Abstract
Context
Besides the generic “basic” vs. “applied” labels, little information is known about the types of life-science research conducted within academic medical centers (AMC).
Objective
To determine the relative proportion, characteristics, funding, and productivity of AMC faculty by the type of research they conduct.
Design
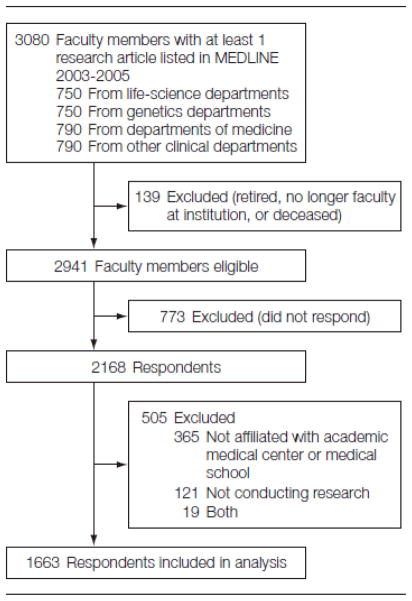
Mailed survey conducted in 2007 of life sciences faculty at the 50 universities with medical schools that received the most NIH funding in 2004. Response rate was 74% (n=2,168)
Setting and Participants
Faculty affiliated with a medical school or teaching hospital who had published at least one research article in the previous three years.
Main outcome measures
Type of research (basic, translational, clinical trials, health services research/clinical epidemiology, multimode, other); total funding; industry funding; publications; professional activities; patenting behavior; and industry relationships.
Results
Nearly one in four (23.4%) of AMC research faculty exclusively conduct basic research. In comparison, translational researchers, clinical trial investigators, and health services research/clinical epidemiologists each comprise roughly ten percent of AMC staff. While principal investigators garnered a mean of $410,755 in total annual research funding, nearly one-fifth of all AMC research faculty are unfunded, a proportion that ranges from 11.5% for basic researchers to 46.8% for health services researchers (p<0.01). The average AMC faculty member received $33,417 in industry-sponsored funding, with most of this money concentrated among clinical trial ($110,869) and multimode ($59,916) principal investigators. Compared to basic science researchers, translational, clinical trial, and multimode researchers are significantly more likely to report a relationship with industry and that these relationships contributed to their most important scientific work (p<0.01 for all comparisons).
Conclusions
These data document many of the stresses in the research arena, especially those of the clinician-researcher. National R&D funding policies should reflect priorities for encouraging the right balance of life-science investigations.
INTRODUCTION
Encompassing 60% of all research money to universities, the academic, life-science research enterprise is large and growing, representing $28.8 billion dollars in R&D expenditures in 1998.1 And while there is general consensus on the need for continued government investment in life-science research2,3, a more nuanced debate has emerged. With limited public funds, what types and kinds of research should take precedence, especially within the field of life sciences where new and more efficient treatments are needed?4,5 Researchers and policy experts disagree about whether we have the correct emphasis, arguing alternatively for more funding of basic research6, translational infrastructure7,8,9,10, or clinical trial capabilities.11,12,13 Most recently, the economic stimulus package of 2009 allocated significant new money for comparative effectiveness and health services research14,15, in an attempt to prioritize the study of “health care practices to try to determine the best treatments, devices, and procedures for almost any ailment or disease.”16
To establish policies and priorities, we first need a better empirical picture of what the AMC research enterprise looks like. But beyond generic classifications such as “basic” and “applied”, these data do not exist. The purpose of this study is to quantitatively document the state of academic research in academic medical centers through a survey of research faculty. We utilize a novel method for categorizing types of researchers and document the relative proportion, characteristics, funding, and productivity of each group. This paper provides the foundation to better argue from empirical evidence whether national research and development priorities are appropriate and whether they are successful in encouraging the right kind of clinical investigations.
METHODS
Sample Selection
The data presented here were collected from a survey of life science faculty conducted between September 2006 and February 2007. A sample of 3080 faculty members was selected in a three-step process similar to that used in previous studies.17,18 First, we identified the 50 U.S. universities and medical centers that received the most extramural research support from the National Institutes of Health in 2004. Second, within these institutions, we identified all life-science departments and programs in four survey strata: departments of medicine, other clinical departments (non-medicine), non-clinical life science departments, and genetics departments or programs. The set of other clinical departments included those receiving the most NIH funding in 2003: Anesthesiology, Neurology, Neurosurgery, Obstetrics/Gynecology, Otolaryngology, Pathology, Pediatrics, Psychiatry, Radiation/Oncology, and Surgery. The nonclinical category consisted of medical school departments of and graduate academic programs in Anatomy/Cell Biology, Biochemistry, Microbiology, Pharmacology, and Physiology/Biophysics. These represented nonclinical departments that received the most NIH funding in 2003. Finally, using Peterson’s Guide to Graduate Programs, we identified all U.S. medical school departments and graduate programs that offer doctoral-level training in genetics. If two programs existed within the same institution, both were selected into the sample.
Third, we selected a stratified, random sample of life-science faculty members in each of the four sample strata. Names and addresses were drawn from departmental websites and from the Association of American Medical Colleges (AAMC) Faculty Roster. To avoid the inclusion of fellows and hospital staff members not truly functioning as clinician-researchers, clinical faculty members were eligible for the sample only if they had a publication other than a review or a letter listed in the National Library of Medicine’s Medline database for the period from 2003 through 2005. This analysis removed the names of 40.2% of clinical faculty because they had not published a research article in the last three years. Because the focus of this paper is to describe the state of research within academic medical centers, the dataset was limited to the 82% of faculty who indicated that they were affiliated with a medical school or teaching hospital.
Survey Design and Administration
The survey instrument was a modified version of an instrument administered to 1238 life-science faculty in 1985 and 3742 life-science faculty in 1995.17,18 While many items were identical to past surveys, new questions were developed using 2 focus groups with scientists at medical schools and 10 confidential personal interviews with scientists across the United States. In addition, the new survey items were pretested using 11 cognitive interviews conducted by experienced survey researchers.
The survey was conducted by mail between September 2006 and February 2007 by the Center for Survey Research at the University of Massachusetts. Sampled faculty members were sent a survey instrument, a cover letter, a fact sheet answering frequently-asked question about the study, a postage-paid return envelope, and an incentive of $20 cash. As in the past, the survey instrument contained no identifying information. Subjects were instructed to return the completed survey in a pre-addressed envelope and return a postcard separately which included the respondent’s identification number. Doing so allowed us to maintain anonymity of a respondent’s survey results and avoid sending additional mailings to subjects who had completed the survey. We conducted telephone reminder calls to all individuals who did not send in a postcard indicating participation. This study was approved by IRBs at both the Massachusetts General Hospital and UMass-Boston.
Response Rates
Of the 3080 faculty researchers in the original sample, 139 were ineligible because they were deceased or retired, did not hold a faculty appointment, or could not be located (no return address was provided on an undelivered questionnaire and telephone follow-up was unsuccessful). Of the remaining 2941 faculty members, 2168 completed the survey, for an overall response rate of 74%.
Key Variables
To gauge the magnitude of funded research, faculty respondents reported their total budget for the fiscal year for grants and contracts from any source on which they were the principal investigator, excluding overhead/indirect costs. Faculty who reported zero dollars were assumed to be unfunded. Respondents were queried on the amount of industry funding in the same manner.
Respondents were asked to self-describe their research by responding to the following question: “Which of the following types of research are you currently conducting? (Check all that apply).” Survey categories included: Basic research; Early clinical/phase I clinical trials; Phase II clinical trials; Phase III clinical trials; Health services research/clinical epidemiology; Other clinical research; and Not currently involved in research.
In addition, these responses were grouped into six mutually-exclusive categories of Researcher Type. Basic Researchers were defined as respondents who report conducting only basic research. Translational Researchers, representing what Sung et al. define as the first “translational block”11, were defined as respondent who conduct: Phase I research only, Phase I/Phase II research only, Basic/Phase I research, or Basic/Phase I/Phase II research. Clinical Trial Researchers were defined as respondents who conduct: Phase II research only, Phase III research only, or both. Health Services/Clinical Epidemiology Researchers and Other Clinical Researchers were respectively defined as respondents who indicated they only conduct this one type of research. Finally, Multimode Researchers were defined as any combination of the above types (for instance, a respondent who indicated she conducted both basic research and Phase III clinical trials). While factor analyses yielded similar groupings, categories were defined as above for ease of interpretation, representing many of the prototypical stages of product development.
Statistical Analysis
The data were analyzed using standard statistical techniques. All data was weighted to adjust for differential non-response and probability of selection within sampling strata. Comparisons across researcher type were made against the reference case of basic science researchers. To determine the characteristics of funded versus unfunded researchers, a logistic regression was conducting using gender, race/ethnicity, academic rank, and academic degree as the independent variables.
RESULTS
Prevalence of research activities
Among the research faculty within academic medical centers, over half (54.7%) conduct basic research as part of their research program (Figure 1). Just under a quarter (22.8%) of all AMC research faculty are currently involved in a Phase III trial, while approximately 15.8% are associated with a Phase II trial and 14.9% and Early Clinical/Phase I trial. In addition, 24.7% conduct health services research or clinical epidemiology. Finally, 27.5% of faculty indicate that they are involved with additional “other clinical research” activities, including nutrition research, informatics studies, medical education, and quality improvement research.
Figure.
Study Sample Flow Chart
Descriptive statistics of researchers
Based on the combination of research activities selected above, research faculty were categorized into six mutually exclusive researcher types (Table 1). Nearly one in four (23.4%) of AMC faculty members exclusively conduct basic research. In comparison, translational researchers, clinical trial investigators, and health services research/clinical epidemiologists each comprise roughly ten percent of AMC staff (9.5%, 8.7%, and 10.5%, respectively). Those who solely conduct “other clinical research” represent 14.4% of research staff, and are generally less focused on research, allocating 10.8 hours to research each week. Women were overrepresented in non-laboratory settings, disproportionately choosing to conduct health services and other clinical research (p=0.01 and p<0.01, respectively).
Table 1.
Descriptive Statistics by Faculty Department and Type of Researcher
| All AMC Faculty | Basic Researchers | Translational Researchers | Clinical Trial Researchers | HSR/Clin Epi Researchers | Other Clinical Researchers | Multi-mode Researchers | |
|---|---|---|---|---|---|---|---|
| Percent of Research Faculty | 100 | 23.4 | 9.5 | 8.7 | 10.5 | 14.4 | 33.6 |
| Individual/Academic Characteristics | |||||||
| Pct Male | 67.0 | 70.4 | 69.5 | 76.3 | 58.3* | 45.4* | 75.2 |
| Years Since Prof Degree | 22.3 | 20.6 | 21.4 | 25.2* | 19.9 | 21.0 | 23.4* |
| Pct holding PhD degree | 37.5 | 70.4 | 24.7* | 6.2* | 21.8* | 15.9* | 20.2* |
| Pct holding MD-PhD degree | 9.5 | 12.2 | 12.6 | 4.3* | 6.1 | 3.2* | 12.4 |
| Pct Full Professor | 34.7 | 27.7 | 42.5* | 41.1* | 28.3 | 25.8 | 45.1* |
| Num of Prof Service Activities | 1.5 | 1.3 | 1.7 | 1.5 | 1.7 | 1.0* | 2.0* |
| Allocation of Work Activities | |||||||
| Research Hours per Week | 23.1 | 36.2 | 24.6* | 12.9* | 18.0* | 10.2* | 22.8* |
| Patient Care Hours per Week | 14.5 | 2.8 | 13.2* | 24.9* | 16.7* | 25.2* | 17.5* |
| Administration Hours per Week | 8.4 | 6.5 | 7.9 | 10.9* | 10.1* | 9.3* | 8.9* |
| Teaching Hours per Week | 7.9 | 7.7 | 7.3 | 7.7 | 6.8 | 8.8 | 7.3 |
| Other Prof Activities Hours per Week | 3.7 | 3.3 | 3.6 | 4.1 | 4.0 | 3.3 | 4.2* |
| Research Characteristics | |||||||
| Lab/Group Members (FTEs) | 6.2 | 5.5 | 6.3 | 6.0 | 5.0 | 3.5* | 8.7* |
| Publications, Career | 59.9 | 59.4 | 72.1 | 65.4 | 48.7 | 34.5* | 80.3* |
| Pubs per Year, last 3 years | 3.5 | 3.7 | 4.2 | 3.2 | 3.7 | 2.0* | 4.3 |
| Journal Impact Score, last 5 pubs | 5.8 | 6.5 | 5.6* | 5.9 | 5.5* | 4.3* | 5.9 |
| Percent Applied for a Patent | 24.0 | 37.2 | 37.5 | 18.8* | 6.5* | 13.5* | 24.8* |
p<0.05 compared to Basic Science Researchers
The largest single group (33.6%) consists of investigators who currently could not be categorized into only one type of research. Of the 331 respondents classified as multimode, 49.2% perform basic research, 66.2% have conducted a clinical trial, and 55.0% work on health services or clinical epidemiological research. Compared to their basic research colleagues, these multi-mode research faculty are older (p<0.01), more likely to be full professors (p<0.01), maintain larger research groups (p=0.03), and have achieved greater publication outputs, both over their careers (p<0.01) and over the last three years (p=0.09).
Publications rates across the research categories were not significantly different, except for staff members conducting “other clinical research” who publish at rates roughly half that of their peers (p<0.01). Faculty in earlier stages of research are significantly more likely to patent; among AMC faculty, basic and translational researchers are approximately twice as likely to patent compared to their peers (p<0.01).
Research funding
In 2007, each AMC faculty member brought in a mean of $410,755 in research funding as a principal investigator from all sources (Table 2). However, this overall average masks large differences in the distribution of funding: nearly a quarter (22.1%) of published AMC faculty conduct research without any grants as a PI. Of AMC researchers, basic science and multimode researchers garnered the most PI funding ($539,455 and $472,541, respectively) followed by their departmental peers who conduct clinical trials ($409,472) and translational research ($403,293), although none of these differences are statistically significant at conventional levels. Health services research and other research faculty brought in significantly less ($303,002 and $73,375, p<0.01 for both compared to AMC basic researchers), nearly half of whom were conducting research without any funding (p<0.01 for both, compared to basic researchers). The average AMC faculty member received $33,417 in industry-sponsored funding, with most of this money concentrated among clinical trial ($110,869) and multimode ($59,916) principal investigators.
Table 2.
Annual Research Funding by Stage
| All AMC Researchers | Basic Researchers | Translational Researchers | Clinical Trial Researchers | HSR, Clin Epi Researchers | Other Clinical Researchers | Multimode Researchers | |
|---|---|---|---|---|---|---|---|
| Total Funding, All Sources | |||||||
| Mean | $410,755 | $472,541 | $403,293 | $409,072 | $303,002* | $73,375† | $539,455 |
| Median | $190,000 | $255,000 | $250,000 | $75,000 | $10,000 | $0 | $186,000 |
| Percent with No Funding | 22.1% | 11.5% | 22.5%† | 20.0% | 46.8%† | 56.6%† | 16.4%† |
| Mean Funding Among Faculty Receiving Funds | $548,711 | $534,079 | $520,402 | $511,053† | $571,703 | $169,106† | $645,396 |
| Mean Funding by Rank | |||||||
| Full Professor | $690,541 | $761,332 | $577,974 | $477,814† | $622,788† | $115,094† | $865,029† |
| Assoc Professor | $254,801 | $310,701 | $363,349 | $355,139 | $193,772† | $78,635† | $275,321† |
| Assistant Professor | $179,931 | $231,495 | $185,316 | $334,671 | $158,858† | $32,658† | $197,814 |
| Industry Funding | |||||||
| Mean | $33,417 | $14,381 | $29,487† | $110,869† | $19,827 | $5,561† | $59,916† |
| Percent with Industry Funding | 20.1% | 8.6% | 25.6%† | 48.2%† | 13.2% | 4.9% | 38.6%† |
p<0.05 compared to Basic Science Researchers
In multivariate logistic regressions controlling for the type of researcher, faculty with and without research funding were not significantly different by gender or race/ethnicity. Compared to full professors, assistant and associate professors were half as likely to be funded (OR=0.45, p<0.01 and OR=0.61, p=0.02, respectively). However, the largest differentiator is found amongst physician-scientists. Compared to researchers with MD degrees, those with PhDs and MD-PhDs were nearly three times as likely to be funded (OR=2.79 and OR=2.99, respectively, p<0.01 for both).
Faculty without funding were associated with significantly fewer hours devoted to research activities (p<0.01) and significantly greater hours devoted to patient care (p<0.01, data not shown). For example, the 22.5% of translational researchers who report no grant support spent an average of 9.0 hours per week on research activities and 29.2 hours per week in patient care, while the remaining translational investigators with support devoted 31.3 hours per week in research (versus 7.3 hours in patient care) and garnered $520,402 in average annual funding.
Industry Relationships
Over half (52.4%) of all AMC research faculty maintain some relationship to industry (Table 3), with roles ranging from a start-up company founder to a scientific advisory board member to an industry consultant. Compared to basic science researchers, translational, clinical trial, and multimode researchers are significantly more likely to report a relationship and to report that these relationships contributed to their most important scientific work (p<0.01 for all comparisons).
TABLE 3.
Involvement with Industry by Type of Researcher
| All AMC Researchers | Basic Researchers | Translational Researchers | Clinical Trial Researchers | HSR/Clin Epi Researchers | Other Clinical Researchers | Multi-mode Researchers | |
|---|---|---|---|---|---|---|---|
| Any relationship with industry* | 52.4% | 40.4% | 58.6%† | 68.9%§ | 38.4% | 35.8% | 71.6%§ |
| If yes, contributed to most important scientific work‡ | 35.7 | 30.6 | 46.5§ | 49.5§ | 21.7 | 21.4 | 40.3 |
| Received research support from industry | 23.3 | 9.6 | 25.7§ | 48.3§ | 13.3 | 4.6 | 39.7§ |
| Shared information, data, expertise, or materials with industry scientists in the last three years | 26.3 | 25.0 | 38.4§ | 31.7 | 15.0§ | 11.9§ | 39.0§ |
| If yes, opened a new line of research | 39.4 | 24.7 | 49.1§ | 42.2 | 26.4 | 4.0 | 47.6§ |
| led to research that would otherwise not have been possible | 47.1 | 42.8 | 47.2 | 39.8 | 43.6 | 31.8 | 54.0 |
| formed collaborations leading to publications | 49.6 | 41.5 | 52.8 | 47.6 | 52.1 | 16.2 | 55.7 |
| formed collaborations leading to sponsored research | 42.5 | 25.6 | 47.2§ | 52.4§ | 47.9 | 24.0 | 48.1§ |
| experienced a negative outcome§ | 15.7 | 15.6 | 11.3 | 15.0 | 0.0 | 4.0 | 19.9 |
Relationships include: corporate founder, member of the board of directors, member of scientific advisory board, officer/executive, employee, consultant, paid speaker, recipient of funding for university research or students/fellows, or recipient of royalties or license fees based on a patent.
p<0.05 compared to Basic Science Researchers
Percent of respondents answering “to some extent” or “to a great extent”
Negative outcomes included: had your ideas appropriated without fair compensation; been “scooped” by another scientist; compromised the ability of a graduate student, post-doctoral fellow, or junior faculty member to publish; or been unable to commercialize your results.
Of the 26.3% of faculty that shared data, expertise, or materials with industry within the past three years, a substantially greater proportion document positive outcomes than negative outcomes. Translational and multimode researchers are more likely to share with industry and report that this cooperation has led to new lines of research and more sponsored research funding. Interestingly, basic researchers experienced a greater ratio of negative consequences to positive outcomes, compared to their peers.
DISCUSSION
Taken in their entirety, these data create a composite view of the current landscape of the AMC research enterprise. The results document the prevalence of several types of clinical research as well as demonstrating that the characteristics and stresses of clinical researchers often differ by the type of research they conduct. As a byproduct, these data also provide national benchmarks for funding and productivity that could be considered for academic promotion. Several specific implications are warranted.
First, contrary to popular myth19, the “valley of death” for translational research appears to be quite fertile within AMCs. At the time of this survey in 2007, 22 of the 50 institutions were participating members of the Clinical and Translational Science Awards (CTSA) Consortium, with another 12 poised to join in 2008.20 In general, translational researchers are well funded and scientifically productive. Data regarding their academic status and funding by rank do not suggest that they are subject to widespread disadvantages for their research or career trajectories.21 Their patenting behavior and relationships with industry both underscore the critical role they serve in developing basic research findings into useful advances for patient care.
Second, multi-mode investigators represent an understudied population. These investigators, who conduct research across the spectrum of research activities, report both substantial scientific and commercializing characteristics. Due to their age and rank, these researchers likely represent the mature product of a successful scientific research career, managing labs that are larger, more productive, and better funded to investigate both the scientific and clinical implications of a research stream. In this current scientific climate where research is too often describe in terms of either basic or applied categories, more study is needed in the operations and outcomes of the multidisciplinary principal investigators and their labs. For example, what is the role of “topic experts” who study a biological problem through all the aspects of development versus “domain experts” who focus on one aspect of development, like some clinical trialists?
Third, the findings also demonstrate the important role of industrial collaboration in scientific advancement. Academic-industry relationships provide substantial, tangible benefits to both the science and the scientist. Among AMC faculty with the greatest involvement with industry (translational, clinical trial, and multimode researchers), nearly half reported that it contributed to their most important scientific work and led to research that would not otherwise have been possible. Even though the relative magnitude of industry funding was one-half to one-tenth of total funding, researchers reported that working with industry opened new lines of research and formed productive collaborations. Current policies and initiatives to ban or restrict academic-industry relationships must balance the advantages to clinical development against the threats to scientific integrity.22,23,24
Several limitations should be noted. Because the survey population was drawn from the AAMC faculty roster, the sample does not include life-science researchers who are not affiliated with a medical school or teaching hospital, likely missing a substantial pool of basic science investigators. Consequently, generalizations of these results are not applicable outside this population of life-science faculty within research-intensive academic medical centers. Further, it included only the subset of faculty that had published a research article in the past three years, which may under-represent new or young researchers and may include many patient-centric physicians who see research as a side pursuit. Like all survey-based analyses, our study likely suffers from the potential biases within the self-reported responses, especially on the amount and nature of industrial and total funding. Faculty members who did not respond to our survey may differ systematically from those who did, although we detected no significant differences by known characteristics of our eligible population (academic rank, employment within a medical school, or level of institutional NIH funding).
Overall, the findings in this paper document many of the stresses in the research arena, especially for the clinician-researcher.25 While 22% of all faculty are unfunded, MDs comprise more than two-thirds (69.2%) of this group.26,27 Unfunded physicians spent on average 7.3 hours per week on research. This finding likely underscores the fact that research publications are the coin of the realm in faculty promotion and tenure decisions. However, it may also reflect the stolen hours available to researchers after the pressures of generating clinical income and the inability to procure funding are considered. More research in this field is needed, including how these faculty are able to leverage a network of funding using the infrastructure of the AMC, the quality of unfunded research compared to funded projects, and the career trajectories of faculty who engage in this activity.28 From a medical school perspective unfunded research, like unfunded clinical care, must be supported from other revenues—a potentially difficult proposition in the current economic climate.
Even the future funding options for clinician-researchers look bleak. MDs make up 90% of all clinical trial investigators within AMCs, and nearly half of their funding (48.2%) is dependent on biopharmaceutical and medical device sponsors. But as Glickman and colleagues point out29, the clinical trials industry is rapidly moving overseas, leaving this once stable funding source for academic medical centers in jeopardy. Clearly additional research must examine the potential implications of this trend for clinical investigators in academic settings as well as for the quality and quantity of clinical research conducted in non-academic settings.
In the end, this study cannot determine whether this represents the right balance of research, but the results provide benchmark data and raise questions for future research. Is a large and well-funded basic-science workforce necessary in early stage, exploratory research to discover and develop new biomedical findings? As the natural funnel of successful projects are narrowed, are fewer resources needed in hypothesis-confirming clinical studies? And what of the “second translational block”11 that seeks to implement clinical studies into medical practice; what role should funding play in documenting which clinical interventions are most effective in everyday use?
In our study, the data show that half of all faculty conduct basic research as part of their portfolio. When generalized, faculty that exclusively conduct basic science research garnered more than $4.7 billion in total research funding across the top 50-funded AMCs. In comparison, $802 thousand were collected by health services researchers and only $250 thousand went toward “other clinical research,” where studies center on patient outcomes and patient care (e.g., nutrition, Phase IV, psychology/behavioral, or quality improvement/safety studies). By sector, these two groups each represent more faculty members than clinical trials, yet half are unfunded. To the extent that this research is being cross-subsidized from other internal funds, these investigations may be a net drain on resources within the AMC.
National policies can have a substantial effect on the nature of research performed in this country. For example, the strong state of translational medicine documented in this paper is likely a reflection of the emphasis placed on this type of research by the NIH recently through its CTSA and Roadmap initiatives. In this vein, the massive investment in clinical effectiveness research put forward American Recovery and Reinvestment Act of 2009 may signal a change in emphasis and a potential new direction of AMC research. Compared to previous eras, research priorities will now stress efficiency in addition to innovation.
Acknowledgments
Funding/Support: This research was supported by a grant from National Human Genome Research Institute of the National Institutes of Health (1R01GM074915-01, PI Eric G. Campbell).
Role of the Sponsors: The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
We would like to acknowledge the contributions of Sandra Feibelmann M.P.H., of the Institute for Health Policy at Massachusetts General Hospital.
Footnotes
Financial Disclosures: None reported.
Author Contributions: Dr Zinner had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Zinner, Campbell.
Acquisition of data: Zinner, Campbell.
Analysis and interpretation of data: Zinner, Campbell.
Drafting of the manuscript: Zinner.
Critical revision of the manuscript for important intellectual content: Zinner, Campbell.
Statistical analysis: Zinner.
Obtained funding: Campbell.
Administrative, technical, or material support: Zinner, Campbell.
Study supervision: Campbell.
Contributor Information
Darren E Zinner, Email: dzinner@braneis.edu, Schneider Institutes for Health Policy, The Heller School, Brandeis University, 415 South St MS 035, Waltham, MA 02454, 781 736-3971, 781 736-3306 (fax).
Eric G Campbell, Email: ecampbell@partners.org, MGH Institute for Health Policy, 50 Staniford Street, Suite 901, Boston, MA 02114.
Works Cited
- 1.National Science Board. Two volumes. Vol. 1. Arlington, VA: National Science Foundation; 2008. Science and Engineering Indicators 2008. NSB 08-01; volume 2, NSB 08-01A), Appendix Table 5-3. [Google Scholar]
- 2.National Science Board. Two volumes. Vol. 1. Arlington, VA: National Science Foundation; 2008. Science and Engineering Indicators 2008. (NSB 08-01; volume 2, NSB 08-01A), Appendix Table 7-18. [Google Scholar]
- 3.Heinig SJ, Krakower JY, Dicker HB, Korn D. Sustaining the Engine of U. S Biomedical Discovery. NEJM. 2007;357(10):1042–1047. doi: 10.1056/NEJMsb071774. [DOI] [PubMed] [Google Scholar]
- 4.Zerhouni EA. NIH in the Post-Doubling Era: Realities and Strategies. Science. 2006 Nov 17;314:1088–1090. doi: 10.1126/science.1136931. [DOI] [PubMed] [Google Scholar]
- 5.Bonetta L. Tough Challenges for the Next NIH Director. Cell. 2008 Nov 14;135 (4):583–585. doi: 10.1016/j.cell.2008.10.038. [DOI] [PubMed] [Google Scholar]
- 6.Mandel HG, Vesell ES. From progress to regression: biomedical research funding. J Clin Inv. 2004 Oct;114(7):872–876. doi: 10.1172/JCI23245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moskowitz J, Thompson JN. Enhancing the clinical research pipeline: Training approaches for a new century. Acad Med. 2001 Apr;76 (4):307–315. doi: 10.1097/00001888-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Moses H, Thier SO, Matheson DHM. Why have academic medical centers survived? JAMA. 2005 Mar 23;293 (12):1495–1500. doi: 10.1001/jama.293.12.1495. [DOI] [PubMed] [Google Scholar]
- 9.Moses H, Dorsey ER, Matheson DHM, et al. Financial anatomy of biomedical research. JAMA. 2005;294(11):1333–1342. doi: 10.1001/jama.294.11.1333. [DOI] [PubMed] [Google Scholar]
- 10.Sabroe I, Dockrell DH, Vogel SN, et al. Opinion - Identifying and hurdling obstacles to translational research. Nat Rev Immun. 2007 Jan;7 (1):77–82. doi: 10.1038/nri1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sung NS, Crowley WF, Genel M, et al. Central Challenges Facing the National Clinical Research Enterprise. JAMA. 2003;289(10):1278–1287. doi: 10.1001/jama.289.10.1278. [DOI] [PubMed] [Google Scholar]
- 12.Nathan DG, Wilson JD. Clinical Research and the NIH – A Report Card. NEJM. 2003 Nov 6;349(19):1860–1865. doi: 10.1056/NEJMsb035066. [DOI] [PubMed] [Google Scholar]
- 13.Druss BG, Marcus SC. Academic medicine: who is it for? Funding gap between clinical and basic science publications is growing. BMJ. 2005 Feb 12;330 (7487):360–361. doi: 10.1136/bmj.330.7487.360-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slutsky JR, Clancy CM. AHRQ’s Effective Health Care Program: Why Comparative Effectiveness Matters. Am J Med Qual. 2009 Jan-Feb;24 (1):67–70. doi: 10.1177/1062860608328567. [DOI] [PubMed] [Google Scholar]
- 15.Tunis SR, Carino TV, Williams RD, et al. Federal initiatives to support rapid learning about new technologies. Health Aff. 2007 Mar-Apr;26 (2):W140–W149. doi: 10.1377/hlthaff.26.2.w140. [DOI] [PubMed] [Google Scholar]
- 16.Hitt G, Weisman J. Congress Strikes $789 Billion Stimulus Deal. Wall Street Journal. 2009 Feb 12; [Google Scholar]
- 17.Blumenthal D, Gluck M, Louis KS, Stoto MA, Wise D. University-industry research relationships in biotechnology: Implications for the university. Science. 1986;232:1361–66. doi: 10.1126/science.3715452. [DOI] [PubMed] [Google Scholar]
- 18.Blumenthal D, Campbell EG, Causino N, Louis KS. Participation of life-science faculty in research relationships with industry. NEJM. 1996;335:1734–1739. doi: 10.1056/NEJM199612053352305. [DOI] [PubMed] [Google Scholar]
- 19.Butler D. Crossing the Valley of Death. Nature. 2008 Jun;453(12):840–842. doi: 10.1038/453840a. [DOI] [PubMed] [Google Scholar]
- 20. [Accessed March 1, 2009.];Clinical and Translational Science Awards (CTSAs) program. http://www.ctsaweb.org/
- 21.Nathan D. Careers in Translational Clinical Research – Historical Perspectives, Future Challenges. JAMA. 2002;287(18):2424–797. doi: 10.1001/jama.287.18.2424. [DOI] [PubMed] [Google Scholar]
- 22.Blumenthal D. Ethics issues in academic-industry relationships in the life sciences: The continuing debate. Acad Med. 1996;71 (12):1291–1296. doi: 10.1097/00001888-199612000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Campbell EG, Powers JB, Blumenthal D, et al. Inside the triple helix: Technology transfer and commercialization in the life sciences. Health Aff. 2004 Jan-Feb;23 (1):64–76. doi: 10.1377/hlthaff.23.1.64. [DOI] [PubMed] [Google Scholar]
- 24.Ehringhaus SH, Weissman JS, Sears JL, Goold SD, Feibelmann S, Campbell EG. Responses of medical schools to institutional conflicts of interest. JAMA. 2008 Feb 13;299(6):665–71. doi: 10.1001/jama.299.6.665. [DOI] [PubMed] [Google Scholar]
- 25.Campbell EG, Weissman JS, Moy E, Blumenthal D. Status of Clinical Research in Academic Health Centers: Views From the Research Leadership. JAMA. 2001;286(7):800–806. doi: 10.1001/jama.286.7.800. [DOI] [PubMed] [Google Scholar]
- 26.Beaty HN, Babbott D, Higgins EJ, et al. Research activities of faculty in academic departments of medicine. Ann Int Med. 1986;104:90–97. doi: 10.7326/0003-4819-104-1-90. [DOI] [PubMed] [Google Scholar]
- 27.Weissman JS, Saglam D, Campbell EG, Causino N, Blumenthal D. Market forces and unsponsored research in academic health centers. JAMA. 1999 Mar 24;281(12):1093–1098. doi: 10.1001/jama.281.12.1093. [DOI] [PubMed] [Google Scholar]
- 28.Dickler HB, Korn D, Gabbe SG. Promoting Translational and Clinical Science: The Critical Role of Medical Schools and Teaching Hospitals. PLoS Med. 2006;3(9):e378 1492–1495. doi: 10.1371/journal.pmed.0030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glickman SW, McHutchison JG, Peterson ED, et al. Ethical and Scientific Implications of the Globalization of Clinical Research. NEJM. 2009 Feb 19;360(8):816–823. doi: 10.1056/NEJMsb0803929. [DOI] [PubMed] [Google Scholar]