Abstract
Maintaining weight is important for better prognosis of breast cancer survivors. The associations between weight and cancer-related symptoms are not known. We examined associations among weight, weight change, inflammation, cancer-related symptoms, and health-related quality of life (HRQOL) in a cohort of stage 0-IIIA breast cancer survivors. Participants were recruited on average 6 months (2–12 months) after diagnosis. Height, weight, and c-reactive protein (CRP) were assessed at approximately 30 months post-diagnosis; cancer-related symptoms (chest wall and arm symptoms, vasomotor symptoms, urinary incontinence, vaginal symptoms, cognition/mood problems, sleep, sexual interest/function), and HRQOL (SF-36) were assessed at approximately 40 months post-diagnosis. Weight was measured at baseline in a subset. Data on 661 participants were evaluable for body mass index (BMI); 483 were evaluable for weight change. We assessed associations between BMI (<25.0, 25.0–29.9, ≥30.0 kg/m2), post-diagnosis weight change (lost ≥5%, weight change <5%, gained ≥5%), and CRP (tertile) with cancer-related symptoms and HRQOL using analysis of covariance (ANCOVA). Higher symptoms scores indicate more frequent or severe symptoms. Higher HRQOL scores indicate better HRQOL. Compared with those with BMI<25kg/m2, women with BMI ≥30 kg/m2 had scores that were: increased for arm symptoms (+25.0%), urinary incontinence (+40.0%), tendency to nap (+18.9%) and poorer physical functioning (−15.6%, all p<0.05). Obese women had lower scores in trouble falling asleep (−9.9%; p<0.05). Compared with weight change <5%, participants with ≥5% weight gain had lower scores in physical functioning (−7.2%), role-physical (−15.5%) and vitality (−11.2%), and those with weight loss ≥5% had lower chest wall (−33.0%) and arm symptom scores (−35.5%, all p<0.05). Increasing CRP tertile was associated with worse scores for chest wall symptoms, urinary incontinence, physical functioning, role-physical, vitality and physical component summary scores (all Ptrend<0.05). Future studies should examine whether interventions to maintain a healthy weight and reduce inflammation could alleviate cancer-related symptoms and improve HRQOL.
Keywords: Breast cancer survivors, body weight, inflammation, cancer-related symptoms, quality of life
Introduction
Cancer-related symptoms such as cognitive problems, pain, insomnia and urinary incontinence are highly prevalent among breast cancer survivors and can significantly decrease health-related quality of life (HRQOL) [1–4]. Up to 65% of breast cancer survivors suffer from persistent pain [3,5] and menopausal symptoms [6] more than a year after diagnosis.
Maintaining normal weight is associated with improved prognosis in breast cancer survivors [7]; however few studies have examined the associations between weight and cancer-related symptoms [8–11]. In the Health, Eating, Activity and Lifestyle (HEAL) cohort, post-diagnosis weight gain was associated with temporary or sustained changes in sleep patterns [8], and increase in body mass index [BMI]>5% from 5- to 10-year follow-up was associated with increased odds of an above-average bodily pain score of the Medical Outcomes Study 36-Item Short Form (SF-36) at 10-year follow-up [10]. A review concluded that obesity is associated with increased risk for cancer-related lymphedema which could cause discomfort and pain in the affected area [11]. In another study of 3088 breast cancer survivors, those who gained >10% of pre-diagnosis weight had an increased risk of hot flashes (odds ratio=1.33, 95% CI=1.11–1.60, vs. maintained weight)[9]. In non-cancer populations, greater weight and adiposity are associated with more severe vasomotor symptoms [12–15], and poorer mental health [16,17] and cognitive function [18,19].
Cancer-related symptoms such as fatigue, insomnia, and depression are inter-correlated in breast cancer patients [1,20,21]. Cytokines produced in inflammation interfere with both central and peripheral nervous systems that regulate sleep cycle, pain sensation and cognition [22]. Because of the similarities between cytokine-induced sickness behaviors (e.g., sleepiness and hyperalgesia)[23] and cancer-related symptoms, it is hypothesized that inflammation could be a common biological mechanism [22,24]. Since obesity is associated with increased levels of inflammatory biomarkers [25], inflammation may account for the excess symptom burden in obese cancer survivors. Although high levels of circulating inflammatory biomarkers are associated with greater fatigue in breast cancer survivors [20,26–29], we found only one study that examined the associations between inflammatory biomarkers and other breast cancer-related symptoms such as sleep problems and depression [20]. The importance of C-reactive protein (CRP) for cancer survivors was supported by our previous finding of increased mortality with higher CRP levels [30].
The primary aim of this study was to investigate whether post-diagnosis weight status (BMI), weight change and the inflammatory biomarker, CRP, were associated with cancer-related symptoms and HRQOL among breast cancer survivors. We hypothesized that high BMI, weight gain, and high levels of CRP would be associated with more severe cancer-related symptoms and worse HRQOL. The secondary aim was to investigate whether BMI and CRP were independently associated with cancer-related symptoms and HRQOL. Understanding these associations could provide important information on potential ways to prevent or alleviate these symptoms in breast cancer survivors.
PATIENTS AND METHODS
Participants and Study Setting
HEAL is a multicenter, multiethnic prospective cohort of 1183 women diagnosed with first primary stage 0-IIIA breast cancer, designed to examine the effects of weight, physical activity, diet, circulating biomarkers, and other exposures on breast cancer prognosis. Study design, recruitment and eligibility are reported elsewhere [31,32]. Participants were recruited between 1996 and 1999 within 2–12 months of diagnosis. Study protocols were approved by the Institutional Review Boards of each participating center. All participants provided written informed consent.
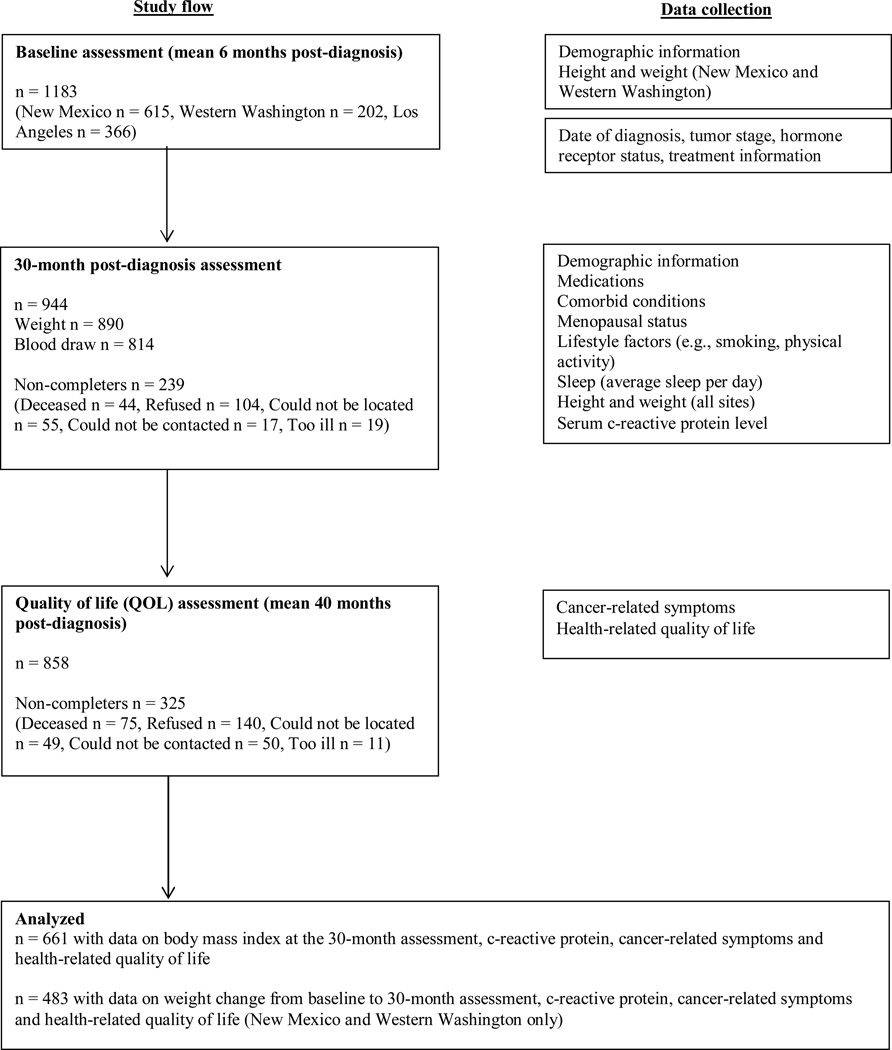
Participants completed 3 assessments: baseline (on average 6 months post-diagnosis), the 30-month (approximately 30 months post-diagnosis) and quality of life (QOL) assessments (approximately 40 months post-diagnosis, Fig 1). Demographic and clinical variables, sleep (average hours/day), and physical activity were assessed at baseline and the 30-month assessments. Weight and height were measured at the baseline and 30-month assessments in New Mexico (NM) and Western Washington (WA), and at the 30-month assessment only in Los Angeles (LA). We collected fasting blood samples at the 30-month assessment. Questionnaires that assessed cancer-related symptoms and HRQOL were completed either at the 30-month assessment (WA), or at the QOL assessment (LA and NM).
Figure 1.
Participants in the HEAL cohort and timing of data collection
A total of 1183 women completed the baseline assessment; 944 women completed the 30-month assessment; 814 provided a serum sample; and 858 completed the QOL assessment. Women who were diagnosed with a recurrence or a new primary breast cancer during the follow-up were excluded from the analysis. A total of 661 participants with data available on CRP, BMI at 30-month assessment and cancer-related symptoms and HRQOL were included in the analysis. Data on weight change from baseline to 30-month assessment were available for 483 participants recruited in WA and NM. Compared with participants not included in this analysis (N=522), those included (N=661) were on average more likely to be non-Hispanic white, have higher education, with lower tumor stage, and less likely to have had chemotherapy (all p<0.01, Online Resource Supplement Table 1).
Body Measures
Height and weight were measured and BMI was computed as kg/m2 [25].
CRP measurement
Fasting blood samples were collected and serum CRP was measured at the University of Washington [25]. The lower detection limit for CRP was 0.2 mg/L. Interassay coefficients of variation were 5–9%.
Cancer-related symptoms and health-related quality of life measures
Vasomotor symptoms, urinary incontinence, cognitive impairment/mood disturbance, vaginal symptoms and sleep quality were assessed using a shortened version of the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial (BCPT) Hormonal Symptom Checklist [33,34]. Response for each symptom ranged from 0 (not at all) to 4 (extremely). We calculated the four hormone-related symptom scales: vasomotor, urinary incontinence, cognitive/mood, and vaginal symptoms [34]. Previous analysis of the HEAL cohort have shown good validity and reliability for these scales [34]. Three items from the BCPT Hormonal Symptom Checklist, interrupted sleep, tendency to nap and trouble getting to sleep, were used to assess sleep problems [33,34]. We also asked participants to rate their degree of feeling awake in a range of 0 (awake) to 10 (sleepy). Single-item sleep questions have been used in other studies [35–37]. Hours of sleep on typical weekdays and weekends in the prior year were assessed using the Modifiable Activity Questionnaire [38,39].
We assessed chest wall and arm symptoms by asking frequencies of 7 physical sensations (i.e., pins and needle sensations, numbness, increased skin sensitivity, swelling, tenderness, tightness and pain) during the past 3 months [40,41]. Response ranged from 0 (never) to 4 (very often). These scales were shown to have adequate internal consistency (i.e., Cronbach’s alphas=0.81 for chest wall symptom and 0.88 for arm symptom) in the HEAL cohort [41].
Sexual function/interest was assessed by the previously validated sexual summary scale from the Cancer Rehabilitation Evaluation System [42]. The score ranged from 0–3; greater scores indicated more problems with sexual function/interest.
HRQOL was assessed by the SF-36 [43]. Eight subscales and two summary scores of SF-36 were calculated [43]. The two summary scores were standardized on a T-score metric. All scores ranged from 0–100; higher scores indicated better HRQOL.
Other variables
Standardized questionnaires were used to collect demographic characteristics, medical history, medication use and smoking habits at baseline and 30-month assessments. A race/study site variable was defined [29]. Tumor stage (in situ, local, and regional) and hormone receptor status (estrogen and progesterone receptors) were obtained from the SEER. Unknown estrogen or progesterone receptor status was coded as ‘unknown’. Cancer treatment data were obtained from medical records and SEER. Menopausal status (pre, post, unclassifiable) was determined using an algorithm [34]. Tamoxifen use was self-reported at the 30-month assessment. Women taking medications that have been demonstrated to reduce CRP or other inflammatory biomarkers, including angiotensin I converting enzyme inhibitors, beta-blockers, anti-hyperlipidemics, anti-gout agents, non-steroidal anti-inflammatory drugs, antipyretics, or respiratory corticosteroids, were classified as “anti-inflammatory medication users” [44,45]. Women taking antidepressants, anxiolytics, or sedatives/hypnotics were classified as “antidepressant/anxiolytic medication users”. A history of medical conditions (i.e., angina/chest pain, heart attack, congestive heart failure, deep venous thrombosis, pulmonary embolism, stroke, arthritis, diabetes/high blood sugar, or other cancer) was self-reported at the 30-month assessment. A number of comorbid conditions was categorized as 0, 1, or ≥ 2 [29]. Type, duration and frequency of physical activity in the past year were collected at the 30-month assessment [46]. Total average metabolic equivalent task (MET) hours/week (an estimate of energy expenditure based on exercise intensity and duration) of moderate-to-vigorous sport and recreational activities were calculated.
Statistical analysis
Characteristics of participants were compared using the χ2-, Fisher’s exact test and t- tests, as appropriate. We created subgroups based on BMI (<25.0, 25.0–29.9, ≥30 kg/m2); weight change since baseline (lost ≥5%, maintained [weight change <5%], and gained ≥5%) [47]; and tertiles of CRP. Linear regression tested the scores and trends (Ptrend) of symptoms/HRQOL scores across these subgroups. The symptom/HRQOL scores were compared between these groups using analysis of covariance. We also tested the associations between weight loss and symptoms/HRQOL (1) stratified by baseline BMI (<25, ≥25 kg/m2) and (2) including both baseline BMI and weight change as independent variables. The covariates in the models other than BMI and CRP were chosen based on their clinical relevance and significant contributions to the model. The models were further adjusted for BMI or CRP to examine potential confounding effects. A change in β coefficients >10% was used as a criterion for confounding. We used Cohen’s d to assess the clinical significance of the differences between the groups [48]. For HRQOL, score differences of 5–10 points for the eight subscales and 2.5–5 points for the two component summary scores were considered clinically meaningful [48–51]. We also performed the analyses excluding participants with BMI<18.5 kg/m2 (N=8) for the analysis examining associations between BMI and symptoms/HRQOL (Online Resource Supplement Table 2), and CRP outliers (N=31), defined by the 95th percentile cutoffs from National Health and Nutrition Examination Survey data [52] for the analysis examining associations between CRP and symptoms/HRQOL (Online Resource Supplement Table 3). These exclusions did not affect most analysis results except the trends of higher CRP associated with better mental component summary scores became significant (Ptrend<0.05). Therefore, these participants were included in the presented analyses. Although the associations between CRP and vitality scores (HRQOL) were previously reported [29], we repeated the analysis to confirm the association in breast cancer survivors included in the present analysis. A two-sided alpha of <0.05 was considered statistically significant. All analyses were performed with SAS 9.2 (SAS Institute, Cary, NC).
RESULTS
Of the 661 women, 407 (61.6%) were non-Hispanic white, 163 (24.7%) were black and 70 (10.6%) were Hispanic (Table 1). The mean (SD) age was 55.5 (10.0) years old at baseline. At the 30-month assessment, 76.7% of the participants were postmenopausal; 45.5% were taking tamoxifen; and 12% were current smokers. The mean (SD) BMI was 28.0 (6.3) kg/m2 and more than 60% were classified as overweight (BMI=25–29.9 kg/m2) or obese (BMI≥30 kg/m2) at the 30-month assessment. Among our subsample with weight change data available [participants recruited in WA and NM (N=483)], 45 (9.3%) lost ≥5% weight, 307 (63.6%) maintained weight, and 131 (27.1%) gained ≥5% weight from baseline to 30-month assessment.
Table 1.
Demographic and clinical characteristics of HEAL study participants
| All participants (N=661) | Participants with weight change data (N=483) |
|||
|---|---|---|---|---|
| Baseline Characteristics | N (%) | Mean (SD) | N (%) | Mean (SD) |
| Race/Ethnicity/Study site | ||||
| Non-Hispanic White - Western Washington | 136 (20.6) | 136 (28.2) | ||
| Non-Hispanic White - New Mexico | 271 (41.0) | 258 (53.4) | ||
| Black | 163 (24.7) | 1 (0.2) | ||
| Hispanic | 70 (10.6) | 67 (13.9) | ||
| Other | 21 (3.2) | 21 (4.4) | ||
| Age, years | ||||
| 29–49 | 195 (29.5) | 123 (25.5) | ||
| 50–59 | 255 (38.6) | 189 (39.1) | ||
| 60–69 | 146 (22.1) | 114 (23.6) | ||
| 70+ | 65 (9.8) | 57 (11.8) | ||
| Mean age | 55.5 (10.0) | 56.6 (10.2) | ||
| Educationa | ||||
| High School or less | 166 (25.2) | 103 (21.3) | ||
| Some college | 241 (36.5) | 166 (34.4) | ||
| College grad | 131 (20.0) | 111 (23.0) | ||
| Grad school | 122 (18.5) | 103 (21.3) | ||
| Stage at diagnosis | ||||
| in situ | 154 (23.3) | 120 (24.8) | ||
| Local | 367 (55.5) | 278 (57.6) | ||
| Regional | 140 (21.2) | 85 (17.6) | ||
| Estrogen and Progesterone Receptor status | ||||
| ER positive / PR positive | 285 (43.1) | 236 (48.9) | ||
| ER positive / PR negative | 56 (8.5) | 46 (9.5) | ||
| ER negative / PR positive | 12 (1.8) | 3 (0.6) | ||
| ER negative / PR negative | 79 (12.0) | 47 (9.7) | ||
| Unknown | 229 (34.6) | 151 (31.3) | ||
| Primary treatment type | ||||
| Any chemotherapy | 198 (30.0) | 132 (27.3) | ||
| Surgery/Radiation | 256 (38.7) | 210 (43.5) | ||
| Surgery only | 207 (31.3) | 141 (29.2) | ||
| Time Since Diagnosis, months* | ||||
| Diagnosis to the 24-month assessment | 30.7 (3.7) range 18.3–48.2 |
29.8 (2.8) range 18.3–43.7 |
||
| Diagnosis to the quality of life (QOL) assessment | 40.8 (6.6) range 23.4–63.8 |
39.8 (6.4) range 23.4–57.6 |
||
| New Mexico | 42.8 (5.4) range 32.4–57.6 |
42.8 (5.3)range 32.4–57.6 | ||
| Los Angeles | 43.7 (6.3) range 33.9–63.8 |
N/A | ||
| Western Washington | 33.9 (3.7) range 23.4–46.0 |
33.8 (3.7) range 23.4–46.0 |
||
| 30-month assessment | ||||
| Marital statusb | ||||
| Partnered | 395 (59.8) | 314 (65.0) | ||
| Not partnered | 263 (39.8) | 166 (34.3) | ||
| Menopausal status | ||||
| Pre | 116 (17.6) | 87 (18.0) | ||
| Post | 506 (76.6) | 372 (77.0) | ||
| Unclassifiable | 39 (5.9) | 24 (5.0) | ||
| Medication use | ||||
| Tamoxifen | 301 (45.5) | 227 (47.0) | ||
| Antidepressants/anxiolytics/hypnotics/sedatives | 135 (20.4) | 115 (23.8) | ||
| Anti-inflammatory drugs | 371 (56.1) | 292 (60.5) | ||
| Number of Comorbid conditions | ||||
| 0 | 228 (34.5) | 178 (36.9) | ||
| 1 | 222 (33.6) | 158 (32.7) | ||
| 2+ | 211 (31.9) | 147 (30.4) | ||
| Diabetes | 68 (10.3) | 42 (8.7) | ||
| Arthritis | 256 (38.7) | 189 (39.1) | ||
| Cardiovascular disease (angina, myocardial infarction, congestive heart failure, stroke) | 79 (12.0) | 50 (10.4) | ||
| Lifestyle factors | ||||
| Smoking | ||||
| Current | 77 (11.7) | 52 (10.8) | ||
| Former | 263 (39.8) | 199 (41.2) | ||
| Never | 321 (48.6) | 232 (48.0) | ||
| Moderate-to-vigorous physical activity, METs /week | 12.4 (18.3) | 13.6 (19.4) | ||
| Anthropometrics | ||||
| Underweight (BMI < 18.5 kg/m2) | 8(1.2) | 6 (1.2) | ||
| Normal weight (18.5 ≤ BMI < 25 kg/m2) | 245 (37.1) | 204 (42.2) | ||
| Overweight (25 ≤ BMI < 30 kg/m2) | 209 (31.6) | 160 (33.1) | ||
| Obese (BMI ≥ 30 kg/m2) | 199 (30.1) | 113 (23.4) | ||
| Mean BMI, kg/m2 | 28.0 (6.3) | 27.0 (5.6) | ||
| Weight change from baseline to 24-mo follow-up† | ||||
| Lost weight (≤ −5%) | N/A | 45 (9.3) | ||
| Maintained weight (weight change < 5%) | N/A | 307 (63.6) | ||
| Gained weight (≥ 5%) | N/A | 131 (27.1) | ||
| Inflammatory biomarker | ||||
| C-reactive protein (mg/L) | 4.4 (8.4) | 3.6 (5.0) | ||
| C-reactive protein > 3 mg/L | 254 (38.4) | 169 (35.0) | ||
n=7 were missing information on 24-month follow-up date
n=1 missing for all participants.
n=3 missing for all participants and for participants with weight change data.
Associations between weight and cancer-related symptoms and health-related quality of life
There was a trend for higher BMI to be associated with increasing arm symptom scores: adjusted means for arm symptoms scores were 0.72 (BMI<25 kg/m2); 0.83 (overweight); 0.90 (obese), Ptrend=0.04, corresponding to a difference in adjusted mean scores of 15.3% (Cohen’s d=0.10) comparing overweight to BMI<25 kg/m2, and 25.0% (Cohen’s d=0.17) comparing obese to BMI<25 kg/m2 (Table 2). Increasing BMI was also associated with more difficulty with bladder control (0.80; 1.09; 1.12 respectively, Ptrend=0.001) corresponding to a difference in scores of 36.3% (Cohen’s d=0.24) and 40.0% (Cohen’s d=0.27) greater difficulty with bladder control, respectively among overweight and obese survivors, vs. those with BMI<25kg/m2. Survivors with higher BMI had greater tendency to nap (1.11; 1.23; 1.32; Ptrend=0.04) corresponding to 18.9% (Cohen’s d=0.14) greater tendency to nap in obese survivors vs. those with BMI<25kg/m2. Greater BMI was associated with poor physical functioning (71.0; 66.9; 59.9 respectively, Ptrend<0.0001) and physical component summary scores (42.0; 40.2; 38.5 respectively, Ptrend<0.0001) but with better mental component summary scores (46.7; 47.7; 48.7 respectively, Ptrend=0.02). In contrast, survivors with higher BMI had less difficulty falling asleep (2.43; 2.29; 2.19; Ptrend=0.02) corresponding to 5.8% and 9.9% (Cohen’s d=0.16) less problems, respectively in overweight and obese survivors vs. those with BMI<25kg/m2. The associations between BMI and symptoms/HRQOL (i.e., urinary incontinence, tendency to nap, difficulty falling asleep, physical functioning, physical and mental component summary scores) were confounded by CRP (Δβ coefficients>10%). However, BMI was independently associated with urinary incontinence and physical functioning after adjusting for CRP.
Table 2.
Associations between cancer-related symptoms, health-related quality of life (HRQOL) and body mass index (BMI) at the 30 months post-diagnosis assessment
| Overall | BMI < 25 | 25 ≤ BMI < 30 | BMI ≥ 30 | ||
|---|---|---|---|---|---|
| N=661 | N=253 | N=209 | N=199 | ||
| Cancer-related symptoms | Mean Scores (SD) | P trend | |||
| Chest wall symptom (Scored 0–4) | |||||
| Unadjusted mean (SD) | 0.97 (0.82) | 0.91 (0.77) | 0.99 (0.86) | 1.03 (0.85) | |
| Adjusted mean (Model 1) | 0.94 | 1.00 | 1.04 | 0.21 | |
| Adjusted mean (Model 1+CRP) | 0.98 | 0.99 | 1.00 | 0.83 | |
| Arm symptom (Scored 0–4) | |||||
| Unadjusted mean (SD) | 0.82 (0.87) | 0.68 (0.79) | 0.84 (0.89) | 0.97 (0.93) | |
| Adjusted mean (Model 1) | 0.72 | 0.83 | 0.90 a | 0.04 | |
| Adjusted mean (Model 1+CRP) | 0.73 | 0.83 | 0.89 | 0.10 | |
| Urinary incontinence (Scored 0–4) | |||||
| Unadjusted mean (SD) | 0.90 (1.03) | 0.73 (0.90) | 1.01 (1.03) | 1.01 (1.16) | |
| Adjusted mean (Model 1) | 0.80 | 1.09 b | 1.12 b | 0.001 | |
| Adjusted mean (Model 1+CRP) | 0.82 | 1.09 b | 1.11 a | 0.01 | |
| Vasomotor symptoms (Scored 0–4) | |||||
| Unadjusted mean (SD) | 1.63 (1.11) | 1.61 (1.10) | 1.63 (1.12) | 1.64 (1.13) | |
| Adjusted mean (Model 2) | 1.66 | 1.66 | 1.56 | 0.35 | |
| Adjusted mean (Model 2+CRP) | 1.68 | 1.66 | 1.54 | 0.28 | |
| Vaginal symptoms (Scored 0–4) | |||||
| Unadjusted mean (SD) | 0.55 (0.74) | 0.55 (0.73) | 0.55 (0.74) | 0.56 (0.76) | |
| Adjusted mean (Model 2) | 0.67 | 0.67 | 0.62 | 0.52 | |
| Adjusted mean (Mode l 2+CRP) | 0.71 | 0.66 | 0.58 | 0.13 | |
| Cognitive/mood problems (Scored 0–4) | |||||
| Unadjusted mean (SD) | 1.07 (0.80) | 1.05 (0.81) | 1.05 (0.78) | 1.11 (0.82) | |
| Adjusted mean (Model 2) | 1.29 | 1.27 | 1.27 | 0.72 | |
| Adjusted mean (Model 2+CRP) | 1.29 | 1.27 | 1.27 | 0.86 | |
| Sexual interest/function (Scored 0–3)* | |||||
| Unadjusted mean (SD) | 0.88 (0.79) | 0.96 (0.83) | 0.86 (0.75) | 0.79 (0.75) | |
| Adjusted mean (Model 2) | 0.91 | 0.81 | 0.79 | 0.23 | |
| Adjusted mean (Model 2+CRP) | 0.89 | 0.82 | 0.82 | 0.54 | |
| Sleep (hrs/d)† | |||||
| Unadjusted mean (SD) | 6.94 (1.21) | 6.99 (1.17) | 6.88 (1.24) | 6.95 (1.22) | |
| Adjusted mean (Model 2) | 6.95 | 6.91 | 7.12 | 0.20 | |
| Adjusted mean (Model 2+CRP) | 6.90 | 6.92 | 7.17 | 0.057 | |
| Interrupted sleep (Scored 0–4) | |||||
| Unadjusted mean (SD) | 1.67 (1.27) | 1.72 (1.22) | 1.67 (1.28) | 1.60 (1.32) | |
| Adjusted mean (Model 2) | 1.83 | 1.74 | 1.60 | 0.07 | |
| Adjusted mean (Model 2+CRP) | 1.85 | 1.74 | 1.58 | 0.057 | |
| Tendency to nap (Scored 0–4) | |||||
| Unadjusted mean (SD) | 0.88 (1.05) | 0.74 (0.90) | 0.88 (1.05) | 1.06 (1.19) | |
| Adjusted mean (Model 2) | 1.11 | 1.23 | 1.32 a | 0.04 | |
| Adjusted mean (Model 2+CRP) | 1.13 | 1.23 | 1.29 | 0.18 | |
| Trouble getting to sleep (Scored 0–4) | |||||
| Unadjusted mean (SD) | 2.35 (1.03) | 2.49 (0.96) | 2.33 (1.06) | 2.20 (1.07) | |
| Adjusted mean (Model 2) | 2.43 | 2.29 | 2.19 a | 0.02 | |
| Adjusted mean (Model 2+CRP) | 2.48 | 2.28 | 2.15 b | 0.055 | |
| Feeling awake (Scored 0–10) † | |||||
| Unadjusted mean (SD) | 3.73 (2.66) | 3.54 (2.69) | 3.73 (2.60) | 3.97 (2.69) | |
| Adjusted mean (Model 2) | 4.07 | 4.11 | 4.11 | 0.85 | |
| Adjusted mean (Model 2+CRP) | 4.19 | 4.08 | 3.98 | 0.48 | |
|
HRQOL (Scored 0–100) | |||||
| Physical functioning | |||||
| Unadjusted mean (SD) | 74.7 (25.2) | 82.3 (21.0) | 75.3 (24.3) | 64.3 (27.4) | |
| Adjusted mean (Model 3) | 71.0 | 66.9 a | 59.9 b | <0.0001 | |
| Adjusted mean (Model 3 + CRP) | 69.2 | 67.2 | 61.7 b | 0.003 | |
| Role-physical | |||||
| Unadjusted mean (SD) | 65.8 (39.8) | 70.4 (37.6) | 65.2 (40.1) | 60.6 (41.6) | |
| Adjusted mean (Model 3) | 60.2 | 57.5 | 54.9 | 0.17 | |
| Adjusted mean (Model 3 + CRP) | 57.3 | 58.0 | 57.8 | 0.91 | |
| Bodily pain | |||||
| Unadjusted mean (SD) | 71.1 (24.8) | 75.8 (23.5) | 69.8 (24.7) | 66.5 (25.6) | |
| Adjusted mean (Model 3) | 65.6 | 61.7 | 61.8 | 0.10 | |
| Adjusted mean (Model 3 + CRP) | 64.7 | 61.9 | 62.7 | 0.44 | |
| Vitality | |||||
| Unadjusted mean (SD) | 56.2 (22.0) | 58.4 (22.1) | 55.1 (21.6) | 54.7 (22.1) | |
| Adjusted mean (Model 3) | 42.1 | 39.3 | 40.5 | 0.42 | |
| Adjusted mean (Model 3 + CRP) | 40.8 | 39.5 | 41.7 | 0.73 | |
| General health | |||||
| Unadjusted mean (SD) | 45.4 (9.7) | 46.5 (9.9) | 45.6 (9.6) | 43.7 (9.4) | |
| Adjusted mean (Model 3) | 42.8 | 42.5 | 42.0 | 0.45 | |
| Adjusted mean (Model 3 + CRP) | 42.7 | 42.5 | 42.1 | 0.58 | |
| Social functioning | |||||
| Unadjusted mean (SD) | 81.1 (23.2) | 82.6 (22.9) | 82.9 (22.2) | 77.5 (24.4) | |
| Adjusted mean (Model 3) | 69.0 | 70.7 | 69.1 | 0.90 | |
| Adjusted mean (Model 3 + CRP) | 67.5 | 71.0 | 70.5 | 0.23 | |
| Role-emotional | |||||
| Unadjusted mean (SD) | 71.8 (36.8) | 72.5 (36.0) | 70.6 (37.1) | 72.2 (37.7) | |
| Adjusted mean (Model 3) | 62.9 | 61.9 | 64.0 | 0.80 | |
| Adjusted mean (Model 3 + CRP) | 62.5 | 62.0 | 64.3 | 0.68 | |
| Mental health | |||||
| Unadjusted mean (SD) | 75.6 (16.9) | 74.9 (17.1) | 76.0 (16.3) | 75.9 (17.2) | |
| Adjusted mean (Model 3) | 68.6 | 69.8 | 69.9 | 0.40 | |
| Adjusted mean (Model 3 + CRP) | 68.8 | 69.8 | 69.8 | 0.58 | |
| Physical component summary score | |||||
| Unadjusted mean (SD) | 43.1 (9.7) | 45.8 (8.5) | 43.0 (9.3) | 39.7 (10.5) | |
| Adjusted mean (Model 3) | 42.0 | 40.2 a | 38.5 b | <0.0001 | |
| Adjusted mean (Model 3 + CRP) | 41.2 | 40.4 | 39.3 | 0.054 | |
| Mental component summary score | |||||
| Unadjusted mean (SD) | 50.5 (9.0) | 49.5 (8.9) | 50.7 (8.6) | 51.7 (9.3) | |
| Adjusted mean (Model 3) | 46.7 | 47.7 | 48.7 a | 0.02 | |
| Adjusted mean (Model 3 + CRP) | 46.8 | 47.7 | 48.5 | 0.09 | |
N=351 (BMI<25 N=151, 25≤BMI<30 N=110, BMI>30 N=90).
N=660
Higher symptoms scores indicate scores indicate more frequent symptoms. Higher HRQOL scores indicate better HRQOL.
Model 1 was adjusted for age, race/study site, cancer treatment, and number of comorbid condition.
Model 2 was adjusted for age, race/study site, cancer treatment, number of comorbid condition, menopausal status, and tamoxifen and antidepressants/anxiolytics use.
Model 3 was adjusted for age, race/study site, education, marital status, cancer treatment, number of comorbid condition, menopausal status, and tamoxifen and antidepressants/anxiolytics use.
P trend tested a trend across the subgroups defined by BMI.
P values for direct comparisons between subgroups are indicated with symbols: p<0.05 vs. ‘BMI < 25’,
p<0.01 vs. ‘BMI<25’.
Associations between weight change and cancer-related symptoms and health-related quality of life
Chest wall and arm symptoms, physical functioning, role-physical and physical component summary scores differed between the subgroups defined by weight change categories (p<0.05; Table 3). Compared with survivors with weight change<5%, participants who gained ≥5% of baseline weight had lower scores in physical functioning (−7.2%, −5.5 points), role-physical (−15.5%, −10.2 points), and physical component summary score (− 4.5%, −1.9 points); weight loss ≥5% was associated with less severe chest wall (−33.0%, Cohen’s d=0.37) and arm (-35.5%, Cohen’s d=0.09) symptom scores (all p<0.05). The associations between weight change with chest wall and arm symptoms and physical functioning scores were independent of CRP.
Table 3.
Associations between cancer-related symptoms, health-related quality of life (HRQOL) and weight change from baseline to the 30 months post-diagnosis assessment
| Weight change (%) | |||||
|---|---|---|---|---|---|
| Overall | Lost ≥ 5% | Weight change <5% |
Gained ≥5% | ||
| N=483 | N=45 | N=307 | N=131 | ||
| Cancer-related symptoms | Mean (SD) | P value1 | |||
| Chest wall symptom (Scored 0–4) | |||||
| Unadjusted mean | 1.01 (0.82) | 0.72 (0.74) | 0.98 (0.81) | 1.18 (0.83) | |
| Adjusted mean (Model 1) | 0.61a | 0.91 | 1.02 | 0.01 | |
| Adjusted mean (Model 1 + CRP) | 0.63a | 0.93 | 1.03 | 0.02 | |
| Arm symptom (Scored 0–4) | |||||
| Unadjusted mean | 0.78 (0.85) | 0.52 (0.71) | 0.75 (0.82) | 0.94 (0.93) | |
| Adjusted mean (Model 1) | 0.49 a | 0.76 | 0.89 | 0.02 | |
| Adjusted mean (Model 1 + CRP) | 0.51 a | 0.78 | 0.90 | 0.03 | |
| Urinary incontinence (Scored 0–4) | |||||
| Unadjusted mean | 1.00 (1.04) | 1.29 (1.33) | 0.94 (0.98) | 1.02 (1.07) | |
| Adjusted mean (Model 1) | 1.01 | 0.74 | 0.84 | 0.22 | |
| Adjusted mean (Model 1 + CRP) | 1.04 | 0.77 | 0.86 | 0.23 | |
| Vasomotor symptoms (Scored 0–4) | |||||
| Unadjusted mean | 1.65 (1.10) | 1.47 (1.04) | 1.60 (1.08) | 1.84 (1.15) | |
| Adjusted mean (Model 2) | 1.44 | 1.63 | 1.64 | 0.47 | |
| Adjusted mean (Model 2 + CRP) | 1.43 | 1.62 | 1.63 | 0.46 | |
| Vaginal symptoms (Scored 0–4) | |||||
| Unadjusted mean | 0.57 (0.76) | 0.57 (0.84) | 0.58 (0.78) | 0.54 (0.68) | |
| Adjusted mean (Model 2) | 0.59 | 0.65 | 0.51 | 0.24 | |
| Adjusted mean (Model 2 + CRP) | 0.66 | 0.71 | 0.54 a | 0.11 | |
| Cognitive/mood problems (Scored 0–4) | |||||
| Unadjusted mean | 1.12 (0.82) | 1.04 (0.95) | 1.05 (0.78) | 1.32 (0.85) | |
| Adjusted mean (Model 2) | 1.03 | 1.12 | 1.23 | 0.26 | |
| Adjusted mean (Model 2 + CRP) | 1.01 | 1.11 | 1.22 | 0.23 | |
| Sexual interest/function (Scored 0–3)* | |||||
| Unadjusted mean | 0.95 (0.78) | 0.91 (0.91) | 0.94 (0.76) | 1.00 (0.80) | |
| Adjusted mean (Model 2) | 0.83 | 0.79 | 0.78 | 0.95 | |
| Adjusted mean (Model 2 + CRP) | 0.78 | 0.75 | 0.75 | 0.98 | |
| Sleep (hours /d) | |||||
| Unadjusted mean | 7.12 (1.15) | 6.87 (1.46) | 7.17 (1.11) | 7.08 (1.16) | |
| Adjusted mean (Model 2) | 6.87 | 7.13 | 6.95 | 0.16 | |
| Adjusted mean (Model 2 + CRP) | 6.83 | 7.10 | 6.93 | 0.20 | |
| Interrupted sleep (Scored 0–4) | |||||
| Unadjusted mean | 1.75 (1.25) | 1.56 (1.29) | 1.69 (1.23) | 1.96 (1.26) | |
| Adjusted mean (Model 2) | 1.39 | 1.56 | 1.68 | 0.40 | |
| Adjusted mean (Model 2 + CRP) | 1.40 | 1.57 | 1.68 | 0.43 | |
| Tendency to nap (Scored 0–4) | |||||
| Unadjusted mean | 0.89 (1.05) | 1.02 (1.14) | 0.80 (0.95) | 1.07 (1.22) | |
| Adjusted mean (Model 2) | 0.88 | 0.81 | 0.96 | 0.36 | |
| Adjusted mean (Model 2 + CRP) | 0.90 | 0.82 | 0.96 | 0.41 | |
| Trouble getting to sleep (Scored 0–4) | |||||
| Unadjusted mean | 2.46 (1.00) | 2.56 (0.94) | 2.40 (1.02) | 2.54 (0.97) | |
| Adjusted mean (Model 2) | 2.43 | 2.23 | 2.35 | 0.29 | |
| Adjusted mean (Model 2 + CRP) | 2.46 | 2.25 | 2.36 | 0.33 | |
| Feeling awake (Scored 0–10) | |||||
| Unadjusted mean | 3.73 (2.62) | 3.50(2.77) | 3.55(2.61) | 4.21 (2.55) | |
| Adjusted mean (Model 2) | 2.89 | 3.14 | 3.42 | 0.44 | |
| Adjusted mean (Model 2 + CRP) | 3.07 | 3.29 | 3.49 | 0.63 | |
|
HRQOL (Scored 0–100) | |||||
| Physical functioning | |||||
| Unadjusted mean | 77.7 (23.1) | 71.6 (25.9) | 78.9 (22.8) | 77.1 (22.6) | |
| Adjusted mean (Model 3) | 73.1 | 76.7 | 71.2 a | 0.03 | |
| Adjusted mean (Model 3 + CRP) | 72.1 | 75.8 | 70.8 a | 0.06 | |
| Role-physical | |||||
| Unadjusted mean | 65.0 (38.9) | 56.7 (39.7) | 68.0 (37.9) | 60.7 (40.6) | |
| Adjusted mean (Model 3) | 58.3 | 66.0 | 55.8 a | 0.03 | |
| Adjusted mean (Model 3 + CRP) | 54.7 | 62.6 | 54.3 | 0.08 | |
| Bodily pain | |||||
| Unadjusted mean | 71.9 (23.4) | 69.6 (27.0) | 72.6 (22.4) | 71.1 (24.4) | |
| Adjusted mean (Model 3) | 66.0 | 65.9 | 64.0 | 0.72 | |
| Adjusted mean (Model 3 + CRP) | 65.2 | 65.2 | 63.7 | 0.82 | |
| Vitality | |||||
| Unadjusted mean | 54.9 (21.6) | 52.4 (22.9) | 57.3 (20.5) | 50.3 (22.9) | |
| Adjusted mean (Model 3) | 43.8 | 45.5 | 40.4 a | 0.08 | |
| Adjusted mean (Model 3 + CRP) | 42.0 | 43.8 | 39.7 | 0.20 | |
| General health | |||||
| Unadjusted mean | 45.7 (9.5) | 44.4 (9.4) | 46.2 (9.3) | 44.9 (10.1) | |
| Adjusted mean (Model 3) | 39.6 | 40.5 | 39.7 | 0.64 | |
| Adjusted mean (Model 3 + CRP) | 39.1 | 40.1 | 36.5 | 0.72 | |
| Social functioning | |||||
| Unadjusted mean | 81.7 (22.3) | 80.0 (23.7) | 83.3 (20.9) | 78.6 (24.7) | |
| Adjusted mean (Model 3) | 72.6 | 73.1 | 70.7 | 0.58 | |
| Adjusted mean (Model 3 + CRP) | 71.1 | 71.7 | 70.1 | 0.79 | |
| Role-emotional | |||||
| Unadjusted mean | 69.7 (36.6) | 74.8 (36.3) | 70.2 (35.4) | 66.7 (39.2) | |
| Adjusted mean (Model 3) | 81.2 | 72.8 | 68.9 | 0.14 | |
| Adjusted mean (Model 3 + CRP) | 80.2 | 71.9 | 68.5 | 0.17 | |
| Mental health | |||||
| Unadjusted mean | 73.7 (16.3) | 76.4 (15.5) | 74.9 (15.6) | 70.0 (17.8) | |
| Adjusted mean (Model 3) | 73.9 | 70.5 | 67.7 | 0.07 | |
| Adjusted mean (Model 3 + CRP) | 73.9 | 70.6 | 67.7 | 0.07 | |
| Physical component summary score | |||||
| Unadjusted mean | 44.0 (9.1) | 40.9 (9.4) | 44.6 (8.9) | 43.8 (9.4) | |
| Adjusted mean (Model 3) | 40.1 | 42.6 | 40.7 a | 0.03 | |
| Adjusted mean (Model 3 + CRP) | 39.4 | 42.0 | 40.4 | 0.056 | |
| Mental component summary score | |||||
| Unadjusted mean | 49.5 (8.8) | 51.7 (8.5) | 49.9 (8.3) | 47.9 (9.7) | |
| Adjusted mean (Model 3) | 50.8 | 48.3 | 47.7 | 0.10 | |
| Adjusted mean (Model 3 + CRP) | 50.8 | 48.2 | 47.6 | 0.10 | |
N=264 (lost ≥5% N=25, no change N=167, gained ≥5% N=72). CRP: c-reactive protein.
Higher symptoms scores indicate scores indicate more frequent symptoms. Higher HRQOL scores indicate better HRQOL.
Model 1 was adjusted for age, race/study site, cancer treatment, number of comorbid condition and baseline BMI.
Model 2 was adjusted for age, race/study site, cancer treatment, number of comorbid condition, menopausal status, tamoxifen and antidepressants/anxiolytics use, and baseline BMI.
Model 3 was adjusted for age, race/study site, education, marital status, cancer treatment, number of comorbid condition, menopausal status, tamoxifen and antidepressants/anxiolytics use, and baseline BMI.
P value tested the differences between the subgroup defined by weight change.
P values for direct comparisons between subgroups are indicated with symbols: p<0.05 vs. ‘no change’,
p<0.01 vs. ‘no change’
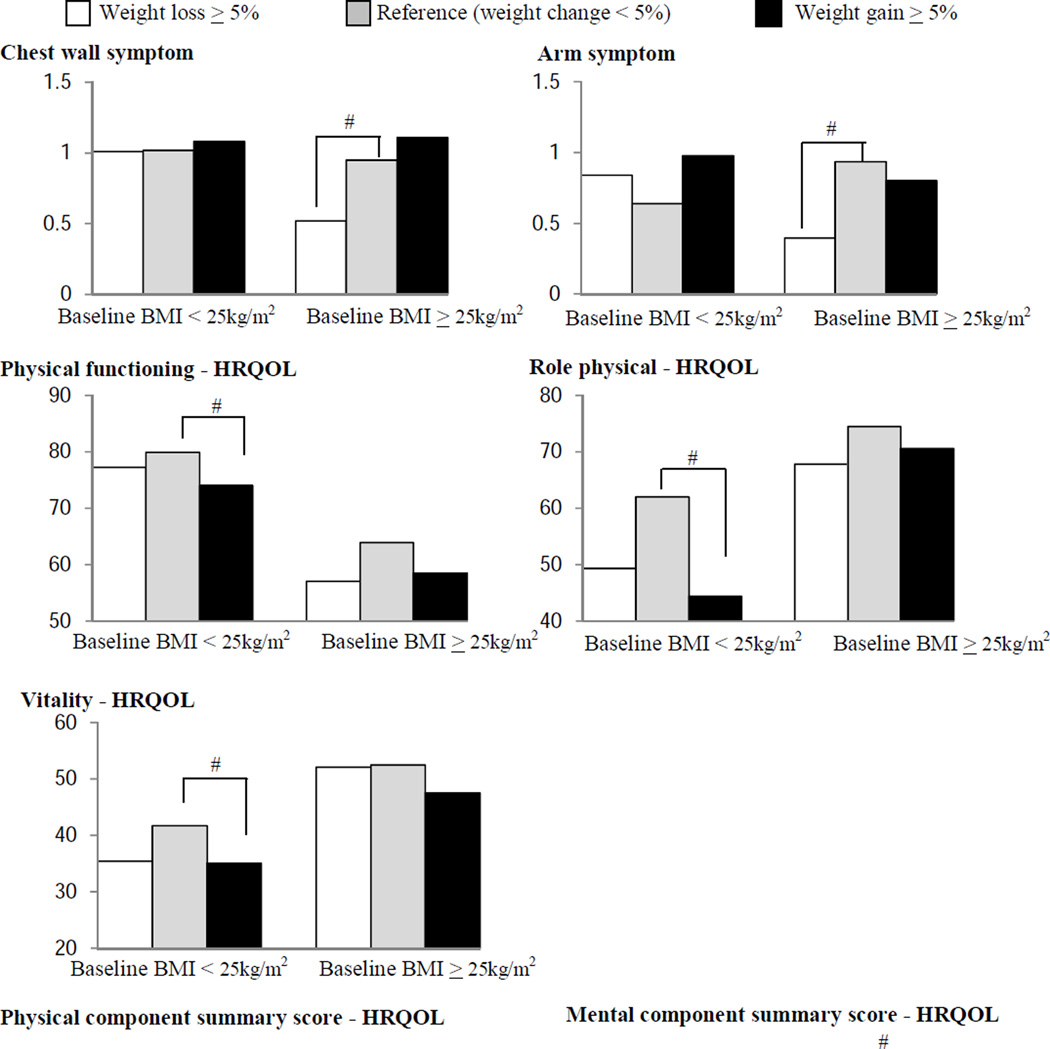
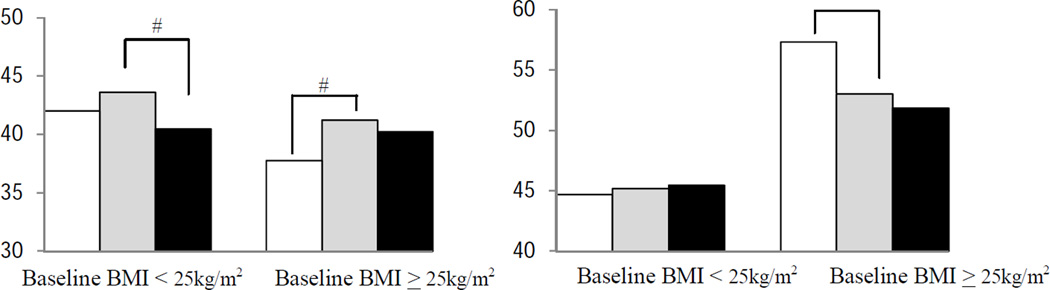
We also examined associations between weight change and symptoms/HRQOL stratified by baseline BMI (<25, ≥25 kg/m2, Fig 2). Among women with baseline BMI<25kg/m2, weight gain>5% was associated with lower physical functioning, role-physical, vitality and physical component summary scores (p<0.05, vs. weight change <5%). Among overweight or obese survivors at baseline (BMI≥25 kg/m2), weight loss ≥5% was associated with less frequent chest wall and arm symptoms, with better mental component summary scores, but with lower physical component summary score (all p<0.05, vs. weight change<5%).
Figure 2.
Associations between weight change and cancer-related symptoms/health-related quality of life (HRQOL) by baseline body mass index (BMI)
# P<0.05 vs. reference (weight change <5%)
Higher symptoms scores indicate scores indicate more frequent symptoms. Higher HRQOL scores indicate better HRQOL.
Models for arm and chest wall symptoms were adjusted for age, race/study site, cancer treatment, and number of comorbid condition. Models for HRQOL were adjusted for age, race/study site, education, marital status, cancer treatment, number of comorbid condition, menopausal status, and tamoxifen and antidepressants/anxiolytics use.
When baseline BMI and weight change were simultaneously included in a model, we found that weight loss was associated with less frequent chest wall and arm symptoms, and weight gain was associated with worsened symptoms (Online Resource Supplement Table 4). Both weight loss and gain were associated with reduced role-physical scores. Being overweight or obese at baseline was associated with more problems with urinary incontinence and tendency to nap, and with poorer physical functioning and bodily pain (vs. BMI<25 kg/m2). Being overweight or obese at baseline as well as weight loss and gain were associated with lower physical component summary scores (p<0.05).
Associations between inflammation and cancer-related symptoms and health-related quality of life
Increasing CRP tertile was associated with more adverse scores for chest wall symptoms, urinary incontinence, and physical functioning, role-physical, vitality and physical component summary score (all Ptrend<0.05, Table 4). Survivors in the highest CRP tertile had 18.4% (Cohen’s d=0.13) greater chest wall symptom scores, 22.4% (Cohen’s d=0.14) worse urinary incontinence, 13.9% (10.6 points) lower physical functioning, 14.5% (9.6 points) lower role-physical, 8.8% (4.8 points) lower vitality and 8.3% (3.6 points) lower physical component summary scores (all p<0.05 vs. lowest CRP tertile). The associations of CRP with urinary incontinence, physical functioning, role-physical, vitality, and physical component summary scores were confounded by BMI; however, higher CRP was independently associated with lower physical functioning, role-physical, and physical component summary after adjusting for BMI.
Table 4.
Associations between cancer-related symptoms, health-related quality of life (HRQOL) and c-reactive protein (CRP)
| CRP, mg/L | |||||
|---|---|---|---|---|---|
| Overall | Lowest tertile | Middle tertile | Highest tertile | ||
| N=661 | N=223 | N=220 | N=218 | ||
| Cancer-related symptoms | Mean (SD) | P trend1 | |||
| Chest wall symptom (Scored 0–4) | |||||
| Unadjusted mean | 0.97 (0.82) | 0.92 (0.76) | 0.92 (0.82) | 1.08 (0.88) | |
| Adjusted mean (Model 1) | 0.98 | 1.02 | 1.16 a | 0.03 | |
| Adjusted mean (Model 1 + BMI) | 0.99 | 1.02 | 1.15 | 0.06 | |
| Arm symptom (scored 0–4) | |||||
| Unadjusted mean | 0.82 (0.87) | 0.74 (0.78) | 0.78 (0.83) | 0.91 (1.04) | |
| Adjusted mean (Model 1) | 0.93 | 0.97 | 1.09 | 0.053 | |
| Adjusted mean (Model 1 + BMI) | 0.96 | 0.98 | 1.06 | 0.27 | |
| Urinary incontinence (scored 0–4) | |||||
| Unadjusted mean | 0.90 (1.03) | 0.77 (0.92) | 0.91 (1.04) | 1.03 (1.12) | |
| Adjusted mean (Model 1) | 1.07 | 1.14 | 1.31 a | 0.02 | |
| Adjusted mean (Model 1 + BMI) | 1.12 | 1.15 | 1.27 | 0.19 | |
| Vasomotor symptoms | |||||
| Unadjusted mean | 1.63 (1.11) | 1.62 (1.05) | 1.65 (1.16) | 1.61 (1.12) | |
| Adjusted mean (Model 1) | 1.79 | 1.84 | 1.81 | 0.84 | |
| Adjusted mean (Model 1 + BMI) | 1.77 | 1.84 | 1.83 | 0.59 | |
| Vaginal symptoms (scored 0–4) | |||||
| Unadjusted mean | 0.55 (0.74) | 0.51 (0.70) | 0.60 (0.77) | 0.56 (0.76) | |
| Adjusted mean (Model 2) | 0.62 | 0.71 | 0.69 | 0.32 | |
| Adjusted mean (Model 2 + BMI) | 0.59 | 0.71 | 0.71 | 0.13 | |
| Cognitive/mood problems (scored 0–4) | |||||
| Unadjusted mean | 1.07 (0.80) | 1.12 (0.78) | 0.99 (0.78) | 1.10 (0.85) | |
| Adjusted mean (Model 2) | 1.43 | 1.28 a | 1.35 | 0.33 | |
| Adjusted mean (Model 2 + BMI) | 1.45 | 1.28 a | 1.33 | 0.13 | |
| Sexual interest/function (Scored 0–3)* | |||||
| Unadjusted mean | 0.88 (0.79) | 0.93 (0.78) | 0.89 (0.79) | 0.80 (0.79) | |
| Adjusted mean (Model 2) | 0.91 | 0.90 | 0.80 | 0.31 | |
| Adjusted mean (Model 2 + BMI) | 0.88 | 0.90 | 0.84 | 0.79 | |
| Sleep hours | |||||
| Unadjusted mean | 6.94 (1.21) | 7.00 (1.12) | 7.04 (1.30) | 6.78 (1.19) | |
| Adjusted mean (Model 2) | 6.89 | 6.97 | 6.78 | 0.35 | |
| Adjusted mean (Model 2 + BMI) | 6.93 | 6.98 | 6.74 | 0.14 | |
| Interrupted sleep (Scored 0–4) | |||||
| Unadjusted mean | 1.67 (1.27) | 1.70 (1.22) | 1.69 (1.31) | 1.61 (1.28) | |
| Adjusted mean (Model 2) | 1.99 | 1.97 | 1.89 | 0.43 | |
| Adjusted mean (Model 2 + BMI) | 1.96 | 1.97 | 1.92 | 0.73 | |
| Tendency to nap (Scored 0–4) | |||||
| Unadjusted mean | 0.88 (1.05) | 0.82 (0.92) | 0.79 (1.03) | 1.03 (1.17) | |
| Adjusted mean (Model 2) | 1.21 | 1.12 | 1.32 | 0.27 | |
| Adjusted mean (Model 2 + BMI) | 1.27 | 1.12 | 1.27 | 0.97 | |
| Trouble getting to sleep (Scored 0–4) | |||||
| Unadjusted mean | 2.35 (1.03) | 2.40 (0.99) | 2.36 (1.07) | 2.29 (1.03) | |
| Adjusted mean (Model 2) | 2.51 | 2.48 | 2.52 | 0.66 | |
| Adjusted mean (Model 2 + BMI) | 2.46 | 2.48 | 2.50 | 0.69 | |
| Feeling awake (Scored 0–10) | |||||
| Unadjusted mean | 3.73 (2.66) | 3.52 (2.60) | 3.54 (2.58) | 4.14 (2.77) | |
| Adjusted mean (Model 2) | 4.34 | 4.30 | 4.69 | 0.18 | |
| Adjusted mean (Model 2 + BMI) | 4.35 | 4.30 | 4.68 | 0.25 | |
|
HRQOL (Scored 0–100) | |||||
| Physical functioning | |||||
| Unadjusted mean | 74.7 (25.2) | 83.5 (21.7) | 75.4 (22.2) | 65.0 (27.9) | |
| Adjusted mean (Model 3) | 76.1 | 73.5 | 65.5 b | <0.0001 | |
| Adjusted mean (Model 3 + BMI) | 74.2 | 73.6 | 67.5 b | 0.005 | |
| Role-physical | |||||
| Unadjusted mean | 65.8 (39.8) | 75.6 (35.3) | 63.1 (39.9) | 58.5 (42.1) | |
| Adjusted mean (Model 3) | 66.2 | 59.3 | 56.6 b | 0.01 | |
| Adjusted mean (Model 3 + BMI) | 65.5 | 59.3 | 57.3 a | 0.048 | |
| Bodily pain | |||||
| Unadjusted mean | 71.1 (24.8) | 75.4 (23.2) | 71.6 (23.7) | 66.3 (26.6) | |
| Adjusted mean (Model 3) | 69.4 | 68.8 | 65.8 | 0.12 | |
| Adjusted mean (Model 3 + BMI) | 68.6 | 68.8 | 66.6 | 0.42 | |
| Vitality | |||||
| Unadjusted mean | 56.2 (22.0) | 59.7 (20.6) | 56.5 (22.1) | 52.4 (22.8) | |
| Adjusted mean (Model 3) | 54.8 | 53.0 | 50.0 a | 0.02 | |
| Adjusted mean (Model 3 + BMI) | 54.2 | 53.0 | 50.6 | 0.12 | |
| General health | |||||
| Unadjusted mean | 45.4 (9.7) | 46.3 (10.1) | 45.7 (9.2) | 44.1 (9.8) | |
| Adjusted mean (Model 3) | 44.6 | 44.9 | 44.3 | 0.74 | |
| Adjusted mean (Model 3 + BMI) | 44.4 | 44.9 | 44.5 | 0.98 | |
| Social functioning | |||||
| Unadjusted mean | 81.1 (23.2) | 84.3 (21.3) | 82.1 (22.2) | 76.9 (25.6) | |
| Adjusted mean (Model 3) | 78. | 78.5 | 75.5 | 0.20 | |
| Adjusted mean (Model 3 + BMI) | 3 78.1 | 78.5 | 75.6 | 0.30 | |
| Role-emotional | |||||
| Unadjusted mean | 71.8 (36.8) | 74.9 (35.2) | 71.7 (37.0) | 68.8 (38.2) | |
| Adjusted mean (Model 3) | 71.3 | 71.6 | 69.8 | 0.66 | |
| Adjusted mean (Model 3 + BMI) | 70.9 | 71.6 | 70.2 | 0.87 | |
| Mental health | |||||
| Unadjusted mean | 75.6 (16.9) | 74.7 (18.0) | 76.6 (15.2) | 75.4 (17.2) | |
| Adjusted mean (Model 3) | 71.8 | 74.1 | 73.6 | 0.26 | |
| Adjusted mean (Model 3 + BMI) | 71.5 | 74.1 | 73.8 | 0.20 | |
| Physical component summary score | |||||
| Unadjusted mean | 43.1 (9.7) | 46.4 (8.6) | 42.9 (8.9) | 39.9 (10.4) | |
| Adjusted mean (Model 3) | 43.5 | 41.9 | 39.9b | <0.0001 | |
| Adjusted mean (Model 3 + BMI) | 43.0 | 42.0 | 40.4 b | 0.005 | |
| Mental component summary score | |||||
| Unadjusted mean | 50.5 (9.0) | 49.7 (9.0) | 50.9 (8.8) | 51.0 (9.0) | |
| Adjusted mean (Model 3) | 49.0 | 50.2 | 50.5 | 0.08 | |
| Adjusted mean (Model 3 + BMI) | 49.1 | 50.2 | 50.4 | 0.19 | |
N=351 (lowest tertile N=138, middle tertile N=121, highest tertile=92). BMI: body mass index
Higher symptoms scores indicate scores indicate more frequent symptoms. Higher HRQOL scores indicate better HRQOL.
Model 1 was adjusted for race/study site, age (continuous), cancer treatment, hormone receptor status, tamoxifen use, number of comorbidities, and menopausal status.
Model 2 was adjusted for race/study site, age (continuous), cancer treatment, hormone receptor status, number of comorbidities, menopausal status, and tamoxifen and antidepressant/anxiolytic use.
Model 3 was adjusted for race/study site, age (continuous), cancer treatment, number of comorbidities, menopausal status, and antidepressant/anxiolytic use.
P trend tested a trend of scores across the subgroups determined by tertiles of CRP.
P values for direct comparisons between subgroups are indicated with symbols: p<0.05 vs. ‘lowest tertile’,
p<0.01 vs. ‘lowest tertile’.
DISCUSSION
In this study, higher BMI and post-diagnosis weight gain were associated with greater degree of cancer-related symptoms including arm and chest wall symptoms, urinary incontinence, and sleep, as well as poorer physical HRQOL. Post-diagnosis weight loss was associated with less chest wall and arm symptoms, while weight gain was associated with poorer HRQOL. The associations between weight change and symptoms/HRQOL differed by baseline BMI (<25 or ≥25 kg/m2): weight gain >5% was associated with lower HRQOL only among women with baseline BMI<25kg/m2, while weight loss ≥5% was associated with less severe chest wall and arm symptoms and with better mental HRQOL among survivors with baseline BMI≥25 kg/m2. Increased CRP was associated with greater chest wall symptom, more difficulty with bladder control, and worse physical functioning, role-physical and vitality scores.
Arm and chest wall symptoms such as pain are common even years after completion of breast cancer treatments [3,53–55] and can be caused by multiple pathologic mechanisms [3]. Our study suggests that elevated CRP is one potential mechanism. We also found a trend that higher BMI was associated with greater arm symptoms, while weight loss ≥5% was associated with less arm and chest wall symptoms (vs. no weight change). The associations between weight change and arm and chest wall symptoms persisted after adjustment for CRP suggesting an independent association of weight with these symptoms.
Consistent with findings in a non-cancer population [56], higher BMI was associated with more difficulties with bladder control in breast cancer survivors. We also found a positive association between CRP and urinary incontinence. The association became non-significant after adjusting for BMI, suggesting that BMI confounded the associations between inflammation and urinary problems or that BMI and CRP share a causal pathway. High levels of CRP among obese women may affect urinary incontinence through increased inflammatory reactions such as oxidative stress and vascular damage which could cause pelvic and bladder muscular damage [57].
Higher BMI was associated with greater tendency to nap but with less trouble getting to sleep among breast cancer survivors. A review reported that obese individuals are more likely than normal-weight persons to suffer from daytime sleepiness [58]. Weight loss through dietary change and surgery has been shown to improve obstructive sleep apnea [59] and daytime hypersomnolence [60] in obese patients. A longitudinal analysis of the Nurse’s Health Study found positive associations between short sleep duration and future weight gain and obesity [61]. The associations between body weight and sleep therefore could be bi-directional. In addition, studies have shown that sleep is affected by various factors including cancer treatment [8], fatigue [21], and menopausal symptoms [6] in breast cancer survivors. Future studies are needed to assess the associations between body weight and different aspects of sleep in this population.
Consistent with previous reports of breast and endometrial cancer survivors [62,63], high BMI and weight gain were associated with lower physical aspects of HRQOL in our study. Furthermore, CRP was inversely associated with physical functioning, role-physical and physical component summary scores, independent of body weight. A recent study in 250 healthy older adults showed that interleukin-6 (an inflammatory biomarker) was inversely associated with physical functioning (HRQOL) adjusting for age, gender, BMI and health conditions [64]. Systemic inflammation is associated with increased risk of cardiovascular disease [65], type 2 diabetes [66], metabolic syndrome[66], cognitive disorders[67] and depression [68] which could have detrimental effects on both physical and mental HRQOL. In addition, in the HEAL cohort, CRP is higher with African-American ethnicity, increased age, presence of co-morbid conditions, current smoking, and lower with higher physical activity and tamoxifen use [25]. Although we adjusted for these variables, it is possible that other factors such as self-report bias, severity of medical conditions, and other medical conditions affecting both CRP and HRQOL could account for the observed association between CRP and physical HRQOL. More studies are needed to determine pathological mechanisms of how CRP affects HRQOL and effects of various medical conditions on these relationships.
Strengths of this study include: a large, multiethnic sample of breast cancer survivors, longitudinal data on weight, availability of anthropometric and biomarker measures, and use of validated measures to assess cancer-related symptoms and HRQOL. There were several limitations. Height, weight and blood samples were collected prior to assessment of cancer-related symptoms and HRQOL. Changes in weight and CRP between these assessments could have affected cancer-related symptoms and HRQOL. However, we believe that the time between CRP and symptoms/HRQOL assessments did not have large effects on the observed associations. For example, the Pearson’s correlation coefficient between the baseline and 12-months CRP levels in 187 middle-aged and postmenopausal women (i.e., the control group of previously completed diet and exercise trials) was 0.81 (p<0.001). Similarly, studies have shown that CRP is a stable biomarker over 1 year [69,70]. The participants included in this analysis were more likely to be non-Hispanic white, have higher education, with lower tumor stage, and less likely to have had chemotherapy compared to those who were not included, limiting the generalizability of our findings. Also, our weight change analysis was limited to Hispanic and non-Hispanic white women. Finally, the observed associations between weight, CRP and cancer-related symptoms do not imply causality. Randomized controlled trials are needed to test effects of weight loss and reduced CRP on these symptoms.
In conclusion, BMI and CRP were associated with several breast cancer-related symptoms. Although the observed associations between weight, CRP and symptoms/HRQOL require further investigation, our findings suggest that interventions to maintain healthy weight and to reduce inflammation could potentially alleviate cancer-related symptoms and improve HRQOL of breast cancer survivors. Given the high prevalence of obesity and post-diagnosis weight gain [71,72] and their associations with poor prognosis among breast cancer survivors [7], our findings underscore the importance of weight control in this population.
Supplementary Material
Acknowledgments
The HEAL study was supported by National Cancer Institute Grants: N01-CN-75036-20, N01-CN-05228, and N01-PC-67010. Portions of this work were conducted through the Clinical Research Laboratory at University of Washington, supported by the National Institutes of Health Grant M01-RR-00037, or through the University of New Mexico Grant NCRR M01-RR-0997.
Footnotes
Disclosures: The authors declare that they have no conflict of interest.
REFERENCES
- 1.Meeske K, Smith AW, Alfano CM, McGregor BA, McTiernan A, Baumgartner KB, Malone KE, Reeve BB, Ballard-Barbash R, Bernstein L. Fatigue in breast cancer survivors two to five years post diagnosis: a HEAL Study report. Qual Life Res. 2007;16:947–960. doi: 10.1007/s11136-007-9215-3. 2007. [DOI] [PubMed] [Google Scholar]
- 2.Bower JE. Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol. 2008;26:768–777. doi: 10.1200/JCO.2007.14.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gartner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302:1985–1992. doi: 10.1001/jama.2009.1568. [DOI] [PubMed] [Google Scholar]
- 4.Donovan KA, Boyington AR, Ismail-Khan R, Wyman JF. Urinary symptoms in breast cancer: a systematic review. Cancer. 2012;118:582–593. doi: 10.1002/cncr.26324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mejdahl MK, Andersen KG, Gartner R, Kroman N, Kehlet H. Persistent pain and sensory disturbances after treatment for breast cancer: six year nationwide follow-up study. BMJ. 2013;346:f1865. doi: 10.1136/bmj.f1865. [DOI] [PubMed] [Google Scholar]
- 6.Couzi RJ, Helzlsouer KJ, Fetting JH. Prevalence of menopausal symptoms among women with a history of breast cancer and attitudes toward estrogen replacement therapy. J Clin Oncol. 1995;13:2737–2744. doi: 10.1200/JCO.1995.13.11.2737. [DOI] [PubMed] [Google Scholar]
- 7.McTiernan A, Irwin M, Vongruenigen V. Weight, physical activity, diet, and prognosis in breast and gynecologic cancers. J Clin Oncol. 2010;28:4074–4080. doi: 10.1200/JCO.2010.27.9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alfano CM, Lichstein KL, Vander Wal GS, Smith AW, Reeve BB, McTiernan A, Bernstein L, Baumgartner KB, Ballard-Barbash R. Sleep duration change across breast cancer survivorship: associations with symptoms and health-related quality of life. Breast Cancer Res Treat. 2011;130:243–254. doi: 10.1007/s10549-011-1530-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caan BJ, Emond JA, Su HI, Patterson RE, Flatt SW, Gold EB, Newman VA, Rock CL, Thomson CA, Pierce JP. Effect of Postdiagnosis Weight Change on Hot Flash Status Among Early-Stage Breast Cancer Survivors. J Clin Oncol. 2012;30:1492–1497. doi: 10.1200/JCO.2011.36.8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forsythe LP, Alfano CM, George SM, McTiernan A, Baumgartner KB, Bernstein L, Ballard-Barbash R. Pain in long-term breast cancer survivors: the role of body mass index, physical activity, and sedentary behavior. Breast Cancer Res Treat. 2013;137:617–630. doi: 10.1007/s10549-012-2335-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paskett ED, Dean JA, Oliveri JM, Harrop JP. Cancer-related lymphedema risk factors, diagnosis, treatment, and impact: a review. J Clin Oncol. 2012;30:3726–3733. doi: 10.1200/JCO.2012.41.8574. [DOI] [PubMed] [Google Scholar]
- 12.Thurston RC, Sowers MR, Sutton-Tyrrell K, Everson-Rose SA, Lewis TT, Edmundowicz D, Matthews KA. Abdominal adiposity and hot flashes among midlife women. Menopause. 2008;15:429–434. doi: 10.1097/gme.0b013e31815879cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thurston RC, Sowers MR, Chang Y, Sternfeld B, Gold EB, Johnston JM, Matthews KA. Adiposity and reporting of vasomotor symptoms among midlife women: the study of women's health across the nation. Am J Epidemiol. 2008;167:78–85. doi: 10.1093/aje/kwm244. [DOI] [PubMed] [Google Scholar]
- 14.Thurston RC, Sowers MR, Sternfeld B, Gold EB, Bromberger J, Chang Y, Joffe H, Crandall CJ, Waetjen LE, Matthews KA. Gains in body fat and vasomotor symptom reporting over the menopausal transition: the study of women's health across the nation. Am J Epidemiol. 2009;170:766–774. doi: 10.1093/aje/kwp203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang AJ, Subak LL, Wing R, West DS, Hernandez AL, Macer J, Grady D. An intensive behavioral weight loss intervention and hot flushes in women. Arch Intern Med. 2010;170:1161–1167. doi: 10.1001/archinternmed.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao G, Ford ES, Dhingra S, Li C, Strine TW, Mokdad AH. Depression and anxiety among US adults: associations with body mass index. Int J Obes (Lond) 2009;33:257–266. doi: 10.1038/ijo.2008.268. [DOI] [PubMed] [Google Scholar]
- 17.McCrea RL, Berger YG, King MB. Body mass index and common mental disorders: exploring the shape of the association and its moderation by age, gender and education. Int J Obes (Lond) 2012;36:414–421. doi: 10.1038/ijo.2011.65. [DOI] [PubMed] [Google Scholar]
- 18.Whitmer RA, Gunderson EP, Quesenberry CP, Jr, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res. 2007;4:103–109. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- 19.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 20.Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29:3517–3522. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 22.Cleeland CS, Bennett GJ, Dantzer R, Dougherty PM, Dunn AJ, Meyers CA, Miller AH, Payne R, Reuben JM, Wang XS, Lee BN. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97:2919–2925. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- 23.Watkins LR, Maier SF. The pain of being sick: implications of immune-to-brain communication for understanding pain. Annu Rev Psychol. 2000;51:29–57. doi: 10.1146/annurev.psych.51.1.29. [DOI] [PubMed] [Google Scholar]
- 24.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierce BL, Neuhouser ML, Wener MH, Bernstein L, Baumgartner RN, Ballard-Barbash R, Gilliland FD, Baumgartner KB, Sorensen B, McTiernan A, Ulrich CM. Correlates of circulating C-reactive protein and serum amyloid A concentrations in breast cancer survivors. Breast Cancer Res Treat. 2009;114:155–167. doi: 10.1007/s10549-008-9985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12:2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- 27.Orre IJ, Reinertsen KV, Aukrust P, Dahl AA, Fossa SD, Ueland T, Murison R. Higher levels of fatigue are associated with higher CRP levels in disease-free breast cancer survivors. J Psychosom Res. 2011;71:136–141. doi: 10.1016/j.jpsychores.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64:604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Alfano CM, Imayama I, Neuhouser ML, Kiecolt-Glaser JK, Smith AW, Meeske K, McTiernan A, Bernstein L, Baumgartner KB, Ulrich CM, Ballard-Barbash R. Fatigue, inflammation, and omega-3 and omega-6 fatty acid intake among breast cancer survivors. J Clin Oncol. 2012;30:1280–1287. doi: 10.1200/JCO.2011.36.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, Baumgartner KB, Gilliland FD, Sorensen BE, McTiernan A, Ulrich CM. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. (2009;27:3437–3444. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McTiernan A, Rajan KB, Tworoger SS, Irwin M, Bernstein L, Baumgartner R, Gilliland F, Stanczyk FZ, Yasui Y, Ballard-Barbash R. Adiposity and sex hormones in postmenopausal breast cancer survivors. J Clin Oncol. 2003;21:1961–1966. doi: 10.1200/JCO.2003.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, Kriska A, Ballard-Barbash R. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer. 2003;97:1746–1757. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ganz PA, Day R, Ware JE, Jr, Redmond C, Fisher B. Base-line quality-of-life assessment in the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial. J Natl Cancer Inst. 1995;87:1372–1382. doi: 10.1093/jnci/87.18.1372. [DOI] [PubMed] [Google Scholar]
- 34.Alfano CM, McGregor BA, Kuniyuki A, Reeve BB, Bowen DJ, Baumgartner KB, Bernstein L, Ballard-Barbash R, Malone KE, Ganz PA, McTiernan A. Psychometric properties of a tool for measuring hormone-related symptoms in breast cancer survivors. Psychooncology. 2006;15:985–1000. doi: 10.1002/pon.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma H, Sullivan-Halley J, Smith AW, Neuhouser ML, Alfano CM, Meeske K, George SM, McTiernan A, McKean-Cowdin R, Baumgartner KB, Bernstein L. Estrogenic botanical supplements, health-related quality of life, fatigue, and hormone-related symptoms in breast cancer survivors: a HEAL study report. BMC Complement Altern Med. 2011;11:109. doi: 10.1186/1472-6882-11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grandner MA, Patel NP, Perlis ML, Gehrman PR, Xie D, Sha D, Pigeon WR, Teff K, Weaver T, Gooneratne NS. Obesity, diabetes, and exercise associated with sleep-related complaints in the American population. Z Gesundh Wiss. 2011;19:463–474. doi: 10.1007/s10389-011-0398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pedraza S, Al Snih S, Ottenbacher KJ, Markides KS, Raji MA. Sleep quality and sleep problems in Mexican Americans aged 75 and older. Aging Clin Exp Res. 2012;24:391–397. doi: 10.3275/8106. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kriska AM, Bennett PH. An epidemiological perspective of the relationship between physical activity and NIDDM: from activity assessment to intervention. Diabetes Metab Rev. 1992;8:355–372. doi: 10.1002/dmr.5610080404. [DOI] [PubMed] [Google Scholar]
- 39.Pereira MA, FitzerGerald SJ, Gregg EW, Joswiak ML, Ryan WJ, Suminski RR, Utter AC, Zmuda JM. A collection of Physical Activity Questionnaires for health-related research. Med Sci Sports Exerc. 1997;29((6 Suppl)):S1–S205. [PubMed] [Google Scholar]
- 40.Shimozuma K, Ganz PA, Petersen L, Hirji K. Quality of life in the first year after breast cancer surgery: rehabilitation needs and patterns of recovery. Breast Cancer Res Treat. 1999;56:45–57. doi: 10.1023/a:1006214830854. [DOI] [PubMed] [Google Scholar]
- 41.Alfano CM, Smith AW, Irwin ML, Bowen DJ, Sorensen B, Reeve BB, Meeske KA, Bernstein L, Baumgartner KB, Ballard-Barbash R, Malone KE, McTiernan A. Physical activity, long-term symptoms, and physical health-related quality of life among breast cancer survivors: a prospective analysis. J Cancer Surviv. 2007;1:116–128. doi: 10.1007/s11764-007-0014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schag CA, Heinrich RL, Aadland RL, Ganz PA. Assessing problems of cancer patients: psychometric properties of the cancer inventory of problem situations. Health Psychol. 1990;9:83–102. doi: 10.1037//0278-6133.9.1.83. [DOI] [PubMed] [Google Scholar]
- 43.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 44.Prasad K. C-reactive protein (CRP)-lowering agents. Cardiovasc Drug Rev. 2006;24:33–50. doi: 10.1111/j.1527-3466.2006.00033.x. [DOI] [PubMed] [Google Scholar]
- 45.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U. S A. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kriska A. Modifiable activity questionnaire. Med Sci Sports Exerc. 1997;29:S73–S78. [Google Scholar]
- 47.Blackburn G. Effect of degree of weight loss on health benefits. Obes Res. 1995;2(3 Suppl):211s–216s. doi: 10.1002/j.1550-8528.1995.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 48.Cohen J. Statistical power anlaysis for the behavioural sciences. 2nd edn. Hillsdale, NJ: Lawrence Earlbaum; 1998. [Google Scholar]
- 49.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 health survey: Manual and interpretation guide. Boston, MA: The Health Institute; 1993. [Google Scholar]
- 50.Hays RD, Woolley JM. The concept of clinically meaningful difference in health-related quality-of-life research. How meaningful is it? Pharmacoeconomics. 2000;18:419–423. doi: 10.2165/00019053-200018050-00001. [DOI] [PubMed] [Google Scholar]
- 51.Hays RD, Farivar SS, Liu H. Approaches and recommendations for estimating minimally important differences for health-related quality of life measures. COPD. 2005;2:63–67. doi: 10.1081/copd-200050663. [DOI] [PubMed] [Google Scholar]
- 52.Wener MH, Daum PR, McQuillan GM. The influence of age, sex, and race on the upper reference limit of serum C-reactive protein concentration. J Rheumatol. 2000;27:2351–2359. [PubMed] [Google Scholar]
- 53.Macdonald L, Bruce J, Scott NW, Smith WC, Chambers WA. Long-term follow-up of breast cancer survivors with post-mastectomy pain syndrome. Br J Cancer. 2005;92:225–230. doi: 10.1038/sj.bjc.6602304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee TS, Kilbreath SL, Refshauge KM, Herbert RD, Beith JM. Prognosis of the upper limb following surgery and radiation for breast cancer. Breast Cancer Res Treat. 2008;110:19–37. doi: 10.1007/s10549-007-9710-9. [DOI] [PubMed] [Google Scholar]
- 55.Peuckmann V, Ekholm O, Rasmussen NK, Groenvold M, Christiansen P, Moller S, Eriksen J, Sjogren P. Chronic pain and other sequelae in long-term breast cancer survivors: nationwide survey in Denmark. Eur J Pain. 2009;13:478–485. doi: 10.1016/j.ejpain.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 56.Subak LL, Richter HE, Hunskaar S. Obesity and urinary incontinence: epidemiology and clinical research update. J Urol. 2009;182(6 Suppl):S2–S7. doi: 10.1016/j.juro.2009.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Richter HE, Creasman JM, Myers DL, Wheeler TL, Burgio KL, Subak LL. Urodynamic characterization of obese women with urinary incontinence undergoing a weight loss program: the Program to Reduce Incontinence by Diet and Exercise (PRIDE) trial. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1653–1658. doi: 10.1007/s00192-008-0694-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 59.Sultan S, Parikh M, Youn H, Kurian M, Fielding G, Ren C. Early U.S. outcomes after laparoscopic adjustable gastric banding in patients with a body mass index less than 35 kg/m2. Surg Endosc. 2009;23:1569–1573. doi: 10.1007/s00464-009-0341-6. [DOI] [PubMed] [Google Scholar]
- 60.Smith PL, Gold AR, Meyers DA, Haponik EF, Bleecker ER. Weight loss in mildly to moderately obese patients with obstructive sleep apnea. Ann Intern Med. 1985;103:850–855. doi: 10.7326/0003-4819-103-6-850. [DOI] [PubMed] [Google Scholar]
- 61.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164:947–954. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Voskuil DW, van Nes JG, Junggeburt JM, van de Velde CJ, van Leeuwen FE, de Haes JC. Maintenance of physical activity and body weight in relation to subsequent quality of life in postmenopausal breast cancer patients. Ann Oncol. 2010;21:2094–2101. doi: 10.1093/annonc/mdq151. [DOI] [PubMed] [Google Scholar]
- 63.von Gruenigen VE, Gibbons HE, Kavanagh MB, Janata JW, Lerner E, Courneya KS. A randomized trial of a lifestyle intervention in obese endometrial cancer survivors: quality of life outcomes and mediators of behavior change. Health Qual Life Outcomes. 2009;7:17. doi: 10.1186/1477-7525-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Christian LM, Glaser R, Porter K, Malarkey WB, Beversdorf D, Kiecolt-Glaser JK. Poorer self-rated health is associated with elevated inflammatory markers among older adults. Psychoneuroendocrinology. 2011;36:1495–1504. doi: 10.1016/j.psyneuen.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kempf K, Rose B, Herder C, Kleophas U, Martin S, Kolb H. Inflammation in metabolic syndrome and type 2 diabetes: Impact of dietary glucose. Ann N Y Acad Sci. 2006;1084:30–48. doi: 10.1196/annals.1372.012. [DOI] [PubMed] [Google Scholar]
- 67.Kuo HK, Yen CJ, Chang CH, Kuo CK, Chen JH, Sorond F. Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population: systematic review and meta-analysis. Lancet Neurol. 2005;4:371–380. doi: 10.1016/S1474-4422(05)70099-5. [DOI] [PubMed] [Google Scholar]
- 68.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 69.Ockene IS, Matthews CE, Rifai N, Ridker PM, Reed G, Stanek E. Variability and classification accuracy of serial high-sensitivity C-reactive protein measurements in healthy adults. Clin Chem. 2001;47:444–450. [PubMed] [Google Scholar]
- 70.Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1999;100:230–235. doi: 10.1161/01.cir.100.3.230. [DOI] [PubMed] [Google Scholar]
- 71.Irwin ML, McTiernan A, Baumgartner RN, Baumgartner KB, Bernstein L, Gilliland FD, Ballard-Barbash R. Changes in body fat and weight after a breast cancer diagnosis: influence of demographic, prognostic, and lifestyle factors. J Clin Oncol. 2005;23:774–782. doi: 10.1200/JCO.2005.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Demark-Wahnefried W, Rimer BK, Winer EP. Weight gain in women diagnosed with breast cancer. J Am Diet Assoc. 1997;97:519–526. doi: 10.1016/s0002-8223(97)00133-8. 529; quiz 527–518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.