Abstract
Background
Attention-deficit/hyperactivity disorder (ADHD) and substance use disorder are often comorbid in adults. The effects of ADHD treatment on comorbid alcohol use disorder have not been extensively studied.
Objective
To assess correlates of ADHD and alcohol use outcomes in ADHD with comorbid alcohol use disorders, via a post-hoc exploratory subgroup analysis of a previously conducted, randomized, double-blind, placebo controlled study of recently abstinent adults.
Methods
Adults who had ADHD and alcohol use disorders and were abstinent for 4–30 days were randomized to daily atomoxetine 25–100 mg (mean final dose=89.9 mg) or placebo for 12 weeks. Changes in ADHD symptoms from baseline to endpoint were assessed using the ADHD Investigator Symptom Rating Scale (AISRS) total score, alcohol use by the timeline followback method, and alcohol cravings by the Obsessive Compulsive Drinking Scale.
Results
Of 147 subjects receiving atomoxetine (n=72) or placebo (n=75) in the primary study, 80 (54%) completed 12 weeks (n=32 atomoxetine; n=48 placebo). Improvements in ADHD symptoms on the AISRS correlated significantly with decreases in alcohol cravings (Pearson’s r=0.28; 95% confidence interval [CI]=0.11–0.43; p=0.002), and the correlation was most notable with atomoxetine (r=0.29; CI [0.04 – 0.51]; p=0.023) rather than with placebo (r=0.24; CI [0.00–0.46]; p=0.055). On-treatment drinking levels correlated with AISRS scores (r=0.12; CI [0.05 –0.19]; p=0.001). Relapse to alcohol abuse significantly correlated with worse ADHD symptoms on 15 of 18 items of the AISRS in the placebo group (p<0.05 for each).
Conclusions
No baseline predictor (other than degree of sobriety) of alcohol use or ADHD outcomes emerged. ADHD symptom improvements correlated significantly with reductions in alcohol cravings, and relapse to alcohol abuse correlated significantly with worsening of most ADHD symptoms in the placebo group, but not in the atomoxetine group. This post-hoc subgroup analysis is of a hypothesis-generating nature, and the generalizability of the findings may be limited by exclusion of adults with common ADHD comorbidities from the base study. Further, prospective clinical trials in larger and more heterogeneous patient populations are warranted to confirm or reject these preliminary associations.
Keywords: Adult, Alcohol, Atomoxetine, Attention-deficit/hyperactivity disorder, Drug therapy, Treatment outcomes
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a chronic disorder, often persisting into adulthood1,2. Adults with ADHD are at increased risk of alcohol and/ or drug abuse or dependence3–6; by some estimates, ADHD doubles the lifetime risk of developing an addiction, with comorbid depressive, anxiety, and conduct disorders potentially further heightening such risk7–9. One-half of adults with untreated ADHD have a substance use disorder (SUD)5,8,10. Conversely, studies also show that approximately 11%–35% of adults with different forms of SUD experience ADHD6,11–14. Substance use disorder that is comorbid with ADHD typically has an earlier onset, is more severe and less responsive to treatment, and has a longer duration than SUD without ADHD10,15,16.
Few studies have assessed ADHD treatment effects in adults with comorbid SUD17–25. Clinical trials involving stimulants, bupropion, and atomoxetine in patients with ADHD and active SUD have produced mixed outcomes in terms of changes in the symptoms of each condition. Most of these studies have shown either no change or minimal improvement in ADHD, without accompanying improvements in SUD18–25. Treatment of ADHD in adults using extended-release formulations of stimulants or the nonstimulant atomoxetine may be associated with lower risks of abuse, misuse, and/or diversion compared with other agents and formulations8,9,14.
In the first controlled trial (to our knowledge) of adults with ADHD and comorbid alcohol use disorder, daily treatment with atomoxetine HCl for 12 weeks significantly decreased both ADHD symptoms (p=0.007; effect size=0.48) and heavy drinking days by 26% (p=0.023) compared with placebo in recently abstinent adult alcoholics. Kaplan–Meier estimates of the time to relapse to heavy drinking did not differ between the atomoxetine and placebo groups, and the overall effects of atomoxetine on drinking behaviors were inconsistent. No major safety or tolerability issues related to the proximity of atomoxetine treatment and previous alcohol use have been identified, and discontinuation rates were low and not significantly different between the atomoxetine and placebo groups24,25. Because improvements in SUD were noted in adults with ADHD and SUD treated with atomoxetine, and responses to ADHD treatment in patients with comorbid SUD were similar to the response observed in the absence of SUD26, data analyses have been conducted to better understand these complex relationships24,25. The chief objective of the present post-hoc subgroup analysis was to determine if any baseline ADHD symptoms or drinking behaviors were predictive of AHD or alcohol use outcomes, and to examine the relationship between ADHD symptoms and drinking behavior.
Patients and Methods
Study design and definition of terms
Details of the primary study design have been published elsewhere24. This was a multicenter (14-site), North American, randomized, double-blind, placebo-controlled study, which included Kaplan–Meier estimates of time to alcohol use relapse among recently abstinent adult study participants randomized to atomoxetine or placebo. Initial screening was conducted at Visit 1. At Visit 2, subjects who met entry criteria were randomly assigned to atomoxetine (25–100 mg daily [mean final dose=89.9 mg]) or placebo for 12 weeks, with weekly study visits. Daily treatment with atomoxetine was initiated at 25 mg (in the morning) for the first week, followed by an increase to 40 mg at the beginning of the second week and 80 mg at the end of the second week. The daily dose could be advanced to 100 mg at any visit after 4 weeks of treatment (either as single or divided doses, according to patient tolerability)24.
Baseline characteristics were assessed at Visit 2, except for baseline drinking behavior, which was assessed from Visit 1 to Visit 2. Because drink numbers were tracked using the timeline followback method, drinking records at Visit 2 comprised the drinking baseline. Before Visit 2, there were approximately 40 days in the timeline followback calendar; the protocol required participants to refrain from alcohol for ≥4 days (i.e., any 4 non-alcohol-use days), but <30 days, before randomization.
In the present post-hoc subgroup analysis, patients were classified into two groups based on duration of abstinence before Visit 2. The ‘limited-sobriety’ group included subjects with ≤4 consecutive days of abstinence, whereas the ‘stable-sobriety’ group included subjects with >4 consecutive days of abstinence. Our analysis also categorized participants as abstainers, nonheavy drinkers, heavy drinkers, or relapsers to alcohol abuse based on drinking behaviors during the double-blind treatment period (Visits 3–14).
Consistent with standard definitions, we categorized 1) abstainers as patients reporting no alcohol consumption; 2) heavy drinkers as women consuming ≥4 alcoholic drinks daily, or men consuming ≥5 drinks daily, for >14 days (cumulative); 3) nonheavy drinkers as subjects who were not abstainers but did not meet criteria for heavy drinking; and 4) relapsers to alcohol abuse were women consuming ≥4 drinks daily for 1 day, men consuming ≥5 drinks daily for 1 day, or any adult consuming ≥3 drinks daily for ≥7 consecutive days, all by patient self-report; also consistent with widely cited definitions, a standard alcoholic drink was defined as 1.5 oz. of 80-proof distilled spirits, 5 oz. of wine, or 12 oz. of beer27,28.
Patients
Adults ≥ 18 years of age meeting criteria from the Diagnostic and Statistical Manual of Mental Disorders IV, Text Revision (DSM-IV-TR)29 and the Adult ADHD Clinical Diagnostic Scale (ACDS) version 1.230,31 for ADHD (any subtype) and for alcohol use disorders (abuse or dependence; see above definition for alcohol abuse) were included. Eligibility criteria related to alcohol and substance use are summarized in Table 1. Psychotherapeutic, pharmacologic, and/or other interventions for substance abuse (other than 12-step programs) were not permitted. Other exclusion criteria comprised significant cognitive impairment, including memory impairment or other consequences of heavy alcohol use or dementia, as well as organic brain disease (e.g. dementia, residua of traumatic brain injury, or a history of seizure disorder aside from childhood febrile seizures). Also excluded were patients with any psychiatric disorder other than ADHD and SUD requiring (or expected to require) use of psychotropic medications. These included diagnoses of, or medications for, bipolar disorder (including mood stabilizers); major depressive disorder, including a baseline Hamilton Depression (HAM-D-17) rating scale score >18; psychotic disorders apart from past psychotic episodes related to alcohol- or drug-induced delirium, including treatment with antipsychotics; anxiety disorders, including a HAM-Anxiety (HAM-A) rating scale score >18 or treatment with anxiolytics; as well as the use of anticonvulsants, disulfiram, or naltrexone32–37. Prior treatment with atomoxetine also precluded study enrollment.
Table 1.
Eligibility criteria related to alcohol and substance use.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| DSM-IV-TR29 criteria for alcohol use disorders (abuse or dependence) | Primary substance of abuse or dependence not alcohol |
| Criteria include: ≥4 standard alcoholic drinks* for women, or ≥5 drinks for men, within 24 hours; or ≥3 standard alcoholic drinks*/day for ≥1 week (i.e., ≥7 consecutive days) in any adult |
Actively abusing substances other than alcohol |
| Recently abstinent: alcohol free (by patient self-report) for ≥4 (but not >30) days** | Minimum 4 days of abstinence did not overlap the week before randomization |
Standard alcoholic drinks included 12oz. of regular beer, 5oz. of wine, or 1.5 oz of 80-proof (40% alcohol) distilled spirits.
Days abstinent were not necessarily consecutive.
DSM-IV-TR – Diagnostic and Statistical Manual of Mental Disorders, 4th edition text revision; oz. - ounces.
The base study24 was conducted in a manner consistent with ethical tenets originating in the Declaration of Helsinki according to International Conference on Harmonization guidelines38. The study was approved by each site’s institutional review board, and subjects provided voluntary, written informed consent before participating in the study. The protocol and informed-consent document were approved by an ethical review board.
Measures
In the base study24, the primary measure of ADHD symptoms was the Adult ADHD Investigator Symptom Rating Scale (AISRS)39, and a secondary measure was the World Health Organization Adult ADHD Self-Report Scale (ASRS) v 1.1 Symptom Checklist40. The 18-item AISRS, which utilizes a semistructured interview methodology, was developed in part to characterize ADHD symptoms (and their responsiveness to treatments) in adults rather than children. The AISRS includes nine hyperactive–impulsive symptoms alternating with nine inattentive symptoms, and is scored on a Likert scale of 0 (‘none’) to 3 (‘severe’); hence, the total possible (worst) score is 54 (27 for each subscale). Improvement in the AISRS was recorded as decreases in total or subscale scores and worsening as increases in these scores39.
The AISRS has high internal consistency (Cronbach’s alpha = 0.74–0.95), more than acceptable test–retest reliability (concordance correlation coefficient value=0.904), good convergent and discriminant validity, as well as low divergent validity and small (0.2%–1.0%) floor and ceiling effects39. The minimum clinically important difference (MCID) is −12.5 for the AISRS total score, −6.8 for the inattentive subscale, and −5.6 for the hyperactive-impulsive subscale within treatment groups, according to data from a 6-month placebo-controlled trial39 of atomoxetine for adults with ADHD. Corresponding data for MCID between treatment groups were −10.1, −5.2, and −4.9, respectively.
The primary measure of alcohol use was the timeline followback method41, and a secondary measure (of alcohol cravings) was the Obsessive Compulsive Drinking Scale (OCDS)42–45.
The OCDS is a 14-item self-rating scale originally developed as a modification of the 10-item Yale–Brown Obsessive Compulsive Scale_heavy drinkers (YBOCShd) and scores on the OCDS have high concurrent validity with data on the YBOCS-hd (correlation coefficient 0.83). The OCDS scale also has high test–retest reliability, with a correlation coefficient of 0.96 for the OCDS total score44. The internal consistency of OCDS items is also high (Cronbach’s alpha of 0.86)44. The timeline followback method has been validated against other instruments for alcohol assessments and exhibits adequate test–retest reliability in both alcohol-dependent and normal populations.
Although OCDS scores correlated with measures of craving, the maximal shared variance between them was only 20%, suggesting that the dimension measured by the OCDS does not completely overlap patients’ subjective ratings of cravings43. Alcohol cravings have obsessive–compulsive features, and factor analysis of the OCDS demonstrated four correlated dimensions: alcohol obsessions, alcohol consumption, automaticity of drinking, and interference due to drinking43,45. Like the AISRS, the OCDS also proved to be responsive to changes associated with effective management of alcohol dependence and also to relapse drinking.
Data Analysis
Alcohol consumption was measured as the average number of drinks per day calculated at each post-baseline visit. No p values were presented for treatment differences in alcohol consumption from baseline to endpoint within sobriety groups. Treatment-group differences in continuous baseline measures were estimated using an analysis-of-variance (ANOVA) model containing terms for treatment and investigator. Treatment-group differences in categorical baseline measures were estimated using Fisher’s exact test.
Changes from baseline in ADHD symptoms were calculated as last-observation-carried-forward (LOCF) changes in AISRS and ASRS scores in study participants. Individual AISRS items and the ASRS total score were calculated at Visits 4, 6, 8, 10, 12, and 14 (endpoint). To measure changes in alcohol cravings, we recorded OCDS total scores at Visits 2 (baseline) and 14 (or LOCF). Descriptive statistics for changes in ADHD symptoms were determined for each treatment group, baseline sobriety group, and drinking level (i.e., abstainers, nonheavy drinkers, heavy drinkers, relapsers to alcohol abuse). Within each sobriety group, treatment differences in changes in AISRS total scores were assessed by ANOVA. A mixed-model repeated-measures analysis was conducted to compare changes in on-treatment drinking behaviors in the two baseline sobriety groups.
Using Pearson correlation-coefficient analyses, we tested potential correlates of changes from baseline to endpoint in the numerical values of ADHD symptoms (on the AISRS), alcohol consumption, and alcohol cravings (on the OCDS). Pearson correlation coefficients were used under the normality assumption because the initial sample size exceeded 70 study participants in each treatment group. To evaluate a predictive relationship between the duration of baseline sobriety and SUD, we compared the mean number of drinks consumed during the study period in the limited-sobriety and stable-sobriety groups. Correlations between changes in AISRS item scores and the presence or absence of relapse to alcohol abuse for the two treatment groups were explored using the point biserial correlations adjusted for multiplicity by the Hochberg method46. The point biserial correlation is a measure of association between a continuous variable and a binary variable47.
Statistical significance was defined a priori at a two-tailed a=0.05. Data are presented as mean standard deviation (SD) unless otherwise stated. Statistical analyses were conducted using SAS Drug Development 3.4_04_C (SAS Institute, Cary, NC, USA).
Results
Patients
Of 72 subjects randomized to atomoxetine in the base study, 32 receiving atomoxetine (44%) completed the 12-week, double-blind period, as did 48 (64%) of 75 subjects randomized to placebo (Table 2)24.
Table 2.
Baseline characteristics.
| Characteristic | Randomized
|
Completers
|
||
|---|---|---|---|---|
| Atomoxetine (n=72) | Placebo (n=75) | Atomoxetine (n = 32) | Placebo (n = 48)* | |
| AISRS, mean (SD) | ||||
| Total score | 40.6 (7.8) | 40.1 (7.9) | 39.3 (7.9) | 39.8 (7.4) |
| Hyperactive/impulsive subscale | 19.0 (5.0) | 18.7 (5.2) | 18.3 (5.1) | 18.5 (4.5) |
| Inattentive subscale | 21.7 (3.9) | 21.4 (4.1) | 21.0 (4.3) | 21.3 (4.2) |
| ASRS, mean (SD) | ||||
| Total score | 48.5 (10.1) | 51.3 (9.3) | 47.3 (8.9) | 50.1 (9.2) |
| Hyperactive/impulsive subscale | 23.6 (6.1) | 24.6 (6.0) | 22.9 (6.1) | 24.0 (6.0) |
| Inattentive subscale | 24.9 (5.5) | 26.7 (5.6) | 24.5 (5.2) | 26.1 (5.5) |
| OCDS, mean (SD) | ||||
| Total score | 20.3 (8.5) | 21.9 (8.3) | 19.1 (7.3) | 21.0 (8.2) |
| Obsessive subscale | 6.6 (4.4) | 7.4 (4.1) | 6.0 (3.8) | 6.8 (4.2) |
| Compulsive subscal | 13.7 (4.9) | 14.4 (4.9) | 13.1 (4.5) | 14.1 (4.8) |
| Mean (SD) number of alcohol-free days at baseline (from Visit 1 to 2) | NA | NA | 7.3 (4.7) | 7.9 (3.8) |
| Alcohol use disorder, n (%) | ||||
| Alcohol abuse | 33 (45.8) | 32 (42.7) | 17 (53.1) | 19 (39.6) |
| Alcohol dependence | 39 (54.2) | 43 (57.3) | 15 (46.9) | 29 (60.4) |
| Drinking-related measures, mean (SD)** | ||||
| Drinks per day | 2.0 (1.5) | 2.0 (1.8) | 2.1 (1.3) | 2.6 (2.1) |
| Proportion of drinking days | 0.3 (0.2) | 0.3 (0.2) | 0.4 (0.2) | 0.4 (0.2) |
| Drinks per drinking day | 6.5 (2.9) | 6.7 (3.5) | 5.9 (2.8) | 6.2 (4.3) |
| Proportion of days using substances other than alcohol | 0.07 (0.2) | 0.04 (0.1) | 0.01 (0.0) | 0.03 (0.1) |
There were no significant between-group differences in baseline characteristics.
Baseline drinking was assessed from Visit 1 to 2 or for 3 weeks either preceding study entry or from the beginning of the current period of sobriety. Because drink numbers were tracked using the timeline followback method, drinking records at Visit 2 comprised the drinking baseline, N values are for study participants completing 12 weeks. Treatment-group differences in continuous measures were compared using an analysis-of-variance model containing terms for treatment and investigator. Treatment-group differences in categorical measures were compared using Fisher’s exact test.
AISRS – Adult ADHD Investigator Symptom Rating Scale; ASRS – Adult ADHD Self-Report Scale; NA – not available; OCDS – Obsessive Compulsive Drinking Scale; SD-standard deviation.
Predictive factors and ADHD/SUD symptom correlations
Symptoms of ADHD were significantly improved in the atomoxetine (vs. placebo) group (p= 0.003 for AISRS total score; p=0.010 for ASRS total score). Apart from lower baseline alcohol use correlating significantly with better ADHD and alcohol use outcomes during the study, no baseline or on-treatment variable significantly predicted either alcohol use or the response of ADHD to treatment. For instance, there was no significant correlation between the visit-to-visit change in ADHD symptoms and drinking behaviors during the study. There was no significant correlation between changes from Visit 1 to study endpoint in total number of drinks and the AISRS score (r=−0.057; p=0.12) or between these variables from Visit 3 to endpoint (r=0.071; p=0.13).
There was also no significant correlation between the AISRS item scores and the presence or absence of relapse to alcohol abuse in the atomoxetine group from Visit 3 to 14. On the other hand, statistically significant (positive) correlations between relapse to alcohol abuse and worsening ADHD symptoms (increased AISRS scores) were observed across 15 of 18 symptoms in the placebo group.
By inclusion criteria, no subject could be drinking at the baseline assessment. The limited-sobriety group (abstinent less than or equal to 4 consecutive days) consumed a greater mean number of drinks per day at each study visit compared with the stable sobriety group (abstinent >4 consecutive days; Figure 1). The mean number of daily drinks increased from baseline in both of these sobriety groups, with larger increases in the placebo (vs. atomoxetine) group (Table 3). Individuals in the stable-sobriety group who received atomoxetine experienced a mean (SD) increase in drinks per day of +0.19±1.66, compared with +0.71±2.01 for placebo within the stable-sobriety group. Corresponding values in the limited-sobriety group were not significantly different between atomoxetine and placebo (Table 2). Changes in drinks per day from baseline to endpoint were not significantly different (p=0.660) between the limited- and stable-sobriety groups or between the atomoxetine and placebo groups (p=0.487).
Figure 1.
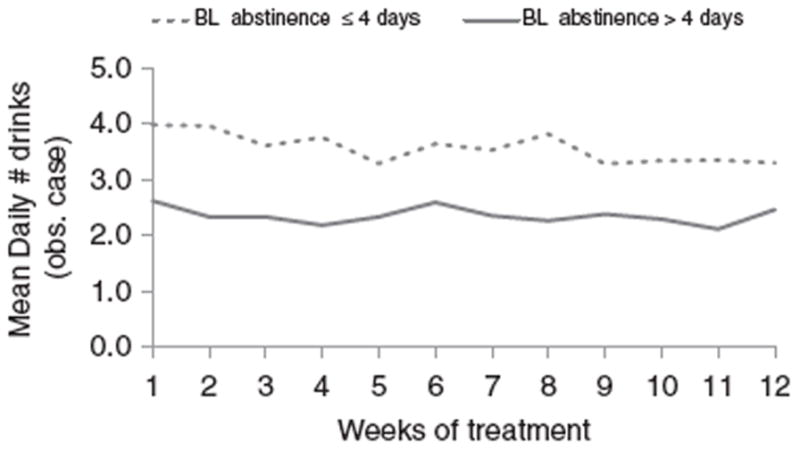
Alcohol use during study treatment stratified by duration of baseline abstinence, among randomized subjects (n = 71 with limited sobriety and n = 69 with stable sobriety). *p < 0.001 for overall abstinence group comparison after mixed-model repeated-measures analysis.
Table 3.
Change from baseline to Visit 14 (end of double-blind phase) in drinks per day and AISRS total score stratified by baseline duration of sobriety.
| Baseline Duration of Sobriety
|
Compare Sobriety. Group (p-value) | ||||||
|---|---|---|---|---|---|---|---|
| ≤4 consecutive days | >4 consecutive days | ||||||
|
| |||||||
| ATX (n = 17) | Placebo (n = 23) | Total (n = 40) | ATX (n = 15) | Placebo (n = 25) | Total (n = 40) | ||
| Drinks per day Baseline Mean (SD) | 2.25 (1.31) | 3.29 (2.51) | 2.85 (2.13) | 1.97 (1.23) | 1.93 (1.45) | 1.94 (1.36) | 0.0566 |
| Drinks per day Change from baseline to V14 Mean(SD) | 0.41 (2.51) | 0.49 (2.55) | 0.45 (2.49) | 0.19 (1.66) | 0.71 (2.01) | 0.51 (1.88) | 0.6602 |
| AISRS, Baseline Mean (SD) | 39.65 (7.01) | 40.43 (7.79) | 40.10 (7.38) | 38.93 (9.12) | 39.12 (7.08) | 39.05 (7.79) | 0.4221 |
| AISRS, Change from baseline to V14 Mean (SD) | −20.53 (11.32) | −12.70 (13.67) | −16.03 (13.16) | −13.27 (11.04) | −6.44 (10.02) | −9.00 (10.80) | 0.0790 |
| ASRS, Baseline Mean (SD) | 44.47 (8.59) | 49.04 (9.14) | 47.10 (9.09) | 50.60 (8.35) | 51.16 (9.39) | 50.95 (8.91) | 0.0781 |
| ASRS, Change from baseline to V14 Mean (SD) | −17.35 (9.43) | −12.78 (16.21) | −14.73 (13.78) | −17.00 (13.85) | −7.64 (11.51) | −11.00 (13.04) | 0.5821 |
ADHD – attention-deficit/hyperactivity disorder; AISRS – Adult ADHD Investigator Symptom Rating Scale; ATX – atomoxetine; SD – standard deviation; V14–Visit 14.
The limited-sobriety group experienced reductions (improvements) in AISRS from baseline to endpoint (Visit 14 or LOCF) of 20.53±11.32 in the atomoxetine group compared with a decrease of 12.70±13.67 in the placebo group (Table 3). Similar findings were noted in the stable-sobriety group, and ASRS values exhibited similar patterns (Table 3), with changes from baseline being nonsignificant across sobriety groups for changes in AISRS (p=0.079) or ASRS (p=0.582).
Improved ADHD symptoms from baseline to endpoint on the AISRS total score significantly correlated with reduced alcohol cravings on the OCDS in all subjects (r=0.28; 95% CI [0.11–0.43], p=0.002; Figure 2). This correlation was also significant in the atomoxetine group (r=0.29; CI [0.04–0.51]; p=0.023) but not the placebo group (r=0.24; CI [0.00–0.46]; p=0.055). However, these positive associations did not translate into a significant correlation in changes from baseline AISRS scores and actual drinking behaviors in the atomoxetine group as assessed by mean numbers of daily drinks at Visit 14. In addition to the significant positive (moderate) correlation between improvements on the AISRS and the OCDS total scores, there was also a significant correlation between improvements in the AISRS total score and the OCDS Obsessive subscale in the atomoxetine group and between the AISRS total score and the Compulsive subscale in the placebo group. In the base study24, the OCDS total score decreased (improved) from baseline to endpoint by 6.0 in subjects randomized to atomoxetine and 3.4 in those randomized to placebo (p=0.025). Corresponding data on the Obsessive subscale were decreases of 2.6 and 1.5 (p=0.023), respectively; and on the Compulsive subscale, decreases of 3.3 and 1.9 (p=0.097), respectively.
Figure 2.
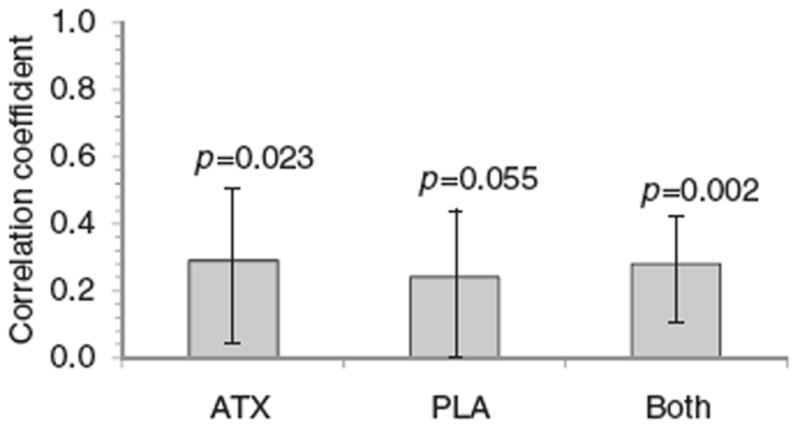
Changes in AISRS vs. changes in OCDS total scores from baseline to double-blind endpoint (or LOCF) among 61 study participants randomized to atomoxetine, and 64 randomized to placebo, who had nonmissing AISRS and OCDS data. Error bars represent 95% confidence intervals for the Pearson r correlation values. AISRS – Adult ADHD Investigator Symptom Rating Scale; ATX – atomoxetine; LOCF – last observation carried forward; OCDS – Obsessive Compulsive Drinking Scale; PLA – placebo.
Assessments of individual ADHD symptoms revealed that relapse to alcohol abuse during the trial correlated significantly (positively) with worsening on 15 of 18 items on the AISRS in the placebo group (Figure 3). All three of the items on which correlations of relapse to alcohol abuse to worsening ADHD symptoms were statistically nonsignificant (Hochberg-adjusted p>0.077) in the placebo group were on the Hyperactive–Impulsive (rather than Inattentive) subscale: item 10 (‘compelled to do things’), 12 (‘talk too much socially’), and 18 (‘interrupt others when they are busy’). There was no significant adjusted correlation between the change in any AISRS item and relapse to alcohol abuse in the atomoxetine group from Visit 3 to 14. In the overall population (both treatment groups), relapse to alcohol abuse correlated significantly with 6 items on the AISRS: difficulty with attention (item 3, on the Inattentive subscale; r=0.13; CI [0.05–0.21]; Hochberg-adjusted p=0.025); leaving seat early (item 4, on the Hyperactive–Impulsive subscale; r=0.15; CI [0.07–0.23]; p=0.004); trouble completing projects (item 7, on the Inattentive subscale; r=0.14; CI [0.06–0.21]; p=0.015); delaying starting tasks (item 11, on the Inattentive subscale; r=0.13; CI [0.05–0.21]; p=0.018); finishing other people’s sentences (item 14, on the Hyperactive–Impulsive subscale; r=0.13; CI [0.05–0.21]; p=0.019); and interrupting others when they are busy (item 18, on the Hyperactive–Impulsive subscale; r=0.13; CI [0.05–0.21]; p=0.027). Increases (worsening) in AISRS total score across all visits correlated significantly with increased drinking level (abstainer, non-heavy, heavy) in the overall population (r=0.12; CI [0.05–0.19]; p=0.001).
Figure 3.
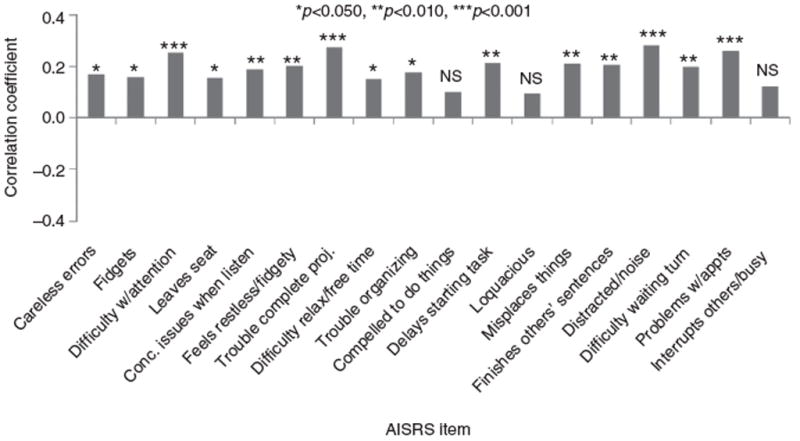
Changes in AISRS item scores vs. the presence or absence of relapse to alcohol abuse from Visit 3 to 14 (Week 12) in the placebo group (n = 63) after adjustment for multiple comparisons (relapse to alcohol abuse: ≥4 drinks/day × 1 day for women and ≥5 drinks/day × 1 day for men, or ≥3 drinks/day for ≥7 consecutive days for any adult). AISRS – Adult ADHD Investigator Symptom Rating Scale; NS – not statistically significant (i.e., p> 0.05); Conc. –concentration; w/– with; appts – appointments; proj. – projects.
Discussion
In this exploratory, hypothesis-generating subgroup analysis, no baseline characteristics emerged as robust predictors of outcomes for ADHD or SUD in adults with ADHD and alcohol abuse or dependence who were randomized in a 12-week, placebo-controlled study of atomoxetine24. There was a significant correlation between improvements in ADHD symptoms and reductions in alcohol cravings, although the associational nature of the present analysis does not permit us to determine directionality or whether cause–effect relationships were present.
Our data suggest a significant correlation between alcohol use by adults and changes in ADHD symptoms. Of note, individuals relapsing to alcohol abuse had significantly more worsening symptoms of ADHD in the placebo group but not the atomoxetine group. Our finding of worsening in 15 of 18 symptoms of ADHD among members of the placebo group who relapsed to alcohol abuse suggests that ADHD symptoms may be exacerbated by heavy drinking (although other causes of symptom worsening are also possible) and may have implications for the diagnosis and course of ADHD in adults with active alcohol use disorders. If replicated, these data might extend the notion of a need for some degree of patient sobriety to more effectively recognize ‘true’ ADHD symptoms in diagnostically naive patients.
The relative lack of symptom changes in heavily drinking adults while receiving atomoxetine compared to placebo suggests that treatment with atomoxetine may ‘uncouple’ the relationship between alcohol use and worsened ADHD symptoms. Moreover, previous studies demonstrated that adults with ADHD exhibited cognitive and behavioral deficits that could mimic alcohol impairment (e.g., on simulated-driving performance tests), and that atomoxetine was effective in attenuating other high-risk behaviors (e.g., tobacco use)48,49. Other studies of ADHD have shown less robust responses to treatment in patients having a dual diagnosis of comorbid ADHD and SUD compared with those experiencing ADHD but not SUD17–21,50. Participants in the present study were required to have had at least brief (≥4-day) abstinence to qualify for randomization, which may have affected the overall baseline severity of SUD. Despite this potential limitation, our data lend support to the clinical utility of stabilizing patients’ SUD before initiating ADHD treatment. However, given the bidirectional nature of the overlap between ADHD and SUD, it is also conceivable that some patients may benefit from having their ADHD stabilized initially or in the same overall time frame as their SUD51,52. Our results also support the need to treat more substantial comorbid conditions associated with ADHD to realize a more substantial effect on ADHD, although eligibility criteria in the base study24 excluded many potential subjects with psychiatric comorbidities.
The present findings share some similarities with data from the Combined Pharmacotherapies and Behavioral Interventions (COMBINE) study, which also analyzed only individuals who were recently abstinent (for 4–21 days) at study entry53. In this 1-year, multicenter, randomized controlled trial involving 1383 alcohol-dependent volunteers (median age=44 years), treatment including naltrexone was associated with significantly attenuated alcohol cravings (vs. other therapies). Other studies, in patients with ADHD, have demonstrated that treatment with stimulants and other agents can reduce cravings for (or abuse of) substances, stabilize substance use, and/or improve ADHD symptoms18,54,55.
One qualifier of the initial description of the base study for the present analysis24 was that it did not address the question of whether atomoxetine affects alcoholic drinking directly or does so indirectly via reductions in ADHD symptoms. The present data do not provide a clear or consistent answer to this important question. Although week-to-week changes in drinking behaviors and ADHD symptoms were not significantly correlated, on-treatment relapse to alcohol abuse was linked to worsening of most ADHD symptoms in the placebo but not the atomoxetine group. This latter finding suggests that atomoxetine’s effects on alcohol use may in part be mediated by improvements in ADHD symptoms (or vice versa). Research has begun to untangle the complex relationships between ADHD and alcohol use7,56–60.
In the present study, lower baseline alcohol use in the pre-randomization period correlated with better treatment outcomes (vs. higher baseline alcohol use). This finding of initially less severe alcohol use predicting better outcomes for ADHD and alcohol use during the study is consistent with other reported data. A dose-related effect of previous alcohol use in ‘priming’ alcohol-dependent or other drinkers to commit a greater degree of disinhibitory attentional control errors (i.e., Stroop interference effect) has been reported61–65.
Potential study limitations
As an exploratory, post-hoc subgroup analysis, this study was of a hypothesis-generating nature and potentially subject to certain biases, including the inherent pitfalls of self report. Most patients in the base study24 were men (85%) and Caucasian (88%). Further potentially limiting the generalizability of our findings, patients who had psychiatric comorbidities, including conduct disorder, bipolar disorder, major depressive disorder, and/or anxiety disorders – or who required psychotropic medications to treat them – were not eligible for inclusion in the base study24. Hence, the present analysis could not determine whether a history of, or treatment for, these conditions predicted later development or relapse to SUD. Of particular importance is the exclusion of patients with a history of conduct or bipolar disorder, which may increase the risk, and/or hasten the development, of SUD in patients with ADHD66. It is also not clear whether working-memory deficits and other cognitive difficulties, reduced selfesteem, and/or dysregulated executive function andmotivation associated with ADHD and comorbid anxiety might be potentiated by abuse of alcohol or other substances67. Anxiety as well as major-depressive, obsessive– compulsive, and bipolar disorders are commonly comorbid (and may share certain features) with ADHD6,68.
Among the 68 (32%) of the total of 215 patients who were screened but failed to be enrolled in the base study24, the mean age was significantly higher (39.7 years) compared with the randomized subjects (35.0 years; p=0.003 from an ANOVA). In the screening failure population, there was also a higher representation (vs. randomized subjects) of ethnic (Hispanics 9% vs. 6%) and racial (African 6% vs. 4%) minorities, but these differences were not statistically significant.
The present analysis evaluated baseline predictors of alcohol cravings but did not collect sufficient data related to age of onset of ADHD or SUD to assess this as a potential predictor or correlate of ADHD or SUD outcomes in adulthood. Other baseline characteristics were ascribed to minorities of study participants and/or were relatively evenly distributed overall in the base study; hence, analyses of these smaller populations were not expected to be informative. For instance, 32% (24/75) of individuals randomized to placebo and 40% (29/72) to atomoxetine had previous experience with stimulants; and a total of approximately 41% (31/75) and 44% (32/72), respectively, had family histories of ADHD24.
The base study also was not designed to compare ADHD treatment outcomes between subjects who had different ADHD subtypes or alcohol abuse or dependence, or between atomoxetine and psychostimulants. It is not surprising that the majority of subjects (123/147; 83.7%) in the base study had the combined subtype because this category subsumes both the hyperactive–impulsive and inattentive subtypes. Subjects with the combined subtype typically have higher degrees of psychiatric comorbidity; those with the hyperactive–impulsive subtype (2/147;1.4% in the base study) may be considered to be, on average, more reckless (or less risk averse) and thus perhaps more likely to experiment with alcohol or other substances; whereas those with the inattentive subtype (21/147; 14.3%; 1 subject did not have a childhood ADHD history) might be expected to have an increased likelihood of using a substance chiefly for stimulation or increased alertness7,69. We did not find any significant associations between changes in ADHD and SUD outcomes as a function of baseline ADHD subtype, although, as mentioned above, numbers of subjects in the hyperactive– impulsive and inattentive subgroups (n=23) were small.
Distributions of subjects with alcohol abuse or dependence in the atomoxetine and placebo groups at baseline were not significantly different, although somewhat higher proportions of patients in each group had alcohol dependence (vs. abuse): 54.2% vs. 45.8% in the atomoxetine group and 57.3% vs. 42.7% in the placebo group24. Among subjects completing the base clinical trial and eligible for analysis in the present study, disparities in proportions of individuals with alcohol dependence (vs. abuse) were wider in the placebo group (60.4% alcohol dependent vs. 39.6% alcohol abusing), and changed direction in the atomoxetine group (53.1% alcohol abusing vs. 46.9% alcohol dependent) compared with baseline. It is not clear how such discrepancies might have affected associations between changes in ADHD symptoms and either alcohol cravings or drinking behaviors in the two treatment groups. If possible, future studies should evaluate such associations in groups with more even baseline distributions of alcohol abuse or dependence at baseline. In addition, categorical analyses of numbers (%) of subjects switching from baseline alcohol abuse to on-treatment alcohol dependence (or vice versa) according to treatment groups might also be warranted in future research as a means of evaluating the potential effects of different treatments (or placebo) on these important alcohol outcome measures.
Some reports have documented favorable ADHD treatment outcomes after stimulant treatment in substance abusing populations, but issues of misuse and diversion may limit the utility of such therapy in adults with dual diagnoses70–73. Cognitive or behavioral interventions apart from traditional 12-step programs were not permitted in the base study24. However, numbers (%) of study participants attending such programs, and/or numbers (%) of 12-step program sessions attended, in the atomoxetine and placebo groups were not recorded.
In general, patient self-reporting bias can result in under-reporting of certain socially unacceptable behaviors or practices. On the other hand, most participants in our study reported substance use at some time during the treatment period, and self-report is considered to be a viable method of assessing SUD outcomes. Other studies (e.g., COMBINE) used objective measures of alcohol use, such as altered production of abnormal serum transferrin protein because of alcohol consumption53, that would have strengthened our study. Subject responder bias (e.g. Hawthorne effect), with potential benefits attributable to participation in a study per se, may have been present, given that subjects with either decreases or increases in AISRS scores drank slightly less: a median of 1.5 fewer drinks per week in those with improvements in ADHD symptoms and 1 fewer in those with worsening symptoms.
Our posthoc definitions of stable-sobriety (>4 consecutive days of abstinence) and limited-sobriety (≤4 consecutive days of abstinence) populations were of an ‘operational’ and, hence, somewhat arbitrary nature. The diagnostic utility of a 4-day hiatus in alcohol use to determine the stability of sobriety needs to be validated in further studies. Other trials involving pharmacologic and behavioral management of alcohol dependence have used a minimum of 4 days of abstinence as a study inclusion criterion53.
Also supporting our definitions of relapse to alcohol abuse is epidemiologic research demonstrating a linear association between the number of days that an individual consumes ≥5 drinks, or the mean number of drinks that he or she consumes per day, and alcohol dependence according to DSM-IV definitions. For instance, in one study, respondents who self-reported daily consumption of ≥5 drinks were at about 6-fold higher risk of being dependent on alcohol compared with their counterparts who consumed less alcohol74. Subjects with relapse drinking – defined as ≥5 drinks for men, or ≥4 drinks for women, on 2 consecutive days – also exhibited initially decreased (improved) OCDS scores on treatment which then increased (worsened) over time, whereas their abstinent counterparts experienced lower OCDS scores throughout the 12-week treatment phase43.
In associational research such as the present analysis, the causal nature of findings, or the direction of causality, cannot be unambiguously inferred. For instance, the present study could not determine whether (or to what extent) symptom worsening on 15 of 18 items of the AISRS in the placebo group was a cause – or result – of increased drinking or some other factor or combination of factors. For the purposes of this correlational analysis, study participants who exhibited heavy drinking or relapse to alcohol abuse on treatment (i.e., overlapping categories) were combined into a single category (heavy drinking). Further, we did not evaluate possible correlations between changes in the total AISRS score and relapse to alcohol abuse (and/or heavy drinking) but rather between changes in each of 18 individual ADHD items and relapse. The finding that subjects receiving placebo who had persistent ADHD symptoms also experienced poorer alcohol related outcomes may be partly ascribed to the fact that many individuals with alcohol use disorders also manifest attentional problems secondary to previous or current heavy drinking.
Although a higher proportion of subjects randomized to atomoxetine in the base study24 experienced adverse events (9.7%) compared with placebo (2.7%), the main reason for premature discontinuation in each treatment group was ‘lost to follow-up’, in 26.4% of subjects randomized to atomoxetine and 24.0% of those randomized to placebo. A higher patient attrition rate from loss to follow-up in both the atomoxetine and placebo groups might be expected in a population of former alcohol users compared with their abstinent counterparts and may be secondary to other, unmeasured or uncontrolled variables (e.g., education level, socioeconomic status). The base study24 determined that there were no significant differences between completers and noncompleters in terms of age, gender, average number of drinks per drinking day, family history of ADHD, or AISRS total score.
The number of individuals abstaining from alcohol during the study (n=4; 2 each in the atomoxetine and placebo groups) was too small to infer trends in ADHD symptoms and either alcohol cravings or drinking behaviors among initial abstainers. Our criteria for relapse to alcohol abuse and heavy drinking overlapped, precluding precise differentiation between individuals in these categories. The base study did not assess potential effects of atomoxetine (vs. placebo) on the use of other substances (e.g. nicotine, caffeine, drugs of abuse, psychoactive drugs) or on ADHD remission in adults with comorbid SUD, although these are potentially viable lines of future inquiry59,75,76. A recent study determined that atomoxetine decreased a range of high-risk behaviors, including nicotine use, in adolescents with ADHD49. Finally, although correlations between changes in AISRS item scores and the presence or absence of relapse to alcohol abuse were adjusted for multiple comparisons by the Hochberg method46, other statistical tests were not adjusted for multiplicity.
Conclusions
Apart from the baseline level of sobriety, no baseline characteristic was identified as a significant predictor of alcohol use or ADHD treatment outcomes. However, reduced cravings for alcohol from baseline significantly correlated with improved ADHD symptoms in all subjects, especially those receiving atomoxetine. On-study relapse to alcohol abuse significantly correlated with worsening ADHD symptoms in the placebo group. Further studies are warranted to: 1) substantiate our findings that atomoxetine is effective in managing ADHD symptoms in adults with comorbid SUD; 2) identify other potential mediators or moderators of a favorable ADHD or alcohol treatment outcome in these patients; 3) elucidate mechanisms by which atomoxetine treatment might attenuate alcohol cravings or use in adults with ADHD; and 4) better understand the confound that active SUD may partake on ADHD symptoms.
Acknowledgments
Editorial assistance was provided by Ann C. Sherwood, PhD, and Lauren Baker, PhD, Rete Biomedical Communications Corp. (Wyckoff, NJ), with financial support from Eli Lilly and Co.
Footnotes
Study guarantor: T.E.W. had been involved in the design and training of the original trial, has had access to all data, drafted and edited the manuscript, and takes responsibility for the study.
Previous presentation: selected findings were presented at the 57th Annual Meeting of the American Academy of Child and Adolescent Psychiatry, New York, NY, October 26–31, 2010.
Author contributions - category 1 (a) conception and design: T.E.W, L.A.A., H.P.U. (b) Acquisition of data: T.E.W., L.A.A., and other clinical investigators. (c) Analysis and interpretation of data: all authors. Category 2 (a) drafting the article: T.E.W. drafted the initial outline and manuscript. S.W.G. further revised the manuscript draft and provided further editorial assistance along with Ann C. Sherwood, PhD, and Lauren Baker, PhD (Rete Biomedical Communications Corp., Wyckoff, NJ, USA). (b) Revising it for intellectual content: all authors. Category 3 (a) final approval of the completed article: All authors.
Declaration of funding
This study and the present communication were supported by Eli Lilly and Co. (Indianapolis, IN, USA) and a National Institute on Drug Abuse grant to T.E.W. (#K24 DA016264).
Declaration of financial/other relationships
T.E.W. has disclosed that he receives or has received grant support from the following sources: Abbott, McNeil, Lilly, Nextwave, National Institutes of Health/National Institute of Drug Addiction (NIH/NIDA), Merck, and Shire; has been a speaker for Lilly, McNeil, Novartis, and Shire; and is or has been a consultant for Abbott, AstraZeneca, McNeil, Lilly, Nextwave, NIH, Novartis, Merck, and Shire. T.E.W. has a published book with Guilford Press: Straight Talk About Psychiatric Medications for Kids. L.A.A. has disclosed that, in the past 3 years, he has received grant/research support from Abbott, Bristol-Myers Squibb, Merck, Novartis, Pfizer, Shire, Lilly, Ortho-McNeil/Janssen/Johnson & Johnson, New River Pharmaceuticals, Cephalon, NIDA, Chelsea Therapeutics, and Organon; served on advisory boards and as a consultant to Abbott, Cortex Pharmaceuticals, Novartis, Pfizer, Shire, Lilly, Ortho McNeil/Janssen/Johnson & Johnson, Merck, Organon, sanofi-aventis, Psychogenics, Mindsite (uncompensated), AstraZeneca, Major League Baseball, and i3 Research; and has served as a consultant to Epi-Q, INC Research, United BioSource, Otsuka, and the Major League Baseball Players Association. L.A.A. has served on speakers bureaus for Shire, Ortho-McNeil/Janssen/Johnson & Johnson, and Lilly (but does not currently serve on any speakers bureaus) and has received royalty payments (as inventor) from NYU for license of adult ADHD scales and training materials. He has no stock ownership. Y.T. and H.P.U. are employees of and shareholders in Lilly. F.X. and D.N.D. are paid consultants to Lilly and/or its affiliates. When the study was conducted and early drafts of the manuscript prepared, D.N.D. was an employee of, and minor shareholder in, the study sponsor. S.W.G. is a paid consultant to Lilly (and its affiliates) and BioBehavioral Diagnostics (Westford, MA, and Plymouth Meeting, PA, USA).
Contributor Information
Timothy E. Wilens, Massachusetts General Hospital, Boston, MA, USA
Lenard A. Adler, New York University School of Medicine and VA New York Harbor Healthcare Service, New York, NY, USA
Yoko Tanaka, Lilly Research Laboratories, Eli Lilly and Company, Indianapolis, IN, USA.
Feng Xiao, 3 Statprobe, Cary, NC, USA.
Deborah N. D’Souza, 3 Statprobe, Cary, NC, USA
Stephen W. Gutkin, Rete Biomedical Communications Corp., Wyckoff, NJ, USA
Himanshu P. Upadhyaya, Lilly Research Laboratories, Eli Lilly and Company, Indianapolis, IN, USA
References
- 1.Barkley RA, Fischer M, Smallish L, et al. Young adult outcome of hyperactive children: adaptive functioning in major life activities. J Am Acad Child Adolesc Psychiatry. 2006;45:192–202. doi: 10.1097/01.chi.0000189134.97436.e2. [DOI] [PubMed] [Google Scholar]
- 2.Wilens TE, Dodson W. A clinical perspective of attention-deficit/hyperactivity disorder into adulthood. J Clin Psychiatry. 2004;65:1301–13. doi: 10.4088/jcp.v65n1003. [DOI] [PubMed] [Google Scholar]
- 3.Mannuzza S, Klein RG, Bonagura N, et al. Hyperactive boys almost grown up. V. Replication of psychiatric status. Arch Gen Psychiatry. 1991;48:77–83. doi: 10.1001/archpsyc.1991.01810250079012. [DOI] [PubMed] [Google Scholar]
- 4.Biederman J, Faraone SV, Spencer T, et al. Patterns of psychiatric comorbidity, cognition, and psychosocial functioning in adults with attention deficit hyperactivity disorder. Am J Psychiatry. 1993;150:1792–8. doi: 10.1176/ajp.150.12.1792. [DOI] [PubMed] [Google Scholar]
- 5.McGough JJ, Smalley SL, McCracken JT, et al. Psychiatric comorbidity in adult attention deficit hyperactivity disorder: findings from multiplex families. Am J Psychiatry. 2005;162:1621–7. doi: 10.1176/appi.ajp.162.9.1621. [DOI] [PubMed] [Google Scholar]
- 6.Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716–23. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohlmeier MD, Peters K, Te Wildt BT, et al. Comorbidity of alcohol and substance dependence with attention-deficit/hyperactivity disorder (ADHD) Alcohol Alcohol. 2008;43:300–4. doi: 10.1093/alcalc/agn014. [DOI] [PubMed] [Google Scholar]
- 8.Biederman J, Wilens T, Mick E, et al. Psychoactive substance use disorders in adults with attention deficit hyperactivity disorder (ADHD): effects of ADHD and psychiatric comorbidity. Am J Psychiatry. 1995;152:1652–8. doi: 10.1176/ajp.152.11.1652. [DOI] [PubMed] [Google Scholar]
- 9.Disney ER, Elkins IJ, McGue M, Iacono WG. Effects of ADHD, conduct disorder, and gender on substance use and abuse in adolescence. Am J Psychiatry. 1999;156:1515–21. doi: 10.1176/ajp.156.10.1515. [DOI] [PubMed] [Google Scholar]
- 10.Wilens TE, Biederman J, Mick E, et al. Attention deficit hyperactivity disorder (ADHD) is associated with early onset substance use disorders. J Nerv Ment Dis. 1997;185:475–82. doi: 10.1097/00005053-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Schubiner H, Tzelepis A, Milberger S, et al. Prevalence of attention-deficit/ hyperactivity disorder and conduct disorder among substance abusers. J Clin Psychiatry. 2000;61:244–51. doi: 10.4088/jcp.v61n0402. [DOI] [PubMed] [Google Scholar]
- 12.Kalpag AS, Levin FR. Adult ADHD and substance abuse: diagnostic and treatment issues. Substance Use Misuse. 2005;40:13–14. doi: 10.1080/10826080500294858. [DOI] [PubMed] [Google Scholar]
- 13.Wilens TE. Attention-deficit/hyperactivity disorder and the substance use disorders: the nature of the relationship, subtypes at risk, and treatment issues. Psychiatr Clin North Am. 2004;27:283–301. doi: 10.1016/S0193-953X(03)00113-8. [DOI] [PubMed] [Google Scholar]
- 14.Wilens TE. Attention deficit hyperactivity disorder and substance use disorders. Am J Psychiatry. 2006;163:2059–63. doi: 10.1176/ajp.2006.163.12.2059. [DOI] [PubMed] [Google Scholar]
- 15.Carroll KM, Rounsaville BJ. History and significance of childhood attention deficit disorder in treatment-seeking cocaine abusers. Compr Psychiatry. 1993;34:75–82. doi: 10.1016/0010-440x(93)90050-e. [DOI] [PubMed] [Google Scholar]
- 16.Wilens TE, Biederman J, Mick E. Does ADHD affect the course of substance abuse? Findings from a sample of adults with and without ADHD. Am J Addict. 1998;7:156–63. [PubMed] [Google Scholar]
- 17.Adler LA, Guida F, Irons S, et al. Open label pilot study of atomoxetine in adults with ADHD and substance use disorder. J Dual Diagn. 2010;6:196–207. [Google Scholar]
- 18.Schubiner H, Saules KK, Arfken CL, et al. Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Exp Clin Psychopharmacol. 2002;10:286–94. doi: 10.1037//1064-1297.10.3.286. [DOI] [PubMed] [Google Scholar]
- 19.Levin FR, Evans SM, Brooks DJ, et al. Treatment of methadone-maintained patients with adult ADHD: double-blind comparison of methylphenidate, bupropion and placebo. Drug Alcohol Depend. 2006;81:137–48. doi: 10.1016/j.drugalcdep.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Levin FR, Evans SM, Brooks DJ, et al. Treatment of cocaine dependent treatment seekers with adult ADHD: double-blind comparison of methylphenidate and placebo. Drug Alcohol Depend. 2007;87:20–9. doi: 10.1016/j.drugalcdep.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Levin FR, Mariani JJ, Secora A, et al. Atomoxetine treatment for cocaine abuse and adult attention-deficit hyperactivity disorder (ADHD): a preliminary open trial. J Dual Diagn. 2009;5:41–56. doi: 10.1080/15504260802628767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carpentier PJ, de Jong CA, Dijkstra BA, et al. A controlled trial of methylphenidate in adults with attention deficit/hyperactivity disorder and substance use disorders. Addiction. 2005;100:1868–74. doi: 10.1111/j.1360-0443.2005.01272.x. [DOI] [PubMed] [Google Scholar]
- 23.Upadhyaya HP, Brady KT, Sethuraman G, et al. Venlafaxine treatment of patients with comorbid alcohol/cocaine abuse and attention-deficit/hyperactivity disorder: a pilot study. J Clin Psychopharmacol. 2001;21:116–18. doi: 10.1097/00004714-200102000-00025. [DOI] [PubMed] [Google Scholar]
- 24.Wilens TE, Adler LA, Weiss MD, et al. Atomoxetine ADHD/SUD Study Group. Atomoxetine treatment of adults with ADHD and comorbid alcohol use disorders. Drug Alcohol Depend. 2008;96:145–54. doi: 10.1016/j.drugalcdep.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Adler L, Wilens T, Zhang S, et al. Retrospective safety analysis of atomoxetine in adult ADHD patients with or without comorbid alcohol abuse and dependence. Am J Addict. 2009;18:393–401. doi: 10.3109/10550490903077663. [DOI] [PubMed] [Google Scholar]
- 26.Michelson D, Adler L, Spencer T, et al. Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry. 2003;53:112–20. doi: 10.1016/s0006-3223(02)01671-2. [DOI] [PubMed] [Google Scholar]
- 27.US Department of Health and Human Services. US Department of Agriculture. Dietary Guidelines for Americans. 6. Washington, DC: US Government Printing Office; 2005. [Google Scholar]
- 28.Borges G, Cherpitel CJ, Mondragón L, et al. Episodic alcohol use and risk of nonfatal injury. Am J Epidemiol. 2004;159:565–71. doi: 10.1093/aje/kwh073. [DOI] [PubMed] [Google Scholar]
- 29.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. 4. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- 30.Adler L, Cohen J. Diagnosis and evaluation of adults with attention-deficit/ hyperactivity disorder. Psychiatr Clin North Am. 2004;27:187–201. doi: 10.1016/j.psc.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Adler L, Spencer T. The Adult ADHD Clinical Diagnostic Scale (ACDS) Vol. 1.2. New York: New York University School of Medicine; 2004. [Google Scholar]
- 32.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 35.Romera I, Perez V, Menchon JM, et al. Optimal cutoff point of the Hamilton Rating Scale for Depression according to normal levels of social and occupational functioning. Psychiatry Res. 2011;186:133–7. doi: 10.1016/j.psychres.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 36.Vaccarino AL, Evans KR, Sills TL, Kalali AH. Symptoms of anxiety in depression: assessment of item performance of the Hamilton Anxiety Rating Scale in patients with depression. Depress Anxiety. 2008;25:1006–13. doi: 10.1002/da.20435. [DOI] [PubMed] [Google Scholar]
- 37.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV TR Axis I Disorders, Research Edition, Patient Version (SCIDI/P) New York, NY: Biometrics Research Dept., New York State Psychiatric Institute; 2002. [Google Scholar]
- 38.World Medical Association. Declaration of Helsinki: Recommendations Guiding Medical Doctors in Biomedical Research Involving Human Subjects. 5. 2000. revision. [Google Scholar]
- 39.Spencer TJ, Adler LA, Qiao M, et al. Validation of the adult ADHD investigator symptom rating scale (AISRS) J Atten Disord. 2010;14:57–68. doi: 10.1177/1087054709347435. [DOI] [PubMed] [Google Scholar]
- 40.Kessler RC, Adler L, Ames M, et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med. 2005;35:245–56. doi: 10.1017/s0033291704002892. [DOI] [PubMed] [Google Scholar]
- 41.Sobell LC, Sobell MB. Timeline followback: a technique for assessing selfreported alcohol consumption. In: Allen J, Litten R, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- 42.Anton RF, Moak D, Latham PK. The obsessive compulsive drinking scale: a new method of assessing outcome in alcoholism treatment studies. Arch Gen Psychiatry. 1996;53:225–31. doi: 10.1001/archpsyc.1996.01830030047008. [DOI] [PubMed] [Google Scholar]
- 43.Anton RF. Obsessive–compulsive aspects of craving: development of the Obsessive Compulsive Drinking Scale. Addiction. 2000;95 (Suppl 2):S211–17. doi: 10.1080/09652140050111771. [DOI] [PubMed] [Google Scholar]
- 44.Anton RF, Moak DH, Latham P. The Obsessive Compulsive Drinking Scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol Clin Exp Res. 1995;19:92–9. doi: 10.1111/j.1530-0277.1995.tb01475.x. [DOI] [PubMed] [Google Scholar]
- 45.Bohn MJ, Barton BA, Barron KE. Psychometric properties and validity of the obsessive–compulsive drinking scale. Alcohol Clin Exp Res. 1996;20:817–23. doi: 10.1111/j.1530-0277.1996.tb05257.x. [DOI] [PubMed] [Google Scholar]
- 46.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–18. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 47.Hopkins K, Glass GV. Statistical Methods in Education and Psychology. 3. Columbus, OH: Pearson Allyn & Bacon; 1995. [Google Scholar]
- 48.Weafer J, Camarillo D, Fillmore MT, et al. Simulated driving performance of adults with ADHD: comparisons with alcohol intoxication. Exp Clin Psychopharmacol. 2008;16:251–63. doi: 10.1037/1064-1297.16.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saylor K, Williams DW, Schuh KJ, et al. Effects of atomoxetine on selfreported high-risk behaviors and health-related quality of life in adolescents with ADHD. Curr Med Res Opin. 2010;26:2087–95. doi: 10.1185/03007995.2010.493747. [DOI] [PubMed] [Google Scholar]
- 50.Thurstone C, Riggs PD, Salomonsen-Sautel S, et al. Randomized, controlled trial of atomoxetine for attention-deficit/hyperactivity disorder in adolescents with substance use disorder. J Am Acad Child Adolesc Psychiatry. 2010;49:573–82. doi: 10.1016/j.jaac.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riggs PD. Clinical approach to treatment of ADHD in adolescents with substance use disorders and conduct disorder. J Am Acad Child Adolesc Psychiatry. 1998;37:331–2. doi: 10.1097/00004583-199803000-00019. [DOI] [PubMed] [Google Scholar]
- 52.Wilens TE. The nature of the relationship between attention-deficit/ hyperactivity disorder and substance use. J Clin Psychiatry. 2007;68 (Suppl 11):4–8. [PubMed] [Google Scholar]
- 53.Anton RF, O’Malley SS, Ciraulo DA, et al. COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–17. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 54.Levin FR, Evans SM, McDowell DM, Kleber HD. Methylphenidate treatment for cocaine abusers with adult attention-deficit/hyperactivity disorder: a pilot study. J Clin Psychiatry. 1998;59:300–5. doi: 10.4088/jcp.v59n0605. [DOI] [PubMed] [Google Scholar]
- 55.Riggs PD, Leon SL, Mikulich SK, Pottle LC. An open trial of bupropion for ADHD in adolescents with substance use disorders and conduct disorder. J Am Acad Child Adolesc Psychiatry. 1998;37:1271–8. doi: 10.1097/00004583-199812000-00010. [DOI] [PubMed] [Google Scholar]
- 56.Weafer J, Filmore MT, Milich R. Increased sensitivity to the disinhibiting effects of alcohol in adults with ADHD. Exp Clin Psychopharmacol. 2009;17:113–21. doi: 10.1037/a0015418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knop J, Penick EC, Nickel EJ, et al. Childhood ADHD and conduct disorder as independent predictors of male alcohol dependence at age 40. J Stud Alcohol Drugs. 2009;70:169–77. doi: 10.15288/jsad.2009.70.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lopez B, Schwartz SJ, Prado G, et al. Correlates of early alcohol and drug use in Hispanic adolescents: examining the role of ADHD with comorbid conduct disorder, family, school, and peers. J Clin Child Adolesc Psychol. 2008;37:820–32. doi: 10.1080/15374410802359676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohlmeier MD, Peters K, Kordon A, et al. Nicotine and alcohol dependence in patients with comorbid attention-deficit/hyperactivity disorder (ADHD) Alcohol Alcohol. 2007;42:539–43. doi: 10.1093/alcalc/agm069. [DOI] [PubMed] [Google Scholar]
- 60.Comings DE. Genetic factors in substance abuse based on studies of Tourette syndrome and ADHD probands and relatives. II. Alcohol abuse Drug Alcohol Depend. 1994;35:17–24. doi: 10.1016/0376-8716(94)90105-8. [DOI] [PubMed] [Google Scholar]
- 61.Field M, Duka T. Cues paired with a low dose of alcohol acquire conditioned incentive properties in social drinkers. Psychopharmacology (Berl) 2002;159:325–34. doi: 10.1007/s00213-001-0923-z. [DOI] [PubMed] [Google Scholar]
- 62.Sharma D, Albery IP, Cook C. Selective attentional bias to alcohol related stimuli in problem drinkers and non-problem drinkers. Addiction. 2001;96:285–95. doi: 10.1046/j.1360-0443.2001.96228512.x. [DOI] [PubMed] [Google Scholar]
- 63.Townshend JM, Duka T. Patterns of alcohol drinking in a population of young social drinkers: a comparison of questionnaires and diary measures. Alcohol Alcohol. 2002;37:187–92. doi: 10.1093/alcalc/37.2.187. [DOI] [PubMed] [Google Scholar]
- 64.Abroms BD, Gottlob LR, Filmore MT. Alcohol effects on inhibitory control of attention: distinguishing between intentional and automatic mechanisms. Psychopharmacology (Berl) 2006;188:324–34. doi: 10.1007/s00213-006-0524-y. [DOI] [PubMed] [Google Scholar]
- 65.Barkley RA, Murphy KR, O’Connell T, et al. Effects of two doses of alcohol on simulator driving performance in adults with attention-deficit/hyperactivity disorder. Neuropsychology. 2006;20:77–87. doi: 10.1037/0894-4105.20.1.77. [DOI] [PubMed] [Google Scholar]
- 66.Biederman J, Wilens T, Mick E, et al. Is ADHD a risk factor for psychoactive substance use disorders? Findings from a four-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1997;36:21–9. doi: 10.1097/00004583-199701000-00013. [DOI] [PubMed] [Google Scholar]
- 67.Schatz DB, Rostain AL. ADHD with comorbid anxiety: a review of the current literature. J Atten Disord. 2006;10:141–9. doi: 10.1177/1087054706286698. [DOI] [PubMed] [Google Scholar]
- 68.Kunwar A, Dewan M, Faraone SV. Treating common psychiatric disorders associated with attention-deficit/hyperactivity disorder. Expert Opin Pharmacother. 2007;8:555–62. doi: 10.1517/14656566.8.5.555. [DOI] [PubMed] [Google Scholar]
- 69.Wilens TE, Biederman J, Faraone SV, et al. Presenting ADHD symptoms, subtypes, and comorbid disorders in clinically referred adults with ADHD. J Clin Psychiatry. 2009;70:1557–62. doi: 10.4088/JCP.08m04785pur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mariani JJ, Levin FR. Treatment strategies for co-occurring ADHD and substance use disorders. Am J Addict. 2007;16 (Suppl 1):45–54. doi: 10.1080/10550490601082783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schubiner H, Tzelepis A, Isaacson JH, et al. The dual diagnosis of attentiondeficit/ hyperactivity disorder and substance abuse: case reports and literature review. J Clin Psychiatry. 1995;56:146–50. [PubMed] [Google Scholar]
- 72.Vitiello B. Long-term effects of stimulant medications on the brain: possible relevance to the treatment of attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol. 2001;11:25–34. doi: 10.1089/104454601750143384. [DOI] [PubMed] [Google Scholar]
- 73.Wilens TE, Adler LA, Adams J, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47:21–31. doi: 10.1097/chi.0b013e31815a56f1. [DOI] [PubMed] [Google Scholar]
- 74.Caetano R, Tam T, Greenfield T, et al. DSM-IV alcohol dependence and drinking in the U.S. population: a risk analysis. Ann Epidemiol. 1997;7:542–9. doi: 10.1016/s1047-2797(97)00114-2. [DOI] [PubMed] [Google Scholar]
- 75.Dickson RA, Maki E, Gibbins C, et al. Time courses of improvement and symptom remission in children treated with atomoxetine for attention-deficit/ hyperactivity disorder: analysis of Canadian open-label studies. Child Adolesc Psychiatry Ment Health. 2011;5:14. doi: 10.1186/1753-2000-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Steele M, Jensen PS, Quinn DM. Remission versus response as the goal of therapy in ADHD: a new standard for the field? Clin Ther. 2006;28:1892–908. doi: 10.1016/j.clinthera.2006.11.006. [DOI] [PubMed] [Google Scholar]


