Abstract
Background
Many elderly cancer patients experience increased cancer-related morbidity and mortality compared to younger patients. In soft tissue sarcoma, adjuvant radiotherapy is an integral part of definitive therapy for limb preservation. We hypothesized that age-related disparities exist in the use of radiation.
Methods
We used SEER data to conduct a retrospective cohort study among patients 25 years or older diagnosed from 1998 to 2004 with non-metastatic, biopsy-proven, high-grade soft tissue sarcoma in the extremities undergoing a limb-sparing procedure. Patients were stratified according to age (<50, 50-70, and >70). Logistic regression was used to determine the association between age and radiotherapy use, adjusting for histology, location, size, surgery, gender, race, and marital status. A Cox proportional hazards model was used to compare disease-specific and all-cause mortality.
Results
Among 1,354 eligible patients; 37.1% were older than age 70, 44.3% female, and 84.4% Caucasian. While almost three-quarters (73.8%) of the cohort received radiotherapy, receipt decreased from 78.2% in patients younger than age 50 to 69.6% in patients older than age 70 (test of trend p=0.006). After adjusting for demographic and tumor factors, older patients remained less likely to receive radiotherapy (odds ratio=0.66, 95% confidence interval (CI)=0.47-0.92) and more likely to experience disease-specific death (hazard ratio=2.4, CI=1.4-4.1) as compared to the youngest group.
Conclusion
Older adults appear less likely to receive definitive therapy for soft tissue sarcoma of the extremities. In the absence of clinical trials and treatment guidelines tailored to this population, under-treatment may disadvantage the elderly with increased cancer-related morbidity and mortality.
Keywords: sarcoma, radiotherapy, age factors, healthcare disparities, treatment
Introduction
The United States (US) Census Bureau indicates that the number of adults over age 65 will increase dramatically from the current estimate of 39 million persons to over 70 million by 2030 1. Cancer disproportionately afflicts older adults, with more than 50% of all new cancer diagnoses from 2000 to 2006 occurring in adults 65 years of age or older 2. The average life expectancy for a healthy 70 year old adult in the United States is 12 to15 years; even those with significant comorbidities are expected to live five to seven years 3.
Despite this significant predicted longevity, many elderly cancer patients experience increased cancer-related morbidity and mortality compared to younger patients 4-7. Surveillance, Epidemiology and End Results (SEER) statistics indicate that the age-adjusted mortality rate for all cancer types combined is 15 times greater for older adults 4, 7. Several studies have suggested that under-representation in clinical trials 8, 9 and administration of sub-standard treatment based on age contributes to this outcome disparity in older adults 10-13.
Approximately 10,000 people will be diagnosed with cancer of the soft tissues in 2008. In those with high-grade extremity tumors, radiotherapy (RT) has been established as an integral component of treatment through several randomized trials 14-16 demonstrating a significant reduction in the rates of local recurrence when radiotherapy is combined with limb-sparing surgery. When no radiotherapy is given, the majority of local failures occur within the first two to four years. Because salvage options are limited, failure to prevent local recurrences with radiation therapy will certainly increase patient morbidity and may increase the probability of dying from sarcoma.
The randomized trials establishing limb preservation in soft tissue sarcoma included very few patients over the age of 70. It is unclear if this standard of care is applied uniformly across age groups in everyday practice. This is particularly relevant as 40% of new soft tissue cancer diagnosis are in adults over the age of 65 2. Thus, potential age-related disparities were explored using an existing database to evaluate the hypothesis that older adults with soft tissue sarcoma of the extremity would be less likely to receive adjuvant radiotherapy when compared to their younger counterparts, and that this would translate into decreased survival.
Methods/Materials
Setting and Design
We conducted a retrospective cohort study using the National Cancer Institute’s SEER database to obtain data on the treatment of patients with localized soft tissue sarcoma in the US. The Institutional Review Board at Wake Forest University determined the use of de-identified data in this analysis did not meet the federal definition of human subject research and thus was exempt from review.
Patients
Our cohort included patients with non-metastatic, biopsy-proven, high-grade soft tissue sarcoma in the extremity undergoing a limb-sparing surgical procedure between the years of 1998 and 2004. The seminal trials establishing the use of adjuvant radiotherapy were published in 1998 or prior and thus we would expect treatment patterns to have been relatively stable during this time period. In addition, we restricted the cohort to adults aged 25 years or older. Tumors occurring in younger patients were felt to represent a distinct population with differing prognoses and treatment options. Other tumors that represent unique biologic entities, such as Kaposi’s sarcoma, were excluded for the same reason. Finally, we excluded patients with missing data on surgical or radiotherapy treatment, age, sex, race/ethnicity, or marital status.
Data
SEER is a population-based tumor registry that contains demographic, tumor, treatment, and vital status information on all incident cancer cases within specific geographic regions. Currently, the data contained in the SEER Program covers approximately a quarter of the US population. Data from the following registries was included as available: Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, Utah, Los Angeles, San Jose-Monterey, Rural Georgia, Alaska, Greater California, Kentucky, Louisiana, and New Jersey.
Our main interest was in the association between age, use of radiotherapy, and correlation with patient outcomes. Patients satisfying the inclusion criteria were stratified into three groups: <50, 50-70, and >70 years of age in order to evaluate the use of radiotherapy and treatment-related outcomes in these three distinct populations. Age brackets were chosen to reflect geriatric oncology practice trends, consistent with recent publications in which age 70 is used to define a geriatric population 17, 18. Radiotherapy was included in any of the following forms: beam radiation, radioactive implants, radioisotopes, combination of the previous three, or radiation not otherwise specified.
We controlled for additional variables as follows. Three racial groups were considered; white, black, and other. Marital status was divided into married or currently not married: single, separated/divorced, and widowed. Histology was categorized using the SEER Histology Validation List. Selected codes are reported in Table 1. High-grade tumors, those defined as moderately, poorly, or undifferentiated, were defined according to the Term Grade SEER code (2/3, 3/3, 2/2). Surgery was considered as limb-sparing when coded as a local excision or radical excision/resection of lesion with limb salvage. Tumors less than or equal to 50mm were recorded as small and those >50mm as large. International Classification of Diseases (ICD) 9/10 codes were utilized to determine cause of death. The codes for death from soft tissue malignancy (164.1, 171, C47, C49, C38.0, C45.2) were selected to determine disease-specific survival.
Table 1.
Histology codes reflective of selected patient cohort.
| Code | Histology | Number (%) |
|---|---|---|
|
| ||
| 8800 | Sarcoma, NOS | 76 (5.6) |
| 8801 | Spindle cell sarcoma | 45 (3.3) |
| 8802 | Giant cell sarcoma | 62 (4.6) |
| 8803 | Small cell sarcoma | 1 (0.1) |
| 8804 | Epithelioid sarcoma | 10 (0.7) |
| 8805 | Undifferentiated sarcoma | 2 (0.2) |
| 8810 | Fibrosarcoma, NOS | 17 (1.3) |
| 8811 | Fibromyxosarcoma | 38 (2.8) |
| 8830 | Fibrous histiocytoma, malignant | 576 (42.5) |
| 8832 | Dermatofibrosarcoma, NOS | 3 (0.2) |
| 8840 | Myxosarcoma | 11 (0.8) |
| 8850 | Liposarcoma, NOS | 20 (1.5) |
| 8851 | Liposarcoma, well differentiated | 1 (0.1) |
| 8852 | Myxoid liposarcoma | 43 (3.2) |
| 8853 | Round cell liposarcoma | 27 (2) |
| 8854 | Pleomorphic liposarcoma | 74 (5.5) |
| 8855 | Mixed type liposarcoma | 13 (1) |
| 8858 | Dedifferentiated liposarcoma | 33 (2.4) |
| 8890 | Leiomyosarcoma, NOS | 141 (10.4) |
| 8891 | Epithelioid leiomyosarcoma | 6 (0.4) |
| 8894 | Angiomyosarcoma | 2 (0.2) |
| 8895 | Myosarcoma | 2 (0.2) |
| 8896 | Myxoid leiomyosarcoma | 3 (0.2) |
| 8900 | Rhabdomyosarcoma, NOS | 1 (0.1) |
| 8901 | Pleomorphic rhabdomyosarcoma, adult type | 12 (0.9) |
| 8910 | Embryonal rhabdomyosarcoma | 2 (0.2) |
| 8920 | Alveolar rhabdomyosarcoma | 4 (0.3) |
| 9040 | Synovial sarcoma, NOS | 31 (2.3) |
| 9041 | Synovial sarcoma, spindle cell | 31 (2.3) |
| 9042 | Synovial sarcoma, epithelioid cell | 2 (0.2) |
| 9043 | Synovial sarcoma, biphasic | 12 (0.9) |
| 9044 | Clear cell sarcoma, NOS (except of kidney M-8964/3) | 2 (0.2) |
| 9120 | Hemangiosarcoma | 18 (1.3) |
| 9133 | Epithelioid hemangioendothelioma, malignant | 1 (0.1) |
| 9150 | Hemangiopericytoma, malignant | 2 (0.2) |
| 9540 | Malignant peripheral nerve sheath tumor | 22 (1.6) |
| 9560 | Neurilemoma, malignant | 2 (0.2) |
| 9581 | Alveolar soft part sarcoma | 6 (0.4) |
Analysis
We used the Cochran-Armitage test of trend to examine the association between age and RT use and Pearson Chi-square tests to examine associations between RT use and other variables of interest. A logistic regression model was used to look at the effect of age on RT use while adjusting for other variables that would also likely influence the use of RT. These other variables included demographic (sex, race, marital status) and tumor (site, histology, size) characteristics, as well as type of surgical procedure. Additional potential confounders such as margin status, comorbidities, and performance status are not recorded in the SEER database and thus, cannot be accounted for in this analysis. Tumor depth can be reported but is poorly captured in the SEER database; only 58% of our patients had data on tumor depth. Similarly, data on local control is not included in the SEER database and thus the impact of radiotherapy on local recurrence rates can not be examined. A Cox proportional hazards model was used to assess age and use of RT on all-cause and disease-specific mortality. All data were analyzed using the SAS Version 9.2 (SAS Institute, Cary, NC) statistical software package. Results were considered to be statistically significant when p < .05.
Results
We identified 1,354 patients who met our eligibility criteria (Figure 1). Within the cohort, 354 (26.1%) were less than 50 years, 497 (36.7%) were 50 to 70 years, and 503 (37.2%) were older than 70 years (Table 2). Men (754, 55.7%) and whites (1134, 84.4%) made up the majority of the patient cohort. Malignant fibrous histiocytoma (MFH) was the most common histologic diagnosis (576, 42.5%): “other” histologies, a heterogeneous collection of tumors, also were common (487, 36%). Large tumors were more prevalent (805, 59.5%) than small.
Figure 1.
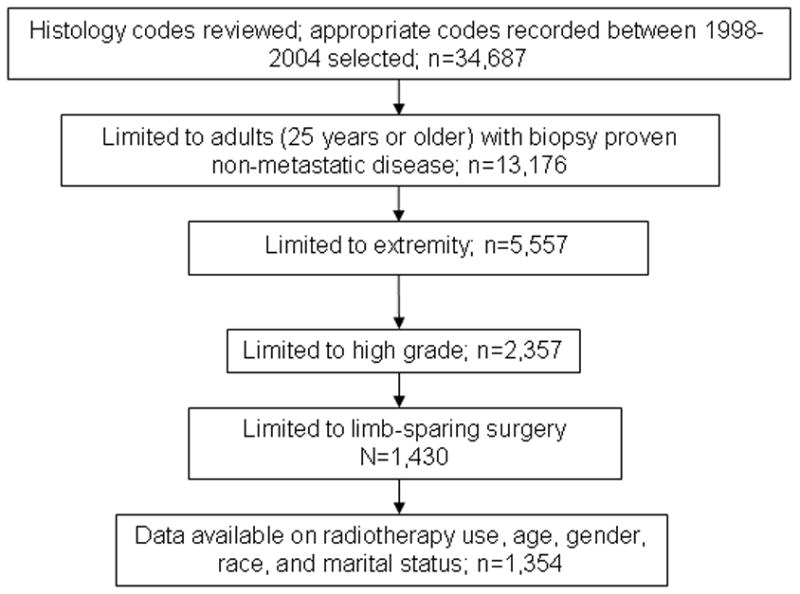
Schema for selection of soft tissue sarcoma patients from the Surveillance, Epidemiology and End Results Database.
Table 2.
Demographic characteristics of patients selected from the SEER database with high-grade soft tissue sarcoma of the extremities.
| Demographic | Age | Total # |
P | ||
|---|---|---|---|---|---|
| <50 # (%) | 50-70 # (%) | >70 # (%) | |||
| N | 354 (26.1%) | 497 (36.7%) | 503 (37.1%) | 1354 | |
| Histology | <0.0001 | ||||
| Fibrous histiocytoma, malignant | 101 (28.5%) | 222 (44.7%) | 253 (50.3%) | 576 | |
| Leiomyosarcoma | 29 (8.2%) | 50 (10.1%) | 62 (12.3%) | 141 | |
| Pleomorphic liposarcoma | 12 (3.4%) | 34 (6.8%) | 28 (5.6%) | 74 | |
| Sarcoma, not otherwise specified | 17 (4.8%) | 29 (5.8%) | 30 (6.0%) | 76 | |
| Other | 195 (55.1%) | 162 (32.6%) | 130 (25.8%) | 487 | |
| Site | 0.9508 | ||||
| Lower extremity | 261 (73.7%) | 363 (73.0%) | 366 (72.8%) | 990 | |
| Upper extremity | 93 (26.3%) | 134 (27.0%) | 137 (27.2%) | 364 | |
| Size | 0.5222 | ||||
| Small (≤5cm) | 142 (40.1%) | 211 (42.5%) | 196 (39.0%) | 549 | |
| Large (>5cm) | 212 (59.9%) | 286 (57.5%) | 307 (61.0%) | 805 | |
| Surgical Procedure | 0.0287 | ||||
| Local Excision | 104 (29.4%) | 173 (34.8%) | 192 (38.2%) | 469 | |
| Radical Resection | 250 (70.6%) | 324 (65.2%) | 311 (61.8%) | 885 | |
| Sex | 0.0399 | ||||
| Male | 186 (52.5%) | 299 (60.2%) | 269 (53.5%) | 754 | |
| Female | 168 (47.5%) | 198 (39.8%) | 234 (46.5%) | 600 | |
| Race | 0.0324 | ||||
| White | 286 (80.8%) | 426 (85.7%) | 431 (85.7%) | 1143 | |
| Black | 47 (13.3%) | 44 (8.9%) | 36 (7.2%) | 127 | |
| Other | 21 (5.9%) | 27 (5.4%) | 36 (7.2%) | 84 | |
| Marital Status | 0.0009 | ||||
| Currently not married | 139 (39.3%) | 150 (30.2%) | 206 (41.0%) | 495 | |
| Married | 215 (60.7%) | 347 (69.8%) | 297 (59.0%) | 859 | |
| Radiotherapy | 0.0141 | ||||
| No radiotherapy | 77 (21.8%) | 125 (25.2%) | 153 (30.4%) | 355 | |
| Radiotherapy | 277 (78.2%) | 372 (74.8%) | 350 (69.6%) | 999 | |
Overall, 999 (73.8%) of the patients in the study population received radiotherapy. The overwhelming majority (960, 96.1%) were treated with external beam RT. However, receipt of radiotherapy decreased from 78.3% in patients less than 50 years old to 74.9% in those aged 50 to 70 and again to 69.6% in patients older than 70 years (test of trend p=0.006). Conversely, use of a local excision as the definitive surgical procedure increased from 29.4% in patients <50 years to 38.2% in patients >70 years of age (p=0.029). Older patients were also more likely to be married, white males, and with malignant fibrous histiocytoma histology.
Age continued to show a strong association with receipt of radiotherapy in a multivariable model (Table 3). Compared to those younger than age 50, patients aged 70 and older were statistically significantly less likely to receive radiotherapy (odds ratio[OR]=0.66, 95% confidence interval[CI]=0.47-0.92). Patients aged 50 to 70 also were less likely to receive radiotherapy, although this difference was only borderline statistically significant (OR=0.81, CI=0.57-1.14). Pleomorphic liposarcoma, radical resection, large size, and being married continued to be independently associated with radiotherapy use in the adjusted model. On further analysis of the 74 patients with pleomorphic sarcomas, 73% were >5cm.
Table 3.
Unadjusted and adjusted analysis of factors affecting the use of radiotherapy in patients with high-grade soft tissue sarcoma of the extremities.
| Characteristics | Unadjusted | Adjusted* | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Age | ||||
| <50 | 1 | referent | ||
| 50-70 | 0.83 | 0.60, 1.14 | 0.81 | 0.57, 1.14 |
| 70 + | 0.64 | 0.46, 0.87 | 0.66 | 0.47, 0.92 |
| Histology | ||||
| Fibrous histiocytoma, malignant (MFH) | 1 | referent | ||
| Pleomorphic liposarcoma | 6.67 | 2.40, 18.58 | 6.04 | 2.15, 17.0 |
| Sarcoma, not otherwise specified | 1.14 | 0.66, 1.98 | 1.28 | 0.73, 2.26 |
| Leiomyosarcoma | 0.61 | 0.42, 0.90 | 0.67 | 0.45, 1.01 |
| Other | 1.18 | 0.89, 1.55 | 1.07 | 0.8, 1.44 |
| Site | ||||
| Lower extremity | 1 | referent | ||
| Upper extremity | 1.01 | 0.77, 1.33 | 1.15 | 0.86, 1.55 |
| Surgical Procedure | ||||
| Local excision | 1 | referent | ||
| Radical resection | 1.95 | 1.52, 2.50 | 1.83 | 1.41, 2.37 |
| Sex | ||||
| Female | 1 | referent | ||
| Male | 1.18 | 0.92, 1.50 | 1.03 | 0.79, 1.33 |
| Race | ||||
| White | 1 | referent | ||
| Black | 0.93 | 0.61, 1.40 | 0.93 | 0.61, 1.44 |
| Other | 0.99 | 0.60, 1.65 | 1.14 | 0.67, 1.93 |
| Marital Status | ||||
| Currently not married | 1 | referent | ||
| Married | 1.65 | 1.29, 2.12 | 1.59 | 1.22, 2.08 |
| Size | ||||
| Small | 1 | referent | ||
| Large | 1.93 | 1.51, 2.46 | 1.91 | 1.48, 2.47 |
Final model adjusted for all variables shown in table.
In an adjusted model, age and receipt of radiation therapy together were associated with survival (Table 4). Regardless of age, not receiving radiotherapy was statistically associated with all-cause death and borderline associated with disease-specific death. Age increased these effects. For example, relative to patients less than 50 years old, patients aged 70 years and older who received radiation therapy appeared to have a slightly elevated chance of disease-specific death (hazard ratio[HR]=1.3, CI=0.9-2.1). However, those older patients not receiving radiation therapy were even more likely to experience disease-specific death (HR=2.4. CI=1.4-4.1).
Table 4.
Cox proportional hazards model of all-cause and disease-specific survival in patients with high-grade soft tissue sarcoma of the extremity.
| Characteristics | All Cause | Disease Specific | ||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Radiation Therapy | ||||
| <50 with RT | 1 | referent | 1 | referent |
| <50 without RT | 1.7 | 1.0, 2.9 | 1.4 | 0.7, 2.9 |
| 50 - 70 with RT | 1.4 | 1.0, 2.1 | 1.5 | 1.0, 2.3 |
| 50-70 without RT | 2.1 | 1.3, 3.4 | 1.8 | 1.0, 3.3 |
| 70 + with RT | 2.0 | 1.4, 2.9 | 1.3 | 0.9, 2.1 |
| 70 + without RT | 4.0 | 2.8, 6.3 | 2.4 | 1.4, 4.1 |
| Histology | ||||
| Fibrous histiocytoma, malignant (MFH) | 1 | referent | 1 | referent |
| Pleomorphic liposarcoma | 0.7 | 0.4, 1.3 | 1.0 | 0.5, 2.0 |
| Sarcoma, not otherwise specified | 1.8 | 1.1, 2.7 | 2.7 | 1.6, 4.5 |
| Leiomyosarcoma | 1.1 | 0.8, 1.7 | 1.5 | 0.9, 2.6 |
| Other | 1.0 | 0.8, 1.3 | 1.5 | 1.1, 2.1 |
| Site | ||||
| Lower extremity | 1 | referent | 1 | referent |
| Upper extremity | 1.1 | 0.9, 1.4 | 1.0 | 0.7, 1.5 |
| Surgical Procedure | ||||
| Local excision | 1 | referent | 1 | referent |
| Radical resection | 0.9 | 0.7, 1.1 | 0.8 | 0.6, 1.1 |
| Sex | ||||
| Female | 1 | referent | 1 | referent |
| Male | 1.5 | 1.2, 1.9 | 1.3 | 0.9, 1.7 |
| Race | ||||
| White | 1 | referent | 1 | referent |
| Black | 1.2 | 0.8, 1.7 | 1.2 | 0.8, 1.9 |
| Other | 0.7 | 0.4, 1.1 | 0.5 | 0.2, 1.1 |
| Marital Status | ||||
| Currently not married | 1 | referent | 1 | referent |
| Married | 0.7 | 0.5, 0.9 | 0.8 | 0.6, 1.1 |
| Size | ||||
| Small | 1 | referent | 1 | referent |
| Large | 2.1 | 1.6, 2.7 | 2.2 | 1.6, 3.1 |
Discussion
As expected, over one-third of the patients in our study diagnosed with high-grade soft tissue sarcoma of the extremity were 70 years of age or older. Factors known to correlate with radiotherapy use were predictive of treatment in our series. For example, married patients with greater family support more frequently received radiotherapy and those with larger tumors, and thus higher risk, were also more frequently treated. In addition, those undergoing more radical surgery, which implies the possibility of both a greater risk and a more robust patient, more commonly received adjuvant radiation. In contrast, and in spite of increasing sarcoma incidence, use of adjuvant radiotherapy declined with age. This trend remained even after adjusting for tumor histology, site, size, and surgical procedure. In turn, use of radiotherapy was closely linked to survival. Across all age groups, overall survival was worse in those not receiving radiotherapy, and a trend towards decreased disease-specific survival was seen with increasing age.
To our knowledge, no other report has focused on age differences and patient outcomes in receipt of radiotherapy for soft tissue sarcoma of the extremity. Our findings suggest that omitting radiotherapy for older adults with high grade sarcoma may result in inferior outcomes. The rationale for differential cancer treatment by increasing age has been challenged in recent years with reports suggesting equal benefit from equal treatment in various tumor types 19-24. For example, Muss et al. performed a meta-analysis of breast cancer trials focused on locally advanced disease and reported an equal benefit for adjuvant chemotherapy in patients greater than 65 years of age compared to those younger than 65 years of age 20. Similar findings have been reported in the treatment of colon cancer with respect to mortality 25. In addition, a few studies have demonstrated that attenuated dose treatment regimens for older patients with Non-Hodgkin’s lymphoma and small cell lung cancer may negatively impact treatment outcomes 22-24, 26. These studies have challenged the previous notion of limiting therapeutic options based on chronologic age alone.
Unfortunately, existing clinical trials provide limited data on the treatment of older adults. Older adults comprise the majority of incident and prevalent cancer cases, but they make up the minority of patients previously and currently enrolled on clinical trials. In a recent publication by Lewis et al., which evaluated participation of the elderly (age ≥ 65 years) in clinical trials sponsored by the National Cancer Institute, only 32% of the participants in trials were elderly 9. Another report, published by Hutchins et al., described the representation of older adults in the Southwest Oncology Group treatment trials between 1993 and 1996 8. Over 16,000 patients were consecutively enrolled during this time frame of which only 25% were 65 years of age or older. Similarly, there were very few patients over the age of 65 in four sentinel randomized trials that established the use of radiotherapy in soft tissue sarcomas 14-16, 27. Thus, while clinical trials provide critical data guiding patient care, this data is difficult to extrapolate to the elderly population and thus, may contribute to treatment disparity.
A key limitation of our study is the lack of local recurrence data. These data are not captured in the SEER database but would be useful in measuring the direct impact of radiotherapy. However, randomized data clearly supports a significant reduction in local recurrence for high grade tumors treated with local radiation. In those trials, a survival benefit was not seen but with the small number of patients, a modest improvement in survival may have been missed. Koshy, et al, using the SEER database in a larger patient population similar to our own, concluded that the use of radiotherapy is associated with improvements in overall survival 28.
Whether or not this extends to an older population with generally poorer health is less clear. The significant decrease in overall survival seen in our study when radiotherapy is not used may be related to poor overall health in the older population. This may strongly influence treatment decision-making on the part of the practitioner or the patient. For example, patients with significant comorbidities or poor performance status may not be selected for aggressive surgical therapy (ie. radical resection) or post-operative radiotherapy. The SEER database does not capture data on overall health and function and it could be that this data would explain some or all of the trends seen in our study. However, the trend towards decreased disease-specific survival with increasing age, suggests that some patients that could benefit from radiotherapy may not be receiving it. Even older patients with significant comorbidities are expected to live five to seven years 3 and local recurrence in high-grade soft tissue sarcomas is often concentrated in the two to four years following surgical excision 16. It may be that even older patients with comorbidities could still benefit from local therapy, particularly when accounting for limited salvage options. It is important to note, however, that specific data on comorbidities and performance status, factors which often shape treatment recommendations, are also not available in the SEER database. Because clinical trials often exclude patients with comorbidities, (7) observational studies incorporating comorbidity and performance status may prove useful in understanding the potential benefit of radiotherapy for older patients with suboptimal health. Clinical trials addressing these issues would also be useful.
Without evidence based treatment options, clinicians are faced with extrapolating results obtained from younger, healthier subjects or tailoring treatment without the benefit of scientific evidence. Improving outcomes and treatment decision-making for older adults with malignancy depends upon a better understanding of the specific factors that contribute to response and adverse outcomes in this population. Our results suggest that future clinical trials and observational studies need to explicitly address appropriate treatment for older adults with and without comorbidities. In the absence of additional data, the growing population of older adults with high-grade soft tissue sarcoma of the extremities may experience increased cancer-related morbidity and mortality due to under-treatment.
Footnotes
Preliminary results presented at the annual meeting of the Radiological Society of North America in Chicago, Illinois on December 3rd, 2008.
Conflicts of Interest/Financial Disclosures: None
References
- 1.United States Census Bureau. Available from URL: http://www.census.gov/
- 2.Surveillance Epidemiology and End Results. Available from URL: http://www.seer.cancer.gov/statistics/
- 3.Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–6. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 4.SEER Cancer Statistics Review 1975-2003. 2006 [Google Scholar]
- 5.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481–5. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mileshkin L, Prince HM. The adverse prognostic impact of advanced age in multiple myeloma. Leuk Lymphoma. 2005;46(7):951–66. doi: 10.1080/10428190500085024. [DOI] [PubMed] [Google Scholar]
- 7.Yancik R. Cancer burden in the aged: an epidemiologic and demographic overview. Cancer. 1997;80(7):1273–83. [PubMed] [Google Scholar]
- 8.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341(27):2061–7. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 9.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21(7):1383–9. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Bouchardy C, Rapiti E, Blagojevic S, Vlastos AT, Vlastos G. Older female cancer patients: importance, causes, and consequences of undertreatment. J Clin Oncol. 2007;25(14):1858–69. doi: 10.1200/JCO.2006.10.4208. [DOI] [PubMed] [Google Scholar]
- 11.Giordano SH, Duan Z, Kuo YF, Hortobagyi GN, Goodwin JS. Use and outcomes of adjuvant chemotherapy in older women with breast cancer. J Clin Oncol. 2006;24(18):2750–6. doi: 10.1200/JCO.2005.02.3028. [DOI] [PubMed] [Google Scholar]
- 12.Hurria A, Naeim A, Elkin E, et al. Adjuvant treatment recommendations in older women with breast cancer: a survey of oncologists. Crit Rev Oncol Hematol. 2007;61(3):255–60. doi: 10.1016/j.critrevonc.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Kohne CH, Grothey A, Bokemeyer C, Bontke N, Aapro M. Chemotherapy in elderly patients with colorectal cancer. Ann Oncol. 2001;12(4):435–42. doi: 10.1023/a:1011170808734. [DOI] [PubMed] [Google Scholar]
- 14.Pisters PW, Harrison LB, Leung DH, Woodruff JM, Casper ES, Brennan MF. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14(3):859–68. doi: 10.1200/JCO.1996.14.3.859. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg SA, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196(3):305–15. doi: 10.1097/00000658-198209000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16(1):197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 17.Feuvret L, Noel G, Mazeron JJ, Bey P. Conformity index: a review. Int J Radiat Oncol Biol Phys. 2006;64(2):333–42. doi: 10.1016/j.ijrobp.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 18.Walter LC, Brand RJ, Counsell SR, et al. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001;285(23):2987–94. doi: 10.1001/jama.285.23.2987. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg RM, Tabah-Fisch I, Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24(25):4085–91. doi: 10.1200/JCO.2006.06.9039. [DOI] [PubMed] [Google Scholar]
- 20.Muss HB, Woolf S, Berry D, et al. Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA. 2005;293(9):1073–81. doi: 10.1001/jama.293.9.1073. [DOI] [PubMed] [Google Scholar]
- 21.Pepe C, Hasan B, Winton TL, et al. Adjuvant vinorelbine and cisplatin in elderly patients: National Cancer Institute of Canada and Intergroup Study JBR.10. J Clin Oncol. 2007;25(12):1553–61. doi: 10.1200/JCO.2006.09.5570. [DOI] [PubMed] [Google Scholar]
- 22.Ardizzoni A, Favaretto A, Boni L, et al. Platinum-etoposide chemotherapy in elderly patients with small-cell lung cancer: results of a randomized multicenter phase II study assessing attenuated-dose or full-dose with lenograstim prophylaxis--a Forza Operativa Nazionale Italiana Carcinoma Polmonare and Gruppo Studio Tumori Polmonari Veneto (FONICAP-GSTPV) study. J Clin Oncol. 2005;23(3):569–75. doi: 10.1200/JCO.2005.11.140. [DOI] [PubMed] [Google Scholar]
- 23.Dixon DO, Neilan B, Jones SE, et al. Effect of age on therapeutic outcome in advanced diffuse histiocytic lymphoma: the Southwest Oncology Group experience. J Clin Oncol. 1986;4(3):295–305. doi: 10.1200/JCO.1986.4.3.295. [DOI] [PubMed] [Google Scholar]
- 24.Gottlieb AJ, Anderson JR, Ginsberg SJ, et al. A randomized comparison of methotrexate dose and the addition of bleomycin to CHOP therapy for diffuse large cell lymphoma and other non-Hodgkin’s lymphomas. Cancer and Leukemia Group B study 7851. Cancer. 1990;66(9):1888–96. doi: 10.1002/1097-0142(19901101)66:9<1888::aid-cncr2820660906>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 25.Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345(15):1091–7. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 26.Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360(20):2055–65. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235–41. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 28.Koshy M, Rich SE, Mohiuddin MM. Improved survival with radiation therapy in high-grade soft tissue sarcomas of the extremities: a SEER analysis. Int J Radiat Oncol Biol Phys. 77(1):203–9. doi: 10.1016/j.ijrobp.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]


