Summary
The structural effects of cocaine on neural systems mediating cognition and motivation are not well known. By comparing thickness of neocortical and paralimbic brain regions between cocaine dependent and matched control subjects, we found that four of 18 a priori regions involved with executive regulation of reward and attention were significantly thinner in addicts. Correlations were significant between thinner prefrontal cortex and reduced keypresses during judgment and decision-making of relative preference in addicts, suggesting one basis for restricted behavioral repertoires in drug dependence. Reduced effortful attention performance in addicts also correlated with thinner paralimbic cortices. Some thickness differences in addicts were correlated with cocaine use independent of nicotine and alcohol, but addicts also showed diminished thickness heterogeneity and altered hemispheric thickness asymmetry. These observations suggest brain structure abnormalities in addicts are related in part to drug use, and in part to predisposition toward addiction.
Introduction
Animal studies of cocaine’s action have implicated brain structures involved with reward/aversion function such as the ventral tegmental area, nucleus accumbens (NAc), amygdala and prefrontal cortex (PFC) (Everitt and Robbins, 2005; Hyman et al., 2006; Kalivas and Volkow, 2005; Koob, 2006). Results of human studies are consistent with the animal literature showing functional activation of subcortical and cortical reward/aversion regions during drug exposure (Breiter et al., 1997; Stein and Fuller, 1992). Human studies have further demonstrated significant alterations in cognitive control in addiction related to functional alterations in the PFC (Bechara, 2005; Goldstein et al., 2007; Volkow and Fowler, 2000). It is unknown, however, whether these observations might parallel findings of structural alterations to the PFC (Fein et al., 2002; Franklin et al., 2002; Matochik et al., 2003; Sim et al., 2007) and amygdala (Makris et al., 2004), despite broad claims that cocaine, potentially in conjunction with alcohol or other drugs, can be neurotoxic (Du et al., 2006; Glauser and Queen, 2007). The absence of quantitative evidence supporting this thesis, beyond the known potential for inducing stroke and seizures, is striking in contrast to what has been reported regarding another psychostimulant, methamphetamine, in animals and humans (Deng et al., 2007; Kuczenski et al., 2007; Thompson et al., 2004).
The present study addressed this issue using T1-weighted MRI of 20 cocaine dependent patients (COC) and 20 matched controls (CON) to study the variation in cortical thickness (Makris et al., 2006b, 2007; Fischl and Dale, 2000; Shaw et al., 2007) of neocortical and paralimbic regions subserving executive control and regulation of reward (to be referred to as “reward regulation” or RWR) and attention function (ATTN). Thickness is a topographical measure that is an indicator of the integrity of cytoarchitecture in the cortex (Makris et al., 2007), and of all the topographical measures that can be made, cortical thickness is the most invariant brain size parameter across mammalian evolution (Prothero and Sundsten, 1984; Mountcastle, 1998). Neocortical enlargement depends primarily on growth of surface area (Rockel et al., 1980; Jones, 1990; Mountcastle, 1998). Cortical surface and brain volume bear a nearly linear relation, and cortical thickness changes minimally for volumes above 3 cm3 (Hofman, 1989; Mountcastle, 1998). Alterations in thickness might thus be an important observation in functional brain illness. The connection of such measures to well-defined behavioral indices that are known to be affected in cocaine addiction, would be a further index of the importance of these findings to addiction. Cortical thickness was therefore assessed in COC and CON subjects using a double blind semi-automated segmentation/parcellation method integrated with a set of automated steps for topographical measures (Caviness et al., 1996; Worth et al., 1997; Makris et al., 2006b, 2007; Fischl and Dale, 2000).
If differences were seen between COC and CON subjects, we then examined hypotheses of etiology secondary to drug exposure or to potential predisposition. Absent associations of any thickness differences with drug exposure, findings of an alteration in normative asymmetry would argue for a potential genetic etiology (e.g., Piedra et al., 1998; Hyatt and Yost, 1998; Supp et al., 1997; see Methods Appendix, Symmetry Analysis). Similarly, alterations in the pattern of thickness measures across the cortical mantle would contrast with the preservation of thickness measures across mammalian evolution (Prothero and Sundsten, 1984; Mountcastle, 1998). Lastly, to interpret the relevance of any cortical thickness, laterality or pattern differences found in COC subjects with regard to their diagnosis, we examined possible behavioral implications of these findings.
Behavioral assessments involved (1) a keypress task measuring relative preference for a validated picture set (i.e., beautiful versus average faces; Aharon et al., 2001), which build upon operant procedures used in animal addiction research (Everitt and Robbins, 2005; Hyman et al., 2006; Kalivas and Volkow, 2005; Koob, 2006), and (2) a continuous performance task (CPT) for three categories of attention function, including an effortful attention condition, which involves high information processing demands (Goldstein et al., 2005; Seidman et al., 1998b, 2007; Thermenos et al., 2004) and recruits brain regions traditionally classified with reward processing (Breiter et al., 2006; Breiter and Rosen, 1999; Everitt and Robbins, 2005; McClure et al., 2004; Rolls et al., 2007).
With these behavioral, clinical, and topographical/morphometric measures, our hypotheses were that COC subjects, in contrast to CON subjects, would show quantitative decreases in cortical thickness and volume in cerebral regions dedicated to the control of relative preference and effortful attention, and these topographical alterations would (i) correlate with behavioral indices, and (ii) provide etiological hypotheses for subsequent animal and/or family/twin studies.
Results
Data were analyzed using a hierarchical process that first tested hypotheses with large volumes of tissue and sequentially followed positive results down to more elementary structural units. We grouped the orbitofrontal cortex (FOC), anterior cingulate gyrus (CGa) and paracingulate cortex (PAC), insular cortices (INS) and dorsolateral prefrontal cortex (DLPFC), as RWR regions controlling subcortical and cortical systems that process reward/aversion information (see Appendix 2, Data Analyses for rationale). Systems mediating the alerting, orienting, and executive control of ATTN were also grouped, including the CGa and posterior cingulate gyrus (CGp), inferior parietal lobule (IPL, comprising regions in the angular gyrus (AG), supramarginal gyrus (anterior and posterior, SGa, SGp), and parietal operculum (PO)) and DLPFC (see Appendix 2, Data Analyses for rationale). Given lateralization of ATTN function in humans, we specifically limited a priori regions for ATTN to the right hemisphere (Heilman and Van Den Abell, 1980; Makris et al., 2007; Mesulam, 1990; Seidman et al., 1998b). Across these groupings we then assessed cortical thickness measurements in COC and CON subjects and related these findings to behavioral measurements.
Cortical Volume and Thickness
Overall cerebral exterior was similar (t(38) = 1.67, p = 0.38) between CON (mean ± SD; 1139.4 ± 147.8cc) and COC subjects (1067.5 ± 123.9cc). Total cortical volume was significantly less (t(38) = 2.21, p = 0.047) in COC subjects (532.3 ± 67.3cc) relative to CON subjects (584.9 ± 82.2cc). Significant cortical thickness effects were observed using a multivariate general linear mixed model (Table 1), and subsequent pair-wise comparisons revealed significantly thinner cortex in COC subjects for the total RWR system, and separately, for the right hemisphere RWR. A similar trend was also seen for the total ATTN system.
Table 1.
Average cortical thickness at the systems level (i.e., RWR, and ATTN), followed by an analysis at the hemispheric level for significant effects seen at the systems level. All regions are weighted by the number of vertices in each PU. General linear mixed model covarying for average cortical thickness (of the entire neocortex) for the total RWR and right ATTN systems: F(2, 40) = 4.9, p = 0.013. General linear mixed model covarying for average cortical thickness for the Right RWR and Left RWR F(2, 40) = 4.0, p = 0.026. Pairwise post-hoc statistics are listed in the table, where * = significance at alpha (0.05) corrected for 2 comparisons = 0.025. Abbreviations: RWR: reward regulation system.
| Means | Std Devs. | ||||||
|---|---|---|---|---|---|---|---|
| Region | # vertices in the network |
Controls | Addicts | Controls | Addicts | T value | P-value |
| T RWR | 26999 | 3.71 | 3.59 | 0.23 | 0.19 | 2.8 | 0.009 * |
| R RWR | 13622 | 3.69 | 3.55 | 0.23 | 0.19 | 2.8 | 0.008 * |
| L RWR | 13377 | 3.73 | 3.64 | 0.25 | 0.21 | 0.6 | 0.60 |
| R Attention | 13927 | 3.36 | 3.25 | 0.23 | 0.18 | 1.5 | 0.15 |
Sub-regions composed of sets of elementary parcellation units or regions of interest (PUs) (Caviness et al., 1996) comprising RWR (FOC, CGa, PAC, INS, DLPFC) and ATTN (CGa, CGp, IPL, DLPFC) were then assessed for cortical thickness. Mean thickness across anatomically linked PUs was significantly thinner in COC subjects for right RWR subregions by multivariate GLM (Table 2). Pairwise contrasts were significant and showed a trend for the right DLPFC and right INS, respectively.
Table 2.
Analysis of the average cortical thickness at the sub-region level in parcellation units (PUs) of the RWR network. The general linear mixed model for RWR sub-regions, covarying for average cortical thickness of the entire neocortex, shows F(5, 40) = 4.9, p < 0.001.
| Means | Std Devs. | ||||||
|---|---|---|---|---|---|---|---|
| Region | # vertices in the PU |
Controls | Addicts | Controls | Addicts | T-value | P-value |
| Right DLPFC | 5551 | 3.65 | 3.41 | 0.31 | 0.21 | 3.0 | 0.005 * |
| Right CGa | 2478 | 2.69 | 2.80 | 0.50 | 0.79 | −0.9 | 0.4 |
| Right FOC | 1702 | 4.22 | 4.08 | 0.28 | 0.28 | 1.2 | 0.2 |
| Right INS | 2553 | 4.14 | 3.96 | 0.28 | 0.21 | 2.5 | 0.017 |
| Right PAC | 1338 | 4.21 | 4.08 | 0.34 | 0.30 | 0.8 | 0.4 |
indicates a significant difference at alpha (0.05) corrected for 5 comparisons = 0.01. Abbreviations: DLPFC: dorsolateral prefrontal cortex; CGa: anterior cingulate gyrus; FOC: orbitofrontal cortex; INS: insula; PAC: paracingulate gyrus.
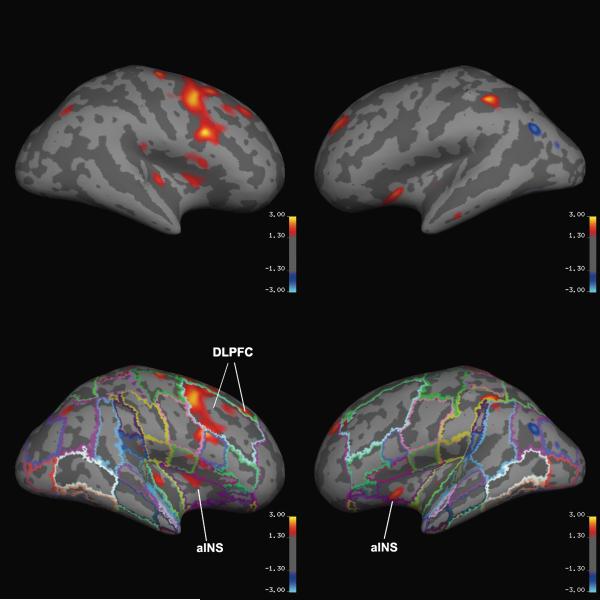
Clusters within right RWR showed significant effects by a multivariate general linear mixed model (Table 3). Pairwise contrasts were significant for the dorsolateral superior frontal gyrus (F1l; BA 9 and 8) and the middle frontal gyrus (F2; BA 46, 9 and 8) of the DLPFC, along with the anterior and posterior insular lobules (Table 3; Figure 1). Given the conservative bias of hierarchical analyses to potentially miss true positives, PUs containing clusters that met the a priori size and significance thresholds, but were not necessarily a priori regions of interest, were also included in Table 3. After correcting for multiple comparisons, the right inferior precentral gyrus (PRG) contained a cluster that was significantly thinner, whereas the dorsolateral aspect of the left occipital lobe (OLs) and angular gyrus (AG) contained clusters that were significantly thicker in the COC subjects.
Table 3.
Mean cortical thickness for clusters within individual parcellation units (PUs). Test statistics show the group effect after controlling for average thickness of the entire neocortex. The RWR network is comprised of five regions (DLPFC, CGa, FOC, INS, PAC), sub-divided by the parcellation schema. There are 26 distinct parcellation units for post-hoc analyses outside of RWR. The overall multivariate general linear mixed model for RWR units, covarying for average cortical thickness, shows F(5, 40) = 6.3, p < 0.001.
| Means | Std Devs. | Cluster statistic | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | # vertices in the cluster |
# vertices in the PU |
%PU | Controls | Addicts | Controls | Addicts | T value | P-value |
| Right RWR | |||||||||
| F1l | 754 | 2189 | 34.4 | 3.75 | 3.43 | 0.38 | 0.26 | 3.1 | 0.004 * |
| F2 | 1810 | 3362 | 53.8 | 3.42 | 3.12 | 0.28 | 0.21 | 4.0 | <0.001 * |
| subgenual_CGa_BA24_32 | 0 | 334 | . | . | . | . | . | . | . |
| pregenual_CGa_BA24 | 0 | 882 | . | . | . | . | . | . | . |
| amCGa_BA24 | 0 | 1262 | . | . | . | . | . | . | . |
| aFOC | 0 | 268 | . | . | . | . | . | . | . |
| pFOC | 15 | 646 | 2.3 | 4.64 | 4.32 | 0.52 | 0.42 | . | . |
| mFOC | 4 | 494 | 0.8 | 4.59 | 4.27 | 0.52 | 0.42 | . | . |
| lFOC | 0 | 294 | . | . | . | . | . | . | . |
| aINS | 245 | 1365 | 17.9 | 4.31 | 4.04 | 0.34 | 0.26 | 2.7 | 0.009 * |
| pINS | 264 | 1188 | 22.2 | 3.57 | 3.24 | 0.46 | 0.30 | 2.8 | 0.008 * |
| PAC_BA32_dorsal | 0 | 358 | . | . | . | . | . | . | . |
| pregenual_PAC_BA32 | 249 | 980 | 25.4 | 4.52 | 4.14 | 0.48 | 0.44 | 2.5 | 0.02 |
| Other Right Hemisphere Regions | |||||||||
| AG | 133 | 1562 | 8.5 | 3.70 | 3.36 | 0.51 | 0.33 | 2.35 | 0.0244 |
| COa | 124 | 585 | 21.2 | 3.79 | 3.56 | 0.32 | 0.27 | 2.33 | 0.0253 |
| F1m | 94 | 993 | 9.5 | 4.46 | 4.16 | 0.45 | 0.36 | 2.17 | 0.0366 |
| F3o | 203 | 629 | 32.3 | 3.47 | 3.22 | 0.24 | 0.24 | 3.27 | 0.0023 |
| FMC | 112 | 431 | 26.0 | 4.58 | 4.18 | 0.52 | 0.50 | 2.29 | 0.0277 |
| FO | 184 | 699 | 26.3 | 3.91 | 3.70 | 0.25 | 0.24 | 2.49 | 0.0172 |
| FP | 113 | 4281 | 2.6 | 4.34 | 4.01 | 0.43 | 0.40 | 2.32 | 0.0262 |
| LG | 142 | 1677 | 8.5 | 2.59 | 2.85 | 0.27 | 0.32 | −2.81 | 0.0078 |
| PO | 64 | 513 | 12.5 | 3.34 | 3.10 | 0.34 | 0.30 | 2.06 | 0.0469 |
| PRG_inf | 278 | 1854 | 15.0 | 3.33 | 3.07 | 0.25 | 0.22 | 3.45 | 0.0014 ** |
| PRG_mid | 284 | 1425 | 19.9 | 2.85 | 2.56 | 0.37 | 0.23 | 2.79 | 0.0083 |
| Other Left Hemiphere Regions | |||||||||
| AG | 137 | 1264 | 10.8 | 3.66 | 3.97 | 0.39 | 0.27 | −3.34 | 0.0019 ** |
| aINS | 180 | 1366 | 13.2 | 4.87 | 4.48 | 0.39 | 0.42 | 2.82 | 0.0076 |
| BFsbcmp | 157 | 944 | 16.6 | 1.59 | 2.19 | 0.48 | 0.72 | −3.18 | 0.0030 |
| F1l | 119 | 2118 | 5.6 | 4.11 | 3.86 | 0.32 | 0.20 | 3.15 | 0.0032 |
| F1m | 101 | 1132 | 8.9 | 4.25 | 4.02 | 0.31 | 0.23 | 2.67 | 0.0111 |
| F2 | 92 | 3652 | 2.5 | 3.94 | 3.66 | 0.42 | 0.28 | 2.32 | 0.0260 |
| FP | 206 | 4829 | 4.3 | 4.03 | 3.71 | 0.45 | 0.26 | 2.98 | 0.0050 |
| OLs | 62 | 1773 | 3.5 | 3.80 | 4.08 | 0.37 | 0.35 | −3.43 | 0.0015 ** |
| PCN | 78 | 2233 | 3.5 | 3.27 | 3.54 | 0.37 | 0.34 | −2.86 | 0.0070 |
| pFOC | 62 | 506 | 12.3 | 4.68 | 4.31 | 0.37 | 0.57 | 2.21 | 0.0337 |
| POG_inf | 156 | 2139 | 7.3 | 3.45 | 3.23 | 0.21 | 0.25 | 2.92 | 0.0059 |
| POG_med | 149 | 874 | 17.0 | 3.82 | 3.45 | 0.47 | 0.43 | 2.47 | 0.0183 |
| SGp | 100 | 2074 | 4.8 | 3.75 | 3.47 | 0.42 | 0.29 | 2.22 | 0.0323 |
| SPL | 65 | 1436 | 4.5 | 3.63 | 3.31 | 0.51 | 0.39 | 2.10 | 0.0429 |
| TOF | 75 | 926 | 8.1 | 3.30 | 3.57 | 0.25 | 0.46 | −2.76 | 0.0089 |
indicates a significant difference at alpha (0.05) corrected for 5 comparisons = 0.01, and
indicates a significant difference at alpha (0.05) corrected for 26 comparisons = 0.0019. Abbreviations: RWR: reward regulation system; BA: Brodmann’s area; F1l: superior fronal gyrus lateral division; F2: middle frontal gyrus; CGa: anterior cingulate gyrus; amCGa: anterior cingulate gyrus, anterior middle division; aFOC, pFOC, mFOC, lFOC: anterior, posterior, medial, lateral fronto-orbital cortex; aINS: anterior insula; pINS: posterior insula; PAC: paracingulate gyrus; AG: angular gyrus; COa: anterior central opercular cortex; F1m: medial superior frontal gyrus; F3o: inferior frontal gyrus, pars opercularis; FMC: frontal medial cortex; FO: frontal operculum cortex; FP: frontal pole; LG: lingual gyrus; PO: parietal operculum cortex; PRG_inf: precentral gyrus inferior division; PRG_mid: precentral gyrus middle division; BFsbcmp: basal forebrain subcomponent; OLs: superior lateral occipital cortex; PCN: precuneus cortex; POG_inf: postcentral gyrus, inferior division; POG_med: postcentral gyrus, medial division; SGp: posterior supramarginal gyrus; SPL: superior parietal lobule; TOF: temporal occipital fusiform cortex.
Figure 1.
Inflated cerebral mantle with (below) and without (above) superimposed parcellation from the MNI 305 average brain. Pseudo-color statistical map overlays (red, p < .05 and yellow, p < .001) illustrate where the cortex of the COC subjects is thinner than in CON subjects. DLPFC, dorsolateral prefrontal cortex; aINS, anterior insula.
Given that education may be a proxy for drug abuse, but has not been definitively correlated with cortical thickness (Im et al., 2006), analyses were run both with and without covarying for years of education; both sets of analyses yielded similar results.
Analysis of Symmetry and Thickness Heterogeneity
As alterations in normative asymmetries in cortical thickness argue for a genetic etiology of behavior, differences in hemispheric symmetry between groups and distribution of cortical thickness were assessed for potential premorbid influences on addiction.
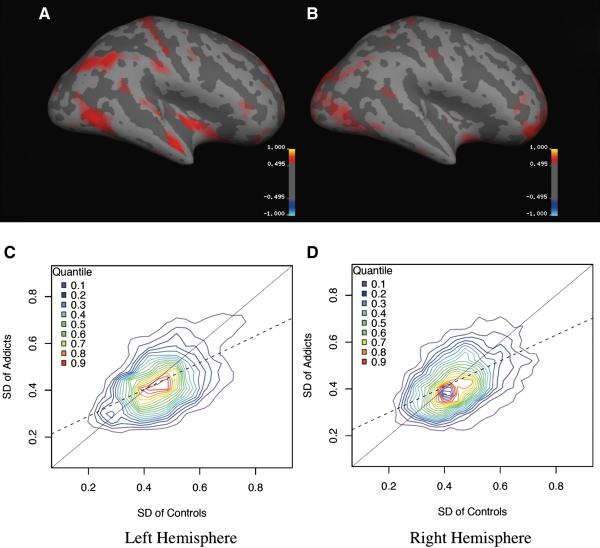
Significant asymmetry effects were observed in the RWR subregion of the DLFPC [2.8% rightward for controls (i.e., right thickness > left thickness), 0.8% leftward for addicts; t(38) = 2.2, p = 0.03]. There were no other significant symmetry alterations observed in average thickness of the systems (i.e., RWR or ATTN), sub-regions (or sets of elementary PUs), or clusters of vertices within the a priori elementary PUs. Additionally, qualitative comparison of standard deviation maps reveals greater thickness heterogeneity in CON relative to COC subjects (Figures 2a, b). Quantitative assessment by bivariate fit of thickness across vertices indicates greater cortical smoothness or less heterogeneity in the average PU thickness for COC subjects (Figures 2c, d).
Figure 2.
Standard deviation maps across the inflated cortex (Fig 2a, b) showing greater thickness heterogeneity in CON versus COC subjects. Quantitative assessment of the cortical thickness heterogeneity, using a bivariate fit of thickness across vertices (Fig 2c, d), shows the quantile density contours are centered on the CON section of the plot for both hemispheres.
Behavioral Effects
Relative preference measures were collected using a dual keypress procedure with beautiful and average faces (see Methods for details). In the attention task (CPT), subjects sought to identify cue-target pairs amidst a string of letters, presented visually one letter per second. Three experimental conditions were utilized where subjects identified cue-target pairs (hits and misses) amidst a string of letters: (i) vigilance/selective attention (qA condition), (ii) sustained attention (q3Ad condition), and (iii) effortful divided attention (q3Ai condition) (Seidman et al., 1998b, 2007; Goldstein et al., 2005; Thermenos et al., 2004).
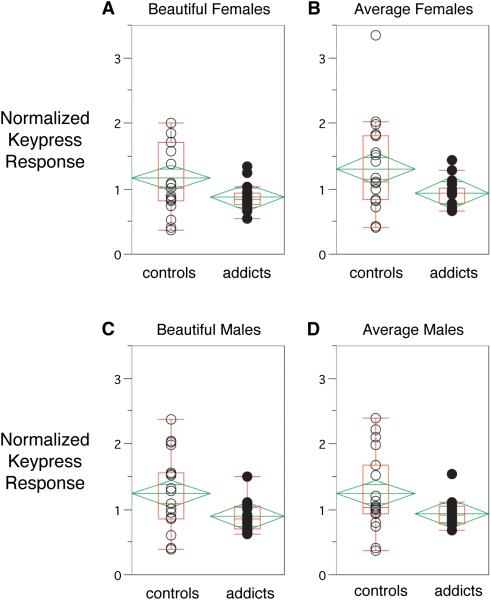
In the relative preference task, COC subjects produced significantly lower keypress responses (i.e., reduced or restricted preference measures), normalized for subject motor capabilities, for all four categories of stimuli (Figure 3, Table 4). COC subjects also produced significantly lower hits and more misses for sustained attention and effortful divided attention (Table 4).
Figure 3.
Box-plots of normalized keypress responses in COC and CON subjects to the BF faces (3A), AF faces (3B), BM faces (3C), and AM faces (3D). The dispersion measure is presented as a diamond, and the horizontal line through the data represents the whole-group mean.
Table 4.
Top: normalized keypress responses in COC and CON subjects to the BF, AF, BM, and AM faces. Bottom: the three task components of the CPT are signified by qA (vigilance condition), a3Ad (a sustained attention condition), and q3Ai (an effortful and divided attention condition). Correct hits are listed as a ratio of hits to possible targets, and thus will range from 0 to 1. Misses are presented as an absolute number. Degrees of freedom are 35 for the normalized keypress response and 34 for performance on the CPT task.
| Means | Std Devs. | Statistic | ||||
|---|---|---|---|---|---|---|
| Test | Controls | Addicts | Controls | Addicts | T-value | P-value |
| Normalized keypress response | ||||||
| beautiful females | 1.17 | 0.87 | 0.54 | 0.19 | 2.2 | 0.03 |
| average females | 1.31 | 0.94 | 0.69 | 0.20 | 2.2 | 0.04 |
| beautiful males | 1.24 | 0.89 | 0.57 | 0.23 | 2.4 | 0.02 |
| average males | 1.24 | 0.94 | 0.58 | 0.20 | 2.1 | 0.048 |
| Performance on CPT task | ||||||
| hits: qA | 0.91 | 0.90 | 0.18 | 0.13 | 0.2 | 0.9 |
| hits: q3Ad | 0.89 | 0.72 | 0.12 | 0.24 | 2.6 | 0.01 |
| hits: q3Ai | 0.77 | 0.61 | 0.18 | 0.20 | 2.4 | 0.02 |
| misses: qA | 2.53 | 4.26 | 4.68 | 5.60 | −1.0 | 0.3 |
| misses: q3Ad | 2.06 | 5.26 | 2.56 | 5.96 | −2.1 | 0.048 |
| misses: q3Ai | 2.76 | 7.21 | 2.41 | 6.91 | −2.5 | 0.02 |
Correlational Analysis
To test whether structural differences in RWR and associated PU’s were potentially related to drug consumption, or had behavioral implications in line with known alterations in attention for stimulant addicts, we examined associations between (a) cortical thickness and drug use, and (b) cortical thickness and behavior. When significant correlations were found with behavior, we further tested the differential contribution of the two systems (RWR and ATTN) by evaluating correlation against sets of PUs that were and were not overlapping between them. It has previously been reported that PUs within RWR but not ATTN (i.e., INS and FOC), demonstrate significant changes in BOLD signal with fMRI during effortful attention (Seidman et al., 1998b; Goldstein et al., 2005), potentially due to both increased allocation of attention resources and motivation/reward processes (Maunsell, 2004).
Measures of Substance Use
Measures of cocaine use were tested a priori, whereas measures of alcohol and nicotine use were assessed post hoc. Correlations were significant for right RWR (r(18) = −0.54, p = 0.014) and right CGa (r(18) = −0.50, p = 0.026) with years of cocaine use. Post hoc correlations with alcohol use did not meet hierarchical constraints for RWR or any sub-region. With a ceiling effect, significant correlations were observed for days of nicotine use and right DLPFC thickness (r(18) = −0.52, p = 0.016) and mean PU thickness for F1l (r(18) = −0.63, p = −0.024).
To sort out polysubstance abuse effects on the a priori cocaine correlations, we performed partial correlations for years of cocaine and right RWR plus right CGa, covarying for the effects of alcohol and nicotine use, and found significant partial correlation results (all p < 0.05; see Appendix 1 for specifics). No significant interaction (p >> 0.05) between days of nicotine use and years of cocaine use was observed, nor was a separate post-hoc correlation between age of drug use onset and thickness indices significant (p >> 0 .05).
Measures of Relative Preference
Significant correlations were observed between the normalized keypress response (see above), for a number of face conditions and the cortical thickness (a) of one sub-region, right DLPFC, and (b) of cluster thickness in right F2, and right F1l. Separately, significant observations were observed between a number of face conditions and right INS cluster thickness. See Table 5 for synopsis of results.
Table 5a.
Correlations between the relative preference measures (normalized keypress response) for all face conditions and the cortical thickness (a) of one subregion, right DLPFC, and (b) of cluster thickness in right F2, right F1l, and INS.
| Controls | Addicts | Slope comparison | ||||||
|---|---|---|---|---|---|---|---|---|
| Normalized keypress response | n | r | p | n | r | p | T-value | P-value |
| DLPFC | ||||||||
| beautiful females | 19 | 0.06 | 0.8 | 19 | −0.54 | 0.02 | −1.9 | 0.07 |
| average females | 19 | −0.02 | 0.9 | 19 | −0.53 | 0.02 | −1.9 | 0.06 |
| beautiful males | 19 | 0.02 | 0.9 | 19 | −0.60 | 0.01 | −2.1 | 0.04 |
| average males | 19 | 0.00 | 0.9 | 19 | −0.62 | 0.01 | −2.3 | 0.03 |
| F2 | ||||||||
| beautiful females | 19 | 0.01 | 0.9 | 19 | −0.66 | 0.002 | −2.2 | 0.03 |
| average females | 19 | −0.03 | 0.9 | 19 | −0.68 | 0.001 | −2.7 | 0.01 |
| beautiful males | 19 | −0.03 | 0.9 | 19 | −0.71 | 0.001 | −2.5 | 0.02 |
| average males | 19 | −0.09 | 0.7 | 19 | −0.71 | 0.001 | −2.6 | 0.01 |
| F1l | ||||||||
| beautiful females | 19 | 0.09 | 0.7 | 19 | −0.51 | 0.02 | −1.9 | 0.07 |
| average females | 19 | −0.04 | 0.9 | 19 | −0.49 | 0.03 | −1.7 | 0.10 |
| beautiful males | 19 | 0.05 | 0.8 | 19 | −0.53 | 0.02 | −1.9 | 0.06 |
| average males | 19 | 0.02 | 0.9 | 19 | −0.55 | 0.02 | −2.0 | 0.05 |
| INS | ||||||||
| beautiful females | 19 | 0.14 | 0.6 | 19 | −0.48 | 0.04 | −2.0 | 0.06 |
| average females | 19 | −0.15 | 0.5 | 19 | −0.47 | 0.04 | −1.4 | 0.16 |
| beautiful males | 19 | 0.09 | 0.7 | 19 | −0.47 | 0.04 | −1.9 | 0.07 |
| average males | 19 | 0.03 | 0.9 | 19 | −0.56 | 0.01 | −2.2 | 0.03 |
Measures of Effortful Attention
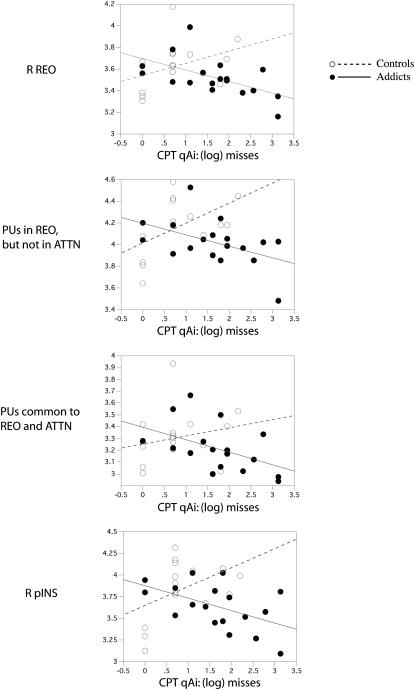
In a hierarchical analysis, significant correlations were only observed between error measures (i.e., misses) for the effortful divided attention condition (q3Ai), and cortical thickness measures in COC but not CON subjects, producing differences in slopes. Significant correlations were observed for right RWR cortical thickness (Figure 4a). Significant effects were then noted for regions not overlapping with ATTN (i.e., RWR_minus_ATTN) but not regions overlapping with ATTN (i.e., RWR_intersect_ATTN; Figures 4b, c). These effects were due to correlations between misses during effortful attention: and (1) orbitofrontal cortex (FOC) PU thickness and (2) posterior insula (pINS) cluster thickness (Figure 4d).
Figure 4.
Correlation plots [CON (dashed lines) and COC (solid lines) subjects] between misses for the effortful divided attention condition (q3Ai task) and average cortical thickness in (i) right RWR, (ii) sets of PUs in RWR but not ATTN, (iii) sets of PUs in RWR and ATTN, and (iv) right posterior INS.
Discussion
Synopsis
COC relative to CON subjects showed reduced total cortical volume, and reduced thickness for the RWR system, including paralimbic cortices such as the INS, and neocortical regions such as the DLPFC. Significant correlation results suggest that some cortical thickness alterations in COC subjects may be partly related to drug use. In contrast, symmetry and variance differences in cortical topography in COC subjects may reflect a predisposition for addiction, a hypothesis that requires future testing.
The functional implications of these cortical topography differences include a set of altered relationships in COC subjects between cortical thickness and (i) relative preference indices plus (ii) effortful attention indices. Specifically, DLPFC thickness and F2, F1l, and anterior INS thickness significantly correlated with altered keypress responses to the four categories of facial stimuli, indicating abnormal cognitive control of preference during judgment and decision-making. The reduction in keypress response to all stimuli in COC subjects is analogous to the restriction in behavioral repertoire that is a defining feature of addiction. Cortical regions correlating with keypress responses did not overlap with regions correlating with performance at an effortful attention task. The effortful attention correlations were consistent with fMRI studies demonstrating activation of the same regions during tasks with high information processing demands (Seidman et al., 1998b, 2007).
Structural Abnormalities in Cocaine Addiction
Prior research comparing brain anatomy between COC and CON subjects has used voxel-based morphometry (VBM) and quantitative multi-spectral tissue characterization techniques, which produce gray matter “density” or “concentration” measures, or volume differences between two groups, respectively. These are relative measures, and not absolute volume or thickness measures, as reported herein with regard to large cortical volumetric effects (50+ cc volume decrease) and regional thickness changes in COC subjects. The topographical differences found in the cingulate cortex support the reports of “concentration” differences in this region by Franklin and colleagues (2002) and Matochik and colleagues (2003), and the report of insula differences by Franklin and colleagues (2002). In the current study, positive correlations with years of cocaine use were also focused on RWR, and the cingulate cortex. We did not find thickness differences in the FOC consistent with reports by VBM (Fein et al., 2002; Franklin et al., 2002; Matochik et al., 2003; Sim et al., 2007), although FOC cortical thickness did correlate with reduced effortful attention performance for the COC subjects in our study, consistent with Fein et al. (2002). Correlations reported herein, between performance in effortful attention and right RWR, FOC, and pINS thickness, are consistent with fMRI studies demonstrating FOC and pINS recruitment during effortful attention tasks with high information processing demands (Seidman et al., 1998b, 2007).
Current insights in the neurobiology of cocaine dependence emphasize the role of RWR and ATTN dysfunction in this disorder (Goldstein et al., 2007; Tomasi et al., 2007; Volkow and Fowler, 2000). A common thread across the two aforementioned neural networks is the right DLPFC, which was thinner in COC subjects in this study, and is known to project to and to receive connections from paralimbic cortices such as the CGa, FOC, and INS (Cohen et al., 2000; Kringelbach, 2005; Mesulam and Mufson 1985; Mesulam, 2000; Petrides and Pandya, 2002; Seo et al., 2007; Tanaka et al., 2004) and subcortical regions (Alheid and Heimer, 1988; Breiter et al., 1997, 2006; McClure et al., 2004) traditionally implicated with reward/aversion function. In current models of judgment and decision-making that consider issues of homeostasis (e.g., Breiter et al., 2006; Paulus, 2007; Verdejo-Garcia et al., 2006), the DLPFC plays an important role in choice and choice implications over time through the biasing of somatic states that endorse some behavioral options and reject others (Verdejo-Garcia et al., 2006). Interconnections between the DLPFC and INS further constitute an important frontal-limbic interaction needed for normative functioning of the reward system (Mayberg, 2002; Mesulam, 2000). Our data are consistent with these models in that observed reductions in choice behavior with a keypress procedure for COC subjects (Figure 3) correlated, across all categories of experimental stimuli, with their reductions in DLPFC thickness, DLPFC sub-unit (i.e., F2 and F1l) thickness, and INS thickness. These statistically significant associations suggest that one factor contributing to the diminished behavioral repertoire observed with drug dependence may be attributed to a regional reduction of cortical thickness.
Functional Implications of Structural Abnormalities
The regions in COC subjects associated with altered attention function, or years of cocaine use, are paralimbic regions reciprocally connected to the DLPFC, such as the INS, FOC, and CGa (see Figure 5a-c), which have been implicated in reward processing by other studies. The DLPFC itself has topographically proximate regions associated with ATTN functions (posterior section, BA 8 and rostral 6), and with reward regulation for planning and execution of behavior (anterior section, BA 46, 9), underscoring the likelihood of their functional affiliation (Maunsell, 2004). The frontolimbic interactions mediated by the DLPFC-aINSlbl/FOC system appear to be crucial for modulating emotional and motivational significance of incoming stimuli, thus influencing cognitive processing and decision-making on the basis of real-time emotional and motivational states (Mesulam and Mufson, 1985; Pandya and Yeterian, 1985; Petrides and Pandya, 2002). The CGa is an important regulator of other cortical and subcortical brain regions as well, and appears to be a key structure for the integration of cognitive and emotional aspects of behavior with drives (Paus, 2001), along with monitoring of conflict and modulation of cognitive control, and modulation of attention allocation in real time (Carter et al., 2000; Cohen et al., 2000; Fan et al., 2005). A failure in the interaction between DLPFC-aINSlbl/FOC and DLPFC-PAC/CGa may account for altered behaviors in cocaine dependence such as antisocial and violent behavior as well as progressive loss of judgment concerning “safe” drug use habits (Bechara, 2005). In the somatic marker model, the FOC, INS, and CGa are fundamental components of distributed systems that trigger emotional (somatic) states from secondary inducers (i.e., thoughts about an action), and provide a substrate for feeling emotional states and biasing decisions (Verdejo-Garcia et al., 2006).
Figure 5.
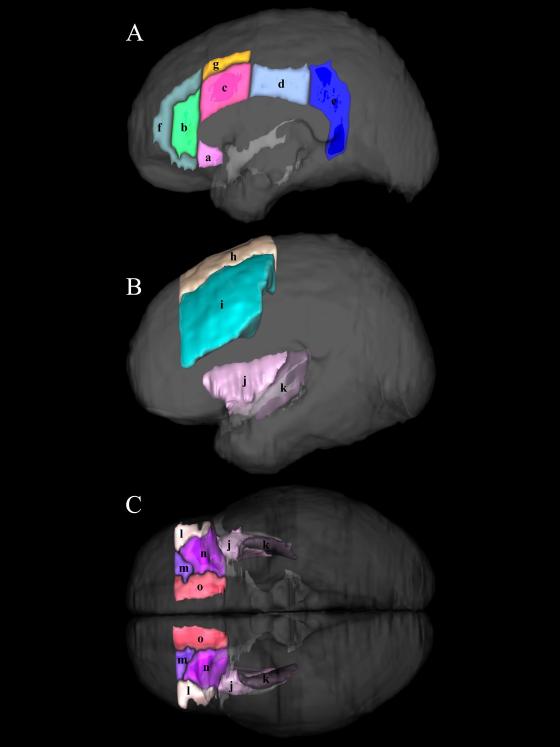
Anatomical PUs in cingulate gyrus (CG; a-e), paracingulate gyrus (PAC; f, g), dorsolateral prefrontal cortex (DLPFC; h, i), insula (INS; j, k), and orbitofrontal cortex (FOC; l-o). (a) subgenual CGa, (b) pregenual CGa, (c) anterior-middle CGa, (d) posterior-middle CGa, (e) posterior cingulate gyrus, (f) pregenual PAC, (g) dorsal PAC, (h) lateral superior frontal gyrus, (i) middle frontal gyrus, (j) anterior insular lobule, (k) posterior insular lobule. (l) lateral FOC, (m) anterior FOC, (n) posterior FOC, (o) medial FOC. (a - c, f - o) were included in RWR.
Etiologic Hypotheses
This study found correlations between cocaine years of use and (i) RWR cortical thickness, plus (ii) CGa PU thickness, but no correlation with age of cocaine use onset and these thickness measures. This correlation suggests a primary or secondary effect of drug use, but not an increased susceptibility due to earlier onset of drug use. A separate correlation between days of nicotine use and DLPFC plus PU (F1l) thickness was also observed, although this post-hoc finding exhibited a ceiling effect, and did not alter partial correlation results for cocaine with the RWR and CGa. Cocaine partial correlations were also not affected by alcohol use. None of these drug use parameters correlated with each other, pointing to the importance of follow-up longitudinal or family studies to confirm or clarify these findings.
The current study provides evidence that cocaine and polysubstance use has a detrimental effect on brain volume and topography, but not a uniform global effect as might otherwise be expected from Ca2+ toxicity (Du et al., 2006) or from transient cerebral ischemia, stroke, and hemorrhage (Glauser and Queen, 2007). It also provides evidence connecting these structural alterations with a functional effect of cocaine/polysubstance use in the form of worsened attentional function and reduced judgment and decision-making regarding relative preference. Further work is warranted to assess (a) anatomical specificity, and (b) susceptibility across the larger cocaine and polysubstance using population to different patterns of psychostimulant-related alterations to the cortical mantle.
A second etiology is supported by the observation of differences in the symmetry of DLPFC thickness between COC and CON subjects. Alterations to normative asymmetries generally have a genetic basis (e.g., Piedra et al., 1998; Hyatt and Yost, 1998; Supp et al., 1997; see Methods Appendix, Symmetry Analysis), as in many forms of situs inversus (Supp et al., 1997). In human and animal studies, cerebral asymmetry has been significantly correlated with handedness, which in turn has a strong genetic basis (Geschwind et al., 2002; Roubertoux et al., 2003). COC subjects also have a consistent decrease in the standard deviation of cortical thickness across the cortex, and less heterogeneity than controls, which does not correlate with years of drug use. A similar relationship between increased heterogeneity/variability and health has been noted for heart rate variability (HRV) studies (e.g., Krstacic et al., 2007), where medical issues ranging from sepsis to hyperthyroidism to traumatic brain injury to sleep disordered breathing can reduce HRV indices (Tateishi et al., 2007; Chen et al., 2007; Riordan et al., 2007). Despite reduced heterogeneity in cortical thickness, our COC subjects reported a broad range of cocaine use/exposure, with an average use duration of 12 years + 9.8 years (Table 6). In contrast to our data, subjects with a broad range of illness duration, such as with Alzheimer’s disease, will demonstrate an increase in structural variance measures (Burton et al., 2006). Variable lesion sizes with stroke will also lead to an increase in brain structural variance measures (Caviness et al., 2002). Our findings of changes in thickness asymmetry in DLPFC and reduced heterogeneity in thickness measures despite a large temporal range in drug exposure, argue for a predisposing (i.e., genetic) component to the differences in cortical topography observed between addicts and controls.
Limitations
Given the inherent limitations of any technique of brain segmentation that is used for extracting thickness measures, we included a comparison of an automated and a semi-automated segmentation method as part of this study to underscore the rigor of the methods underlying our results (Appendix 1, Figures A1-A28). The fully automated method (i.e., FreeSurfer) was less time consuming, lower cost, but less accurate for our a priori regions than a semi-automated method (i.e., Cardviews). Purely automated approaches are occasionally prone to systematic errors that may obscure subtle neuroanatomic effects; this issue has become an important topic of recent discussion (e.g., Wiegand et al., 2004; Devlin and Poldrack, 2007).
Limitations for group analysis are due in part to registration errors inherent to intersubject mapping and the transformation procedure, as well as the potentially ill-posed nature of intersubject correspondence in topography. Another potential limitation is sample size. Whereas 40 subjects is not a particularly large sample population, the case-control matching used in this study is expected to reduce spurious sources of between group variance. Lastly, the degree of comorbid use (nicotine 90%, alcohol 80%) in COC subjects was consistent with other reports (Simpson et al., 1999; Tang et al., 2007); nevertheless, it raises questions as to their role in the brain differences observed herein.
Conclusion
This study found salient cortical volumetric reduction and cortical thickness decreases in COC subjects for cortical regions regulating reward/aversion function, and regions involved with effortful attention. The correlation of cocaine and nicotine use measures with alterations in regional thickness supports an etiological hypothesis centered on drug effects, whereas the reversal of symmetry findings in DLPFC and the diminished cortical thickness heterogeneity found in COC subjects suggests an alternate predisposition hypothesis. These hypotheses can be directly tested by a combination of animal experiments and family/twin studies (e.g., with nicotine addiction). Relative preference behavior was also uniformly reduced in addicts, and correlated with thinner cortices for regions involved with the organization of behavior; this association may partly underlie the restriction in behavioral repertoire or range in behavioral preferences observed with drug dependence. Keypress and attention findings from this study, and their relation with cortical topography, can be directly tested in animal models of chronic drug self-administration.
Since cortical thickness is the most invariant brain size parameter across the model of mammalian evolution (Prothero and Sundsten, 1984; Mountcastle, 1998), some of the findings reported herein may have causal relevance. A fundamental component of addiction may involve neuroadaptations and/or developmental predispositions involving brain regions necessary for cognitive control of somatic states (Verdejo-Garcia et al., 2006), and judgment and decision-making regarding complex rewards and attention toward goal-objects. Addiction thus may represent a complex syndromic phenotype (Aylsworth, 1998), with multiple effects necessary for compulsive drug use.
Methods
Subjects
Subjects were participants in the Phenotype Genotype Project (PGP) in Addiction and Mood Disorders, recruited by direct advertisement and clinical referrals. They signed consent following the approval of the Massachusetts General Hospital Institutional Review Board. Cohorts were matched, after excluding subjects with residual motion artifacts from motion correction of structural MRI data, on a one-by-one basis across groups with respect to age, handedness, gender, race and educational history. See Appendices for details.
In each group of 20 matched cocaine dependent (COC) and healthy control (CON) subjects, 11 subjects were women (all scanned during their mid-follicular menstrual phase per hormonal testing), 16 subjects were right-handed (Oldfield, 1971), and 13 subjects were Caucasians, 5 subjects were African Americans, and 1 subject was Asian (Benson & Marano, 1998). Compared with CON subjects, COC subjects were not significantly different on age, sex distribution, handedness or race (Table 6, appendices), and within group there were no gender-based age or education differences. However, COC subjects had on average 1.9 fewer years of education (t (38) = 3.5, p = 0.001), and thus analyses were run both with and without co-varying for years of education, producing the same findings.
Behavioral Measures of Relative Preference and Effortful Attention
Relative Preference The task quantified units of keypress that subjects traded for viewing time of a set of normalized face pictures, comprising model and non-model faces of both genders. This task defined a subject’s relative preferences for these stimuli ((Aharon et al., 2001; i.e., their utility for the set of faces (Breiter et al., 2006)), as has been done with angry and other facial expressions (Strauss et al., 2005). Keypress procedures were implemented using MatLab software on a PC computer.
The dependent measures of interest, were the amount of work in units of key press that subjects exerted to (a) approach (positive keypress), (b) avoid (negative keypress), (c) approach and avoid if they overshot or undershot a target view time, or (d) do nothing about to the different categories of stimuli. The key press procedure quantified (i) decision-making regarding the valence of preference and (ii) judgment regarding the amount of value that each picture had relative to the default position of 6 seconds of viewing time (see Appendix I). Keypress behavior was normalized for potential differences in motor coordination or resiliency across groups, using a measure of maximum keypress capacity/speed.
Attention
Three measures of attention were collected during a separate functional MRI sequence, during which subjects performed a continuous performance task (CPT) using visual rather than auditory stimuli (Seidman et al., 1998b, 2007; Goldstein et al., 2005; Thermenos et al., 2004). In the baseline vigilance or selective attention task, subjects were required to respond to “A” immediately following a “Q” (qA). In the lowest-load working memory condition (a sustained attention condition, “q3Ad”), subjects were required to respond to an “A” following a “Q” after three intervening letters (e.g. QxyzA), with no nested sequences. A third task added interference and divided attention load by nesting a subset of QxyzA sequences within each other (e.g., QxQyAzA). Stimuli were presented in blocks, with the three block conditions completely counterbalanced forward and backward one block. Subjects responded to targets with a button press and did not respond to non-targets. Correct responses (“hits”) and misses (i.e., errors of commission) at target detection were used in statistical analyses.
Magnetic Resonance Imaging
Whole brain MR images were collected on a Siemens Avanto 1.5 T scanner at the MGH Martinos Center (Charlestown, MA). Three sagittal 3D Magnetization Prepared Rapid Gradient Echo (MP-RAGE) T1-weighted sequences were collected: TR = 2730 ms, TE = 3.31 ms, T1 = 1,000 ms, flip angle = 7°, bandwidth = 195 Hz/pixel, FOV = 256 × 256 mm2, sampling matrix = 256 × 192 pixels, 128 contiguous 1.33 mm slices, averages = 3.
Image Preprocessing and MRI-based Segmentation
Images were re-sampled into a standard coordinate system (Filipek et al., 1994; Makris et al., 2004). A new set of coronal images, not rescaled, were reconstructed at the slice thickness of the original acquisition.
Segmentation was performed with double blinding to group category and study hypotheses for all individuals performing data analysis at the MGH Center for Morphometric Analysis (CMA). Neuroanatomic segmentation was performed on coronal images (Figure 6A) using a semi-automated morphometric technique (Caviness et al., 1996; Filipek et al., 1994; Makris et al., 2004; Makris et al., 2006b; Worth et al., 1997). The cerebrum was segmented into its principal gray matter and white matter structures and total cerebral white matter. Specifically, the cortical ribbon was defined by two outlines, one external outline between the subarachnoid CSF and the cerebral cortex, and the other between the cerebral cortex and the underlying cerebral white matter (Makris et al., 2006b; Worth et al., 1997) as illustrated in Figure 6B. The total number of voxels in each brain region determined its volume.
Figure 6.
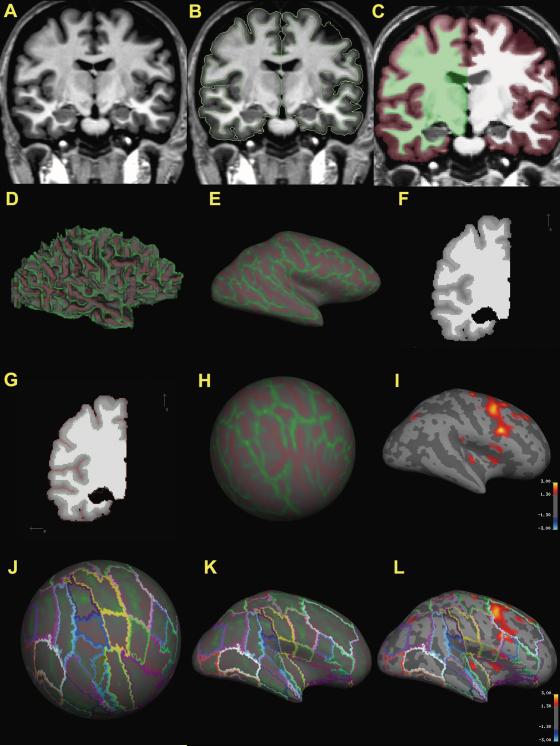
Topological cortical parcellation (TCP) system. 6A shows an intensity normalized T1-weighted MR coronal image. Segmentation (6B) is executed in Cardviews by a semi-automated procedure. Outline files created by Cardviews segmentation and parcellation are converted to a FreeSurfer volume segmentation (6C). The surface is tessellated, smoothed (6D) and inflated (6E) from the converted FreeSurfer volume. An intensity gradient is created throughout the cortex as a function of the distance from the white matter surface (6F). The exterior surface is generated to be consistent with the manual segmentation (6G). The white matter surface of each subject is transferred to spherical coordinates and registered to the average MNI 305 brain (6H). Inter-subject averaging and mapping of cortical thickness differences between groups of subjects; pseudo-color statistical maps where red represents p < .05 and yellow p < .001 (6I). The registration subject, (MNI 305) (Caviness et al., 1996; Evans et al., 1993; Makris et al., 2007), was segmented, parcellated and overlaid on the spherical surface (6J) and inflated surface (6K). Cortical thickness statistical results are overlain with the parcellation scheme to localize results (6L).
In contrast to semi-automated and manual routines, one source of error in fully automated cortical thickness measurements (e.g., Fischl and Dale 2000), stems from the placement of the cerebral exterior within the layers of the cortical mantle as opposed to the level of the meningeal pia (Figure 7b; Figures A1- A26, Appendix 2). Another source of error results from the placement of the gray-white border within the cortical layers as opposed to the floor of the sixth layer. Thus using fully automated techniques for cortical segmentation results in an inconsistent estimation of the cortical ribbon (Figures A1- A26, Appendix 2), producing statistically significant differences in derived properties such as thickness (Figures A27-A28, Appendix 2), and undershooting validated estimates of volume (Filipek et al., 1994; Caviness et al., 1996; Seidman et al., 1999; Goldstein et al., 1999; Seidman et al., 2002). The semi-automated approach implemented in the TCP method (see next section) approximates the pial level and the floor of the sixth cortical layer (Figure 7a), which is only partially accomplished by the fully automated segmentation. Thus the implementation of semi-automated or manual routines is currently necessary to represent an anatomically appropriate cortical ribbon, which is a relevant prerequisite for the determination of locations of cortical thinning and the connectional networks in which affected regions belong (Wiegand et al., 2004; Devlin & Poldrack, 2007; Amunts et al., 2007; Passingham, 2007).
Figure 7.
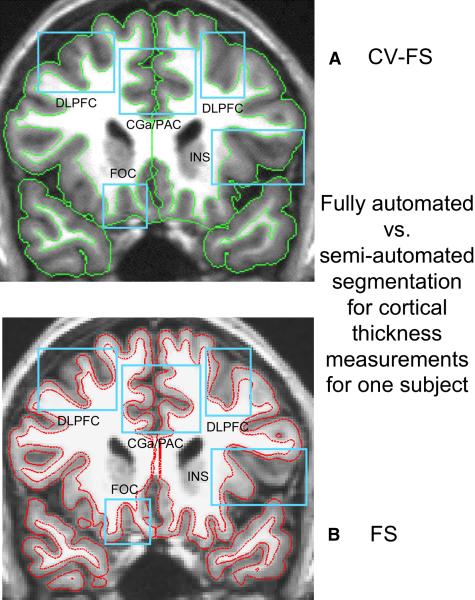
Segmentations from the FreeSurfer automated system (b) and the semi-automated Cardviews (TCP) system (a). Slices are from the same individual in their native anatomic space. Brain exterior and gray matter – white matter boundaries are shown in green for Cardviews, and in red for the FreeSurfer. Blue boxes are placed where errors are apparent for a priori anatomic regions (e.g., FOC, CGa/PAC, INS, DLPFC). Specifically, where (a) the white matter-gray matter boundary and/or (b) the cortical exterior is observed to extend into the cortical ribbon and exclude a block of tissue from the cortical ribbon. CGa/PAC – anterior cingulate gyrus/paracingulate cortex; DLPFC, dorsolateral prefrontal cortex; FOC, orbitofrontal cortex; INS, insula.
The Topological Cortical Parcellation (TCP) System
The TCP system computes measurements of cortical surface topography such as cortical thickness (Makris et al., 2006b, 2007). The overall approach is to segment and/or parcellate the cerebral cortex using Cardviews and then use FreeSurfer to compute cortical thickness differences (Makris et al., 2006b, 2007). The derived measurements can be regionally specific and integrated with systems of cortical parcellation that subdivide the neocortex into gyral-based parcellation units (PUs) (Rademacher et al., 1992; Caviness et al., 1996). See Figure 6 for details.
Data Analyses
The primary analyses tested a priori hypotheses derived from published data on the network subserving executive control and regulation of reward functions (RWR) (Alheid and Heimer, 1988; Breiter et al., 1997, 2006; Breiter and Rosen, 1999; Bush et al., 2002; Cohen et al., 2000; Gemba et al., 1997; Kringelbach, 2005; McClure et al., 2004; Mesulam and Mufson, 1985; Rolls et al., 2007; Schoenbaum et al., 1998; Seo et al., 2007; Tanaka et al., 2004; see Results and Methods Appendices for full set of references) and attention (ATTN) (Fan et al., 2005; Heilman and Van Den Abell, 1980; Makris et al., 2007; Mesulam, 2000; Posner and Peterson, 1990; Seidman et al., 1998b, 2007; Thermenos et al., 2004; see Results and Methods Appendices for full set of references) in humans. We implemented a hierarchical set of analyses to probe the thickness effects in the RWR and ATTN systems across three levels of organization. We first evaluated mean thickness in all regions of the two systems, and tested hemispheric contributions if relevant. These systems were further broken down to gyral-based sub-regions comprised of sets of elementary parcellation units (PUs). Lastly, we evaluated clusters of surface vertices at the systems level and within each PU.
For a priori analyses of the targeted systems and sub-regions, we focused on group differences within thirty-one [thirteen homotypic regions in the two hemispheres and five additional regions in the right hemisphere (AG, SGa, SGp PO, and CGp)] fine-grained regions of interest (ROIs) or parcellation units (PUs). Sub-region subdivisions or PUs are illustrated in Figure 5. The following PUs were considered for a priori analyses in sub-regions of the right and left hemispheres: orbitofrontal cortex (FOC; i.e., anterior (aFOC), posterior (pFOC), medial (mFOC) and lateral FOC (lFOC)); anterior cingulate cortex (CGa; i.e., subgenual CGa, pregenual CGa, and amCGa (anterior-middle CGa)); paracingulate gyrus (PAC; i.e., pregenual PAC and dorsal PAC); insula (INS; i.e., anterior (aINSlbl) and posterior insular lobules (pINSlbl)); and dorsolateral prefrontal cortex (DLPFC; i.e., lateral superior frontal gyrus (F1l) and middle frontal gyrus (F2)) (Caviness et al., 1996, Makris et al., 2006a). The AG (angular gyrus), two subdivisions of the supramarginal gyrus (SG), i.e., SGa (anterior SG) and SGp (posterior SG), the parietal operculum (PO), and posterior cingulate gyrus (CGp) were considered only in the right hemisphere. We considered the inferior parietal lobule (IPL) to comprise AG, SGa, SGp and PO PUs.
Systems
At a systems level, the RWR system included the following sub-regions: FOC (BA 11, 12, 13, 14, 25, 47), CGa (BA 24), PAC (BA 32), INS, and DLPFC (F1l; BA 9 and 8; and F2; BA 46, 9 and 8) bilaterally. The ATTN system included these sub-regions: CGa (BA 24), CGp (BA 23, 26, 29, 30, 31), IPL [AG (BA 39), SG (BA 40), PO (BA 40)], and the DLPFC in the right hemisphere (Heilman and Van Den Abell, 1980).
Gyral-based sub-regions comprised of sets of elementary PUs
At the level of the individual gyrus, we investigated each of the PUs within sub-regions of the RWR (FOC, CGa, PAC, INS, DLPFC) and ATTN systems (CGa, CGp, IPL, DLPFC).
Clusters of thickness differences
Within the REO and attention systems, clusters were identified if they comprised at least 58 contiguous vertices, with each vertex showing group differences at p < 0.05. If clusters were in PUs outside of a priori regions of interest, they were evaluated as exploratory analyses. A cluster of 58 vertices corresponded to a surface area of 31.77mm2 and a volume of 135mm3, corresponding to cluster sizes used in fMRI analyses (Aharon et al., 2001, Breiter et al., 1997).
Symmetry Analysis
Cortical symmetry was computed using a standard formula (Makris et al., 2004) (see Appendix 2).
Statistical Analysis of Cortical Thickness/Volume and Correlations
At each level of the hierarchy (systems, sub-regions or sets of PUs, and clusters), tests on the average thickness in a priori regions of interest were performed with a multivariate general linear mixed model (GLM) for correlated data (Makris et al., 2006a, 2007). The multivariate GLM included the average thickness of the entire neocortex as a covariate. If the multivariate GLM showed a general effect, post-hoc pairwise comparisons were then performed for the measures used in the GLM. Post-hoc pairwise comparisons were evaluated against a correction for the number of post-hoc comparisons performed at each level of analysis. Exploratory analyses of clusters within PUs outside a priori systems were corrected for the total number of clusters where at least 58 vertices were observed.
Systems (i.e., RWR) or subregions showing significant group effects were examined with Pearson and Spearman correlations to explore relationships with drug-seeking behavior. We used both parametric and nonparametric correlations to increase our confidence that any findings were not just an effect of non-normal distributions; in results, only Spearman results are reported. If a system (i.e., RWR) or sub-region (DLPFC) demonstrated a significant correlation corrected for the number of comparisons performed regarding cocaine use (p < 0.05/3 = 0.017), we then examined if this effect were present for a PU within a sub-region or cluster of vertices with p < 0.05. To be reported, a correlation with a drug use measure had to be present for RWR (or sub-region) and a constituent PU/cluster. Correlations were also assessed against alcohol and nicotine use on a post hoc basis. If significant correlations were observed for multiple drugs of abuse besides cocaine, we further performed partial correlations to explore relative contributions of these substances.
Similar procedures, with modification, were implemented for assessing correlations between regions showing significant group effects and behavioral measures (see Appendices).
Supplementary Material
Table 5b.
Correlations between the effortful divided attention measures (misses) and cortical thickness measures.
| Controls | Addicts | Slope comparison | ||||||
|---|---|---|---|---|---|---|---|---|
| Region | n | r | p | n | r | p | T-value | P-value |
| right RWR | 16 | 0.37 | 0.16 | 18 | −0.56 | 0.01 | 2.6 | 0.016 |
| RWR minus ATTN | 16 | 0.52 | 0.04 | 18 | −0.48 | 0.05 | 3.1 | 0.004 |
| RWR intersect ATTN | 16 | 0.22 | 0.42 | 18 | −0.50 | 0.03 | 1.8 | 0.075 |
| right FOC (PU) | 16 | 0.71 | 0.002 | 18 | −0.41 | 0.09 | 4.1 | 0.0003 |
| right pINS (cluster) | 16 | 0.53 | 0.04 | 18 | −0.57 | 0.01 | 3.3 | 0.002 |
Acknolwedgements
This work was supported by a grant to H.C.B. (#14118) from the National Institute on Drug Abuse, Bethesda, MD, and a grant (DABK39-03-C-0098; The Phenotype Genotype Project in Addiction and Depression) from the Office of National Drug Control Policy – Counterdrug Technology Assessment Center (ONDCP-CTAC), Washington, D.C. Further support, in part, was provided to H.C.B. by the MGH Department of Radiology, the National Center for Research Resources (P41RR14075), and the Mental Illness and Neuroscience Discovery Institute.
Other support was provided by in part by grants from: the National Institutes of Health National Center for Complementary and Alternative Medicine (NCAM) to N.M., and NINDS (#34189) plus the Fairway Trust to D.K.
A subset of authors served on the PGP Publications Committee, entailing significant oversight of study and manuscript: R.H.P. (Chair), J.W.S., M.F., A.J.B., G.P.G., H.C.B. This manuscript was written conjointly by N.M. and H.C.B. The authors also wish to thank Jill Goldstein, PhD, for her technical, statistical, and experimental psychology guidance, and Alan Sinai, PhD, of Decisions Economic Inc, for microeconomic framing of the relative preference keypress task.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aharon I, Etcoff N, Ariely D, Chabris CF, O’Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Amunt K, Scheleicher A, Zilles K. Neuroimage. 2007;37:1061–1065. doi: 10.1016/j.neuroimage.2007.02.037. [DOI] [PubMed] [Google Scholar]
- Aylsworth AS. Defining disease phenotypes. In: Haines JL, Pericak-Vance MA, editors. Approaches to gene mapping in complex human diseases. Wiley-Liss; New York: 1998. pp. 53–76. [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Benson V, Marano MA. Current Estimates from the National Health Interview Survey, 1995. National Center for Health Statistics. Vital and Health Statistics. 1998;10 [PubMed] [Google Scholar]
- Breiter HC, Gasic GP. A general circuitry processing reward/aversion information and its implications for neuropsychiatric illness. In: Gazzaniga M, editor. The Cognitive Neurosciences III. MIT Press; Cambridge: 2004. pp. 1043–1065. [Google Scholar]
- Breiter HC, Gasic GP, Makris N. Imaging the neural systems for motivated behavior and their dysfunction in neuropsychiatric illness. In: Deisboeck TS, Kresh JY, editors. Complex Systems Science in Biomedicine. Springer Verlag; 2006. pp. 763–810. [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Rosen BR. Functional magnetic resonance imaging of brain reward circuitry in the human. Ann N Y Acad Sci. 1999;877:523–547. doi: 10.1111/j.1749-6632.1999.tb09287.x. [DOI] [PubMed] [Google Scholar]
- Burton EJ, McKeith IG, Burn DJ, Firbank MJ, O’Brien JT. Progression of white matter hyperintensities in Alzheimer disease, dementia with lewy bodies, and Parkinson disease dementia: a comparison with normal aging. Am J Geriatr Psychiatry. 2006;14:842–849. doi: 10.1097/01.JGP.0000236596.56982.1c. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness VS, Jr., Makris N, Meyer JW, Kennedy DN. MRI-based parcellation of human neocortex: an anatomically specified method with estimate of reliability. J Cogn Neurosci. 1996;8:566–588. doi: 10.1162/jocn.1996.8.6.566. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Makris N, Montinaro E, Sahin NT, Bates JF, Schwamm L, Caplan D, Kennedy DN. Anatomy of stroke, Part II: volumetric characteristics with implications for the local architecture of the cerebral perfusion system. Stroke. 2002;33:2557–2564. doi: 10.1161/01.str.0000036084.82955.c7. [DOI] [PubMed] [Google Scholar]
- Chen JL, Tseng YJ, Chiu HW, Hsiao TC, Chu WC. Nonlinear analysis of heart rate dynamics in hyperthyroidism. Physiol Meas. 2007;28:427–437. doi: 10.1088/0967-3334/28/4/008. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Botvinick M, Carter CS. Anterior cingulate and prefrontal cortex: who’s in control? Nat Neurosci. 2000;3:421–423. doi: 10.1038/74783. [DOI] [PubMed] [Google Scholar]
- Deng X, Ladenheim B, Jayanthi S, Cadet JL. Methamphetamine administration causes death of dopaminergic neurons in the mouse olfactory bulb. Biol Psychiatry. 2007;61:1235–1243. doi: 10.1016/j.biopsych.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Poldrack RA. In praise of tedious anatomy. Neuroimage. 2007;37:1033–1041. doi: 10.1016/j.neuroimage.2006.09.055. discussion 1050-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C, Yu M, Volkow ND, Koretsky AP, Fowler JS, Benveniste H. Cocaine increases the intracellular calcium concentration in brain independently of its cerebrovascular effects. J Neurosci. 2006;26:11522–11531. doi: 10.1523/JNEUROSCI.3612-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AC, Collins DL, Mills SR, Brown ED, Kelly RL, Peters TM. 3D statistical neuroanatomical model from 305 MRI volumes. Nuclear Science Symposium and Medical Imaging Conference, 1993 IEEE Conference Record 3.1993. pp. 1813–1817. [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68:87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Caviness VS., Jr. The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex. 1994;4:344–360. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Gemba H, Miki N, Sasaki K, Kyuhou S, Matsuzaki R, Yoshimura H. Motivation-dependent activity in the dorsolateral part of the prefrontal cortex in the monkey. Neurosci Lett. 1997;230(2):133–6. doi: 10.1016/s0304-3940(97)00493-x. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Miller BL, DeCarli C, Carmelli D. Heritability of lobar brain volumes in twins supports genetic models of cerebral laterality and handedness. Proc Natl Acad Sci U S A. 2002;99:3176–3181. doi: 10.1073/pnas.052494999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser J, Queen JR. An overview of non-cardiac cocaine toxicity. J Emerg Med. 2007;32:181–186. doi: 10.1016/j.jemermed.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Anagnoson R, Breiter HC, Makris N, Goodman JM, Tsuang MT, Seidman LJ. Sex differences in prefrontal cortical brain activity during fMRI of auditory verbal working memory. Neuropsychology. 2005;19:509–519. doi: 10.1037/0894-4105.19.4.509. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, Telang F, Caparelli EC, Chang L, Ernst T, et al. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am J Psychiatry. 2007;164:43–51. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KM, Van Den Abell T. Right hemisphere dominance for attention: the mechanism underlying hemispheric asymmetries of inattention (neglect) Neurology. 1980;30:327–330. doi: 10.1212/wnl.30.3.327. [DOI] [PubMed] [Google Scholar]
- Hofman MA. On the evolution and geometry of the brain in mammals. Prog. Neurobiol. 1989;32:137–158. doi: 10.1016/0301-0082(89)90013-0. [DOI] [PubMed] [Google Scholar]
- Hyatt BA, Yost HJ. The left-right coordinator: the role of Vg1 in organizing left-right axis formation. Cell. 1998;93:37–46. doi: 10.1016/s0092-8674(00)81144-7. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Im K, Lee JM, Yoon U, Shin YW, Hong SB, Kim IY, Kwon JS, Kim SI. Fractal dimension in human cortical surface: multiple regression analysis with cortical thickness, sulcal depth, and folding area. Hum Brain Mapp. 2006;27:994–1003. doi: 10.1002/hbm.20238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. Modulatory events in the development and evolution of the primate neocortex. In: Jones E, Peters A, editors. Cerebral Cortex: Comparative Structure and Evolution of Cerebral Cortex: Part I. Plenum; New York: 1990. [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction. 2006;101(Suppl 1):23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Krstacic G, Krstacic A, Smalcelj A, Milicic D, Jembrek-Gostovic M. The “Chaos Theory” and nonlinear dynamics in heart rate variability analysis: does it work in short-time series in patients with coronary heart disease? Ann Noninvasive Electrocardiol. 2007;12:130–136. doi: 10.1111/j.1542-474X.2007.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Everall IP, Crews L, Adame A, Grant I, Masliah E. Escalating dose-multiple binge methamphetamine exposure results in degeneration of the neocortex and limbic system in the rat. Exp Neurol. 2007;207:42–51. doi: 10.1016/j.expneurol.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Biederman J, Valera EM, Bush G, Kaiser J, Kennedy DN, Caviness VS, Faraone SV, Seidman LJ. Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb Cortex. 2007;17:1364–1375. doi: 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- Makris N, Gasic GP, Seidman LJ, Goldstein JM, Gastfriend DR, Elman I, Albaugh MD, Hodge SM, Ziegler DA, Sheahan FS, et al. Decreased absolute amygdala volume in cocaine addicts. Neuron. 2004;44:729–740. doi: 10.1016/j.neuron.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Makris N, Goldstein JM, Kennedy D, Hodge SM, Caviness VS, Faraone SV, Tsuang MT, Seidman LJ. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr Res. 2006a;83:155–171. doi: 10.1016/j.schres.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Makris N, Kaiser J, Haselgrove C, Seidman LJ, Biederman J, Boriel D, Valera EM, Papadimitriou GM, Fischl B, Caviness VS, Jr., Kennedy DN. Human cerebral cortex: a system for the integration of volume- and surface-based representations. Neuroimage. 2006b;33:139–153. doi: 10.1016/j.neuroimage.2006.04.220. [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- Maunsell JH. Neuronal representations of cognitive state: reward or attention? Trends Cogn Sci. 2004;8:261–265. doi: 10.1016/j.tics.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Mayberg H. Mapping mood: an evolving emphasis on frontal-limbic interactions. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. Oxford University Press; New York: 2002. [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Mesulam M. Brain, mind, and the evolution of connectivity. Brain Cogn. 2000;42:4–6. doi: 10.1006/brcg.1999.1145. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. The insula of Reil in man and monkey. Architectonics, connectivity and function. In: Peters A, Jones EG, editors. Cerebral Cortex. Plenum Press; New York: 1985. pp. 179–226. [Google Scholar]
- Mountcastle V. Perceptual Neuroscience: The Cerebral Cortex. Harvard University Press; Cambridge, MA: 1998. [Google Scholar]
- Oldfield R. The assessment and analysis of handedness. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Yeterian EH. Architecture and connections of cortical association areas. In: Peters A, Jones EG, editors. Association and Auditory Cortices. Plenum Press; New York: 1985. pp. 3–61. [Google Scholar]
- Paulus MP. Decision-making dysfunctions in psychiatry--altered homeostatic processing? Science. 2007;318:602–606. doi: 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]
- Passingham R. Neuroimage. 2007;37:1552–1056. doi: 10.1016/j.neuroimage.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Association pathways of the prefrontal cortex and functional observations. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. Oxford University Press; New York: 2002. pp. 31–50. [Google Scholar]
- Piedra ME, Icardo JM, Albajar M, Rodriguez-Rey JC, Ros MA. Pitx2 participates in the late phase of the pathway controlling left-right asymmetry. Cell. 1998;94:319–324. doi: 10.1016/s0092-8674(00)81475-0. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Prothero JW, Sundsten JW. Folding of the cerebral cortex in mammals. A scaling model. Brain Behav. Evol. 1984;24:152–167. doi: 10.1159/000121313. [DOI] [PubMed] [Google Scholar]
- Rademacher J, Galaburda AM, Kennedy DN, Filipek PA, Caviness VS., Jr. Human cerebral cortex: Localization, parcellation and morphometry with magnetic resonance imaging. J Cogn Neurosci. 1992;4:352–374. doi: 10.1162/jocn.1992.4.4.352. [DOI] [PubMed] [Google Scholar]
- Riordan WP, Jr., Cotton BA, Norris PR, Waitman LR, Jenkins JM, Morris JA., Jr. Beta-blocker exposure in patients with severe traumatic brain injury (TBI) and cardiac uncoupling. J Trauma. 2007;63:503–510. doi: 10.1097/TA.0b013e3181271c34. discussion 510-501. [DOI] [PubMed] [Google Scholar]
- Rockel AJ, Horns RW, Powell TPS. The basic uniformity of structure of the neocortex. Brain. 1980;103:221–244. doi: 10.1093/brain/103.2.221. [DOI] [PubMed] [Google Scholar]
- Rolls ET, McCabe C, Redoute J. Expected Value, Reward Outcome, and Temporal Difference Error Representations in a Probabilistic Decision Task. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm097. [DOI] [PubMed] [Google Scholar]
- Roubertoux PL, Le Roy I, Tordjman S, Cherfou A, Migliore-Samour D. Analysis of quantitative trait loci for behavioral laterality in mice. Genetics. 2003;163:1023–1030. doi: 10.1093/genetics/163.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Breiter HC, Goodman JM, Goldstein JM, Woodruff PW, O’Craven K, Savoy R, Tsuang MT, Rosen BR. A functional magnetic resonance imaging study of auditory vigilance with low and high information processing demands. Neuropsychology. 1998b;12:505–518. doi: 10.1037//0894-4105.12.4.505. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Thermenos HW, Koch JK, Ward M, Breiter H, Goldstein JM, Goodman JM, Faraone SV, Tsuang MT. Auditory verbal working memory load and thalamic activation in nonpsychotic relatives of persons with schizophrenia: an fMRI replication. Neuropsychology. 2007;21:599–610. doi: 10.1037/0894-4105.21.5.599. [DOI] [PubMed] [Google Scholar]
- Seo H, Barraclough DJ, Lee D. Dynamic Signals Related to Choices and Outcomes in the Dorsolateral Prefrontal Cortex. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm064. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lerch JP, Pruessner JC, Taylor KN, Rose AB, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurol. 2007;6:494–500. doi: 10.1016/S1474-4422(07)70106-0. [DOI] [PubMed] [Google Scholar]
- Sim ME, Lyoo IK, Streeter CC, Covell J, Sarid-Segal O, Ciraulo DA, Kim MJ, Kaufman MJ, Yurgelun-Todd DA, Renshaw PF. Cerebellar Gray Matter Volume Correlates with Duration of Cocaine Use in Cocaine-Dependent Subjects. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301346. [DOI] [PubMed] [Google Scholar]
- Simpson DD, Joe GW, Fletcher BW, Hubbard RL, Anglin MD. A national evaluation of treatment outcomes for cocaine dependence. Arch Gen Psychiatry. 1999;56:507–514. doi: 10.1001/archpsyc.56.6.507. [DOI] [PubMed] [Google Scholar]
- Stein EA, Fuller SA. Selective effects of cocaine on regional cerebral blood flow in the rat. J Pharmacol Exp Ther. 1992;262:327–334. [PubMed] [Google Scholar]
- Strauss MM, Makris N, Aharon I, Vangel MG, Goodman J, Kennedy DN, Gasic GP, Breiter HC. fMRI of sensitization to angry faces. Neuroimage. 2005;26:389–413. doi: 10.1016/j.neuroimage.2005.01.053. [DOI] [PubMed] [Google Scholar]
- Supp DM, Witte DP, Potter SS, Brueckner M. Mutation of an axonemal dynein affects left-right asymmetry in inversus viscerum mice. Nature. 1997;389:963–966. doi: 10.1038/40140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka SC, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S. Prediction of immediate and future reward differentially recruits cortical-basal ganglia loops. Nat Neurosci. 2004;7(8):887–93. doi: 10.1038/nn1279. [DOI] [PubMed] [Google Scholar]
- Tang YL, Kranzler HR, Gelernter J, Farrer LA, Cubells JF. Comorbid psychiatric diagnoses and their association with cocaine-induced psychosis in cocaine-dependent subjects. Am J Addict. 2007;16:343–351. doi: 10.1080/10550490701525723. [DOI] [PubMed] [Google Scholar]
- Tateishi Y, Oda S, Nakamura M, Watanabe K, Kuwaki T, Moriguchi T, Hirasawa H. Depressed heart rate variability is associated with high IL-6 blood level and decline in the blood pressure in septic patients. Shock. 2007;28:549–553. doi: 10.1097/shk.0b013e3180638d1. [DOI] [PubMed] [Google Scholar]
- Thermenos HW, Seidman LJ, Breiter H, Goldstein JM, Goodman JM, Poldrack R, Faraone SV, Tsuang MT. Functional magnetic resonance imaging during auditory verbal working memory in nonpsychotic relatives of persons with schizophrenia: a pilot study. Biol Psychiatry. 2004;55:490–500. doi: 10.1016/j.biopsych.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Schultz W, Roelcke U, Nienhusmeier M, Missimer J, Maguire RP, Leenders KL. Activation of the human brain by monetary reward. Neuroreport. 1997;8:1225–8. doi: 10.1097/00001756-199703240-00033. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Thalamo-cortical dysfunction in cocaine abusers: implications in attention and perception. Psychiatry Res. 2007;155:189–201. doi: 10.1016/j.pscychresns.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Perez-Garcia M, Bechara A. Emotion, decision-making and substance dependence: a somatic-marker model of addiction. Current Neuropharmacology. 2006;4:17–31. doi: 10.2174/157015906775203057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur J Neurosci. 2003;18(7):2069–81. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- Wiegand LC, Warfield SK, Levitt JJ, Hirayasu U, Salisbury DF, Heckers S, Dickey CC, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. Prefrontal cortical thickness in first episode psychosis: A magnetic resonance imaging study. Biolog Psych. 2004;55:131–140. doi: 10.1016/j.biopsych.2003.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worth AJ, Makris N, Caviness VS, Jr., Kennedy DN. Neuroanatomical Segmentation in MRI: Technological Objectives. Int J Pattern Recogn. 1997;11:1161–1187. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.