Abstract
While considerable effort has been made to investigate the neural mechanisms of pain, much less effort has been devoted to itch, at least until recently. However, itch is now gaining increasing recognition as a widespread and costly medical and socioeconomic issue. This is accompanied by increasing interest in the underlying neural mechanisms of itch, which has become a vibrant and rapidly-advancing field of research. The goal of the present forefront review is to describe the recent progress that has been made in our understanding of itch mechanisms.
1. Introduction
Itch and pain are defined as “an unpleasant cutaneous sensation which provokes the desire to scratch” (Rothman, 1941) and “unpleasant sensory and emotional experience associated with actual or potential tissue damage” (McCracken et al., 2004), respectively. Itch and pain are similar in that they signal the organism of potentially dangerous stimuli, and are associated with protective motor responses. Itch and pain might share a common pathway, based on following observations. 1) Both sensory qualities are transmitted via spinothalamic tract. 2) Itch is absent in patients congenitally insensitive to pain. 3) Light touch surrounding a region of itch or pain elicits a sensation of itch (alloknesis) or pain (allodynia), respectively. 4) Many spinal neurons respond to both pruritic and algesic stimuli. 5) Brain imaging studies have revealed considerable overlap in areas that are active during itch or pain, such as prefrontal areas, supplementary motor areas (SMA), premotor cortex, anterior insular cortex, anterior midcingulate cortex, primary (S1) and secondary (S2) somatosensory cortices, thalamus, basal ganglia, and cerebellum (Pfab et al., 2012). However, itch and pain differ on a number of points. Firstly, itch-inducing stimuli typically elicit scratching to remove an irritant from the skin surface or to dig out parasites invading the skin, whereas algogenic stimuli typically elicit withdrawal of the stimulated body area away from the stimulus, and/or other integrated escape or aggressive motor responses. Secondly, pain is attenuated by μ-opioids which can elicit or exacerbate itch (Staender and Schmelz, 2006). Conversely, μ-opioid antagonists suppress itch (Heyer et al., 1997) while sometimes inducing hyperalgesia (Levine et al., 1978; Gracely et al., 1983). Thirdly, painful counterstimuli (scratch, cold, heat) inhibit itch. These differences have been used to differentiate between itch and pain in animal models (Shimada & LaMotte, 2008; Akiyama et al., 2010a; LaMotte et al., 2011) (see section 3). Fourthly, while pain occurs on the body surface as well as in deep tissues (e.g. muscle, joints, or inner organs), itch only occurs at the body surface of the body including skin, cornea, and other mucosal surfaces.
Itch (pruritus) is distinguished as acute or chronic, with the latter defined as pruritus lasting more than 6 weeks (Staender et al., 2007). Chronic pruritus is associated with inflammatory skin diseases as well as systemic diseases and has been classified by several groups. An early classification scheme was based on the origin of itch (Twycross et al., 2003). Later, in 2007, the International Forum for the Study of Itch (IFSI) proposed a clinically-oriented classification scheme (Ständer et al., 2007) consisting of 6 categories: 1) Dermatological (atopic dermatitis, psoriasis, etc.), 2) Systemic (kidney dialysis, liver cholestasis, etc.), 3) Neurological (postherpetic neuralgia, etc.), 4) Psychogenic (e.g., delusional parasitosis), 5) Mixed (overlapping and coexistence of several diseases) and 6) others (undetermined origin). Epidemiological data for each classification of chronic pruritus have been reported by various groups. Among patients with atopic dermatitis, 83–87% reported daily itch (Yosipovitch et al., 2002, Chrostowska-Plak et al., 2009). The incidence of patients with psoriasis reporting itch was 64–85% (Yosipovitch et al., 2000, Sampogna et al., 2004, Prignano et al., 2009). Between 22–90% of haemodialysis patients suffered from uremic itch (Feramisco et al., 2010). In a large epidemiological study of 18,801 hemodialysis patients, moderate to extreme itch was experienced by 42% (Pisoni et al., 2006). The prevalence of itch in primary biliary cirrhosis was variable, ranging from 25–70% (Rishe et al., 2008). Of patients with hepatitis C, 24% reported having itch (Bonacini, 2000). The prevalence of pruritus at 2 years following burn injury was 73% (Carrougher et al., 2013) while another study reported that 87% of burn survivors experience itch on a daily basis (Laura et al., 2012). The prevalence of shingles-associated itch is 17–58% (Oaklander et al., 2003). Among psychiatric inpatients, 36–42% reported idiopathic itch (Kretzmer et al., 2008, Mazeh et al., 2008). Overall, the incidence of chronic itch is high under a variety of different conditions. A population-based cohort study revealed that one out of four people experience chronic itch during their lifetime (Matterne et al., 2013). While the economic costs of chronic pain have been estimated as $560–635 billion per year in the US (Institute of Medicine of the National Academies, 2011), the exact economic costs of chronic itch have not been estimated. NIAMS reported that direct costs of chronic itch (atopic dermatitis) may exceed $3 billion per year (NIAMS, 2009). Considering the high incidence of chronic itch under many different conditions, the economic costs of chronic itch are likely to be much higher. Treatment is challenging, with no current universally accepted therapy for itch (Patel and Yosipovitch, 2010). Although some topical and systemic antipruritic drugs are available, the optimal therapy is not easy to classify due to a lack of knowledge about the mechanisms underlying the various sub-types of itch (Steinhoff et al., 2011).
Pain pathways have been investigated extensively. The spinal cord plays a central role, receiving ascending sensory input from peripheral afferents as well as descending input from supraspinal modulatory curcuits that include the periaqueductal gray (PAG) and rostral ventromedial medulla (RVM) (Basbaum et al., 2009, Heinricher et al., 2009, Dubin and Patapoutian, 2010). In contrast to pain, there have been until recently few studies of the spinal processing and modulation of itch, despite the fact that chronic itch is difficult to treat and can significantly reduce the quality of life as much as chronic pain. Recent studies indicate that itch appears to be transmitted by subsets of spinal nociceptive neurons (see below). Thus, a better understanding of basic mechanisms of itch will not only lead to novel mechanisms-based strategies to treat itch, but will also move forward our understanding of pain signaling.
2 Pruritogens
A list of pruritogens is shown in table 1.
Table 1.
Pruritogens and mechanisms
| category | pruritogen | Scratching | itch humans | mechanisms | ||
|---|---|---|---|---|---|---|
| CD-1 mice | C57BL/6 mice | SD rats | ||||
| amines | histamine | + | + | − | + | H1/H4 receptor, PLC-β3, TRPV1 |
| 5-HT | + | + | + | + | 5-HT2 receptor | |
|
| ||||||
| proteases (tethered ligands) | SLIGRL | + | + | − | + | PAR-2, MrgprC11 |
| AYPGKF | + | + | − | N.T. | PAR-4 | |
| tryptase | + | N.T. | N.T. | N.T. | PAR-2 | |
| mucunain | + | N.T. | N.T. | + | PAR-2, PAR-4 | |
| cathepsin S | N.T. | N.T. | N.T. | + | PAR-2, PAR-4, MgprC11 | |
|
| ||||||
| neuropeptides | substance P | + | N.T. | − | + | mast cell degranulation, NK1 receptor, LTB4, NO |
| endothelin-1 | + | + | + | + | ETA receptor | |
|
| ||||||
| lipid mediators | PAF | N.T. | N.T. | + | + | PAF receptor |
| LPA | + | N.T. | N.T. | N.T. | histamine, Rho-ROCK | |
| LTB4 | + | N.T. | N.T. | N.T. | BLT1 receptor | |
| TXA2 | + | N.T. | N.T. | N.T. | TP receptor | |
| SPC | + | N.T. | N.T. | N.T. | Rho-ROCK, LTB4 | |
| 12-HETE | + | N.T. | N.T. | N.T. | BLT2 receptor | |
|
| ||||||
| cytokines | IL-2 | N.T. | N.T. | N.T. | + | mast cell degranulation? |
| IL-31 | N.T. | + | N.T. | N.T. | IL31RA/OSMR | |
|
| ||||||
| Mrgpr agonists | chloroquine | + | + | − | +* | MrgprA3, TRPA1, mast cell degranulation |
| BAM8-22 | N.T. | + | N.T. | + | MrgprC11, TRPA1 | |
| β-alanine | N.T. | + | N.T. | + | MrgprD | |
|
| ||||||
| uncategorized | compound 48/80 | + | + | − | + | mast cell degranulation |
| Bile acids | N.T. | + | N.T. | + | TGR5 | |
| oxidative stress | N.T. | + | N.T. | N.T. | TRPA1 | |
| TLR7 agonists | N.T. | + | N.T. | N.T. | TLR7, K2P, Kv1.1, Kv1.2 | |
+, induction of itch or scratching; −, no induction of itch or scratching; N.T., not tested;
oral administration
Abbreviations: 5-HT, 5-hydroxytryptamine (serotonin); PAF, platelet activating factor; LPA, lysophosphatidic acid, LTB4, leukotriene B4; TXA2, thromboxane A2; SPC, sphingosylphosphorylcholine; 12-HETE, 12-Hydroxyeicosatetraenoic acid; BAM8-22, bovine adrenal medullary peptide 8-22; TLR7, toll-like receptor 7; H, histamine; PAR, protease-activated receptor; NO, nitric oxide; ETA, endothelin-1 A, Rho-ROCK, Rho-associated protein kinase; BLT, LTB4 receptor; IL31, interleukin 31; OSMR, oncostatin M receptor; Mrgpr, Mas-related G-protein-coupled receptor; TP, thromboxane receptor; TGR5, G protein-coupled bile acid receptor 1 (GPBAR1); TRPA1, transient receptor potential ankyrin 1; K2P, two-pore-domain potassium channels; Kv, voltage-gated potassium channel.
2.1. Amines
Histamine is one of the best-evaluated itch mediators. Histamine produces itch accompanied by skin reactions (wheal and flare) in humans (Lewis, 1927; Weisshaar et al., 1997, Hosogi et al., 2006). Intradermal injection of histamine elicits scratching in most strains of mice (Inagaki et al., 1999, Han et al., 2006b). Histamine in the same dose range elicited little or no scratching in Sprague-Dawley rats (Thomsen et al., 2001; Jinks & Carstens, 2002), except at a high dose that elicited both hindlimb scratching and forelimb wiping following cheek injection (Klein et al., 2011) that may reflect both itch and pain (see section 3). Histamine type 1 and type 4 receptors are involved in histamine-evoked itch (Inagaki et al., 1999, Rossbach et al., 2009). Phospholipase Cβ3, which is downstream of Gq/G11 coupled with the histamine type1 receptor, is activated by histamine and contributes to itch (Han et al., 2006b). 5-HT is another amine showing pruritogenic activity. When applied iontophorecally in humans, 5-HT provokes itch accompanied by flare (Weisshaar et al., 1997, Hosogi et al., 2006). Intradermal injection of 5-HT evokes scratching in rodents via the 5-HT2 receptor (Yamaguchi et al., 1999, Thomsen et al., 2001; Jinks & Carstens, 2002; Nojima and Carstens, 2003a).
2.2. Proteases and tethered ligands
Protease-activated receptors (PARs) are activated by protease-induced cleavage of part of the extracellular domain that acts as a tethered ligand. PARs have been identified in afferent nerves (Steinhoff et al., 2000, Steinhoff et al., 2003) and their role in hyperalgesia and itch has received considerable attention. Cowhage spicules from the bean plant, Mucuna pruriens, have long been known to induce itch (Arthur & Shelley, 1955). The active component is mucunain, a cysteine protease, which acts at PAR-2 and PAR-4 subtypes to produce itch (Reddy et al., 2008). Cowhage spicules elicit histamine-independent itch with little or no accompanying flare (Johanek et al., 2007, Sikand et al., 2009). Tryptase, a serine protease, is stored in mast cell granules and activates PAR-2. Intradermal injection of tryptase elicits scratching in mice (Ui et al., 2006). Tethered ligands, such as SLIGRL (agonist of PAR-2) and AYPGKF (PAR-4 agonist) are also known to elicit scratching in mice, but not rats (Klein et al., 2011). Interestingly, a recent study reported that SLIGRL elicited scratching via Mas-related G-protein-coupled receptors C11 (MrgprC11) rather than PAR-2 (Liu et al., 2011a). Moreover, cathepsin S cysteine protease cleaves PAR-2 and PAR-4 as well as MrgprC11 to produce itch (Reddy et al., 2010 Reddy et al., 2013).
2.3. Neuropeptides
Substance P (SP) produces itch in humans as well as scratching in mice (Hagermark et al., 1978, Andoh and Kuraishi, 1998a, Andoh et al., 2001, Andoh and Kuraishi, 2003). In human skin, substance P liberates histamine through mast cell degranulation (Hagermark et al., 1978). In mice, SP elicits scratching through a direct action on primary sensory neurons, as well as by release of NO and leukotriene B4 (LTB4) from keratinocytes, rather than mast cell degranulation (Andoh and Kuraishi, 1998a, Andoh et al., 2001, Andoh and Kuraishi, 2003). Intradermal injection of endothelin-1 (ET-1) elicits itch accompanied by a flare response in humans (Ferreira et al., 1989, Katugampola et. al., 2000). ET-1 is produced by mast cells, endothelial cells and keratinocytes in the skin and is a potent pruritogen which can elicit scratching at low concentration (10–100 pmol/site), implying that ET-1 might act as an endogenous pruritogen (McQueen et al., 2007, Gomes et al., 2012, Tsugunobu et al., 2012). ET-1-evoked scratching is mediated by the ETA receptor, but not via TRPV1 or TRPA1 (McQueen et al., 2007, Jiexian et al., 2011).
2.4. Lipid mediators
Intradermal injection of platelet activating factor (PAF) induced histamine release through degranulation of mast cells, contributing to itch accompanied by flare and wheal reactions (Fjellner and Hagermark, 1985, Petersen et al., 1997, Thomsen et al., 2002). Intradermal injection of PAF elicits scratching in rats (Thomsen et al., 2001). Lysophosphatidic acid (LPA) elicits scratching through mast cell degranulation and/or a Rho/Rho-associated protein kinase (ROCK)-mediated pathway (Hashimoto et al., 2004). LPA produced by autotaxin may contribute to pruritus of cholestasis (Kremer et al., 2012).
Leukotriene B4 (LTB4), a 5-lipoxygenase metabolite, is increased in the skin in an atopic dermatitis mouse model (Andoh et al., 2011a). Intradermal injection of LTB4 elicits scratching in mice through the LTB4 receptor-1 (BLT1) receptor (Andoh and Kuraishi, 1998b, Andoh and Kuraishi, 2005), while 12-lipoxigenase metabolites elicit scratching via the LTB4 receptor-2 (BLT2) receptor (Dae-Kwon et al., 2007, Kim et al., 2008a). LTB4 is a downstream mediator of scratching evoked by SP as well as sphingosylphosphorylcholine (SPC) (Andoh et al., 2009). SPC is increased in the stratum corneum of patients with atopic dermatitis (Okamoto, 2002) and elicits scratching through a Rho/ROCK-mediated pathway (Kim et al., 2008b). Thromboxane A2, a cyclooxygenase metabolite, is synthesized by keratinocytes (Andoh et al., 2007). Intradermal injection of a stable analogue of Thromboxane A2 elicits scratching through the thromboxane (TP) receptor expressed in nerve fibers as well as keratinocytes (Andoh et al., 2007).
2.5. Cytokines
Intradermal injection of interleukin-2 (IL-2) elicits transient weak pruritus in healthy humans as well as atopic dermatitis patients (Wahlgren et al., 1995, Darsow et al., 1997). IL-31 is produced by T helper type 2 cells and is overexpressed in pruritic skin compared with non-pruritic skin (Sonkoly et al., 2006). Thus, IL-31 is a promising endogenous pruritogen in inflammatory skin diseases, in particular atopic dermatitis. Injection of IL-31 elicits scratching through a heterodimeric receptor composed of IL-31 receptor A (IL-31RA) and oncostatin M receptor (OSMR) (Dillon et al., 2004).
2.6. Mrgpr agonists
Mrgprs consist of over 50 members, in which MrgprAs, MrgprB4-5, MrgprC11 and MrgprD are restricted to small diameter dorsal root ganglion (DRG) neurons in mice (Dong et al., 2001) and are involved in histamine-independent itch. Chloroquine, the bovine adrenal medulla peptide 8-22 (BAM8-22), and β-alanine elicited itch-related scratching through MrgprA3, MrgprC11, and MrgprD, respectively, in mice (Liu et al., 2009, Liu et al., 2012a), and all compounds elicit itch in humans (Abila et al., 1994, Sikand et al., 2011, Liu et al., 2012a). The precursor of BAM8-22, proenkephalin A, is expressed in fibroblasts and keratinocytes (Slominski et al., 2011). This expression is increased under pathological conditions, such as psoriasis, and may contribute to chronic itch.
2.7. Uncategorized
Compound 48/80 is known as a mast cell degranulating agent and elicits itch in humans as well as rodents (Inagaki et al., 2002, Roman et al., 2002). Compound 48/80-evoked itch is mediated through mast cell degranulation as well as a mast cell-independent pathway, such as direct activation of capsaicin-sensitive primary sensory neurons (Eglezos et al., 1992, Inagaki et al., 2002).
The pruritogenic activity of bile acids has been debated. A recent study reported that bile acids selectively act at the G-protein-coupled bile acid receptor-1, also known as TGR5, expressed in small size sensory neurons, to elicit itch (Kirby et al., 1974, Alemi et al., 2013a). This mechanism could contribute to itch in patients with cholestatic liver diseases.
Oxidative stress, which occurs during many pathophysiological conditions such as inflammation, elicits pain as well as itch through TRPA1 (Andersson et al., 2008, Tong and Ru-Rong, 2012).
Toll-like receptors (TLRs) are important for innate immunity. One member, TLR7, is expressed in small size sensory neurons and its agonist, imiquimod, elicits scratching through the activation of TLR7 as well as the inhibition of background (K2P) and voltage-gated (Kv1.1 and Kv1.2) potassium channels (Liu et al., 2010a, Kim et al., 2011, Lee et al., 2012).
3. Animal models of itch
The close association between itch and scratching has led to the use of scratching behavior as a readout of itch in most animal models. However, itch may also be associated with other behaviors such as biting or licking the itchy area. These models are discussed, below.
3.1. Rostral back model
Intradermal injection of itch mediators into skin in the rostral back (nape of the neck) in mice and rats elicits bouts of hindlimb scratches directed to the injection site (Kuraishi et al., 1995). Scratch bouts consist of one or more rapid back-and-forth hindpaw motions with the toe claws contacting the site of itch, at a rate of ~12 Hz in mice and ~8 Hz in rats, and lasting approximately 0.5–2 sec (Cuellar et al., 2003, Nojima and Carstens, 2003b; Klein et al., 2011). Scratching is typically quantified as the number or cumulative duration of scratch bouts over time. Because of the constant within-bout scratch frequency, there have been various attempts to automate counts of scratch bouts, based on cage vibration (Brash et al., 2005), magnetic induction (Elliott et al., 2000; Inagaki et al., 2003; Marino et al., 2012), high speed vision (Nie et al., 2009) or scratch sounds (Umeda et al., 2006) with fairly good concordance between automated and direct visual assessments. Attempts have also been made to automate the assessment of scratching in humans as an additional outcome measure of chronic itch (e.g., (Talbot et al., 1991; Benjamin et al., 2004, Murray and Rees, 2011).
3.2. Cheek model
In the rodent rostral back model, hindlimb scratches are the only biomechanically available motor response that can be directed toward the site of itch since the forepaws cannot access this skin location. Thus, hindlimb scratching might reflect other sensations in addition to itch. In contrast, animals can readily access the face with both hindpaws and forepaws. Intradermal microinjection of histamine in the cheek of mice elicited hindlimb scratch bouts directed toward injection site, whereas microinjection of capsaicin elicited singular forelimb wiping motions directed caudo-rostrally across the cheek injection site (Shimada and LaMotte, 2008; Akiyama et al., 2010c, a). Thus, this “cheek” model appears to distinguish between chemical stimuli that elicit itch vs. pain in humans (LaMotte et al., 2011). The parameters of hindlimb scratching were similar between the rostral back and cheek models in mice and rats (Akiyama et al., 2010a; Klein et al., 2011). Forepaw wipes were singular and of much shorter duration (Akiyama et al., 2010a, Klein et al., 2011). The μ-opioid ligands are useful tools to distinguish between itch and pain. The μ-opioid agonist morphine inhibited wiping but not scratching, while the μ-antagonists naltrexone or naloxone inhibited scratching but not wiping, respectively (Akiyama et al., 2010a, Spradley et al., 2012a). This is further evidence that the cheek model can discriminate between itch and pain. The cheek model has the added advantage that behavior can be correlated with responses of trigeminal subnucleus caudalis (Vc) neurons elicited by identical cheek stimuli (Akiyama et al., 2010c).
There may be differences in sensory responses elicited by stimulation of facial skin vs. skin of the lower body (Hunt and Mantyh, 2001, Dussor et al., 2008). The cheek appears to be less sensitive to certain itch mediators compared to the rostral back (Bay et al., 2009), and rats exhibited fewer scratch bouts when 5-HT was delivered to the cheek compared to rostral back (Spradley et al., 2012c). Thus, caution is warranted in estimating the potency of chemically-evoked behavioral responses between the cheek and rostral back models.
3.3. Hindpaw/calf models
Intradermal injection of 5-HT in the mouse hindpaw elicited biting behaviors directed to the injection site, while formalin elicited almost exclusively licking behavior (Hagiwara et al., 1999). Moreover, the 5-HT-evoked biting was suppressed by naloxone, suggesting that it reflects itch. Mice exhibited enhanced biting of the hindpaw in a model of chronic dry skin itch, with no change in pain sensitivity (Nojima et al., 2004; Akiyama et al., 2010d). Spontaneous biting of dry skin-treated hindpaw skin was attenuated by naltrexone but not morphine (Akiyama et al., 2010d). This suggests that biting is a surrogate for scratching to relieve itch in the distal extremities of rodents. In this model, licking of the hindpaw was observed in control mice suggesting that licking is a subtype of grooming behavior (Akiyama et al., 2010d). It is cautioned that use of the plantar surface may not be ideal to assess itch or pain due to weight-bearing and locomotion.
Intradermal injection of pruritogens or algogens into hairy skin on the calf of the lower leg elicits respective biting or licking behaviors that appear to discriminate between itch and pain (LaMotte et al., 2011). An additional advantage of the hindlimb models is that second-order spinal cord neurons activated from the distal hindlimb are located in the lumbar spinal cord and thus readily accessible. However, discrimination between biting and licking requires high-definition video recording and careful visual analysis at slow playback speed. Biting involves high-frequency low-amplitude jaw movements compared to the lower-frequency high-amplitude licking motion of the tongue; these movement parameters may prove useful in developing automated means to distinguish between these two behaviors. Thus, we believe that the “calf” model holds great promise for investigating behavioral-neural correlates of itch.
4. Primary sensory afferents
Itch is mediated by unmyelinated C-fiber afferents as well as thinly myelinated Aδ-fiber afferents. In microneurographic recordings in humans, mechano-insensitive C-fibers preferentially respond to histamine but not cowhage (Schmelz et al., 1997, Namer et al., 2008). In contrast, mechano-sensitive, polymodal C-fibers readily respond to cowhage with lesser or no responses to histamine in humans and primates (Johanek et al., 2008, Namer et al., 2008). Thus, cowhage and histamine appear to activate largely separate populations of C-fibers. While pruritogen-responsive polymodal C-fibers can respond to noxious mechanical stimuli and thus are not pruritogen-specific, histamine-responsive mechano-insensitive C-fibers may be pruritogen-specific although most of them additionally respond to algogens such as capsaicin or bradykinin in humans (Schmelz et al., 2003b). Mechano-sensitive A-fibers responded more vigorously to cowhage than to histamine, but some exclusively responded to histamine in monkeys (Ringkamp et al., 2011). C-fibers and Aδ-fibers may convey two distinct qualities of itch, a slow burning component and a faster pricking component, respectively (Graham et al., 1951). Local anesthesia by procaine abolished the slow component without affecting the fast component. Either local pressure-evoked ischemia or anesthesia by cold produced an area where slow, but not fast, itch was elicited. However, other studies are inconsistent with these observations. The local anesthetic, chloroprocaine, enhanced itch following an intradermal injection of histamine (Atanassoff et al., 1999). Conduction block of myelinated fibers by nerve compression reduced pricking as well as burning sensations (Ringkamp et al., 2011).
Murine C fibers have been divided into peptidergic and nonpeptidergic subsets mainly on the basis of neurochemical criteria, although there is some overlap (Han et al., 2012, McCoy et al., 2013). The peptidergic neurons typically contain SP and calcotonin gene-related peptide (CGRP), while nonpeptidergic neurons commonly express the purinergic P2X3 receptor and the plant lectin isolectin B4 (IB4) (Hunt and Mantyh, 2001, Dussor et al., 2008). Peptidergic and nonpeptidergic neurons exhibit anatomically distinct distribution patterns in the dorsal horn of the spinal cord as well as in skin. A large subset of nonpeptidergic neurons expresses MrgprD whose central projections terminate mainly in inner lamina II (IIi), while peptidergic neurons mainly terminate in lamina I and outer lamina II (IIo) (Zylka et al., 2005). Within the skin, MrgprD-expressing neurons innervate the most superficial layer of epidermis, the stratum granulosum, while peptidergic neurons innervate the underlying stratum spinosum (Zylka et al., 2005). The sensory function of these two subsets of neurons has been examined and is apparently different. Ablation of MrgprD-expressing neurons selectively reduced mechanical but not thermal nociception, even though they respond to noxious thermal and mechanical stimuli and innervate diverse types of spinal neurons (Cavanaugh et al., 2009, Rau et al., 2009, Wang and Zylka, 2009). Conversely, a large subset of peptidergic neurons expresses TRPV1 and removal of TRPV1-expressing neurons results in a reduction in thermal nociception but not mechanical nociception (Cavanaugh et al., 2009, Rau et al., 2009). Consistent with these observations, genetic ablation of CGRPα-expressing neurons impaired thermal but not mechanical nociception (McCoy et al., 2013). Itch is likely to be transmitted by both a unique population of peptidergic neurons expressing MrgprA3, as well as a small subset of nonpeptidergic neurons expressing MrgprD. Genetic ablation of CGRPα-expressing neurons reduced MrgprA3 expression by 70%, and impaired scratching evoked by chloroquine (MrgprA3 agonist) and histamine (McCoy et al., 2013). Impaired scratching in these animals is likely attributed to the reduction in MrgprA3-expressing neurons. Genetic ablation of MrgprA3-expressing neurons attenuated scratch responses to chloroquine, BAM8-22 (MrgprC11 agonist) and histamine without affecting pain behaviors (Han et al., 2012). MrgprA3-expressing neurons represent a unique population of DRG neurons, with 63% co-expressing CGRP and IB4 (Han et al., 2012). MrgprA3-expressing sensory neurons are polymodal nociceptors responding to noxious mechanical and chemical stimuli (Han et al., 2012). The involvement of nonpeptidergic neurons in itch was reported by Dong et al (Liu et al., 2012a). The MrgprD agonist, β-alanine, elicited itch in humans and scratching in mice; the latter was abolished in MrgprD knockout mice. All MrgprD-expressing neurons exhibited responses to noxious mechanical stimuli. Within this population, about 40% exhibited responses to β-alanine as well as noxious heat stimuli, while the remainder responded to neither stimulus. Genetic ablation of neither MrgprA3- nor CGRPα-expressing neurons affected β-alanine-evoked scratching, implicating the involvement of an additional supopulation of sensory neurons in β-alanine-elicited itch (Han et al., 2012, McCoy et al., 2013).
MrgprA3- and MrgprD-expressing neurons that respond to pruritogens are also sensitive to noxious stimuli. This indicates that most primary afferent pruriceptors are not specifically responsive only to pruritogens, but additionally respond to noxious stimuli. Consistent with this, pruriceptors apparently share some of the same signal transduction molecules with nociceptors. TRPV1 and TRPA1 are responsible for mediating burning pain elicited by capsaicin and AITC, respectively. TRPV1 knockout mice show deficits in histamine-evoked scratching (Han et al., 2006a, Imamachi et al., 2009). In contrast, TRPA1 is expressed by a subset of TRPV1-expressing neurons, and TRPA1 knockout mice exhibited a reduced scratching responses to chloroquine and BAM8-22 in a histamine-independent manner (Liu et al., 2009, Wilson et al., 2011). Thus, histaminergic and non-histaminergic itch pathway utilize distinct channels, namely TRPV1 and TRPA1, respectively. However, cellular and molecular mechanisms underlying chloroquine-evoked itch might be more complicated than previously reported (Than et al., 2013). Phospholipase C (PLC) plays a key role in intracellular signaling by G-protein-coupled receptors (GPCRs). PLCβ3 contributes to certain types of itch as well as inflammatory and neuropathic, but not thermal, pain (Xie et al., 1999, Han et al., 2006a, Joseph et al., 2007, Shi et al., 2008, Imamachi et al., 2009). A membrane protein expressed in nociceptors, phosphoinositide interacting regulator of TRP (Pirt)(Kim et al., 2008), binds to phosphatidylinositol (4,5)-bisphosphate (PIP2), TRPV1, and other ion channels and plays a role in thermal nociception through regulation of TRPV1. Pirt knockout mice exhibited significant reductions in scratch responses elicited by a variety of pruritogens (Patel et al., 2011).
5. Spinal Neurotransmitters
5.1. Gastrin releasing peptide (GRP), substance P (SP) and glutamate
The neurotransmitters involved in spinal or trigeminal transmission of itch have recently come under investigation, with particular emphasis on GRP, SP, and glutamate. Neurotoxic ablation of neurokinin-1 (NK-1) receptor-expressing neurons in the superficial dorsal horn of rats attenuated 5-HT-evoked scratching (Carstens et al., 2010), and selective NK-1 antagonists reduced scratching elicited by chloroquine, but not histamine, in mice, implying a role for SP in non-histaminergic itch (Akiyama et al., 2012c). Neurotoxic ablation of GRP receptor-expressing neurons in mice attenuated scratching elicited by a variety of pruritogens without affecting pain-related behaviors (Sun et al., 2009). The GRP receptor is extensively colocalized with MOR1D in the superficial spinal cord, and antagonism of the GRP receptor abolished morphine-induced scratching (Liu et al., 2011b), implicating the GRP receptor in opioid-induced itch. Selective GRP receptor antagonists and knock out of the GRP receptor partially reduced scratching evoked by chloroquine, but not histamine, implying a partial role for GRP in non-histaminergic itch (Sun and Chen, 2007, Akiyama et al., 2012c). Although GRP is expressed in DRG neurons (Sun and Chen, 2007, Liu et al., 2009, Lagerstrom et al., 2010, Liu et al., 2010b, Akiyama et al., 2012c, Alemi et al., 2013b), it is still debatable whether GRP is released from central terminals of primary sensory neurons. MrgprA3-expressing sensory neurons expressed GRP, and their central terminals made synaptic contact with GRPR-expressing spinal neurons (Liu et al., 2009, Han et al., 2012). However, recent in vitro studies have shown that GRP-sensitive spinal neurons utilize glutamate rather than GRP as a neurotransmitter (Koga et al., 2011). GRP staining of the rhizotomized spinal cord revealed that the majority of GRP is synthesized locally within the spinal cord (Fleming et al., 2012). Additionally, recent in vitro studies using segments of spinal cord with attached dorsal roots have shown that pruritogen-evoked release of GRP was not blocked by pretreatment of capsaicin, which causes depletion of neuropeptides such as CGRP in the central terminals (Alemi et al., 2013b). Thus, the source of GRP acting to promote itch transmission within the spinal cord may be from local spinal neuronal circuits, rather than (or in addition to) release from the intraspinal terminals of non-histaminergic primary afferent pruriceptors. GRP was reported to be expressed in peripheral nerve terminals of primary sensory neurons (Tominaga et al., 2009, Kagami et al., 2013, Nattkemper et al., 2013). Peripheral intradermal injection of GRP elicited scratching through mast cell degranulation (Andoh et al., 2011b). Serum GRP levels in atopic dermatitis patients positively correlated with the itch score (Kagami et al., 2013). Thus, expression of GRP in DRG neurons may reflect a peripheral role of GRP in itch. Moreover, the GRP receptor (GRPR) is expressed in immune cells, such as macrophages, T-cells and neutrophils, and contributes to development of inflammatory diseases (Zhou et al., 2011, Czepielewski et al., 2012). GRP might play a key role in transmission of itch signaling as well as modulating neuroimmune interactions. Glutamate acting at the AMPA/kainate receptor is also a likely candidate for spinal itch transmission (Koga et al., 2011). Synaptic input to neurons that responded to application of GRP could be blocked with AMPA/kainate receptor antagonists, suggesting that the prominent primary afferent input to these GRP-responsive neurons is glutamatergic. Thus, SP, GRP, and glutamate are good targets for developing novel treatments for itch.
5.2. Nautriuretic polypeptide b (Nppb)
A very recent, striking finding was reported by Mishra and Hoon (2013). Nppb, previously known to be released upon stretching of cardiomyocytes in the heart, was found to be expressed in a subset of small diameter sensory neurons that co-express TRPV1, PLCβ3, and MrgprA3 (Mishra and Hoon, 2013). Nppb knockout mice exhibited lack of scratching responses to histamine, chloroquine, ET-1, 5-HT, SLIGRL-NH2, and compound48/80, but exhibited normal pain behaviors. Moreover, intrathecal injection of Nppb elicited scratching behaviors. Mice in which the natriuretic peptide receptor A (Npra) was ablated, exhibited impaired scratching responses to histamine as well as Nppb. Npra was expressed primarily in lamina I. Collectively, these data suggest that Nppb plays a major role in spinal itch transmission. The authors proposed that Nppb is released from primary afferent pruriceptors to excite second-order Npra-expressing spinal neurons and ultimately excite downstream GRPR-expressing spinal neurons that are required for the transmission of itch signals to higher centers. The exact neurocircuitry and relative roles of glutamate, Nppb, SP and GRPR-expressing neurons in itch are important questions that remain to be answered in the exciting and rapidly-advancing field of itch mechanisms research.
5.3. Neurokinins and neuromedins
Neurokinin B encoded by the tachykinin 2 gene is a member of the tachykinin peptides along with SP, and is a possible candidate as a neuropeptide transmitter in spinal itch transmission. However, a recent study revealed that tachykinin 2 null mice exhibited normal scratch responses to compound 48/80, chloroquine, SLIGRL-NH2 and α-methyl-5-HT as well as normal responses to noxious heat and mechanical stimuli, implying that neurokinin B does not appear to be essential for the spinal transmission of itch or pain signaling (Mar et al., 2012). Neuromedin B is a member of the mammalian bombesin family of peptides along with GRP, and is another candidate as a neuropeptide transmitter in spinal itch transmission. Intrathecal or intracerebroventricular administration of neuromedin B elicited marked scratching (Van Wimersma Greidanus and Maigret, 1991, Cridland and Henry, 1992, Su and Ko, 2011), while intrathecal administration of neuromedin B produced a transient decrease followed by a delayed increase to above baseline in tail flick latency (Cridland and Henry, 1992). Neurotoxic ablation of neuromedin B receptor-expressing neurons in the superficial dorsal horn did not affect histamine H1 receptor agonist-evoked scratching, but reduced noxious heat-evoked behavioral responses (Mishra et al., 2012), implying a role for neuromedin B in thermal pain. It would be interesting to test whether neuromedin B is involved in the spinal transmission of non-histaminergic itch. CGRP may contribute to the spinal transmission of pain as well as itch. CGRPα was expressed by 61–73% of pruritogen-responsive DRG neurons, and 27–83% of algogen-responsive DRG neurons (McCoy et al., 2012). Overall, pathways for itch and pain signaling appear to use the same neurotransmitters. Moreover, it may be expected that nociceptive primary afferents activate pruritogen-responsive spinal neurons as discussed in the next section.
6. Pruritogen-responsive spinal neurons
The dorsal horn is the major site processing information from primary sensory afferents. Superficial dorsal horn neurons (laminae I–II) receive direct input from most nociceptive Aδ- and C-fibers, while deep dorsal horn neurons (laminae III–V) receive direct input from Aβ-fibers (Todd, 2002). Recent molecular studies have further categorized the central projections of nociceptive C-fibers and low-threshold mechanoreceptors (LTMRs) in the spinal cord (Basbaum et al., 2009, Li et al., 2011). Spinal cord neurons in lamina I-IIo, IIm, IIi, III, or III-V receive projections from peptidergic C-fibers, nonpeptidergic C-fibers, C- low-threshold mechanorecptors (LTMRs), Aδ-LTMRs, or Aβ-LTMRs, respectively.
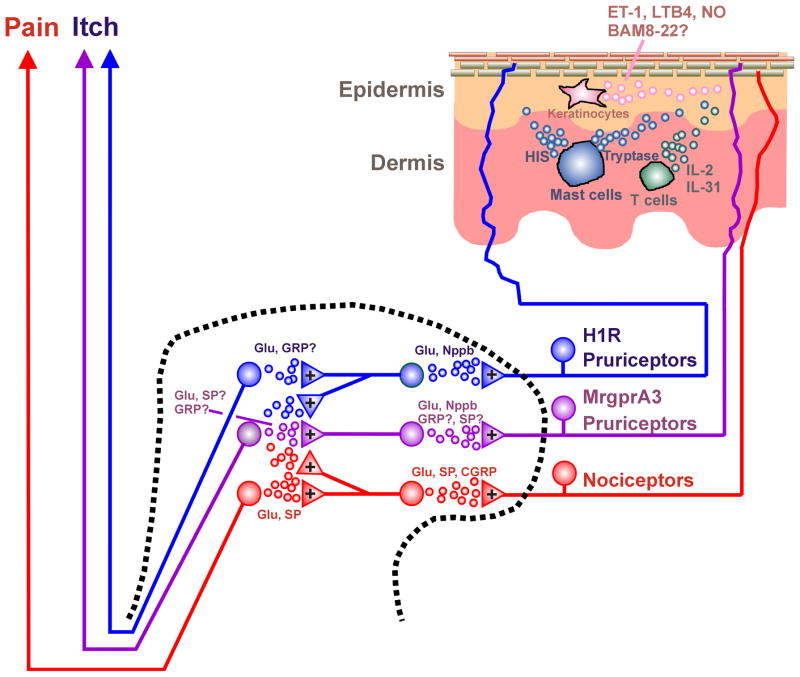
A schematic diagram of excitatory spinal circuits mediating itch is shown in Fig. 1. Approximately 80% of ascending projection neurons express the NK-1 receptor in rat spinal cord, with those located in lamina I receiving input mostly both SP- and CGRP-containing afferents and a few SP-containing afferents lacking CGRP (Todd et al., 2002). Since 5-HT-evoked scratching was reduced in rats following ablation of NK-1-expressing superficial dorsal horn neurons, the latter plausibly play the role in ascending transmission of itch as well as pain signals (Carstens et al., 2010). A later study reported that lamina I projection neurons are innervated by CGRP- as well as IB4-labeled afferents (with a small population expressing both), consistent with the unique characteristics of MgrprA3-expressing neurons (Han et al., 2012, Saeed and Ribeiro-da-Silva, 2012). Since MrgprA3-expressing neurons synapse with GRPR-expressing spinal neurons, some of the latter may be ascending projection neurons. Alternatively, GRPR-expressing spinal neurons might be excitatory interneurons which receive GRP released from Npra-expressing spinal neurons (Mishra and Hoon, 2013). Genetic ablation of testicular orphan nuclear receptor 4 (TR4) resulted in the loss of neurons expressing SP, the vesicular glutamate transporter-2 (VGLUT2), and GRPR, without loss of ascending projection neurons, suggesting that neurons expressing SP, VGLUT2 and GRPR are mainly excitatory interneurons (Todd et al., 2003, Maxwell et al., 2007, Wang et al., 2013). TR4 knockout mice exhibited impaired scratch responses, implying that those excitatory interneurons play a role in spinal excitatory circuits for itch (Wang et al., 2013).
Fig. 1.
Schematic diagram of excitatory circuits for itch. Dashed line is cross-section of spinal cord dorsal horn. Upper right shows cross-section through skin. + denotes excitatory synapse.
Dorsal horn neurons can be classified into four general categories according to their responses to mechanical stimuli: mechano-insensitive (MI) neurons that respond to neither noxious nor innocuous mechanical stimuli, low-threshold (LT) neurons that do not respond to noxious mechanical stimuli, wide dynamic range (WDR) neurons that respond at higher firing rate to noxious than to innocuous mechanical stimuli, and nociceptive specific (NS) or high-threshold (HT) neurons that respond to noxious but not innocuous mechanical stimuli. WDR and NS neurons are located in both superficial and deep dorsal horn (Price et al., 1978, Chudler et al., 1991, Dado et al., 1994). LT neurons are located mainly in the deep dorsal horn (laminae III-IV) (Price et al., 1978, Chudler et al., 1991).
Itch-signaling spinal neurons should respond to cutaneous application of a pruritogen over a time course matching that of itch sensation. Some studies have taken a non-biased approach to identify ascending projection neurons by antidromic stimulation, and then test if they respond to pruritogens (e.g., Andrew & Craig, 2001; Simone et al., 2004; Davidson et al., 2007, 2012). Other studies have used intradermal injection of a pruritogen as a search stimulus to identify spinal neurons (Carstens, 1997; Jinks & Carstens, 2002; Akiyama et al., 2009a,b). By either approach, pruritogen-responsive neurons were mostly either NS or WDR, with fewer of them being MI (Andrew and Craig, 2001, Jinks and Carstens, 2002, Davidson et al., 2007, Akiyama et al., 2009a, Akiyama et al., 2009b, Akiyama et al., 2010c, Davidson et al., 2012). Andrew and Craig recorded from 190 identified spinothalamic tract (STT) neurons in lamina I of cats; 18 were mechanically- and thermally-insensitive. Interestingly, 10 of the latter responded to iontophoretically applied histamine and 2 of 4 histamine-responsive STT neurons tested did not respond to the algogen, AITC (capsaicin was not tested in this study). Davidson et al. (2012) recorded from 111 STT neurons in adult macaques; 32 responded to either histamine or cowhage and 2 responded to both. All of the histamine- and cowhage-responsive STT neurons were either WDR or NS. Overall, approximately 30% of nociceptive STT neurons were pruritogen-responsive as well. An important implication of these latter studies (Davidson et al., 2007; 2012) is that itch elicited by histamine vs. cowhage may be mediated via largely separate subpopulations of ascending spinothalamic tract neurons.
Using a pruritogen search stimulus, Jinks & Carstens (2002) identified 21 5-HT-responsive neurons in the superficial dorsal horn of rats, of which 15 were classified as WDR, 5 as NS and 1 as MI. The MI unit responded to noxious chemical and heat stimuli. Using a pruritogen search strategy in mice, we have collectively recorded from 17 histamine-, 7 5-HT-, 58 SLIGRL-NH2-, and 10 chloroquine-responsive lumber spinal neurons; 5 histamine- and 3 SLIGRL-NH2-responsive neurons were MI and the remainder were WDR or NS (Akiyama et al., 2009a, Akiyama et al., 2009b). Overall, the vast majority of pruritogen-responsive spinal neurons, including WDR, NS and MI (but not LT) subtypes, additionally responded to noxious mechanical, thermal, and/or chemical stimuli and thus appears to be a subset of nociceptive spinal neurons.
7. Trigeminal processing of itch
Using calcium imaging of trigeminal ganglion (TG) cells, 15.4% and 5.8% responded to histamine and SLIGRL-NH2, respectively (Akiyama et al., 2010c). Of these, more than 70% additionally responded to capsaicin or AITC. We also recorded from 58 neurons in trigeminal subnucleus caudalis (Vc) with afferent input from the cheek (Akiyama et al., 2010c). Out of 32 pruritogen-responsive Vc neurons, 4 were MI and responded to either capsaicin or AITC. In this study, a subpopulation of nociceptive neurons was isolated using an algogen (AITC) search stimulus and subsequently tested with several pruritogens. Only a minority of these nociceptive neurons (13–41%) additionally responded to the pruritogens histamine, SLIGRL-NH2, or 5-HT. Overall, the vast majority of pruritogen-responsive medullary dorsal horn neurons additionally responds to noxious mechanical, thermal, and/or chemical stimuli and thus appears to be a subset of nociceptive spinal neurons, similar to pruritogen-responsive spinal neurons. There appears to be a larger population of nociceptive neurons, such as those isolated using an AITC search stimulus, the majority or which does not respond to pruritogens. This is consistent with our population-coding model in which itch is proposed to be signaled by pruritogen-sensitive nociceptive neurons, while pain is signaled by the larger population of puritogen-insensitive nociceptive neurons (Fig. 1).
A recent study identified two populations of antidromically identified trigeminothalamic tract neurons in rats (Moser and Giesler, 2013). Pruritogen-responsive neurons were activated by intrathecal application of morphine, while nociceptive neurons which did not respond to pruritogen were inhibited by morphine (Moser and Giesler, 2013). Morphine thus appears to be a convenient tool to identify pruritogen-responsive medullary dorsal horn neurons (see also below).
8. Inhibitory interneurons
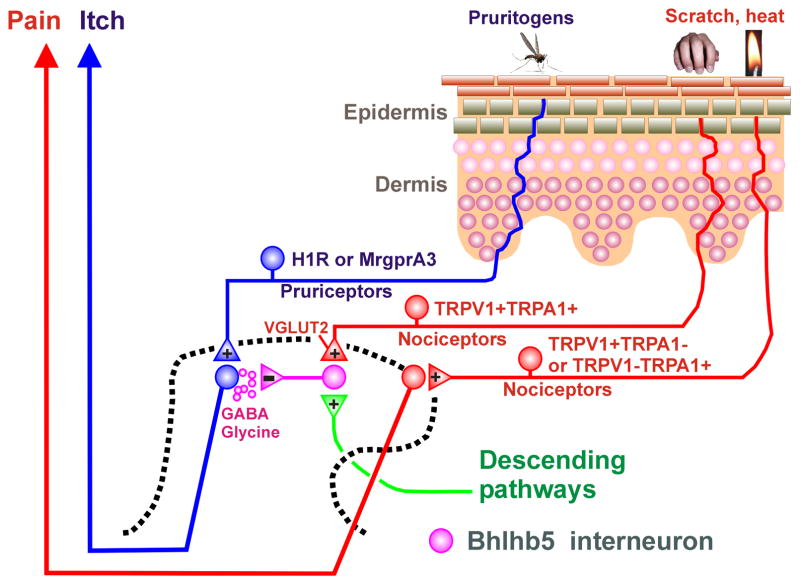
Inhibitory interneurons in laminae I–III consist of four distinct neurochemical populations containing neuropeptide Y (NPY), galanin, parvalbumin and neuronal nitric oxide synthase (nNOS) (Tiong et al., 2011). The transcription factor Bhlhb5 is transiently expressed in the dorsal horn of the developing spinal cord to regulate a unique population of inhibitory interneurons that inhibit itch (Ross et al., 2010). Approximately 65% of inhibitory interneurons are innervated by Aδ-fibers and/or C-fibers (Daniele and MacDermott, 2009). This implies that noxious (and possibly pruritic) stimuli activate inhibitory interneurons, which are capable of modulating the spinal transmission of various somatosensory submodalities. Itch can be inhibited by various types of noxious thermal, mechanical, chemical or electrical counterstimuli (Ward et al., 1996; Nillson et al., 1997). Scratching relieves itch, and recent studies support a spinal site of action by which scratching inhibits pruritogen-responsive neurons. Monkey STT neurons are modulated in a state-dependent manner by cutaneous scratching; scratching inhibited responses elicited by the pruritogen histamine but did not inhibit responses of the same neurons to the algogen capsaicin (Davidson et al., 2009). Consistent with this, pruritogen-responsive mouse spinal neurons are modulated in a state- and also site-dependent manner by cutaneous scratching (Akiyama et al., 2012b). Pruritogen- but not algogen-evoked neuronal responses were inhibited following scratching delivered to the stimulus site (state-dependency), and also during scratching at a distance away (site dependency). Spinal application of glycine and GABA-A and GABA-B receptor antagonists attenuated or abolished scratch-evoked inhibition of spontaneous activity in dorsal horn neurons with input from dry skin (Akiyama et al., 2011b), suggesting that GABA and glycine mediate inhibition of itch-signaling spinal neurons.
Conceivably, spinal inhibitory interneurons are tonically active, based on recent studies showing that decreased activity in, or deletion of, inhibitory spinal interneurons is associated with enhanced itch. Loss of a population of inhibitory interneurons in the superficial dorsal horn of knockout mice lacking the transcription factor Bhlhb5 (Ross et al., 2010), as well as knockout of the glutamate transporter VGLUT2 in certain types of nociceptors (Lagerstrom et al., 2010, Liu et al., 2010c), both resulted in excessive scratching behavior. These findings suggest that a reduction in nociceptive input decreases spinal inhibition, resulting in disinhibition of itch transmission. This is further supported by a recent study (Roberson et al., 2013). When TRPV1- or TRPA1-expressing nociceptors were electrically silenced by entry of a local anesthetic through the open ion channel, cheek application of capsaicin or AITC, which normally elicit forelimb wiping behavior, instead elicited hindlimb scratching. This implies that TRPV1- and TRPA1-expressing nociceptor afferents exert a tonic inhibitory effect on trigeminal itch transmission, and that removal of this tonic inhibition by electrical silencing disinhibited itch-transmitting Vc neurons to result in scratching behavior. A schematic diagram of spinal itch-inhibitory circuits is presented in Fig. 2. In this scenario, decreased input from nociceptors expressing TRPV1 and/or TRPA1 reduced the excitation of Bhlhb5 inhibitory interneurons, thus disinhibiting itch-signaling neurons. Such neurons may be excited via capsaicin or AITC activation of H1R and/or MrgprA3-expressing pruriceptors to elicit scratching (Fig. 1).
Fig. 2.
Schematic diagram of inhibitory spinal circuits for itch. +, − denote excitatory and inhibitory synapses, respectively.
9. Opioid modulation of itch
As noted earlier, morphine inhibits pain but can induce or enhance itch, whereas μ-opiate antagonists suppress itch but not pain. One possible explanation for morphine-induced itch is that opioid peptide-expressing inhibitory interneurons in the spinal cord might synapse onto the Bhlhb5 interneurons; activity in the opioid interneurons (or exogenous application of μ-agonists) would inhibit the Bhlhb5 interneurons to disinhbit itch-signaling neurons (Handwerker, 2010). An alternative explanation is that the morphine binds to the μ-opioid receptor isoform MOR1D which heterodimerizes with GRPR co-expressed in itch-signaling spinal neurons (Liu et al., 2011b). In either case, exogenous spinal application of morphine was recently reported to excite pruritogen-responsive trigeminothalamic projection neurons in rats, while inhibiting nociceptive trigeminothalamic neurons (Moser & Giesler, 2013), providing a functional explanation for how systemic morphine induces itch.
The κ-agonist TRK-820 (Nalfurafine) inhibited pruritogen-evoked scratching in mice (Togashi et al., 2002) and morphine-induced scratching in primates (Ko et al., 2003), indicating a role for the κ-opioid receptor in the modulation of itch that is worthy of further investigation. This κ-agonist has proven effective in relieving intractable itch in kidney dialysis patients (Kumagi et al., 2012).
10. Descending modulation of itch
Scratch-evoked inhibition of spinal itch-signaling neurons involves both segmental and supraspinal circuits. Cold-block or complete transection of the upper cervical spinal cord reduced scratch-evoked inhibition of spontaneous activity in dorsal horn neurons with input from dry skin by 30% and 50%, respectively. This implies that scratch-evoked inhibition is mediated partially via activation of supraspinal neurons that, in turn, engage descending pathways to result in spinal release of glycine and GABA (Fig. 2). The supraspinal circuit is unknown but may involve the same descending pathways that modulate pain. In a human brain imaging study, the midbrain periaqueductal gray (PAG) was activated during the inhibition of histamine-evoked itch by a noxious cold stimulus (Mochizuki et al., 2003), suggesting that the PAG, a well-known center for descending modulation of pain, may also be involved in modulating itch. The locus coeruleus is a major source of descending noradrenergic projections (Ossipov et al., 2010). Neurotoxic destruction of catecholaminergic neurons in the spinal cord enhanced itch-related behaviors, implying that descending noradrenergic neurons inhibit spinal itch signaling (Gotoh et al., 2011). Moreover, swim stress-induced analgesia, which is thought to be mediated by descending antinociceptive pathways, attenuated 5-HT-evoked scratching behavior in rats (Spradley et al., 2012b). The otherwise scant information regarding supraspinal modulation of spinal and trigeminal itch transmission makes this a fruitful area for future research.
11. Sensitization of itch-signaling pathways
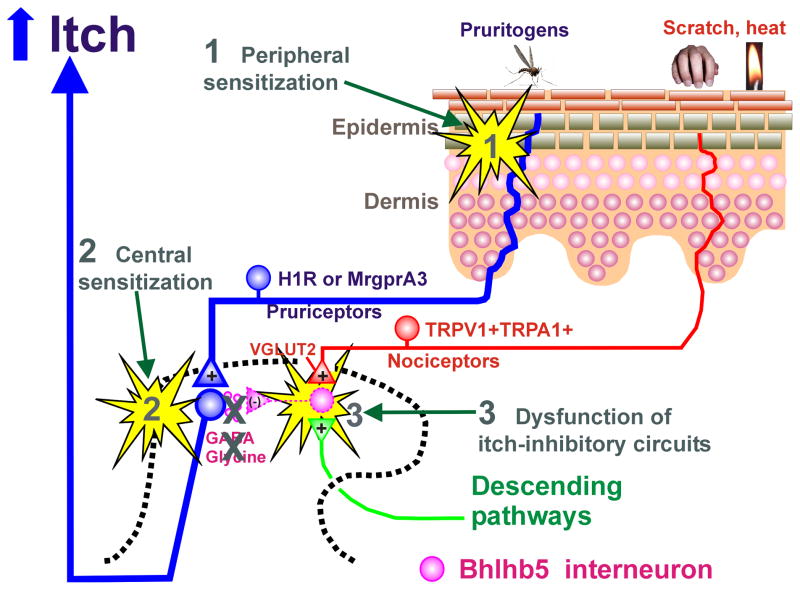
Peripheral and central sensitization play important roles in the establishment of chronic pain, and the same processes may contribute to various types of chronic itch. Chronic pain is often associated with ongoing spontaneous pain, hyperalgesia, and allodynia (touch-evoked pain). These conditions can also be experimentally reproduced in human skin by intradermal injection of capsaicin. In primates, capsaicin enhanced the responses of monkey STT neurons to touch and noxious heat, as well as electrical nerve stimulation, suggesting sensitization of the STT neurons (Simone et al., 1991). Chronic itch has parallels with chronic pain (Yosipovitch et al., 2007). Chronic itch can be associated with spontaneous itch, hyperknesis (enhanced itch to a normally itchy stimulus), and alloknesis (itch elicited by an innocuous touch stimulus). Three general mechanisms possibly contributing to chronic itch are shown in Fig. 3, as discussed further below.
Fig. 3.
Schematic diagram of mechanisms underlying itch sensitization. 1. Peripheral sensitization may occur through PAR-2. 2. Central sensitization may occur through TLR3. 3. Dysfunction of itch inhibitory circuits may contribute to itch sensitization. See text for details.
11.1. Peripheral Sensitization
Few studies have addressed whether primary afferent pruriceptors are sensitized under chronic itch conditions. Pruritogen-sensitive C-fibers recorded in atopic dermatitis patients exhibited high levels of spontaneous firing (Schmelz et al., 2003a). Using the dry skin model of chronic itch, mice exhibited significantly greater scratching (hyperknesis) following intradermal injections of 5-HT and SLIGRL, but not histamine, within the treatment area (Akiyama et al., 2010b). DRG cells taken from the dry skin-treated mice exhibited significantly greater responses to 5-HT and SLIGRL, but not histamine, consistent with the behavioral results (Akiyama et al., 2010b). A potential mediator of peripheral sensitization is nerve growth factor (NGF), which is elevated in dry skin and might contribute to peripheral sensitization of pruriceptors (Tominaga et al., 2007). Intradermally administered NGF enhanced itch induced by cowhage but not histamine in humans (Rukwied et al., 2013). A possible role for PAR-2 in peripheral sensitization comes from our study showing that SLIGRL, but not BAM8-22, enhanced the response of DRG neurons to subsequently-applied chloroquine and BAM8-22 (Akiyama et al., 2012c).
11.2. Central Sensitization
To investigate if chronic dry skin itch sensitizes spinal neurons, we recorded from superficial dorsal horn neurons receiving afferent input from a dry skin-treated hindpaw (Akiyama et al., 2011a). These neurons exhibited heightened spontaneous activity and enhanced responses to SLIGRL, but not histamine, compared to units recorded in control animals. However, mechanically-evoked responses were not enhanced, suggesting that the enhanced response to SLIGRL was due to peripheral sensitization of pruriceptors in the dry skin area and consistent with our results noted in the previous section.
A possible central mechanism of enhanced itch transmission is long-term potentiation (LTP). Knockout mice lacking toll-like receptor 3 (TLR3) exhibited significantly reduced scratching to histamine and variety of other pruritogens, as well as impaired LTP in spinal neurons (Liu et al., 2012b), supporting a role for TLR3 in central sensitization of spinal itch-transmitting neurons. In addition, TLR3 knockout mice with experimental dry skin exhibited almost no spontaneous scratching behavior compared to the robust scratching observed in wildtype mice (Liu et al., 2012b). Interestingly, the dry skin-treated wild type mice showed a 25-fold increase in TLR3 expression in the skin, suggesting that TLR3 may also be involved in peripheral sensitization of dry skin itch (Liu et al., 2012b).
Alloknesis is a common and often distressing symptom of many chronic itch patients. Alloknesis has been suggested to be mediated by mechanoreceptor afferent input to sensitized itch-signaling spinal neurons, but there is currently no evidence for this. We recently developed a novel animal model of alloknesis involving innocuous mechanical stimulation of rostral back skin in the C57BL/6 mouse (Akiyama et al., 2012a). C57BL/6 mice do not normally respond to this low-threshold mechanical stimulus. However, following intradermal injection of histamine and certain other pruritogens, lightly touching skin surrounding the injection site reliably elicited discrete hindlimb scratch bouts directed to the stimulus. The touch-evoked scratching developed more slowly and lasted longer compared to the scratching that began shortly after the pruritogen injection and usually ceased within 30 min. Touch-evoked scratching was observed following histamine, 5-HT, a PAR-4 agonist, and BAM8-22, but not SLIGRL or chloroquine. We also observed touch-evoked scratching in dry skin-treated animals, suggesting that dry skin itch is associated with alloknesis. In recordings from primate STT neurons, innocuous mechanical stimuli elicited greater responses after vs. before intradermal histamine injection (Davidson et al., 2012). In contrast, cowhage did not affect mechanically-evoked responses. Innocuous mechanical stimuli elicited greater responses in pruritogen-responsive rat trigeminothalamic tract neurons after compared to before the intrathecal application of morphine (Moser and Giesler, 2013). The enhancement of mechanically-evoked responses of ascending sensory neurons may thus represent a mechanism by which certain pruritogens induce alloknesis.
11.3. Dysfunction of Inhibitory Interneurons
As noted above, loss of Bhlhb5 inhibitory interneurons resulted in spontaneous scratching (Ross et al., 2010), consistent with disinhibition of itch-signaling spinal neurons. Future studies are needed to determine if the number of Bhlhb5 inhibitory interneurons is reduced under conditions of chronic itch.
As more animal models of chronic itch conditions become available, it will be important to determine if the animals exhibit ongoing spontaneous scratching, hyperknesis and alloknesis, if peripheral and/or central itch-signaling neurons exhibit sensitization, and if there are functional changes in spinal inhibitory interneruons. The identification of the molecular players involved in these various processes will provide important targets for the future development of treatments for chronic itch.
12. Theories of itch
It has been debated for over a century whether itch and pain are mediated via distinct pathways, a concept known as specificity theory or labeled-line coding, or if itch is a low-level form of pain on the same sensory continuum, a concept known as the intensity (or frequency) theory (von Frey, 1922). Intensity theory holds that a common population of sensory neurons responds to both pruritic and noxious stimuli, with itch being signaled by a low firing rate and pain by a higher firing rate in these neurons. Indeed, many spinal neurons in our studies exhibited relatively lower firing rates to pruritogens and higher-frequency responses to algogens. However, the concept of intensity coding is not supported by observations that electrical stimulation at certain points on the skin surface elicits a distinct sensation of itch that increases in intensity, but does change to pain, with increasing stimulus frequency (Tuckett, 1982). Instead, it is the authors’ opinion that current evidence supports the concept of specificity or labeled-line coding for distinct sensations of itch and pain. However, it must be recognized that most pruritogen-sensitive primary afferents and second-order spinal and trigeminal neurons also respond to noxious stimuli, rather than being pruritogen-specific, thereby introducing complications for a simple labeled-line theory of neural coding. The interested reader is directed to additional references describing alternative theories for itch and pain coding (McMahon & Koltzenburg, 1992; Handwerker, 2010; Namer & Reeh, 2013).
The concept of labeled-line coding for itch is supported by studies implicating GRPR-expressing dorsal horn neurons in selectively mediating itch but not pain (Sun et al., 2009), as noted above. Further support comes from recent observations of human mechano-insensitive C-fibers that responded to histamine over a time course matching that of concomitant itch sensation (Schmelz et al., 1996), and mechano-insensitive lamina I STT neurons in cats that similarly responded to histamine (Andrew and Craig, 2001). A recent striking observation supports labeled-line coding. In knockout mice lacking TRPV1 globally, TRPV1 was selectively re-expressed in MrgprA3-expressing sensory neurons. In these mice, cheek injection of capsaicin, which normally evokes pain-related wiping, instead elicited itch-related scratching behavior (Han et al., 2012). This implies that MrgprA3-expressing pruriceptors are linked to a labeled line for itch sensation, even though they can be excited by other types of stimuli including capsaicin and noxious pinch (Han et al., 2012). Consistent with this, application of capsaicin-impregnated cowhage spicules within the superficial epidermis (Sikand et al., 2009), where MrgprA3-expressing primary afferent terminals are located, or topical application of capsaicin (Green, 1990; Green & Shaffer, 1993), elicited a dominant sensation of itch in humans. However, intradermal injection of capsaicin elicits burning pain (LaMotte et al., 1991), and it remains a challenge to explain how the nervous system discriminates between capsaicin-evoked itch and pain sensations, as discussed further, below.
Nevertheless, many of the available molecular-genetic, behavioral and electrophysiological studies suggest that pruriceptors are a subset of nociceptors that respond to noxious mechanical, chemical, and/or thermal stimuli. This raises the question as to how noxious stimuli elicit pain without simultaneously eliciting itch. One possibility is that noxious stimuli activate inhibitory interneurons that suppress itch transmission. Although noxious stimuli can inhibit responses of spinal neurons to pruritogens, this inhibition is state-dependent (Davidson et al., 2009, Akiyama et al., 2011b). Most pruritogen-sensitive spinal neurons are also excited by capsaicin, so that scratching becomes an excitatory rather than inhibitory stimulus to further excite the neuron. If such neurons are dedicated to only signal itch, then it remains difficult to explain how a noxious stimulus only elicits pain. Another possibility is that noxious stimuli do elicit both itch and pain simultaneously, whereby the sensation of itch is masked or occluded by the larger pain signal in order to discriminate between the two sensory qualities. An additional related concept is population coding (Akiyama et al., 2009a,b; Ma, 2010). Itch is postulated to be signaled by the activation of a subset of spinal neurons that responds to both pruritogens and noxious stimuli. Noxious stimulation activates a larger population of nociceptive spinal neurons, including those responsive to pruritogens, to signal pain. The CNS decodes activity in the former and latter neuronal populations as itch and pain, respectively. According to this idea, MrgprA3-expressing sensory neurons would be expected to project to a subset of GRPR-expressing spinal neurons that respond to both pruritogens and noxious stimuli. MrgprA3-expressing sensory neurons do respond to noxious mechanical and chemical stimuli, and their central spinal terminals make synaptic contact with GRPR-expressing spinal neurons (Han et al., 2012). Since ablation of neither MrgprA3-expressing neurons nor GRPR-expressing spinal neurons has any effect on nociception (Sun et al., 2009, Han et al., 2012), the CNS may decode activity in a larger population of nociceptive spinal neurons as pain regardless of activity in a smaller subset of spinal neurons responsive to both pruritogens and noxious stimuli.
Highlights.
There are histamine-dependent and –independent types of itch
Molecular detectors of pruritogens include Mrgprs, PARs, and many others
Spinal transmitters include gastrin releasing peptide and natriuretic polypeptide B
Pruritogen-sensitive sensory neurons usually also respond to pain-producing stimuli
Pruritogen-responsive sensory neurons connect to an itch-specific central pathway
Acknowledgments
The work was supported by supported by grants from the National Institutes of Health DE013685, AR057194 and AR063228.
List of Abbreviations
- 5-HT
5-hydroxytryptamine (serotonin)
- 5-HT2
5-HT receptor subtype 2
- 12-HETE
12-Hydroxyeicosatetraenoic acid
- AITC
allyl isothiocyanate
- BAM8-22
bovine adrenal medullary peptide 8-22
- BLT
LTB4 receptor
- DRG
dorsal root ganglion
- ET-1
endothelin-1
- ETA-1
endothelin receptor A-1
- GABA
gamma aminobutyric acid
- Glu
glutamate
- GRP
gastrin releasing peptide
- GRPR
gastrin releasing peptide receptor
- H1R
histamine receptor-1
- IB-4
isolectin B4
- IL31
interleukin 31
- K2P
two-pore-domain potassium channels
- Kv
voltage-gated potassium channel
- LPA
lysophosphatidic acid
- LTB4
leukotriene B4
- LT
low threshold
- LTP
long-term potentiation
- MI
mechanically insensitive
- MOR1D
morphine receptor-1D isoform
- Mrgpr
Mas-related G-protein-coupled receptor
- NGF
nerve growth factor
- NK-1
neurokinin-1 receptor
- NO
nitric oxide
- Nppb
natriuretic polypeptide B
- NS
nociceptive specific
- OSMR
oncostatin M receptor
- PAF
platelet activating factor
- PAG
periaqueductal gray
- PAR
protease-activated receptor
- Rho-ROCK
Rho-associated protein kinase
- RVM
rostral ventromedial medulla
- SP
substance P
- SPC
sphingosylphosphorylcholine
- STT
spinothalamic tract
- TG
trigeminal ganglion
- TGR5
G protein-coupled bile acid receptor 1 (GPBAR1)
- TLR3
toll-like receptor 3
- TLR7
toll-like receptor 7
- TP
thromboxane receptor
- TR4
testicular orphan nuclear receptor-4
- TRPA1
transient receptor potential ankyrin 1
- TRPV1
transient receptor potential vanilloid 1
- TXA2
thromboxane A2
- Vc
trigeminal subnucleus caudalis
- VGLUT2
vesicular glutamate transporter-2
- WDR
wide dynamic range
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abila B, Ezeamuzie IC, Igbigbi PS, Ambakederemo AW, Asomugha L. Effects of two antihistamines on chloroquine and histamine induced weal and flare in healthy African volunteers. African journal of medicine and medical sciences. 1994;23:139–142. [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E. Excitation of mouse superficial dorsal horn neurons by histamine and/or PAR-2 agonist: potential role in itch. Journal of neurophysiology. 2009a;102:2176–2183. doi: 10.1152/jn.00463.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E. Differential itch- and pain-related behavioral responses and μ-opoid modulation in mice. Acta dermato-venereologica. 2010a;90:575–581. doi: 10.2340/00015555-0962. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E. Enhanced scratching evoked by PAR-2 agonist and 5-HT but not histamine in a mouse model of chronic dry skin itch. Pain. 2010b;151:378–383. doi: 10.1016/j.pain.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E. Facial injections of pruritogens and algogens excite partly overlapping populations of primary and second-order trigeminal neurons in mice. Journal of neurophysiology. 2010c;104:2442–2450. doi: 10.1152/jn.00563.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E. Spontaneous itch in the absence of hyperalgesia in a mouse hindpaw dry skin model. Neuroscience letters. 2010d;484:62–65. doi: 10.1016/j.neulet.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E. Enhanced responses of lumbar superficial dorsal horn neurons to intradermal PAR-2 agonist but not histamine in a mouse hindpaw dry skin itch model. Journal of neurophysiology. 2011a;105:2811–2817. doi: 10.1152/jn.01124.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Ikoma A, Cevikbas F, Steinhoff M, Carstens E. Mouse Model of Touch-Evoked Itch (Alloknesis) The Journal of investigative dermatology. 2012a;132(7):1886–1891. doi: 10.1038/jid.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Iodi Carstens M, Carstens E. Transmitters and pathways mediating inhibition of spinal itch-signaling neurons by scratching and other counterstimuli. PloS one. 2011b;6:e22665. doi: 10.1371/journal.pone.0022665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Merrill AW, Carstens MI, Carstens E. Activation of superficial dorsal horn neurons in the mouse by a PAR-2 agonist and 5-HT: potential role in itch. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009b;29:6691–6699. doi: 10.1523/JNEUROSCI.6103-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Tominaga M, Carstens MI, Carstens EE. Site-dependent and state-dependent inhibition of pruritogen-responsive spinal neurons by scratching. Eur J Neurosci. 2012b;36(3):2311–2316. doi: 10.1111/j.1460-9568.2012.08136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Tominaga M, Davoodi A, Nagamine M, Blansit K, Horwitz A, Carstens MI, Carstens E. Cross-sensitization of histamine-independent itch in mouse primary sensory neurons. Neuroscience. 2012c;226:305–312. doi: 10.1016/j.neuroscience.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Tominaga M, Davoodi A, Nagamine M, Blansit K, Horwitz A, Iodi Carstens M, Carstens E. Roles for substance P and gastrin releasing peptide as neurotransmitters released by primary afferent pruriceptors. Journal of neurophysiology. 2012c;109(3):742–748. doi: 10.1152/jn.00539.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemi F, Kwon E, Poole D, Lieu T, Lyo V, Cattaruzza F, Cevikbas F, Steinhoff M, Nassini R, Materazzi S, Guerrero-Alba R, Valdez-Morales E, Cottrell G, Schoonjans K, Geppetti P, Vanner S, Bunnett N, Corvera C. The TGR5 receptor mediates bile acid-induced itch and analgesia. The Journal of clinical investigation. 2013a;123:1513–1530. doi: 10.1172/JCI64551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemi F, Kwon E, Poole DP, Lieu T, Lyo V, Cattaruzza F, Cevikbas F, Steinhoff M, Nassini R, Materazzi S, Guerrero-Alba R, Valdez-Morales E, Cottrell GS, Schoonjans K, Geppetti P, Vanner SJ, Bunnett NW, Corvera CU. The TGR5 receptor mediates bile acid-induced itch and analgesia. The Journal of clinical investigation. 2013b;123:1513–1530. doi: 10.1172/JCI64551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson D, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh T, Haza S, Saito A, Kuraishi Y. Involvement of leukotriene B4 in spontaneous itch-related behaviour in NC mice with atopic dermatitis-like skin lesions. Experimental dermatology. 2011a;20:894–898. doi: 10.1111/j.1600-0625.2011.01346.x. [DOI] [PubMed] [Google Scholar]
- Andoh T, Katsube N, Maruyama M, Kuraishi Y. Involvement of leukotriene B(4) in substance P-induced itch-associated response in mice. The Journal of investigative dermatology. 2001;117:1621–1626. doi: 10.1046/j.0022-202x.2001.01585.x. [DOI] [PubMed] [Google Scholar]
- Andoh T, Nagasawa T, Satoh M, Kuraishi Y. Substance P induction of itch-associated response mediated by cutaneous NK1 tachykinin receptors in mice. J Pharmacol Exp Ther. 1998a;286(3):1140–1145. [PubMed] [Google Scholar]
- Andoh T, Kuraishi Y. Intradermal leukotriene B4, but not prostaglandin E2, induces itch-associated responses in mice. European journal of pharmacology. 1998b;353(1):93–96. doi: 10.1016/s0014-2999(98)00440-3. [DOI] [PubMed] [Google Scholar]
- Andoh T, Kuraishi Y. Nitric oxide enhances substance P-induced itch-associated responses in mice. British journal of pharmacology. 2003;138:202–208. doi: 10.1038/sj.bjp.0705004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh T, Kuraishi Y. Expression of BLT1 leukotriene B4 receptor on the dorsal root ganglion neurons in mice. Brain research Molecular brain research. 2005;137:263–266. doi: 10.1016/j.molbrainres.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Andoh T, Kuwazono T, Lee JB, Kuraishi Y. Gastrin-releasing peptide induces itch-related responses through mast cell degranulation in mice. Peptides. 2011b;32:2098–2103. doi: 10.1016/j.peptides.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Andoh T, Nishikawa Y, Yamaguchi-Miyamoto T, Nojima H, Narumiya S, Kuraishi Y. Thromboxane A2 induces itch-associated responses through TP receptors in the skin in mice. The Journal of investigative dermatology. 2007;127:2042–2047. doi: 10.1038/sj.jid.5700810. [DOI] [PubMed] [Google Scholar]
- Andoh T, Saito A, Kuraishi Y. Leukotriene B(4) mediates sphingosylphosphorylcholine-induced itch-associated responses in mouse skin. The Journal of investigative dermatology. 2009;129:2854–2860. doi: 10.1038/jid.2009.155. [DOI] [PubMed] [Google Scholar]
- Andoh T, Tetsuro Y, Jung-Bum L, Yasushi K. Cathepsin E induces itch-related response through the production of endothelin-1 in mice. European journal of pharmacology. 2012;686(1–3):16–21. doi: 10.1016/j.ejphar.2012.04.024. [DOI] [PubMed] [Google Scholar]
- Andrew D, Craig A. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nature neuroscience. 2001;4:72–77. doi: 10.1038/82924. [DOI] [PubMed] [Google Scholar]
- Atanassoff P, Brull S, Zhang J, Greenquist K, Silverman D, Lamotte R. Enhancement of experimental pruritus and mechanically evoked dysesthesiae with local anesthesia. Somatosensory & motor research. 1999;16:291–298. doi: 10.1080/08990229970357. [DOI] [PubMed] [Google Scholar]
- Bachmann GA, Arnold LD. Recurrent vulvar itching. Obstetrics and gynecology. 2005;106:639. doi: 10.1097/01.AOG.0000177658.06354.bf. author reply 639. [DOI] [PubMed] [Google Scholar]
- Basbaum A, Bautista D, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bay B, Hilliges M, Weidner C, Sandborgh-Englund G. Response of human oral mucosa and skin to histamine provocation: laser Doppler perfusion imaging discloses differences in the nociceptive nervous system. Acta odontologica Scandinavica. 2009;67:99–105. doi: 10.1080/00016350802698622. [DOI] [PubMed] [Google Scholar]
- Benjamin K, Waterston K, Russell M, Schofield O, Diffey B, Rees JL. The development of an objective method for measuring scratch in children with atopic dermatitis suitable for clinical use. Journal of the American Academy of Dermatology. 2004;50:33–40. doi: 10.1016/s0190-9622(03)02480-0. [DOI] [PubMed] [Google Scholar]
- Bonacini M. Pruritus in patients with chronic human immunodeficiency virus, hepatitis B and C virus infections. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2000;32:621–625. doi: 10.1016/s1590-8658(00)80847-6. [DOI] [PubMed] [Google Scholar]
- Brash HM, McQueen DS, Christie D, Bell JK, Bond SM, Rees JL. A repetitive movement detector used for automatic monitoring and quantification of scratching in mice. Journal of neuroscience methods. 2005;142:107–114. doi: 10.1016/j.jneumeth.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Carrougher G, Martinez E, McMullen K, Fauerbach J, Holavanahalli R, Herndon D, Wiechman S, Engrav L, Gibran N. Pruritus in adult burn survivors: postburn prevalence and risk factors associated with increased intensity. J Burn Care Res. 2013;34:94–101. doi: 10.1097/BCR.0b013e3182644c25. [DOI] [PubMed] [Google Scholar]
- Carstens E. Responses of rat spinal dorsal horn neurons to intracutaneous microinjection of histamine, capsaicin, and other irritants. J Neurophysiol. 1997;77(5):2499–2514. doi: 10.1152/jn.1997.77.5.2499. [DOI] [PubMed] [Google Scholar]
- Carstens EE, Carstens MI, Simons CT, Jinks SL. Dorsal horn neurons expressing NK-1 receptors mediate scratching in rats. Neuroreport. 2010;21:303–308. doi: 10.1097/WNR.0b013e328337310a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh D, Lee H, Lo L, Shields S, Zylka M, Basbaum A, Anderson D. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrostowska-Plak D, Salomon J, Reich A, Szepietowski JC. Clinical Aspects of Itch in Adult Atopic Dermatitis Patients. Acta Dermato Venereologica. 2009;89(4):379–383. doi: 10.2340/00015555-0676. [DOI] [PubMed] [Google Scholar]
- Chudler E, Foote W, Poletti C. Responses of cat C1 spinal cord dorsal and ventral horn neurons to noxious and non-noxious stimulation of the head and face. Brain research. 1991;555:181–192. doi: 10.1016/0006-8993(91)90341-r. [DOI] [PubMed] [Google Scholar]
- Cridland R, Henry J. Bombesin, neuromedin C and neuromedin B given intrathecally facilitate the tail flick reflex in the rat. Brain research. 1992;584(1–2):163–168. doi: 10.1016/0006-8993(92)90890-l. [DOI] [PubMed] [Google Scholar]
- Cuellar JM, Jinks SL, Simons CT, Carstens E. Deletion of the preprotachykinin A gene in mice does not reduce scratching behavior elicited by intradermal serotonin. Neuroscience letters. 2003;339:72–76. doi: 10.1016/s0304-3940(02)01458-1. [DOI] [PubMed] [Google Scholar]
- Czepielewski RS, Porto BN, Rizzo LB, Roesler R, Abujamra AL, Pinto LG, Schwartsmann G, de Cunha FQ, Bonorino C. Gastrin-releasing peptide receptor (GRPR) mediates chemotaxis in neutrophils. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:547–552. doi: 10.1073/pnas.1110996109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dado R, Katter J, Giesler G. Spinothalamic and spinohypothalamic tract neurons in the cervical enlargement of rats. II. Responses to innocuous and noxious mechanical and thermal stimuli. Journal of neurophysiology. 1994;71:981–1002. doi: 10.1152/jn.1994.71.3.981. [DOI] [PubMed] [Google Scholar]
- Dae-Kwon K, Hyung-June K, Ki-Sa S, Hyuk K, Sun AC, Kwang-Mi K, Chang-Hoon L, Jung-Ju K. 12(S)-HPETE induces itch-associated scratchings in mice. European journal of pharmacology. 2007;554(1):30–33. doi: 10.1016/j.ejphar.2006.09.057. [DOI] [PubMed] [Google Scholar]
- Daniele CA, MacDermott AB. Low-threshold primary afferent drive onto GABAergic interneurons in the superficial dorsal horn of the mouse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:686–695. doi: 10.1523/JNEUROSCI.5120-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsow U, Scharein E, Bromm B, Ring J. Skin testing of the pruritogenic activity of histamine and cytokines (interleukin-2 and tumour necrosis factor-alpha) at the dermal-epidermal junction. The British journal of dermatology. 1997;137:415–417. [PubMed] [Google Scholar]
- Davidson S, Zhang X, Khasabov SG, Moser HR, Honda CN, Simone DA, Giesler GJ. Pruriceptive spinothalamic tract neurons: physiological properties and projection targets in the primate. Journal of neurophysiology. 2012;108(6):1711–1723. doi: 10.1152/jn.00206.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Khasabov SG, Simone DA, Giesler GJ., Jr Relief of itch by scratching: state-dependent inhibition of primate spinothalamic tract neurons. Nature neuroscience. 2009;12:544–546. doi: 10.1038/nn.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ., Jr The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:10007–10014. doi: 10.1523/JNEUROSCI.2862-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon S, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell S, Haugen H, Maurer M, Harder B, Johnston J, Bort S, Mudri S, Kuijper J, Bukowski T, Shea P, Dong D, Dasovich M, Grant F, Lockwood L, Levin S, LeCiel C, Waggie K, Day H, Topouzis S, Kramer J, Kuestner R, Chen Z, Foster D, Parrish-Novak J, Gross J. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nature immunology. 2004;5:752–760. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–632. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. The Journal of clinical investigation. 2010;120:3760–3772. doi: 10.1172/JCI42843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussor G, Zylka M, Anderson D, McCleskey E. Cutaneous sensory neurons expressing the Mrgprd receptor sense extracellular ATP and are putative nociceptors. Journal of neurophysiology. 2008;99:1581–1589. doi: 10.1152/jn.01396.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglezos A, Lecci A, Santicioli P, Giuliani S, Tramontana M, Del Bianco E, Maggi CA. Activation of capsaicin-sensitive primary afferents in the rat urinary bladder by compound 48/80: a direct action on sensory nerves? Archives internationales de pharmacodynamie et de therapie. 1992;315:96–109. [PubMed] [Google Scholar]
- Elliott GR, Vanwersch RA, Bruijnzeel PL. An automated method for registering and quantifying scratching activity in mice: use for drug evaluation. Journal of pharmacological and toxicological methods. 2000;44:453–459. doi: 10.1016/s1056-8719(01)00111-3. [DOI] [PubMed] [Google Scholar]
- Feramisco J, Berger T, Steinhoff M. Innovative management of pruritus. Dermatologic clinics. 2010;28:467–478. doi: 10.1016/j.det.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Ferreira S, Romitelli M, de Nucci G. Endothelin-1 participation in overt and inflammatory pain. Journal of cardiovascular pharmacology. 1989;13(Suppl 5):2. doi: 10.1097/00005344-198900135-00065. [DOI] [PubMed] [Google Scholar]
- Fjellner B, Hagermark O. Experimental pruritus evoked by platelet activating factor (PAF-acether) in human skin. Acta dermato-venereologica. 1985;65:409–412. [PubMed] [Google Scholar]
- Fleming M, Ramos D, Han S, Zhao J, Son Y-J, Luo W. The majority of dorsal spinal cord gastrin releasing peptide is synthesized locally whereas neuromedin B is highly expressed in pain- and itch-sensing somatosensory neurons. Molecular pain. 2012;8:52. doi: 10.1186/1744-8069-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes L, Hara D, Rae G. Endothelin-1 induces itch and pain in the mouse cheek model. Life sciences. 2012;91:628–633. doi: 10.1016/j.lfs.2012.03.020. [DOI] [PubMed] [Google Scholar]
- Gotoh Y, Omori Y, Andoh T, Kuraishi Y. Tonic inhibition of allergic itch signaling by the descending noradrenergic system in mice. J Pharmacol Sci. 2011;115(3):417–420. doi: 10.1254/jphs.10305sc. [DOI] [PubMed] [Google Scholar]
- Gracely RH, Dubner R, Wolskee PJ, Deeter WR. Placebo and naloxone can alter post-surgical pain by separate mechanisms. Nature. 1983;306(5940):264–265. doi: 10.1038/306264a0. [DOI] [PubMed] [Google Scholar]
- Graham D, Goodell H, Wolff H. Neural mechanisms involved in itch, itchy skin, and tickle sensations. The Journal of clinical investigation. 1951;30:37–49. doi: 10.1172/JCI102414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BG. Spatial summation of chemical irritation and itch produced by topical application of capsaicin. Percept Psychophys. 1990;48(1):12–18. doi: 10.3758/bf03205007. [DOI] [PubMed] [Google Scholar]
- Green BG, Shaffer GS. The sensory response to capsaicin during repeated topical exposures: differential effects on sensations of itching and pungency. Pain. 1993;53(3):323–34. doi: 10.1016/0304-3959(93)90228-H. [DOI] [PubMed] [Google Scholar]
- Hagermark O, Hokfelt T, Pernow B. Flare and itch induced by substance P in human skin. The Journal of investigative dermatology. 1978;71:233–235. doi: 10.1111/1523-1747.ep12515092. [DOI] [PubMed] [Google Scholar]
- Hagiwara K, Nojima H, Kuraishi Y. Serotonin-induced biting of the hind paw is itch-related response in mice. Pain Research. 1999;14:53–59. [Google Scholar]
- Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, Li Z, McNeil B, He S, Guan Y, Xiao B, Lamotte RH, Dong X. A subpopulation of nociceptors specifically linked to itch. Nature neuroscience. 2012;16(2):174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S-K, Mancino V, Simon M. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron. 2006a;52:691–703. doi: 10.1016/j.neuron.2006.09.036. [DOI] [PubMed] [Google Scholar]
- Han SK, Mancino V, Simon MI. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron. 2006b;52:691–703. doi: 10.1016/j.neuron.2006.09.036. [DOI] [PubMed] [Google Scholar]
- Handwerker HO. Microneurography of pruritus. Neurosci Lett. 2010;470(3):193–196. doi: 10.1016/j.neulet.2009.06.092. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Ohata H, Momose K. Itch-scratch responses induced by lysophosphatidic acid in mice. Pharmacology. 2004;72:51–56. doi: 10.1159/000078632. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain research reviews. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer G, Dotzer M, Diepgen TL, Handwerker HO. Opiate and H1 antagonist effects on histamine induced pruritus and alloknesis. Pain. 1997;73:239–243. doi: 10.1016/S0304-3959(97)00098-5. [DOI] [PubMed] [Google Scholar]
- Hosogi M, Schmelz M, Miyachi Y, Ikoma A. Bradykinin is a potent pruritogen in atopic dermatitis: a switch from pain to itch. Pain. 2006;126:16–23. doi: 10.1016/j.pain.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Hunt S, Mantyh P. The molecular dynamics of pain control. Nature reviews Neuroscience. 2001;2:83–91. doi: 10.1038/35053509. [DOI] [PubMed] [Google Scholar]
- Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N, Igeta K, Kim J, Nagao M, Shiraishi N, Nakamura N, Nagai H. Involvement of unique mechanisms in the induction of scratching behavior in BALB/c mice by compound 48/80. European journal of pharmacology. 2002;448:175–183. doi: 10.1016/s0014-2999(02)01933-7. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Igeta K, Shiraishi N, Kim JF, Nagao M, Nakamura N, Nagai H. Evaluation and characterization of mouse scratching behavior by a new apparatus, MicroAct. Skin pharmacology and applied skin physiology. 2003;16:165–175. doi: 10.1159/000069755. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Nakamura N, Nagao M, Musoh K, Kawasaki H, Nagai H. Participation of histamine H1 and H2 receptors in passive cutaneous anaphylaxis-induced scratching behavior in ICR mice. European journal of pharmacology. 1999;367:361–371. doi: 10.1016/s0014-2999(98)00974-1. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine of the National Academies. Relieving Pain in America 2011 [Google Scholar]
- Jiexian L, Qing J, Wenjin J. Role of transient receptor potential ankyrin subfamily member 1 in pruritus induced by endothelin-1. Neuroscience letters. 2011;492(3):175–178. doi: 10.1016/j.neulet.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Jinks S, Carstens E. Responses of superficial dorsal horn neurons to intradermal serotonin and other irritants: comparison with scratching behavior. Journal of neurophysiology. 2002;87:1280–1289. doi: 10.1152/jn.00431.2001. [DOI] [PubMed] [Google Scholar]
- Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, Hartke T, LaMotte RH, Ringkamp M. A role for polymodal C-fiber afferents in nonhistaminergic itch. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:7659–7669. doi: 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanek LM, Meyer RA, Hartke T, Hobelmann JG, Maine DN, LaMotte RH, Ringkamp M. Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:7490–7497. doi: 10.1523/JNEUROSCI.1249-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph E, Bogen O, Alessandri-Haber N, Levine J. PLC-beta 3 signals upstream of PKC epsilon in acute and chronic inflammatory hyperalgesia. Pain. 2007;132:67–73. doi: 10.1016/j.pain.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Kagami S, Sugaya M, Suga H, Morimura S, Kai H, Ohmatsu H, Fujita H, Tsunemi Y, Sato S. Serum Gastrin-Releasing Peptide Levels Correlate with Pruritus in Patients with Atopic Dermatitis. The Journal of investigative dermatology. 2013;133(6):1673–1675. doi: 10.1038/jid.2013.38. [DOI] [PubMed] [Google Scholar]
- Katugampola R, Church MK, Clough GF. The neurogenic vasodilator response to endothelin-1: a study in human skin in vivo. Exp Physiol. 2000;85(6):839–846. [PubMed] [Google Scholar]
- Kelso JM. Application of topical corticosteroids to sites of positive immediate-type allergy skin tests to relieve itching: results of a double-blind, placebo-controlled trial. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2007;98:182–184. doi: 10.1016/S1081-1206(10)60694-1. [DOI] [PubMed] [Google Scholar]
- Kim H, Kim D, Koh J, Kim K, Noh M, Lee S, Kim S, Park S, Kim J, Lee C. Involvement of the BLT2 receptor in the itch-associated scratching induced by 12-(S)-lipoxygenase products in ICR mice. British journal of pharmacology. 2008a;154:1073–1078. doi: 10.1038/bjp.2008.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Kim H, Han E-S, Park S-M, Koh J-Y, Kim K-M, Noh M-S, Kim J-J, Lee C-H. Characterizations of sphingosylphosphorylcholine-induced scratching responses in ICR mice using naltrexon, capsaicin, ketotifen and Y-27632. European journal of pharmacology. 2008b;583:92–96. doi: 10.1016/j.ejphar.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Kim S-J, Park G, Kim D, Lee J, Min H, Wall E, Lee C, Simon M, Lee S, Han S-K. Analysis of cellular and behavioral responses to imiquimod reveals a unique itch pathway in transient receptor potential vanilloid 1 (TRPV1)-expressing neurons. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3371–3376. doi: 10.1073/pnas.1019755108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AY, Tang Z, Liu Q, Patel KN, Maag D, Geng Y, Dong X. Pirt, a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1. Cell. 2008;133(3):475–485. doi: 10.1016/j.cell.2008.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby J, Heaton K, Burton J. Pruritic effect of bile salts. British medical journal. 1974;4(5946):693–695. doi: 10.1136/bmj.4.5946.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Carstens MI, Carstens E. Facial injections of pruritogens or algogens elicit distinct behavior responses in rats and excite overlapping populations of primary sensory and trigeminal subnucleus caudalis neurons. Journal of neurophysiology. 2011;106:1078–1088. doi: 10.1152/jn.00302.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MCH, Lee H, Song MS, Sobczyk-Kojiro K, Mosberg HI, Kishioka S, Woods JH, Naughton NN. Activation of kappa-Opioid Receptors Inhibits Pruritus Evoked by Subcutaneous or Intrathecal Administration of Morphine in Monkeys. J Pharmacol Exp Ther. 2003;305:173–179. doi: 10.1124/jpet.102.044909. [DOI] [PubMed] [Google Scholar]
- Koga K, Chen T, Li XY, Descalzi G, Ling J, Gu J, Zhuo M. Glutamate acts as a neurotransmitter for gastrin releasing peptide-sensitive and insensitive itch-related synaptic transmission in mammalian spinal cord. Molecular pain. 2011;7:47. doi: 10.1186/1744-8069-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer A, van Dijk R, Leckie P, Schaap F, Kuiper EM, Mettang T, Reiners K, Raap U, van Buuren H, van Erpecum K, Davies N, Rust C, Engert A, Jalan R, Oude Elferink R, Beuers U. Serum autotaxin is increased in pruritus of cholestasis, but not of other origin, and responds to therapeutic interventions. Hepatology (Baltimore, Md) 2012;56:1391–1400. doi: 10.1002/hep.25748. [DOI] [PubMed] [Google Scholar]
- Kretzmer GE, Gelkopf M, Kretzmer G, Melamed Y. Idiopathic pruritus in psychiatric inpatients: an explorative study. Gen Hosp Psychiatry. 2008;30(4):344–348. doi: 10.1016/j.genhosppsych.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Kumagai H, Ebata T, Takamori K, Miyasato K, Muramatsu T, Nakamoto H, Kurihara M, Yanagita T, Suzuki H. Efficacy and safety of a novel k-agonist for managing intractable pruritus in dialysis patients. Am J Nephrol. 2012;36:175–183. doi: 10.1159/000341268. [DOI] [PubMed] [Google Scholar]
- Kuraishi Y, Nagasawa T, Hayashi K, Satoh M. Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. European journal of pharmacology. 1995;275:229–233. doi: 10.1016/0014-2999(94)00780-b. [DOI] [PubMed] [Google Scholar]
- Lagerstrom MC, Rogoz K, Abrahamsen B, Persson E, Reinius B, Nordenankar K, Olund C, Smith C, Mendez JA, Chen ZF, Wood JN, Wallen-Mackenzie A, Kullander K. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron. 2010;68:529–542. doi: 10.1016/j.neuron.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH, Shain CN, Simone DA, Tsai EF. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol. 1991;66(1):190–211. doi: 10.1152/jn.1991.66.1.190. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Shimada SG, Sikand P. Mouse models of acute, chemical itch and pain in humans. Experimental dermatology. 2011;20:778–782. doi: 10.1111/j.1600-0625.2011.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laura KSP, Bernadette N, Grazyna R, Léo L. Assessment of Pruritus Characteristics and Impact on Burn Survivors. Journal of Burn Care & Research. 2012;33(3):407–418. doi: 10.1097/BCR.0b013e318239d206. [DOI] [PubMed] [Google Scholar]
- Lee J, Kim T, Hong J, Woo J, Min H, Hwang E, Lee SJ, Lee CJ. Imiquimod enhances excitability of dorsal root ganglion neurons by inhibiting background (K2P) and voltage-gated (Kv1. 1 and Kv1. 2) potassium channels. Mol Pain. 2012;8:2. doi: 10.1186/1744-8069-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JD, Gordon NC, Jones RT, Fields HL. The narcotic antagonist naloxone enhances clinical pain. Nature. 1978;272(5656):826–827. doi: 10.1038/272826a0. [DOI] [PubMed] [Google Scholar]
- Lewis T. The Blood Vessels of the Human Skin and their Responses. Shaw and Sons, Ltd; London: 1927. [Google Scholar]
- Li L, Rutlin M, Abraira V, Cassidy C, Kus L, Gong S, Jankowski M, Luo W, Heintz N, Koerber H, Woodbury C, Ginty D. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147:1615–1627. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Sikand P, Ma C, Tang Z, Han L, Li Z, Sun S, LaMotte R, Dong X. Mechanisms of itch evoked by β-alanine. The Journal of neuroscience. 2012a;32:14532–14537. doi: 10.1523/JNEUROSCI.3509-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Weng HJ, Patel KN, Tang Z, Bai H, Steinhoff M, Dong X. The distinct roles of two GPCRs, MrgprC11 and PAR2, in itch and hyperalgesia. Science signaling. 2011a;4:ra45. doi: 10.1126/scisignal.2001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Berta T, Xu ZZ, Park CK, Zhang L, Lu N, Liu Q, Liu Y, Gao YJ, Liu YC, Ma Q, Dong X, Ji RR. TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. The Journal of clinical investigation. 2012b;122:2195–2207. doi: 10.1172/JCI45414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Xu Z-Z, Park C-K, Berta T, Ji R-R. Toll-like receptor 7 mediates pruritus. Nature neuroscience. 2010a;13:1460–1462. doi: 10.1038/nn.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Liu ZC, Sun YG, Ross M, Kim S, Tsai FF, Li QF, Jeffry J, Kim JY, Loh HH, Chen ZF. Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell. 2011b;147:447–458. doi: 10.1016/j.cell.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Abdel Samad O, Zhang L, Duan B, Tong Q, Lopes C, Ji RR, Lowell BB, Ma Q. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron. 2010c;68:543–556. doi: 10.1016/j.neuron.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. Labeled lines meet and talk: population coding of somatic sensations. J Clin Invest. 2010;120(11):3773–3778. doi: 10.1172/JCI43426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar L, Yang F-C, Ma Q. Genetic marking and characterization of Tac2-expressing neurons in the central and peripheral nervous system. Molecular brain. 2012;5:3. doi: 10.1186/1756-6606-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino M, Huang P, Malkmus S, Robertshaw E, Mac EA, Shatterman Y, Yaksh TL. Development and validation of an automated system for detection and assessment of scratching in the rodent. J Neurosci Methods. 2012;211(1):1–10. doi: 10.1016/j.jneumeth.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matterne U, Apfelbacher C, Vogelgsang L, Loerbroks A, Weisshaar E. Incidence and Determinants of Chronic Pruritus: A Population-based Cohort Study. Acta dermato-venereologica. 2013 Feb 28; doi: 10.2340/00015555-1572. [DOI] [PubMed] [Google Scholar]
- Maxwell DJ, Belle MD, Cheunsuang O, Stewart A, Morris R. Morphology of inhibitory and excitatory interneurons in superficial laminae of the rat dorsal horn. The Journal of physiology. 2007;584:521–533. doi: 10.1113/jphysiol.2007.140996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazeh D, Melamed Y, Cholostoy A, Aharonovitzch V, Weizman A, Yosipovitch G. Itching in the Psychiatric Ward. Acta dermato-venereologica. 2008;88(2):128–131. doi: 10.2340/00015555-0406. [DOI] [PubMed] [Google Scholar]
- McCoy E, Taylor-Blake B, Zylka M. CGRPα-expressing sensory neurons respond to stimuli that evoke sensations of pain and itch. PloS One. 2012;7(5):e36355. doi: 10.1371/journal.pone.0036355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy ES, Taylor-Blake B, Street SE, Pribisko AL, Zheng J, Zylka MJ. Peptidergic CGRPalpha Primary Sensory Neurons Encode Heat and Itch and Tonically Suppress Sensitivity to Cold. Neuron. 2013;78(1):138–151. doi: 10.1016/j.neuron.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken LM, Carson JW, Eccleston C, Keefe FJ. Acceptance and change in the context of chronic pain. Pain. 2004;109:4–7. doi: 10.1016/j.pain.2004.02.006. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Koltzenburg M. Itching for an explanation. Trends Neurosci. 1992;15(12):497–501. doi: 10.1016/0166-2236(92)90102-e. [DOI] [PubMed] [Google Scholar]
- McQueen DS, Noble MAH, Bond SM. Endothelin-1 activates ETA receptors to cause reflex scratching in BALB/c mice. British journal of pharmacology. 2007;151(2):278–284. doi: 10.1038/sj.bjp.0707216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Holzman S, Hoon M. A nociceptive signaling role for neuromedin B. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:8686–8695. doi: 10.1523/JNEUROSCI.1533-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science. 2013;340:968–971. doi: 10.1126/science.1233765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki H, Tashiro M, Kano M, Sakurada Y, Itoh M, Yanai K. Imaging of central itch modulation in the human brain using positron emission tomography. Pain. 2003;105:339–346. doi: 10.1016/s0304-3959(03)00249-5. [DOI] [PubMed] [Google Scholar]
- Moser HR, Giesler GJ., Jr Itch and analgesia resulting from intrathecal application of morphine: contrasting effects on different populations of trigeminothalamic tract neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:6093–6101. doi: 10.1523/JNEUROSCI.0216-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CS, Rees JL. Are subjective accounts of itch to be relied on? The lack of relation between visual analogue itch scores and actigraphic measures of scratch. Acta dermato-venereologica. 2011;91:18–23. doi: 10.2340/00015555-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M. Separate peripheral pathways for pruritus in man. Journal of neurophysiology. 2008;100:2062–2069. doi: 10.1152/jn.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namer B, Reeh P. Scratching an itch. Nat Neurosci. 2013;16(2):117–118. doi: 10.1038/nn.3316. [DOI] [PubMed] [Google Scholar]
- Nattkemper LA, Zhao ZQ, Nichols AJ, Papoiu AD, Shively CA, Chen ZF, Yosipovitch G. Over-Expression of the Gastrin-Releasing Peptide in Cutaneous Nerve Fibers and its Receptor in Spinal Cord in Primates with Chronic Itch. The Journal of investigative dermatology. 2013 Apr 4; doi: 10.1038/jid.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAMS. Pub.#09-4272. 2009 http://www.niams.nih.gov/Health_Info/Atopic_Dermatitis/default.asp.
- Nie Y, Ishii I, Yamamoto K, Orito K, Matsuda H. Real-time scratching behavior quantification system for laboratory mice using high-speed vision. Journal of Real-Time Image Processing. 2009;4:181–190. [Google Scholar]
- Nilsson HJ, Levinsson A, Schouenborg J. Cutaneous field stimulation (CFS): a new powerful method to combat itch. Pain. 1997;71(1):49–55. doi: 10.1016/s0304-3959(97)03339-3. [DOI] [PubMed] [Google Scholar]
- Nojima H, Carstens E. 5-Hydroxytryptamine (5-HT)2 receptor involvement in acute 5-HT-evoked scratching but not in allergic pruritus induced by dinitrofluorobenzene in rats. The Journal of pharmacology and experimental therapeutics. 2003a;306:245–252. doi: 10.1124/jpet.103.049239. [DOI] [PubMed] [Google Scholar]
- Nojima H, Carstens E. Quantitative assessment of directed hind limb scratching behavior as a rodent itch model. Journal of neuroscience methods. 2003b;126:137–143. doi: 10.1016/s0165-0270(03)00074-8. [DOI] [PubMed] [Google Scholar]
- Nojima H, Cuellar JM, Simons CT, Carstens MI, Carstens E. Spinal c-fos expression associated with spontaneous biting in a mouse model of dry skin pruritus. Neurosci Lett. 2004;361(1–3):79–82. doi: 10.1016/j.neulet.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Oaklander AL, David B, Bradley G, Maija H, Mark PJ. Herpes zoster itch: preliminary epidemiologic data. The Journal of Pain. 2003;4(6):338–343. doi: 10.1016/s1526-5900(03)00637-0. [DOI] [PubMed] [Google Scholar]
- Okamoto R. Sphingosylphosphorylcholine is upregulated in the stratum corneum of patients with atopic dermatitis. The Journal of Lipid Research. 2002;44 doi: 10.1194/jlr.m200225-jlr200. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. The Journal of clinical investigation. 2010;120:3779–3787. doi: 10.1172/JCI43766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K, Liu Q, Meeker S, Undem B, Dong X. Pirt, a TRPV1 modulator, is required for histamine-dependent and -independent itch. PloS one. 2011;6(5):e20559. doi: 10.1371/journal.pone.0020559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel T, Yosipovitch G. Therapy of pruritus. Expert opinion on pharmacotherapy. 2010;11:1673–1682. doi: 10.1517/14656566.2010.484420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen LJ, Church MK, Skov PS. Platelet-activating factor induces histamine release from human skin mast cells in vivo, which is reduced by local nerve blockade. The Journal of allergy and clinical immunology. 1997;99:640–647. doi: 10.1016/s0091-6749(97)70026-5. [DOI] [PubMed] [Google Scholar]
- Pfab F, Valet M, Napadow V, Tölle TR, Behrendt H, Ring J, Darsow U. Itch and the brain. Chem Immunol Allergy. 2012;98:253–65. doi: 10.1159/000336529. [DOI] [PubMed] [Google Scholar]
- Pisoni RL, Wikström B, Elder SJ, Akizawa T, Asano Y, Keen ML, Saran R, Mendelssohn DC, Young EW, Port FK. Pruritus in haemodialysis patients: International results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2006;21(12):3495–505. doi: 10.1093/ndt/gfl461. [DOI] [PubMed] [Google Scholar]
- Postovit VA. Role of cholacidemia in the occurrence of skin itching during parenchymatous and obstructive jaundice. Vrachebnoe delo. 1971;7:150–152. [PubMed] [Google Scholar]
- Price D, Hayes R, Ruda M, Dubner R. Spatial and temporal transformations of input to spinothalamic tract neurons and their relation to somatic sensations. Journal of neurophysiology. 1978;41:933–947. doi: 10.1152/jn.1978.41.4.933. [DOI] [PubMed] [Google Scholar]
- Prignano F, Ricceri F, Pescitelli L, Lotti T. Itch in psoriasis: epidemiology, clinical aspects and treatment options. Clin Cosmet Investig Dermatol. 2009;2:9–13. doi: 10.2147/ccid.s4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau K, McIlwrath S, Wang H, Lawson J, Jankowski M, Zylka M, Anderson D, Koerber H. Mrgprd enhances excitability in specific populations of cutaneous murine polymodal nociceptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:8612–8619. doi: 10.1523/JNEUROSCI.1057-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V, Iuga A, Shimada S, LaMotte R, Lerner E. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. The Journal of neuroscience. 2008;28:4331–4335. doi: 10.1523/JNEUROSCI.0716-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V, Shimada S, Sikand P, Lamotte R, Lerner E. Cathepsin S elicits itch and signals via protease-activated receptors. The Journal of investigative dermatology. 2010;130:1468–1470. doi: 10.1038/jid.2009.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V, Elmariah S, Azimi E, Luo T, Lerner EA. Mechanisms of itch: Proteases activate Mas-related G-protein coupled receptors. The Journal of investigative dermatology. 2013;133:S41. doi: 10.1016/j.jid.2017.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringkamp M, Schepers R, Shimada S, Johanek L, Hartke T, Borzan J, Shim B, LaMotte R, Meyer R. A role for nociceptive, myelinated nerve fibers in itch sensation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:14841–14849. doi: 10.1523/JNEUROSCI.3005-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rishe E, Azarm A, Bergasa NV. Itch in Primary Biliary Cirrhosis: A Patients’ Perspective. Acta dermato-venereologica. 2008;88(1):34–37. doi: 10.2340/00015555-0350. [DOI] [PubMed] [Google Scholar]
- Roberson DP, Gudes S, Sprague JM, Patoski HA, Robson VK, Blasl F, Duan B, Oh SB, Bean BP, Ma Q, Binshtok AM, Woolf CJ. Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons. Nature neuroscience. 2013 May 19; doi: 10.1038/nn.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukwied R, Zeck S, Schmelz M, McGlone F. Sensitivity of human scalp skin to pruritic stimuli investigated by intradermal microdialysis in vivo. J Am Acad Dermatol. 2002;47(2):245–250. doi: 10.1067/mjd.2002.120461. [DOI] [PubMed] [Google Scholar]
- Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, Tolias C, Corfas R, Chen S, Inquimbert P, Xu Y, McInnes RR, Rice FL, Corfas G, Ma Q, Woolf CJ, Greenberg ME. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–898. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossbach K, Wendorff S, Sander K, Stark H, Gutzmer R, Werfel T, Kietzmann M, Baumer W. Histamine H4 receptor antagonism reduces hapten-induced scratching behaviour but not inflammation. Experimental dermatology. 2009;18:57–63. doi: 10.1111/j.1600-0625.2008.00762.x. [DOI] [PubMed] [Google Scholar]
- Rothman S. Physiology of itching. Physiological reviews. 1941;21:357–381. [Google Scholar]
- Rukwied RR, Main M, Weinkauf B, Schmelz M. NGF sensitizes nociceptors for cowhage- but not histamine-induced itch in human skin. The Journal of investigative dermatology. 2013;133:268–270. doi: 10.1038/jid.2012.242. [DOI] [PubMed] [Google Scholar]
- Saeed AW, Ribeiro-da-Silva A. Non-peptidergic primary afferents are presynaptic to neurokinin-1 receptor immunoreactive lamina I projection neurons in rat spinal cord. Molecular pain. 2012;8:64. doi: 10.1186/1744-8069-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampogna F, Gisondi P, Melchi C, Amerio P, Girolomoni G, Abeni D Investigators IDIMPRoVE. Prevalence of symptoms experienced by patients with different clinical types of psoriasis. The British journal of dermatology. 2004;151:594–599. doi: 10.1111/j.1365-2133.2004.06093.x. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Hilliges M, Schmidt R, Orstavik K, Vahlquist C, Weidner C, Handwerker HO, Torebjork HE. Active “itch fibers” in chronic pruritus. Neurology. 2003a;61:564–566. doi: 10.1212/01.wnl.0000078193.64949.08. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjork HE. Specific C-receptors for itch in human skin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjork H, Handwerker H. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. Journal of neurophysiology. 2003b;89:2441–2448. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]
- Shi T-JS, Liu S-XL, Hammarberg H, Watanabe M, Xu Z-QD, Hökfelt T. Phospholipase C{beta}3 in mouse and human dorsal root ganglia and spinal cord is a possible target for treatment of neuropathic pain. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20004–20008. doi: 10.1073/pnas.0810899105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain. 2008;139:681–687. doi: 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley WB, Arthur RP. Mucunain, the active pruritogenic proteinase of cowhage. Science. 1955;122(3167):469–470. doi: 10.1126/science.122.3167.469. [DOI] [PubMed] [Google Scholar]
- Sikand P, Dong X, LaMotte RH. BAM8-22 peptide produces itch and nociceptive sensations in humans independent of histamine release. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:7563–7567. doi: 10.1523/JNEUROSCI.1192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikand P, Shimada SG, Green BG, LaMotte RH. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66–75. doi: 10.1016/j.pain.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone DA, Sorkin LS, Oh U, Chung JM, Owens C, LaMotte RH, Willis WD. Neurogenic hyperalgesia: central neural correlates in responses of spinothalamic tract neurons. Journal of neurophysiology. 1991;66:228–246. doi: 10.1152/jn.1991.66.1.228. [DOI] [PubMed] [Google Scholar]
- Simone DA, Zhang X, Li J, Zhang JM, Honda CN, LaMotte RH, Giesler GJ., Jr Comparison of responses of primate spinothalamic tract neurons to pruritic and algogenic stimuli. J Neurophysiol. 2004;91(1):213–222. doi: 10.1152/jn.00527.2003. [DOI] [PubMed] [Google Scholar]
- Slominski AT, Zmijewski MA, Zbytek B, Brozyna AA, Granese J, Pisarchik A, Szczesniewski A, Tobin DJ. Regulated proenkephalin expression in human skin and cultured skin cells. The Journal of investigative dermatology. 2011;131:613–622. doi: 10.1038/jid.2010.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkoly E, Muller A, Lauerma A, Pivarcsi A, Soto H, Kemeny L, Alenius H, Dieu-Nosjean M-C, Meller S, Rieker J, Steinhoff M, Hoffmann T, Ruzicka T, Zlotnik A, Homey B. IL-31: a new link between T cells and pruritus in atopic skin inflammation. The Journal of allergy and clinical immunology. 2006;117:411–417. doi: 10.1016/j.jaci.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Spradley JM, Davoodi A, Carstens MI, Carstens E. Opioid Modulation of Facial Itch- and Pain-related Responses and Grooming Behavior in Rats. Acta dermato-venereologica. 2012a;92(5):515–520. doi: 10.2340/00015555-1364. [DOI] [PubMed] [Google Scholar]
- Spradley JM, Davoodi A, Carstens MI, Carstens E. Effects of acute stressors on itch- and pain-related behaviors in rats. Pain. 2012b;153(9):1890–1897. doi: 10.1016/j.pain.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradley JM, Davoodi A, Gee LB, Carstens MI, Carstens E. Differences in peripheral endocannabinoid modulation of scratching behavior in facial vs. spinally-innervated skin. Neuropharmacology. 2012c;63(4):743–749. doi: 10.1016/j.neuropharm.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stander S, Schmelz M. Chronic itch and pain--similarities and differences. Eur J Pain. 2006;10:473–478. doi: 10.1016/j.ejpain.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Ständer S, Weisshaar E, Mettang T, Szepietowski J, Carstens E, Ikoma A, Bergasa N, Gieler U, Misery L, Wallengren J, Darsow U, Streit M, Metze D, Luger T, Greaves M, Schmelz M, Yosipovitch G, Bernhard J. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta dermato-venereologica. 2007;87:291–294. doi: 10.2340/00015555-0305. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Cevikbas F, Ikoma A, Berger T. Pruritus: management algorithms and experimental therapies. Seminars in cutaneous medicine and surgery. 2011;30:127–137. doi: 10.1016/j.sder.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov P, Luger T, Schmelz M. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:6176–6180. doi: 10.1523/JNEUROSCI.23-15-06176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff M, Vergnolle N, Young S, Tognetto M, Amadesi S, Ennes H, Trevisani M, Hollenberg M, Wallace J, Caughey G, Mitchell S, Williams L, Geppetti P, Mayer E, Bunnett N. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nature medicine. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- Su P-Y, Ko M-C. The role of central gastrin-releasing peptide and neuromedin B receptors in the modulation of scratching behavior in rats. The Journal of pharmacology and experimental therapeutics. 2011;337:822–829. doi: 10.1124/jpet.111.178970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot TL, Schmitt JM, Bergasa NV, Jones EA, Walker EC. Scratching behavior assessed by application of piezo film technology for the quantitative assessment of pruritus. Biomedical Instrumentation & Technology. 1991;25:400–403. [PubMed] [Google Scholar]
- Than JY, Li L, Hasan R, Zhang X. The excitation and modulation of TRPV1-, TRPA1-and TRPM8-expressing sensory neurons by the pruritogen chloroquine. The Journal of biological chemistry. 2013 doi: 10.1074/jbc.M113.450072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen J, Petersen M, Benfeldt E, Jensen S, Serup J. Scratch induction in the rat by intradermal serotonin: a model for pruritus. Acta dermato-venereologica. 2001;81:250–254. doi: 10.1080/00015550152572868. [DOI] [PubMed] [Google Scholar]
- Thomsen J, Sonne M, Benfeldt E, Jensen S, Serup J, Menné T. Experimental itch in sodium lauryl sulphate-inflamed and normal skin in humans: a randomized, double-blind, placebo-controlled study of histamine and other inducers of itch. The British journal of dermatology. 2002;146:792–800. doi: 10.1046/j.1365-2133.2002.04722.x. [DOI] [PubMed] [Google Scholar]
- Tiong SY, Polgar E, van Kralingen JC, Watanabe M, Todd AJ. Galanin-immunoreactivity identifies a distinct population of inhibitory interneurons in laminae I-III of the rat spinal cord. Molecular pain. 2011;7:36. doi: 10.1186/1744-8069-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd A. Anatomy of primary afferents and projection neurones in the rat spinal dorsal horn with particular emphasis on substance P and the neurokinin 1 receptor. Experimental physiology. 2002;87:245–249. doi: 10.1113/eph8702351. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Hughes DI, Polgar E, Nagy GG, Mackie M, Ottersen OP, Maxwell DJ. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. The European journal of neuroscience. 2003;17:13–27. doi: 10.1046/j.1460-9568.2003.02406.x. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Puskar Z, Spike RC, Hughes C, Watt C, Forrest L. Projection neurons in lamina I of rat spinal cord with the neurokinin 1 receptor are selectively innervated by substance p-containing afferents and respond to noxious stimulation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:4103–4113. doi: 10.1523/JNEUROSCI.22-10-04103.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi Y, Umeuchi H, Okano K, Ando N, Yoshizawa Y, Honda T, Kawamura K, Endoh T, Utsumi J, Kamei J, Tanaka T, Nagase H. Antipruritic activity of the kappa-opioid receptor agonist, TRK-820. Eur J Pharmacol. 2002;435:259–264. doi: 10.1016/s0014-2999(01)01588-6. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Ogawa H, Takamori K. Histological characterization of cutaneous nerve fibers containing gastrin-releasing peptide in NC/Nga mice: an atopic dermatitis model. The Journal of investigative dermatology. 2009;129:2901–2905. doi: 10.1038/jid.2009.188. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Ozawa S, Tengara S, Ogawa H, Takamori K. Intraepidermal nerve fibers increase in dry skin of acetone-treated mice. Journal of dermatological science. 2007;48:103–111. doi: 10.1016/j.jdermsci.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Tong L, Ru-Rong J. Oxidative stress induces itch via activation of transient receptor potential subtype ankyrin 1 in mice. Neuroscience bulletin. 2012;28(2):145–154. doi: 10.1007/s12264-012-1207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckett RP. Itch evoked by electrical stimulation of the skin. J Invest Dermatol. 1982;79(6):368–373. doi: 10.1111/1523-1747.ep12529734. [DOI] [PubMed] [Google Scholar]
- Twycross R, Greaves M, Handwerker H, Jones E, Libretto S, Szepietowski J, Zylicz Z. Itch: scratching more than the surface. QJM : monthly journal of the Association of Physicians. 2003;96:7–26. doi: 10.1093/qjmed/hcg002. [DOI] [PubMed] [Google Scholar]
- Ui H, Andoh T, Lee JB, Nojima H, Kuraishi Y. Potent pruritogenic action of tryptase mediated by PAR-2 receptor and its involvement in anti-pruritic effect of nafamostat mesilate in mice. European journal of pharmacology. 2006;530:172–178. doi: 10.1016/j.ejphar.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Umeda K, Noro Y, Murakami T, Tokime K, Sugisaki H, Yamanaka K, Kurokawa I, Kuno K, Tsutsui H, Nakanishi K, Mizutani H. A novel acoustic evaluation system of scratching in mouse dermatitis: rapid and specific detection of invisibly rapid scratch in an atopic dermatitis model mouse. Life sciences. 2006;79:2144–2150. doi: 10.1016/j.lfs.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Van Wimersma Greidanus T, Maigret C. Neuromedin-induced excessive grooming/scratching behavior is suppressed by naloxone, neurotensin and a dopamine D1 receptor antagonist. European journal of pharmacology. 1991;209:57–61. doi: 10.1016/0014-2999(91)90010-n. [DOI] [PubMed] [Google Scholar]
- Von Frey M. Zur Physiologie der Juckempfindung. Arch Neerl Physiol. 1922;7:142–145. [Google Scholar]
- Wahlgren CF, Tengvall Linder M, Hagermark O, Scheynius A. Itch and inflammation induced by intradermally injected interleukin-2 in atopic dermatitis patients and healthy subjects. Archives of dermatological research. 1995;287:572–580. doi: 10.1007/BF00374079. [DOI] [PubMed] [Google Scholar]
- Wang H, Zylka M. Mrgprd-expressing polymodal nociceptive neurons innervate most known classes of substantia gelatinosa neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:13202–13209. doi: 10.1523/JNEUROSCI.3248-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang J, Eberhart D, Urban R, Meda K, Solorzano C, Yamanaka H, Rice D, Basbaum AI. Excitatory superficial dorsal horn interneurons are functionally heterogeneous and required for the full behavioral expression of pain and itch. Neuron. 2013;78:312–324. doi: 10.1016/j.neuron.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward L, Wright E, McMahon SB. A comparison of the effects of noxious and innocuous counterstimuli on experimentally induced itch and pain. Pain. 1996;64(1):129–138. doi: 10.1016/0304-3959(95)00080-1. [DOI] [PubMed] [Google Scholar]
- Weisshaar E, Ziethen B, Gollnick H. Can a serotonin type 3 (5-HT3) receptor antagonist reduce experimentally-induced itch? Inflamm Res. 1997;46:412–416. doi: 10.1007/s000110050213. [DOI] [PubMed] [Google Scholar]
- Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nature neuroscience. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Samoriski G, McLaughlin J, Romoser V, Smrcka A, Hinkle P, Bidlack J, Gross R, Jiang H, Wu D. Genetic alteration of phospholipase C beta3 expression modulates behavioral and cellular responses to mu opioids. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:10385–10390. doi: 10.1073/pnas.96.18.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Nagasawa T, Satoh M, Kuraishi Y. Itch-associated response induced by intradermal serotonin through 5-HT2 receptors in mice. Neuroscience research. 1999;35:77–83. doi: 10.1016/s0168-0102(99)00070-x. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Carstens E, McGlone F. Chronic itch and chronic pain: Analogous mechanisms. Pain. 2007;131(1–2):4–7. doi: 10.1016/j.pain.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Goon A, Wee J, Chan Y, Goh C. The prevalence and clinical characteristics of pruritus among patients with extensive psoriasis. The British journal of dermatology. 2000;143:969–973. doi: 10.1046/j.1365-2133.2000.03829.x. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Goon ATJ, Wee J, Chan YH, Zucker I, Goh CL. Itch characteristics in Chinese patients with atopic dermatitis using a new questionnaire for the assessment of pruritus. International journal of dermatology. 2002;41(4):212–216. doi: 10.1046/j.1365-4362.2002.01460.x. [DOI] [PubMed] [Google Scholar]
- Zhou S, Potts EN, Cuttitta F, Foster WM, Sunday ME. Gastrin-releasing peptide blockade as a broad-spectrum anti-inflammatory therapy for asthma. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2100–2105. doi: 10.1073/pnas.1014792108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zylka M, Rice F, Anderson D. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]