-ana19>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 16.Seppala TT, Herukka SK, Hanninen T, et al. Plasma A{beta}42 and A{beta}40 as markers of cognitive change in follow-up: a prospective, longitudinal, population-based cohort study. J Neurol Neurosurg Psychiatry. May 16; doi: 10.1136/jnnp.2010.205757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viswanathan A, Raj S, Greenberg SM, et al. Plasma Abeta, homocysteine, and cognition: the Vitamin Intervention for Stroke Prevention (VISP) trial. Neurology. 2009 Jan 20;72(3):268–272. doi: 10.1212/01.wnl.0000339486.63862.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992 Jun 1;135(11):1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 19.Okereke OI, Xia W, Irizarry MC, et al. Performance characteristics of plasma amyloid-beta 40 and 42 assays. J Alzheimers Dis. 2009 Feb;16(2):277–285. doi: 10.3233/JAD-2009-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986 Sep;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Smith GD, Altman DG. Systematic reviews in health care: meta-analysis in context. London: BMJ; 2001. [Google Scholar]
- 22.Egger M, DaveySmith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997 Sep 13;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seppala TT, Herukka SK, Hanninen T, et al. Plasma A{beta}42 and A{beta}40 as markers of cognitive change in follow-up: a prospective, longitudinal, population-based cohort study. J Neurol Neurosurg Psychiatry. 2010 May 16; doi: 10.1136/jnnp.2010.205757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cosentino SA, Stern Y, Sokolov E, et al. Plasma {beta}-Amyloid and Cognitive Decline. Arch Neurol. 2010 Aug 9; doi: 10.1001/archneurol.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yaffe K, Weston A, Graff-Radford N, et al. Association of Plasma β-Amyloid Level and Cognitive Reserve With Subsequent Cognitive Decline. JAMA. 2011;305(3):261–266. doi: 10.1001/jama.2010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blasko I, Kemmler G, Jungwirth S, et al. Plasma Amyloid Beta-42 Independently Predicts Both Late-Onset Depression and Alzheimer Disease. Am J Geriatr Psychiatry. 2010 May 7; doi: 10.1097/JGP.0b013e3181df48be. [DOI] [PubMed] [Google Scholar]
- 27.Berlin JA. Does blinding of readers affect the results of meta-analyses? University of Pennsylvania Meta-analysis Blinding Study Group. Lancet. 1997 Jul 19;350(9072):185–186. doi: 10.1016/s0140-6736(05)62352-5. [DOI] [PubMed] [Google Scholar]
- 28.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002 Jul 19;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.