Abstract
Background
The peripheral nervous system has an inherent capability to tolerate the gliding (excursion), stretching (increased strain), and compression associated with limb motions necessary for functional activities. The biomechanical properties during joint movements are well studied but the influence of other factors such as limb pre-positioning, age and the effects of diabetes mellitus are not well established for the lower extremity. The purposes of this pilot study were to compare the impact of two different hip positions on lower extremity nerve biomechanics during an active ankle dorsiflexion motion in healthy individuals and to determine whether nerve biomechanics are altered in older individuals with diabetes mellitus.
Methods
Ultrasound imaging was used to quantify longitudinal motion of the tibial nerve and transverse plane motion of the tibial and common fibular nerves in the popliteal fossa during active ankle movements.
Findings
In healthy individuals, ankle dorsiflexion created mean tibial nerve movement of 2.18 millimeters distally, 1.36 millimeters medially and 3.98 millimeters superficially. When the hip was in a flexed position there was a mean three-fold reduction in distal movement. In people with diabetes mellitus there was significantly less distal movement of the tibial nerve in the neutral hip position and less superficial movement of the nerve in both hip positions compared to healthy individuals.
Interpretation
We have documented reductions in tibial nerve excursion due to limb pre-positioning thought to pre-load the nervous system using a non-invasive methodology. Thus, lower limb pre-positioning impacts nerve biomechanics during ankle motions common in functional activities. Additionally, our findings indicate that nerve biomechanics have the potential to be altered in older individuals with diabetes mellitus compared to younger healthy individuals.
Keywords: Nerve Biomechanics, Ultrasound Imaging, Diabetes Mellitus
INTRODUCTION
The peripheral nervous system has an inherent capacity to tolerate significant limb motion. In part, the nerves are capable of gliding (excursion) relative to adjacent tissues, stretching (increased strain) when exposed to tensile stress and deforming in shape when exposed to compressive stresses. These properties are often referred to as nerve biomechanics (Grewal et al., 1996, Topp and Boyd, 2006). Functional activities require significant lower extremity motion of the hips, knees and ankles. Nerve biomechanics during hip flexion includes increased strain in L4-S1 spinal nerves (Smith et al., 1993), sciatic nerve in the posterior thigh (Babbage et al., 2007, Boyd et al., 2005, Coppieters et al., 2006), and tibial nerve at the knee and ankle (Coppieters et al., 2006). In addition, hip flexion induces measureable distal excursion (means 0.48-2.5 mm) of the L4-S1 spinal nerves (Brieg and Troup, 1979, Smith et al., 1993, Gilbert et al., 2007) and proximal excursion (means 2.4-28.0 mm) of the sciatic nerve in the thigh (Boyd et al., 2005, Coppieters et al., 2006) and tibial nerve at the knee (mean 12.2 mm) and ankle (mean 6.4 mm) (Coppieters et al., 2006).
Ankle dorsiflexion has also been shown to increase strain in the tibial nerve at the ankle (Boyd et al., 2005, Coppieters et al., 2006, Alshami et al., 2008), while creating distal excursion of the tibial nerve at the knee (Coppieters et al., 2006, Ellis et al., 2008) and ankle (Coppieters et al., 2006). Several studies have investigated the impact of pre-positioning the limb on nerve biomechanics in the upper limb (Julius et al., 2004, Coppieters and Butler, 2008, Dilley et al., 2003), however, little is known about how limb pre-positioning affects nerve biomechanics in the lower limb. While numerous studies have examined the clinical alterations associated with limb pre-positioning (Boyd et al., 2009, Boyd et al., 2010, Herrington et al., 2008), there is additional value in understanding the underlying impact on nerve biomechanics.
In addition to limb positioning, it can be hypothesized that other factors may alter nerve biomechanics such as age or Diabetes Mellitus (DM). If there was inadequate nerve mobility, functional activities that require these lower limb movements might expose the nervous system to excessive mechanical stress. For example, if the tibial nerve does not have adequate capacity to glide with hip flexion and ankle dorsiflexion, the nerve may be exposed to excessive levels of strain (tension) during activities such as walking when the limb is advanced during stride, which may contribute to movement induced neuropathic symptoms. Such a biomechanical influence is purely speculative at this stage. However, understanding the impact of limb positioning on nerve biomechanics in various groups of individuals may help to support or negate this hypothesis. The purpose of this study was to compare the impact of two different hip positions on lower extremity nerve biomechanics during an active ankle dorsiflexion motion in healthy individuals and then to determine whether nerve biomechanics are altered in older individuals with DM.
METHODS
Participants included people with Type 2 DM (n=5) and people without diabetes (n=5). Participants were required to have hip flexion ≥90°, full knee extension, ankle dorsiflexion ≥0° and plantar flexion ≥30°, assessed by visual inspection of active range of motion. Exclusion criteria included low back or leg pain lasting >3 consecutive days in the past 6 months, complex regional pain syndrome, lumbar spine surgeries, chemical dependence or alcohol abuse, a history of nerve trauma, or chemotherapy in the past year. Institutional ethical review boards approved the study. Written informed consent was obtained prior to testing.
The participant was positioned in left side lying with the spine supported in neutral with pillows and folded towels as necessary to ensure the spine was straight in the coronal plane and that the cervical spine was in line with the trunk. The participant’s upper extremities were resting in front of his/her chest. The first hip position (neutral) included the right limb passively supported by a second tester in 20° hip flexion, full knee extension and a relaxed ankle position. The second hip position (flexion), performed passively by a second tester, included hip flexion to the point the participant reported the first onset of any sensory response to movement, such as stretch, pulling, pain or tightness. The hip flexion angle was measured with a standard goniometer. The participants then performed active ankle motions from plantar flexion (>30°) to dorsiflexion (>0°) over approximately a 5-7 second time frame. Ultrasound images of the right tibial nerve (TibN) and common fibular nerve (CFibN) in the popliteal fossa were acquired at 7 frames/second with an 8-15 MHz linear probe and an Acuson Sequoia Ultrasound Machine (Siemens Medical Solutions, Malvern, PA, USA). Both longitudinal and transverse plane images were obtained for the TibN. Only transverse plane images were obtained for the CFibN due to the oblique course of the nerve in the popliteal fossa as it angles laterally towards the neck of the fibula.
Longitudinal excursion was quantified using a cross-correlation analysis previously described where images were converted to sequential bitmap files and analyzed using software developed in Matlab (Dilley et al., 2001). This method was used to track the fine speckle pattern of up to three selected areas of interest within the nerve (Figure 1) and quantify relative nerve movement based upon the pixel shift that produced the highest correlation coefficient between adjacent frames (Dilley et al., 2001). Transverse plane images were imported into Image J (National Institutes of Health, Bethesda, MD, USA) where the perimeter of the nerves were manually traced (Figure 1) (Alshami et al., 2009, Watanabe et al., 2010). The central pixel of the nerve (centroid) was calculated based upon the average x and y coordinates of all pixels selected and utilized to quantify nerve movement. To eliminate any impact of transducer movement, motion of the subcutaneous layers was measured and subtracted from the nerve motion to isolate true nerve movement for longitudinal and transverse plane analysis. Measurements were repeated three times and the averages were utilized for statistical analysis.
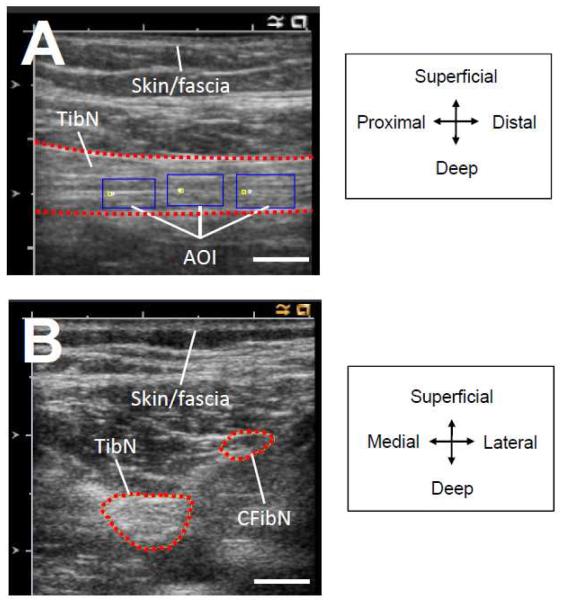
Figure 1. Ultrasound images of nerves in popliteal fossa.
Longitudinal ultrasound image of tibial nerve (TibN) in the popliteal fossa (A). Three areas of interest (AOI) are indicated by the rectangular boxes and were used for cross-correlational analysis for quantifying longitudinal excursion. Transverse plane ultrasound images of TibN and CFibN in the popliteal fossa (B). Directional orientation is presented in legends on the right. Skin/fascia represents the location of the skin and subcutaneous layers in the popliteal fossa. White bar in bottom right of images represents 5 mm.
Statistical analyses were performed with SPSS, v.19 (IBM Corporation, Somers, NY, USA). Due to the small sample size, non-parametric statistics were utilized to examine between group differences (independent samples Mann-Whitney U test) and differences between limb positions (related samples Friedman’s test). Repeated measurements were used to determine reliability using Intraclass Correlation Coefficient (ICC2,1) analysis and standard error of measurement (SEM) using the formula: (Weir, 2005). Data are presented as mean (SD). Alpha was set at 0.05.
RESULTS
Participant demographics are presented in Table 1. The neutral hip position was 19.0° (2.5°) for the healthy control group and 20.8° (2.3°) for people with DM (P=0.26). The hip flexion position was 62.4° (16.4°) flexion for the healthy control group and 65.0° (11.2°) for people with DM (P=0.78). Reliability (ICC2,1) for measuring TibN excursion in all participants was 0.97 for longitudinal motion (95% CI: 0.94, 0.99), 0.97 for medial-lateral motion (95% CI: 0.94, 0.99), and 0.98 for superficial-deep motion (95% CI: 0.96, 0.99). For the CFibN, reliability (ICC2,1) was 0.98 for medial-lateral motion (95% CI: 0.95, 0.99) and 0.98 for superficial-deep motion (95% CI: 0.97, 0.99). The SEM for the TibN was 0.23 mm for longitudinal motion, 0.42 mm for medial-lateral motion and 0.47 mm for superficial-deep motion while the SEM for the CFibN was 0.44 mm for medial-lateral motion and 0.34 mm for superficial-deep motion. An individual’s nerve movement was considered to have occurred only when the magnitude was greater than the appropriate SEM value.
TABLE 1.
Subject demographics
|
| |||
| Demographic characteristic |
Healthy Controls Mean (SD) |
People with DM Mean (SD) |
P value |
|
| |||
| Age (years) | 40.0 (11.8) | 57.0 (10.1) | 0.036 * |
| Height (m) | 1.7 (0.1) | 1.7 (0.1) | 0.117 |
| Weight (kg) | 66.1 (13.2) | 88.8 (4.3) | 0.009 * |
| Body mass index (kg/m2) | 23.5 (2.7) | 30.2 (5.6) | 0.028 * |
| Years with diagnosis of DM | n/a | 4.1 (5.7) | --- |
| Sex | 4 female / 1 male | 1 female / 4 males | --- |
| HbA1c | (%) 5.2 (0.2) | 8.4 (3.0) | 0.009 * |
| Mean plasma glucose | 109.2 (6.2) | 193.0 (102.0) | 0.014 * |
| Vibration perception threshold – halluces (V) | 11.6 (4.4) | 36.4 (16.8) | 0.016 * |
HbA1c = Hemoglobin A1c blood test
Alpha was set at 0.05.
Asterisk (*) = denotes statistically significant difference between groups.
Healthy control group: within group comparisons
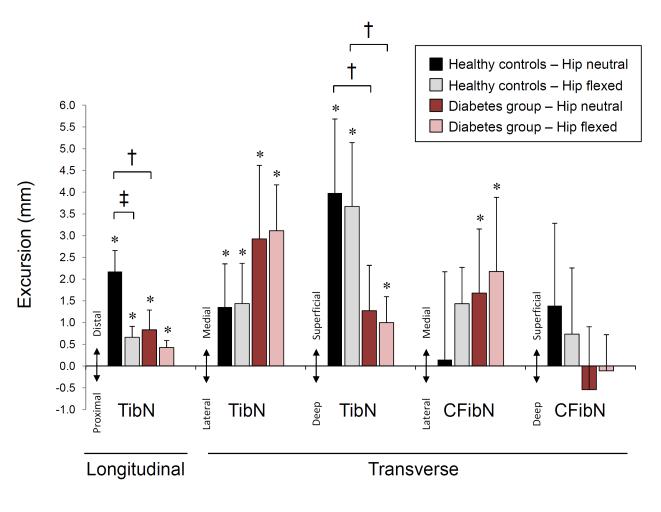
In the neutral hip position, ankle dorsiflexion produced distal longitudinal TibN movement in all subjects (5/5) with a mean of 2.18 mm (SD 0.48 mm, P=0.001) (Figure 2). In the transverse plane, 4/5 subjects had a medial TibN movement with a group mean of 1.36 mm (SD 0.99 mm, P=0.038) and all subjects (5/5) had superficial TibN movement with a mean of 3.98 mm (SD 1.70 mm, P=0.006) (Figure 2).
Figure 2. Tibial and common fibular nerve excursion during active ankle dorsiflexion.
Tibial nerve (TibN) and common fibular nerve (CFibN) excursion is presented in millimeters for longitudinal and transverse plane motions during the active ankle dorsiflexion; proximal-to-distal, medial-to-lateral, and superficial-to-deep motion. Data are presented as group means with standard deviation error bars for each hip position. Asterisk (*) denotes statistically significant nerve excursion (compared to 0 mm movement), † indicates statistical significant difference between groups and ‡ denotes statistically significant difference between hip positions within the group. Alpha was set at 0.05.
In hip flexion, ankle dorsiflexion produced distal longitudinal TibN movement in all subjects (5/5) with a mean of 0.66 mm (SD 0.25 mm, P=0.004) (Figure 2), which was a threefold decrease compared to the neutral hip position (P=0.043). In the transverse plane, there was medial TibN movement in 4/5 subjects with a mean of 1.44 mm (SD 0.93 mm, P=0.026) and superficial TibN movement in all subjects (5/5) with a mean of 3.67 mm (SD 1.47 mm, P=0.005) (Figure 2).
Excursion of the CFibN exhibited a less consistent trend with ankle dorsiflexion (Figure 2). In the neutral hip position, CFibN motion was medial in 3, lateral in 1 and absent in 1 subject with a mean of 0.15 mm medial motion (SD 2.02 mm, P=0.878). CFibN motion was superficial in 4 and deep in 1 subject with a mean of 1.39 mm superficial motion (SD 1.89 mm, P=0.176). In the flexed hip position, CFibN motion was medial in all 5 subjects with a mean of 1.43 mm (SD 0.84 mm, P=0.018) and superficial in 2, deep in 2 and absent in 1 subject with a mean of 0.73 mm superficial motion (SD 1.52 mm, P=0.342).
DM group: within group comparisons
A similar pattern was seen in older people with DM with respect to the effect of hip pre-positioning (Figure 2). In the neutral hip position, ankle dorsiflexion produced distal longitudinal TibN movement in 4/5 subjects with a mean of 0.83 mm (SD 0.45 mm, P=0.015). In the transverse plane, there was medial TibN movement in all subjects (5/5) with a mean of 2.92 mm (SD 1.69 mm, P=0.018). In hip flexion, ankle dorsiflexion produced distal longitudinal TibN movement in all subjects (5/5) with a mean of 0.42 mm (SD 0.16 mm, P=0.004). In the transverse plane, ankle dorsiflexion produced medial TibN movement in all subjects (5/5) with a mean of 3.11 mm (SD 1.06 mm, P=0.003) and superficial TibN movement in 4/5 subjects with a mean of 1.00 mm (SD 0.60 mm, P=0.020).
Excursion of the CFibN with ankle dorsiflexion was less consistent than that seen in the TibN (Figure 2). In the neutral hip position, CFibN motion was medial in 3 and absent in 2 subjects with a mean of 1.68 mm medial motion (SD 1.47 mm, P=0.063). CFibN motion was superficial in 2, deep in 2 and absent in 1 subject with a mean of 0.54 mm deep motion (SD 1.45 mm, P=0.449). In the flexed hip position, CFibN motion was medial in all 5 subjects with a mean of 2.17 mm (SD 1.71 mm, P=0.046) and superficial in 2, deep in 2 and absent in 1 subject with a mean of 0.11 mm deep motion (SD 0.83 mm, P=0.781).
Between group analyses revealed that people with DM had significantly less distal TibN movement in the neutral hip position (P=0.009) and less superficial TibN movement in both hip positions (P=0.016 each). While the magnitude of TibN motion was different between the groups, the overall trend with respect to the impact of limb pre-positioning was similar between groups.
DISCUSSION
This is the first study to document an in vivo reduction of TibN excursion with alterations in adjacent joint positioning using non-invasive methodology. Pre-positioning the lower extremity into hip flexion produced a threefold reduction in the amount of longitudinal TibN movement during ankle dorsiflexion. The magnitude of mean TibN motion exceeds the SEM in all conditions measured, and thus we can be confident that the nerve motions measured are not due to measurement error. The present findings support the premise that pre-positioning the limb in a manner that increases the pre-loading on the nervous system will diminish the available nerve excursion during subsequent active limb movements.
The three-dimensional pattern of nerve excursion identified in this study improves our understanding of normal nerve biomechanics. The direction of excursion is consistently moving towards the nerve that is theoretically being placed under tension; medial towards the TibN with dorsiflexion and lateral towards the CFibN with the return to plantar flexion (reverse sequence not shown). Ankle dorsiflexion also caused the TibN to move superficially from a deep resting position in the popliteal fossa. Previous findings support this pattern of distal, superficial and medial movement of the TibN in the popliteal crease during ankle dorsiflexion either with or without simultaneous neck extension (Ellis et al., 2008, Schafhalter-Zoppoth et al., 2004). Ankle motions are often utilized during lower extremity exercises aimed at improving the tolerance of the nervous system to limb movements. Our findings can inform decisions regarding limb pre-positioning during exercise prescription when the goal is to facilitate maximal TibN excursion.
Interestingly nerve biomechanics were different in the group of individuals with DM. Limited distal and superficial TibN excursion during ankle dorsiflexion suggests that nerve biomechanics have potential to be diminished in older individuals with DM. It is possible that this lost distal and superficial mobility can be compensated for in the medial-lateral direction based upon the trend of between group mean comparisons, although this difference was not statistically significant. Possible explanations include increased quantity or stiffening of connective tissue within the nerve and between the nerve and surrounding tissues. As has been previously hypothesized in the median nerve of the upper limb (LaBan et al., 1986), a thicker nerve or a nerve tethered to the surrounding nerve bed may decrease the nerves capacity to glide smoothly with limb movement. Increases in collagen in the endoneurium and perineurium have been identified in human lower extremity nerves in people with Type 1 and Type 2 diabetes (Bradley et al., 2000, Hill, 2009). Increased collagen quantity and fibril diameter have also been identified in the epineurium of the sciatic nerve in rat models of experimentally induced diabetes (Wang et al., 2003, Layton et al., 2004). Greater cross sectional area of the TibN has been demonstrated in people with Type 1 and Type 2 diabetes (Watanabe et al., 2010). Age is also associated with increased quantity of neural connective tissue within the sciatic nerve in the thigh (Sladjana et al., 2008). Additionally, subcutaneous adipose tissue associated with increased body mass may alter nerve biomechanics, which may provide an alternative explanation for the limited superficial movement observed in the group with DM. As limitations to this pilot study include significant between-group baseline differences with respect to age, sex and BMI as well as the use of manual goniometry for hip positioning and the lack of standardization for the amount of ankle range of motion utilized, future studies with larger samples and more precise measurement instruments are necessary to delineate which factors are responsible for the alterations in nerve biomechanics observed in this study.
CONCLUSIONS
We have demonstrated that lower limb pre-positioning impacts nerve biomechanics during ankle motions commonly utilized for functional activities. This is the first study to document differences in TibN biomechanics during lower extremity movements in older people with DM.
ACKNOWLEDGEMENTS
This project was supported by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Additional funding for this study was provided by a Graduate Student Research Award from the University of California, San Francisco awarded to BSB and Mary McMillan Doctoral Scholarship from the Foundation of Physical Therapy awarded to BSB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no competing interests. The authors would like to thank Isabel Belkind, Ryan Broms, Sean Darling, Romy Havard, Alyssa Keeney-Roe, and Stephanie Klitgord, for their assistance with data collection and processing.
REFERENCES
- ALSHAMI AM, BABRI AS, SOUVLIS T, COPPIETERS MW. Strain in the tibial and plantar nerves with foot and ankle movements and the influence of adjacent joint positions. J. Appl. Biomech. 2008;24:368–376. doi: 10.1123/jab.24.4.368. [DOI] [PubMed] [Google Scholar]
- ALSHAMI AM, CAIRNS CW, WYLIE BK, SOUVLIS T, COPPIETERS MW. Reliability and size of the measurement error when determining the cross-sectional area of the tibial nerve at the tarsal tunnel with ultrasonography. Ultrasound Med. Biol. 2009;35:1098–1102. doi: 10.1016/j.ultrasmedbio.2009.01.011. [DOI] [PubMed] [Google Scholar]
- BABBAGE CS, COPPIETERS MW, MCGOWAN CM. Strain and excursion of the sciatic nerve in the dog: biomechanical considerations in the development of a clinical test for increased neural mechanosensitivity. Vet. J. 2007;174:330–336. doi: 10.1016/j.tvjl.2006.07.005. [DOI] [PubMed] [Google Scholar]
- BOYD BS, PUTTLITZ C, GAN J, TOPP KS. Strain and excursion in the rat sciatic nerve during a modified straight leg raise are altered after traumatic nerve injury. J. Orthop. Res. 2005;23:764–770. doi: 10.1016/j.orthres.2004.11.008. [DOI] [PubMed] [Google Scholar]
- BOYD BS, WANEK L, GRAY AT, TOPP KS. Mechanosensitivity of the lower extremity nervous system during straight leg raise neurodynamic testing in healthy individuals. J. Ortho. Sports Phys. Ther. 2009;39:780–790. doi: 10.2519/jospt.2009.3002. [DOI] [PubMed] [Google Scholar]
- BOYD BS, WANEK L, GRAY AT, TOPP KS. Mechanosensitivity during lower extremity neurodynamic testing is diminished in individuals with Type 2 Diabetes Mellitus and peripheral neuropathy: a cross sectional study. BMC Neurol. 2010;10:75. doi: 10.1186/1471-2377-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADLEY JL, KING RH, MUDDLE JR, THOMAS PK. The extracellular matrix of peripheral nerve in diabetic polyneuropathy. Acta Neuropathol. 2000;99:539–46. doi: 10.1007/s004010051158. [DOI] [PubMed] [Google Scholar]
- BRIEG A, TROUP JDG. Biomechanical considerations in the straight-leg-raising test: cadaveric and clinical studies of the effects of medial hip rotation. Spine. 1979;4:242–250. doi: 10.1097/00007632-197905000-00011. [DOI] [PubMed] [Google Scholar]
- COPPIETERS MW, ALSHAMI AM, BABRI AS, SOUVLIS T, KIPPERS V, HODGES PW. Strain and excursion of the sciatic, tibial, and plantar nerves during a modified straight leg raising test. J. Orthop. Res. 2006;24:1883–1889. doi: 10.1002/jor.20210. [DOI] [PubMed] [Google Scholar]
- COPPIETERS MW, BUTLER DS. Do ‘sliders’ slide and ‘tensioners’ tension? An analysis of neurodynamic techniques and considerations regarding their application. 2008;13:213–221. doi: 10.1016/j.math.2006.12.008. [DOI] [PubMed] [Google Scholar]
- DILLEY A, GREENING J, LYNN B, LEARY R, MORRIS V. The use of cross-correlation analysis between high-frequency ultrasound images to measure longitudinal median nerve movement. Ultrasound Med. Biol. 2001;27:1211–1218. doi: 10.1016/s0301-5629(01)00413-6. [DOI] [PubMed] [Google Scholar]
- DILLEY A, LYNN B, GREENING J, DELEON N. Quantitative in vivo studies of median nerve sliding in response to wrist, elbow, shoulder and neck movements. Clin. Biomech. 2003;18:899–907. doi: 10.1016/s0268-0033(03)00176-1. [DOI] [PubMed] [Google Scholar]
- ELLIS R, HING W, DILLEY A, MCNAIR P. Reliability of measuring sciatic and tibial nerve movement with diagnostic ultrasound during a neural mobilisation technique. Ultrasound Med. Biol. 2008;34:1209–1216. doi: 10.1016/j.ultrasmedbio.2008.01.003. [DOI] [PubMed] [Google Scholar]
- GILBERT KK, BRISMEE JM, COLLINS DL, JAMES CR, SHAH RV, SAWYER SF, SIZER PS., JR 2006 Young Investigator Award Winner: lumbosacral nerve root displacement and strain: part 1. A novel measurement technique during straight leg raise in unembalmed cadavers. Spine. 2007;32:1513–1520. doi: 10.1097/BRS.0b013e318067dd55. [DOI] [PubMed] [Google Scholar]
- GREWAL R, XU J, SOTEREANOS DG, WOO SL-Y. Biomechanical properties of peripheral nerves. Hand Clin. 1996;12:195–204. [PubMed] [Google Scholar]
- HERRINGTON L, BENDIX K, CORNWELL C, FIELDEN N, HANKEY K. What is the normal response to structural differentiation within the slump and straight leg raise tests? Man. Ther. 2008;13:289–294. doi: 10.1016/j.math.2007.01.013. [DOI] [PubMed] [Google Scholar]
- HILL R. Extracellular matrix remodelling in human diabetic neuropathy. J. Anat. 2009;214:219–225. doi: 10.1111/j.1469-7580.2008.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JULIUS A, LEES R, DILLEY A, LYNN B. Shoulder posture and median nerve sliding. BMC Musculoskelet. Disord. 2004;5:23. doi: 10.1186/1471-2474-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LABAN MM, FRIEDMAN NA, ZEMENICK GA. “Tethered” median nerve stress test in chronic carpal tunnel syndrome. Arch. Phys. Med. Rehab. 1986;67:803–804. [PubMed] [Google Scholar]
- LAYTON BE, SASTRY AM, WANG H, SULLIVAN KA, FELDMAN EL, KOMOROWSKI TE, PHILBERT MA. Differences between collagen morphologies, properties and distribution in diabetic and normal biobreeding and Sprague-Dawley rat sciatic nerves. J. Biomech. 2004;37:879–888. doi: 10.1016/j.jbiomech.2003.11.008. [DOI] [PubMed] [Google Scholar]
- SCHAFHALTER-ZOPPOTH I, YOUNGER SJ, COLLINS AB, GRAY AT. The “seesaw” sign: improved sonographic identification of the sciatic nerve. Anesthesiology. 2004;101:808–809. doi: 10.1097/00000542-200409000-00045. [DOI] [PubMed] [Google Scholar]
- SLADJANA UZ, IVAN JD, BRATISLAV SD. Microanatomical structure of the human sciatic nerve. Surg. Radiol. Anat. 2008;30:619–626. doi: 10.1007/s00276-008-0386-6. [DOI] [PubMed] [Google Scholar]
- SMITH SA, MASSIE JB, CHESNUT R, GARFIN SR. Staight leg raise: anatomical effects on the spinal nerve root without and with fusion. Spine. 1993;18:992–999. [PubMed] [Google Scholar]
- TOPP KS, BOYD BS. Structure and biomechanics of peripheral nerves: nerve responses to physical stresses and implications for physical therapist practice. Phys. Ther. 2006;86:92–109. doi: 10.1093/ptj/86.1.92. [DOI] [PubMed] [Google Scholar]
- WANG H, LAYTON BE, SASTRY AM. Nerve collagens from diabetic and nondiabetic Sprague-Dawley and biobreeding rats: an atomic force microscopy study. Diabetes Metab. Res. Rev. 2003;19:288–298. doi: 10.1002/dmrr.372. [DOI] [PubMed] [Google Scholar]
- WATANABE T, ITO H, SEKINE A, KATANO Y, NISHIMURA T, KATO Y, TAKEDA J, SEISHIMA M, MATSUOKA T. Sonographic evaluation of the peripheral nerve in diabetic patients: the relationship between nerve conduction studies, echo intensity, and cross-sectional area. J. Ultrasound Med. 2010;29:697–708. doi: 10.7863/jum.2010.29.5.697. [DOI] [PubMed] [Google Scholar]
- WEIR JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J. Strength Cond. Res. 2005;19:231–240. doi: 10.1519/15184.1. [DOI] [PubMed] [Google Scholar]