Abstract
Purpose
Nitric oxide mediates urethral smooth muscle relaxation and may also be involved in detrusor activity control. Mice with mutation in the Immp2l gene have high superoxide ion levels and a consequent decrease in the bioavailable amount of nitric oxide. We studied bladder function in this mouse model.
Material and Methods
Young male mutants at ages 4 to 6 months, old female mutants at age 18 months and healthy WT age matched controls were used. The detrusor contractile response to carbachol and electrical field stimulation was tested in isolated detrusor strips in organ baths. In vivo bladder function was evaluated by cystometry in conscious animals.
Results
Young male mutants had significantly lower micturition and higher post-void residual volume than WT controls. They had pronounced voiding difficulty and strained when initiating micturition. Detrusor contractile responses to carbachol and electrical field stimulation were similar in mutant and WT mice. Old female mutant mice had lower bladder capacity and micturition volume, and higher micturition frequency and bladder-to-body weight ratio than WT controls. In the in vitro study detrusor strips from mutants showed a lower maximum response to carbachol.
Conclusions
Mice with mutation in the Immp2l gene have bladder dysfunction, mainly characterized by emptying abnormalities in young males and increased detrusor activity in old females. Detrusor function was preserved in young males and impaired in old females. These animals are a natural model of oxidative stress with low bioavailable nitric oxide. Thus, they are interesting tools in which to evaluate the role of these conditions on bladder dysfunction.
Keywords: urinary bladder, urination disorders, nitric oxide, muscle contraction, mutation
Reactive oxygen species are normal products of aerobic metabolism. When ROS production overcomes the antioxidant defense mechanism due to pathophysiological conditions, the result is oxidative stress. ROS can react with DNA, proteins and lipids, leading to strand break, protein oxidation and lipid peroxidation, respectively.1 The reaction between ROS and NO leads to NO scavenging and production of intermediates such as RNS associated with indirect effects.
ROS and RNS are involved in the pathophysiological mechanisms of different conditions affecting bladder function, such as diabetes, aging and bladder outlet obstruction. Rats with diabetes induced by streptozotocin showed increased catalase-like enzyme activity and thiobarbituric acid reactive substance levels as well as an increased number of apoptotic cells in the detrusor.2 These findings suggest that oxidative stress in these animals contributes to bladder dysfunction. With aging there seems to be a decrease in antioxidant mechanisms, leading to increased sensitivity to ROS and RNS mediated bladder damage.3,4 Partial bladder outlet obstruction in various animal species is associated with higher protein oxidation, and decreased antioxidant capacity, nerve density and detrusor contractile responses.5–7
Mitochondria have a central role in the production of cellular energy by adenosine triphosphate synthesis via oxidative phosphorylation. In this process mitochondria produce most endogenous ROS and RNS. In mitochondrial dysfunction cases the production of these radicals is increased, leading to damage to the mitochondrial respiratory chain and consequently a further increase in oxidant generation.8
Recently the new Immp2lTg(Tyr)979Ove mutant mouse with mitochondrial dysfunction was described.2 These animals have a deficiency of Immp2l protein and show abnormal signal peptide sequence processing of the mitochondrial proteins cytochrome c1 and glycerol-3-phosphate dehydrogenase. Mitochondria in these mutant mice manifest hyperpolarization, increased superoxide ion generation and higher adenosine triphosphate, probably due to a high mitochondrial respiration rate. Homozygous mutant females are infertile due to defects in folliculogenesis and ovulation, and mutant males are severely subfertile and manifest erectile dysfunction. Young mutant male mice show normal spermatogenesis but older animals show a higher rate of germ cell apoptosis. It was hypothesized that the ROS scavenger system in young animals can partly compensate for the increased superoxide while in older mice decreased ROS scavenger activity and/or chronic exposure to mitochondrial ROS generation leads to apoptosis.9
We used mutant Immp2lTg(Tyr)979Ove mice to study bladder function in vivo and in vitro. Due to increased superoxide ion generation these animals may be an interesting model in which to evaluate the effects of oxidative stress and low bioavailability of NO on the urinary tract.
MATERIALS AND METHODS
Animals
The Immp2lTg(Tyr)979Ove mouse mutant studied was generated by a transgenic insertional mutagenesis strategy. By injecting a tyrosinase minigene into fertilized eggs of albino FVB mice and screening for offspring with phenotypes in the reproductive system a mutant line with impaired fertility in each gender was obtained. The genomic lesion of the mutant line was mapped to chromosome 12, disrupting the Immp2l gene.9 Animals were bred and housed at the Wake Forest University Health Sciences animal facilities. Experiments were approved by the local animal care and use committee and done in accordance with the National Research Council Guide for Care and Use of Laboratory Animal. Animals older than 18 months were considered old and animals 4 to 6 months old were considered young. Due to limited availability of mutant animals only young males and old females were used in the study.
Organ Bath Techniques
Five WT and 6 homozygous young male mutants were used as well as 6 WT and 6 homozygous old female mutants. They were sacrificed by CO2 suffocation, followed by cervical dislocation. Bladders were removed with the bladder neck as the resection margin. Adherent fat was removed. The mucosa was removed by gently scraping the luminal surface with a scalpel. Two circular (transverse right to left) strips approximately 3 × 6 mm were prepared from each bladder. All strips were mounted immediately after removal in a 750 TOBS (Danish Myo Technology, Aarhus, Denmark) organ bath containing 37C 95% oxygenated Krebs solution composed of 119 mM NaCl, 4.6 mM KCl, 1.5 mM CaCl2, 1.2 mM MgCl2, 15 mM NaHCO3, 1.2 mM NaH2PO4 and 5 mM glucose, resulting in pH 7.4. They were suspended between 2 hooks (1 fixed and 1 connected to a force transducer) using silk ligatures, stretched to 2 gm tension, allowed to equilibrate for 60 minutes and washed once with fresh Krebs solution. Data were collected via compatible PowerLab™ data acquisition software. The system was calibrated before each experiment. Krebs solution was tested with pH indicator paper to verify pH 7.4.
Each experiment was started by exposing strips to a high K+ (60 mM) Krebs solution until 2 reproducible contractions were obtained. Nonreacting strips were discarded. Between exposures strips were washed several times with changes of fresh Krebs solution. Carbachol concentration-response curves were obtained by cumulative adding at half increments (10–8.5 to 10–4 M). After pharmacological stimulation strips were washed with several changes of fresh Krebs solution and allowed to rest for 30 minutes before electrical stimulation. EFS was done by parallel platinum electrodes positioned at either side of the strips and connected to an S88 square pulse stimulator (Grass Instruments, Quincey, Massachusetts). Square pulse duration was 0.5 milliseconds, voltage was 20 V and train duration was 10 seconds. Between stimuli the strips were allowed to rest for 2 minutes. Contractile responses to EFS were studied at a frequency of 1, 2, 4, 8, 16 and 32 Hz. After experiments the strips were removed from the organ bath and weighed. Data were corrected by weight and, thus, results are expressed in gm tension per gm of tissue.
Cystometry
Bladder catheter implantation
Five young male WT, 5 young male homozygous mutant, 5 old female WT and 5 old female homozygous mutant mice were anesthetized with 3% isoflurane inhalation. A small incision was made in the bladder dome and a PE-10 polyethylene catheter (Clay Adams, Parsippany, New Jersey) with a cuff was inserted and anchored with 6-zero silk purse-string suture. Saline was injected via the catheter to exclude bladder leakage. The catheter was tunneled subcutaneously to the neck of the animal and thermally sealed.
Cystometry
Cystometry was done 3 days after bladder catheterization without anesthesia. The bladder catheter was connected via a T tube to a pressure transducer and an infusion pump. Room temperature saline was infused into the bladder at a rate of 2 ml per hour. The pressure transducer was connected via a transducer amplifier to PowerLab data acquisition software. Conscious mice were placed in metabolic cages without restraint. Urine volume was measured by a fluid collector connected to a force displacement transducer, which was connected via a transducer amplifier to the software. Intravesical pressure and micturition volumes were recorded synchronously and continuously. After a 30-minute equilibration phase data were collected for 60 minutes. The system was calibrated before each experiment. The bladder was emptied by manual compression before measurement.
The cystometric parameters investigated were maximum pressure (maximum bladder pressure during micturition), threshold pressure (bladder pressure at micturition onset), baseline pressure (minimum bladder pressure between 2 micturitions), intermicturition pressure (mean bladder pressure between 2 micturitions), spontaneous activity (intermicturition pressure minus baseline pressure), micturition frequency per hour, bladder capacity (infusion rate divided by micturition frequency), micturition volume, post-void residual volume (bladder capacity minus micturition volume), bladder compliance (bladder capacity divided by threshold minus baseline pressure) and time from the first bladder pressure increase over baseline to maximum pressure during micturition. After the procedure the animals were sacrificed and the bladders were removed and weighed.
Statistical Analysis
Comparisons between groups were done by Student's t test. Pharmacological concentration-response curves were generated by nonlinear regression and analyzed by the F test. Statistical significance was considered at p <0.05. We used SigmaStat® for Windows®, version 2.0 and Prism® for Windows, version 4.03.
RESULTS
The bladder weight/body weight ratio was significantly higher in the mutant old female group compared to matched controls (mean ± SD 1.6 ± 0.44 vs 0.94 ± 0.15 mg/gm). This difference was not observed in young males.
Organ Bath Studies
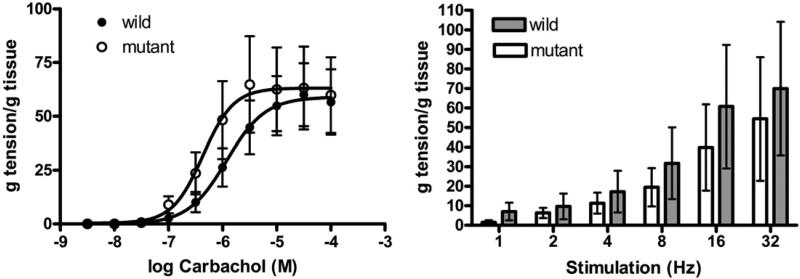
In young male mice detrusor muscle contractile responses to carbachol were not statistically different in WT vs mutant animals. The maximum response was similar in the WT and mutant groups (58.95 ± 6.73 and 63.16 ± 7.63 gm/gm, –logEC50 5.92 ± 0.28 and 6.36 ± 0.24 gm/gm, respectively). Likewise detrusor contractions evoked by EFS were not statistically different (fig. 1).
Figure 1.
Mean ± SEM contractile response to carbachol and EFS in isolated detrusor strips in organ bath studies in WT and Immp2l mutant mice. g, gm.
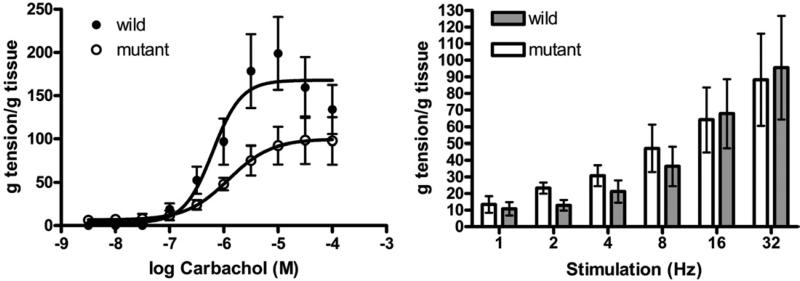
In bladder strips from old female mutant mice exposure to progressive concentrations of carbachol resulted in a weaker response than in strips from age matched WT mice. The maximum response in mutant mice was significantly lower than in WT mice (99.9 ± 12.07 vs 169.2 ± 14.42 gm/gm, p <0.001) but –logEC50 was similar (5.91 ± 0.23 and 6.19 ± 0.16 gm/gm, respectively). EFS produced similar responses in these groups (fig. 2).
Figure 2.
Mean ± SEM contractile response to carbachol and EFS in isolated detrusor strips in organ bath studies in WT and Immp2l mutant mice. g, gm.
Cystometry
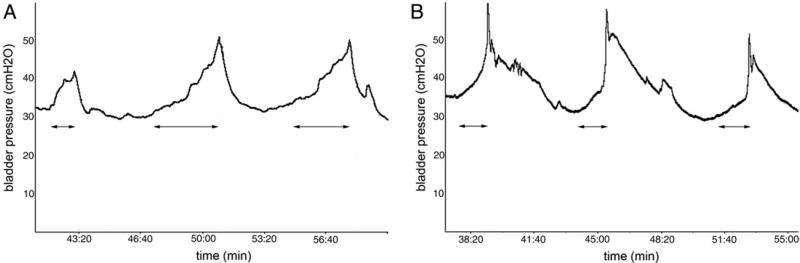
Young male mutant mice showed significantly lower micturition volume and higher post-void residual volume than WT mice. During cystometry and simultaneously with the increase in bladder pressure mutant mice showed abnormal behavior, had voiding difficulty and strained when initiating micturition. This behavior ceased after voiding. This observation was objectified by calculating the variation in time between the first bladder pressure increase and maximum pressure during voiding (fig. 3). This was significantly higher in mutant mice (see table).
Figure 3.
Representative cystometry traces in young male mice. A, Immp2l mutant. B, WT.
Cystometric findings in WT and mutant young male and old female mice
| Mean ± SD WT | Mean ± SD Mutant | p Value (Student's t test) | |
|---|---|---|---|
| Young male | |||
| Bladder capacity (ml) | 0.24 ± 0.11 | 0.25 ± 0.07 | |
| Bladder compliance (ml/cm H2O) | 0.02 ± 0.01 | 0.02 ± 0.01 | |
| Voiding frequency (No.) | 9.8 ± 4.32 | 9.6 ± 2.07 | |
| Vol (ml): | |||
| Voided | 0.2 ± 0.04 | 0.08 ± 0.07 | <0.05 |
| Post-void residual | 0.04 ± 0.03 | 0.17 ± 0.03 | <0.001 |
| Pressure (cm H2O): | |||
| Baseline | 29.9 ± 6.95 | 32.08 ± 1.46 | |
| Threshold | 42.2 ± 2.49 | 49.66 ± 8.74 | |
| Max | 58.98 ± 5.44 | 60.12 ± 13.58 | |
| Intermicturition | 35.52 ± 4.66 | 37.62 ± 3.28 | |
| Spontaneous activity | 5.62 ± 2.99 | 5.54 ± 1.94 | |
| Time to max pressure (secs) | 24.18 ± 15.16 | 77.9 ± 29.38 | <0.05 |
| Bladder/body wt (mg/gm) | 1.08 ± 0.20 | 1.18 ± 0.17 | |
| Old female | |||
| Bladder capacity (ml) | 0.2 ± 0.06 | 0.07 ± 0.01 | <0.05 |
| Bladder compliance (ml/cm H2O) | 0.012 ± 0.005 | 0.004 ± 0.001 | <0.05 |
| Voiding frequency (No.) | 10.75 ± 4.27 | 29.5 ± 3.79 | <0.001 |
| Vol (ml): | |||
| Voided | 0.17 ± 0.06 | 0.02 ± 0.01 | <0.05 |
| Post-void residual | 0.04 ± 0.04 | 0.05 ± 0 | |
| Pressure (cm H2O): | |||
| Baseline | 29.85 ± 2.04 | 40.45 ± 6.59 | <0.05 |
| Threshold | 47.28 ± 2.14 | 60.45 ± 13.70 | |
| Max | 64.54 ± 15.72 | 88.28 ± 19.85 | |
| Intermicturition | 35.15 ± 1.86 | 46.35 ± 8.34 | <0.05 |
| Spontaneous activity | 5.30 ± 0.43 | 6.20 ± 2.21 | |
| Time to max pressure (secs) | 28.36 ± 17.82 | 20.55 ± 14.67 | |
| Bladder/body wt (mg/gm) | 0.94 ± 0.15 | 1.6 ± 0.44 | |
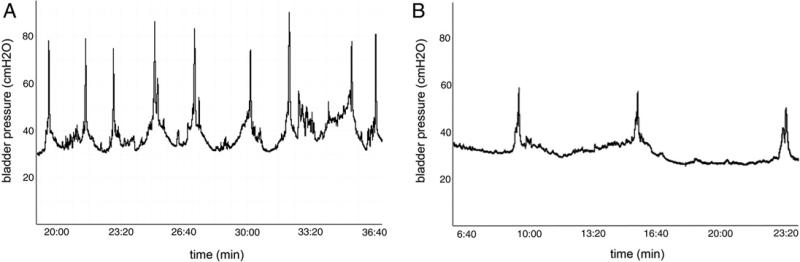
Old female mutant mice showed several significant differences on cystometry compared to WT mice. Bladder capacity and compliance were significantly lower in mutants, which also showed higher micturition frequency and lower micturition volume. Baseline and intermicturition pressure was increased (see table). Figure 4 shows representative cystometric recordings in the 2 groups.
Figure 4.
Representative cystometry traces in old female mice. A, Immp2l mutant. B, WT.
DISCUSSION
NO is an important neurotransmitter and cell mediator in the lower urinary tract, acting as a nonadrenergic, noncholinergic inhibitory factor that is possibly responsible for bladder relaxation during the filling phase and urethral relaxation during voiding.10 The role of this system can be studied by interfering with any steps of the NO-guanylate cyclase-cGMP pathway. In rats inhibition of NO synthesis by N-nitro-L-arginine-methyl ester HCl caused bladder overactivity.11 Intravesical administration of the NO scavenger oxy-hemoglobin induced bladder overactivity, and increased micturition pressure and residual urine, suggesting an increase in outflow resistance. These findings were attributable to possible inhibition of NO generated in the bladder and urethra urothelium or suburothelium.12 Mice lacking cGMP-dependent protein kinase had a decreased urethral relaxant re sponse to nerve derived NO.13 Cystometric analysis revealed bladder overactivity, characterized by decreased intercontraction intervals and nonvoiding contractions. The distribution of NO synthase containing nerves was similar in mutant and WT mice in that study, showing that the source of NO production was present. Burnett et al evaluated bladder and urethral sphincter function in mice with targeted deletion of the gene for neuronal NO synthase.14 The animals showed dilated bladders, increased micturition frequency and loss of neurally mediated relaxation of bladder and urethral muscle.
Our study is in accordance with the mentioned studies concerning findings of increased outflow resistance in young males and increased detrusor activity in old females. Cystometry revealed that young male mutants had lower micturition volume and higher post-void residual volume than WT mice. During voiding contractions these animals seemed perturbed and showed cyclic downward pelvic movements until achieving micturition. This behavior was interpreted as straining and objectified by the longer duration of contraction curves in these animals. Since Immp2lTg(Tyr)979Ove mice have high superoxide levels, NO bioavailability is probably low. This may lead to impaired urethral relaxation, which could explain the apparently increased outflow resistance. This group did not show any cystometric signs of increased activity and the bladder-to-body weight ratio was similar to that in controls. This is in contrast to old females, who had a significantly higher bladder-to-body weight ratio than controls. Likewise in young male mutants detrusor contractile responses to carbachol and to EFS were not affected.
Old female mutant mice showed cystometric signs of increased detrusor activity, characterized by low bladder capacity, and high micturition frequency and intermicturition pressure. These findings in old female mutants may be a consequence of chronic exposure to ROS, assuming that with aging the ROS scavenging mechanism may not be as efficient as in young animals.9 Furthermore, low NO bioavailability in these mutants may be a factor contributing to increased detrusor activity. NO may hyperpolarize the afferent nerves, decreasing nerve firing during filling and, thus, increasing the volume threshold to trigger bladder contraction.12 Another possible factor for this behavior is increased outflow resistance. Female mutant mice did not have higher post-void residual volume, which may have been due to high micturition frequency. Although threshold and maximum pressures were not statistically significant, they were increased in these animals compared to WT controls and, as mentioned, the bladder-to-body weight ratio was significantly higher. It remains to be determined whether this is a gender or age related phenomenon. In in vitro studies isolated detrusor strips from mutants showed a lower maximum response to carbachol. Damage to cholinergic receptors by oxidative stress may be a mechanism underlying these changes.3,15 However, the detrusor contractile response to EFS was similar in mutant and WT animals. This may be attributable to purinergic transmission, which is well established in rodents. An age related increase in purinergic transmission in the detrusor was also described in humans.16 Such a possible compensatory mechanism may explain our finding.
The effect of ROS on the lower urinary tract has been studied in animal models. Intravesical H2O2 administration caused detrusor overactivity in rats in a dose dependent manner.17 Detrusor contractile responses to KCl, carbachol and to a lesser extent EFS were decreased when H2O2 was applied to the organ bath.3 These changes were more pronounced in old than in young rats, suggesting that aging may increase bladder sensitivity to oxidative damage.
Because homozygous mutants are infertile, obtaining enough mutant animals, especially old animals, is time consuming. We could not study each gender at young and old ages. Thus, we do not know whether findings in young males would also be observed in young females or findings in old females would also be observed in old males. Nevertheless, results clearly show that mutant animals manifested bladder dysfunction compared with their age matched controls. Further study is needed to determine whether the observed phenomena are age and/or gender related.
Disregarding the age factor, the Immp2lTg(Tyr)979Ove mouse proved to be an interesting tool in which to study the effect of natural, noninduced oxidative stress and low NO bioavailability on bladder function. These animals can be included in the list of models in which changes in the NO-guanylate cyclase-cGMP pathway lead to loss of effect on the target organs/tissues. They may represent an interesting model of disease in which increased superoxide levels are involved in patho-physiological changes, such as diabetes and aging.
CONCLUSIONS
Mice with a mutation in the Immp2l gene, which leads to high superoxide ion production, show bladder dysfunction. In young male mutant mice emptying abnormalities were seen on conscious cystometry. Old female mutant mice had increased detrusor activity, a higher bladder-to-body weight ratio and a lower maximum detrusor contractile response to carbachol than WT controls. These animals are a natural model of oxidative stress and consequent low bioavailability of NO. Thus, they are an interesting tool in which to study the role of these conditions on bladder function.
Acknowledgments
Supported by a post-doctoral scholarship from the National Counsel of Technological and Scientific Development, Brazil (RS).
Abbreviations and Acronyms
- cGMP
cyclic guanosine monophosphate
- EFS
electrical field stimulation
- Immp2l
inner mitochondrial membrane peptidase 2-like
- NO
nitric oxide
- NOS
NO synthase
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
REFERENCES
- 1.Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 2.Beshay E, Carrier S. Oxidative stress plays a role in diabetes-induced bladder dysfunction in a rat model. Urology. 2004;64:1062. doi: 10.1016/j.urology.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Aikawa K, Leggett RE, Levin RM. Effect of age on hydrogen peroxide mediated contraction damage in the male rat bladder. J Urol. 2003;170:2082. doi: 10.1097/01.ju.0000081461.73156.48. [DOI] [PubMed] [Google Scholar]
- 4.Gomez-Pinilla PJ, Gomez MF, Sward K, et al. Melatonin restores impaired contractility in aged guinea pig urinary bladder. J Pineal Res. 2008;44:416. doi: 10.1111/j.1600-079X.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- 5.de Jongh R, Dambros M, Haenen GR, et al. Partial bladder outlet obstruction reduces the tissue antioxidant capacity and muscle nerve density of the guinea pig bladder. Neurourol Urodyn. 2009;28:461. doi: 10.1002/nau.20677. [DOI] [PubMed] [Google Scholar]
- 6.Siflinger-Birnboim A, Levin RM, Hass MA. Partial outlet obstruction of the rabbit urinary bladder induces selective protein oxidation. Neurourol Urodyn. 2008;27:532. doi: 10.1002/nau.20557. [DOI] [PubMed] [Google Scholar]
- 7.Lin WY, Guven A, Juan YS, et al. Free radical damage as a biomarker of bladder dysfunction after partial outlet obstruction and reversal. BJU Int. 2008;101:621. doi: 10.1111/j.1464-410X.2007.07389.x. [DOI] [PubMed] [Google Scholar]
- 8.Leon J, Acuna-Castroviejo D, Sainz RM, et al. Melatonin and mitochondrial function. Life Sci. 2004;75:765. doi: 10.1016/j.lfs.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Lu B, Poirier C, Gaspar T, et al. A mutation in the inner mitochondrial membrane peptidase 2-like gene (Immp2l) affects mitochondrial function and impairs fertility in mice. Biol Reprod. 2008;78:601. doi: 10.1095/biolreprod.107.065987. [DOI] [PubMed] [Google Scholar]
- 10.Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev. 2004;84:935. doi: 10.1152/physrev.00038.2003. [DOI] [PubMed] [Google Scholar]
- 11.Persson K, Igawa Y, Mattiasson A, et al. Effects of inhibition of the L-arginine/nitric oxide pathway in the rat lower urinary tract in vivo and in vitro. Br J Pharmacol. 1992;107:178. doi: 10.1111/j.1476-5381.1992.tb14483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandita RK, Mizusawa H, Andersson KE. Intravesical oxyhemoglobin initiates bladder over-activity in conscious, normal rats. J Urol. 2000;164:545. [PubMed] [Google Scholar]
- 13.Persson K, Pandita RK, Aszodi A, et al. Functional characteristics of urinary tract smooth muscles in mice lacking cGMP protein kinase type I. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1112. doi: 10.1152/ajpregu.2000.279.3.R1112. [DOI] [PubMed] [Google Scholar]
- 14.Burnett AL, Calvin DC, Chamness SL, et al. Urinary bladder-urethral sphincter dysfunction in mice with targeted disruption of neuronal nitric oxide synthase models idiopathic voiding disorders in humans. Nat Med. 1997;3:571. doi: 10.1038/nm0597-571. [DOI] [PubMed] [Google Scholar]
- 15.de Jongh R, Haenen GR, van Koeveringe GA, et al. Oxidative stress reduces the muscarinic receptor function in the urinary bladder. Neurourol Urodyn. 2007;26:302. doi: 10.1002/nau.20298. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida M, Miyamae K, Iwashita H, et al. Management of detrusor dysfunction in the elderly: changes in acetylcholine and adenosine triphosphate release during aging. Urology. 2004;63:17. doi: 10.1016/j.urology.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Masuda H, Kihara K, Saito K, et al. Reactive oxygen species mediate detrusor overactivity via sensitization of afferent pathway in the bladder of anaesthetized rats. BJU Int. 2008;101:775. doi: 10.1111/j.1464-410X.2007.07310.x. [DOI] [PubMed] [Google Scholar]