Abstract
The location of glucocorticoid receptors (GR) implicated in depression symptoms and antidepressant action remains unclear. Forebrain glucocorticoid receptor deletion on a C57B/6×129×CBA background (FBGRKO-T50) reportedly produces increased depression-like behavior and elevated glucocorticoids. We further hypothesized that forebrain GR deletion would reduce behavioral sensitivity to glucocorticoids and to antidepressants. We have tested this hypothesis in mice with calcium calmodulin kinase IIα-Cre-mediated forebrain GR deletion derived from a new founder on a pure C57BL/6 background (FBGRKO-T29-1). We measured immobility in forced swim or tail suspension tests after manipulating glucocorticoids or after dose response experiments with tricyclic or monoamine oxidase inhibitor antidepressants. Despite forebrain GR deletion that was at least as rapid and more extensive than reported in the mixed-strain FBGRKO-T50 mice (Boyle et al. 2005), and possibly because of their different founder, our FBGRKO-T29-1 mice did not exhibit increases in depression-like behavior or adrenocortical axis hormones. Nevertheless, FBGRKO-T29-1 mice were at least as sensitive as floxed GR controls to the depressive effects of glucocorticoids and the effects of two different classes of antidepressants. FBGRKO-T29-1 mice also unexpectedly exhibited increased mineralocorticoid receptor (MR) gene expression. Our results reinforce prior evidence that antidepressant action does not require forebrain GR, and suggest a correlation between the absence of depression-like phenotype and combined MR up-regulation and central amygdala GR deficiency. Our findings demonstrate that GR outside the areas targeted in FBGRKO-T29-1 mice are involved in the depressive effects of glucocorticoids, and leave open the possibility that these GR populations also contribute to antidepressant action.
Keywords: depression, glucocorticoid receptor, mineralocorticoid receptor, hypothalamic-pituitary-adrenal axis, antidepressant
1. INTRODUCTION
Activity of the hypothalamic-pituitary-adrenocortical (HPA) axis is often increased in depression, and has garnered considerable interest as a possible biomarker of this disease. HPA activity is elevated or resistant to normal glucocorticoid feedback inhibition in almost 80% of depressed patients (Heuser et al., 1994). HPA dysregulation can be reversed by successful antidepressant treatment, but patients exhibiting continued HPA hyperactivity after antidepressant treatment are more likely to relapse (Hatzinger et al., 2002). These findings have suggested that HPA activity could be a useful predictor of response to antidepressants.
Glucocorticoids, the end product of the HPA axis, also have complex effects on mood, which raises the question of whether elevated HPA activity might be contributing to, as well as reflecting, the state of depression. Glucocorticoid excess, which can result from Cushing's syndrome or immunosuppressive therapy, can induce depression or symptoms associated with depression, including psychosis, anxiety, mania, sleep and appetite disturbances, and cognitive impairment. The impact of glucocorticoids on mood is unrelated to the psychiatric history of the individual or the severity of any immunologic disease for which glucocorticoids may have been prescribed (Brown et al., 1999; Loosen, 2002; Sirois, 2003; Starkman and Schteingart, 1981). Glucocorticoid receptor antagonists, such as RU 38486, or glucocorticoid synthesis inhibitors, such as ketoconazole and metyrapone, have been reported to improve mood in major depression and Cushing's syndrome (Jahn et al., 2004; Murphy, 1997; O'Dwyer et al., 1995; Wolkowitz et al., 1999), suggesting that glucocorticoid secretion might be contributing to depression symptoms in these patients. Depression-like behaviors in rodents are increased by glucocorticoids and reduced by glucocorticoid antagonists (DeKloet et al., 1988; Mitchell and Meaney, 1991; Sterner and Kalynchuk, 2010). Glucocorticoids also have inhibitory effects on the expression of norepinephrine- and serotonin-synthesizing enzymes that could contribute to the monoamine deficiencies hypothesized to underlie depression (reviewed in (Gass et al., 2001; Heydendael and Jacobson, 2010)). Thus, there is intriguing, if limited, evidence to suggest that HPA activity could be relevant to the causes as well as the treatment of depression.
Excess glucocorticoid secretion is normally prevented by glucocorticoid negative feedback (Keller-Wood and Dallman, 1984), which is mediated by high-affinity mineralocorticoid receptors (MR) and lower affinity glucocorticoid receptors (GR) in the brain (DeKloet et al., 1998; Gass et al., 2001; Kellendonk et al., 2002; Pace and Spencer, 2005; Pariante and Miller, 2001; Reichardt et al., 1998). HPA hyperactivity in depressed patients has been attributed to deficits in glucocorticoid receptor levels or function (Barden, 2004). This interpretation is supported by the dexamethasone suppression test, in which dexamethasone, a synthetic glucocorticoid receptor ligand, causes inhibition of endogenous glucocorticoid release in healthy patients, but is frequently less effective in inhibiting glucocorticoid release in depressed patients (Holsboer et al., 1982). Furthermore, depressed patients who previously were dexamethasone nonsuppressors often pass the dexamethasone test after antidepressant treatment (Gass et al., 2001; Holsboer and Barden, 1996; Zobel et al., 2001). These findings have formed the basis for the corticosteroid receptor hypothesis of depression, which posits that the impaired glucocorticoid negative feedback system observed in depressed patients is due to decreases in the levels or function of glucocorticoid receptors in the brain, and that antidepressant treatment can enhance glucocorticoid receptor function or expression, thus normalizing glucocorticoid negative feedback and HPA activity (Gass et al., 2001; Holsboer, 2000; Jefferys D, 1983; Pariante CM, 2001). In support of this hypothesis, post-mortem studies of depressed patients and suicide victims have found decreased glucocorticoid receptor expression, while rodents treated with antidepressants often exhibit increases in GR expression (Pariante and Miller, 2001). Animal models with glucocorticoid receptor deficits have also been reported to show a depression-like phenotype and HPA hyperactivity, further supporting the idea that decreases in glucocorticoid receptor function may be responsible for the abnormal HPA activity observed in depressed patients (Boyle et al., 2005; Montkowski et al., 1995; Ridder et al., 2005).
Notably, the best correlation to date of GR deficits with depression-like behavior has been found in models of partial GR deletion in brain. Complete GR deficiency in brain has been more clearly linked with effects on anxiety; although brain GR knockout mice exhibit less depression-like immobility in the forced swim test, it is unclear if this behavior is attributable to memory impairment also demonstrated in this model (Gass et al., 2001; Kellendonk et al., 2002). In models of partial brain GR deletion, GR expression was knocked out only in forebrain (Boyle et al., 2005), or was reduced in neurons or all tissues by targeted knockdown (Montkowski et al., 1995) or haploinsufficiency (Ridder et al., 2005). Thus, GR still remain in these models for occupancy by glucocorticoids, and all models exhibited some degree of corticosteroid overproduction resulting from the loss of GR-mediated feedback (Boyle et al., 2005; Montkowski et al., 1995; Ridder et al., 2005).
Since elevated glucocorticoids can cause depression-like behavior (DeKloet et al., 1988; Mitchell and Meaney, 1991; Sterner and Kalynchuk, 2010), these models raise the question whether the primary significance of HPA hyperactivity in depression is related to GR impairment or to glucocorticoid oversecretion. It is similarly unclear whether antidepressant normalization of HPA activity might have additional benefits by mitigating the depressive effects of glucocorticoid excess. Further complicating this issue, glucocorticoids have also been found to have mood-elevating effects, evident either as positive responses to glucocorticoid administration or as difficulty in weaning from glucocorticoid treatment (Arana et al., 1995; Bouwer et al., 2000; DeBattista et al., 2000; Dinan et al., 1997; Sirois, 2003). Thus, the GR loss postulated in the corticosteroid hypothesis of depression could alone have depressive effects by abrogating the positive mood effects of glucocorticoids. Consequently, it is unclear whether GR or glucocorticoid levels are more important to depression symptoms.
The forebrain glucocorticoid receptor knockout (FBGRKO) mouse developed by the Muglia lab (Boyle et al., 2005) offers a convenient model to address this question. In this model, forebrain glucocorticoid receptor deletion on a mixed C57BL/6 × 129 × CBA background was achieved by breeding in floxed GR mice with mice transgenic for Cre recombinase driven by the calcium calmodulin kinase IIα (CamKIIα) promoter (T50 founder line; (Tsien et al., 1996)). GR deletion in this model, henceforth referred to as FBGRKO-T50, was nearly complete in corticolimbic areas such as the cerebral cortex, nucleus accumbens, hippocampus and the most subdivisions of the amygdala, with partial (~ 50%) deletion in the bed nucleus of the stria terminalis (Boyle et al., 2006). FBGRKO-T50 mice were reported to exhibit a depression-like phenotype, with increased depression-like behavior (measured by forced swim, tail suspension, and sucrose preference tests), increased HPA activity, and impaired dexamethasone suppression (Boyle et al., 2005; Solomon et al., 2012). Supporting the relevance of the FBGRKO-T50 phenotype to depression, both HPA activity and depression behaviors are normalized by chronic treatment of FBGRKO-T50 mice with the antidepressant imipramine (Boyle et al., 2005). Questions remaining unanswered with this model, however, include whether GR loss or glucocorticoid excess account for the elevated depression-like behavior in FBGRKO-T50 mice. In addition, since forebrain GR are targeted by antidepressants, it is unknown if forebrain GR loss diminishes antidepressant sensitivity, even if, as shown by Boyle et al., forebrain GR deletion does not eliminate responsiveness to a relatively high dose of antidepressant (Boyle et al., 2005).
To test if forebrain GR are required for full behavioral sensitivity to glucocorticoids or antidepressants, we began experiments with FBGRKO-T50 mice. When the depression-like phenotype of these mice did not appear to be robust, we derived a new line of mice with CamKIIα-Cre-mediated forebrain GR deletion on a pure C57BL/6 background (henceforth referred to as FBGRKO-T29-1) using a new, commercially-available CamKIIα-Cre transgenic founder. Possibly as a result of their different founder, FBGRKO-T29-1 mice did not display increases in baseline depression-like behavior or in HPA hormones, even though their forebrain GR deletion resembled that reported in FBGRKO-T50 mice (Boyle et al., 2005; Boyle et al., 2006). However, our FBGRKO-T29-1 were as least as sensitive as C57BL/6 floxed GR controls to the depressive effects of glucocorticoids and the behaviorally-activating effects of two different classes of antidepressants. Our results suggest that the GR, and possibly other factors, influencing depression-like behavior and antidepressant response are separate from the GR targeted in FBGRKO-T29-1 mice.
2. RESULTS
2.1. Forebrain GR deletion does not ensure a robust depression-like phenotype
Initial experiments on FBGRKO-T50 mice of the mixed C57BL/6 × 129 × CBA background obtained from the Muglia lab did not reveal robust behavioral or hormonal differences resembling the depression-like phenotype originally reported by Boyle et al. or Solomon et al., even though our preliminary assessment of GR expression (not shown) revealed GR deletion consistent with that reported by these authors (Boyle et al., 2005; Solomon et al., 2012). Although FBGRKO-T50 mice exhibited trends in behavior and hormones resembling those previously reported, none of these were significant, with the most marked difference being in depression-like immobility in the forced swim test (P= 0.056). In the tail suspension test, a related test of depression-like behavior, there was no significant difference in immobility (P= 0.15), and sucrose preference, a rodent correlate of pleasure-seeking behavior, also did not differ between floxed GR and FBGRKO-T50 mice (P= 0.25; Table 1). Plasma corticosterone measured after the stress of the forced swim test was likewise similar between genotypes (P= 0.43; Table 1).
Table 1.
Depression-like behavior and plasma corticosterone in floxed GR and FBGRKO-T50 mice on the original C57BL/6 × 129 × CBA background (Boyle et al., 2005; Furay et al., 2008; Solomon et al., 2012).
| Forced Swim Immobility (5 sec bins/15 min) | Tail Suspension Immobility (5 sec bins/15 min) | Sucrose Preference (% total fluid intake) | Plasma Corticosterone (μg/dl) | |
|---|---|---|---|---|
| Floxed GR | 21±9 (5) | 27±5 (10) | 76.00±2.43 (8) | 50.5±7.7 (5) |
| FBGRKO-T50 | 55±12 (5) | 39±7 (7) | 70.01±4.38 (8) | 58.8±6.5 (5) |
Immobility was measured, as reported by Boyle et al., as the number of 5 sec bins the mouse was completely immobile during an 8 min tail suspension or a 15 min forced swim test (Boyle et al., 2005). Sucrose preference was measured as a percentage of daily total fluid intake and averaged over 10 days, as reported by Boyle et al. (Boyle et al., 2005). Plasma corticosterone was measured immediately after the 15 min forced swim test. Group sizes are indicated by the number in parentheses.
To address the possibility that genetic drift within the mixed strains of the original FBGRKO-T50 line might have obscured the depression-like phenotype, we established the new line of FBGRKO-T29-1 mice on a pure C57BL/6 background by breeding C57BL/6 floxed GR females with a commercially-available CamKIIα-Cre transgenic male from the T29-1 founder line ((Tsien et al., 1996); stock number 005359, Jackson Laboratories, Bar Harbor, ME), respectively provided and suggested by Dr. Louis Muglia (University of Cincinnati, OH). Offspring of these matings were selected for homozygosity of the floxed GR allele (Table 2). Because GR haploinsufficiency has been reported to produce a depression-like phenotype of its own (Ridder et al., 2005), we also developed a PCR screen (Table 2) to test all offspring for heterozygous GR deletion (homozygous GR deletion is lethal and would not be detected in surviving mice (Kellendonk et al., 2002)). Approximately 10% of the mice screened exhibited heterozygous germline GR deletion and were immediately killed. However, even after we derived the C57BL/6 FBGRKO-T29-1 mice and eliminated the possibility of GR haploinsufficiency, we did not detect any differences in depression-like immobility in the forced swim test (6-month old floxed GR vs. FBGRKO-T29-1 mice, 468 ± 83 vs. 582 ± 37 sec, P =0.24, n=6/group).
Table 2.
Parameters for PCR screening of floxed GR and FBGRKO mice.
| Cre Recombinase | Germline deletion of floxed GR allele | Homozygosity of floxed GR allele | |
|---|---|---|---|
| 5' primer | AAGTGCCTTCTCTACA CCTG* | CTCCTCCATTTTGCGA GC | AATCAGAATTGCTCAC TCACAA* |
| 3' Primer | TGCTTATAACACCCTG TTACG* | AAGGAGCAGTCACCA ACTGTC | CAGTGTTACTACTTCC AGTTC* |
| Mouse CRH Internal Control Primer, 5' | GCTCAGCAAGCTCACA GCAA* | AAACGGAGTAAGGGC AGGAA | |
| Mouse CRH Internal Control Primer, 3' | ACTGGATGACTCCCAT CTGCT | CTCCGGCTGCAAGAA ATTCA | |
| Cycles | 1 min, 95° 1 min, 52° 1 min, 72°* |
1 min, 95° 1 min, 55° 1 min, 72° |
1 min, 95° 1 min, 55° 1 min, 72°* |
| Cycle number | 33* | 30 | 30* |
PCR products, along with appropriate positive and negative controls, were separated on 1% (Cre recombinase) or 1.2% agarose gels (other PCR reactions).
communicated by Louis Muglia (University of Cincinnati, Cincinnati, OH).
2.2. Confirmation of GR deletion in FBGRKO-T29-1 and floxed GR mice
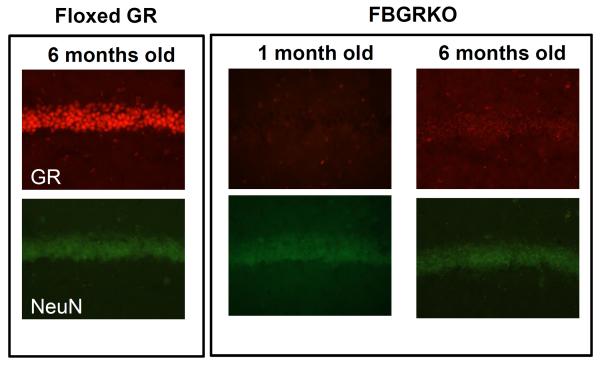
Because the CaMKIIα-Cre male founder of our FBGRKO-T29-1 line was derived from a different transgenic line than that of the mice used by Boyle et al., we confirmed appropriate GR deletion in these mice using the same reagents and techniques as Boyle et al. (L. Muglia and B. Kolber, personal communication). Coronal brain sections of 6-month-old FBGRKO-T29-1 and floxed GR mice were double-labeled for GR and the neuron-specific marker NeuN (Figure 1). Because Boyle et al. reported that FBGRKO-T50 GR deletion was age-dependent, we also tested if the lack of a depression-like phenotype in our FBGRKO-T29-1 mice might be due to delayed GR deletion. We found that as early as 1 month of age, FBGRKO-T29-1 mice exhibit GR deletion in CA1 region hippocampus that is extensive as that at 6 months of age (Figure 1); GR deletion at 2 and 4 months of age was similarly complete (not shown).
Figure 1.
Representative immunofluorescence images of GR (top) and neuronal marker NeuN (bottom) in the CA1 region of the hippocampus in 1- (n = 5) and 6-month old FBGRKO-T29-1 mice vs. 6 month old floxed GR mice (n =6 each) on a pure C57/BL6 background. Brain regions were identified using the Franklin and Paxinos mouse brain atlas (Franklin and Paxinos, 2004).
In agreement with the results reported by Boyle et al. (Boyle et al., 2006), we found extensive (>90%) loss of GR -positive cells in the cerebral cortex, striatum, nucleus accumbens, basolateral and basomedial regions of the amygdala, the dentate gyrus, and CA1 hippocampus of 6-month-old FBGRKO-T29-1 mice (Figure 2). Similar to Boyle et al. (Boyle et al., 2005), we found partial (~50%) GR deletion in the bed nucleus of the stria terminalis (Figure 2) and no significant GR deletion in the paraventricular hypothalamus (data not shown). Notably, although FBGRKO-T50 mice were originally described as having no GR loss in the central amygdaloid nucleus (Boyle et al., 2005; Boyle et al., 2006; Furay et al., 2008; Solomon et al., 2012), our age-matched FBGRKO-T29-1 mice showed approximately 50% decrease in GR-positive neurons in the central amygdala (Figure 2). Thus, GR deletion in our FBGRKO-T29-1 mice is at least as extensive as that previously reported in FBGRKO-T50 mice (Boyle et al., 2005; Boyle et al., 2006; Furay et al., 2008; Solomon et al., 2012) but occurs at an earlier age.
Figure 2.
GR expression in floxed GR and FBGRKO-T29-1 mice (n = 5–6/ group). Quantitation of GR-immunoreactive neurons was performed as described in Experimental Procedures and followed the techniques originally used by Boyle et al. (B. Kolber and L. Muglia, personal communication).
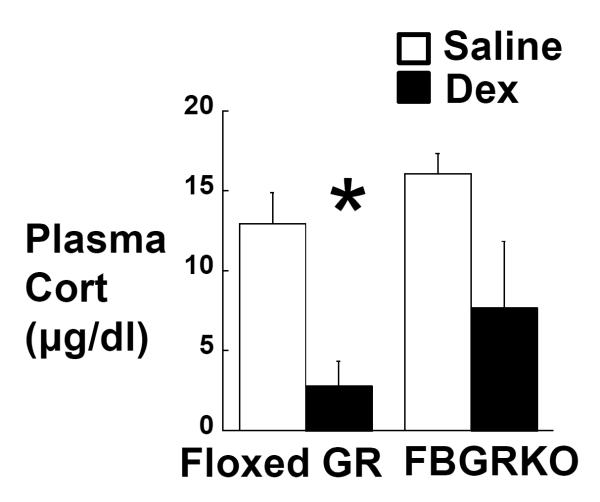
Boyle et al. used the dexamethasone suppression test to demonstrate that 6 month-old FBGRKO-T50 mice exhibit impaired HPA negative feedback similar to that observed in depressed patients (Boyle et al., 2005). We hypothesized that the earlier onset of GR deletion in our FBGRKO-T29-1 mice would result in an impaired corticosterone negative feedback at an earlier age. To test this hypothesis, 2-month-old FBGRKO-T29-1 and floxed GR mice were injected ip with 100 µg/kg dexamethasone or saline, and blood was collected by submandibular puncture 6h later, within 1h of lights-off. Post-hoc analysis revealed that dexamethasone significantly suppressed corticosterone release in 2- month-old floxed GR mice (Figure 3). While dexamethasone appeared to decrease corticosterone in 2-month-old FBGRKO-T29-1 mice, this effect was not significant (P = 0.091; Figure 3).
Figure 3.
Plasma corticosterone 6h after injection of 100 μg/kg dexamethasone (black bars or saline (white bars) in 2-month old floxed GR (n =6) and FBGRKO-T29-1 mice (n =7). *, P<0.05 vs. saline.
2.3. Re-examination of the effects of forebrain GR deletion on depression-like behavior and HPA activity in FBGRKO-T29-1 mice
Having confirmed that GR deletion in our FBGRKO-T29-1 mice was neither incomplete nor delayed compared to that reported by Boyle et al., we evaluated additional measures of depression-like behavior to determine if we could detect the depression-like phenotype originally reported for FBGRKO-T50 mice (Boyle et al., 2005). We measured tail suspension immobility and sucrose preference, both measures of depression- and hedonic-like behavior that had been reported to be altered in FBGRKO-T50 mice (Boyle et al., 2005; Solomon et al., 2012). We found no significant effects of genotype on tail suspension immobility or the percent of sucrose consumed over a 10-day period (Table 3). We also measured social interaction, which has been used to model the social withdrawal symptoms of human affective disorders such as depression (Berton et al., 2006). We hypothesized that our FBGRKO-T29-1 mice would show a decreased motivation for social interaction in comparison to control floxed GR mice. However, there was no significant effect of genotype on the latency to enter (data not shown) or time spent in the interaction zone when a novel mouse was the interaction target (Table 3).
Table 3.
Immobility time during a tail suspension test (TST), sucrose preference, and social interaction time in C57BL/6 floxed GR and FBGRKO-T29-1 mice.
| Floxed GR | FBGRKO-T29-1 | |
|---|---|---|
| TST Immobility (sec) | 200.7± 23.1 N = 13 | 204.3 ± 19.7 N = 11 |
| Sucrose Preference (% fluid intake) | 76.9 ± 3.5 N = 10 | 73.6 ± 4.0 N = 9 |
| Social Interaction Time (sec with mouse as interaction target) | 19.6 ± 11.4 N = 9 | 10.4 ± 3.3 N = 10 |
Behaviors were measured as described in Experimental Procedures.
We further measured HPA hormones under additional conditions to determine if we could detect the elevated HPA activity reported by Boyle et al. (Boyle et al., 2005). We did not find any significant effects of genotype on diurnal plasma corticosterone levels measured at the circadian nadir or circadian peak (Table 4). To verify that we weren't missing more rapid changes in activity of higher levels of the HPA axis, we also analyzed plasma ACTH under the stimulated conditions of the circadian peak and following the stress of tail suspension. Again, we did not find any differences between C57BL/6 floxed GR and FBGRKO-T29-1 mice (Table 4).
Table 4.
Plasma corticosterone (μg/dl) and ACTH (pg/ml) in 6-month-old C57BL/6 floxed GR and FBGRKO-T29-1 mice.
| Floxed GR | FBGRKO-T29-1 | |
|---|---|---|
| AM plasma corticosterone (μg/dl) | 0.59 ± 0.17 N = 6 | 0.84 ± 0.28 N = 6 |
| PM plasma corticosterone (μg/dl) | 12.4 ± 2.2 N = 6 | 15.3 ± 1.9 N = 6 |
| PM plasma ACTH (pg/ml) | 134.4 ± 31.1 N = 6 | 161.5 ±19.6 N = 6 |
| Plasma ACTH post-TST (pg/ml) | 448.2 ± 47.2 N = 7 | 463.4 ± 81.1 N = 7 |
| Plasma corticosterone 30 min after restraint (μg/dl) | 63.4 ± 13.0 N = 9–10 | 40.5 ± 6.2 N = 9–10 |
| Plasma corticosterone 120 min after restraint (μg/dl) | 49.0 ± 3.8 N = 9–10 | 46.3 ± 5.6 N = 9–10 |
Circadian nadir (AM) and peak (PM) plasma corticosterone was measured in samples collected within 2h of lights-on and 1h of lights-off, respectively. Plasma ACTH was measured in the same samples as PM corticosterone and also after an 8 min tail suspension test (TST). Plasma corticosterone after 30 min restraint was measured in samples collected by tail nick immediately (30 min) and 90 min after release (120 min). All other blood samples were collected via submandibular venipuncture.
To rule out possible differences in testing and facilities among institutions, we shipped C57BL/6 FBGRKO-T29-1 and floxed GR mice to the laboratory of Dr. James Herman (University of Cincinnati, OH), which has also reported depression-like phenotypic difference in male FBGRKO-T50 vs. floxed GR mice (Solomon et al., 2012). We sent 4-month-old mice to ensure a two month period where the mice could acclimate to the new animal facility before being tested at the 6 month age at which Boyle et al. found maximal phenotypic differences (Boyle et al., 2005). Mice were tested for circadian and post-stress corticosterone levels, forced swim immobility, and sucrose preference. Our respective labs used similar protocols with the following exceptions. The Herman lab analyzed immobility behavior by scoring 5 second bins of activity from videotapes taken above the swim chamber; our lab videotaped a side view of the mice and analyzed immobility behavior as the total amount of time the mouse spent immobile. The Herman lab collected tail vein blood to measure nadir and peak corticosterone, while we used submandibular venipuncture. In addition to measuring circadian nadir and peak corticosterone, the Herman lab also analyzed plasma corticosterone immediately and 90 min after a 30 min restraint (Table 4). Regardless of the institution where the mice were tested or the methods used for analysis, there was no difference between C57BL/6 FBGRKO-T29-1 and floxed GR mice in Herman lab assessments of HPA activity or depression-like behavior (Table 4 and data not shown). To test if depression-like phenotype was present transiently at a younger age due to early GR deletion (Figure 1), we measured circadian plasma corticosterone levels and forced swim immobility behavior in 2-month-old C57BL/6 floxed GR and FBGRKO-T29-1 mice. There was no significant effect of genotype on circadian plasma corticosterone levels or forced swim immobility (data not shown).
2.4. Glucocorticoid effects on depression-like immobility in FBGRKO-T29-1 and floxed GR mice
The lack of baseline depression-like characteristics in our FBGRKO-T29-1 mice precluded testing whether the depression phenotype originally described for forebrain GR deletion (Boyle et al., 2005) could be attributed to loss of positive GR-mediated effects on mood or to the effects of elevated glucocorticoids signaling through remaining GR or MR. However, since glucocorticoids have well-recognized depressive effects in humans that can be readily modeled in rodents, it was of interest to determine if these effects require forebrain GR. To address this question, we tested the sensitivity of immobility during a forced swim to glucocorticoid manipulation in C57BL/6 floxed GR and FBGRKO-T29-1 mice. We chose forced swim rather than tail suspension in order to reproduce a well-characterized paradigm in which the presence of glucocorticoids during a 15 min swim has been shown to increase depression-like immobility in a forced swim 24h later (DeKloet et al., 1988; Mitchell and Meaney, 1991). We compared the effects of acute corticosterone (5 mg/kg) or vehicle injection immediately before a 15 min swim on immobility in adrenalectomized (ADX) mice during a 5 min test swim the next day. Sham-ADX mice were included to control for the effects of corticosterone manipulation and to detect any genotype-related differences in immobility or plasma corticosterone originally reported for FBGRKO-T50 mice with a mixed strain background (Boyle et al., 2005; Solomon et al., 2012). We hypothesized that the presence or absence of acute corticosteroid treatment would affect d2 immobility behavior in floxed GR mice, whereas glucocorticoid receptor deletion would render FBGRKO-T29-1 mice insensitive to the effects of either adrenalectomy or acute corticosterone treatment on d2 immobility. There was a significant main effect of glucocorticoid group (F2,32= 8.033; P= 0.0015) and a significant genotype × glucocorticoid group interaction (F2,32= 4.593; P= 0.0176) on time spent immobile during the d2 swim. Post-hoc analysis showed immobility time was significantly lower in ADX floxed GR mice previously injected with vehicle (ADX + Acute Vehicle) compared to Sham-ADX floxed GR mice (Figure 4, top). There was a similar trend between the Sham-ADX and ADX + Acute Vehicle groups of FBGRKO-T29-1 mice that was not significant at the post-hoc level. However, although prior corticosterone treatment did not markedly alter immobility in floxed GR mice, there was a robust increase in immobility between adrenalectomized FBGRKO-T29-1 mice that did and did not receive corticosterone (Figure 4, top).
Figure 4.
Effect of glucocorticoid manipulation on FST immobility behavior of C57BL/6 FBGRKO-T29-1 and floxed GR mice. Mice were adrenalectomized and given an acute injection of either 5 mg/kg corticosterone (ADX + Acute Cort) or vehicle (ADX + Acute Vehicle) before a 15 min test swim 24 h earlier. Sham-adrenalectomized FBGRKO-T29-1 and floxed GR mice injected with vehicle and included as controls for the effects of adrenalectomy and forebrain GR deletion. Total time immobile and latency to first immobility were scored in a 5 min swim 24h later. *, P<0.05 vs. Sham-ADX; #, P<0.05 vs. ADX + Acute Vehicle. Ns are shown within the data bars.
There was also a significant main effect of glucocorticoid group (F2,32 = 5.569; P= 0.0084), without any significant main effect of or interaction with genotype, on latency to become immobile during the d2 swim. Post-hoc analysis showed latency to immobility was significantly higher in adrenalectomized FBGRKO-T29-1 mice (ADX + Acute Vehicle) compared to Sham-ADX FBGRKO-T29-1 mice (Figure 4, bottom). Similar trends in the latency to immobility were evident in floxed GR mice, although these were not significant. Completeness of adrenalectomy was confirmed by plasma corticosterone levels below 1 μg/dl in all ADX mice after the second swim (Table 5). Notably, we again did not observe any differences between Sham-ADX FBGRKO-T29-1 and floxed GR mice in total immobility, latency to immobility (Figure 4), or post-swim plasma corticosterone (Table 5).
Table 5.
Plasma corticosterone (μg/dl) in C57BL/6 floxed GR and FBGRKO-T29-1 mice immediately after a 5 min test swim, 24h after a 15 min pre-swim with corticosterone or vehicle administration, as described in Experimental Procedures.
| Floxed GR | FBGRKO-T29-1 | |
|---|---|---|
| Sham | 12.4 ± 1.0 N = 10 | 10.8 ± 0.9 N = 8 |
| ADX + Acute Vehicle | 0.8 ± 0.2 N = 6 | 0.7 ± 0.2 N = 8 |
| ADX + Acute Cort | 0.4 ± 0.03 N = 3 | 0.9 ± 0.1 N = 3 |
2.5. Effects of forebrain GR deletion on responsiveness to antidepressants in FBGRKO-T29-1 mice
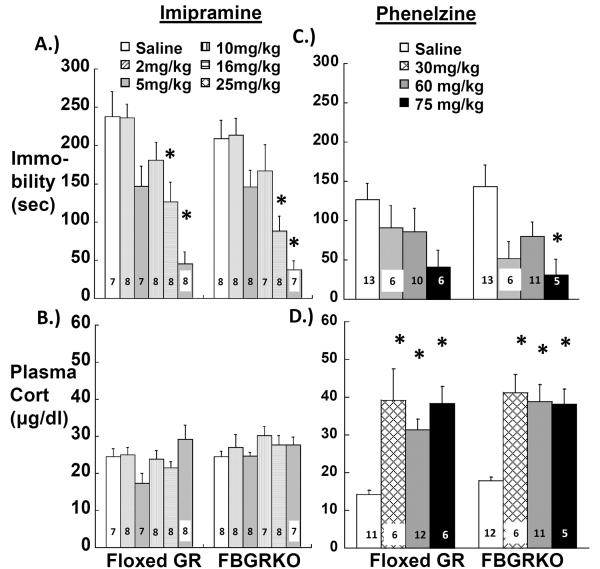
There is considerable evidence that antidepressants change glucocorticoid receptor expression (Heydendael and Jacobson, 2010; Pariante and Miller, 2001) and that glucocorticoid-dependent endpoints, such as HPA activity, can be predictors of antidepressant response (Hatzinger et al., 2002). Despite reports that antagonists or synthesis inhibitors that modify glucocorticoid signaling can have antidepressant effects (Jahn et al., 2004; Murphy, 1997; O'Dwyer et al., 1995; Wolkowitz et al., 1999), it is yet unknown if changes in glucocorticoid receptors are important to antidepressant action. The report by Boyle et al. that imipramine can reverse the depression-like increases in immobility behavior and HPA activity in FBGRKO-T50 mice (Boyle et al., 2005) suggests that forebrain GR are not required for antidepressant response. However, since Boyle et al. only tested a single dose of imipramine, it is unclear whether GR deletion might alter the sensitivity of behavior or HPA activity to lower doses or other types of antidepressants. We have compared acute doses of the tricyclic antidepressant imipramine and the monoamine oxidase inhibitor phenelzine on immobility in a tail suspension test (TST) and on post-TST plasma corticosterone levels in our C57BL/6 FBGRKO-T29-1 mice and floxed GR mice. The 16 mg/kg imipramine dose was included to replicate the dose used by Boyle et al. (Boyle et al., 2005). We hypothesized that GR gene deletion would render depression-like behavior and HPA activity in FBGRKO-T29-1 mice less sensitive to antidepressant treatment, meaning that FBGRKO-T29-1 mice would only respond to doses that were greater than those reducing immobility behavior in floxed GR mice. Although we observed significant main effects of both antidepressants to reduce immobility (imipramine, F5,80= 17.753; P< 0.0001; phenelzine, F3,61= 5.124; P= 0.0032), there were no significant effects of genotype or antidepressant × genotype interaction (Figure 5, top). Post-hoc analysis revealed a significant decrease in immobility in both genotypes after 16 and 25 mg/kg imipramine (Figure 5, top left). FBGRKO-T29-1 mice exhibited significant decreases in immobility after treatment with 75 mg/kg phenelzine, and there was a trend for a similar effect in floxed GR mice (P=0.056; Figure 5, top right). Lower acute doses of phenelzine (2, 5, 10, 16, 25, and 50 mg/kg) did not significantly decrease TST immobility in either genotype (data not shown).
Figure 5.
Effect of acute imipramine (A, B) or phenelzine treatment (C, D) on immobility behavior in C57BL/6 floxed GR or FBGRKO-T29-1 mice during (A, C) and corticosterone levels (B, D) immediately after an 8 min TST. Group ns are shown within the data bars. Group sizes differ between immobility behavior (C) and corticosterone (D) graphs because we were unable to collect plasma in some mice for corticosterone assay. *, P=<0.05 vs. saline-treated mice within the same genotype.
Antidepressant treatment has been reported to inhibit corticosterone responses to the stress of forced swim (Conti et al., 2004), so we tested if imipramine or phenelzine would affect corticosterone secretion stimulated by the stress of tail suspension in our C57BL/6 FBGRKO-T29-1 and floxed GR mice. While there was no main effect of imipramine or imipramine × genotype interaction, there was a main effect of genotype on (F5,80= 5.541; P= 0.021) on post-TST corticosterone levels. However, corticosterone levels did not differ significantly at the post-hoc level between floxed GR and FBGRKO-T29-1 mice given the same dose of imipramine or saline (Figure 5, bottom left). In contrast to the lack of effect of imipramine on post-TST plasma corticosterone levels, phenelzine had a significant main effect to increase post-TST corticosterone levels (F3,62= 19.939; P< 0.0001). However, there was no significant effect of genotype or genotype × phenelzine interaction on post-TST corticosterone levels between FBGRKO-T29-1 and floxed GR mice at the time examined (Figure 5, bottom right).
To determine if sensitivity to chronic antidepressant treatment was altered by forebrain GR deletion, we repeated the chronic imipramine treatment used by Boyle et al. and included an intermediate imipramine dose. Floxed GR and FBGRKO-T29-1 mice were injected daily ip for 3 weeks with either saline or 8 or 16 mg/kg imipramine. At the end of treatment we also found no genotype-related differences in TST immobility or post-TST corticosterone levels (data not shown).
2.6. MR gene expression in FBGRKO-T29-1 mice
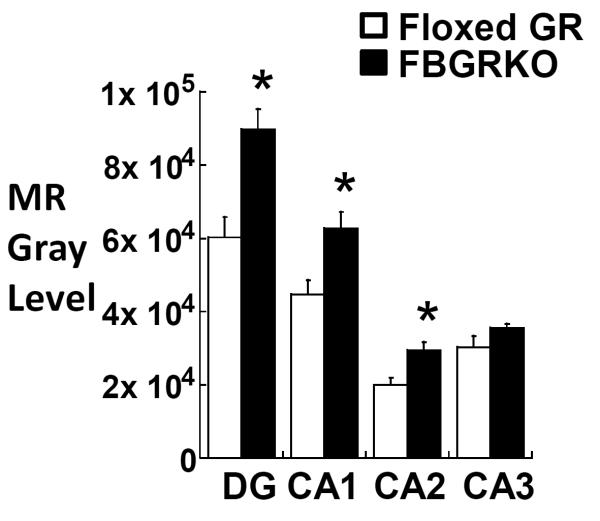
HPA activity is regulated by mineralocorticoid receptors (MR) as well as GR (DeKloet et al., 1975; Gass et al., 2001; Jacobson, 2005; Kellendonk et al., 2002; Reichardt et al., 1998). Because of its higher affinity for corticosterone, MR is the principal regulator of HPA activity when corticosterone levels are low, such as the trough of the circadian rhythm (early light phase in rodents). Although GR has a major role in controlling HPA activity during the peak of the circadian rhythm or stressful situations when corticosterone levels are high, MR have also been shown to contribute to feedback inhibition of HPA activity under these conditions (Jacobson, 2005; Pace and Spencer, 2005). Chronic forebrain MR deletion and overexpression has respectively been associated with increases and decreases in GR expression in the hippocampus, suggesting that changes in MR expression cause reciprocal changes in GR levels (Berger et al., 2006; Rozeboom et al., 2007). We hypothesized that a similar reciprocal regulation might result from chronic changes in GR levels, such that a decrease in GR expression would correlate with increased MR expression. Although MR expression was originally reported to be unaltered in FBGRKO-T50 mice (Boyle et al., 2005), we hypothesized that the earlier GR deletion in our FBGRKO-T29-1 line might leave more time for reciprocal changes in MR to occur. We used in situ hybridization in the dentate gyrus and CA1, CA2, and CA3 regions of the hippocampus to determine if MR gene expression was increased in FBGRKO-T29-1 mice compared to floxed GR controls. FBGRKO-T29-1 mice showed a significant increase in MR gene expression compared to C57BL/6 floxed GR mice in the dentate gyrus and CA1 and CA2 regions of the hippocampus, but not in the CA3 region (Figure 6).
Figure 6.
Semi-quantitative analysis of MR mRNA by in situ hybridization in the dentate gyrus and CA1, CA2, and CA3 regions of the hippocampus of C57BL/6 floxed GR (n =6) and FBGRKO-T29-1 mice (n =4). *, P<0.05 vs. floxed GR mice.
3. DISCUSSION
We have found that forebrain glucocorticoid receptor deletion does not reduce behavioral sensitivity to glucocorticoids or antidepressants. Complicating our results, we did not observe the baseline depression-like phenotype reported by Boyle et al. of greater despair- and anhedonia-like behavior combined with increased HPA activity in either FBGRKO-T50 mice on a mixed background (Boyle et al., 2005) or FBGRKO-T29-1 mice on a pure C57BL/6 background. In contrast to results reported by Boyle et al., our FBGRKO-T29-1 mice show 1.) forebrain specific GR deletion that includes the central amygdala, 2.) GR deletion and impaired glucocorticoid feedback at an earlier age, and 3.) increased MR gene expression. Nevertheless, despite the lack of a robust depression-like phenotype from extensive forebrain GR deletion, our antidepressant response data support those of Boyle et al. in confirming that antidepressant action does not require forebrain GR. In addition, we have demonstrated that the depressive effects of glucocorticoids are mediated by corticosteroid receptors other than those missing in our FBGRKO-T29-1 mice.
3.1. Loss of depression-like phenotype in FBGRKO-T29-1 mice is associated with decreased central amygdala GR and increased hippocampal MR expression
Unexpectedly, neither FRGRKO-T50 mice from the original lineage characterized by Boyle et al. nor the FBGRKO-T29-1 mice we derived on a pure C57BL/6 background exhibited a depression-like behavioral or endocrine phenotype. However, we did confirm that GR loss that was at least as extensive and rapid as that reported by Boyle et al. (Boyle et al., 2005). The apparent loss of this phenotype is unlikely to be due to our inability to detect it, since we assessed multiple rodent correlates of the despair, withdrawal, and anhedonic aspects of depression in two separate labs, and since we have detected changes in behavior induced by antidepressant treatment (e.g., Figure 5 and (Kier et al., 2005; Mukherjee et al., 2004)). We also measured HPA activity at two separate circadian times and after a variety of stressful stimuli such as tail suspension or restraint. While it is possible that different time points might have revealed genotypic differences in HPA hormones, we sampled within the time frames in which Boyle et al. detected genotypic differences in plasma corticosterone (Boyle et al., 2005; Boyle et al., 2006). Furthermore, since Solomon et al. and Boyle et al. did not detect increased peak circadian HPA activity consistently in FBGRKO-T50 with the original mixed background, this also suggests that forebrain GR deletion does not robustly alter HPA circadian activity.
Our FBGRKO-T29-1 mice differed from those originally reported both in being on a pure C57BL/6 background and in being derived from a different CamKIIα-Cre transgenic founder (Boyle et al., 2005; Tsien et al., 1996). Strain differences can affect CamKIIα promoter activity (Berger et al., 2006; Rozeboom et al., 2007; Tanaka et al., 2012), so strain difference might account for the absence of depression-like features in our FBGRKO mice on a pure C57BL/6 background. In addition, within the same strain, transgene expression can depend on its integration site in the founder genome. Integration locus can influence the efficiency and cell specificity of transgene expression (Ristevski, 2005), which might account for the more rapid and extensive GR deletion in our FBGRKO-T29-1 line. Lack of a depression phenotype in the FBGRKO-T29-1 mouse might further be attributable to effects on genes around the integration site that influence behavior and HPA activity. Nevertheless, since we also did not observe a depression-like phenotype in FBGRKO-T50 mice from the Muglia lab (Boyle et al., 2005), we suspect instead that some type of genetic drift occurred to alter the consequences of forebrain GR deletion.
Our FBGRKO-T29-1 mice exhibited significant up-regulation of hippocampal MR gene expression and, in contrast to the results reported by Boyle et al., Furay et al., and Solomon et al., marked GR loss in the central amygdala (Boyle et al., 2005; Furay et al., 2008; Solomon et al., 2012). Central amygdala GR are targeted by antidepressants (Heydendael and Jacobson, 2010) and have also been shown to be involved in anxiety-like behavior and fear conditioning (Kolber et al., 2008; Shepard et al., 2003; Weiser et al., 2010). Unlike their inhibitory effects on HPA activity at the hippocampus, hypothalamus, or prefrontal cortex, glucocorticoids acting in the central amygdala enhance HPA activity, an effect that appears to involve central amygdala GR (Kolber et al., 2008; Shepard et al., 2003). Therefore, loss of CeA GR in our FBGRKO-T29-1 line could reduce dysphoric behavior and compensate for or prevent elevations in HPA activity. MR have been shown in both animal and human studies to suppress stimulated as well as basal HPA activity (Pace and Spencer, 2005; Wellhoener et al., 2004), so the augmented MR expression observed in our FBGRKO-T29-1 line might also have prevented the increase in HPA activity originally reported for this model (Boyle et al., 2005). While we did not measure MR protein levels, it has been shown that increases in MR mRNA levels match the increase in MR protein levels in the hippocampus of rats (Herman and Spencer, 1998). Increases in MR expression have also been reported in preclinical studies after antidepressant treatment (reviewed in (Heydendael and Jacobson, 2008)), and have been suggested to contribute to antidepressant reversal of the original depression-like phenotype of FBGRKO-T50 mice (Boyle et al., 2005). It would be of interest in future studies to determine if pharmacological blockade of MR in our FBGRKO-T29-1 mice would be sufficient to reveal a depression-like phenotype.
It is therefore possible that up-regulated MR hippocampal expression, combined with central amygdala GR loss, could account for the loss of genotype-related differences in both baseline depression-like behavior and HPA activity in our FBGRKOT29-1 line. These possibilities notwithstanding, it is still remarkable that widespread corticolimbic GR loss, validated in the same manner as Boyle et al., does not produce a depression-like phenotype as they reported. Although this discrepancy may suggest that the mouse is a poor model for human depression, the inconsistencies we have observed more likely reflect the limitations in the understanding of depression pathology and translating these features to any animal model.
3.2. Glucocorticoids affect depression-like behavior despite forebrain GR deletion in FBGRKO-T29-1 mice
Despite the absence of a baseline depression-like phenotype in our FBGRKO-T29-1 mice, we were able to alter depression-like behavior in these mice by manipulating glucocorticoids. Compared to floxed GR mice, FBGRKO-T29-1 mice actually exhibited more dramatic behavioral changes in response to changes in corticosterone, including increases in latency to immobility and increases in total immobility when respectively deprived of or treated with corticosterone. Because the ability of corticosterone to increase depression-like behavior in this paradigm has been shown to require GR activation in both mice and rats (Ago et al., 2008; Korte et al., 1996; Veldhuis et al., 1985), our results indicate that GR in the brainstem or non-targeted areas of the forebrain (possibly including the areas of partial deletion in the bed nucleus of the stria terminalis, accumbens, or central amygdala) mediate the depressive effects of glucocorticoids. Notably, the behavioral sensitivity of FBGRKO-T29-1 mice to glucocorticoids contrasts with their impaired dexamethasone suppression and suggests that different GR populations are responsible for endocrine feedback inhibition and control of emotional behaviors. Our results are also consistent with the possibility that glucocorticoid oversecretion contributed to the depression-like phenotype previously reported for forebrain GR deletion (Boyle et al., 2005; Solomon et al., 2012).
3.3. Forebrain GR deletion does not affect behavioral sensitivity to antidepressant treatment in FBGRKO-T29-1 mice
We found that our FBGRKO-T29-1 mice appear at least as sensitive as C57BL/6 floxed GR controls to the behavioral effects of acute treatment with two markedly different antidepressants, imipramine (a tricyclic antidepressant) and phenelzine (a monoamine oxidase inhibitor). Our dose response data for both acute and chronic treatment reinforce the conclusion of Boyle et al. (2005) that forebrain GR are not required for behavioral responses to antidepressants. Although antidepressant treatment has been hypothesized to normalize HPA activity by enhancing forebrain GR or MR function (Heydendael and Jacobson, 2010; Holsboer and Barden, 1996; Pariante and Miller, 2001; Zobel et al., 2001), antidepressant effects on baseline behavior do not seem to have a similar dependence on forebrain GR.
We found consistent differences in the acute effects of imipramine and phenelzine on HPA activity that corroborate our previous studies using chronic treatment with these drugs. Phenelzine but not imipramine had consistent, acute effects to increase plasma corticosterone, similar to our prior findings that phenelzine but not imipramine consistently stimulated HPA activity in C57BL/6 mice (Kier et al., 2005). We did not observe any effects of forebrain GR deletion on HPA responses to either antidepressant. Although it is possible that additional time points might have detected genotype-related differences in HPA activity, our samples were collected within the time frame during which Boyle et al. reported altered HPA hormones in FBGRKO-T50 mice (Boyle et al., 2005). Thus, our results are consistent with the likelihood that antidepressants have forebrain GR-independent mechanisms to control glucocorticoid secretion as well as behavior. If, as there is evidence to suggest, glucocorticoid levels contribute to depression symptoms (Jahn et al., 2004; Murphy, 1997; O'Dwyer et al., 1995; Wolkowitz et al., 1999), such effects on glucocorticoid secretion could constitute part of the therapeutic actions of antidepressants.
We have extended the literature on depressive effects of glucocorticoids by showing that these effects do not require the forebrain GR missing in our FBGRKO-T29-1 mice. Our results suggest that targets for glucocorticoid effects on mood reside either in nonforebrain areas or in forebrain areas not targeted in the FBGRKO-T29-1 mouse. Even independently of forebrain GR, antidepressants could still have glucocorticoid-dependent effects on depression symptoms by controlling glucocorticoid secretion and/or action in these areas.
4. EXPERIMENTAL PROCEDURES
4.1. Animals
All animal use was approved by the Institutional Animal Care and Use Committee of Albany Medical College and followed the standards of the NIH Guide for the Care and Use of Animals (Institute of Laboratory Animal Resources, 2010). Mice were housed on a 12 hour light/ 12 hour dark cycle (lights on at 7:00 a.m.) with ad libitum access to rodent chow. Mice were group-housed until 1–2 days before experimentation, at which time they were individually housed to minimize disturbance prior to behavioral testing or blood sampling. All experiments were conducted within 3h of lights-on, with the exception of circadian peak blood sampling, which was performed within 1h of lights-off. Unless noted, all mice were at least 6 months old at the time of experimentation.
Male floxed GR mice expressing a Cre recombinase transgene controlled by the calcium calmodulin Kinase II α (CaMKIIα) promoter were bred to female floxed GR mice lacking the Cre transgene to produce Cre+ (FBGRKO) and Cre− (floxed GR) littermates. Breeding for initial experiments (Table 1) used floxed GR females and CaMKIIα-Cre males derived from the T50 founder line that were on a mixed C57BL/6 × 129 × CBA background and provided by Louis Muglia (University of Cincinnati, OH). Pure C57BL/6 FBGRKO-T29-1 mice used for all other experiments (Figures 1–6 and Tables 3–5) were derived from C57BL/6 floxed GR females (generously provided by Louis Muglia) and a C57BL/6 CaMKIIα-Cre male from the T29-1 founder line ((Tsien et al., 1996); Jackson Laboratories stock number 005359; Bar Harbor, ME). Offspring of these matings were screened for the floxed GR allele by PCR (see below) and used to generate Cre+ (FBGRKO) and Cre− (floxed GR) mice that were homozygous for the floxed GR allele. Male littermate- or age-matched FBGRKO and floxed GR mice were used for all experiments.
Tail DNA was isolated (Invitrogen PureLink Genomic DNA kit; Carlsbad, CA) from all offspring and screened by PCR for (1) the presence or absence of the Cre recombinase transgene, (2) homozygosity of the floxed GR allele; and, because some germline activity has been reported for the CaMIIα-Cre transgene that could result in global GR deletion (http://cre.jax.org/Camk2a/Camk2a-creNano.html), (3) integrity of the floxed GR allele. Primers and parameters for each PCR reaction are given in Table 2.
4.2. Behavioral tests
Forced Swim Test (FST)
Mice were placed in a 1000ml beaker (10-cm wide × 19.5-cm high) filled with 25± 1°C water for 15 minutes, and immobility was scored for the entire 15-min period. For the two-day FST paradigm, FBGRKO and floxed GR mice were subjected to a 15-minute pre-swim on day 1, and immobility was scored in a 5-min test swim on day 2. Immobility was scored from Ethovision videotapes as the time the mouse spent motionless or used minimal, non-rhythmical activity of the paws or tail to maintain balance. The beakers were rinsed with MB-10 disinfectant (Quip Labs, Wilmington, DE) between each FST trial.
For FST and glucocorticoid manipulation, FBGRKO-T29-1 and floxed GR mice were anesthetized with isoflurane (2% in 7 l/min oxygen) and were either adrenalectomized (ADX) or Sham-ADX. Following surgery, all mice received water bottles with 10 μg/ml corticosterone in 0.9% saline for 5 days. Starting on d6, the corticosterone drinking fluid was replaced with 0.9% saline for all ADX mice. Sham-ADX mice received water to drink at all times. On d7, all mice underwent an acute injection followed immediately by a 15 min swim. ADX mice were injected sc with either 5 mg/kg corticosterone (ADX + Acute Cort) or 5% ethanol, 0.9% saline vehicle (ADX + Acute Vehicle). Sham-ADX mice were injected with vehicle. Following the pre-swim on d7, all mice were subjected 24h later to a 5 min swim followed immediately by a submandibular blood sample for plasma corticosterone. Immobility was scored from videotapes of the 5 min day 2 swim.
Tail Suspension Test (TST)
Mice were taped by their tails to a metal rod and allowed to hang out upside-down for 8 minutes. Imipramine (MP Biomedical, Solon, OH) or phenelzine (Sigma, St. Louis, MO) were administered in saline by ip injection 1h before the TST. The TST was videotaped using Ethovision XT 8.0 software (Noldus Information Technology, Leesburg, VA). Immobility was manually scored from the videos by an observer blinded to genotype and treatment groups of the mice. Immobility was measured as the time the mouse spent entirely motionless or motionless but still swinging from the momentum of previous activity. The TST was selected to measure immobility behavior in response to antidepressant treatment because we have found fewer ambiguities in scoring immobility during tail suspension than during forced swim.
Sucrose Preference Test
Following a 2-day adaptation period in which they had access to two bottles of water, FBGRKO and floxed GR mice were given a choice between two bottles containing either 1% sucrose or water. Every day the position of the 1% sucrose- and water-filled bottles was switched to control for side preference. The tubes were weighed daily to determine intake. The data presented as % sucrose intake = sucrose consumed (g) / [water consumed (g) + sucrose consumed (g)] across genotypes.
Social Interaction Test
Mice were put into a 36 × 43 cm basin across from a 8 × 6 × 12cm test box in one corner of the basin. Each experimental mouse was videotaped using Ethovision XT 8.0 software (Noldus Information Technology, Leesburg, VA) for two consecutive sessions of 2.5 min. In the first session the mouse was exposed to the empty box, while in the second session a social target (an unfamiliar CD1 male breeder mouse) was placed in the corner box, similar to the paradigm used by Berton et al (Berton et al., 2006). Mice had visual and olfactory but no physical contact during the second session. The basin and test chamber were rinsed with MB-10 disinfectant and dried thoroughly in between each test. Ethovision tracking software was used to determine the latency to enter and the amount of time the experimental mouse spent in the interaction zone (within 10 cm of the test chamber, both with and without the presence of the mouse in the test box).
Restraint
Mice were restrained in ventilated tubes for 30 min. Blood samples for plasma corticosterone assay were collected immediately after release (30 min) and again 90 min later (120 min).
4.3. Plasma hormone assay
Mice were individually housed at least 12h prior to blood sampling. Blood was collected by submandibular puncture with 5 mm Goldenrod animal lancets (Medipoint Inc., Mineola, NY) within 45 sec of touching the mouse's cage. Blood was collected through heparinized capillary tubes into microfuge tubes, and plasma was subsequently separated by centrifugation for determination of plasma corticosterone and adrenocorticotrophic hormone (ACTH). Each hormone was assayed using corresponding radioimmunoassay kits from MPBiomedical (Solon, OH) with all reagents and plasma volumes reduced by half as previously described (Kier et al., 2005). Adrenalectomized mice with plasma corticosterone levels above 1.0 μg/dl after the stress of forced swim were excluded from analysis (Figure 4).
4.4. GR and NeuN Immunofluorescence
Mice were given lethal injections of sodium pentobarbital (70–80 mg/kg) and perfused intracardially via a peristaltic pump (Harvard Apparatus, Holliston, Massachusetts) with 0.9% saline and then 4% paraformaldehyde. The brains were collected and stored in 4% paraformaldehyde overnight at 4°C and then cryoprotected in 30 % sucrose at 4°C. Cryoprotected brains were frozen in molds (Fisher Scientific, Pittsburgh, PA) containing embedding medium (OCT, Sakura Finetek, Torrance, CA) in a dry ice-ethanol bath and stored at −80°C. Coronal sections (25μm) were cut using a cryostat (Leica, Buffalo Grove, IL), distributed sequentially over the wells of a 6-well tissue culture plate (Corning Incorporated Life Sciences, Lowell, MA), and stored in cryoprotectant solution (50% 0.05M, Tris buffered saline (pH 7.5), 30% ethylene glycol, 20% glycerol) at −20°C.
Sections were washed with TBS-T (0.01M Tris buffered saline, 0.15 NaCl, 0.3% Triton-X-100), blocked for nonspecific binding with 3% normal goat serum in TBS-T, and incubated in primary antibodies overnight at 4°C. GR was detected with a rabbit anti-GR primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at a 1:1000 dilution, and NeuN was detected with a biotinylated mouse monoclonal antibody (Millipore, Billerica, MA) at a 1:500 dilution. Sections were then washed with TBS-T and incubated for 1 hour at room temperature in cyanine-3-conjugated goat anti-rabbit antibody at 1:200 for GR (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and 1:500 fluoresceinated avidin (Vector Laboratories, Burlingame, CA) for NeuN. The avidin signal for NeuN was amplified by washing in TBS-T, incubating with biotinylated goat anti-avidin antibody 1:1000 (Vector Laboratories) for 30 minutes at room temperature, washing in TBS-T, and incubating 1 hour in fluorescein avidin.
GR expression in the central amygdala was analyzed after Tyramide Single Amplification (TSA; Perkin Elmer, Boston, Massachusetts), in which the GR signal was amplified with the TSA Cyanine 5 kit (day 1 and 2) and the NeuN signal was amplified with the TSA Fluorescein kit (day 2 and 3). The dilutions and primary antibodies used to detect GR and NeuN were identical to those in the previous paragraph (unless otherwise noted) and all sections were washed with TBS-T according to manufacturer instructions. In brief, sections were incubated in 1% hydrogen peroxide in TBS, blocked for nonspecific binding with filtered TNB (0.1M TRIS-HCl, 0.15 NaCl, 0.5% blocking reagent, pH 7; Perkin Elmer), and incubated overnight at 4°C in primary antibody (1:2000) to detect GR positive cells. On day 2, sections were incubated for 1 hour at room temperature in horseradish peroxidase (HRP)-goat anti-rabbit (Perkin Elmer Boston, Massachusetts) in TNB (1:1000), and incubated for 10 minutes at room temperature in TSA-Cy5 antibody in amplification diluent (1:50; Perkin Elmer). A similar protocol was used to amplify NeuN immunoreactivity, except that biotinylated anti-NeuN was detected with streptavidin-horseradish peroxidase (1:100 in TNB: Perkin Elmer) and a TSA-FITC antibody in (1:50; Perkin Elmer). Slides were coverslipped with N-propyl-gallate and stored at 4°C.
4.5. Mineralocorticoid Receptor in situ Hybridization
Fresh-frozen brains were collected from 6–15 month old floxed GR and FBGRKO-T29-1 mice decapitated within 2 h of lights-on. In situ hybridization for MR mRNA was performed in 10 μm coronal brain sections as previously described (Jacobson, 2000). In brief, sections were fixed with 4% phosphate-buffered paraformaldehyde, washed with 2X standard saline citrate buffer (SSC), acetylated in 0.25% acetic anhydride/0.1M triethanolamine and dehydrated with increasing concentrations of ethanol. The mineralocorticoid receptor probe was transcribed from a 750 bp Pst I fragment of exon 2 of the mouse mineralocorticoid receptor (generously provided by Gunther Schutz, Cancer Research Center, Heidelberg, Germany). Slides were incubated in hybridization solution (107 cpm/ml of 35-S-labeled cRNA probe in 50% deionized formamide, 10% dextran sulfate, 0.4M NaCl, 1× Denhardt's, 10mM Tris (pH 7.5), 1mM EDTA, 500ug/ml yeast tRNA, 10mM dithiothreitol) for 16 hours at 55°C. After hybridization, sections were washed in 2X SSC, incubated in 20μg/ml RNAase A at 37°C, washed in 0.5X SSC, and dehydrated with increasing ethanol concentrations. Slides were exposed to phosphorimager screens (GE Healthcare, Niskayuna, NY) for 15 hours. Slides from floxed GR and FBGRKO mice were exposed together on each screen along with identical 14C standards (American Radiolabeled Chemicals, St. Louis Missouri) and scanned at a 50μm resolution using the Typhoon 9210 phosphorimager (GE Healthcare, Niskayuna, NY).
4.6. Data Analysis
GR immunocytochemistry
GR and NeuN images were taken at 40X magnification on an Olympus BX50 fluorescence microscope (Center Valley, PA). GR-and NeuN-positive cells were counted using ImageJ (http://rsbweb.nih.gov/ij/). 40 NeuN-positive nuclei were labeled, and the number of NeuN-positive cells that were also GR positive was determined after merging the corresponding GR image. Counts for each anatomical region were made in at least 2 different sections and averaged for each mouse. The percent of GR and NeuN positive cells was calculated as (the number of GR + NeuN positive cells out of 40 NeuN-positive cells) × 100. This approach, including the primary antibodies used for GR and NeuN detection, was based on that used by the Muglia lab (B. Kolber and L. Muglia, personal communication).
MR in situ hybridization
Audioradiographic images were analyzed using Imagequant 5.0 software (GE Healthcare, Niskayuna, NY). Densitometric readings were collected from hand-drawn outlines of the dentate gyrus and CA1, CA2, and CA3 hippocampus. Readings for each region were corrected for background readings obtained from a non-expressing region within the same section and scaled to account for differences in template size for a given region. To compare data between screens, values were normalized to 14C standards exposed on each screen (146B, American Radiolabeled Chemicals, St. Louis, MO).
Statistics
Results are expressed as a mean ± SEM. Two-way independent measures ANOVAs were performed to identify main effects of genotype and treatment (Statview 5.0, SAS Institute, Cary, NC). Post-hoc comparisons were performed by Dunnett's test for the antidepressant dose-response experiments in Figure 5; all other post-hoc comparisons were unpaired t-test, with Bonferroni correction when multiple comparisons were performed. Significance was set at P<0.05.
Highlights
FBGRKO mice did not show the depression-like phenotype despite forebrain GR deletion.
Our FBGRKO mice had central amygdala GR deletion and increased MR gene expression.
Our FBGRKO mice remain sensitive to glucocorticoids and antidepressant treatment.
Non-forebrain corticosteroid receptors may play a role in depression symptoms.
ACKNOWLEDGEMENTS
Supported by MH-080394 to LJ. We are grateful to Scott Purga for expert assistance with the imipramine dose-response experiment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ago Y, et al. Antidepressant-like effects of the glucocorticoid receptor antagonist RU-43044 are associated with changes in prefrontal dopamine in mouse models of depression. Neuropharmacology. 2008;55:1355–63. doi: 10.1016/j.neuropharm.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Arana GW, et al. Dexamethasone for the treatment of depression: a randomized, placebo-controlled, double-blind trial. Am J Psychiatry. 1995;152:265–7. doi: 10.1176/ajp.152.2.265. [DOI] [PubMed] [Google Scholar]
- Barden N. Implication of the hypothalamic-pituitary-adrenal axis in the physiopathology of depression. J Psychiatry Neurosci. 2004;29:185–93. [PMC free article] [PubMed] [Google Scholar]
- Berger S, et al. Loss of the limbic mineralocorticoid receptor impairs behavioral plasticity. Proc. Natl. Acad. Sci. U S A. 2006;103:195–200. doi: 10.1073/pnas.0503878102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, et al. Essential Role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Bouwer C, et al. Prednisone augmentation in treatment-resistant depression with fatigue and hypocortisolaemia: a case series. Depress Anxiety. 2000;12:44–50. doi: 10.1002/1520-6394(2000)12:1<44::AID-DA6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Boyle MP, et al. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci U S A. 2005;102:473–8. doi: 10.1073/pnas.0406458102. Epub 2004 Dec 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle MP, et al. Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. J. Neurosci. 2006;26:1971–8. doi: 10.1523/JNEUROSCI.2173-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ES, Khan DA, Nejtek VA. The psychiatric side effects of corticosteroids. Ann Allergy Asthma Immunol. 1999;83:495–503. doi: 10.1016/S1081-1206(10)62858-X. [DOI] [PubMed] [Google Scholar]
- Conti AC, Kuo Y-C, Blendy JA. Inducible cAMP early repressor regulates corticosterone suppression after tricyclic antidepressant treatment. J. Neuroscience. 2004;24:1967–75. doi: 10.1523/JNEUROSCI.4804-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBattista C, et al. Acute antidepressant effects of intravenous hydrocortisone and CRH in depressed patients: a double-blind, placebo-controlled study. Am J Psychiatry. 2000;157:1334–7. doi: 10.1176/appi.ajp.157.8.1334. [DOI] [PubMed] [Google Scholar]
- DeKloet ER, et al. Antiglucocorticoid RU 38486 attenuates retention of a behavior and disinhibits the hypothalamic-pituitary-adrenal axis at different brain sites. Neuroendocrinology. 1988;47:109–15. doi: 10.1159/000124900. [DOI] [PubMed] [Google Scholar]
- DeKloet ER, et al. Brain corticosteroid receptor balance in health and disease. Endocrine Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- DeKloet R, Wallach G, McEwen BS. Differences in corticosterone and dexamethasone binding to rat brain and pituitary. Endocrinology. 1975;96:598–609. doi: 10.1210/endo-96-3-598. [DOI] [PubMed] [Google Scholar]
- Dinan TG, et al. Dexamethasone augmentation in treatment-resistant depression. Acta Psychiatr Scand. 1997;95:58–61. doi: 10.1111/j.1600-0447.1997.tb00374.x. [DOI] [PubMed] [Google Scholar]
- Furay AR, Bruestle AE, Herman JP. The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology. 2008;149:5482–90. doi: 10.1210/en.2008-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass P, et al. Mice with targeted mutations of glucocorticoid and mineralocorticoid receptors: models for depression and anxiety? Physiol. Behav. 2001;73:811–25. doi: 10.1016/s0031-9384(01)00518-2. [DOI] [PubMed] [Google Scholar]
- Hatzinger M, et al. The combined DEX-CRH test in treatment course and long-term outcome of major depression. J Psychiatr Res. 2002;36:287–97. doi: 10.1016/s0022-3956(02)00021-3. [DOI] [PubMed] [Google Scholar]
- Herman JP, Spencer R. Regulation of hippocampal glucocorticoid receptor gene transcription and protein expression in vivo. J Neurosci. 1998;18:7462–73. doi: 10.1523/JNEUROSCI.18-18-07462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J Psychiatr Res. 1994;28:341–56. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Heydendael W, Jacobson L. Differential effects of imipramine and phenelzine on corticosteroid receptor gene expression in mouse brain: Potential relevance to antidepressant response. Brain Research. 2008;1238:93–107. doi: 10.1016/j.brainres.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Heydendael W, Jacobson L. Widespread hypothalamic-pituitary-adrenocortical axis-relevant and mood-relevant effects of chronic fluoxetine treatment on glucocorticoid receptor gene expression in mice. Eur. J. Neurosci. 2010;31:892–902. doi: 10.1111/j.1460-9568.2010.07131.x. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Liebl R, Hofschuster E. Repeated dexamethasone suppression test during depressive illness. Normalisation of test result compared with clinical improvement. J Affect Disord. 1982;4:93–101. doi: 10.1016/0165-0327(82)90039-8. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Barden N. Antidepressants and hypothalamic-pituitary-adrenocortical regulation. Endocr Rev. 1996;17:187–205. doi: 10.1210/edrv-17-2-187. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources . Guide for the care and use of laboratory animals. National Academies Press; Washington, DC: 2010. [Google Scholar]
- Jacobson L. CRH and NPY are not decreased in hypophagic, protein-deprived rats. Peptides. 2000;21:1487–93. doi: 10.1016/s0196-9781(00)00302-8. [DOI] [PubMed] [Google Scholar]
- Jacobson L. Hypothalamic-pituitary-adrenocortical axis regulation. Endocrinol Metab Clin North Am. 2005;34:271–92. doi: 10.1016/j.ecl.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Jahn H, et al. Metyrapone as additive treatment in major depression: a double-blind and placebo-controlled trial. Arch Gen Psychiatry. 2004;61:1235–44. doi: 10.1001/archpsyc.61.12.1235. [DOI] [PubMed] [Google Scholar]
- Jefferys D, C.D., Irby D, Funder J. Behavioural effect of adrenalectomy: reversal by glucocorticoids or [D-Ala2,Met5] enkephalinamide. Eur J Pharmacol. 1983;92 doi: 10.1016/0014-2999(83)90113-9. [DOI] [PubMed] [Google Scholar]
- Kellendonk C, et al. Corticosteroid receptors in the brain: gene targeting studies. Brain Res. Bull. 2002;57:73–83. doi: 10.1016/s0361-9230(01)00638-4. [DOI] [PubMed] [Google Scholar]
- Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocrine Rev. 1984;5:1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- Kier A, Han J, Jacobson L. Chronic treatment with the monoamine oxidase inhibitor phenelzine increases hypothalamic-pituitary-adrenocortical activity in male C57BL/6 mice: relevance to atypical depression. Endocrinology. 2005;146:1338–47. doi: 10.1210/en.2004-0650. [DOI] [PubMed] [Google Scholar]
- Kolber BJ, et al. Central amygdala glucocorticoid receptor action promotes fear-associated CRH activation and conditioning. Proc. Natl. Acad. Sci. USA. 2008;105:12004–9. doi: 10.1073/pnas.0803216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte SM, et al. Antisense to the glucocorticoid receptor in hippocampal dentate gyrus reduces immobility in the forced swim test. Eur J Pharmacol. 1996;301:19–25. doi: 10.1016/0014-2999(96)00064-7. [DOI] [PubMed] [Google Scholar]
- Loosen PT. Psychiatric manifestations of Cushing's syndrome. In: Blevins LS, editor. Cushing's Syndrome. Kluwer Academic; Boston: 2002. pp. 45–64. [Google Scholar]
- Mitchell JB, Meaney MJ. Effects of corticosterone on response consolidation and retrieval in the forced swim test. Behav Neuroscience. 1991;105:798–803. doi: 10.1037//0735-7044.105.6.798. [DOI] [PubMed] [Google Scholar]
- Montkowski A, et al. Long-term antidepressant treatment reduces behavioral deficits in transgenic mice with impaired glucocorticoid receptor function. J Neuroendocrinol. 1995;7:841–5. doi: 10.1111/j.1365-2826.1995.tb00724.x. [DOI] [PubMed] [Google Scholar]
- Mukherjee K, Knisely A, Jacobson L. Partial glucocorticoid agonist-like effects of imipramine on hypothalamic-pituitary-adrenocortical activity, thymus weight, and hippocampal glucocorticoid receptors in male C57BL/6 mice. Endocrinology. 2004;145:4185–91. doi: 10.1210/en.2004-0147. [DOI] [PubMed] [Google Scholar]
- Murphy BE. Antiglucocorticoid therapies in major depression: a review. Psychoneuroendocrinology. 1997;22:S125–32. [PubMed] [Google Scholar]
- O'Dwyer AM, et al. Treatment of major depression with metyrapone and hydrocortisone. J, Affective Disord. 1995;33:123–9. doi: 10.1016/0165-0327(94)00082-k. [DOI] [PubMed] [Google Scholar]
- Pace TWW, Spencer RL. Disruption of mineralocorticoid receptor function increases corticosterone responding to a mild, but not moderate, psychological stressor. Am. J. Physiol. 2005;288:E1082–8. doi: 10.1152/ajpendo.00521.2004. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- Pariante CM, M.A. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- Reichardt HM, et al. Analysis of glucocorticoid signalling by gene targeting. J. Steroid Biochem. Mol. Biol. 1998;65:111–5. doi: 10.1016/s0960-0760(97)00181-7. [DOI] [PubMed] [Google Scholar]
- Ridder S, et al. Mice with genetically altered glucocorticoid receptor expression how altered sensitivity for stress-induced depressive reactions. J Neurosci. 2005;25:6243–50. doi: 10.1523/JNEUROSCI.0736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristevski S. Making better transgenic models: conditional, temporal, and spatial pproaches. Mol Biotechnol. 2005;29:153–63. doi: 10.1385/MB:29:2:153. [DOI] [PubMed] [Google Scholar]
- Rozeboom AM, Akil H, Seasholtz AF. Mineralocorticoid receptor overexpression in forebrain decreases anxiety-like behavior and alters the stress response in mice. Proc. Natl. Acad. Sci. 2007;104:4688–93. doi: 10.1073/pnas.0606067104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Barron KW, Myers DA. Stereotaxic localization of corticosterone to the amygdala enhances hypothalamo-pituitary-adrenal responses to behavioral stress. Brain Res. 2003;963:203–13. doi: 10.1016/s0006-8993(02)03978-1. [DOI] [PubMed] [Google Scholar]
- Sirois F. Steroid psychosis: a review. Gen Hosp Psychiatry. 2003;25:27–33. doi: 10.1016/s0163-8343(02)00241-4. [DOI] [PubMed] [Google Scholar]
- Solomon MB, et al. Deletion of forebrain glucocorticoid receptors impairs neuroendocrine stress responses and induces depression-like behavior in males but not females. Neuroscience. 2012;203:135–43. doi: 10.1016/j.neuroscience.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkman MN, Schteingart DE. Neuropsychiatric manifestations of patients with Cushing's syndrome. Relationship to cortisol and adrenocorticotropic hormone levels. Arch Intern Med. 1981;141:215–9. [PubMed] [Google Scholar]
- Sterner EY, Kalynchuk LE. Behavioral and neurobiological consequences of prolonged glucocorticoid exposure in rats: relevance to depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:777–90. doi: 10.1016/j.pnpbp.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Tanaka K, et al. Prostaglandin E2-Mediated Attenuation of Mesocortical Dopaminergic Pathway Is Critical for Susceptibility to Repeated Social Defeat Stress in Mice. J. Neurosci. 2012;32:4319–29. doi: 10.1523/JNEUROSCI.5952-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien JZ, et al. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996;87:1317–26. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- Veldhuis HD, DeKorte CCMM, DeKloet ER. Glucocorticoids facilitate the retention of acquired immobility during forced swimming. Eur J Pharmacol. 1985;115:211–7. doi: 10.1016/0014-2999(85)90693-4. [DOI] [PubMed] [Google Scholar]
- Weiser MJ, Foradori CD, Handa RJ. Estrogen receptor beta activation prevents glucocorticoid receptor-dependent effects of the central nucleus of the amygdala on behavior and neuroendocrine function. Brain Res. 2010;1336:78–88. doi: 10.1016/j.brainres.2010.03.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellhoener P, et al. Elevated resting and exercise-induced cortisol levels after mineralocorticoid receptor blockade with canrenoate in healthy humans. J Clin Endocrinol Metab. 2004;89:5048–52. doi: 10.1210/jc.2004-0086. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, et al. Antiglucocorticoid treatment of depression: double-blind ketoconazole. Biol Psychiatry. 1999;45:1070–4. doi: 10.1016/s0006-3223(98)00267-4. [DOI] [PubMed] [Google Scholar]
- Zobel AW, et al. Cortisol response in the combined dexamethasone/CRH test as predictor of relapse in patients with remitted depression. a prospective study. J Psychiatr Res. 2001;35:83–94. doi: 10.1016/s0022-3956(01)00013-9. [DOI] [PubMed] [Google Scholar]