Abstract
OBJECTIVES
The benefit of neoadjuvant methotrexate, vinblastine, adriamycin and cisplatin (MVAC) for muscle-invasive bladder cancer (MIBC) has been prospectively demonstrated in a phase III study. Extrapolating from comparative data in the metastatic setting, platinum doublets such as cisplatin/gemcitabine (CG) have been adopted. We sought to compare clinical outcomes in patients treated for MIBC with neoadjuvant CG and MVAC at our institution.
PATIENTS AND METHODS
Patients with MIBC were identified from a prospectively maintained registry. Clinicopathologic information and clinical outcome data were obtained directly from the registry. When available, pharmacy records were reviewed to ascertain use of growth factors and chemotherapy dose intensity. Survival was compared in subgroups divided by the regimen of chemotherapy rendered (i.e., CG vs MVAC) using the Kaplan-Meier method.
RESULTS
Median OS in the overall cohort (n=61) was 23 months. OS was improved in patients receiving either MVAC or CG chemotherapy compared to patients receiving “other” chemotherapy (35.3 months vs 16.3 months, P=0.055; Figure 2). Although the median OS associated with neoadjuvant CG numerically exceeded the survival associated with neoadjuvant MVAC (104.3 months and 21.8 months, respectively), this was not statistically significant (P=0.73). Pathologic down-staging predicted improved OS with both neoadjuvant CG and MVAC, and the rates of down-staging were similar with both regimens.
CONCLUSIONS
Although warranting prospective validation, our data suggest that CG is a possible alternative neoadjuvant approach to traditional regimens such as MVAC for patients with MIBC.
Keywords: Survival, response, cisplatin, gemcitabine, methotrexate, vinblastine, adriamycin, doxorubicin, neoadjuvant, muscle-invasive, bladder cancer
Introduction
Numerous studies have assessed the role of neoadjuvant chemotherapy in muscle-invasive bladder cancer (MIBC), and a meta-analysis of these efforts suggest a 5% absolute benefit in 5-year survival in association with cisplatin-based regimens.1–2 Amongst the chemotherapy regimens considered in this setting, the Southwest Oncology Group (SWOG) trial 8710 supports a four drug regimen comprised of methotrexate, vinblastine, adriamycin and cisplatin (MVAC).3 In this study, use of neoadjuvant MVAC was associated with an improvement in survival from 46 months to 77 months (P=0.06). Although effective, MVAC was associated with an increased rate of toxicity, with 72% of patients experiencing grade 3/4 adverse events.
In the metastatic setting, a phase III trial compared cisplatin/gemcitabine (CG) to MVAC.4 This direct comparison suggested equivalent clinical benefit with lesser toxicity associated with CG therapy. This dataset is frequently invoked to justify use of CG as neoadjuvant treatment; however, the benefit of neoadjuvant CG for MIBC has yet to be defined in a randomized, phase III study. A retrospective experience from the Memorial Sloan-Kettering Cancer Center assessed this regimen in 42 patients – 39 of which received 4 cycles of 3-weekly CG, and dose intensities of 91% and 90% were achieved with cisplatin and gemcitabine, respectively.5 The proportion of patients achieving pT0 status at the time of cystectomy was 26%, and 36% of patients achieved < pT2 stage. Survival in these cohorts was not reported. A more recent retrospective review of 114 patients with MIBC treated with perioperative chemotherapy at Columbia University included 16 patients who had received neoadjuvant CG chemotherapy.6 Four patients (25%) achieved pT0 status, and on multivariate analysis, it did not appear that the choice of neoadjuvant chemotherapy (whether CG or MVAC) influenced OS or cancer-specific survival (CSS).
Although a phase III neoadjuvant trial directly comparing CG and MVAC could reconcile the dilemma of choosing between these regimens, to the author’s knowledge, no such trial is currently planned. Furthermore, clinical trials assessing neoadjuvant regimens for MIBC have been plagued by slow accrual, and thusly, such a study may take decades to yield a result.7 In the interim, medical oncologists and urologists must rely on existing retrospective data to guide their decision regarding neoadjuvant approach. Herein, we focus on clinical outcomes in a cohort of patients with MIBC treated with either neoadjuvant CG or MVAC. Analogous to phase III studies exploring neoadjuvant regimens, we focus on overall survival (OS). Furthermore, we attempt to characterize the rate of pathologic down-staging and the implications of down-staging on clinical outcomes.
Patients & Methods
Patients
Patients were identified through a prospectively maintained, IRB-approved database. The database houses clinical information pertaining to patients who received cystectomy at City of Hope from 1995 to the present. Open radical cystectomy was performed prior to 2003 with robot-assisted radical cystectomy from 2003 onwards. Extended pelvic lymph node dissection accompanied all operations. Clinical variables collected in the database include demographic factors, past medical history, past surgical history, surgical morbidity, post-operative morbidity, disease status and vital status. Receipt of chemotherapy rendered in any setting (neoadjuvant, adjuvant and metastatic) is also recorded, with further details including regimen type and number of cycles rendered. Pathologic variables collected in the database include complete pathologic descriptions and histologic diagnosis (established by a City of Hope genitourinary pathologist). Patients selected for the current study included those with a pathologically-verified diagnosis of urothelial carcinoma at the time of cystectomy.
To ascertain more detail related to chemotherapy administration, a chart review was performed. We obtained pharmacy records and calculated the dose intensity (DI) of patients receiving MVAC or CG chemotherapy. A relative DI (RDI) was first calculated for each agent administered to each patient by dividing the target dose by the actual dose rendered. The target dose was generated using the expectation that patients would receive a total of 3 months of neoadjuvant chemotherapy (i.e., 4 cycles of a 3-weekly regimen, or 3 cycles of a 4-weekly regimen. An average DI for each patient was calculated by averaging the RDI for each agent rendered to the patient. Use or non-use of white blood cell growth factors (i.e., G-CSF or GM-CSF) was also recorded for each patient. Notably, these analyses were limited to patients who received neoadjuvant chemotherapy at City of Hope, as pharmacy records could not be consistently obtained for patients who had received their neoadjuvant treatment at outside institutions.
Statistical Analysis
Patients were divided into subgroups based on the nature of chemotherapy rendered. Three principal subgroups were characterized: (1) CG, (2) MVAC, and (3) “other”. Descriptive statistics were used to compare clinicopathologic characteristics amongst subgroups. The Kaplan-Meier method with the log-rank test was used to compare survival in subgroups.
Results
Patient Characteristics
A total of 61 patients were identified that had received neoadjuvant chemotherapy for MIBC. Patient characteristics divided by the type of neoadjuvant chemotherapy rendered are listed in Table 1. The median age of the population was 69 (range: 37–99). Patients who received “other” neoadjuvant chemotherapy were significantly older than patients receiving MVAC or CG neoadjuvant chemotherapy (median: 77 vs 64, P<0.0001), and had a higher Charlson Comorbidity index (CCI) score (median: 6 vs 4, P=0.0008). All other clinical and pathologic descriptors were similar among the three treatment groups.
Table 1.
Patient characteristics of patients receiving neoadjuvant chemotherapy with methotrexate/vinblastine/adriamycin/cisplatin (MVAC), cisplatin/gemcitabine (CG), or other regimens.
| MVAC (n=22) | CG (n=24) | Other (n=15) | |
|---|---|---|---|
| Gender, n (%) | |||
| Female | 2 (9.1%) | 5 (20.8%) | 2 (13.3%) |
| Male | 20 (90.9%) | 19 (79.2%) | 13 (86.7%) |
| Age, median (IQR) † | 60.1 (52.7 – 68.1) | 68.6 (64.4 – 71.4) | 77.3 (69.0 – 82.6) |
| CCI, median (IQR) † | 4.0 (3.0 – 5.0) | 5.0 (4.0 – 6.0) | 6.0 (6.0 – 6.0) |
| ASA, n (%) | |||
| II | 2 (9.1%) | 2 (8.3%) | 1 (6.7%) |
| III | 18 (81.8%) | 14 (58.3%) | 7 (46.7%) |
| IV | 2 (9.1%) | 8 (33.3%) | 4 (26.7%) |
| Clinical T Stage, n (%) | |||
| ≤T2 | 18 (81.8%) | 19 (91.7%) | 7 (73.3%) |
| T3 | 1 (4.5%) | 2 (8.3%) | 3 (20.0%) |
| T4 | 2 (9.1%) | 0 (0.0%) | 1 (6.7%) |
| Clinical Grade, n (%) | |||
| II (Intermediate) | 1 (4.6%) | 0 (0.0%) | 1 (6.7%) |
| III (High) | 21 (95.4%) | 24 (100.0%) | 14 (93.3%) |
| Pathologic T Stage, n (%) | |||
| <pT1 | 9 (40.9%) | 9 (37.5%) | 5 (33.3%) |
| pT1 | 2 (9.1%) | 1 (4.2%) | 0 (0.0%) |
| pT2 | 5 (22.7%) | 4 (16.7%) | 1 (6.7%) |
| pT3 | 4 (18.2%) | 5 (20.8%) | 6 (40.0%) |
| pT4 | 2 (9.1%) | 5 (20.8%) | 3 (20.0%) |
| Nodal yield, n (IQR)† | 25 (20 – 41) | 27 (18 – 34) | 17 (6 – 20) |
| Pathologic node status, n (%) | |||
| NX or N0 | 23 (54.5%) | 16 (66.6%) | 14 (93.3%) |
| N1 | 4 (18.2%) | 2 (8.3%) | 1 (6.7%) |
| N2 | 5 (22.7%) | 5 (20.8%) | 0 (0.0%) |
| N3 | 1 (4.5%) | 1 (4.2%) | 0 (0.0%) |
| Positive surgical margins, n (%) | 1 (4.5%) | 6 (25.0%) | 3 (20.0%) |
p<0.05 from comparison testing; chi-square or fisher’s exact test used for categorical variables; student’s t-test or Wilcoxon test for non-parametric data used to compare continuous variables.
Dose Intensity and Use of WBC Growth Factors
Pharmacy records were available for 12 of 22 patients (54%) receiving neoadjuvant MVAC chemotherapy, and for 13 of 24 patients (54%) receiving neoadjuvant CG chemotherapy. The average DI for MVAC and CG were 92% and 93%, respectively. While 10 of 12 patients (83%) treated with neoadjuvant MVAC also received WBC growth factors, only 2 of 13 patients (15%) treated with neoadjuvant CG received these agents.
Clinical Outcome
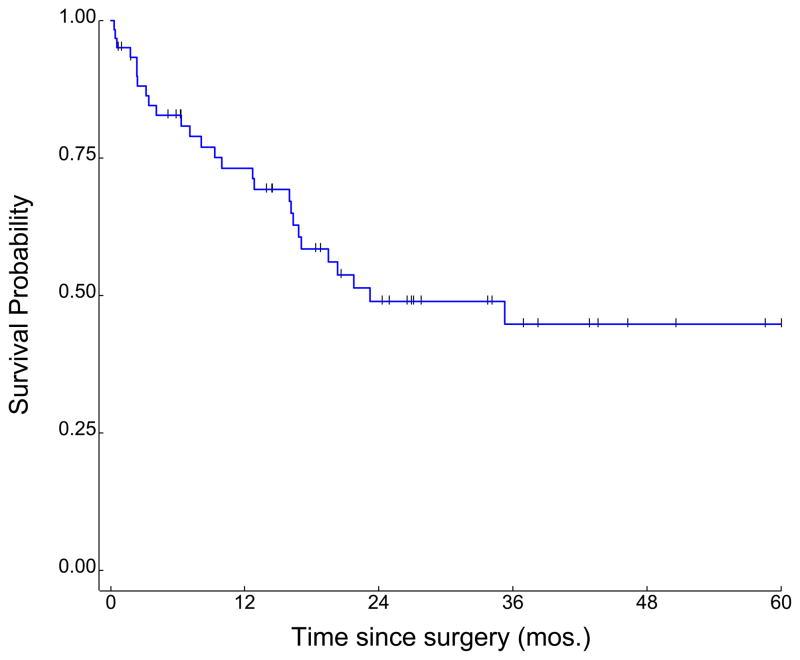
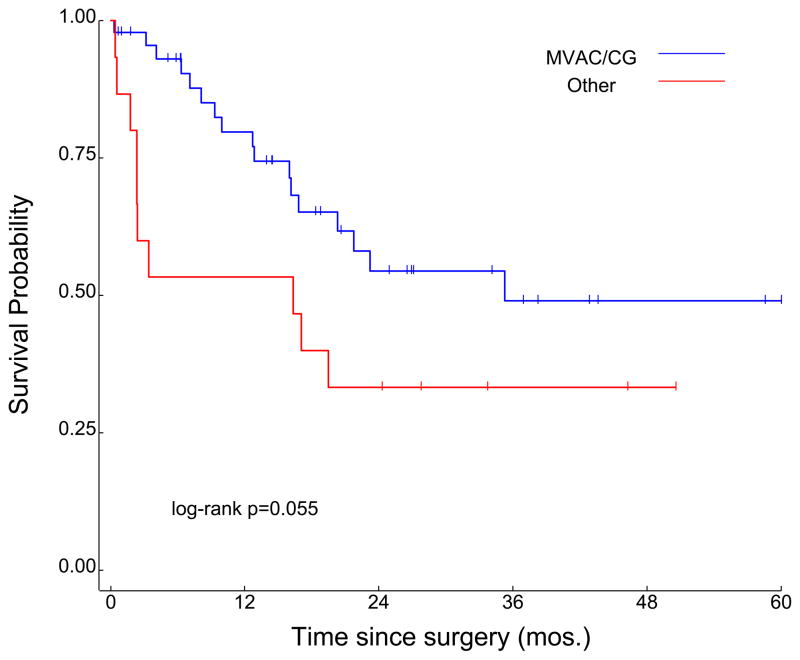
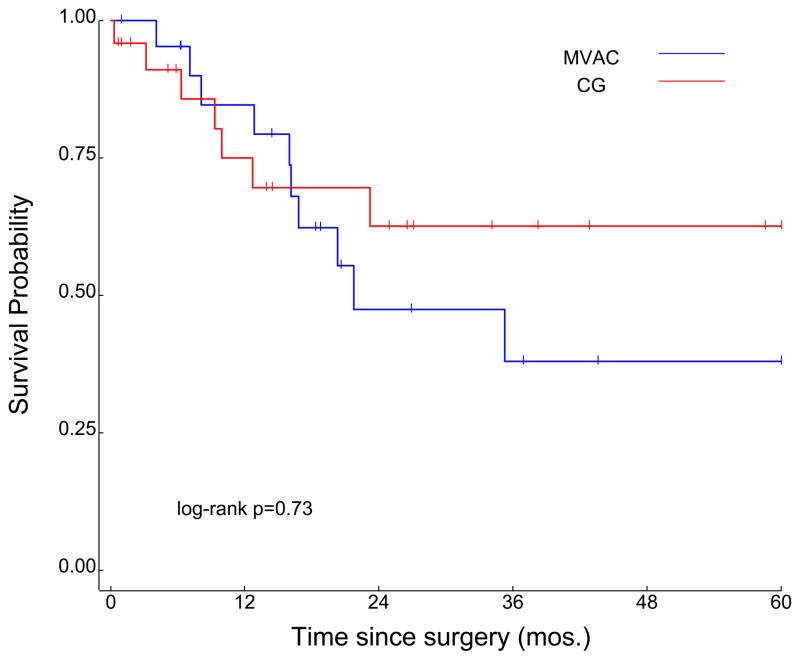
In the overall cohort (n=61), median duration of follow-up was 28.7 months, and median OS was 23 months (Figure 1). Survival was improved in patients receiving either MVAC or CG chemotherapy as compared to patients receiving “other” chemotherapy, at a level approaching statistical significance (35.3 months vs 16.3 months, P=0.055; Figure 2). Although the median OS associated with neoadjuvant CG numerically exceeded the survival associated with neoadjuvant MVAC (104.3 months and 21.8 months, respectively), this was not statistically significant (P=0.73; Figure 3).
Figure 1.
Overall survival in a cohort of 61 patients receiving neoadjuvant chemotherapy for muscle-invasive bladder cancer.
Figure 2.
Survival in cohorts divided by receipt of MVAC or GC chemotherapy as compared to receipt of “other” chemotherapy regimens.
Figure 3.
Survival in cohorts divided by receipt of MVAC or GC chemotherapy.
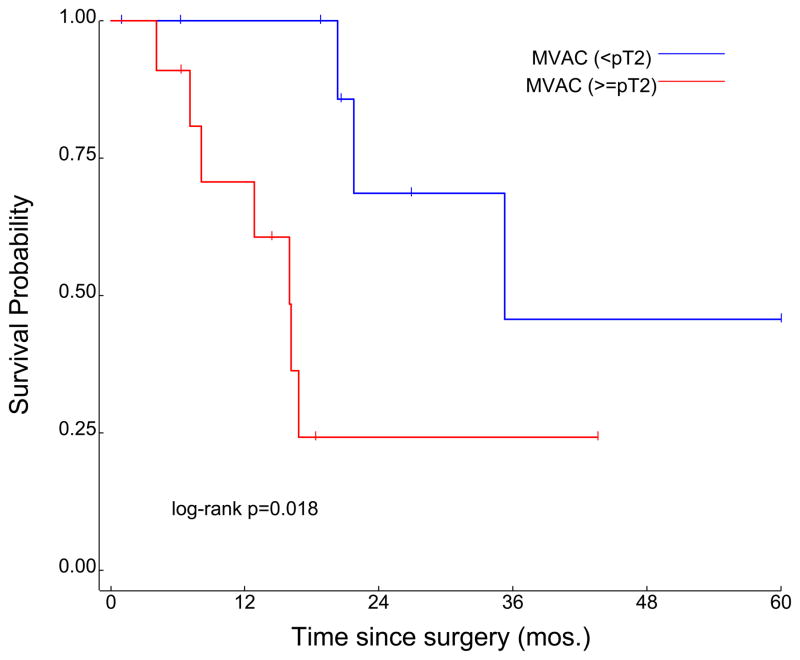
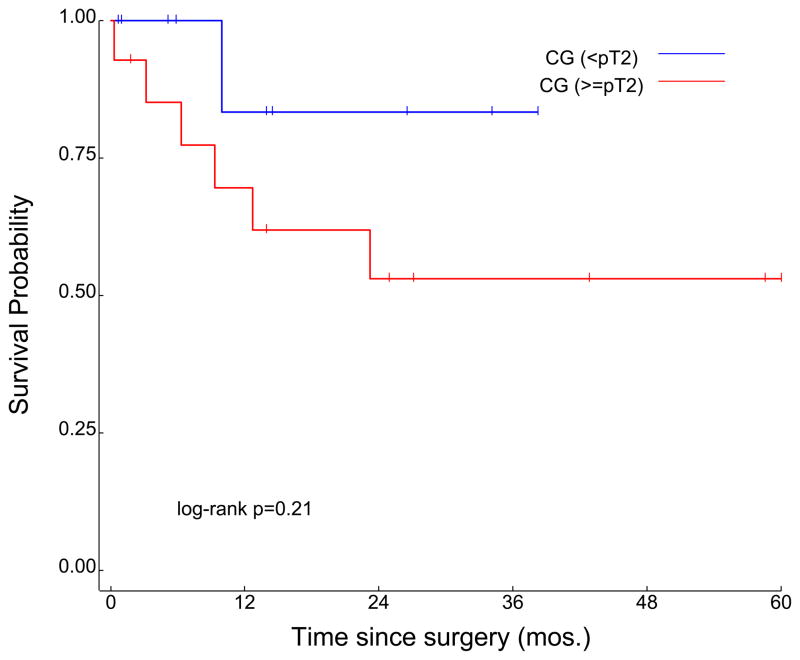
Patients were further stratified into subgroups on the basis of whether or not pathologic down-staging was achieved. Specifically, patients were divided into cohorts based on whether or not their final pathologic stage (on assessment of the cystectomy specimen) was < pT2. Amongst patients receiving neoadjuvant MVAC, 11 of 22 patients (50%) were down-staged; median OS was significantly improved in patients who were < pT2 as compared to ≥ pT2 (35.3 months vs 16.0 months, P=0.018) (Figure 4a). A slightly higher proportion of patients receiving neoadjuvant CG were down-staged (14 of 24, or 58%). Median OS in the subset of patients who were < pT2 was not reached (NR), as compared to 104.3 months in patients who were ≥ pT2 (P=0.21; Figure 4b). With respect to patients who achieved pT0 status at the time of cystectomy, the proportion was again slightly higher amongst patients who received neoadjuvant CG (6 of 24 patients, or 25%) relative to patients receiving neoadjuvant MVAC (4 of 22 patients, or 22.5%).
Figure 4.
Survival according to pathologic T-stage determined at the time of surgery in subgroups receiving neoadjuvant MVAC (a) and neoadjuvant CG (b) chemotherapy.
Discussion
Although neoadjuvant chemotherapy is supported by Level 1 evidence (i.e., positive phase III clinical trials), the widespread utilization of these regimens appears to be relatively infrequent. Estimates from the National Cancer Database (sponsored by both the American Cancer Society and the American College of Surgeons) suggest that as recently as 2007, neoadjuvant chemotherapy was rendered in only 13% of patients with MIBC.8 The reasons for this are multi-fold, and may include (1) a lack of consistent survival benefit across all studies of neoadjuvant chemotherapy, (2) apprehension for delaying definitive surgical intervention with use of neoadjuvant treatment, and (3) reluctance to encounter potential toxicities associated with neoadjuvant treatment. Although the first two reasons are challenging to contest, the latter concern may be mitigated by use of regimens that are better tolerated but equally as efficacious. In our retrospective analysis, we have demonstrated similar survival outcomes with neoadjuvant CG (a more tolerable regimen in the metastatic setting) and MVAC chemotherapy. We have further demonstrated that both regimens can be administered with similar dose intensity, with the caveat that a substantially higher proportion of patients receiving neoadjuvant MVAC required growth factor support.
Several of the tenets yielded from the pivotal phase III trials evaluating neoadjuvant treatment were maintained in our retrospective analysis. For instance, in SWOG 8710, median OS was not reached in patients who achieved pT0 status, while median OS was 45.6 months in patients who had residual disease detected in their specimens at cystectomy.3 Similarly, a retrospective analysis from the Nordic Cystectomy Trials 1 and 2 showed that clinical down-staging (as compared to no down-staging) with neoadjuvant chemotherapy led to dramatic improvements in OS.9 Analogous to these findings, we similarly saw a substantial improvement in median OS in patients who were down-staged to < pT2 disease with neoadjuvant MVAC (P=0.018). To our knowledge, our study is the first to suggest that pathologic down-staging also has prognostic implications for patients receiving neoadjuvant CG, specifically with respect to OS. Although a statistically significant difference was not observed, median OS was not reached in those patients who were down-staged, as compared to 104.3 months in patients who were not down-staged.
Notably, the rate of pathologic down-staging appeared to be similar in patients receiving neoadjuvant MVAC and CG (50% and 58%, respectively). The pT0 rate observed in our study with neoadjuvant MVAC was lower than that in SWOG 8710 (22.5% vs 43%, respectively).3 As others have aptly pointed out, the rate of 43% in SWOG 8710 is not derived from an intent-to-treat analysis – if this approach is taken, the revised pT0 rate is 32%.5 Akin to the rates of pathologic down-staging, the pT0 rates observed in our study for neoadjuvant MVAC and CG were similar (22.5% and 25%, respectively). To date, highly variable pT0 rates have been reported in association with neoadjuvant CG, ranging from 7% to 50%.10–11 The University of Rochester has published the most recent account of neoadjuvant CG in MIBC, with pT0 rates very similar to those observed in the current study (20%).12
Several limitations exist in our study. As with any retrospective analysis, the ability to acquire comprehensive data is limited. For instance, many patients received their neoadjuvant regimens at outside institutions. Because pharmacy records could not be obtained from outside institutions to verify the dose and schedule of chemotherapy administration, our efforts to characterize dose intensity in each patient were hindered. The retrospective nature of the study also prohibited any formal analysis of quality of life or toxicity associated with CG and MVAC chemotherapy. Also, as the treatment arms are not randomized, there are some imbalances in patient characteristics. Factors not reported herein, such as the nature of surgical intervention (i.e., robotic versus open), could potentially affect clinical outcome. Notably, we have previously reported our experience including 196 patients receiving robotic cystectomy and have noted few differences in operative morbidity as compared to contemporary open series.13 Clearly, these are issues that may underscore the selection of chemotherapy regimen in the neoadjuvant setting. The limited sample size in our study also challenges the ability to make far-reaching conclusions about our results – our comparisons of survival in neoadjuvant CG and MVAC cohorts are hypothesis generating, but ultimately would require prospective validation. Finally, our study is focused on just two potential neoadjuvant regimens for MIBC. A recent report of the BA06 30894 study (comparing 3 cycles of cisplatin, methotrexate and vinblastine [CMV] chemotherapy followed by surgery to surgery alone) suggested a statistically significant OS advantage with neoadjuvant CMV (P=0.037).14 These mature data (with a median follow-up of 8 years) provide an alternative neoadjuvant strategy that must be considered. A total of 15 patients in our cohort received “other” neoadjuvant chemotherapy regimens. Two-thirds of these patients (n=10) received carboplatin with gemcitabine; admittedly, limited data exists to support this neoadjuvant strategy. Overall, patients receiving “other” regimens were noted to have inferior survival as compared to patients who received neoadjuvant CG or MVAC – however, interpretation of this data is confounded by higher CCI scores and increased age in the cohort receiving these alternative regimens.
Despite these limitations, our study provides important insights for patients with MIBC. The data presented herein suggests superior outcomes with traditional regimens, such as CG and MVAC. In our series, no significant difference in OS was observed amongst patients receiving neoadjuvant CG and MVAC. Paralleling results from previous assessments of neoadjuvant MVAC, survival was improved with down-staging – a similar trend was observed with neoadjuvant CG. Although a prospective, phase III study could establish a gold standard amongst these regimens, it is unlikely that such a trial will take place in the near future. In the meantime, our data can provide insight for the clinical decision-making of medical oncologists and urologists.
Clinical Practice Points.
Neoadjuvant cisplatin-based chemotherapy is known to confer a survival advantage in patients with muscle-invasive bladder cancer
Phase III data support use of regimens such as methotrexate, vinblastine, doxorubicin and cisplatin (MVAC) or cisplatin, methotrexate, and vinblastine (CMV)
The data presented herein supports consideration of regimens such as cisplatin/gemcitabine (CG) as neoadjuvant chemotherapy
Neoadjuvant CG leads to appreciable rates of pathologic down-staging in our cohort, and with the caveat of a limited sample size, overall survival (OS) with this regimen was not significantly different from neoadjuvant MVAC
Larger prospective evaluations of neoadjuvant CG (although difficult to manifest) may help establish this as an alternative neoadjuvant regimen
Acknowledgments
Dr. Pal’s efforts are supported by the NIH Loan Repayment Plan (LRP) and NIH K12 2K12CA001727-16A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet. 2003;361:1927–34. doi: 10.1016/s0140-6736(03)13580-5. [DOI] [PubMed] [Google Scholar]
- 2.Sternberg CN, Donat SM, Bellmunt J, et al. Chemotherapy for Bladder Cancer: Treatment Guidelines for Neoadjuvant Chemotherapy, Bladder Preservation, Adjuvant Chemotherapy, and Metastatic Cancer. Urology. 2007;69:62–79. doi: 10.1016/j.urology.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 3.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 4.von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–8. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 5.Dash A, Pettus JAt, Herr HW, et al. A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder: a retrospective experience. Cancer. 2008;113:2471–7. doi: 10.1002/cncr.23848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeshchina O, Badalato GM, Wosnitzer MS, et al. Relative efficacy of perioperative gemcitabine and Cisplatin versus methotrexate, vinblastine, adriamycin, and Cisplatin in the management of locally advanced urothelial carcinoma of the bladder. Urology. 2012;79:384–90. doi: 10.1016/j.urology.2011.10.050. [DOI] [PubMed] [Google Scholar]
- 7.Castellano D, Carles J, Esteban E, et al. Recommendations for the optimal management of early and advanced urothelial carcinoma. Cancer Treat Rev. 2011 doi: 10.1016/j.ctrv.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Fedeli U, Fedewa SA, Ward EM. Treatment of Muscle Invasive Bladder Cancer: Evidence From the National Cancer Database, 2003 to 2007. The Journal of urology. 2011;185:72–8. doi: 10.1016/j.juro.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Rosenblatt R, Sherif A, Rintala E, et al. Pathologic Downstaging Is a Surrogate Marker for Efficacy and Increased Survival Following Neoadjuvant Chemotherapy and Radical Cystectomy for Muscle-Invasive Urothelial Bladder Cancer. Eur Urol. 2011 doi: 10.1016/j.eururo.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Weight CJ, Garcia JA, Hansel DE, et al. Lack of pathologic down-staging with neoadjuvant chemotherapy for muscle-invasive urothelial carcinoma of the bladder: a contemporary series. Cancer. 2009;115:792–9. doi: 10.1002/cncr.24106. [DOI] [PubMed] [Google Scholar]
- 11.Kaneko G, Kikuchi E, Matsumoto K, et al. Neoadjuvant gemcitabine plus cisplatin for muscle-invasive bladder cancer. Jpn J Clin Oncol. 2011;41:908–14. doi: 10.1093/jjco/hyr068. [DOI] [PubMed] [Google Scholar]
- 12.Scosyrev E, Messing EM, van Wijngaarden E, et al. Neoadjuvant gemcitabine and cisplatin chemotherapy for locally advanced urothelial cancer of the bladder. Cancer. 2012;118:72–81. doi: 10.1002/cncr.26238. [DOI] [PubMed] [Google Scholar]
- 13.Yuh BE, Nazmy M, Ruel NH, et al. Standardized Analysis of Frequency and Severity of Complications After Robot-assisted Radical Cystectomy. Eur Urol. 2012 doi: 10.1016/j.eururo.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Griffiths G, Hall R, Sylvester R, Raghavan D, Parmar MK. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29:2171–7. doi: 10.1200/JCO.2010.32.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]