Abstract
The coumarin (benzopyran-2-one, or chromen-2-one) ring system, present in natural products (such as the anticoagulant warfarin) that display interesting pharmacological properties, has intrigued chemists and medicinal chemists for decades to explore the natural coumarins or synthetic analogs for their applicability as drugs. Many molecules based on the coumarin ring system have been synthesized utilizing innovative synthetic techniques. The diversity oriented synthetic routes have led to interesting derivatives including the furanocoumarins, pyranocoumarins, and coumarin sulfamates (COUMATES), which have been found to be useful in photochemotherapy, antitumor and anti-HIV therapy, and as stimulants for central nervous system, antibacterials, anti-inflammatory, anti-coagulants, and dyes. Of particular interest in breast cancer chemotherapy, some coumarins and their active metabolite 7-hydroxycoumarin analogs have shown sulfatase and aromatase inhibitory activities. Coumarin based selective estrogen receptor modulators (SERMs) and coumarin-estrogen conjugates have also been described as potential antibreast cancer agents. Since breast cancer is the second leading cause of death in American women behind lung cancer, there is a strong impetus to identify potential new drug treatments for breast cancer. Therefore, the objective of this review is to focus on important coumarin analogs with antibreast cancer activities, highlight their mechanisms of action and structure-activity relationships on selected receptors in breast tissues, and the different methods that have been applied in the construction of these pharmacologically important coumarin analogs.
Keywords: Coumarin, breast cancer, sulfatase inhibitor, aromatase inhibitor
1. INTRODUCTION
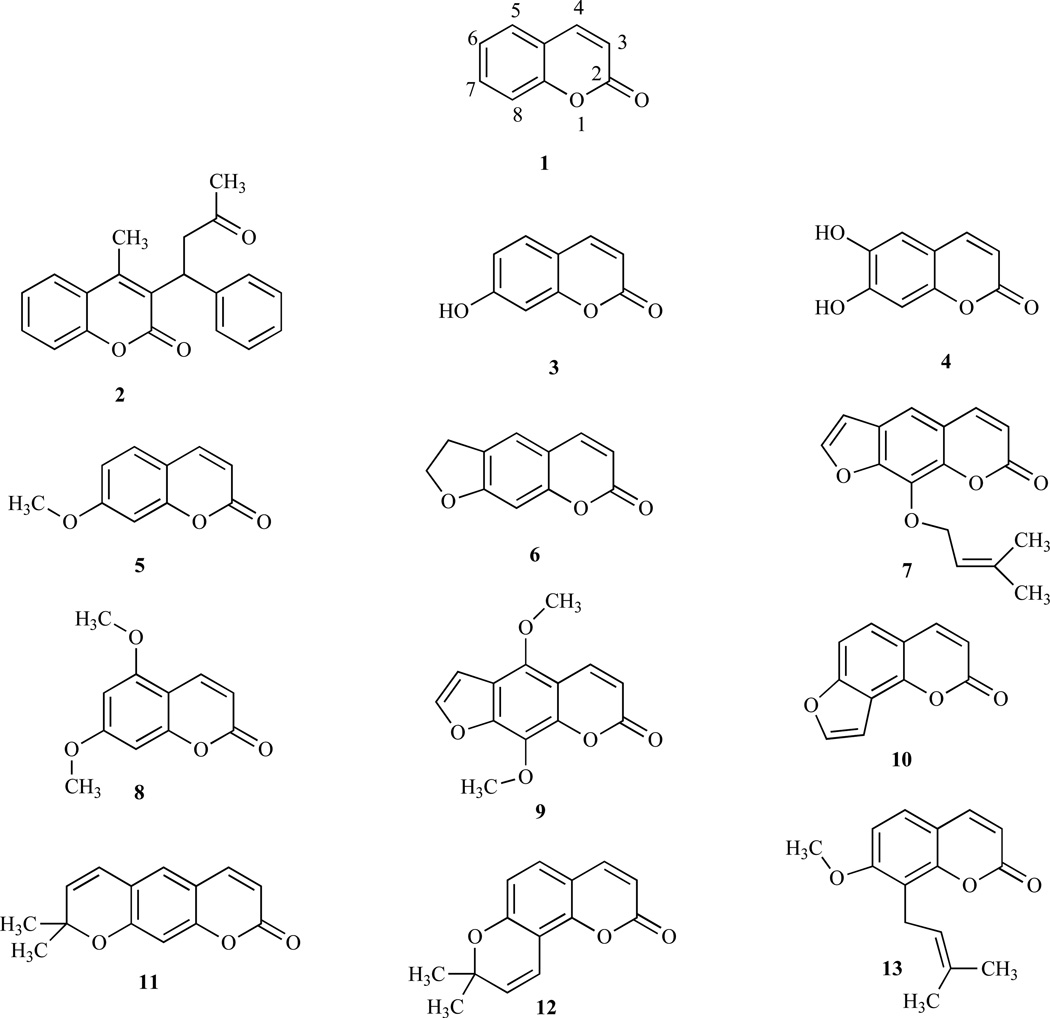
Coumarin (1,2-Benzopyrone or 2H-1-benzopyran-2-one, or phenylpropanoids, 1) and its derivatives (coumarins) are widely distributed throughout nature and many exhibit useful and diverse biological activities [1, 2]. Coumarins occur as secondary metabolites in the seeds, roots and leaves of many plant species, notably in high concentration in the tonka bean and thus the name comes from a French word, coumarou, for the tonka bean. Their function is far from clear, although suggestions include plant growth regulations, fungistasis, bacteriostasis and, even, waste products [3]. Some naturally occurring coumarin derivatives include warfarin (2), umbelliferone (7-hydroxycoumarin, 3), aesculetin (6,7-dihydroxycoumarin, 4), herniarin (7-methoxycoumarin, 5), psoralen (6) and imperatorin (7). Now the diversity of coumarin derivatives, both natural and synthetic, has grown and are thus divided into several subclasses. Most reviews classify coumarins according to whether particular compounds are simple coumarins (e.g. coumarin, 1 and limettin, 8), linear furanocoumarins (e.g. imperatorin, 7 and isopimpinellin, 9), angular furanocoumarins (e.g. angelicin, 10), linear pyranocoumarins (e.g. xanthyletin, 11) or angular pyranocoumarins (e.g. seselin, 12) [4]. Murray et al. [5], however, used a biogenetic approach based upon the number of nuclear oxygen atoms in classifying coumarin-containing compounds.
Coumarin derivatives have been found to have numerous therapeutic applications including photochemotherapy, antitumor and anti-HIV therapy [6, 7], and as central nervous system (CNS) stimulants [8], antibacterials [9, 10] antiinflammatory [11], anti-coagulants [12] and dyes [13]. In addition, coumarins are known to be lipid lowering agents with moderate triglyceride lowering activity [14]. Furthermore, hydroxycoumarins, powerful chain-breaking antioxidants and can prevent free radical injury by scavenging reactive oxygen species [15]. Some of the coumarin derivatives formerly used as fixative and flavoring agents, are now regulated as food adulterants by the Food and Drug Administration (FDA) in the United States due to their adverse effects such as mild nausea, diarrhea, and hepatotoxicity when used in certain amounts [16–19]. Although currently marketed in several European countries, coumarin type drugs such as coumarin (1), used for the treatment of lymphoedema, has not been approved for therapeutic purposes in the United States, due to their hepatotoxicity. However, recent discovery of coumarins having weak estrogenic activity resulted in the use of such derivatives as therapeutic agents in preventing the emergence of menopause related diseases, such as osteoporosis, increased risk for cardiovascular event / disease and cognitive deficiencies [20].
The pattern of substitutions on the basic chemical structure is said to influence both the coumarin’s pharmacological and biochemical properties, including the therapeutic applications, and can beneficially affect toxicity (Table 1) [21–23]. For example, introduction of a methoxy group at the 7-position and a 3-methyl-2-butenyl group at the 8-position of osthole (13) led to a strong reduction of plasma alkaline tranferase (ALT) level in hepatitis and inhibition of caspase-3 activation [24]. Some coumarins display cytostatic properties (growth-inhibitory) while others have cytotoxic activities [25]. For example, coumarin and its active metabolite, 7-hydroxycoumarin, demonstrated growth-inhibitory cytostatic activity in human cancer cell lines, such as A549 (lung), ACHN (renal), H727 (lung), MCF-7 (breast) and HL-60 (leukemia), and have also been reported to demonstrate activity against prostate cancer, malignant melanoma, and metastatic renal cell carcinoma in clinical trials [26–29]. Furthermore, the substituted benzopyranobenzothiazinones expressed estrogenic activity on MCF-7 breast carcinoma cells [30]. It has been demonstrated that the incorporation of a catechol to the basic structure of coumarin would increase cytotoxic activity in tumor cell lines [31]. Naturally occurring coumarins (NOCs), (e.g. 3 and 8) induce mouse skin tumor initiation in a well-established model of multistage carcinogenesis [32–36].
Table 1.
Some Biologically Active Coumarins
| Examples | Biological Properties |
|---|---|
 |
Anticoagulant |
 |
Anti-HIV (Reverse transcriptase inhibitors) |
 |
Antibiotic and Antibacterial (DNA gyrase inhibitors) |
 |
Anticancer (Breast cancer, Stomach cancer, Colon cancer and Renal cancer) |
Among the diverse biological activities of coumarins, the most intriguing is the notable effect of some of the coumarins against breast cancer. Studies have shown that compound 3 as well as 4-hydroxycoumarin inhibited cell proliferation in a gastric carcinoma cell line [37]. An in vitro proliferation assay of the mechanism of action of coumarins on the growth and metabolism of human tumor cells e.g. MCF-7 and A549 confirmed that coumarin itself is not responsible for the observed in vivo effects, but it is a prodrug of other active metabolites [38]. Previous studies have indicated that the ortho-dihydroxycoumarins (e.g. 4) or meta-dihydroxycoumarins possessed more potent cytotoxicity in human tumor cell lines than any of the mono-hydroxycoumarins [39,40].
2. COUMARINS AND BREAST CANCER
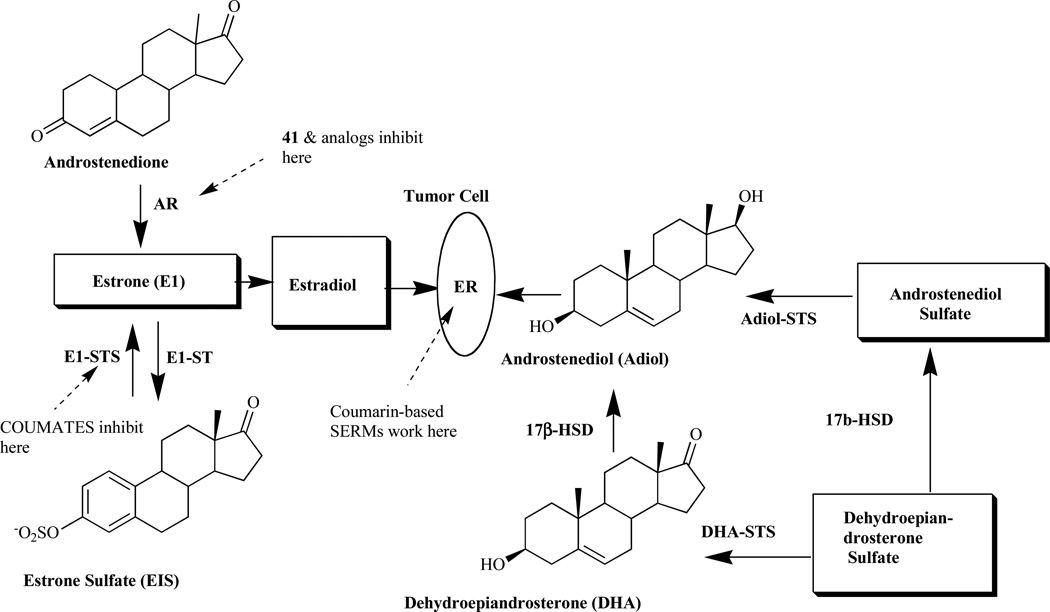
Breast cancer is a major cause of mortality in western countries and it has been reported that about one-third of postmenopausal breast cancer patients have hormone-dependent tumors involving the stimulation of estrogen receptor [41]. Treatment as well as prevention has been the focus of much laboratory work and clinical trials over the past 30 years. Clinical studies focusing on the use of therapeutic agents that prevent the synthesis and action of estrogens (ER antagonists) are known to be very successful in the treatment of breast cancer [42]. The current strategy thus involves the development of ER antagonists as a new approach for the treatment of postmenopausal women with hormone-dependent breast tumors. The high levels of estrogen as a result of its in situ synthesis are associated with the growth of tumors in endocrine-dependent tissues. Estrogens are formed exclusively in peripheral tissues, and there are two pathways associated with their synthesis in such tissues, the aromatase and sulfatase pathways (Fig. 2).
Fig. (2).
Estrogenic steroid origins showing the sites of action of coumarins AR, aromatase; ST, sulfotransferase; STS, sulfatase; 17(β-HSD, 17β-hydroxysteroid dehydrogenase; ER, estrogen receptor) [43].
The aromatase pathway involves the conversion of androgen precursor, androstenedione, secreted mainly by the adrenal cortex, to estrone by the aromatase (AR) enzyme complex, while the estrone sulfatase pathways (E1-STS) involves the conversion of estrone formed via the aromatase route to estrone sulfate (E1S) by the sulfotransferase enzymes [44]. In the breast tumors, activity of the latter enzyme is higher than that of the former, resulting in poor prognosis [45–48]. The E1-STS pathway is considered the major source leading to estrogen formation, causing low response rate in ER+ breast tumor patients to highly potent AR inhibitor [49–52]. Furthermore, studies have shown that endocrine therapy involving the inhibition of enzymes within the steroid biosynthetic cascade may be one route to controlling the disease. This approach has led to the development of novel coumarins as STS [53] as well as AR inhibitors [54].
2.1. Sulfatase Inhibitor
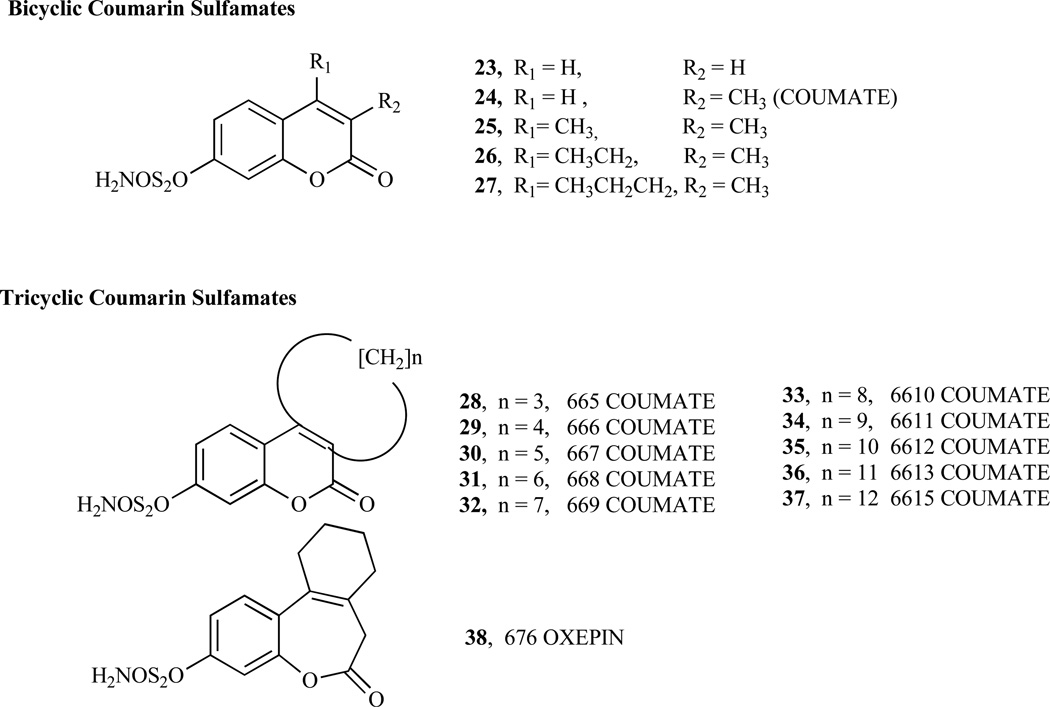
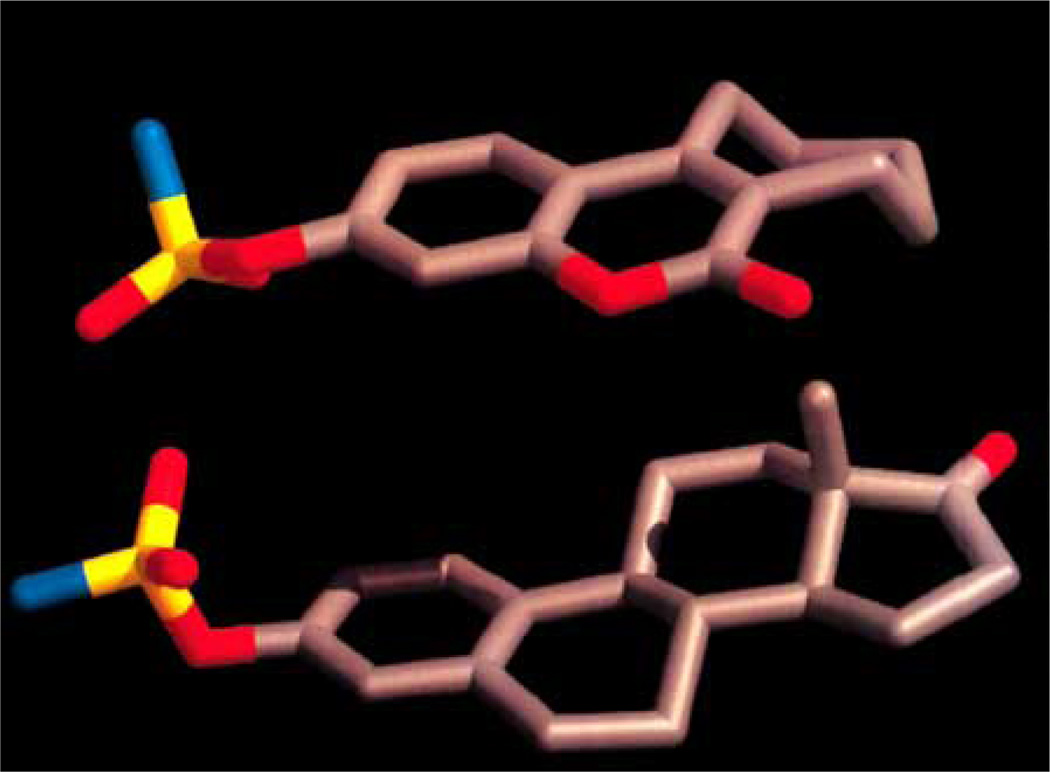
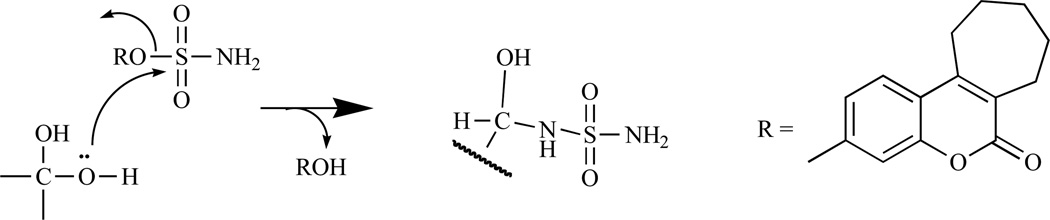
As illustrated in Fig. (2), the cleavage of sulfated steroid hormone precursors, e.g. estrone sulfate, to the active hormones by STS represents the first step in the local production of estrogen and androgens. Therefore, the inhibition of this enzyme (STS), which should decrease the biosynthesis of active hormones, has been a new therapeutic option in the treatment for hormone-dependent diseases [55–58] such as breast, endometrial and prostate cancers, acne and androgenic alopecia [53, 59–61]. Since STS catalyzes the hydrolysis of sulfate monoester bonds in a range of physiological substrates, the incorporation of a sulfamate ester group linked to an aryl ring was considered to be key strategy in the development of potent STS inhibitors [42, 62–65]. Furthermore, attempt to identify non-steroidal STS inhibitors led to the development of various bicyclic and tricyclic coumarin sulfamates, which are active both in vitro and in vivo (Fig. 3) [66–70]. A number of potential STS inhibitors are still in preclinical phases of development with only 667 COUMATE (30) [71] set to enter clinical trials for the treatment of hormone-dependent breast cancer in postmenopausal women.
Fig. (3).
Structures of coumarin sulfamates and tricyclic coumarin sulfamates.
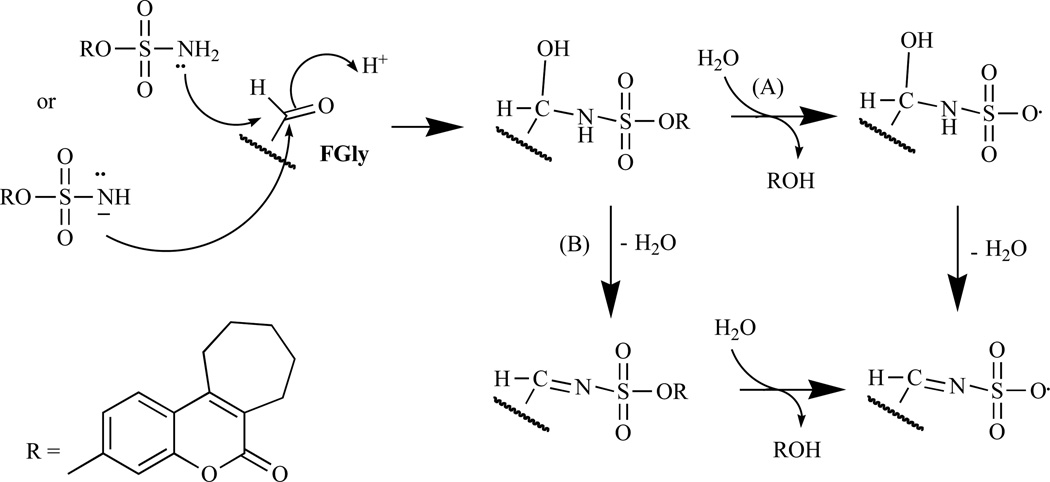
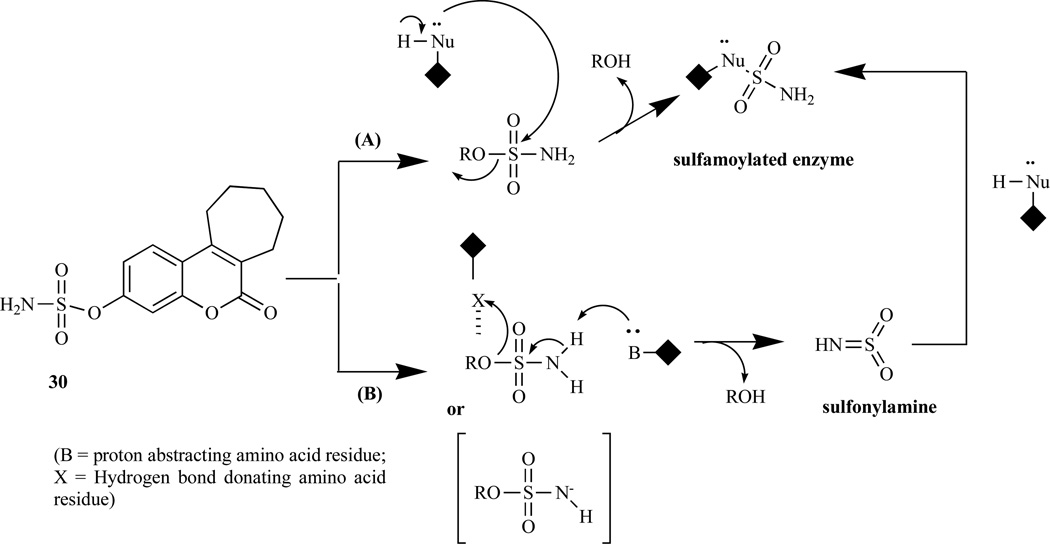
Chemically, bicyclic and tricyclic COUMATES (23–37) and their close relatives the 676 OXEPINs (e.g. 38) are aryl sulfamates (Ar-SO2NH2), which are irreversible inhibitors of STS. The crystal structures of the soluble enzymes arylsulfatase A (ASA) and arylsulfatase B (ASB) [72, 73] have provided an insight in the mechanism through which sulfamate group irreversibly inactivates steroid STS. The mechanisms for irreversibly inhibiting STS activity by COUMATES and its congeners (Fig. 4–6) involve: i) the L-Cα-formylglycine (FGly), ii) the aldehyde hydrate (gem-diol residue), and iii) random specific or nonspecific sulfamoylation of amino acid residues in the active site [62, 63, 74–78]. Random specific or nonspecific sulfamoylation is proposed to occur via two mechanisms – a direct nucleophilic attack by the amino acid residue at the sulfur atom of, for example, compound 30 and elimination of sulfamic acid by an E1cB mechanism, assisted by the extended conjugation present in the coumarin ring [63,79].
Fig. (4).
The proposed mechanism of STS inhibition by (30) involving Fgly in the enzyme active site [63].
Fig. (6).
The proposed random specific or non-specific sulfamoylation by (30). Path A: a direct nucleophilic attack by the amino acid residue at the sulfur atom. Path B: elimination of sulfamic acid by an E1cB mechanism [63].
Structure-activity relationship (SAR) studies of the “locking effect” of the lactone ring structure of the COU-MATE by Ahmed et al. confirmed that conformational restriction of the conjugated C=C bond plays an important role in the potency inhibitory activity displayed by coumarinbased compounds, in addition to the overall size of the inhibitors [80]. The pKa value of compound 30 is 9.1 in methanol/water (1:1), making it possible to exist in monoanionic form under physiological condition [63]. Compound 30 (Fig. 3) is a non-steroidal irreversible STS inhibitor and is the most potent of a series of tricyclic COUMATE developed with annulated carbocylic ring systems at positions 3 and 4 [41, 61, 68] with a very acceptable toxicological profile. It has an equipotent in vivo STS activity compared to estrone-3-O-sulfamate (EMATE), a potent, active sitedirected, irreversible steroidal STS inhibitor [81]. Moreover, Compound 30 is reported to be active both in vitro and in vivo [67] in causing significant regression of E1S-stimulated tumor growth without any sign for estrogenic effect [82].
SAR studies involving bicyclic COUMATES revealed that compound 25 (Fig. 3) displays stronger binding affinity for the enzyme active site via a hydrophobic interaction provided by the methyl groups at the 3- and 4- positions, thereby mimicking the A/B ring of EMATE, [81, 82]. Bilban et al. [83] demonstrated that the oxygen functionality substitution at position 7 of the coumarin core structure also mimics the A/B ring of EMATE. Previous computer-modelling study has shown that the seven-membered ring (third ring) of COUMATE (30) could not be described as closely mimicking the C/D ring regions of EMATE, attributed to its chair conformation form which is similar to the cycloheptene ring structure (Fig. 7) [63, 84]. However, recent finding indicated that the third ring appears to be predominately in the boat conformation rather than previously proposed chair conformation based on temperature factors (B-factors) and electron density map results [79]. With the advent of this new finding regarding the conformation of the 7-membered ring on compound 30 it can now be explained that its higher potency (IC50 value of 8 nM and Ki value of 40 nM) than EMATE (IC50 = 25 nM and Ki = 670 nM) is attributed to the tendency to mimic the steroidal CD-ring; perhaps, better hydrophobic interactions due to favorable binding to the active site of the enzyme are in play [61, 85, 86].
Fig. (7).
Molecular modelling of 667 COUMATE (top) and EMATE (bottom) (adapted from [63] with permission).
Benzocoumarin sulfamates (e.g. 39 and 40), another group of aryl sulfamates closely related to compound 30, mimics the ABC-ring of the steroidal skeleton. Although less active than EMATE, these sulfamates show high inhibitory potency due to strong binding of the benzocoumarin core structure to the enzyme. Removal or disruption of the coumarin ring conjugation results in lower potency [42, 69, 87] due to the resulting higher pKa value for the parent phenol. The extended conjugation present in the coumarin ring structure assists in the S-O-Ar bond breakage during E1-STS catalyzed sulfamoylation by improving the leaving ability of the coumarin compound as a result of lower pKa value of the phenol [63]. On the contrary, an extension of the coumarin conjugation core structure and the relocation of the sulfamate group to the 6-position of the ring resulted in lower potency exhibited by the COUMATE analogs [42].
2.2. Aromatase Inhibitor
Aromatase inhibitors (AIs) include drugs that are currently used for the treatment of hormone-dependent breast cancer, which involves blocking the estrogen action on tumor cells by preventing the biosynthesis of estrogen [88]. AI prevents breast cancer via reduction of cell proliferation, which involves reduction of estrogen level and thus prevention of the formation of genotoxic metabolites of estrogen. The genotoxic estrogen metabolites include i) catechol estrogens, which bind covalently to DNA and induce mutations that initiate cancer; ii) 2-hydroxyl-estradiol, which forms stable DNA adduct; and iii) 4-hydroxy-estradiol, which is a potential carcinogenic metabolite forming depurinating estrogen-DNA adducts with guanine base that are unstable and are rapidly lost from the DNA [89–91]. Clinical trial results have shown AIs to be of superior efficacy to tamoxifen as anti-estrogenic compounds with more favorable toxicity profiles [92–94]. In postmenopausal women, AIs have the potential to suppress circulating estrogen levels by approximately >96.7–98.9% and also abrogate autocrine and paracrine estrogen production by peri-tumoral stromal cells located in both primary and metastatic sites of the tumor [95–101]. The FDA has approved a number of AIs, e.g., anastrozole (Arimidex), exemestana (Femara) and letrozole (Aromasin), as first-line agents for the treatment of postmenopausal women with hormone receptor positive breast cancer [102–106]. In postmenopausal women, clinical results have shown that AIs used only as monotherapy are very effective in treating estrogen-dependent and aromatase-mediated diseases including breast cancer [107]. However, in premenopausal women there is an incomplete blockade of estrogen synthesis resulting in a reflux rise in gonadotrophin level, which in turn can stimulate ovarian aromatase and overcome the estrogen suppression [108].
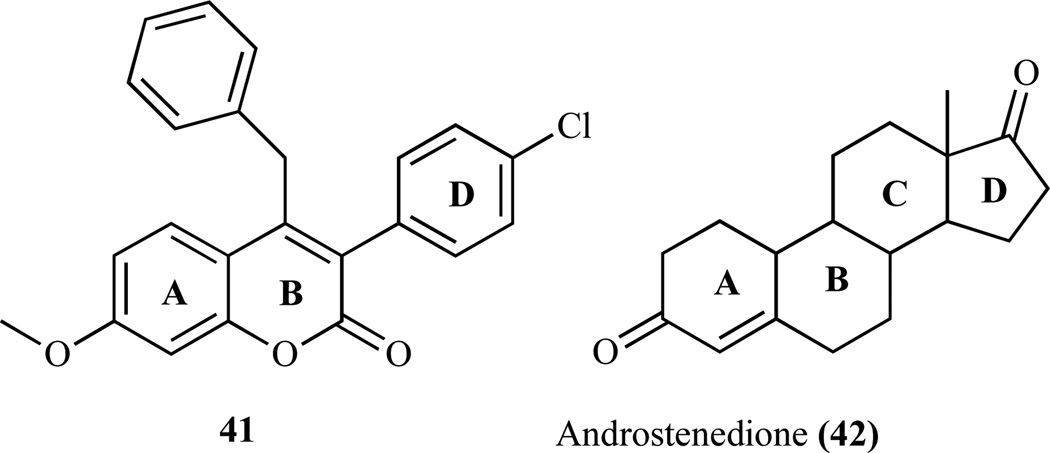
As discussed in previous section, several coumarin derivatives have been reported to be steroid STS inhibitors and evaluated for breast cancer therapy [53, 109]; however, clear evidence of AIs as antibreast cancer agent has not been demonstrated yet. Studies have shown benzopyranone substrates such as 4-benzyl-3-(4’-chlorophenyl)-7-methoxy-coumarin (41) to be a more potent competitive AI than several known AIs such as aminoglutethimide with respect to the androgen substrate. The specific interaction of compound 41 with the AR shows a further reduction by several mutations in binding at the active site region of AR. In addition, compound 41 suppressed the proliferation of AR and ER positive MCF-7 breast cancer cells. It is neither cytotoxic at concentrations up to 40 µM nor an inhibitor of steroid 5α-reductase and also is not a ligand of ERs, ERα and ERβ, or androgen receptor. Thus demonstrating that coumarin derivatives, which are potent inhibitors of aromatase, may not be cytotoxic yet can be useful in the suppression of AR and ER-positive breast tumors.
The SAR studies of (41) revealed that coumarin rings mimic the A and B rings, while the 3-(4-chlorophenyl) group 2670 mimics the D ring of the androgen (42) (Fig. 8) [54]. The methyl group at the C-19 of the androgen substrate, where the first and second hydroxylation reactions take place, points toward the heme group of AR [110]. The spatial orientation of the 4-benzyl group of the coumarin showed that it is aligned very closely to the methyl group at the C-19 position of the substrate. This behavior is attributed to the bending of the benzyl group (through the methylene group), resulting in overlay of the ring on top of the 3-(4-chlorophenyl) group. Furthermore, the 7-methoxyl group of coumarin (41) also aligns very closely with the C-3 keto oxygen of the substrate. These groups- 3-(4-chlorophenyl), 7-methoxyl and 4-benzyl- share the same physicochemical feature that is being hydrogen bond donor groups and are superimposable. However, result has demonstrated that the replacement of the 7-methoxyl group with 7-hydroxyl group on the coumarin significantly decreased the inhibitory activity against AR. A recent study has demonstrated that the presence of an electron-withdrawing group in the phenyl group, e.g. 3-(4-chlorophenyl) group, increase activity as a result of such group alignment that mimics the C-17 keto oxygen of estrone [54]. Thus it is strongly suggested that 7-methoxyl, 3-(4-chlorophenyl) and 4-benzyl functional groups are very critical for the anti-AR activity of the coumarin derivative [54, 111, 112]. For example, the IC50 value of 4-benzyl-3-(4-chlorophenyl)-7-methoxycoumarin is 80 nM and the replacements of any of the three mentioned functional groups resulted to reduction in potency, e.g. 4-benzyl-3-(4’-chlorophenyl)-7-hydroxycoumarin (IC50 300 nM). A proposed pharmacopore hypothesis indicated that the methoxy group might act as hydrogen bonding acceptor depending on its spatial position relative to the nitrogen atom coordinating the iron (II) [112], although there are varieties of possible binding modes, which may differ from those of the unsubstituted coumarin derivatives. The carbonyl group of the coumarin lactone ring is another structural determinant for the observed coumarins’ AR inhibitory activity. Also, a favorable interaction at the 7-position of the coumarin ring is due to the formation of a hydrogen bond by the 7-oxygen atom and the lipophilic or π-π stacking interaction of the phenoxy or benzyloxy group.
Fig. (8).
Proposed pharmacophores of AR inhibiting coumarins [54].
2.3. Coumarin-based SERMs
Selective estrogen receptor modulators (SERMs) are a new category of therapeutic agents that are used for the prevention and in the treatment of diseases such as osteoporosis and uterus and breast cancers [113, 114]. They are known to have high affinity for ER, but no specific affinity for any other steroid hormone receptors. In addition, SERMs are known to stimulate estrogenic actions (ER agonist) in tissues, such as the bone, liver, and cardiovascular system but block estrogen action at other sites (ER antagonist) where stimulation is considered undesirable, such as the breast and uterus [115–119]. This agonistic or antagonistic activity causes different conformational changes of the receptors particularly at the helix 12 [119–121], resulting in activation (transactivation) or repression (transrepression) of the estrogen target genes [120]. Examples of drugs classified as SERMs are: estrogen metabolites, clomiphene, tamoxifen, toremifene, idoxifene and droloxifene [121–123]. Tamoxifen is the most widely used hormonal therapy for breast cancer today.
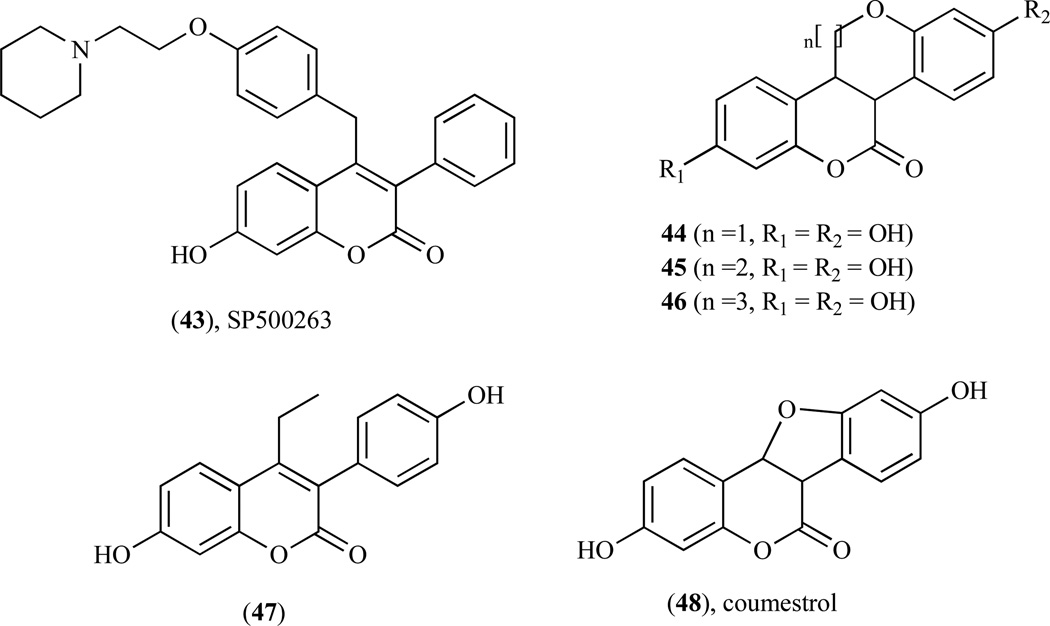
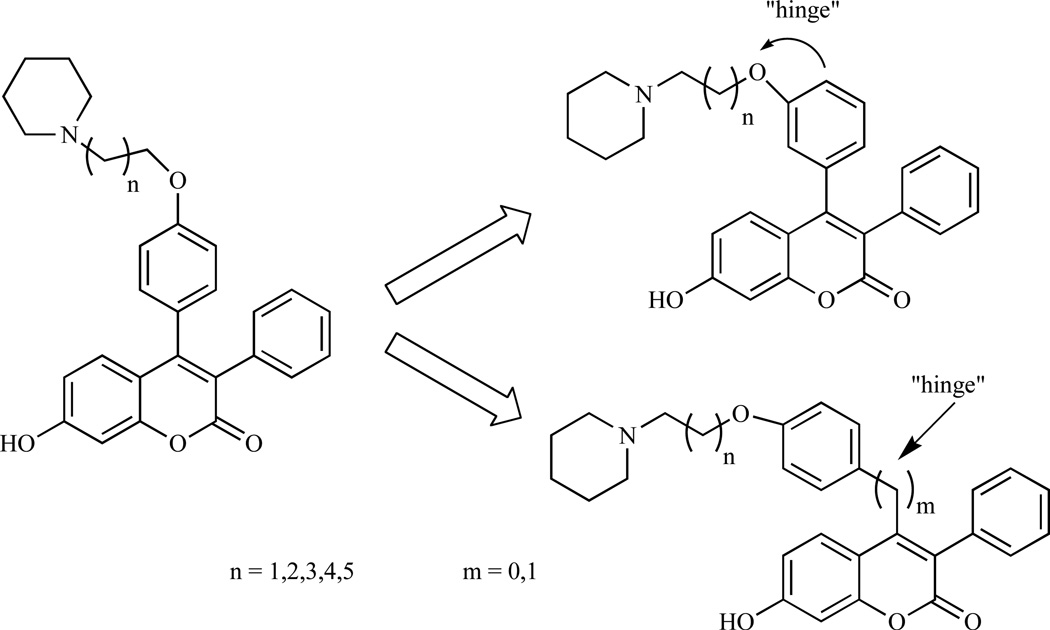
Compound 43 (Fig. 9) belongs to a new category of SERM called benzopyranone molecules or coumarin-based SERM, producing similar effects as other SERMs indicated above [124, 125]. However, it possesses a different structural feature from those of an ideal SERM such as tamoxifen and thus may induce a different conformational change to the ER, resulting in different cofactor recruitment [126–128]. Furthermore, compound 43 does not activate genes through estrogen response element (ERE). It is known to bind with high affinity through ERα, effectively antagonize estrogen action in ERα–expressing breast cancer cells by inhibiting IL-6 and GM-CSF gene expression and thus functions as potent antiestrogen in both in vitro and in vivo models of breast cancer [125, 129]. Previous studies have demonstrated that compound 43 acts as an antiestrogen in the breast and potently inhibits estrogen-dependent MCF-7 proliferation with similar IC50 value to tamoxifen [129]. Furthermore, compound 43 is more potent than estrogen, behaved as an estrogen agonist in the bone cells in vivo, as effective as raloxifene in decreasing the formation of osteoclast-like cells and partially protected animals against ovariectomy-induced osteopenia [124]. In vitro U2OS/IL-6 and MCF-7 assays revealed that i) movement of the basic amine side chain on the phenyl group at C-4 to the C-3 in the 4-phenyl of the benzopyranone series provided a significant improvement in antiproliferative activity; ii) manipulation of the side chain was effective in mimicking the hinge substituent found in the benzopyranone 4-benzyl series and iii) an extension of the side-chain length improved receptor-binding affinity (Fig. 10) [130]. Tetracylic benzopyranone ring system such as benzopyranobenzopyran (44, n=l), benzopyranobenzoxa-pane (45, n=2) and benzopyranobenzoxacane (46, n=3) are excellent in mimicking natural ligands as estrogen receptor modulators [131]. These molecules differ from ideal SERMs in that they do not contain the basic side-chain group critical for providing selective tissue antagonist properties as seen with tamoxifen and raloxifene [132].
Fig. (9).
Structures of coumarin-based SERMs.
Fig. (10).
SARs of the benzopyranone series (43) incorporating the critical hinge feature 130].
Study aimed at the development of newer SERMs revealed that 3-substituted coumarin (47) [133] and coumestrol (48) [134] possess estrogenic activity. Compound 48, a plant derived tetracyclic coumarin, bears similar chemical features as estrogen attributed to its apparently crude mimicry of the steroid skeleton, thus making it possible for the coumarin moiety of the molecule to mimic the A/B ring of estrogen. SAR studies of the structure and relative binding affinities (RBAs) of various 3-substituted coumarins revealed that substituents at positions 3 and 7 resulted to an increase in RBA for the ERa [135–137]. In comparison to estradiol, 3-phenyl-4-ethyl-7-hydroxycoumarins and 3-(4-hydroxy-phenyl)-4,7-dihydroxycoumarin showed weak RBA and lack of selectivity toward both ERs. Furthermore, substitution with a second phenyl group at position 4 resulted in 3,4-diphenyl-7-hydroxycoumarin showing an increase in RBA to both ERs but with more selectivity for ERα than ERβ [138].
2.4. Coumarin-Estrogen Conjugates
There is an over-expressed ER in breast tumor cell in the earlier stage and during hormonal treatment [139, 140]. The non-selectivity and acute toxicity of many antitumor agents have been the major deterrent in their usage for treating human cancer [141]. Among the current cancer therapy focusing on the improvement of drug selectivity, conjugation of cytotoxic drug components to a carrier with selectivity toward the tumor tissues has proven to be an effective strategy in the development of efficient antitumor drugs with high therapeutic indices [142–147]. Studies have shown that coupling of cytotoxic agent with steroid hormones results in improvement of antitumor activity and in the target selectivity of the conjugate as the result of sufficient binding to the ER, allowing selective accumulation of the conjugates in ER-rich cells [148–152]. During the past decades, the application of bioconjugates (i.e. biomolecules bearing unnatural organic structures) in molecular and cell biology has significantly increased [148].
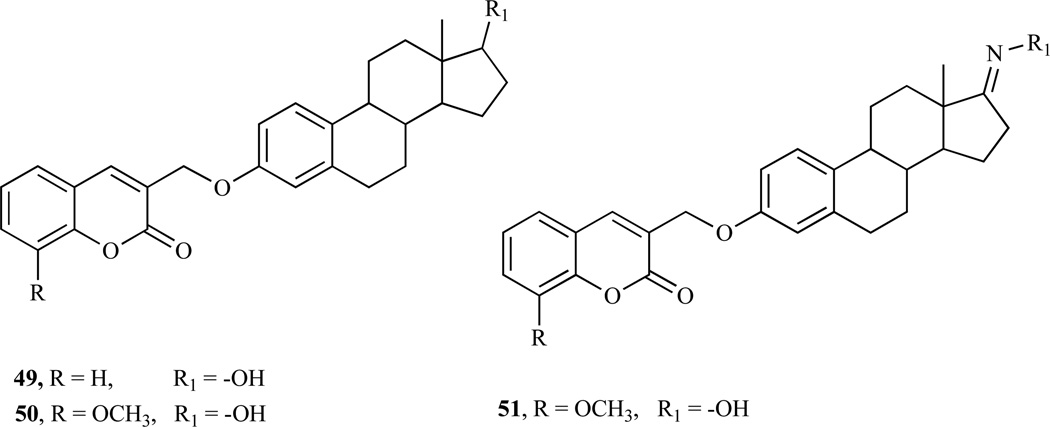
We have recently extended this novel concept of bioconjugation involving 3-substituted coumarins and estradiol (49–51) (Fig. 11) to show antiproliferative activity in NCI-7 human breast cancer cell lines. Comparisons of the GI50 values among the conjugates showed that conjugate 49 has the highest antiproliferation activity against MDA-MB-435 breast cancer cell lines while conjugates (50–51) displayed the highest antiproliferative activities against MDA-MB-231/ATCC. As far as the distinction between noninvasive and invasive breast cancer cell lines, overall conjugate 50 appears to be active against both types while conjugate 49 has the least inhibitory activities against noninvasive MDA-MB-231/ATCC and NCI/ADR-RES cell lines among the conjugates. Moreover, conjugate 49 was surprisingly inactive against the estrogen receptor enriched MCF-7. In general, it was shown that cytotoxicity occurred at around 100 µM for all of the conjugates. It was also observed that conjugate 50 displayed the most cytostatic properties based upon TGI values being less than LC50 values. The detailed results will be published elsewhere and is available on request from the author.
Fig. (11).
Structures of coumarin-estradiol conjugates.
3. SYNTHESIS OF COUMARIN DERIVATIVES WITH ANTICANCER ACTIVITY
Coumarins have been the subject of extensive studies because of their interesting biological activities and have, in fact, been used as therapeutic agents for the treatment of various diseases including breast cancer. There are various methods for preparing coumarin derivatives; these include the classic Pechmann [153],Claisen [154, 155],Perkin [156], Knoevenagel [157], and Wittig [158] reactions, to mention but a few. Recently, Musa [159] explored the intramolecular Baylis-Hillman reaction reported by Drewes et al. [160] as an alternative route to the synthesis of substituted coumarins, which has been further explored in the synthesis of coumarin-estrogen conjugates as anticancer agents. There is, nevertheless, a continuing interest in the synthesis of these important coumarin systems. Of particular interest to this review, the synthesis of only those coumarins with anticancer potentials, namely the coumarin sulfamates, benzopyrano- benzopyran ring systems, coumarin 3-carboxamides, coumarin 3-sulfonamides and some others that have been outlined here.
3.1. Coumarin Sulfamates
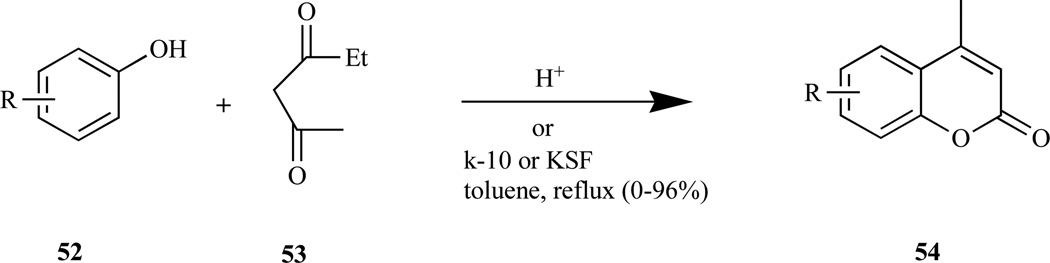
The Pechmann reaction (Scheme 1), widely used method for preparing coumarins (54) in good yield, involves reacting phenol (52) with β-oxo ester (53) in the presence of either homogeneous acid catalysts e.g. sulfuric [154, 161], phosphoric and trifluoroacetic acids [162], and Lewis acids, or heterogeneous catalysts e.g. Nafion-H, zeolite-HBEA and other solid acids [163]. The reaction is considered to involve the following steps: (i) addition across the double bond of the enolic form of the β-keto ester; (ii) ring closure; and (iii) dehydration [164].
Scheme 1.
Pechmann reaction.
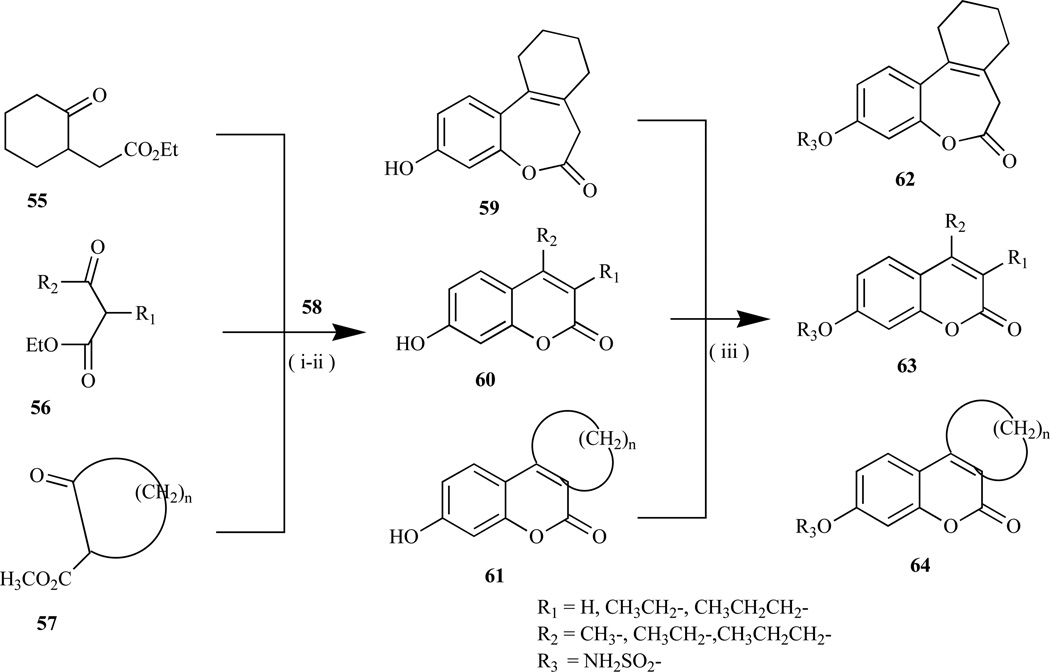
Previous studies demonstrating the estrogenicity of coumestrol (48) led to other coumarin ring systems being explored as an alternative phenolic-based non-steroidal sulfamate compound. Wool et al. reported the synthesis of bicyclic and tricylic coumarin sulfamates, e.g. COUMATE (24) (Fig. 3), a non-estrogenic active site-directed E1-STS inhibitor [43, 69, 70] via the Pechmann reaction between resorcinol and either of the corresponding L-ketoester (55–57) in presence of an equimolar mixture of trifluoroacetic acid and concentrated sulfuric acid as the condensing agent to afford the coumarins (59–61) in > 60% yield [165, 166] (Scheme 2). The lowest yield (25–33%) was obtained for tricyclic coumarin (61, n= 6), which was attributed to the ring size of its starting 8-membered cyclic L-ketoester.
Scheme 2.
Reagents and conditions: (i) resorcinol (58), CF3COOH/conc. H2SO4 (1:1), 0°C; (ii) NaH/DMF, H2NSO2C1; (iii) K2CO3/(Bu)4N+Cl-.xH20/H20/CHC13, r.t.
3.2. Benzopyranobenzopyran Ring Systems
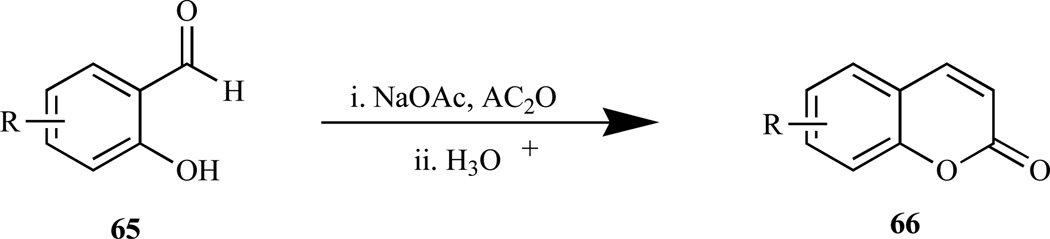
Perkin reaction (Scheme 3) [156], involves heating an O-hydroxybenzaldehyde (65) with acetic anhydride in the presence of sodium acetate at a high temperature (ca. 200°C) to afford a traws-cinnamic acid, followed by irradiation or treatment with iodine and then cyclization to afford the coumarin (66). This reaction is also carried out by reacting acetic anhydride and salicylaldehyde in the presence of trimeth-ylamine as the base catalyst [167]. However, the obvious advantage is that the formation of isomeric chromones is not possible, as is the case with the Pechmann reaction [168].
Scheme 3.
Perkin reaction.
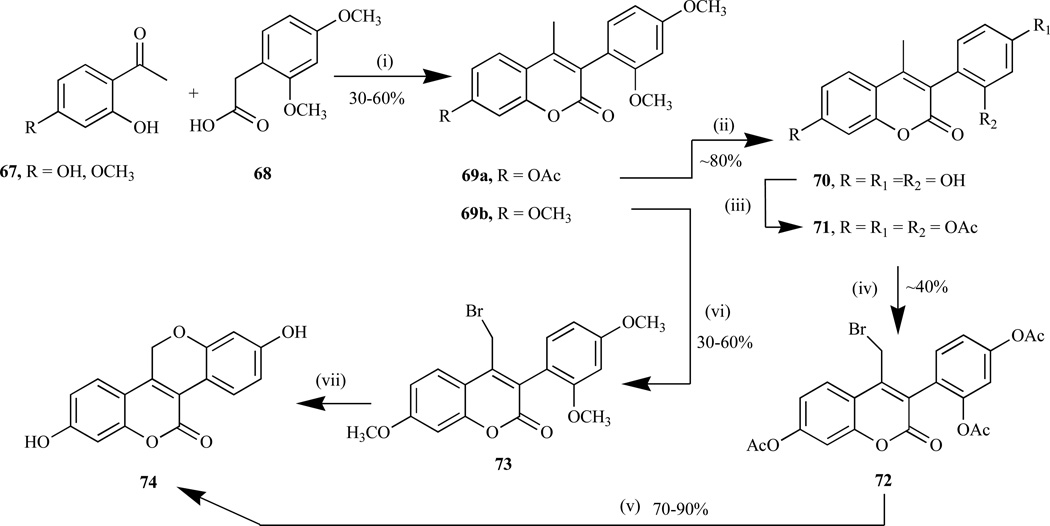
Efforts to synthesize the unsymmetrical tetracyclic benzopyranobenzopyran ring system (74), novel selective estrogen receptor modulators (SERMs) [169–171], by Kanojia et al. led to the synthesis of 4-methyl-3,7-substituted coumarins (69) as the key intermediates, via base-catalyzed Perkin condensation reaction in moderate to good yield (Scheme 4) [170]. These were furnished by reacting a mixture of 2-hydroxyacetophenones (67) and 2,4-dimethoxyphenylacetic acid (68) in the presence of triethylamine in refluxing acetic anhydride. The next step, which is known as the key step is said to involve the conversion of the 4-methyl functional group of coumarin (69) to the 4-halogenated methyl moiety, followed by ring closure to give the benzopyranobenzopyran compounds.
Scheme 4.
Reagents and conditions: (i) Et3N, Ac2O/reflux; ii) pyridine, HC1/200°C; iii) Ac2O/pyridine; iv) NBS /benzoyl peroxide, CCl4/hv; v) K2CO3/MeOH/acetone; vi) LiHMDS/THF/−32°C, then NBS/THF/78°C; vii) BBr3/ DCM, then NaOH/H+.
3.3. Coumarin 3-Carboxamides
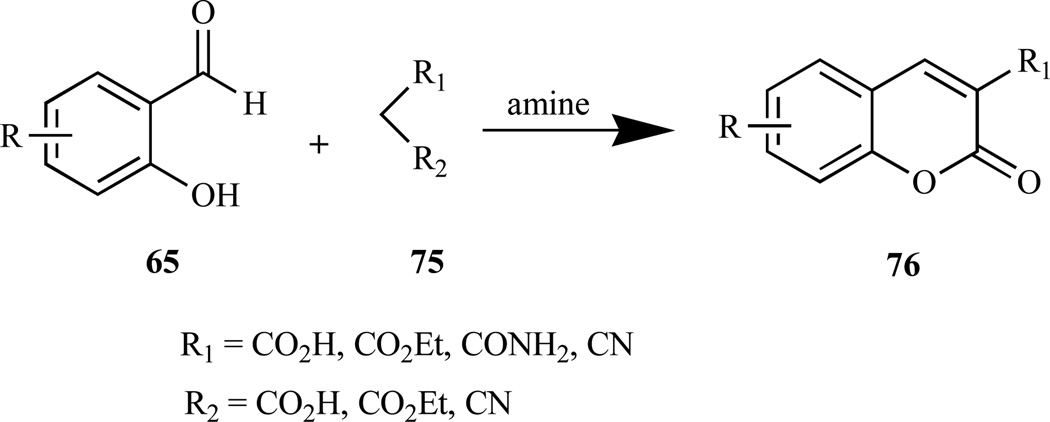
Various coumarins (76) have been prepared via Knoevenagel condensation reaction (Scheme 5) by reacting 2-hydroxybenzaldehydes (65) with activated methylene com- pounds (75) in the presence of an amine (e.g piperidine) [168]. Two different mechanisms have been proposed for Knoevenagel reaction [172]: 1) formation of an imine or iminium salt, followed by reaction with the enolate of the active methylene compound, then elimination of the amine and intramolecular ring closure to give the coumarin, ii) attack by the carbanion formed from the deprotonation of the active methylene compound by the amine, on the carbonyl group to give the intermediate follow by proton transfer, and ring-closure via acyl substitution, and finally dehydration to give the coumarin.
Scheme 5.
Knoevenagel reaction.
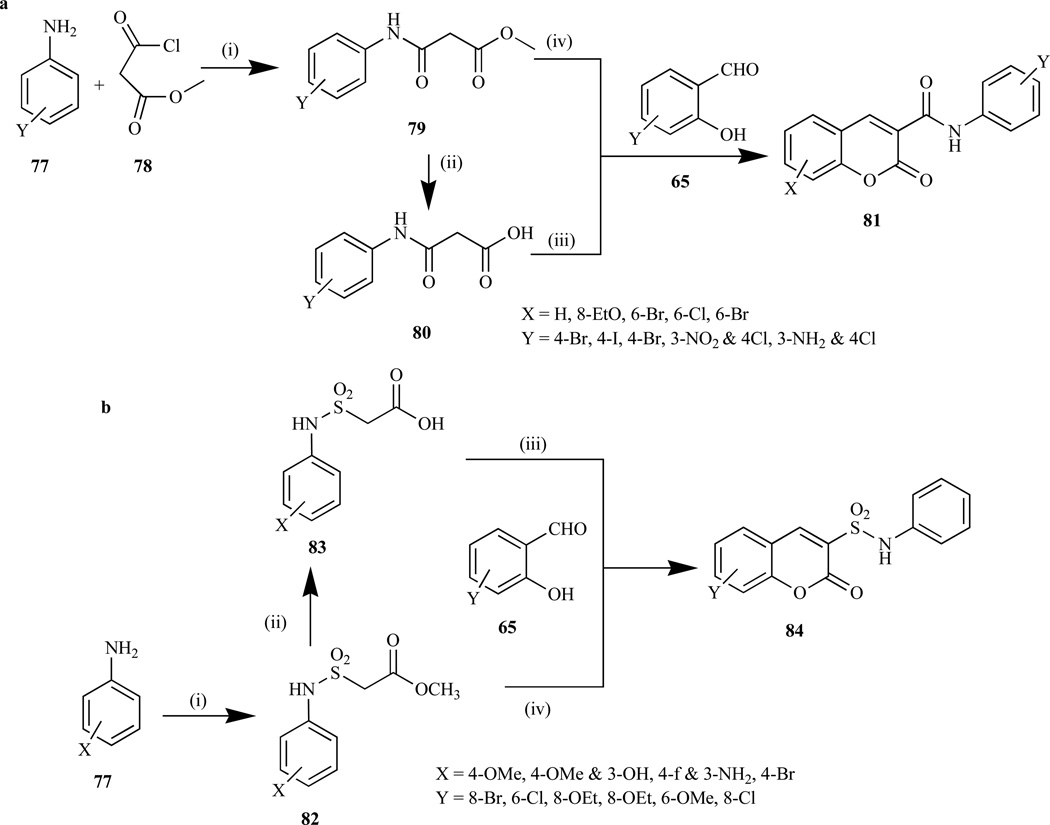
Intrigued by the recent discovery demonstrating that coumarin-3-carboxamide (81) inhibits the growth of cancer cell lines by up-regulating the activity of c-Jun NH2 terminal kinase 1 (JNK1) [173], Reddy et al. explored two novel routes to synthesize it via Knoevenagel condensation reaction. These synthetic routes involved: i) the condensation of 3-anilino-3-oxopropionic acid (80) with substituted salicylaldehydes (65) in glacial acetic acid in the presence of a catalytic amount of benzylamine, to afford (81) in 60–80% isolated yield [174, 175], and ii) condensation of methyl 3-anilino-3-oxopropionates (79) with substituted salicylaldehydes (65) in the presence of piperidine in ethanol to give coumarin (81) in 68–88% isolated yield (Scheme 6) [174].
Scheme 6.
Reagents and conditions: i) Et3N, CH2C12, 0°C to rt; ii) 10% NaOH, HC1; iii) PhCH2NH2, HOAc, reflux, iv) piperidine, ethanol, reflux 1 h.
b. Reagents and conditions: (i) C1SO2CH2COOCH3, triethylamine/ THF, N2, rt, 3 h; (ii) 10% NaOH in H2O; (iii) CH3COOH, C6H5CH2NH2; (iv) piperidine, ethanol, reflux, 5 min.
3.4. Coumarin 3-Sulfonamides
Some coumarin-3-sulfonamides have shown to be potent inhibitors of various cancer cell lines, which are used in the treatment of diseases arising from abnormal cell growth and proliferation [176, 177]. Previously, coumarin-3-sulfonamides was synthesized via the condensation of coumarin-3-sulfonyl chloride with different aromatic amines, which posed some limitations as the result of the difficulty of introducing a variety of substituents on the aromatic rings, thus making it impossible to create a library of new broadly un-substituted coumarin-3-arylsulfonamides [178–181]. Most recently, Reddy et al. [173] reported the synthesis of coumarin-3-sulfonamides (84) in their investigation involving the antitumor activity of novel compounds bearing coumarin and sulfonamide entities via Knoevenagel condensation reaction. This reaction involved reacting methyl-2-chloro-sulfonyl acetate with aromatic amines to afford intermediates (82), which were then hydrolyzed to anilinosulfonylacetic acids (83) and finally, condensation with substituted salicylaldehydes (65) in the presence of glacial acetic acid and a catalytic amount of benzylamine afforded coumarin-3-sulfonamides (84) in quantitative yields. An alternative route for the synthesis of (84) was also reported that involved the condensation of methyl anilinosulfonylacetates (82) with substituted salicylaldehydes (65) in the presence of piperidine and ethanol (Scheme 6) [173].
3.5. Miscellaneous
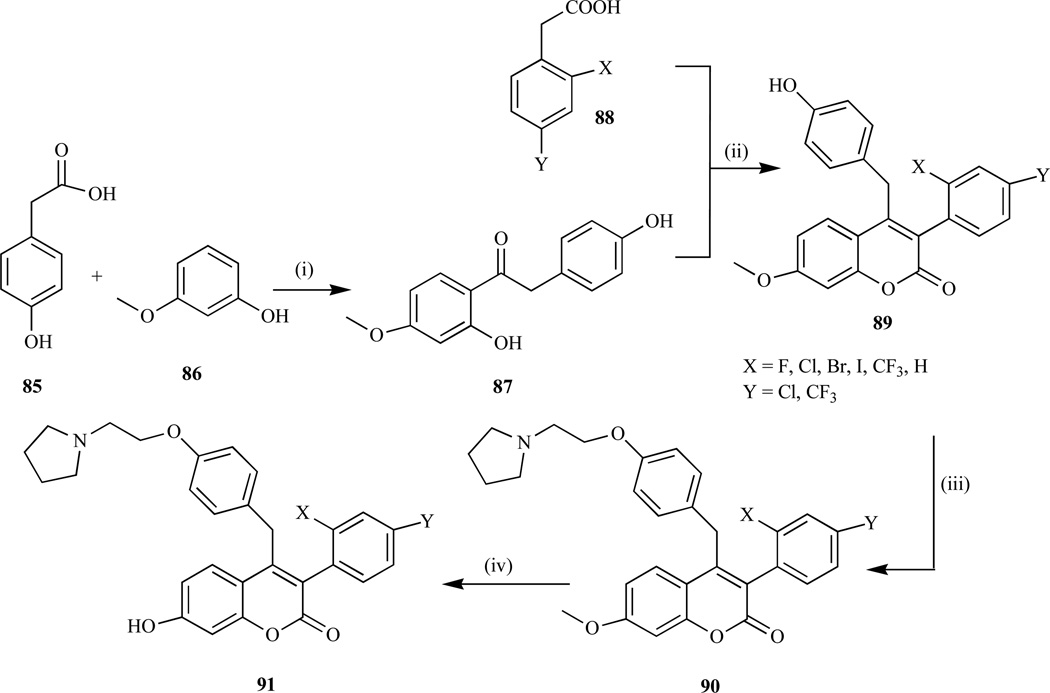
These methods involved the formation of isolable intermediate followed by cyclization using either acid or coupling agents to afford the coumarin derivatives. For example, McKe et al. [182] reported the synthesis of coumarin-based benzopyranone compounds and their salts used for treating bone-resorption disease, cancer, arthritis via the following steps: i) the reaction of 3-methoxyphenol (86) and 4-hydroxyphenylacetic acid (85) in the presence of boron trifluoride in diethyl etherate (Fries reaction) to give (87) in 40–50% yield; ii) coupling reaction using 1,1’-carbonyl-diimidazole to afford the desired coumarin (89) in 10–90% yield; iii) the introduction of the amino basic side-chain to yield coumarin-7-methyl ether (90) in 30–70% yield; and iv) demethylation of 90 using HBr to afford 60–75% of the desired coumarin (91) (Scheme 7).
Scheme 7.
Reagents and conditions: i) BF3 diethyletherate (Fries reaction); ii) CDI, K2CO3, DMAP, 10–90%; iii) K2CO3, l-(2-chloroethyl)pyrrolidine, 30–75%; iv) 30–50% HBr, AcOH.
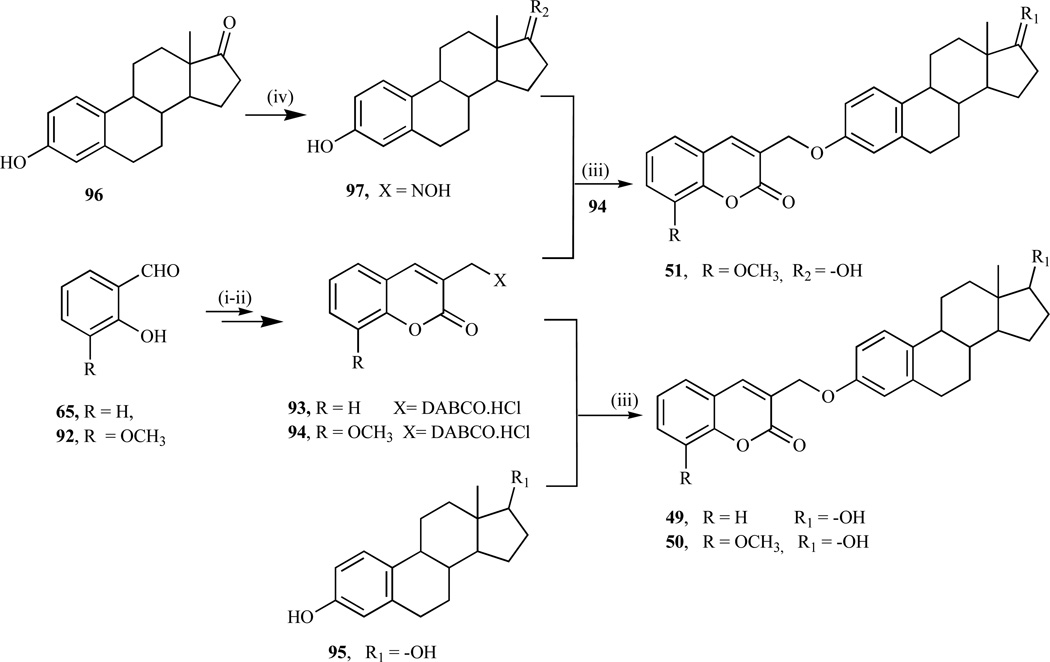
Recent investigation by our research group involving the antitumor activity of coumarin derivatives revealed that the conjugation of coumarin substrate to 17β-estradiol, afforded coumarin-estrogen conjugates, which demonstrate growth inhibitory activities in various breast cancer cell lines. Using the generality of the methodology outlined by Drewes et al., [183], which was further explored and reported [159], various 3-substituted coumarin quaternary salts were prepared via the intramolecular Baylis-Hillmann reaction using DABCO as a catalyst and then coupled to 17β-estradiol and estrone oxime to afford coumarin-estrogen conjugates (49–51, Scheme 8).
Scheme 8.
Reagents: i) Acryloychloride, NaH, reflux; ii) DABCO, CH2C12, rt, iii) K2CO3, CH3CN, reflux, overnight, iv) NH2OH, THF, reflux.
4. CONCLUSIONS
The widespread distribution and numerous bioactivities of coumarins have intrigued scientists for decades to conduct research involving this ring system. This review has outlined such research approaches before focusing on the coumarins with antibreast cancer activity. Although coumarins have vast pharmacological significance, their antibreast cancer potential have been the main focus of this review. A number of innovative synthetic techniques, including the classic Pechmann, Claisen, Perkin, Knoevenagel, and Wittig reactions, have been described in the synthesis of coumarin. With accessibility of such synthetic methods, scientists were able to synthesize numerous analogs with antibreast cancer activity with diverse mechanisms of action, among which aromatase and sulfatase inhibitions were the most notable. However, the coumarin-based SERMs and most recently coumarin-estrogen conjugates have also shown to be reasonable approaches in the development of antibreast cancer agents. Although most natural coumarins exhibited major limitations due to hepatotoxicity, molecular manipulations afforded relatively safer analogs with higher potency and thus better therapeutic index. Some of these derivatives are in clinical trial, which might emerge as new therapeutic option for the treatment of breast cancer. Interestingly, in all these approaches coumarins have been viewed to be antiestrogenic due to the structural similarity with natural female sex hormone estrogen, which is believed to stimulate breast cancer growth.
Fig. (1).
Structures of some naturally occurring coumarins.
Fig. (5).
Proposed mechanism of STS inhibition by (30) involving the aldehyde hydrate in the enzyme active site [63].
ACKNOWLEDGEMENTS
This review article was supported by NIH/NCRR grant G12 RR0 3020, NIH/NCRR grant 1 C06 RR12512-01 and NIH/DHHS grant 1 S11 ES011182 01.
REFERENCE
- 1.Egan D, O'Kennedy R, Moran E, Cox D, Prosser E, Thornes RD. Drug Metab. Rev. 1990;22:503. doi: 10.3109/03602539008991449. [DOI] [PubMed] [Google Scholar]
- 2.Borges F, Roleira F, Milhazes N, Santana L, Uriarte E. Curr. Med. Chem. 2005;12:887. doi: 10.2174/0929867053507315. [DOI] [PubMed] [Google Scholar]
- 3.Murray RDH, Mendez J, Brown SA. The Natural Coumarins: Occurrence, Chemistry and Biochemistry. New York: Wiley; 1982. [Google Scholar]
- 4.Lacy A, O'Kennedy R. Curr. Pharm. Des. 2004;10:3797. doi: 10.2174/1381612043382693. [DOI] [PubMed] [Google Scholar]
- 5.Gottlieb OR, Herrmann K, Murray RDH, Ohloff G, Pattenden G. Progress in the Chemistry of Organic Natural Products. New York: Springer-Verlag; 1978. [Google Scholar]
- 6.Harvey RG, Cortex C, Ananthanarayan TP, Schmolka S. J. Org. Chem. 1988;53:3936. [Google Scholar]
- 7.Kostova I, Raleva S, Genova P, Argirova R. Bioinorg. Chem. Appl. 2006:68274. doi: 10.1155/BCA/2006/68274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moffet RS. J. Med. Chem. 1964;7:446. doi: 10.1021/jm00334a010. [DOI] [PubMed] [Google Scholar]
- 9.Al-Haiza MA, Mostafa MS, El-Kady MY. Molecules. 2003;8:275. [Google Scholar]
- 10.Musiciki B, Periers AM, Laurin P, Ferroud D, Benedetti Y, Lachaud S, Chatreaux F, Haesslein JL, LLtis A, Pierre C, Khider J, Tessol N, Airault M, Demassey J, Dupuis-Hamelin C, Lassaigne P, Bonnefoy A, Vicat P, Klich M. Bioorg. Med. Chem. Lett. 2000;10:1695. doi: 10.1016/s0960-894x(00)00304-8. [DOI] [PubMed] [Google Scholar]
- 11.Fylaktakidou KC, Hadipavlou-Litina DJ, Litinas KE, Nicolaides DN. Curr. Pharm. Des. 2004;10:3813. doi: 10.2174/1381612043382710. [DOI] [PubMed] [Google Scholar]
- 12.Jung J, Kin J, Park OS. Synth. Commun. 2001;31:1195. [Google Scholar]
- 13.Wang Z, Hara K, Dan-oh Y, Kasada C, Shinpo A, Suga S, Arakawa H, Sugihara H. J. Phys. Chem. B. 2005;109:3907. doi: 10.1021/jp044851v. [DOI] [PubMed] [Google Scholar]
- 14.Madhavan GR, Balraju V, Malleshasm B, Chakrabarti R, Lohray VB. Bioorg. Med. Chem. Lett. 2003;13:2547. doi: 10.1016/s0960-894x(03)00490-6. [DOI] [PubMed] [Google Scholar]
- 15.Paya M, Halliwell B, Hoult JR. Biochem. Pharmacol. 1992;44:205. doi: 10.1016/0006-2952(92)90002-z. [DOI] [PubMed] [Google Scholar]
- 16.Casley-Smith JR, Morgan RG, Piller NB. N. Engl. J. Med. 1993;76:1158. doi: 10.1056/NEJM199310143291604. [DOI] [PubMed] [Google Scholar]
- 17.Casley-Smith JR, Casley-Smith JR. Med. J. Aus. 1995;162:391. doi: 10.5694/j.1326-5377.1995.tb139958.x. [DOI] [PubMed] [Google Scholar]
- 18.Beinssen AP. Med. J. Aus. 1994;167:725. [PubMed] [Google Scholar]
- 19.Cox D, O’Kennedy R, Thornes RD. Hum. Toxicol. 1989;8:501. doi: 10.1177/096032718900800612. [DOI] [PubMed] [Google Scholar]
- 20.Usui T. Endocrine J. 2006;53:7. doi: 10.1507/endocrj.53.7. [DOI] [PubMed] [Google Scholar]
- 21.Murray Rd. Fortschr. Chem. Org. Naturst. 2002;83:1. doi: 10.1007/978-3-7091-6172-2_1. [DOI] [PubMed] [Google Scholar]
- 22.Houlr JRS, Paya M. Gen. Pharmacol. 1996;27:713. doi: 10.1016/0306-3623(95)02112-4. [DOI] [PubMed] [Google Scholar]
- 23.Carotti A, Carriri A, Chimichi S, Boccalini M, Cosimelli B, Gnerre C, Carrupt PA, Testa B. Bioorg. Med. Chem. Lett. 2002;72:3551. doi: 10.1016/s0960-894x(02)00798-9. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto T, Kobayashi T, Yoshida S. Curr. Med. Chem. Anticancer Agents. 2005;5:47. doi: 10.2174/1568011053352622. [DOI] [PubMed] [Google Scholar]
- 25.Marshall ME, Ryles M, Butler K, Weiss L. J. Cancer Res.Clin. Oncol. 1994;120:535. doi: 10.1007/BF01377124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanchev S, Momekov G, Jensen F, Manolov I. Eur. J. Med. Chem. 2008;43:694. doi: 10.1016/j.ejmech.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Thornes RD, Daly L, Lynch G, Breslin B, Browne H, Browne HY, Corrigan T, Daly P, Edwards G, Gaffney E, Henley J, Healy F, Keane F, Lennon F, McMurray N, O’Loughlin S, Shine M, Tanner A. J. Cancer Res. Clin. Oncol. 1994;(120 Suppl):S32. doi: 10.1007/BF01377122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall ME, Butler K, Fried A. Mol. Biother. 1991;3:170. [PubMed] [Google Scholar]
- 29.Mohler JL, Gomella LG, Crawford ED, Glode LM, Zippe CD, Fair WR, Marshall ME. Prostate. 1992;20:123. doi: 10.1002/pros.2990200208. [DOI] [PubMed] [Google Scholar]
- 30.Yacquot Y, Bermont L, Giorgi H, Refouvelet B, Adessi G, Daubrosse E, Xicluna A. Eur. J. Med. Chem. 2001;36:127. doi: 10.1016/s0223-5234(00)01207-1. [DOI] [PubMed] [Google Scholar]
- 31.Kolodziej H, Kayser O, Woerdenbag HJ, van Uden W, Pras NZ. Naturforsch. [C] 1997;52:240. doi: 10.1515/znc-1997-3-416. [DOI] [PubMed] [Google Scholar]
- 32.Cai Y, Kleiner H, Johnston D, Dubowski A, Bostic S, Ivie W, DiGiovanni J. Carcinogenesis. 1997;18:1521. doi: 10.1093/carcin/18.8.1521. [DOI] [PubMed] [Google Scholar]
- 33.Diiovanni J. Pharmacol. Ther. 1992;54:63. [Google Scholar]
- 34.Feuer G, Kellen JA. Int. J. Clin. Pharmacol. 1974;9:62. [PubMed] [Google Scholar]
- 35.Feuer G, Kellen JA, Kovacs K. Oncology. 1976;33:35. doi: 10.1159/000225098. [DOI] [PubMed] [Google Scholar]
- 36.Wattenberg LW, Lam LK, Fladmoe AV. Cancer Res. 1979;39:1651. [PubMed] [Google Scholar]
- 37.Budzisz E, Brzezinska E, Krajewska U, Rozalski M. Eur. J. Med. Chem. 2003;38:597. doi: 10.1016/s0223-5234(03)00086-2. [DOI] [PubMed] [Google Scholar]
- 38.Lacy A, O'Kennedy R. Curr. Pharm. Des. 2004;10:3797. doi: 10.2174/1381612043382693. [DOI] [PubMed] [Google Scholar]
- 39.Kostova I, Momekov G, Tzanova T, Karaivanova M. Bioinorg. Chem. Appl. 2006;25651:1. doi: 10.1155/BCA/2006/25651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber US, Steffen B, Siegers CP. Res. Commun. Mol. Pathol. Pharmacol. 1998;99:193. [PubMed] [Google Scholar]
- 41.Henderson IC, Canellos GP. N. Engl. J. Med. 1980;302:17. doi: 10.1056/NEJM198001033020104. [DOI] [PubMed] [Google Scholar]
- 42.Hamelers I, Schaik R, Sussenbach JS, Steenbergh PH. Cancer Cell Int. 2003;3:10. doi: 10.1186/1475-2867-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woo LW, Howarth NM, Purohit A, Hejaz HA, Reed MJ, Potter BV. J. Med. Chem. 1998;41:1068. doi: 10.1021/jm970527v. [DOI] [PubMed] [Google Scholar]
- 44.Strott CA. Endocr. Rev. 2002;23:703. doi: 10.1210/er.2001-0040. [DOI] [PubMed] [Google Scholar]
- 45.Utsumi T, Yoshimura N, Takeuchi S, Ando J, Maruta M, Maeda K, Harada N. Cancer Res. 1999;59:377. [PubMed] [Google Scholar]
- 46.Miyoshi Y, Ando A, Hasegawa S, Ishitobi M, Taguchi T, Tamaki Y, Noguchi S. Clin. Cancer Res. 2003;9:2288. [PubMed] [Google Scholar]
- 47.Suzuki T, Nakata T, Miki Y, Kaneko C, Moriya T, Ishida T, Akinaga S, Hirakawa H, Kimura M, Sasano H. Cancer Res. 2003;63:2762. [PubMed] [Google Scholar]
- 48.Yoshimura N, Harada N, Bukholm I, Karesen R, Borresen-Dale AL, Kristensen VN. Breast Cancer Res. 2004;6:R46. doi: 10.1186/bcr746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castiglione-Gertsch M. Eur. J. Cancer. 1996;32A:393. doi: 10.1016/0959-8049(95)00013-5. [DOI] [PubMed] [Google Scholar]
- 50.Jonat W, Howell A, Blomqvist C, Eiermann W, Winblad G, Tyrrell C, Mauriac L, Roche H, Lundgren S, Hellmund R, Azab M. Eur. J. Cancer. 1996;32A:404. doi: 10.1016/0959-8049(95)00014-3. [DOI] [PubMed] [Google Scholar]
- 51.Santner SJ, Feil PD, Santen RJ. J. Clin. Endocrinol. Metab. 1984;59:29. doi: 10.1210/jcem-59-1-29. [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto T, Kitawaki J, Urabe M, Honjo H, Tamura T, Noguchi T, Okada H, Sasaki H, Tada A, Yoshiteru T, Junji N, Makoto Y. J. Steroid Biochem. Mol. Biol. 1993;44:463. doi: 10.1016/0960-0760(93)90251-q. [DOI] [PubMed] [Google Scholar]
- 53.Purohit A, Woo LW, Chander SK, Newman SP, Ireson C, Ho Y, Grasso A, Leese MP, Potter BV, Reed MJ. J. Steroid Biochem. Mol. Biol. 2003;86:423. doi: 10.1016/s0960-0760(03)00353-4. [DOI] [PubMed] [Google Scholar]
- 54.Chen S, Cho M, Karlsberg K, Zhou D, Yuan YC. J. Biol. Chem. 2004;279:48071. doi: 10.1074/jbc.M406847200. [DOI] [PubMed] [Google Scholar]
- 55.Pasqualini JR, Gelly C, Nguyen BL, Vella C. J. Steroid Biochem. 1989;34:155. doi: 10.1016/0022-4731(89)90077-0. [DOI] [PubMed] [Google Scholar]
- 56.Reed MJ, Purohit A. Rev. Endocrine Relat. Cancer. 1993;45:51. [Google Scholar]
- 57.Poirier D, Ciobanu LC, Maltais R. Exp. Opin. Ther. Pat. 1999;9:1083. [Google Scholar]
- 58.Nussbaumer P, Billich A. Exp. Opin. Ther. Pat. 2003;13:605. [Google Scholar]
- 59.Winum JY, Scozzafava A, Montero JL, Supuran CT. Expert Opin. Ther. Pat. 2004;14:1273. doi: 10.1517/13543776.16.1.27. [DOI] [PubMed] [Google Scholar]
- 60.Scozzafava A, Owa T, Mastrolorenzo A, Supuran CT. Curr. Med. Chem. 2003;70:925. doi: 10.2174/0929867033457647. [DOI] [PubMed] [Google Scholar]
- 61.Nussbaumer P, Billich A. Med. Res. Rev. 2004;24:529. doi: 10.1002/med.20008. [DOI] [PubMed] [Google Scholar]
- 62.Reed MJ, Purohit A, Woo LW, Newman SP, Potter BV. Endocr. Rev. 2005;26:171. doi: 10.1210/er.2004-0003. [DOI] [PubMed] [Google Scholar]
- 63.Woo LL, Purohit A, Malini B, Reed MJ, Potter BV. Chem. Biol. 2000;7:773. doi: 10.1016/s1074-5521(00)00023-5. [DOI] [PubMed] [Google Scholar]
- 64.Purohit A, Vernon KA, Hummelinck AE, Woo LW, Hejaz HA, Potter BV, Reed MJ. J. Steroid Biochem. Mol. Biol. 1998;64:269. doi: 10.1016/s0960-0760(97)00196-9. [DOI] [PubMed] [Google Scholar]
- 65.Li PK, Milano S, Kluth L, Rhodes ME. J. Steroid Biochem. Mol. Biol. 1996;59:41. doi: 10.1016/s0960-0760(96)00093-3. [DOI] [PubMed] [Google Scholar]
- 66.Purohit A, Woo LW, Potter BV, Reed MJ. Cancer Res. 2000;60:3394. [PubMed] [Google Scholar]
- 67.Purohit A, Woo LW, Barrow D, Hejaz HA, Nicholson RI, Potter BV, Reed MJ. Mol. Cell Endocrinol. 2001;171:129. doi: 10.1016/s0303-7207(00)00428-7. [DOI] [PubMed] [Google Scholar]
- 68.Malini B, Purohit A, Ganeshapillai D, Woo LW, Potter BV, Reed MJ. J. Steroid Biochem. Mol. Biol. 2000;75:253. doi: 10.1016/s0960-0760(00)00178-3. [DOI] [PubMed] [Google Scholar]
- 69.Woo LW, Purohit A, Reed MJ, Potter BV. J. Med. Chem. 1996;39:1349. doi: 10.1021/jm950931z. [DOI] [PubMed] [Google Scholar]
- 70.Purohit A, Woo LW, Singh A, Winterborn CJ, Potter BV, Reed MJ. Cancer Res. 1996;56:4950. [PubMed] [Google Scholar]
- 71.Stanway SJ, Purohit A, Woo LW, Sufi S, Vigushin D, Ward R, Wilson RH, Stanczyk FZ, Dobbs N, Kulinskaya E, Elliott M, Potter BV, Reed MJ, Coombes RC. Clin. Cancer Res. 2006;12:1585. doi: 10.1158/1078-0432.CCR-05-1996. [DOI] [PubMed] [Google Scholar]
- 72.Bond CS, Clements PR, Ashby SJ, Collyer CA, Harrop SJ, Hopwood JJ, Guss JM. Structure. 1997;5:277. doi: 10.1016/s0969-2126(97)00185-8. [DOI] [PubMed] [Google Scholar]
- 73.Lukatela G, Krauss N, Theis K, Selmer T, Gieselmann V, von Figura K, Saenger W. Biochemistry. 1998;37:3654. doi: 10.1021/bi9714924. [DOI] [PubMed] [Google Scholar]
- 74.Schmidt B, Selmer T, Ingendoh A, von Figura K. Cell. 1995;82:271. doi: 10.1016/0092-8674(95)90314-3. [DOI] [PubMed] [Google Scholar]
- 75.Recksiek M, Selmer T, Dierks T, Schmidt B, von Figura K. J. Biol. Chem. 1998;273:6096. doi: 10.1074/jbc.273.11.6096. [DOI] [PubMed] [Google Scholar]
- 76.Knaust A, Schmidt B, Dierks T, von Bulow R, von Figura K. Biochemistry. 1998;37:13941. doi: 10.1021/bi9810205. [DOI] [PubMed] [Google Scholar]
- 77.von Bulow R, Schmidt B, Dierks T, von Figura K, Uson I. J. Mol. Biol. 2001;305:269. doi: 10.1006/jmbi.2000.4297. [DOI] [PubMed] [Google Scholar]
- 78.Uhlhorn-Dierks G, Kolter T, Sandhoff K. Angew. Chem. Int. Ed. 1998;57:2453. doi: 10.1002/(SICI)1521-3773(19981002)37:18<2453::AID-ANIE2453>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 79.Lloyd MD, Pederick RL, Natesh R, Woo LW, Purohit A, Reed MJ, Acharya KR, Potter BV. Biochem. J. 2005;385:715. doi: 10.1042/BJ20041037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ahmed S, James K, Owen CP, Patel CK. J. Steroid Biochem. Mol. Biol. 2002;80:419. doi: 10.1016/s0960-0760(02)00037-7. [DOI] [PubMed] [Google Scholar]
- 81.Raobaikady B, Purohit A, Chander SK, Woo LW, Leese MP, Potter BV, Reed MJ. J. Steroid Biochem. Mol. Biol. 2003;84:351. doi: 10.1016/s0960-0760(03)00049-9. [DOI] [PubMed] [Google Scholar]
- 82.Wolf OT, Kirschbaum C. Brain Res. Brain Res. Rev. 1999;30:264. doi: 10.1016/s0165-0173(99)00021-1. [DOI] [PubMed] [Google Scholar]
- 83.Bilban M, Billich A, Auer M, Nussbaumer P. Bioorg. Med. Chem. Lett. 2000;10:967. doi: 10.1016/s0960-894x(00)00144-x. [DOI] [PubMed] [Google Scholar]
- 84.Vicker N, Ho Y, Robinson J, Woo LL, Purohit A, Reed MJ, Potter BV. Bioorg. Med. Chem. Lett. 2003;13:863. doi: 10.1016/s0960-894x(03)00009-x. [DOI] [PubMed] [Google Scholar]
- 85.Purohit A, Williams GJ, Howarth NM, Potter BV, Reed MJ. Biochemistry. 1995;34:11508. doi: 10.1021/bi00036a025. [DOI] [PubMed] [Google Scholar]
- 86.Kitz R, Wilson IB. J. Biol. Chem. 1962;237:3245. [PubMed] [Google Scholar]
- 87.Ahmed S, Owen CP, James K, Sampson L, Patel CK. Curr. Med. Chem. 2002;9:263. doi: 10.2174/0929867023371210. [DOI] [PubMed] [Google Scholar]
- 88.Coombes RC, Gibson L, Hall E, Emson M, Bliss J. J. Steroid Biochem. Mol. Biol. 2003;86:309. doi: 10.1016/s0960-0760(03)00372-8. [DOI] [PubMed] [Google Scholar]
- 89.Roy D, Liehr JG. Mutat. Res. 1999;424:107. doi: 10.1016/s0027-5107(99)00012-3. [DOI] [PubMed] [Google Scholar]
- 90.Liehr JG. Hum. Reprod. Update. 2001;7:273. doi: 10.1093/humupd/7.3.273. [DOI] [PubMed] [Google Scholar]
- 91.Russo J, Russo IH. Trends Endocrinol. Metab. 2004;15:211. doi: 10.1016/j.tem.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 92.Eiermann W, Paepke S, Appfelstaedt J, Llombart-Cussac A, Eremin J, Vinholes J, Mauriac L, Ellis M, Lassus M, Chau-dri-Ross HA, Dugan M, Borgs M. Ann. Oncol. 2001;12:1527. doi: 10.1023/a:1013128213451. [DOI] [PubMed] [Google Scholar]
- 93.Mouridsen HT, Robert NJ. MedGenMed. 2005;7:20. [PMC free article] [PubMed] [Google Scholar]
- 94.Brodie A, Lu Q, Liu Y, Long B, Wang JP, Yue W. Oncology. 1998;12:36. [PubMed] [Google Scholar]
- 95.Geisler J, King N, Anker G, Ornati G, Di Salle E, Lonning PE, Dowsett M. Clin. Cancer Res. 1998;4:2089. [PubMed] [Google Scholar]
- 96.Geisler J, King N, Dowsett M, Ottestad L, Lundgren S, Walton P, Kormeset PO, Lonning PE. Br. J. Cancer. 1996;74:1286. doi: 10.1038/bjc.1996.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Santen RJ, Yue W, Naftolin F, Mor G, Berstein L. Endocr. Relat. Cancer. 1999;6:235. doi: 10.1677/erc.0.0060235. [DOI] [PubMed] [Google Scholar]
- 98.Brodie A, Lu Q, Liu Y, Long B. Endocr. Relat. Cancer. 1999;6:205. doi: 10.1677/erc.0.0060205. [DOI] [PubMed] [Google Scholar]
- 99.Tekmal RR, Ramachandra N, Gubba S, Durgam VR, Mantione J, Toda K, Shizuta Y, Dillehay DL. Cancer Res. 1996;56:3180. [PubMed] [Google Scholar]
- 100.Tekmal RR, Kirma N, Gill K, Fowler K. Endocr. Relat. Cancer. 1999;6:307. doi: 10.1677/erc.0.0060307. [DOI] [PubMed] [Google Scholar]
- 101.Santner SJ, Pauley RJ, Tait L, Kaseta J, Santen RJ. J. Clin. Endocrinol. Metab. 1997;82:200. doi: 10.1210/jcem.82.1.3672. [DOI] [PubMed] [Google Scholar]
- 102.Plourde PV, Dyroff M, Dukes M. Breast Cancer Res. Treat. 1994;30:103. doi: 10.1007/BF00682745. [DOI] [PubMed] [Google Scholar]
- 103.Chen S, Masri S, Wang X, Phung S, Yuan YC, Wu X. J. Steroid Biochem. Mol. Biol. 2006;102:232. doi: 10.1016/j.jsbmb.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bhatnagar AS, Hausler A, Schieweck K, Lang M, Bowman R. J. Steroid Biochem. Mol. Biol. 1990;37:1021. doi: 10.1016/0960-0760(90)90460-3. [DOI] [PubMed] [Google Scholar]
- 105.Demers LM. Breast Cancer Res. Treat. 1994;30:95. doi: 10.1007/BF00682744. [DOI] [PubMed] [Google Scholar]
- 106.Janicke F. Breast. 2004;13(Suppl. 1):S10. doi: 10.1016/j.breast.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 107.Johnston SRD, Smith IE, Dowsett M. In: In Aromatase Inhibition and Breast Cancer. Miller WR, Santen RJ, editors. New York: Marcel Dekker Inc; 2001. p. 29. [Google Scholar]
- 108.Goss P, von Eichel L. J. Steroid Biochem. Mol. Biol. 2007;106:40. doi: 10.1016/j.jsbmb.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 109.Shields-Botella J, Bonnet P, Duc I, Duranti E, Meschi S, Cardinali S, Prouheze P, Chaigneau AM, Duranti V, Gri-baudo S, Riviere A, Mengual L, Carniato D, Cecchet L, Lafay J, Rondot B, Sandri J, Pascal JC, Delansorne R. J. Steroid Biochem. Mol. Biol. 2003;84:327. doi: 10.1016/s0960-0760(03)00046-3. [DOI] [PubMed] [Google Scholar]
- 110.Kao YC, Korzekwa KR, Laughton CA, Chen S. Eur. J. Biochem. 2001;268:243. doi: 10.1046/j.1432-1033.2001.01886.x. [DOI] [PubMed] [Google Scholar]
- 111.Leonetti F, Favia A, Rao A, Aliano R, Paluszcak A, Hartmann RW, Carotti A. J. Med. Chem. 2004;47:6792. doi: 10.1021/jm049535j. [DOI] [PubMed] [Google Scholar]
- 112.Recanatini M, Cavalli A. Bioorg. Med. Chem. 1998;6:377. doi: 10.1016/s0968-0896(97)10053-0. [DOI] [PubMed] [Google Scholar]
- 113.Jordan VC. Cancer Cell. 2004;5:207. doi: 10.1016/s1535-6108(04)00059-5. [DOI] [PubMed] [Google Scholar]
- 114.Meegan MJ, Hughes RB, Lloyd DG, Williams DC, Zisterer DM. J. Med. Chem. 2001;44:1072. doi: 10.1021/jm001119l. [DOI] [PubMed] [Google Scholar]
- 115.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Endocrinology. 1997;138:863. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 116.Grese TA, Dodge JA. Curr. Pharm. Des. 1998;4:71. [PubMed] [Google Scholar]
- 117.Muchmore DB. Oncologist. 2000;5:388. doi: 10.1634/theoncologist.5-5-388. [DOI] [PubMed] [Google Scholar]
- 118.Wenger NK, Grady D. J. Endocrinol. Invest. 1999;22:616. doi: 10.1007/BF03343619. [DOI] [PubMed] [Google Scholar]
- 119.Taras TL, Wurz GT, DeGregorio MW. J. Steroid Biochem. Mol. Biol. 2001;77:271. doi: 10.1016/s0960-0760(01)00066-8. [DOI] [PubMed] [Google Scholar]
- 120.Osborne CK, Zhao H, Fuqua SA. J. Clin. Oncol. 2000;18:3172. doi: 10.1200/JCO.2000.18.17.3172. [DOI] [PubMed] [Google Scholar]
- 121.Draper MW, Eur J. Cancer. 2002;38(Suppl 6):S35. doi: 10.1016/s0959-8049(02)00278-2. [DOI] [PubMed] [Google Scholar]
- 122.McCague R, Parr IB, Haynes BP. Biochem. Pharmacol. 1990;40:2277. doi: 10.1016/0006-2952(90)90723-x. [DOI] [PubMed] [Google Scholar]
- 123.Howell SJ, Johnston SR, Howell A. Best Pract. Res. Clin. Endocrinol. Metab. 2004;18:47. doi: 10.1016/j.beem.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 124.Kung Sutherland MS, Lipps SG, Patnaik N, Gayo-Fung LM, Khammungkune S, Xie W, Brady HA, Barbosa MS, Anderson DW, Stein B. Cytokine. 2003;23:1. doi: 10.1016/s1043-4666(03)00179-0. [DOI] [PubMed] [Google Scholar]
- 125.Brady H, Desai S, Gayo-Fung LM, Khammungkhune S, McKie JA, O'Leary E, Pascasio L, Sutherland MK, Anderson DW, Bhagwat SS, Stein B. Cancer Res. 2002;62:1439. [PubMed] [Google Scholar]
- 126.McDonnell DP. Trends Endocrinol. Metab. 1999;10:301. doi: 10.1016/s1043-2760(99)00177-0. [DOI] [PubMed] [Google Scholar]
- 127.Wijayaratne AL, Nagel SC, Paige LA, Christensen DJ, Norris JD, Fowlkes DM, McDonnell DP. Endocrinology. 1999;140:5828. doi: 10.1210/endo.140.12.7164. [DOI] [PubMed] [Google Scholar]
- 128.McDonnell DP. Biochem. Soc. Trans. 1998;26:54. doi: 10.1042/bst0260054. [DOI] [PubMed] [Google Scholar]
- 129.Brady H, Doubleday M, Gayo-Fung LM, Hickman M, Khammungkhune S, Kois A, Lipps S, Pierce S, Richard N, Shevlin G, Sutherland MK, Anderson DW, Bhagwat SS, Stein B. Mol. Pharmacol. 2002;61:562. doi: 10.1124/mol.61.3.562. [DOI] [PubMed] [Google Scholar]
- 130.McKie JA, Bhagwat SS, Brady H, Doubleday M, Gayo L, Hickman M, Jalluri RK, Khammungkhune S, Kois A, Mortensen D, Richard N, Sapienza J, Shevlin G, Stein B, Sutherland M. Bioorg. Med. Chem. Lett. 2004;14:3407. doi: 10.1016/j.bmcl.2004.04.081. [DOI] [PubMed] [Google Scholar]
- 131.Chen N, Jain N, Xu J, Reuman M, ; Li X, Russell KR, Sui Z. tetrahedron left. 2006;47:5909. [Google Scholar]
- 132.Pollard I, Martin L. Steroids. 1968;11:897. doi: 10.1016/s0039-128x(68)80103-5. [DOI] [PubMed] [Google Scholar]
- 133.Mentzer C, Gley P, Billet D, Molho D. Bull. Soc. Chim. Fr. 1946:271. [Google Scholar]
- 134.Bickoff EM, Booth AN, Lyman RL, Livingston AL, Thompson CR, Deeds F. Science. 1957;126:969. doi: 10.1126/science.126.3280.969-a. [DOI] [PubMed] [Google Scholar]
- 135.Kirkiacharian S, Chidiack H, Philibert D, Van De Velde P, Bouchoux F. Ann. Pharm. Fr. 1999;57:332. [PubMed] [Google Scholar]
- 136.Bakhchinian R, Terrier F, Kirkiacharian S, Resche-Rigon M, Bouchoux F, Cerede E. FARMACO. 2003;58:1201. doi: 10.1016/j.farmac.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 137.Kirkiacharian S, Lormier AT, Resche-Rigon M, Bouchoux F, Cerede E. Ann. Pharm. Fr. 2003;61:51. [PubMed] [Google Scholar]
- 138.Kirkiacharian S, Lormier AT, Chidiack H, Bouchoux F, Cerede E. FARMACO. 2004;59:981. doi: 10.1016/j.farmac.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 139.Gotteland M, May E, May-Levin F, Contesso G, Delarue JC, Mouriesse H. Cancer. 1994;74:864. doi: 10.1002/1097-0142(19940801)74:3<864::aid-cncr2820740312>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 140.Soubeyran I, Quenel N, Mauriac L, Durand M, Bonichon F, Coindre JM. Br. J. Cancer. 1996;73:735. doi: 10.1038/bjc.1996.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Devraj R, Barrett JF, Fernandez JA, Katzenellenbogen JA, Cushman M. J. Med. Chem. 1996;39:3367. doi: 10.1021/jm9602930. [DOI] [PubMed] [Google Scholar]
- 142.Upeslacis J, Hinman L. Annu. Rep. Med. Chem. 1988;23:151. [Google Scholar]
- 143.Blatter WA, Lambert JM, Goldmacher VS. Cancer Cells. 1989;1:50. [PubMed] [Google Scholar]
- 144.Krohn K, Kulikowski K, Leclercq G. J. Med. Chem. 1989;32:1532. doi: 10.1021/jm00127a022. [DOI] [PubMed] [Google Scholar]
- 145.Schmidt BF, Hernandez L, Rouzer C, Czerwinski G, Chmurny G, Michejda CJ. J. Med. Chem. 1994;37:3812. doi: 10.1021/jm00048a016. [DOI] [PubMed] [Google Scholar]
- 146.Varga JM, Asato N, Lande S, Lerner AB. Nature. 1977;267:56. doi: 10.1038/267056a0. [DOI] [PubMed] [Google Scholar]
- 147.Nakagawa-Goto K, Yamada K, Nakamura S, Chen TH, Chiang PC, Bastow KF, Wang SC, Spohn B, Hung MC, Lee FY, Lee FC, Lee KH. Bioorg. Med. Chem. Lett. 2007;17:5204. doi: 10.1016/j.bmcl.2007.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ahmed N, Dubuc C, Rousseau J, Benard F, van Lier JE. Bioorg. Med. Chem. Lett. 2007;17:3212. doi: 10.1016/j.bmcl.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 149.Ali H, Ahmed N, Tessier G, van Lier JE. Bioorg. Med. Chem. Lett. 2006;16:317. doi: 10.1016/j.bmcl.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 150.James DA, Swamy N, Paz N, Hanson RN, Ray R. Bioorg. Med. Chem. Lett. 1999;9:2379. doi: 10.1016/s0960-894x(99)00390-x. [DOI] [PubMed] [Google Scholar]
- 151.Swamy N, James DA, Mohr SC, Hanson RN, Ray R. Bioorg. Med. Chem. 2002;10:3237. doi: 10.1016/s0968-0896(02)00242-0. [DOI] [PubMed] [Google Scholar]
- 152.Liu C, Strobl JS, Bane S, Schilling JK, McCracken M, Chatterjee SK, Rahim-Bata R, Kingston DG. J. Nat. Prod. 2004;67:152. doi: 10.1021/np030296x. [DOI] [PubMed] [Google Scholar]
- 153.De SK, Gibbs RA. Synthesis. 2005;8:1231. [Google Scholar]
- 154.Panetta JA, Rapoport HJ. J. Org. Chem. 1982;47:946. [Google Scholar]
- 155.Drewes SE, Emslie ND, Karodia N, Loizou G. Synth.Commun. 1990;20:1437. [Google Scholar]
- 156.Johnson JR. Org. React. 1942;1:210. [Google Scholar]
- 157.Bogdal D. J. Chem. Res. (S) 1998;8:468. [Google Scholar]
- 158.Yavari I, Hekmatt-Shoar R, Zonouzi A. Tetrahedron Lett. 1998;59:2391. [Google Scholar]
- 159.Musa MA. PhD thesis. South Africa: Rhodes University, Grahamstown; 2003. [Google Scholar]
- 160.Drewes SE, Njamela OL, Emslie ND, Ramesar N, Field JS. Synth. Commun. 1993;23:2807. [Google Scholar]
- 161.Miyano M, Dorn CR. J. Org. Chem. 1972;37:259. doi: 10.1021/jo00967a017. [DOI] [PubMed] [Google Scholar]
- 162.Woods LL, Sapp J. J. Org. Chem. 1962;27:3703. [Google Scholar]
- 163.Hoefnagel AJ, Gunnewegh EA, Downing RS, Bekkum H. van. J. Chem. Soc. Chem. Commun. 1995:225. [Google Scholar]
- 164.Holder MS, Crouch RDD. J. Chem. Ed. 1998;75:1631. [Google Scholar]
- 165.Hua DH, Saha S, Roche D, Maeng JC, Iguch S, Baldwin C. J. Org. Chem. 1992;57:399. [Google Scholar]
- 166.Woo LL, Purohit A, Malini B, Reed MJ, Potter BV. Chem. Biol. 2000;7:773. doi: 10.1016/s1074-5521(00)00023-5. [DOI] [PubMed] [Google Scholar]
- 167.Furniss BS, Hannaford AJ, Smith PWG, Tatchel AR. Vogel ‘s Textbook of Practical Organic Chemistry. UK: Wiley, Pearson Education Ltd; 1989. [Google Scholar]
- 168.Hepwarh JD. In: Comprehensive Heterocyclic Chemistry. Boulton AJ, McKillop A, editors. vol. 3. Pergamon: Oxford; 1984. p. 881. [Google Scholar]
- 169.Kanojia RM, Jain FN, Ng R, Sui Z, Xu J. WO2003053977. 2003:1–306.
- 170.Kanojia RM, Jain N, Xu J, Sui Z. Tetrahedron Lett. 2004;45:5837. [Google Scholar]
- 171.Horvath A, De Smet K, Ormerod D, Depre′ D, Pe′rez-Balado C, Govaerts T, Van den Heuvel D, Schorpion I. Org. Process Res. Dev. 2005;9:356. [Google Scholar]
- 172.Jones G. Org. React. 1967;15:204. [Google Scholar]
- 173.Reddy NS, Mallireddigari MR, Cosenza S, Gumireddy K, Bell SC, Reddy EP, Reddy MVR. Bioorg. Med. Chem. Lett. 2004;14:4093. doi: 10.1016/j.bmcl.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 174.Reddy NS, Gumireddy K, Mallireddigari MR, Cosenza SC, Venkatapuram P, Bell SC, Reddy EP, Reddy MV. Bioorg. Med. Chem. 2005;13:3141. doi: 10.1016/j.bmc.2005.02.051. [DOI] [PubMed] [Google Scholar]
- 175.Reddy MVR, Reddy S. Acta. Chim. Hung. 1984;115:269. [Google Scholar]
- 176.Shan B, Medina JC, Santha E, Frankmoelle WP, Chou TC, Learned RM, Narbut MR, Stott D, Wu P, Jaen JC, Rosen T, Timmermans PBMWM, Beckmann H. Proc. Natl. Acad. Sci. USA. 1999;96:5686. doi: 10.1073/pnas.96.10.5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Medina JC, Shan B, Beckmann H, Farrell RP, Clark DL, Learned RM, Roche D, Li A, Baichwal V, Case C, Baeuerle PA, Rosen T, Jaen JC. Bioorg. Med. Chem. Lett. 1998;8:2653. doi: 10.1016/s0960-894x(98)00477-6. [DOI] [PubMed] [Google Scholar]
- 178.Silvio C, Lorenzo P, Alberti B. Gazz. Chim. Ital. 1967;97:1749. [Google Scholar]
- 179.Mandour AH, Ahmed Kh. M, Nassar MI, El-Bazza ZE. Egyptian J. Pharm. Sci. 1995;36:71. [Google Scholar]
- 180.Imam I, Aleem El, Abd AH, Bary El, Abd H, Hossny AM. Afinidad. 2000;57:217. [Google Scholar]
- 181.Ismail II, El-Hafiz OM, Nassar MI. Afinidad. 2002;59:211. [Google Scholar]
- 182.Mckie JA, Bhagwat SS, Renaud J, Missbach M. US 6620838. 2003;B1:1–22.
- 183.Drewes SE, Njamela OL, Emslie ND, Ramesar N, Field JS. Synth. Commun. 1993;23:2807. [Google Scholar]