Abstract
Cerebellar Purkinje cells (PCs) are particularly sensitive to cerebral ischemia, and decreased GABAA receptor function following injury is thought to contribute to PC sensitivity to ischemia-induced excitotoxicity. Here we examined the functional properties of the GABAA receptors that are spared following ischemia in cultured Purkinje cells from rat and in vivo ischemia in mouse. Using subunit-specific positive modulators of GABAA receptors, we observed that oxygen and glucose deprivation (OGD) and cardiac arrest-induced cerebral ischemia cause a decrease in sensitivity to the β2/3-subunit-preferring compound, etomidate. However, sensitivity to propofol, a β-subunit-acting compound that modulates β1–3-subunits, was not affected by OGD. The α/γ-subunit-act-ing compounds, diazepam and zolpidem, were also unaffected by OGD. We performed single-cell reverse transcription–polymerase chain reaction on isolated PCs from acutely dissociated cerebellar tissue and observed that PCs expressed the β1-subunit, contrary to previous reports examining GABAA receptor subunit expression in PCs. GABAA receptor β1-subunit protein was also detected in cultured PCs by western blot and by immunohistochemistry in the adult mouse cerebellum and levels remained unaffected by ischemia. High concentrations of loreclezole (30 µm) inhibited PC GABA-mediated currents, as previously demonstrated with β1-subunit-containing GABAA receptors expressed in heterologous systems. From our data we conclude that PCs express the β1-subunit and that there is a greater contribution of β1-subunit-containing GABAA receptors following OGD.
Keywords: electrophysiology, GABAA receptor, mouse, oxygen–glucose deprivation, Purkinje cells, rat
Introduction
Cerebellar Purkinje cells (PCs) are the output neurons of the cerebellar cortex and are particularly susceptible to ischemic injury. The large number of excitatory glutamatergic synapses onto PCs contributes to their vulnerability to excitotoxic damage after ischemia (Horn & Schlote, 1992; Brasko et al., 1995; Fonnum & Lock, 2000; Ardeshiri et al., 2006; Kelley et al., 2008). Many neuroprotective strategies have been explored using PCs as a model system, with variable success. One approach is to increase the inhibitory tone by enhancing GABAA receptor activity, thus hyperpolarising cells and leaving them less vulnerable to excitotoxic damage (Schwartz-Bloom & Sah, 2001). One consideration in targeting GABA receptors is that, following cerebral ischemia, as experienced during a stroke or cardiac arrest (CA), GABAA receptors are down-regulated in various brain regions (Li et al., 1993; Schiene et al., 1996; Qu et al., 1998; Kelley et al., 2008; Arancibia-Carcamo et al., 2009), resulting in decreased inhibitory potential and increased susceptibility to excitotoxic damage. We also observed that oxygen and glucose deprivation (OGD) causes a rapid and sustained reduction in GABA-mediated current and GABAA receptor α1-subunit protein (Kelley et al., 2008). Therefore, preserving GABAA receptor function may play an important role in neuroprotection from ischemia. We have recently demonstrated that the neurosteroid allopregnanolone protects PCs from ischemic damage both in vitro and in vivo via a GABAA receptor-dependent mechanism (Ardeshiri et al., 2006; Kelley et al., 2008, 2011). Although it is clear that GABA receptor function is altered by ischemia, there is currently limited information regarding GABAA receptor structural changes and trafficking in pathophysiological states, making the full nature and consequences of these changes to GABAA receptors unknown. This apparent disease-induced plasticity could contribute to the difficulty in finding GABAA receptor-modulating agents that are effective neuroprotectants. Pharmacotherapies targeting GABAA receptors may be effective if we improve our understanding of receptor subunit composition in neurons that have undergone ischemia.
The GABAA receptors are pentameric proteins thought to be composed of two α-subunits, two β-subunits, and one γ- or δ-subunit (or less commonly an ε-, π-, or θ-subunit). Sixteen subtypes of GABAA receptor subunits have been cloned (Olsen & Sieghart, 2009), allowing for heterogeneous expression profiles across brain regions with distinct channel properties. The subunit composition dictates the specific properties of the receptor, including ligand binding, channel kinetics, and response to allosteric modulators. Additionally, GABAA receptor phosphorylation and association with trafficking proteins are subunit dependent. Cerebellar PCs are thought to express a homogeneous population of GABAA receptors, consisting of α1β2/3γ2 (Wisden et al., 1992). Following ischemia, PC GABAA receptor function and protein levels are significantly reduced (Kelley et al., 2008). In the current set of experiments, we examined the function of PC GABAA receptors that remain following ischemia using subunit-specific allosteric modulators. Single-cell polymerase chain reaction (PCR) from isolated PCs and western blot were also used to study GABAA receptor subunit expression. Our findings suggest that PCs express a more heterogeneous population of GABAA receptors than previously believed and that the relative subunit contribution is altered by ischemia.
Materials and methods
Primary cerebellar culture
All experiments were performed in accordance with National Institute of Health guidelines and experimental protocols approved by the institutional animal care and use committee (IACUC). Cerebellar neurons were cultured from embryonic day 18 Sprague-Dawley rats as previously described (Kelley et al., 2008). In brief, time-pregnant Sprague-Dawley rats (Charles River, Wilmington, MA, USA) were killed with CO2 and embryos removed by caesarean section. Embryonic brains were isolated and placed in ice-cold dissection solution composed of Hank’s balanced salt solution (Gibco/Invitrogen, Carlsbad, CA, USA) supplemented with 0.03% bovine serum albumin and 10 mm MgSO4 (Sigma-Aldrich, St Louis, MO, USA). Cerebella were dissected, meninges removed, and tissue digested with 0.2% trypsin in dissection solution at 37 °C. Digestion was halted by washing with cerebellar culture medium composed of Dulbecco’s modified Eagle’s medium/F-12 (1 : 1) mix supplemented with 1.4 mm L-glutamine, 50 units*µg/mL penicillin–streptomycin, b-27 supplement (1 ×), and 10% fetal bovine serum (Hyclone, Logan, UT, USA). Tissue was triturated, filtered through a 70 lm cell sorting nylon mesh, and the suspension centrifuged at 1000 g for 10 min to isolate cells. The cell pellet was resuspended in cerebellar culture medium without fetal bovine serum. Cells were plated at a density of 2.8 × 105 cells per round 12 mm glass coverslip and grown at 37 ° C in a humidified incubator containing 5% CO2. Cultured neurons were grown for 10–14 days in vitro to allow maturation and formation of synapses.
Oxygen and glucose deprivation
Cells were transferred to a glucose-free saline solution composed of (in mm): 140 NaCl, 5 KCl, 0.8 MgCl2, 1 CaCl2, 10 HEPES, pH 7.35, with NaOH, and placed in an anaerobic incubator (at 37 °C) containing an oxygen-reacting catalyst and 5% CO2, 95% N2 (Coy Laboratory Products, Grass Lake, MI, USA) for 2 h. This duration of OGD was previously determined to cause significant levels of cell death in our cultures following 24 h of reperfusion (Ardeshiri et al., 2006). Reoxygenation was initiated by transferring cells to glucose-containing saline for electrophysiological recordings (see below).
Electrophysiology
Cells were transferred to a recording chamber mounted on an inverted microscope (DM IRB; Leica, Houston, TX, USA) containing normal saline composed of (in mm): 140 NaCl, 5 KCl, 0.8 MgCl2, 1 CaCl2, 10 HEPES, 10 glucose, pH 7.35, with NaOH, and a gravity-fed bath flow rate of 2–5 mL/min. Whole-cell voltage-clamp recordings were made from the somas of PCs using an Axopatch 200B amplifier (Axon Instruments, Union City, CA, USA) interfaced to a computer (Dell, Round Rock, TX, USA). PCs were selected by their large soma, low input resistance, and extensive dendritic arbor. Data were excluded from cells in which the series resistance changed more than 20% throughout the course of the experiment. Electrodes were pulled from borosilicate glass capillaries with inner filaments using a Flaming Brown electrode puller (Sutter Instrument Co., Novato, CA, USA) and had resistances of 2–3 MΩ when filled with internal pipette solution composed of (in mM): 140 CsCl, 1 EGTA, 10 HEPES, 1 MgCl2, 5 MgATP, pH 7.3 with CsOH. Data were collected at a sample frequency of 20 kHz and using pCLAMP9 (Molecular Devices, Sunnyvale, CA, USA). Whole-cell capacitance and series resistance were electronically compensated to 60–70%. Adequate whole cell access (Ra < 20 MΩ) was achieved prior to measuring responses to compounds and verified at the end of recording. A pressurised microperfusion system with a wide barrel manifold (250 µm) was used for rapid solution exchange around the entire cell body and dendritic processes (ALA Scientific Instruments, Westbury, NY, USA) to measure PC response to GABA and subunit-specific modulators. The amplitude of currents, in response to GABA and test compounds, was measured using Clampfit analysis software (Axon Instruments). For concentration–response analysis, only cells where a complete concentration–response was obtained were included. Data from each cell were plotted and fit with the following equation to determine EC50 using Igor Pro software (WaveMetrics, Lake Oswego, OR, USA): f(x) = (Imax–I0)/(1+(EC50/x)n)+I0, where x equals the concentration of drug and n equals the Hill coefficient. The EC50 is presented as the mean ± SEM for individual cells. Data were expressed as the percent potentiation of 2 or 5 µm GABA [the concentration of GABA required to activate 20% of total GABAA receptors (GABA20)]. The maximum GABA-mediated current, achieved by applying a saturating concentration of GABA (1 mm, 1 s), was measured at the end of each recording to ensure that the effect of drugs was submaximal and to verify that the concentration of GABA being used would activate 20% of the total GABAA receptors. The concentrations of drugs used for dose–response analysis were based on previous pharmacological studies using heterologous expression systems (Mihic et al., 1994; Sanna et al., 1995; Hill-Venning et al., 1997; Neelands et al., 1998). For loreclezole inhibition experiments, 1 s pulses of 20 µm GABA were applied every 30 s to establish a stable baseline response, 30 µm loreclezole was then preapplied by local perfusion for 30 s and remained present through three subsequent pulses of 20 µm GABA every 30 s. Washout of drug was achieved by local perfusion of saline in addition to bath perfusion.
Cardiac arrest and slice electrophysiology
Briefly, adult male mice (7–9 weeks) were anesthetised with isoflurane (1–3%) via face mask. Temperature probes were placed into the left temporalis muscle and rectum. The rectal temperature was controlled at 37 °C during surgery. For drug administration, the right jugular vein was cannulated with a PE-10 catheter. The electrocardiogram was monitored throughout the experimental procedures. Mice were endotracheally intubated with a 22G intravenous catheter and mechanically ventilated (Minivent; Hugo Sachs Elektronik, March-Hugstetten, Germany). CA was induced by intravenous injection of 50 µL of 0.5 m KCl and was confirmed by the appearance of asystole on the electrocardiography monitor and no spontaneous breathing. Anesthesia was then stopped and the endotracheal tube was disconnected from the ventilator. During CA, the pericranial temperature was maintained at 37.5 ± 0.2 °C and the body temperature at 36 °C. Cardiopulmonary resuscitation (CPR) was initiated at 8 min after the induction of CA by slow injection of 0.5 mL of epinephrine (8 µg), chest compressions (approximately 300/min), and ventilation with 100% oxygen. As soon as return of spontaneous circulation was achieved, defined as electrocardiographic activity with visible cardiac contractions, chest compression was stopped. If return of spontaneous circulation could not be achieved within 2.5 min of CPR, resuscitation was stopped and the animal was excluded from the study. At 25 min after return of spontaneous circulation, temperature probes were removed, skin incisions were closed, and animals were weaned from the ventilator. Animals were extubated after confirmation of spontaneous ventilation/breathing and mice were returned to their housing cage.
At 3 h after CA/CPR, mice were anesthetised and decapitated, and brains were removed. Parasagittal cerebellar sections (300 µm) were prepared using a vibratome. Slices were prepared and maintained in artificial cerebrospinal fluid containing (in mM): 126 NaCl, 2.5 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 26 NaHCO3, and 10 D-glucose. Slices were transferred to a recording chamber with perfused artificial cerebrospinal fluid (1.5 mL/min) and Purkinje neurons were identified by their large soma and location in the PC layer. Whole-cell recordings were obtained using a 2–3 MΩ pipette containing (in mm): 135 K-gluconate, 8 NaCl, 10 HEPES, 0.05 EGTA, 2 MgCl2, 4 MgATP, and 0.3 NaGTP.
The series resistance was < 20 MΩ and did not change more than 20% during the experiment. Cells were voltage clamped at −60 mV and a stimulating electrode placed in the inner molecular layer was used to stimulate GABA release. Stimulation resulted in an outward inhibitory postsynaptic current (IPSC). GABA IPSCs were recorded in the presence of 10 µm NBQX (2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide) to block glutamatergic transmission.
Solutions and drugs
Etomidate and zolpidem were dissolved in EtOH at a stock concentration of 10 mm. Loreclezole was dissolved in dimethylsulfoxide at a stock concentration of 5 mm. Diazepam was dissolved in dimethylsulfoxide at a stock concentration of 10 mm. Propofol was dissolved in EtOH at a stock concentration of 20 mm. GABA was dissolved in water at a stock concentration of 500 mm. Working solutions were prepared by careful serial dilution of stock compounds in saline. GABA currents were unaffected by vehicle controls containing EtOH or dimethylsulfoxide (data not shown). Components of the internal and external solutions, propofol and diazepam were obtained from Sigma-Aldrich. Etomidate, loreclezole, GABA and zolpidem were obtained from Tocris (Ellisville, MO, USA).
Immunohistochemistry
Following CA/CPR (or sham control) and 24 h recovery, mice were perfused through the left ventricle with saline followed by 4% paraformaldehyde, then removed and postfixed for an additional 8–12 h. Cerebelli were dehydrated and embedded in paraffin. Coronal sections (6 µm) were cut and three sections, 100 µm apart (beginning at approximately −5.6 from Bregma), were mounted for immunohistochemistry. Briefly, sections underwent deparaffinisation and antigen retrieval using citrate buffer (10 mm, pH 6.0) at 95 °C for 30 min. Sections were incubated in blocking solution (10% normal goat serum and 0.5% triton x-100) for 2 h at room temperature (22 °C). GABAA beta 1 antibody and beta 2 antibody (Novus Biologicals, Littleton, CO, USA) were diluted 1 : 100 in blocking solution and incubated for 8–12 h at 4 °C. The secondary antibody goat anti-rabbit Alexa-488 (Molecular Probes, Eugene, OR, USA) was diluted 1 : 500 in blocking solution to visualise GABAA receptors and incubated for 2 h at room temperature. A total of nine images were captured per animal (three per level) and analysed by a separate investigator. For analysis, the mean intensity of the soma was measured and background was subtracted. Soma intensities from all nine images were averaged to generate a mean intensity (arbitrary units) per animal (n = 4 in control and CA/CPR).
Acute cerebellar dissociation and Purkinje cell collection
Cerebellar dissociation for the isolation of PCs was performed according to Raman and Bean (Raman & Bean, 1997; Benton & Raman, 2009) with the modification of the use of RNAse-free H2O for all solutions. Postnatal day 14–20 C57BL/6 mice (Charles River) were anesthetised with isoflurane, decapitated, and superficial layers of the vermis of the cerebellum were dissected for dissociation. Tissue was minced in ice-cold, oxygenated dissociation solution composed of (in mm): 82 Na2SO4, 30 K2SO4, 5 MgCl2, 10 HEPES, 10 glucose, and 0.001% phenol red (buffered to pH 7.4 with NaOH). Tissue was then incubated in 10 mL oxygenated dissociation solution containing 3 mg/mL protease XXIII (Sigma-Aldrich), pH 7.4 with NaOH, at 37 °C for 7 min. Enzymatic digestion was halted by washing tissue in warmed, oxygenated dissociation solution containing 1 mg/mL bovine serum albumin and 1 mg/mL trypsin inhibitor, tissue was microdissected to roughly 1 mm pieces, and transferred to oxygenated Tyrode’s solution composed of (in mm): 150 NaCl, 4 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES, and 10 glucose, pH 7.4 with NaOH, at room temperature. To disperse cells, tissue was triturated with fire-polished RNAse-free Pasteur pipettes. Dissociated cells were plated onto 35 mm Petri dishes and allowed to settle for 20–30 min. PCs were visualised using an inverted microscope (DM IRB; Leica) and identified by their large size, teardrop shape, and proximal dendritic stump. Pipettes (2–3 MΩ) were pulled from RNAse-free borosilicate glass, filled with RNAse-free Tyrode’s solution and manipulated with an oocyte injector. Pipettes were positioned near the cell and the tip was broken, by lowering the pipette into the Petri dish, to create a large enough opening to capture PCs with applied negative pressure. Immediately following isolation, four to six cells were expelled into each siliconised microcentrifuge tube containing 1.6 µL of 5 × Superscript buffer (Invitrogen, Carlsbad, CA, USA), 18 U of RNAsin, 0.8 µL of 100 mm dithiothreitol, and diethylpyrocarbonate-treated water to 8 µL. The negative control consisted of Tyrode’s solution collected from the vicinity of PCs. Samples were frozen at −70 °C until used for reverse transcription– PCR.
Single-cell polymerase chain reaction
Each sample, containing four to six cells, was thawed on ice and 0.2 µm dNTPs, 100 ng of random hexamers, and 400 ng OligodT were added. Samples were heat denatured for 5 min at 65 °C and then cooled on ice for 5 min. Cellular RNA was reverse transcribed by adding 100 U of Superscript III reverse transcriptase, 3.4 µL of 5 × Superscript buffer, 15.2 U of RNAsin, 1.2 µL of 100 mm dithiothreitol, and diethylpyrocarbonate-treated water to 14 µL, together with cells for a final volume of 25 µL. Reverse transcription reactions were incubated at 25 °C for 5 min, transcribed at 50 °C for 60 min, denatured at 70 °C for 15 min, and cooled on ice.
Nested PCR was used for all samples, using degenerate outer and inner primers for α- and β-subunits, with the exception of the reaction for the β1b (from the Allen Mouse Brain Atlas; www.brain-map.org) primer set, for which conventional PCR was used. PCR primers were designed using DNA Star software (Madison, WI, USA). For α degenerate primers, sequences corresponded to the α1-subunit [accession number NM_010250, forward primer 347–365 nucleotides (nts), reverse primer 1000–1020 nt] and α6-subunit (accession number NM_008068, forward primer 290–308 nt, reverse primer 853–872 nt). For β degenerate primers, sequences corresponded to the β1-subunit (accession number NM_008069, forward primer 201–223 nt, reverse primer 907–929 nt), β2-subunit (accession number NM_008070, forward primer 206–227 nt, reverse primer 911–933 nt), and β3-subunit (accession number NM_008071, forward primer 305–326 nt, reverse primer 1010–1032 nt). All other primers were as follows: α1 (273 nt product, accession number NM_010250, forward primer 413–434 nt, reverse primer 674– 686 nt), α6 (287 nt product, accession number NM_008068, forward primer 395–417 nt, reverse primer 661–682 nt), β1 (206 nt product, accession number NM_008069, forward primer 464–483 nt, reverse primer 651–670 nt), β1b (669 nt product, accession number NM_008069, forward primer 1145–1166 nt, reverse primer 1792– 1814 nt), β2 (271 nt product, accession number NM_008070, forward primer 428–445 nt, reverse primer 679–699 nt), and β3 (260 nt product, accession number NM_008071, forward primer 519 –535 nt, reverse primer 760–779 nt). PCR was performed using 2 µL of cDNA template from each reverse transcription reaction in a 25 µL PCR volume containing 5 µL 5 × Mg-free buffer, 2 µL 25 mm MgCl2, 0.5 µL 10 mm dNTPs, 0.4 µL GoTaq Flexi DNA polymerase (Promega), and 0.2 µm each of the forward and reverse primers. Thirty-five cycles of amplification were performed using a Gene Amp PCR system 9700 thermal cycler (Applied Biosystems) in 0.2 mL thin-walled PCR tubes with the following protocol: 94 °C for 2 min; 35 cycles of 96 °C for 30 s, 54–57 °C for 0.5– 1 min, and 72 °C for 1 min; with a final 72 °C extension for 5 min. PCR products were visualised with ethidium bromide on a 2% agarose gel. Reverse transcription–PCR experiments revealed the presence of all β-subunits; however, not every reaction on PCs yielded a signal, with four out of six PC reactions exhibiting β2, three out of six showing β3 and two out of six showing β1.
Western blot
Total protein was collected from cultures by lysing cells with Cel-Lytic-M lysis buffer (Sigma-Aldrich), centrifuging at 10 000 g for 5 min at 4 °C and discarding the pellet to remove nuclei and debris. The total protein content of the supernatant was measured using a BCA (bicinchoninic acid) protein assay (Pierce/Thermo Scientific, Rockford, IL, USA) and 30 µg of total protein from each sample was run on a precast 4–12% stacking polyacrylamide gel (Invitrogen) for 1 h at 200 V. Protein was transferred to a polyvinylidene difluoride membrane (Invitrogen) for 1 h at 30 V. Blots were incubated in primary antibody (1 : 1000 dilution of rabbit anti-GABAA receptor β1-subunit) (Novus Biologicals) overnight at 4 °C, followed by secondary antibody (1 : 1000 donkey anti-rabbit IgG ECL-HRP; enhanced chemiluminescence-horseradish peroxidase) (GE Healthcare, Piscataway, NJ, USA) for 1 h at room temperature. Blots were then stripped and reprobed with primary antibody (1 : 7000 mouse anti-β-actin) (Sigma-Aldrich) for 1 h at room temperature, followed by secondary antibody (1 : 1000 goat anti-mouse IgG ECL HRP-linked) (GE Healthcare) for 1 h at room temperature. The SuperSignal West Dura chemiluminescent detection system (Thermo Scientific) was used to visualise protein and blots were imaged and analysed using a FluorChem FC2 gel imager (Alpha Innotech, San Leandro, CA, USA). GABAA receptor β1-subunit protein levels were normalised to β-actin and expressed as arbitrary units.
Statistical analysis
All data are presented as mean ± SEM. Each n represents an individual cell for electrophysiology experiments and an individual culture for western blot experiments. For pharmacological studies, EC50 was calculated for each individual cell and mean EC50S were compared. Statistical significance was determined using Student’s t-test (unpaired, two-tailed, if P < 0.05).
Results
Purkinje cells have decreased sensitivity to etomidate following oxygen and glucose deprivation
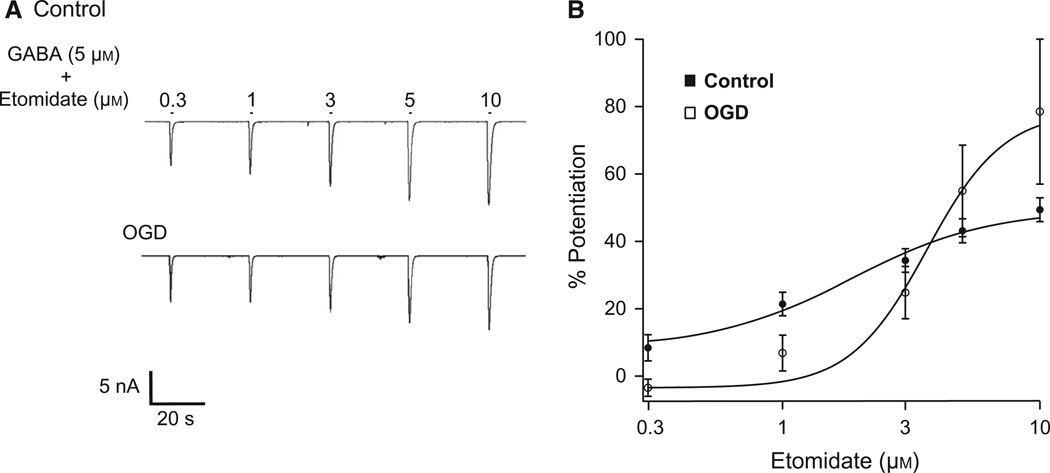
Whole-cell voltage-clamp recordings were made from cultured cerebellar PCs under control conditions or within 1 h of reoxygenation following 2 h OGD, a time point following ischemic insult that reflects data from neurons that are undergoing excitotoxicity and a duration of OGD that produces PC injury with a high probability of cell death (Ardeshiri et al., 2006). We tested the effect of ischemia on GABAA receptor sensitivity to etomidate by measuring peak current amplitudes in response to 1 s pulses of GABA20 (2–5 µm) plus increasing concentrations of etomidate (0.3–10 µm) before and after OGD (Fig. 1A; (Table 1)). Etomidate is a positive modulator of GABAA receptor activity that preferentially binds to β2/3-containing receptors. Concentration–response relationships were plotted and the EC50 for each individual cell was determined. For control cells, the average EC50 was 2.9 ± 0.3 µm (n = 8). Two hours of OGD caused a significant increase in the average EC50 to 3.8 ± 0.2 µM (P < 0.05; n = 6; Fig. 1B).
Fig. 1.
OGD decreases PC GABAA receptor sensitivity to etomidate. (A) Representative recordings from cultured PCs illustrating responses to 1 s applications of 5 µm GABA plus 0.3, 1, 3, 5, or 10 µm etomidate (bars above trace indicate application). (B) Average concentration-response relationships for etomidate from control and OGD PCs. The average EC50 for control cells was 2.9 ± 0.3 µm. The OGD cell average EC50 was 3.8 ± 0.2 µm (P < 0.05). Data presented are the average of eight control cells and six OGD cells.
Table 1.
PC GABAA receptor sensitivity to subunit-specific positive modulators
| Drug | Subunit | Control EC50 | OGD EC50 | Control vs. OGD P < 0.05 |
|---|---|---|---|---|
| Etomidate | β2–3 | 2.9 ± 0.3 µm (8) | 3.8 ± 0.2 µm (6) | * |
| Propofol | β1–3 | 5.6 ± 0.5 µm (9) | 5.8 ± 0.5 µm (12) | – |
| Zolpidem | α1, γ2 | 466.1 ± 75.8 nm (9) | 491.5 ± 55.6 nm (8) | – |
| Diazepam | α1,2,3,5, γ | 2.3 ± 0.4 µm (7) | 2.8 ± 0.3 µm (7) | – |
Note significant change in sensitivity to etomidate. Numbers inside parentheses indicate number of cells/condition. Summary of PC sensitivities to various drugs before and after OGD. Value are given as the mean ± SEM.
Significant difference.
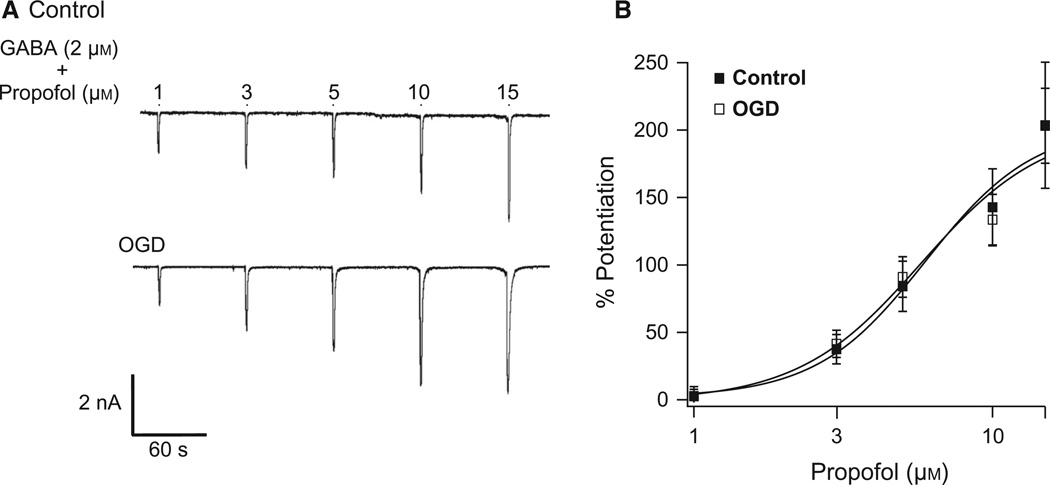
Oxygen and glucose deprivation does not alter Purkinje cell GABAA receptor sensitivity to propofol
The shift in etomidate sensitivity following ischemia led us to hypothesise that the β-subunit of GABAA receptors is altered following ischemia. We tested propofol, another GABAA receptor modulator that binds to the β-subunit, enhancing the activity of all β-subunit-containing receptors (β1–3) equally. Peak current amplitudes in response to 1 s pulses of GABA20 plus increasing concentrations of propofol (1–15 µm) were measured before and after OGD (Fig. 2A). The sensitivity and efficacy of propofol potentiation of GABAA receptors remained unchanged by ischemia (control EC50, 5.6 ± 0.5 µm, n = 9; OGD EC50, 5.8 ± 0.5 µm, n = 12; Fig. 2B).
Fig. 2.
OGD does not affect PC GABAA receptor sensitivity to propofol. (A) Representative recordings from cultured PCs illustrating responses to 1 s applications of 2 µm GABA plus 1, 3, 5, 10, or 15 µm propofol (bars above trace indicate application). (B) Average concentration-response relationships for propofol from control and OGD PCs. The average EC50 for control cells was 5.6 ± 0.5 µm (n = 9). The OGD cell EC50 was 5.8 ± 0.5 µm (n = 12).
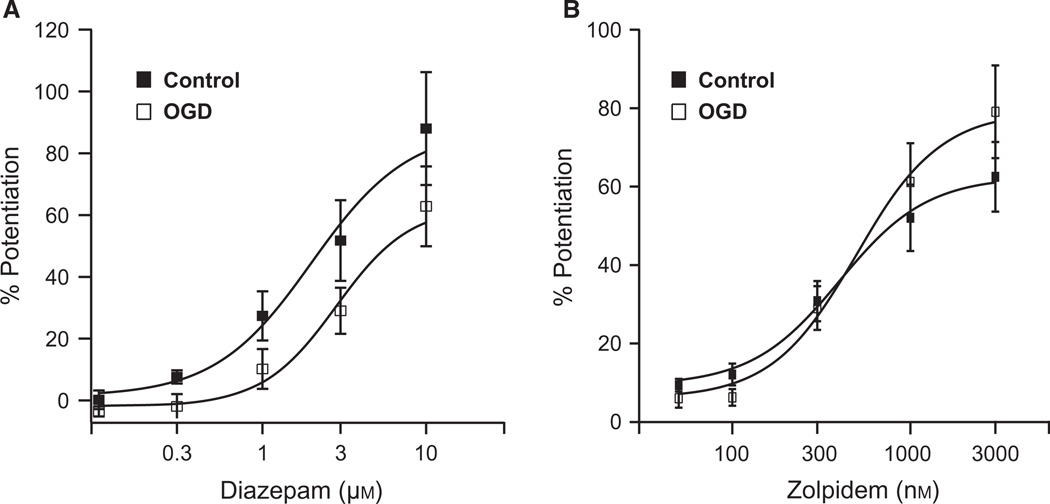
Oxygen and glucose deprivation does not alter GABAA receptor sensitivity to zolpidem and diazepam
We tested the effect of ischemia on the α- and γ-subunits by measuring responses to diazepam and zolpidem, respectively. These compounds bind the α-subunit and potentiate GABAA receptor response to GABA by increasing the current amplitude (Sigel & Buhr, 1997). Zolpidem is more selective for α1-containing receptors and requires the γ2-subunit for potentiation (Buhr & Sigel, 1997). Therefore, we utilised diazepam as an indicator of α-subunit function and zolpidem to more specifically assess α1βxγ2-subunit function. Responses to 1s pulses of GABA20 plus increasing concentrations of diazepam (0.1–10 µm) or zolpidem (50–3000 nm) were measured before and after OGD. OGD did not significantly alter the efficacy or affinity for diazepam (control EC50, 2.27 ± 0.4 µm, n = 7; OGD EC50, 2.81 ± 0.4 µm, n = 6; Fig. 3A) or zolpidem (control EC50, 465.7 ± 75.9 nm, n = 9; OGD EC50, 482.6 ± 62.4 nm, n = 8; Fig. 3B).
Fig. 3.
OGD does not affect PC GABAA receptor sensitivity to diazepam or zolpidem. (A) Average concentration-response relationships for diazepam from control and OGD PCs. The average EC50 for control PCs was 2.27 ± 0.4 µm (n = 7). For OGD cells the average EC50 was 2.81 ± 0.4 µM (n = 6). (B) Average concentration–response relationships for zolpidem from control and OGD PCs. The average EC50 for control cells was 465.7 ± 75.9 nm (n = 9). The average OGD cell EC50 was 482.6 ± 62.4 nm (n = 8).
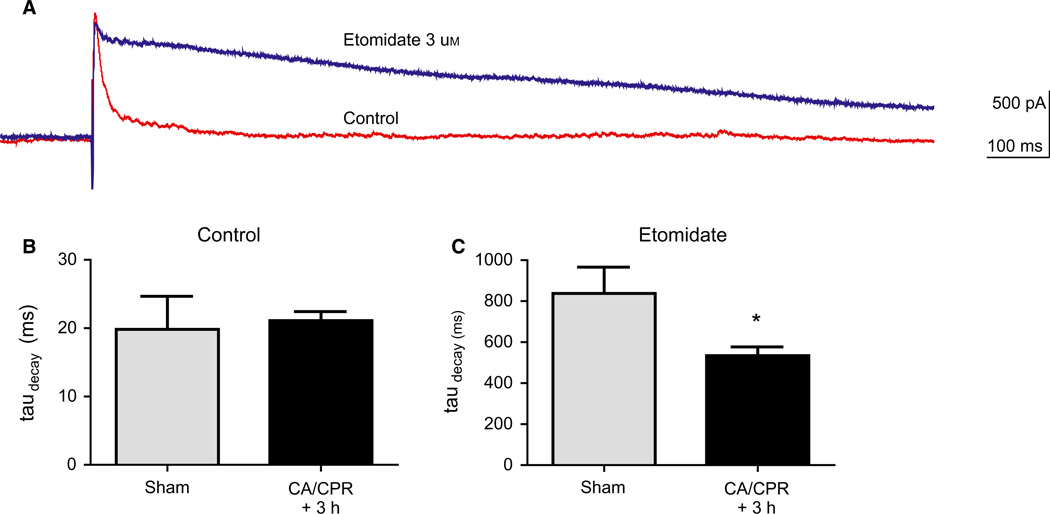
Loss of β2/3-containing receptors following in vivo global ischemia
Global ischemia was induced with 8 min of CA followed by CPR. At 3 h after resuscitation, brains were removed and the cerebellum was sectioned for whole-cell electrophysiology experiments. Stimulation in the inner molecular layer resulted in an outward GABAergic IPSC in Purkinje neurons that was blocked by picrotoxin (data not shown). The decay of the IPSC was fit with a single exponential that was not different between sham and mice that had undergone CA/CPR (Fig. 4) (19.8 ± 4.8 vs. 21.1 ± 1.3 ms). To confirm the loss of β2/3-containing receptors after ischemia, etomidate was applied to slices (3 µm, 10 min) and decay constants were measured. Etomidate prolonged the decay kinetics of the IPSC to 837 ± 128 ms in slices from control sham-operated mice. In contrast, etomidate produced a significantly smaller prolongation after CA/CPR (534.5 ± 42.3 ms) compared with sham (P < 0.05), suggesting a loss of β2/3-containing receptors after in vivo global ischemia.
Fig. 4.
In vivo global ischemia results in reduced potentiation of IPSC decay kinetics. (A) Representative recording showing prolongation of the decay kinetics of the IPSC before (control) and after application of etomidate (3 µm). (B) Summary of IPSC decay time constant (ms) in control conditions in sham and CA/CPR. (C) Summary of IPSC decay time constant in the presence of etomidate. Mean ± SEM; *P < 0.05.
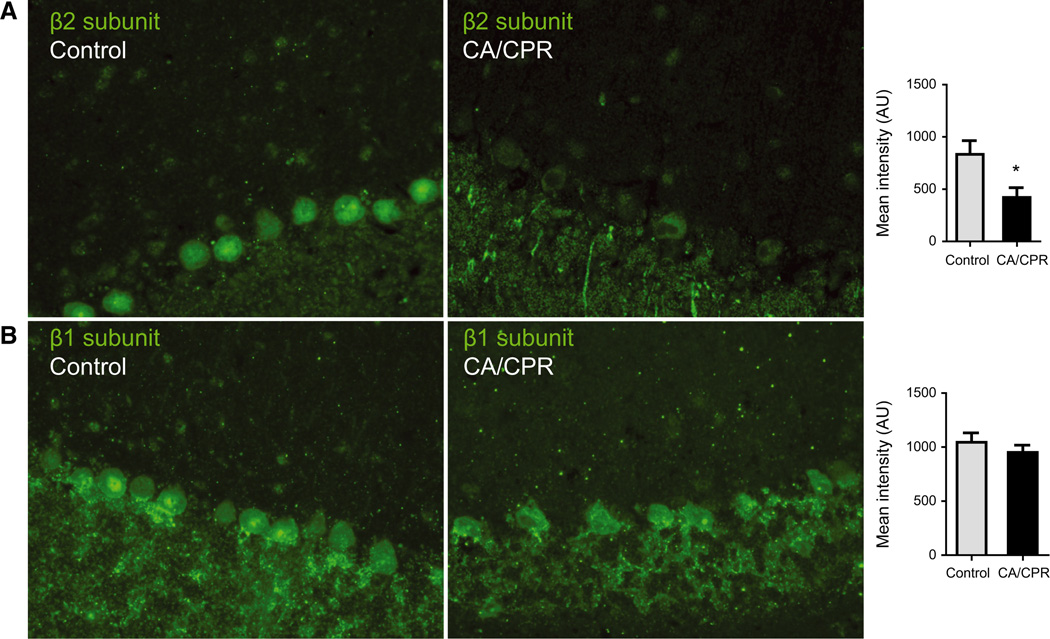
To confirm the loss of β2/3 protein, immunohistochemistry was performed in sections from control mice and after CA with an antibody recognising the β2-subunit. GABAA β2-subunit was clearly detectable in PCs from control and there was a significant reduction after CA/CPR (Fig. 5A). In contrast, GABAA β1-subunit was detected at similar levels in sections from control and CA/CPR (Fig. 5B).
Fig. 5.
Reduced β2- but not β1-subunit expression following in vivo global ischemia. (A) Immunohistochemistry using GABAA receptor β2-subunit antibody on paraffin-embedded sections from control (left) or after CA (middle). Mean intensity of fluorescence [arbitrary units (AUs)] in the soma was significantly reduced (* indicates P < 0.05, n = 4) 24 h after CA (right). (B) Immunohistochemistry using GABAA receptor β1-subunit antibody on sections from control (left) or after CA (middle). Mean intensity of fluorescence was not different between control and CA/CPR.
GABAA receptor β1-subunit is expressed in Purkinje cells
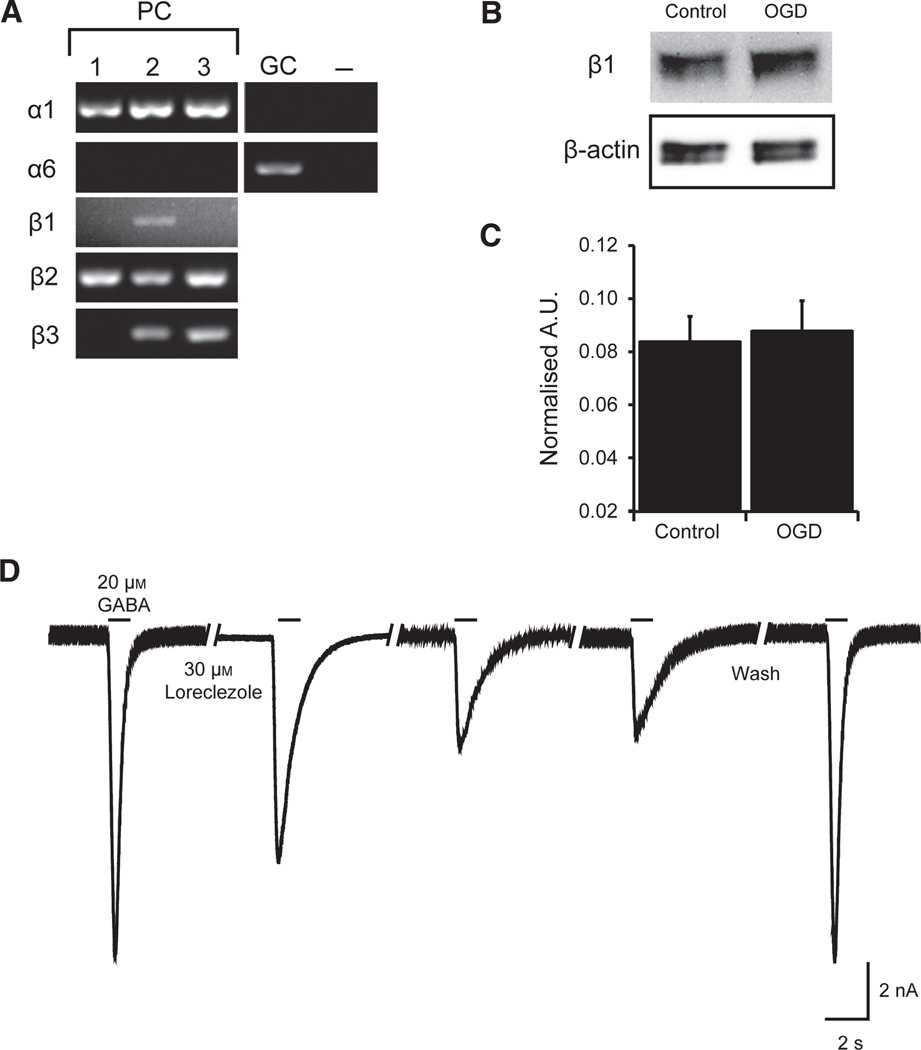
Purkinje cells are thought to express a homogeneous population of GABAA receptors composed of α1-, β2/3-, and γ2-subunits (Wisden et al., 1992; McKernan & Whiting, 1996). However, the pharmacological shift in sensitivity that we observed with etomidate, but not propofol, is consistent with the expression of the β1-subunit. We used single-cell PCR (four to six pooled isolated PCs) to test for the presence β1 mRNA in PCs isolated from acutely dissociated mouse cerebellum (Mintz et al., 1992; Raman & Bean, 1997). The presence of PCs and purity of samples were confirmed by examining α-subunit mRNA expression. In order to verify that we did not contaminate our PC samples with granule cells, the most abundant cell type in the cerebellum, we tested for the presence of α6-subunits. The presence of α1 and absence of α6 are indicative of PCs, whereas the presence of α6 indicates granule cells (Fig. 6A). PC pure samples (lacking α6) were then tested for β-subunit expression. Our PCR results indicate the presence of all three β-subunits (β1–3) (Fig. 6A). Western blot analysis of protein collected from cultured cerebellar neurons confirmed the expression of β1-subunit protein (Fig. 6B). Unlike the α1-subunit, which is degraded following simulated ischemia (Kelley et al., 2008), β1-subunit protein remained unaffected by OGD (Fig. 6C).
Fig. 6.
PCs express the GABAA receptor β1-subunit and protein expression of β1 is not affected by OGD. (A) Representative gels illustrating the mRNA expression of α1-, α6-β1-, β2-, and β3-subunits in isolated PCs by single-cell reverse transcription–PCR. Each lane (1–3) represents one sample containing four to six PCs. Purity of PC samples was confirmed by the absence of α6. Granule cells (GCs) were the positive control for α6. Negative control (−) was Tyrode’s solution collected from the vicinity of PCs. (B) Representative western blot of cerebellar GABAA receptor β1-subunit protein from control cultured PCs and cells that received 2 h OGD and 1 h reoxygenation. β-actin loading control shown in lower panel. (C) Quantification of GABAA receptor β1-subunit protein, normalised to β-actin loading control and expressed as optical density units (n = 5 for each group). Data are mean ± SEM. (D) Representative recording depicting loreclezole inhibition of GABAA receptor-mediated currents. Each bar represents application of 20 µm GABA. After stable peak amplitude response to GABA was achieved, 30 µm loreclezole was preapplied by local perfusion onto the cell for 30 s and loreclezole remained present through three subsequent GABA pulses applied in 30 s intervals. Following 60 s wash out of loreclezole, peak amplitude of GABA response was restored. A.U., arbitrary unit.
To confirm functional expression of the β1-subunit, we tested the effect of loreclezole, a GABAA receptor modulator with both agonist and antagonistic properties. Loreclezole, at low doses, can act as a positive modulator of GABAA receptors containing the β2–3-subunits (Wafford et al., 1994; Wingrove et al., 1994). Conversely, at high concentrations (> 6 µm), loreclezole inhibits GABAA receptors containing the β1-subunit by decreasing channel open time and increasing the occurrence of a closed channel state (Fisher et al., 2000). Consistent with PC expression of β1-subunits, we observed that pre-application of loreclezole (30 µm, 30 s) caused an initial 30% reduction in GABA-mediated current that maximally decreased with subsequent pulses of GABA by 65.5 ± 6.8% (n = 4), and almost completely reversed following 60–120 s washout (92 ± 5.2% recovery) (Fig. 6D).
Discussion
Summary of findings
Ischemia significantly decreased PC GABAA receptor sensitivity to etomidate with no change in propofol sensitivity, thus revealing the presence of β1-containing receptors. We verified β-subunit expression in PCs by single-cell PCR analysis and detected β1-subunit transcripts. Western blot analysis also confirmed that β1-subunit protein was expressed in cultured cerebellar neurons and we observed that loreclezole inhibited PC GABA-mediated current. This is further supported by our immunohistochemical data in adult mouse cerebellum. Taken together, our data indicate the presence of β1-containing GABAA receptors in Purkinje neurons, which challenges current dogma that only β2/3-subunits are expressed in this cell type (Wisden et al., 1992; McKernan & Whiting, 1996). The shift in etomidate sensitivity observed following OGD in PCs suggested a reduced contribution of β2/3-subunit-containing GABAA receptors. The sensitivity to other GABAA receptor modulators, propofol, diazepam, and zolpidem, remained unaffected by ischemia.
Etomidate sensitivity was altered by ischemia but propofol was not
Etomidate acts on the β-subunit of GABAA receptors with a higher affinity for the β2- and β3-subunits compared with β1 (Belelli et al., 1997). Using heterologous expression systems, the EC50 for etomi-date has been reported to range between 0.6 and 1.2 µm for GABAA receptors composed of α1β2γ2 (Hill-Venning et al., 1997), and 4 µm for receptors composed of α1β3γ2 (Belelli et al., 2003). The presence of β1 increases the EC50 to a range between 6 and 11 µm (Hill-Venning et al., 1997). Our data are consistent with several previous reports indicating that PCs predominantly express β2/3-containing receptors, exhibiting an EC50 for etomidate of 2.9 µm under control conditions. Following OGD, the EC50 for etomidate significantly increased to 3.8 µm with no change in propofol sensitivity. Similarly, following CA/CPR, the efficacy of etomidate is reduced in synaptic recordings of PC GABAergic IPSCs. The simplest explanation is that ischemia preferentially decreases expression of β2/3-containing receptors, sparing β1-containing receptors. Indeed, OGD had no effect on β1-subunit protein levels, suggesting that this subunit is spared from ischemia-induced downregulation, in contrast to our previous observation of OGD-induced degradation of α1-subunits (Kelley et al., 2008). Our immunohistochemistry data also support our finding that there is a preferential loss of β2-subunit with no detectable change in β1-subunit expression following in vivo ischemia. Interestingly, ischemia did not alter the sensitivity of GABAA receptors to compounds known to interact with α- and γ-subunits. In total, these data lead to the conclusion that ischemia causes decreased total GABAA receptor levels (Kelley et al., 2008) and that α1β2/3γ2 are preferentially silenced/degraded, leaving a larger relative complement of α1β1γ2 receptors.
Single-cell polymerase chain reaction demonstrated β1 expression
Contrary to our findings, the literature indicates that cerebellar PCs do not express β1-subunits (Wisden et al., 1992; Sergeeva et al., 2010). The β1-subunit has not been detected in PCs by single-cell PCR (Ruano et al., 1997; Sim et al., 2000) or in situ hybridisation (Laurie et al., 1992; Wisden et al., 1992). However, there are reports describing β1 mRNA labeling a distinct band at the interface of the granule cell layer and molecular layer, where PCs reside (Zhang et al., 1991; Zdilar et al., 1992; Lein et al., 2007). One explanation for this discrepancy is that PCs express the β1-subunit transcript at low levels. Indeed, in our hands, PC β-subunit transcripts were more difficult to detect compared with α1. Additional experimental measures were employed to accurately detect low-copy genes by single-cell PCR. First, we tested multiple sets of primers to find those optimised for low-copy transcripts, including utilising a primer sequence for the β1-subunit from the Allen Mouse Brain Atlas (www.brain-map.org) that positively labeled PCs by in situ hybridisation (Lein et al., 2007). We utilised the nested PCR technique to enhance our amplicon levels from dilute cDNA and avoid false negatives from non-specific amplification. Consistent with our single-cell PCR data, the actions and immunohistochemistry data of lorecelezole suggest that cerebellar PCs express GABAA receptors containing functional β1-subunits.
The molecular mechanism of the selective loss of β2/3 observed after ischemia remains unclear. β-subunits are substrates for several protein kinases, including protein kinase A and C and Ca2+/calmodulin-dependent protein kinase II, and phosphorylation of the various β-subunits has been shown to modulate receptor function and trafficking. It was recently demonstrated that etomidate sensitivity is not altered by acute phosphorylation (Drexler et al., 2009), indicating that altered trafficking is a more likely explanation for the ischemia-induced decrease in etomidate sensitivity. In vitro ischemia in hippocampal neurons results in a loss of surface expression of GABAA receptors that is dependent on an interaction of the β-subunit with the clathrin adaptor protein 2 (AP2) (Smith et al., 2012) and previous work has demonstrated that the interaction of AP2 with GABAA receptors is modulated by phosphorylation (Kittler et al., 2005). Thus, further work is needed to explore the possibility that ischemia simulates differential phosphorylation of β1- compared with β2/3-containing receptors, and consequent changes in trafficking and GABAA receptor subunit composition.
Conclusion
From our data we conclude that PCs express the β1-subunit, contrary to previous reports. The relative contribution of β1-containing GABAA receptors appears to increase following ischemia, thereby decreasing sensitivity to etomidate. Although β1 transcript levels may be lower than β2/3 levels, β1-subunit protein is easily detectable by western blot and protein levels remain unaffected by ischemia. β1-subunit expression in PCs is novel and controversial, but is important for understanding inhibition in the cerebellum and GABAA receptor expression and trafficking in ischemia. We provide molecular and functional data indicating that PCs express β1-subunits and the re-evaluation of previous findings may be warranted. Recently, a new GABAA receptor β1-subunit antagonist was found to reduce ataxia in mice (Gee et al., 2010). Motor coordination deficits and ataxia are commonly experienced following cerebral ischemia (Lim et al., 2004; Venkatesan & Frucht, 2006; Lu-Emerson & Khot, 2010), likely due to PC dysfunction and cell death. Therefore, the increased propensity for ataxia following ischemia may be due to the increased relative contribution of β1-containing GABAA receptors in PCs. The physiological and pathophysiological significance of β1-subunit expression in PCs remains to be determined.
Acknowledgements
We would like to thank the laboratory of I.M. Raman (Chicago, IL, USA) for supplying the PC dissociation protocol and training in the method assisted by Mark Benton. Chris Bond provided assistance with primer design for single-cell PCR. Martha Bosch and Oline Ronnekleiv provided assistance with the single-cell PCR protocol. This work was supported by NIH R01NS058792. J.O. was funded by NIH T32GM082770. M.H.K. was funded by NIH F31NS060220.
Abbreviations
- CA
cardiac arrest
- CPR
cardiopulmonary resuscitation
- IPSC
inhibitory postsynaptic current
- nt
nucleotide
- OGD
oxygen and glucose deprivation
- PC
Purkinje cell
- PCR
polymerase chain reaction
Footnotes
The authors have no conflicts of interest.
References
- Arancibia-Carcamo IL, Yuen EY, Muir J, Lumb MJ, Michels G, Saliba RS, Smart TG, Yan Z, Kittler JT, Moss SJ. Ubiquitin-dependent lysosomal targeting of GABA(A) receptors regulates neuronal inhibition. Proc. Natl. Acad. Sci. USA. 2009;106:17552–17557. doi: 10.1073/pnas.0905502106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardeshiri A, Kelley MH, Korner IP, Hurn PD, Herson PS. Mechanism of progesterone neuroprotection of rat cerebellar Purkinje cells following oxygen-glucose deprivation. Eur. J. Neurosci. 2006;24:2567–2574. doi: 10.1111/j.1460-9568.2006.05142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ, Peters JA, Wafford K, Whiting PJ. The interaction of the general anesthetic etomidate with the gamma-aminobutyric acid type A receptor is influenced by a single amino acid. Proc. Natl. Acad. Sci. USA. 1997;94:11031–11036. doi: 10.1073/pnas.94.20.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Muntoni AL, Merrywest SD, Gentet LJ, Casula A, Callachan H, Madau P, Gemmell DK, Hamilton NM, Lambert JJ, Sillar KT, Peters JA. The in vitro and in vivo enantioselectivity of etomidate implicates the GABAA receptor in general anaesthesia. Neuropharmacology. 2003;45:57–71. doi: 10.1016/s0028-3908(03)00144-8. [DOI] [PubMed] [Google Scholar]
- Benton MD, Raman IM. Stabilization of Ca current in Purkinje neurons during high-frequency firing by a balance of Ca-dependent facilitation and inactivation. Channels. 2009;3:393–401. doi: 10.4161/chan.3.6.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasko J, Rai P, Sabol MK, Patrikios P, Ross DT. The AMPA antagonist NBQX provides partial protection of rat cerebellar Purkinje cells after cardiac arrest and resuscitation. Brain Res. 1995;699:133–138. doi: 10.1016/0006-8993(95)01015-n. [DOI] [PubMed] [Google Scholar]
- Buhr A, Sigel E. A point mutation in the gamma2 subunit of gamma-aminobutyric acid type A receptors results in altered benzodiazepine binding site specificity. Proc. Natl. Acad. Sci. USA. 1997;94:8824–8829. doi: 10.1073/pnas.94.16.8824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler B, Jurd R, Rudolph U, Antkowiak B. Distinct actions of etomidate and propofol at β3-containing γ-aminobutyric acid type A receptors. Neuropharmacology. 2009;57:446–455. doi: 10.1016/j.neuropharm.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Fisher JL, Hinkle DJ, Macdonald RL. Loreclezole inhibition of recombinant alpha1beta1gamma2L GABA(A) receptor single channel currents. Neuropharmacology. 2000;39:235–245. doi: 10.1016/s0028-3908(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Fonnum F, Lock EA. Cerebellum as a target for toxic substances. Toxicol. Lett. 2000;112–113:9–16. doi: 10.1016/s0378-4274(99)00246-5. [DOI] [PubMed] [Google Scholar]
- Gee KW, Tran MB, Hogenkamp DJ, Johnstone TB, Bagnera RE, Yoshimura RF, Huang JC, Belluzzi JD, Whittemore ER. Limiting activity at beta1-subunit-containing GABAA receptor subtypes reduces ataxia. J. Pharmacol. Exp. Ther. 2010;332:1040–1053. doi: 10.1124/jpet.109.161885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill-Venning C, Belelli D, Peters JA, Lambert JJ. Subunit-dependent interaction of the general anaesthetic etomidate with the gamma-aminobutyric acid type A receptor. Br. J. Pharmacol. 1997;120:749–756. doi: 10.1038/sj.bjp.0700927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn M, Schlote W. Delayed neuronal death and delayed neuronal recovery in the human brain following global ischemia. Acta Neuropathol. 1992;85:79–87. doi: 10.1007/BF00304636. [DOI] [PubMed] [Google Scholar]
- Kelley MH, Taguchi N, Ardeshiri A, Kuroiwa M, Hurn PD, Trayst-man RJ, Herson PS. Ischemic insult to cerebellar Purkinje cells causes diminished GABAA receptor function and allopregnanolone neuroprotection is associated with GABAA receptor stabilization. J. Neurochem. 2008;107:668–678. doi: 10.1111/j.1471-4159.2008.05617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley MH, Kuroiwa M, Taguchi N, Herson PS. Sex difference in sensitivity to allopregnanolone neuroprotection in mice correlates with effect on spontaneous inhibitory post synaptic currents. Neuropharmacology. 2011;61:724–729. doi: 10.1016/j.neuropharm.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Chen G, Honing S, Bogdanov Y, McAinsh K, Aranci-bia-Carcamo IL, Jovanovic JN, Pangalos MN, Haucke V, Yan Z, Moss SJ. Phospho-dependent binding of the clathrin AP2 adaptor complex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proc. Natl. Acad. Sci. USA. 2005;102:14871–14876. doi: 10.1073/pnas.0506653102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH, Wisden W. The distribution of 13 GA-BAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J. Neurosci. 1992;12:1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Young-strom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Li H, Siegel RE, Schwartz RD. Rapid decline of GABAA receptor subunit mRNA expression in hippocampus following transient cerebral ischemia in the gerbil. Hippocampus. 1993;3:527–537. doi: 10.1002/hipo.450030412. [DOI] [PubMed] [Google Scholar]
- Lim C, Alexander MP, LaFleche G, Schnyer DM, Verfaellie M. The neurological and cognitive sequelae of cardiac arrest. Neurology. 2004;63:1774–1778. doi: 10.1212/01.wnl.0000144189.83077.8e. [DOI] [PubMed] [Google Scholar]
- Lu-Emerson C, Khot S. Neurological sequelae of hypoxicischemic brain injury. NeuroRehabilitation. 2010;26:35–45. doi: 10.3233/NRE-2010-0534. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Mihic SJ, Whiting PJ, Klein RL, Wafford KA, Harris RA. A single amino acid of the human gamma-aminobutyric acid type A receptor gamma 2 subunit determines benzodiazepine efficacy. J. Biol. Chem. 1994;269:32768–32773. [PubMed] [Google Scholar]
- Mintz IM, Adams ME, Bean BP. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992;9:85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- Neelands TR, Greenfield LJ, Jr., Zhang J, Turner RS, Macdonald RL. GABAA receptor pharmacology and subtype mRNA expression in human neuronal NT2-N cells. J. Neurosci. 1998;18:4993–5007. doi: 10.1523/JNEUROSCI.18-13-04993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABAA receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu M, Buchkremer-Ratzmann I, Schiene K, Schroeter M, Witte OW, Zilles K. Bihemispheric reduction of GABAA receptor binding following focal cortical photothrombotic lesions in the rat brain. Brain Res. 1998;813:374–380. doi: 10.1016/s0006-8993(98)01063-4. [DOI] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J. Neurosci. 1997;17:4517–4526. doi: 10.1523/JNEUROSCI.17-12-04517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruano D, Perrais D, Rossier J, Ropert N. Expression of GABA (A) receptor subunit mRNAs by layer V pyramidal cells of the rat primary visual cortex. Eur. J. Neurosci. 1997;9:857–862. doi: 10.1111/j.1460-9568.1997.tb01435.x. [DOI] [PubMed] [Google Scholar]
- Sanna E, Mascia MP, Klein RL, Whiting PJ, Biggio G, Harris RA. Actions of the general anesthetic propofol on recombinant human GABAA receptors: influence of receptor subunits. J. Pharmacol. Exp. Ther. 1995;274:353–360. [PubMed] [Google Scholar]
- Schiene K, Bruehl C, Zilles K, Qu M, Hagemann G, Kraemer M, Witte OW. Neuronal hyperexcitability and reduction of GABAA-receptor expression in the surround of cerebral photothrombosis. J. Cereb. Blood Flow Metab. 1996;16:906–914. doi: 10.1097/00004647-199609000-00014. [DOI] [PubMed] [Google Scholar]
- Schwartz-Bloom RD, Sah R. Gamma-aminobutyric acid(A) neurotransmission and cerebral ischemia. J. Neurochem. 2001;77:353–371. doi: 10.1046/j.1471-4159.2001.00274.x. [DOI] [PubMed] [Google Scholar]
- Sergeeva OA, Kletke O, Kragler A, Poppek A, Fleischer W, Schubring SR, Görg B, Haas HL, Zhu X-R, Lübbert H, Gisselmann G, Hatt H. Fragrant dioxane derivatives identify β1-subunit-containing GABAA receptors. J. Biol. Chem. 2010;285:23985–23993. doi: 10.1074/jbc.M110.103309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel E, Buhr A. The benzodiazepine binding site of GABAA receptors. Trends Pharmacol. Sci. 1997;18:425–429. doi: 10.1016/s0165-6147(97)01118-8. [DOI] [PubMed] [Google Scholar]
- Sim JA, Skynner MJ, Pape JR, Herbison AE. Late postnatal reorganization of GABA(A) receptor signalling in native GnRH neurons. Eur. J. Neurosci. 2000;12:3497–3504. doi: 10.1046/j.1460-9568.2000.00261.x. [DOI] [PubMed] [Google Scholar]
- Smith KR, Muir J, Rao Y, Browarski M, Gruenig MC, Sheehan DF, Haucke V, Kittler JT. Stabilization of GABAA receptors at endocytic zones is mediated by an AP2 binding motif within the GABAA receptor β3 subunit. J. Neurosci. 2012;32:2485–2498. doi: 10.1523/JNEUROSCI.1622-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan A, Frucht S. Movement disorders after resuscitation from cardiac arrest. Neurol. Clin. 2006;24:123–132. doi: 10.1016/j.ncl.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Bain CJ, Quirk K, McKernan RM, Wingrove PB, Whiting PJ, Kemp JA. A novel allosteric modulatory site on the GABAA receptor beta subunit. Neuron. 1994;12:775–782. doi: 10.1016/0896-6273(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Wingrove PB, Wafford KA, Bain C, Whiting PJ. The modulatory action of loreclezole at the gamma-aminobutyric acid type A receptor is determined by a single amino acid in the beta 2 and beta 3 subunit. Proc. Natl. Acad. Sci. USA. 1994;91:4569–4573. doi: 10.1073/pnas.91.10.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Laurie D, Monyer H, Seeburg P. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J. Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdilar D, Luntz-Leybman V, Frostholm A, Rotter A. Differential expression of GABAA/benzodiazepine receptor beta 1, beta 2, and beta 3 subunit mRNAs in the developing mouse cerebellum. J. Comp. Neurol. 1992;326:580–594. doi: 10.1002/cne.903260407. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Sato M, Tohyama M. Region-specific expression of the mRNAs encoding beta subunits (beta 1, beta 2, and beta 3) of GA-BAA receptor in the rat brain. J. Comp. Neurol. 1991;303:637–657. doi: 10.1002/cne.903030409. [DOI] [PubMed] [Google Scholar]