Abstract
Purpose
To determine whether Carbamazepine (CBZ) is a radiation protector and/or mitigator.
Materials and Methods
urine hematopoietic progenitor 32D cl 3 cells were incubated in 1, 10, or 100 μM CBZ 1 hour before or immediately after 0 – 8 Gy irradiation and assayed for clonogenic survival. Autophagy was assayed by immunoblot for microtubule-associated protein light chain 3 (LC3). In vivo radioprotection and mitigation were determined with C57BL/6NTac mice.
Results
CBZ treatment at 1, 10 or 100 μM for 1 hour prior to irradiation increased radioresistance (the dose for 37% survival or D0) from control 1.5 ± 0.1 Gy to 2.1 ± 0.2 Gy (p = 0.012), 2.3 ± 0.1 Gy (p = 0.010), and 3.6 ± 0.7 Gy (p = 0.003), respectively; after irradiation increased the extrapolation number (ñ) from 1.5 ± 0.3 to 10.1 ± 4.2 (p = 0.011), 5.5 ± 1.7 (p = 0.019), and 3.6 ± 0.8 (p = 0.014), respectively, and increased autophagy. CBZ treated mice 10 min or 24 hrs before or 10 min or 12 hrs after 9.25 Gy total body irradiation (TBI) showed increased survival (p = 0.012, 0.011, 0.0002, and 0.017, respectively).
Conclusion
CBZ may be a useful radiation protector and mitigator.
Keywords: Carbamazepine, radiation protection, radiation mitigation, autophagy
Introduction
During autophagy, cells recycle macromolecules by encapsulating proteins and organelles in double membrane autophagic vacuoles that fuse with lysosomes for content degradation (Kondo et al. 2005). Autophagy is important in the normal cellular physiology of differentiation, tissue remodeling and adaptation to environmental stresses such as nutrient deprivation and infection (Shintani and Klionsky 2004). Aberrant autophagy can result in hepatic, muscular and neurodegenerative disorders (Hippert et al. 2006). The role of autophagy in the cellular response to ionizing radiation is controversial (Kondo et al. 2005; Robert et al. 2011).
Recent insight into autophagy comes from investigation of its role in cancer biology and therapy (Dalby et al. 2010). Autophagy may be involved in irradiation induced cancer cell death (Paglin et al. 2001), consistent with the effect of other treatments including tamoxifen, histone deacetylase inhibitors, EB1089, tyrosine kinase inhibitors (Imatinib), Resveratrol and alkylating agents (Høyer-Hansen and Jäättelä 2008). There is evidence to suggest that autophagy may be involved in cancer cell death (Type II programmed cell death) independent of other cell death mechanisms including apoptosis and necrosis (Kondo et al. 2005).
There is also data that indicate autophagy is a response that promotes cell survival. Both cancer and normal cells in nutrient deplete environments avoid apoptosis by increased autophagy (Boya et al. 2005). Autophagy may promote cellular survival by removing depolarized mitochondria or free-radical damaged organelles that otherwise may activate cell death pathways (Elmore et al. 2001; Alva et al. 2004). Some evidence suggests that autophagy may be a protective mechanism for cells exposed to ionizing radiation (Paglin et al. 2001). We reasoned that promoting cell survival by upregulation of autophagy might be useful in acute radioprotection.
We evaluated the autophagy-enhancing drug Carbamazepine (CBZ), currently approved for the clinical treatment of bipolar disorder, trigeminal neuralgia and epilepsy, as a radiation protector and mitigator. While the known action of CBZ is enhancing sodium-channel inactivation in the plasma membrane (MacDonald and Kelly 1995), the drug has recently been shown to increase effective clearance of mutant α1-antitrypsin in hepatocytes via upregulating autophagy both in vitro and in vivo (Hidvegi et al. 2010). Given its extensive historical use and clinical safety profile, evaluation of CBZ for its effectiveness as a radiation protector and mitigator might facilitate efficient and cost-effective clinical translation of its use in radiation protection. We provide evidence that CBZ functions as a radiation protector and mitigator in vitro and in vivo.
Materials and methods
Cell Culture
The 32D cl 3, murine, interleukin-3 (IL-3) dependent hematopoietic progenitor cell line was cultured as described previously (Epperly et al. 2002; Epperly et al. 2003). Briefly, cells were grown at 37° C in high humidity incubators with 5% CO2 and maintained in suspension in Iscove’s modified medium containing 10% WEHI-3 (Walter and Elizabeth Hall Institute-3) cell conditioned medium as a source of Interleukin-3 (murine IL-3). This is a well-described mouse monomyeloid leukemia cell line that produces IL-3, a multipotential hematopoietic growth factor C (Ralph et al. 1976; Greenberger et al. 1983).
In Vitro Irradiation Experiments
CBZ (Tegretol) (Sigma Chemical Company, St. Louis, MO, USA) was prepared in dimethyl sulfoxide (DMSO) (stock 10 mM) and 32D cl 3 cells were incubated with 1, 10, or 100 μM CBZ either 1 hour before or immediately after irradiation. Glyburide (Sigma Chemical Company), a known radioprotector (Jiang et al. 2009), was dissolved in dimethyl sulfoxide (DMSO) (stock 10 mM) and added to the cell culture as a positive control at a final concentration of 10 μM, 1 hour prior to or immediately after irradiation. While DMSO has been shown to be itself a radioprotector in vivo, the concentrations used (< 1%) have no effect on the radiobiology of 32D cl 3 cells (Epperly et al. 2002).
Cells were irradiated in plastic flasks at a dose rate of 70 cGy / minute in logarithmic phase (Epperly et al. 2002), using a Shepherd Mark 1 irradiator with a Cesium source (J. L. Shepherd, San Fernando, CA, USA). Cells were then centrifuged, washed in complete medium and plated at 500 or 1000 cells per mL in tissue culture plates in 0.5% methylcellulose for survival curve assays (Epperly et al. 2003).
Colonies of greater than or equal to 50 cells were scored on day 7. Cells were plated in quadruplicate and experiments repeated at least in triplicate. The data were plotted using linear quadratic (LQ) and single-hit, multi-target models (SHMT) according to published methods (Epperly et al. 2002; Epperly et al. 2003). Student’s t-test was used to compare the different treatment groups in the in vitro survival curves.
In Vivo Irradiation Experiments
In first experiments, C57BL/6NTac (Taconic Laboratories, Hudson, NY, USA) adult female mice weighing 20 – 22 grams received intraperitoneal (IP) injections before or after 9.25 Gy total body irradiation (TBI) as follows: 1) Cremphor/ethanol vehicle only immediately after, 2) CBZ (10 mg/kg) in vehicle immediately before or 3) CBZ immediately after TBI (N = 15 per group). The irradiation dose 9.25 Gy TBI was delivered at 70 cGy/min with a Gamma Cell Cesium irradiator. Preliminary experiments with 5 mg/kg, 10 mg/kg, and 20 mg/kg drug showed optimal survival after TBI with the 10 mg/kg doses, therefore, this drug dose was used in all the in vivo experiments with each of several irradiation doses. In second experiments mice received one of the following: 1) Cremphor/ethanol vehicle only immediately after 9.25 Gy TBI; for protection 2) CBZ (10 mg/kg) 24 hours before TBI or 3) 10 minutes before TBI; for mitigation 4) CBZ (10 mg/kg) 10 minutes after TBI, 5) 12 hours after TBI or 6) 24 hours after TBI (N = 15-30 per group). Mice were housed 5 per cage and fed standard laboratory Purina chow (Test Diet, Richmond, IN, USA). Survival was calculated according to a Log-rank method (Jiang et al. 2009). All animal protocols were approved by the Institutional Animal Care and Use Committee. Veterinary care was provided by the Division of Laboratory Animal Research, University of Pittsburgh.
Calculation of In Vivo TBI Dose Modifying Factor
The dose-modifying factor (DMF) is the ratio of doses with and without a radiation-modifying agent that produce the same biologic effect (Thames and Rasmussen 1978). Groups of 15 C57BL/6NTac female mice were irradiated to 9.25, 9.5, 10, 10.5, 11, or 11.5 Gy TBI and given IP injections of 10 mg/kg of CBZ 10 min after irradiation. Survival of mice treated with each irradiation dose plus CBZ was compared to survival of mice receiving 9.25 Gy only (no CBZ) using a log rank test. The DMF for in vivo TBI mitigation measuring survival was calculated by dividing the highest dose of irradiation given to mice that received CBZ, which yielded the same survival as mice that received 9.25 Gy only, by 9.25 (Brown et al. 2010). In this experiment mice receiving 10.5 Gy + CBZ had statistically the same survival as irradiation only to 9.25 Gy (p = 0.6850) and both CBZ plus 10.5 Gy treatment and control 9.25 Gy irradiation groups had a median survival time of 18 days. Therefore we divided 10.5 Gy by 9.25 Gy to obtain the DMF of 1.13.
Western Blot Analysis for Autophagy
We measured autophagy induced by irradiation using an assay for microtubule-associated protein light chain 3 (LC3), a marker for autophagosomes (Kabeya et al. 2000). 32D cl 3 cells were treated with 1, 10, 50, or 100 μM CBZ for 1 hour and then irradiated to 5 or 10 Gy (Gamma Cell, 70 cGy/min). At 12, 24 or 48 hours, cells were centrifuged, washed in cold phosphate buffer solution (PBS) and resuspended in NP-40 lysis buffer (50 mM Tris, pH 7.8, 10 mM ethylenediaminetetraacetic acid (EDTA), 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1% NP-40 and a protease inhibitor cocktail tablet (Roche Diagnostics, Indianapolis, IN, USA)). Protein was quantified by Bradford assay (Bio-Rad Laboratories, Hercules, CA, USA) and 10-20 μg samples fractionated in precast 15% polyacrylamide gels. Nitrocellulose membranes were blocked with Tris-Buffered Saline (TBS), 0.1% Tween 20 and 5% milk (TBST) and incubated with primary LC3 (1:250) (Novus Biologicals, Littleton, CO, USA) or α-Tubulin antibody (1:20,000) (Sigma Aldrich, St. Louis, MO) in 5% milk TBST solution (Hidvegi et al. 2010). Horseradish peroxidase anti-rabbit or anti-mouse (1:50,000 in TBST) was used as secondary antibody (Promega, Pittsburgh, PA, USA) and blots were visualized with Super Signal West Dura (Thermo Scientific, Rockford, IL, USA). Band densities were quantified with Image J (National Institutes of Health, www.rsbweb.nih.gov/ij) by published methods (Miller 2010). The proportion of LC3II (membrane bound LC3) to LC3I (cytosolic), which correlates with the extent of autophagosome formation (Kabeya et al. 2000) was compared between each CBZ treated and/or irradiated sample to the ratio in the sample that received 0 Gy and 0 μM CBZ for each respective time point.
Results
CBZ is a radiation protector and mitigator for 32D cl 3 cells
We first tested each of three concentrations of CBZ added prior to irradiation of 32D cl 3 cells. Adding 1, 10, or 100 μM CBZ resulted in radiation protection as reflected in an increase in the D0 (dose resulting in 37% survival) by SHMT model (Table I, Table II, and Figure 1B, D, F, H). The combined results of three separate experiments from three separate days demonstrated a statistically significant increase in D0 when the drug was added prior to irradiation for each of three concentrations (Table I). We also present data plotted by the LQ method (Fig. 1A, C, E, G). There were several interesting observations. In Figure 1A, radioprotection was better with 1 μM CBZ added after irradiation than if added before irradiation. This, observation may reflect two different mechanisms of radiation dose modification by CBZ, one of which (protection) is not fully effective at 1 μM CBZ compared to effectiveness at 10 μM (Figure 1C) and 100 μM (Figure 1E). When CBZ was added both before and after irradiation, protection (Figure 1G) was not as effective as when drug was added only before irradiation. This observation may reflect two biochemical mechanisms of action with the post-irradiation effect overriding or reducing the pre-irradiation effect.
Table I.
Effects of Carbamazepine on 32D cl 3 Cell Clonogenic Radiation Survival
| Drug Concentration Added | D0 (Gy) | ñ |
|---|---|---|
| Control | 1.5 ± 0.1 | 1.5 ± 0.3 |
| 1 μM Before Irradiation | 2.1 ± 0.2 (p = 0.012) | 1.0 ± 0.1 |
| 1 μM After Irradiation | 1.6 ± 0.2 | 10.1 ± 4.2 (p = 0.011) |
| 10 μM Before Irradiation | 2.3 ± 0.1 (p = 0.010) | 1.0 ± 0.1 |
| 10 μM After Irradiation | 1.2 ± 0.1 | 5.5 ± 1.7 (p = 0.019) |
| 100 μM Before Irradiation | 3.6 ± 0.7 (p = 0.003) | 1.0 ± 0.1 |
| 100 μM After Irradiation | 1.3 ± 0.8 | 3.6 ± 0.8 (p = 0.014) |
| Glyburide 10 μM Before Irradiation (Jiang et al. 2009) | 1.1 ± 0.2 | 34.9 ± 0.5 (p = 0.002) |
Four-well plates with 500 or 1000 cells per plate were scored at day 7 for colonies of ≥ 50 cells as described in Materials and Methods. Drugs were dissolved DMSO as described in the Methods. The p values compare each drug concentration above to non-drug treated irradiated cells using the SHMT model (Epperly et al. 2003). DMSO (< 1%) was added to control irradiated cultures and had no effect on the radiation survival compared to irradiation alone.
Table II.
Effects of Carbamazepine on Radiosensitivity of 32D cl 3 Wells When Administered Both Before and After Irradiation
| Drug Concentration Added | D0 (Gy) | Ñ |
|---|---|---|
| Control | 1.2 ± 0.1 | 1.0 ± 0.1 |
| 10 μM Before Irradiation | 1.5 ± 0.1 (p = 0.040) | 1.1 ± 0.1 |
| 10 μM After Irradiation | 1.1 ± 0.1 | 2.3 ± 0.3 (p = 0.032) |
| 10 μM Before and After Irradiation | 1.1 ± 0.1 | 1.9 ± 0.2 (p = 0.033) |
4-well plates with 500 or 1000 cells per plate were scored at day 7 for colonies of ≥ 50 cells as described in the Materials and Methods. The p values compare each drug above to non-drug treated irradiated cells using the SHMT model (Epperly et al. 2003).
Figure 1.
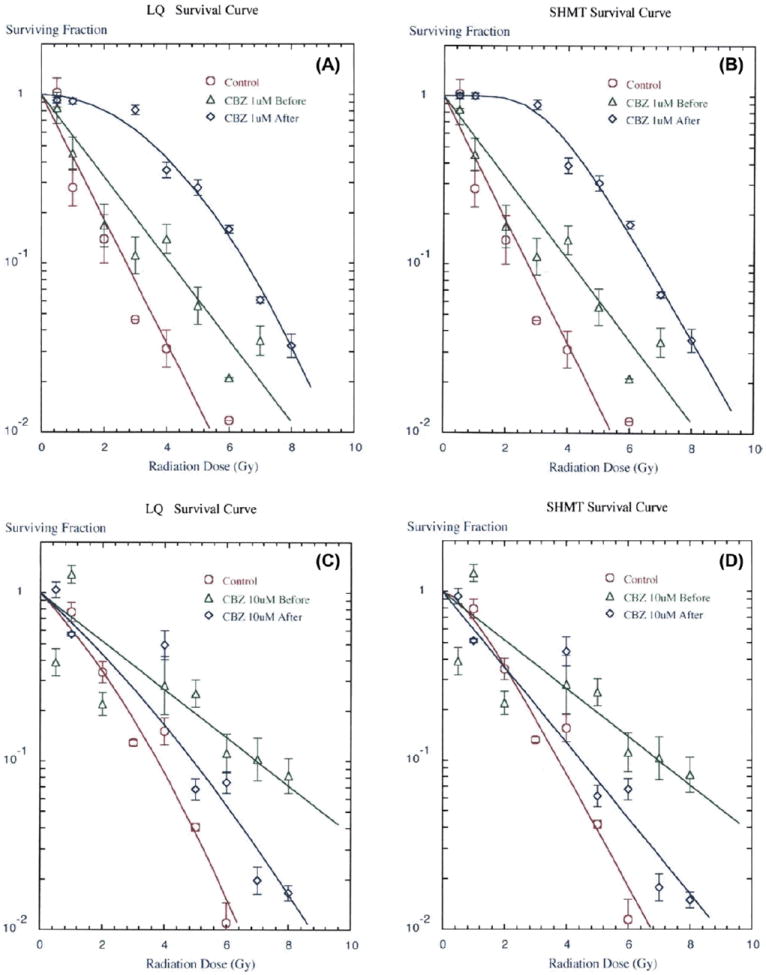
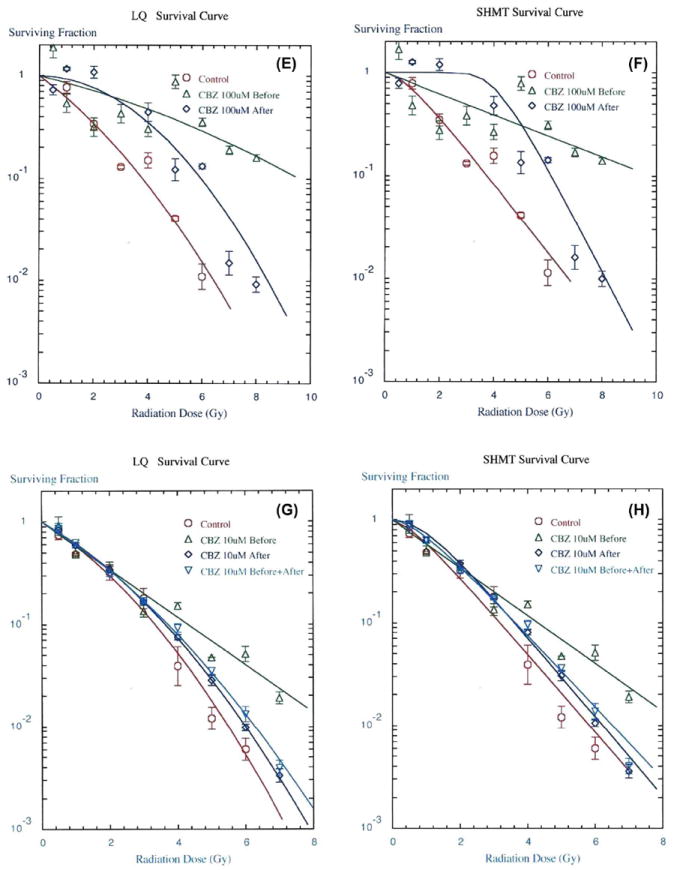
CBZ results in a radioprotective and mitigative effect on 32D cl 3 cells in clonogenic survival curves. Representative LQ (panels A, C, E, G) and SHMT (panels B, D, F, H) plot of colony formation by irradiated and quantified cells treated with CBZ in DMSO (dimethyl sulfoxide) added to culture medium 1 hour before or immediately after irradiation at A,B) 1 μM (p = 0.012 or 0.011, respectively), C,D) 10 μM (p = 0.010 or 0.019, respectively), E,F) 100 μM (p = 0.003 or 0.014, respectively), or G,H) CBZ in DMSO added to culture medium 1 hour before, immediately after, or both 1 hour before and immediately after irradiation at 10 μM (p = 0.040, 0.032 or 0.033, respectively). P values are for SHMT plots in reference to control, which was irradiation plus vehicle only, comparing Do values for protection and ñ values for mitigation. Error bars represent the standard error of the mean (SEM) for the 4 wells quantified for each irradiation dose in each experiment. Experiments were repeated in triplicate. DMSO was added to control irradiated cells and had no effect compared to irradiation alone.
By comparison, the known radiation protector Glyburide had a less significant effect on the D0 but resulted in an increased shoulder on the radiation survival curve (Table I). These data provide evidence that CBZ is a radioprotector of hematopoietic cells in vitro over a 100-fold range of drug concentration, with no detectable cytotoxic effects.
We next tested CBZ as a radiation mitigator. Drug was added in each of the three concentrations to 32D cl 3 cells immediately after irradiation and was present in the semisolid medium for 7 days. CBZ resulted in radiation mitigation as reflected in an increase in the extrapolation number (ñ) on the radiation survival curve (Tables I and II, Figure 1B, D, F, H). In contrast to the protective effect of CBZ the mitigating effect was reflected in the shoulder of the survival curve rather than the D0, which was not altered by incubation in drug after irradiation. Glyburide did not have a detectable mitigating effect in vitro or in vivo (Jiang et al. 2009). These results establish that CBZ has a radiation protective and mitigating effect on 32D cl 3 cells reflected by different changes in D0 and ñ, respectively.
CBZ added both before and after irradiation acts as a mitigator
To determine whether the radioprotective effect of CBZ was independent of its radiation mitigating effect, cells were incubated in each concentration of CBZ before irradiation and then also plated in semisolid medium in the same drug concentration. The results, shown in Figure 1G, H and Table II, demonstrate that the treatment of cells with CBZ both before and after irradiation resulted in an increased shoulder on the survival curve (p = 0.033), more similar to mitigation.
CBZ upregulates autophagy (LC3 conversion) in irradiated 32D cl 3 cells
The effect of CBZ on autophagy was assessed by quantifying the LC3I to LC3II conversion (represented as a LC3II/LC3I ratio) (Mizushima et al. 2010) after irradiation, in the presence or absence of CBZ. Initial assessment revealed that 5 and 10 Gy induced a 1.2 and 1.8 fold increase, respectively, in LC3 conversion at 24 hours (Figure 2A). The addition of 50 μM CBZ to 5 or 10 Gy irradiated cells resulted in a 3.3 and 6.2 fold increase, respectively. Irradiated cells showed elevated LC3 conversion, with or without CBZ, at 48 hours after irradiation. At this point autophagy may have reached a plateau maximum such that further induction by CBZ was not detectable.
Figure 2.
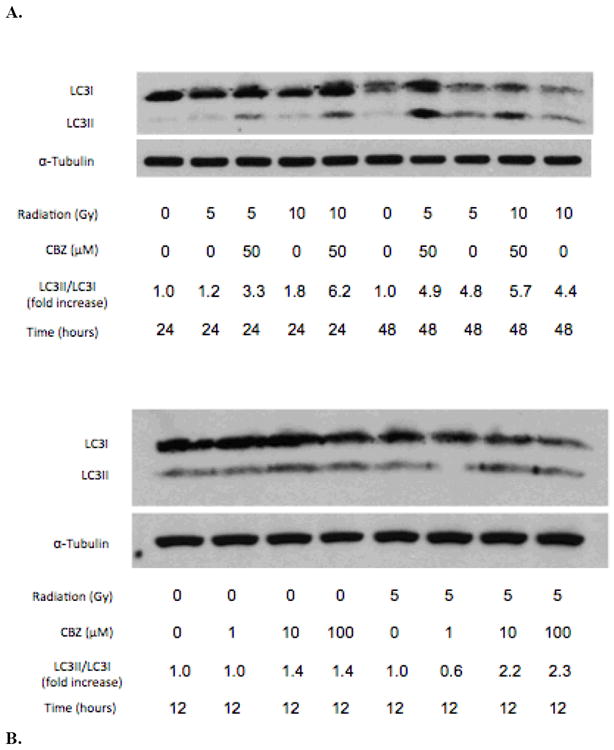
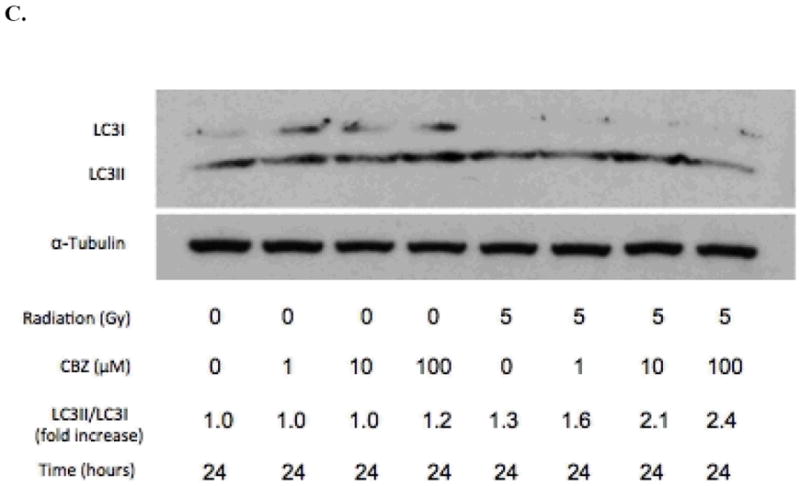
CBZ promotes autophagic activity after irradiation in 32D cl 3 cells. A) Immunoblot analysis of LC3 conversion in cells irradiated with 5 or 10 Gy and then incubated with or without 50 μM CBZ. Cells were incubated with 1, 10, or 100 μM CBZ for B) 12 hours or C) 24 hours after 0 or 5 Gy of irradiation. Experiments were repeated in duplicate.
Comparing CBZ doses used in prior experiments, at 12 hours, 10 and 100 μM CBZ each induced a 1.4 fold increase in LC3 conversion in non-irradiated cells; cells that received 5 Gy irradiation and 10 or 100 μM CBZ had a 2.2 and 2.3 fold increase in LC3 conversion, respectively (Figure 2B). At 24 hours, non-irradiated cells incubated in 100 μM showed a modest increase in LC3II/LC3I ratio of 1.2 fold; cells irradiated with 5 Gy and incubated with 0, 1, 10 or 100 μM had a 1.3, 1.6, 2.1 and 2.4 fold increase, respectively (Figure 2C). These data indicate that CBZ upregulates autophagy in irradiated cells.
CBZ functions as a radiation protector and mitigator in vivo
We tested the effect of CBZ given IP to C57BL/6Tac mice immediately prior to or after each of several TBI doses. Mice were followed for evidence of weight loss, lethargy, and other markers of the hematopoietic syndrome. The survival data with 9.25 Gy TBI demonstrate a statistically significant protective and mitigative effect of 10 mg/kg CBZ in vivo (p = 0.014 and 0.006, respectively) (Figure 3). We next evaluated the effect of added CBZ at several times before and after TBI. Mice injected with CBZ 10 minutes or 24 hours before 9.25 Gy TBI had increased survival compared to vehicle injected controls (Fig. 4A). Furthermore, injection with CBZ 10 minutes or 12 hours after irradiation significantly increased survival compared to vehicle injected controls (Figure B). Thus, CBZ was effective as a radiation protector and mitigator when delivered to mice at different times before or after TBI. The DMF was calculated as described in the Methods and data for multiple radiation doses are shown in Figure 4C - D. The survival of mice receiving 10.5 Gy + CBZ was equal to those that received 9.25 Gy only. The DMF was thus, 10.5 Gy divided by 9.25 Gy, or 1.13.
Figure 3.
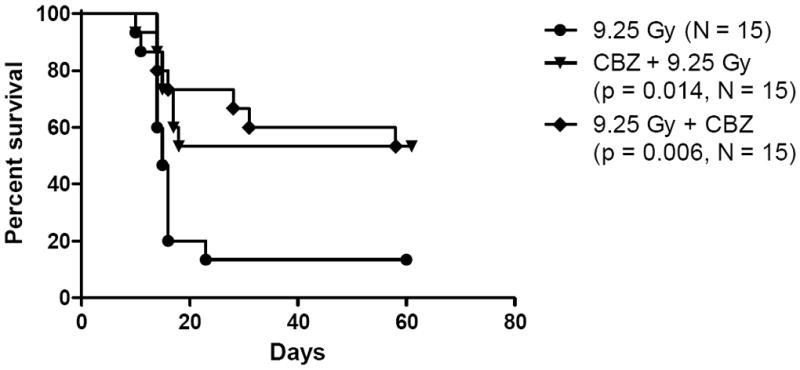
CBZ protects and mitigates against TBI. C57BL/6NTac female mice were injected IP with 10 mg/kg CBZ either 10 min before or 10 min after 9.25 Gy TBI (N = 15 per group). Mice injected with CBZ had significantly increased survival compared to the radiation only group.
Figure 4.

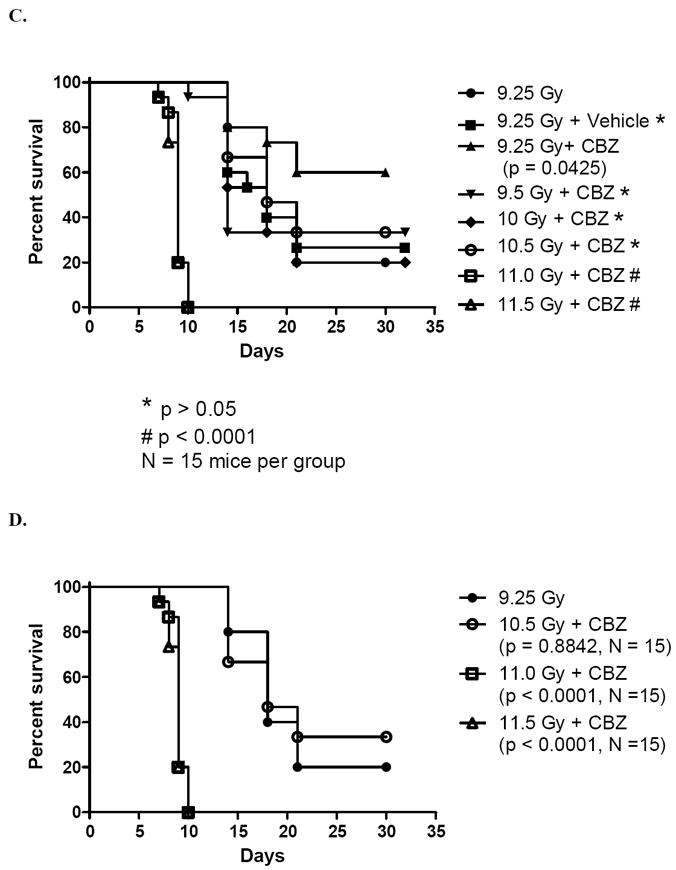
CBZ provides protection (A) and mitigation (B) if administered up to 24 hours before or 12 hours after irradiation. A) C57BL/6NTac female mice were injected IP with 10 mg/kg of CBZ 10 min or 24 hrs before 9.25 Gy TBI. B) Mice were injected with CBZ 10 min, 12 hrs or 24 hrs after 9.25 Gy TBI (N = 15 to 30 mice per group). CBZ resulted in significantly increased survival following irradiation if injected 24 hrs or 10 min before irradiation, or 10 min or 12 hrs after irradiation. The p values are in comparison to the radiation only group. C) DMF of CBZ delivered after each of 6 TBI doses. D) Effect of CBZ delivered after greater than or equal to 10.5 Gy TBI. Survival of mice receiving 10.5 Gy + CBZ was not significantly different than survival of mice receiving 9.5 Gy irradiation only (p < 0.05). Dividing 10.5 Gy by 9.25 Gy resulted in a DMF of 1.13.
Discussion
The present results demonstrate that CBZ is a radiation protector and mitigator in vitro and in vivo. CBZ resulted in radioprotection of 32D cl 3 cells, seen by an increase in D0. In contrast, when CBZ was added after irradiation, cells demonstrated an increased shoulder on the survival curve (Hall and Giaccia 2005). Given both before and after irradiation, the mitigative effect was predominant. These data imply that CBZ may have multiple radiobiologic effects on cells in culture. The sodium ion transport channel is a known target of CBZ and the short 1 hour pre-irradiation incubation may have been inadequate for the drug to affect cell survival beyond initially altering the ionic homeostasis at the time of and shortly after irradiation. In contrast, cells incubated in CBZ in semisolid medium for 7 days after irradiation could have upregulated autophagy at critical decision points hours after irradiation, promoting cell survival. Further, the mechanism by which radioprotection was conferred may require greater than 1 μM CBZ for full effect, while this drug concentration appears to be effective in mitigation. Additional studies will be required to distinguish these possibilities.
The Western blot data demonstrate that autophagy was induced in irradiated cells and upregulated by CBZ as early as 12 hours after irradiation. While the LC3 immunoblot is an accepted assay for autophagy, other assays such as electron microscopy and green fluorescent protein-LC3 will be required to strengthen these findings (Mizushima et al. 2010). It is also possible that upregulation of autophagy by CBZ is not related to the biology of radiation protection and mitigation but is independent of the mechanism promoting cell survival.
The 32D cl 3 line was derived from a C3H/HeJ mouse long-term bone marrow culture and has radiobiologic properties similar to freshly isolated hematopoietic cells from a variety of mouse strains (Epperly et al. 2002; Epperly et al. 2003). The radiation biology of 32D cl 3 cells has been demonstrated to correlate with that of primitive multilineage hematopoietic progenitor cells derived from mouse and human bone marrow (Epperly et al. 2002; Jiang et al. 2009). While there was some experimental variability (e.g. the differences in ñ for 10 μM CBZ), differences may have been attributable to cell growth conditions on different days and cell colony counting variation. However, the present in vitro studies suggesting that CBZ functions as a radiation protector and/or mitigator warranted tests in vivo.
The present in vivo studies demonstrated that IP injection of CBZ 10 minutes or 24 hours before 9.25 Gy TBI increased survival. Furthermore, mice injected with CBZ 10 minutes or 12 hours after irradiation demonstrated increased survival. The irradiation dose of 9.25 Gy was in effect an LD 85/30 (15% survival at 30 days) and LD 95/30 (5% survival at 30 days) dose in these experiments. Due to the size of the Cesium source and construction of the irradiator there is an estimated 5% error with each dose delivery (Shepherd JL and Associates 2005). Given the steepness of the in vivo TBI survival curve, this 5% variation may have accounted for the increased death. In another experiment, groups of mice received TBI doses of 9.25, 9.5, 10.0, 10.5, or 11.0 Gy. Sub-groups were given 10 mg/kg CBZ, 10 minutes after irradiation. In this experiment the DMF was calculated to be 1.13. Further experiments are in progress to deliver CBZ at earlier time points after irradiation both in the current delivery vehicle, and in a novel formulation designed to keep active drug in the circulation and in tissues for prolonged intervals. Experiments are also in progress to measure CBZ levels by mass spectroscopy analysis in plasma and tissues at serial time points after TBI. These experiments are designed to test the effect of interval after irradiation when drug is delivered, and the possibility that the pharmacokinetics and/or pharmacodynamics of CBZ may be altered by TBI. These studies may also lead to optimization of delivery of CBZ to achieve a higher DMF.
Determining the molecular target of action of CBZ that confers its effect may identify other target specific drugs with equivalent or better potency and efficacy. Other mood stabilizing drugs including lithium chloride and valproic acid induce autophagy (Sarkar et al. 2005; Fu et al. 2010). Tests of these commonly prescribed drugs as radioprotectors or mitigators may aid in the determination of the pathways involved in promoting radioresistance.
Improved survival after TBI of the CBZ treated mice indicates that CBZ may be a useful radiation protector and mitigator for potential use in the event of a radiation accident or terrorist event. Of note, an inherent risk with radioprotection is that it may permit the survival of cells containing DNA damage, possibly resulting in mutations or neoplastic transformation. However, as the immediate goal in a radiation accident or terrorist event is to prevent immediate effects of radiation injury such as death, the possibility of long-term malignancy is considered an acceptable risk. CBZ may also be potentially useful in the clinical setting to prevent damage to normal tissues during radiation therapy. Future experiments with multiple systemic and organ restricted delivery of CBZ during fractionated irradiation will be required to evaluate its utility in the clinic.
The historical Food and Drug Administration (FDA) approval of CBZ and its widespread use as an anti-convulsant gives it a definite advantage with respect to potential expedited FDA approval as a radiation mitigator in a counter terrorism or radiation accident scenario (Jiang et al. 2009). Though there are potentially serious side effects of CBZ, including neutropenia and aplastic anemia with chronic CBZ use, these side effects are extremely rare, usually reversible and would not be a consideration for its use in the acute delivery setting (Oyesanmi et al. 1999). Commonly, 10% of CBZ treated patients may experience a transient drop in white blood cell count during the first 4 months of chronic use (Oyesanmi et al. 1999). Our data indicate that a single CBZ dose delivered before or 12 hrs after radiation exposure may improve survival from TBI. Future experiments will be required to demonstrate protection or mitigation with multiple CBZ administrations. Clinical applications will require that patients undergo routine CBZ blood level testing to ensure therapeutic blood levels.
Acknowledgments
Supported by National Institute of Allergy and Infectious Diseases/National Institutes of Health (NIAID/NIH) Center for Medical Counter Measures (CMCR) Grant 1U19 A168021 and by NIH T32AG21885.
Footnotes
Declaration of Interest:
The authors report no declarations of interest.
References
- Alva AS, Gultekin SH, Baehrecke EH. Autophagy in human tumors: cell survival or death? Cell Death and Differentiation. 2004;11:1046–1048. doi: 10.1038/sj.cdd.4401445. [DOI] [PubMed] [Google Scholar]
- Boya P, Gonzalez-Polo R, Casares N, Perfettini J, Dessen P, Larochette N, Metivier D, Meley D, Souquere S, Yoshimori T, Pierron G, Codogno P, Kroemer G. Inhibition of macroautophagy triggers apoptosis. Molecular and Cellular Biology. 2005;15:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AP, Chung EJ, Urick ME, Shield WP, 3rd, Sowers AL, Thetford A, Shankavaram UT, Mitchell JB, Citrin DE. Evaluation of the fullerene compound DF-1 as a radioprotector. Radiation Oncology. 2010;5:34. doi: 10.1186/1748-717X-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby KN, Tekedereli I, Lopez-Berestein G, Ozpolat B. Targeting the prodeath and prosurvival functions of autophagy as novel therapeutic strategies in cancer. Autophagy. 2010;6:322–329. doi: 10.4161/auto.6.3.11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore SP, Qian T, Grissom SF, Lemasters JJ. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. Federation of American Societies for Experimental Biology. 2001;15:2286–2287. doi: 10.1096/fj.01-0206fje. [DOI] [PubMed] [Google Scholar]
- Epperly MW, Gretton JE, Bernarding M, Nie S, Rasul B, Greenberger JS. Mitochondrial localization of copper/zinc superoxide dismutase (Cu/ZnSOD) confers radioprotective functions in vitro and in vivo. Radiation Research. 2003;160:568–578. doi: 10.1667/rr3081. [DOI] [PubMed] [Google Scholar]
- Epperly MW, Sikora C, Defilippi S, Gretton J, Zhan Q, Kufe DW, Greenberger JS. MnSOD inhibits irradiation-induced apoptosis by stabilization of the mitochondrial membrane against the effects of SAP kinases p38 and Jnk1 translocation. Radiation Research. 2002;157:568–577. doi: 10.1667/0033-7587(2002)157[0568:msdsir]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Fu J, Shao CJ, Chen FR, Ng HK, Chen ZP. Autophagy induced by valproic acid is associated with oxidative stress in glioma cell lines. Neuro-Oncology. 2009;12:328–40. doi: 10.1093/neuonc/nop005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberger JS, Eckner RJ, Sakakeeny MA, Reid D, Nabel G, Hapel A, Ihle JN, Humphries KC. Effects of murine leukemia virus on generation of Interleukin-3 dependent hematopoietic progenitor cell lines from continuous marrow cultures. Federation Proceedings. 1983;42:106–115. [PubMed] [Google Scholar]
- Hall EJ, Giaccia AJ. Radiobiology for the radiologist. 6. Lippincott Williams and Wilkins; 2005. [Google Scholar]
- Høyer-Hansen M, Jäättela M. Autophagy: an emerging target for cancer therapy. Autophagy. 2008;4:574–580. doi: 10.4161/auto.5921. [DOI] [PubMed] [Google Scholar]
- Hidvegi T, Ewing M, Hale P, Dippold C, Beckett C, Kemp C, Maurice N, Mukherjee A, Goldbach C, Watkins S, Michalopoulos G, Perlmutter DH. An autophagy-enhanding drug promotes degradation of mutant α1-Antitrypsin Z and reduces hepatic fibrosis. Science. 2010;329:229–235. doi: 10.1126/science.1190354. [DOI] [PubMed] [Google Scholar]
- Hippert MM, O’Toole PS, Thorburn A. Autophagy in cancer: good, bad, or both? Cancer Research. 2006;66:9349–9351. doi: 10.1158/0008-5472.CAN-06-1597. [DOI] [PubMed] [Google Scholar]
- Jiang J, McDonald P, Dixon TM, Franicola D, Zhang X, Nie S, Epperly LD, Kagan VE, Lazo JS, Epperly MW, Greenberger JS. Druggable genome siRNA library screening identifies Glybencamide as a novel radioprotector. Radiation Research. 2009;172:414–422. doi: 10.1667/RR1674.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. The European Molecular Biology Organization Journal. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nature Reviews Cancer. 2005;5:726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- MacDonald RL, Kelly KM. Antiepileptic drug mechanisms of action. Epilepsia. 1995;36(Supplemental 2):S2–12. doi: 10.1111/j.1528-1157.1995.tb05996.x. [DOI] [PubMed] [Google Scholar]
- Miller LP. Quantifying western blots without expensive commercial quantification software. 2010 http://lukemiller.org/index.php/2010/11/analyzing-gels-and-western-blots-with-image-j/
- Mizushima N, Yoshimori T, Levine B. Methods in Mammalian Autophagy Research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyesanmi O, Kunkel EJS, Monti DA, Field HL. Hematologic side effects of psychotropics. Psychosomatics. 1999;40:414–421. doi: 10.1016/S0033-3182(99)71206-5. [DOI] [PubMed] [Google Scholar]
- Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, Domingo D, Yahalom J. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Research. 2001;61:439–444. [PubMed] [Google Scholar]
- Rajagopalan MS, Gupta K, Epperly MW, Franicola D, Zhang X, Wang H, Zhao H, Tyurin VA, Kagan VE, Wipf P, Kanai A, Greenberger JS. The mitochondria-targeted nitroxide JP4-039 augments potentially lethal irradiation damage repair. In Vivo. 2009;23:717–726. [PMC free article] [PubMed] [Google Scholar]
- Ralph P, Moore MAS, Nilsson K. Lysozyme synthesis by established human and murine histiocytic lymphoma cell lines. Journal Experimental Medicine. 1976;143:1528–1533. doi: 10.1084/jem.143.6.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert T, Vanoli F, Chiolo I, Shubassi G, Bernstein KA, Rothstein R, Botrugno OA, Parazzoli D, Oldani A, Minucci S, Foiani M. HDACs link the DNA damage response, processing of double-strand breaks and autophagy. Nature. 2011;471:74–80. doi: 10.1038/nature09803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Flato RA, Berger Z, Imarisio S, Cordenier A, Pasco M, Cook LJ, Rubinsztein DC. Lithium induces autophagy by inhibiting inositol monophosphatase. Journal of Cell Biology. 2005;170:1101–1111. doi: 10.1083/jcb.200504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JL Associates. Isodose curve specifications for Shepherd Mark 1 Cesium Irradiator. 1010 Arroyo Street, San Fernando, CA 91340-1822: 2005. [Google Scholar]
- Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edge sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames HD, Jr, Rasmussen SL. A test for dose-modifying factors. Radiation Research. 1978;76:308–324. [PubMed] [Google Scholar]


