Abstract
Neuronal loss in Parkinson’s Disease (PD) is seen in a number of brain regions in addition to the substantia nigra (SN). Among these is the thalamic parafascicular nucleus (PF), which sends glutamatergic projections to the striatum and receives GABAergic inputs from the SN. Recent data suggest that lesions of nigrostriatal dopamine axons cause a loss of PF neurons, which has been interpreted to suggest that the PF cell loss seen in PD is secondary to dopamine denervation. However, the extent of a PF dopamine innervation in the rat is unclear, and it is possible that PF cell loss in parkinsonism is independent of nigrostriatal dopamine degeneration. We characterized the dopamine innervation of the PF in the rat and determined if 6-hydroxydopamine SN lesions cause PF neuron degeneration. Dual-label immunohistochemistry revealed that almost all tyrosine hydroxylase-immunoreactive (TH-ir) axons in the PF also expressed dopamine-beta-hydroxylase and were therefore noradrenergic or adrenergic. Moreover, an antibody directed against dopamine revealed only very rare PF dopaminergic axons. Retrograde-tract tracing—immunohistochemistry did not uncover an innervation of the PF from midbrain dopamine neurons. Nigrostriatal dopamine neuron lesions did not elicit degeneration of PF cells, as reflected by a lack of FluoroJade C staining. Similarly, neither unilateral 6-OHDA lesions of nigrostriatal axons nor the dorsal noradrenergic bundle decreased the number of PF neurons or the number of PF neurons retrogradely-labeled from the striatum. These data suggest that the loss of thalamostriatal PF neurons in Parkinson’s Disease is a primary event rather than secondary to nigrostriatal dopamine degeneration.
Keywords: Centromedian, Parkinson’s Disease, Substantia nigra, Thalamostriatal, Thalamus
1. Introduction
The loss of dopamine neurons and presence of Lewy bodies in surviving dopamine neurons of the substantia nigra (SN) are two of the pathological features that have classically defined Parkinson’s Disease (PD; Halliday et al., 2011). However, it is evident that the pathology in PD also extends to brain regions that contain non-dopaminergic neurons (Braak et al., 2003; Halliday et al., 2011). Among these is the thalamic centromedian-parafascicular complex (CM-PF), which sends a glutamatergic projection to the striatum (Berendse and Groenewegen, 1990; Dubé et al., 1988; Lapper and Bolam, 1992; Smith et al., 2004). Using stereological methods, Henderson et al. (2000) found that more than 30% of CM-PF neurons degenerate in PD, but that the extent of CM-PF cell loss does not correlate with disease severity, age at onset, or duration of illness.
In rodents, which lack a clearly-defined CM nucleus, anatomical studies have suggested that the lateral PF corresponds to the CM of primates, while the medial PF is homologous with the primate PF (Smith et al., 2004). Neurons in the thalamic anterior intralaminar complex of the rat, which includes the central medial (CeM), paracentral (PC), and central lateral (CL) nuclei, also project to the striatum (Smith et al., 2004) but differ from PF axons in the extent of their axonal arbors (Deschênes et al., 1995).
The functional consequence of the loss of CM-PF neurons on the symptoms associated with PD is poorly understood. Clinico-pathological correlations have not been revealing, and thus the few extant studies are in animal models of parkinsonism. In rodents, lesions of PF neurons do not alter body axis bias, head position, sensorimotor responses, grooming, or apomorphine-induced rotational behavior, and lesions of the PF in 6-hydroxydopamine (6-OHDA)-treated rats did not yield motor changes beyond those already present in the 6-OHDA lesioned animals (Henderson et al., 2005). However, this study did note that animals with combined 6-OHDA lesions and PF lesions performed better in a simple motivation task (latency to retrieve a reward) relative to rats with 6-OHDA or PF lesions alone. In MPTP-treated primates, unilateral excitotoxic lesions of the caudal intralaminar nuclei (centromedian) initially resulted in very modest changes in parkinsonian motor scale that resolved quickly and did not modify levodopa-induced dyskinesias (Lanciego et al., 2008). More positive effects have been noted in studies of molecular changes seen in the dopamine-denervated striatum. Thus, Bacci et al. (2004) reported that large excitoxic lesions of the posterior intralaminar nuclei in the rat prevented the increase in striatal preproenkephalin and GAD67 mRNAs seen after dopamine denervation, but not the changes in preprotachykinin or GAD67 mRNAs in the substantia nigra, pars reticulata. These data suggest that PF lesions may modify the effects of striatal dopamine denervation of the indirect but not direct pathway neurons. Orieux et al. (2000) found that striatal dopamine denervation increased the activity, as reflected by cytochrome oxidase I mRNA, of PF neurons innervating the subthalamic nucleus.
The cause of degeneration of CM-PF cells in PD is also unknown. Sedaghat et al. (2009), on the basis of qualitative examination of Nissl-stained material, suggested that degeneration of SN dopamine neurons results in the loss of PF neurons in rats and decreased the density of leucine-rich repeat containing GPCR 8 (LGR8) mRNA in “surviving” PF neurons (Sedaghat et al., 2009). In addition, neurotoxic lesions of the median forebrain bundle (MFB), in which dopaminergic axons ascend to innervate di- and telencephalic sites, have been reported to selectively decrease the number of PF, but not CeM, PC, or CL, neurons that are retrogradely labeled from the striatum (Aymerich et al., 2006).
The ability of lesions that target nigrostriatal dopamine neurons to cause the loss of PF neurons presupposes either a direct dopamine innervation of the PF or actions on the PF through a complex multisynaptic pathway. Neurons in the pars reticulata of the substantia nigra (SNpr), the majority of which are GABAergic, have long been known to project to the PF (Beckstead et al., 1979; François et al., 2002; Sidibé et al., 2002; Tsumori et al., 2002). However, the presence of a PF innervation derived from dopamine neurons of the SN pars compacta (SNpc) has not been rigorously characterized, although some studies have suggested the presence of such an innervation (Cebrián and Prensa, 2010). In order to determine if the loss of nigrostriatal dopamine neurons can elicit a secondary loss of thalamostriatal PF neurons, we first determined if there is a dopamine innervation of the PF and related anterior intralaminar nuclei in the rat. We then determined if lesions of SN dopamine neurons or their axons elicit degeneration of PF neurons and decrease the number of PF neurons retrogradely-labeled from the striatum.
2. Results
2.1. Assessment of a nigral dopaminergic innervation of the PF
As an initial means of determining if there is a dopamine innervation of the rat PF, we determined if axons in the PF colocalized tyrosine hydroxylase (TH) and dopamine β-hydroxylase (DBH), key enzymes necessary for the synthesis of dopamine and norepinephrine (NE), respectively. We observed a relatively dense innervation of the PF by axons expressing both TH and DBH immunoreactivity (ir), consistent with the presence of a NE or adrenergic innervation of the PF. However, axons that expressed TH but not DBH ir, and were thus dopaminergic, were rarely observed in the PF or in the CeM, PC, and CL nuclei (see Fig. 1). Both single-labeled (TH-ir) dopaminergic and double-labeled (TH-and DBH-ir) noradrenergic axons in the PF were of fine caliber with frequent varicosities, and very infrequently branched.
Fig. 1.
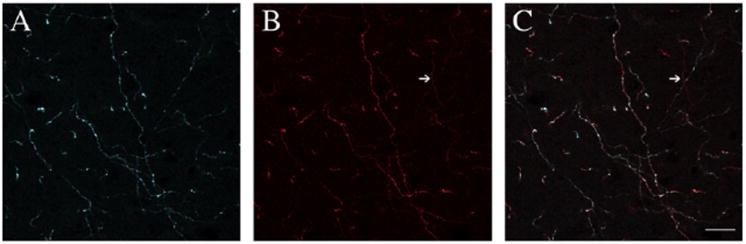
A–B, Immunolabeling for dopamine β-hydroxylase- (DBH; blue; panel A) and tyrosine hydroxylase- (TH; red; panel B) ir axons in the parafascicular nucleus. Almost all axons were double-labeled for both enzymes and thus appear to be noradrenergic. C, Rare single-labeled TH-ir (dopaminergic) axons were seen in the merged image (arrow). Scale bar, 20 μm.
We also determined if dopamine axons invade the PF by an immunohistochemical approach using an anti-dopamine antibody (Chagnaud et al., 1987; Geffard et al., 1984a,b). In the dorsal thalamus, the greatest density of dopamine axons was observed in the paraventricular thalamic nucleus (PVT; Fig. 2). In contrast, only rare DA-ir axons were seen in the PF (see Fig. 2). These DA-ir axons were generally of fine caliber but quite varicose. A very sparse dopamine innervation of the paracentral and centrolateral thalamic nuclei was also present, with the CeM being essentially devoid of dopaminergic axons.
Fig. 2.
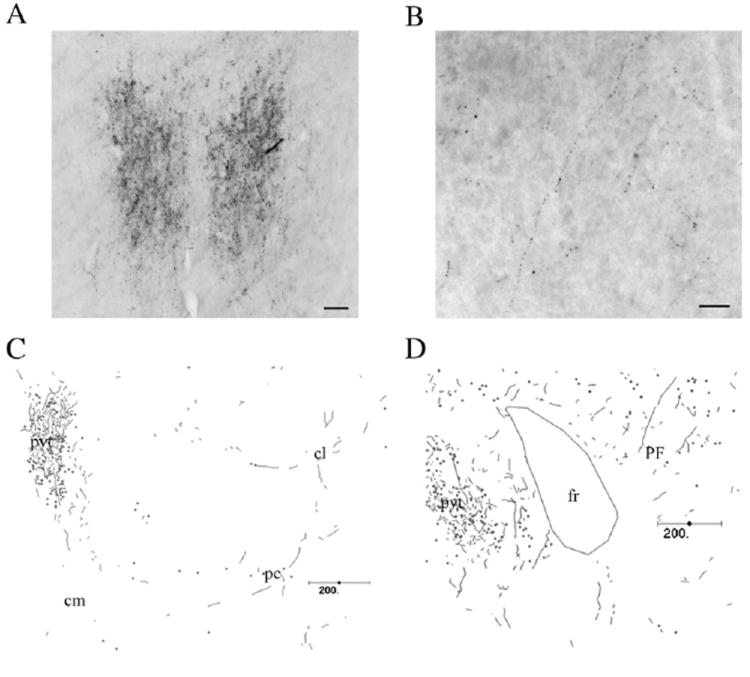
Distribution of dopamine-immunoreactive axons in the dorsomedial thalamus. Photomicrographs of dopamine immunolabeling in the thalamic paraventricular nucleus (A) and the parafasicular nucleus (B). C–D, Chartings of the distribution of dopamine-ir axons in the central medial/central lateral/paracentral (C) and PF (D) nuclei. Abbreviations: cm, central medial nucleus; cl, central lateral nucleus; pc, paracentral nucleus; fr, fasciculus retroflexus; pvt, paraventricular thalamic nucleus; PF, parafascicular thalamic nucleus. Scale bars: A, 50 μm; B, 25 μm; C–D, 200 μm.
In order to gain some appreciation of the relative density of the non-dopaminergic innervation of the PF from the SNpr, we deposited the anterograde tracer biotinylated dextran amine (BDA; Supplemental Fig. 1) into the SN. In contrast to the paucity of dopamine axons, a moderately dense innervation of the PC and CL was seen, with a less dense innervation of the PF (Fig. 3).
Fig. 3.
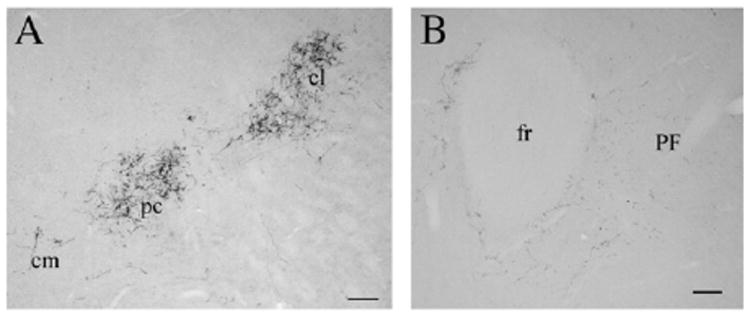
The substantia nigra projects to the anterior intralaminar thalamic nuclei and the PF. Biotinylated dextran amine-ir axons in the central medial (cm), paracentral (pc), and central lateral nuclei (cl; A) and in the parafascicular nucleus (PF; B). The innervation of the PF by the SNpr is appreciably less dense than that of the more anterior intralaminar nuclei. Scale bars: 100 μm.
We also used retrograde-labeling combined with immunohistochemistry to determine if dopamine neurons in the SN innervated the PF. Discrete iontophoretic deposits of Fluor-oGold (FG) into the PF resulted in the presence of a moderate number of retrogradely-labeled cells in the pars reticulata of the SN, with rare FG-positive cells seen in the pars compacta. We did not observe any retrogradely-labeled SN neurons that also expressed TH-ir (data not shown), nor did we observe any double-labeled dopaminergic cells in the ventral tegmental area or retrorubral field (A10 and A8 dopamine cell group regions).
2.2. 6-OHDA lesions of nigral dopamine neurons do not elicit degeneration of PF neurons
We next determined if 6-hydroxydopamine (6-OHDA) lesions of the SN resulted in degeneration of PF cells, using FluoroJade C (FJC) to label degenerating neurons (Schmued et al., 2005) in the thalamus. The 6-OHDA lesion resulted in a nearly complete loss of dopaminergic neurons in the SN (see Fig. 4). However, we observed no FJC-positive cell bodies in the thalamus at 2, 4, 7, or 42 days after the 6-OHDA lesion, although degenerating neurons could be seen in the SN at 2 days after 6-OHDA infusion. Interestingly, we did observe a few degenerating (FJC-positive) axons in the PF and CL ipsilateral to the SN lesion (Fig. 4).
Fig. 4.
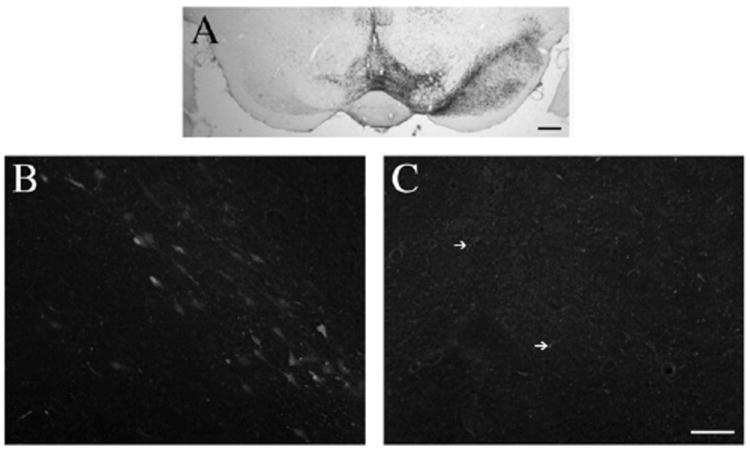
Parafascicular nucleus neurons do not degenerate in response to dopamine lesions. A) The extent of the 6-OHDA SN lesion can be seen in sections stained for tyrosine hydroxylase. There was an almost complete loss of SN dopamine neurons, with a substantial involvement of the lateral ventral tegmental area dopamine neurons. B) FluoroJade C (FJC) accumulates in degenerating neurons in the substantia nigra at 2 days after the surgery. C) No degenerating cell bodies were seen in the thalamus at any of the time points assessed. However, a few FJC-positive processes (arrows) were detected at 4 days post-operatively. Scale bars: A, 400 μm; B–C, 50 μm.
2.3. Effect of 6-OHDA lesions of the ascending dopaminergic and noradrenergic innervations of the di- and telencephalon on the number of PF neurons innervating the striatum
Aymerich et al. (2006) reported that the number of PF neurons that were retrogradely labeled from the striatum was substantially decreased in animals with 6-OHDA lesions of the MFB. While one interpretation of this observation is that PF cells degenerate in response to dopamine denervation, it is also possible the retrograde transport functions of PF cells are compromised. We determined the number of PF cells that were retrogradely-labeled after unilateral 6-OHDA lesions of the SN and the absolute number of PF neurons. Because Aymerich et al. (2006) used 6-OHDA lesions of the median forebrain bundle (MFB) to disrupt the striatal dopamine innervation, we compared the effects of 6-OHDA lesions of the SN and MFB. In addition, because the noradrenergic innervation of the forebrain also ascends in the MFB, we performed 6-OHDA lesions of the dorsal noradrenergic bundle (DNAB) at the level of the pontomesencephalic juncture as a control procedure. One month after 6-OHDA or sham lesions were made, animals received bilateral iontophoretic deposits of FG into the dorsolateral striatum and were sacrificed approximately one week thereafter.
In animals sustaining 6-OHDA SN or MFB lesions there was a massive loss of striatal dopaminergic (TH-ir) axons ipsilateral to the lesion (Fig. 5). The DNAB-lesioned group showed a marked but incomplete loss of DBH-ir axons in the PF ipsilateral to the lesion (see Supplemental Fig. 2). We also examined several cortical sites in these DNAB-lesioned animals, where we saw a marked but not complete loss of the noradrenergic innervation.
Fig. 5.
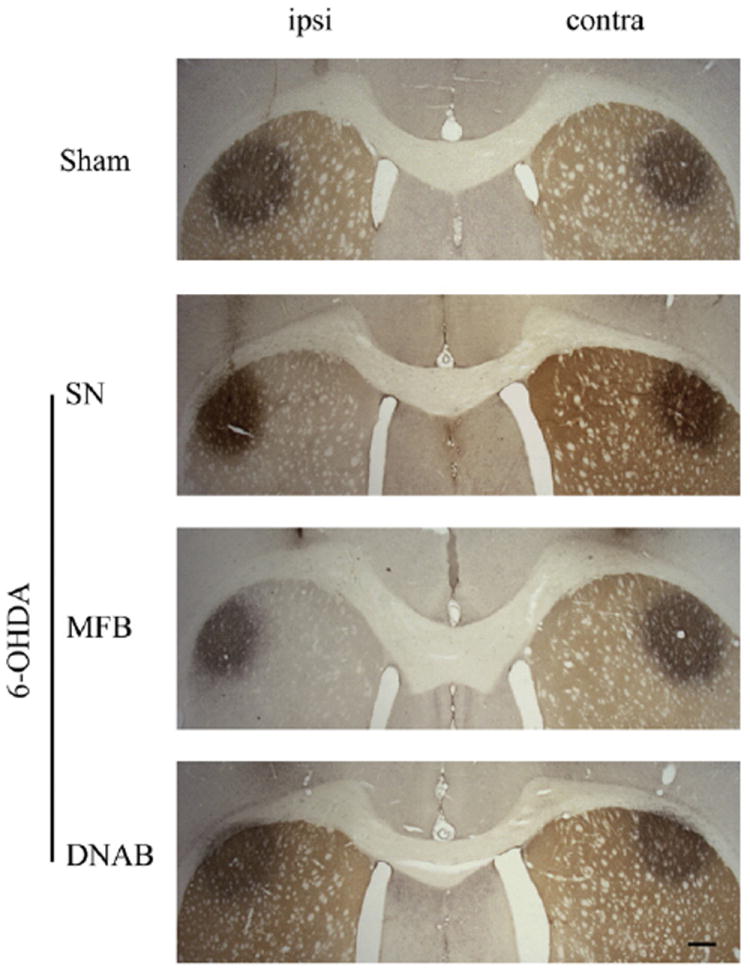
Illustration of FluoroGold (FG) deposits in the intact and dopamine-denervated striatum. Representative FG deposits in the dorsolateral striatum of animals in the sham-operated, 6-OHDA substantia nigra (SN)-, 60OHDA median forebrain bundle (MFB)-, and dorsal noradrenergic bundle (DNAB)-lesioned groups. The dopamine innervation of the striatum is counterstained using TH immunohistochemistry and is extensively lost in one (left) striatum of rats with 6-OHDA SN or MFB-lesioned animals. Scale bar: 400 μm.
The bilateral deposits of FG were confined to the dorsolateral striatum and were largely symmetric in both size and position (see Fig. 5). These retrograde tracer deposits resulted in dense labeling of lateral PF neurons. No differences in the number of retrogradely-labeled PF neurons were seen in any of the experimental groups (SN, MFB, or DNAB-lesions) relative to sham-lesioned animals (F3,28 = 1.836, NS; Fig. 6). Moreover, the number of retrogradely-labeled PF neurons on the ipsilateral side of 6-OHDA lesions of the MFB was not different from that seen in the contralateral PF (t12 =0.1113, NS; Fig. 6). Finally, stereological assessment revealed that the number of Nissl-stained PF cells did not differ across any of the experimental and control groups (F3,24 =0.56, NS; Fig. 7).
Fig. 6.
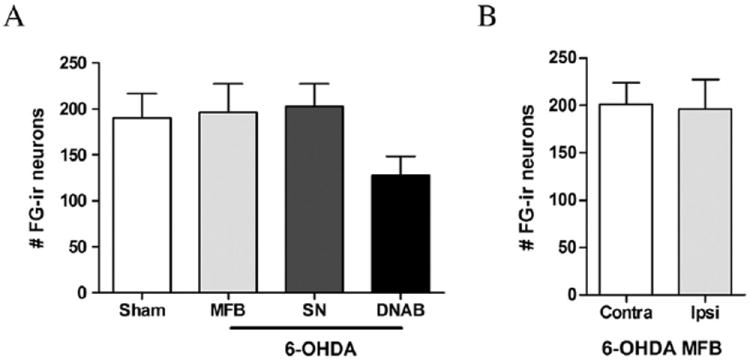
A) The number of retrogradely-labeled FG neurons in the PF ipsilateral to the 6-OHDA lesion did not differ between sham-lesioned animals and those with 6-OHDA MFB, 6-OHDA SN, or 6-OHDA DNAB lesions. B) There was no difference in the number of retrogradely-labeled FG neurons in the parafascicular nucleus (PF) ipsilateral to the lesion relative to the contralateral (contra) hemisphere of the 6-hydroxydopamine median forebrain bundle (MFB)-lesioned animals.
Fig. 7.
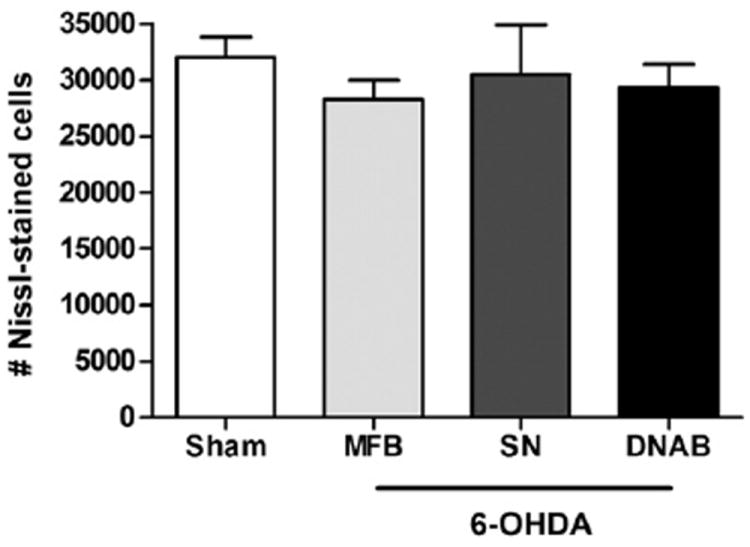
The number of PF Nissl-stained neurons did not differ between sham lesioned animals and those animals with 6-OHDA lesions of the SN, MFB, or DNAB.
3. Discussion
3.1. The PF receives noradrenergic but not dopaminergic inputs
We used three different approaches to determine if the PF receives a dopamine innervation: dual TH–DBH immunohistochemistry, dopamine immunohistochemistry, and retrograde tract tracing combined with immunohistochemistry. All three approaches failed to uncover any evidence of a significant dopamine innervation of the PF or anterior intralaminar nuclei.
We used an antibody generated against a dopamine–glutaraldehyde–BSA conjugate to specifically stain dopaminergic axons. This antibody specifically recognizes dopamine (Geffard et al., 1984a) but not L-DOPA, NE, or trace amines (Geffard et al., 1984a,b, 1985), and has been used extensively to document the presence of dopaminergic elements in the central nervous system (Geffard et al., 1984a,b, 1985; Séguéla et al., 1990; Sesack et al., 1995; Smiley et al., 1992; Williams and Goldman-Rakic, 1993). We observed rare DA-ir axons in the anterior intralaminar nuclei and even fewer DA-ir axons in the PF. In contrast, in the same brain sections we observed a moderately dense dopamine innervation of the thalamic paraventricular nucleus, consistent with previous reports in both the rodent and primate (García-Cabezas et al., 2007, 2009; Groenewegen, 1988; Sánchez-González et al., 2005).
We are not aware of any detailed report on the distribution of dopamine-ir fibers in the PF of the rat. Although Freeman et al. (2001) reported that there was a moderate density of dopamine transporter (DAT)-ir axons in the anterior intralaminar nuclei (CeM and PC) of the rat, more recently García-Cabezas et al. (2009) observed only rare DAT-ir axons in the CL and PF. However, DAT abundance appears to be below detectable levels or not present in some mesotelencephalic dopaminergic axons Ciliax et al., 1995; Sesack et al., 1998), and as such the absence of DAT-ir axons in certain sites does not reflect dopamine denervation. During a detailed study of the mediodorsal nucleus of the thalamus, Groenewegen (1988) noted that the CeM and CL nuclei contain a few dopamine-ir axons, consistent with our observations.
Although our data uncovered no evidence of a significant dopamine innervation of the PF and anterior intralaminar nuclei in the rat, the thalamic dopamine innervation of the primate appears to be expanded considerably relative to rodents. Cavada and colleagues (García-Cabezas et al., 2007; Sánchez-González et al., 2005) have documented a relatively widespread dopamine innervation of the primate thalamus. Using DAT immunohistochemistry, they found a moderately dense plexus of DAT-ir axons in the macaque and human PF, and, using a dopamine antibody, also uncovered a dopamine-ir innervation of the CeM and posterior PF. Because Cavada and colleagues did not show chartings through the human and macaque thalamus at frequent anteroposterior intervals, additional studies will be needed to clarify the precise contribution of dopamine to the PF monoamine innervation in primate species.
In addition to dopamine immunohistochemistry, we also used tract tracing studies to determine if midbrain dopamine neurons project to the PF. Iontophoretic deposits of the anterograde tracer BDA into the SN revealed a dense innervation of the PC and CL and a moderate innervation of the PF, as has been noted in earlier studies (Beckstead et al., 1979; Tsumori et al., 2002). Our combined retrograde tracer-TH immunohistochemistry study did not find any dopamine neurons in the midbrain that were retrogradely labeled from the PF, suggesting that the moderately dense innervation of the PF from the SNpr corresponds to the well-characterized GABAergic nigrothalamic projection (Tsumori et al., 2002). Taken together, our anatomical studies suggest that there is not a significant dopamine innervation of the PF in the rat.
3.2. 6-OHDA lesions of midbrain dopamine neurons and neuronal degeneration in PF
Freyaldenhoven et al. (1997) reported that acute administration of the dopaminergic neurotoxin N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) to mice maintained at a low ambient temperature (6° C) resulted in degeneration of CeM, PC, and CL neurons, as reflected by expression of the marker FluoroJade; MPTP administered to mice maintained at ambient temperature (23 °C) had no effect on PF neurons. It is difficult to interpret the physiological relevance of this observation in light of the dependence of thalamic degeneration on ambient temperature and the high dose of MPTP used (50 mg/kg, ip).
We also used FluoroJade staining to determine if 6-OHDA SN lesions elicit degeneration of PF neurons, examining animals across a wide range of post-lesion survival intervals to capture neurons accumulating FluoroJade C, the latest iteration of FluoroJade, with better signal-to-background ratio and sensitivity than previous FluoroJade compounds (see Schmued and Hopkins, 2000; Schmued et al., 1997, 2005). At no time point between 2 days and 42 days after the lesion did we observe FJC-stained cell bodies in the PF, although at the earliest time point we did see a large number of FJC-positive cells in the SNpc. While we did not observe any FJC-positive perikarya in the PF, at 4 days post-lesion we did see what appeared to be sparse axonal FJC labeling in the PF and CL; we surmise that these degenerating axon profiles in the PF may represent some minor non-specific damage to nigrothalamic neurons in the SNpr caused by the 6-OHDA infusion. The failure to see FJC accumulation in PF neurons after 6-OHDA lesions of the SN suggests that nigrostriatal dopamine neuron loss does not cause direct monosynaptic or transsynaptic degeneration of PF neurons.
However, although FluoroJade labels neurons that degenerate in response to a variety of insults, including excitotoxic events (Schmued et al., 2005), apoptotic cell loss is not detected using this method (Schmued et al., 1997). Given this limitation of FJC, we determined the number of neurons present in the ipsilateral PF after 6-OHDA lesions of the SN. These cell counts were done six weeks after the 6-OHDA lesion, which encompassed the time at which Sedaghat et al. (2009) reported that 6-OHDA lesions of the MFB caused a loss of Nissl-stained PF cells. We failed to detect any decrease in the total number of cresyl violet-stained PF neurons in response to 6-OHDA SN lesions.
3.3. Effects of lesions of brainstem-derived catecholaminergic axons on the number of PF neurons projecting to the striatum
Although nigrostriatal dopamine depletion does not cause a loss of PF neurons, it may nonetheless have a functional impact on PF neurons. Aymerich et al. (2006) reported that unilateral 6-OHDA lesions of the MFB decreased the number of retrogradely-labeled PF cells seen after tracer deposit into the dopamine-denervated striatum. We therefore compared the effects of unilateral 6-OHDA lesions of the SN, MFB, or DNAB on the number of ipsilateral PF cells labeled after Fluor-oGold injections of the dorsolateral striatum. An ANOVA comparing the number of PF neurons retrogradely-labeled from the striatum in animals with sham lesions relative to rats with 6-OHDA lesions of the SN, MFB, or DNAB did not reveal a significant lesion effect (F3,28 =1.836, NS). We did not observe a decrease in the number of FG-positive PF cells ipsilateral to the 6-OHDA MFB lesion relative to the (intact) contralateral side.
One possible explanation for our failure to observe any loss of retrogradely-labeled PF cells after 6-OHDA MFB lesions while Aymerich et al. (2006) found a significant decrease is that we infused a smaller volume of 6-OHDA (1.5 μL) than did Aymerich and associates (4.0 μL). However, our MFB lesion resulted in essentially complete striatal dopamine denervation, suggesting that our lesions were as effective as those of Aymerich and colleagues in disrupting forebrain dopamine innervations. Moreover, Henderson et al. (2005) also found that large (4.0 μL) injections of 6-OHDA into the MFB do not alter the number of PF neurons. Thus, both our data and those of Henderson and colleagues suggest that disruption of ascending dopaminergic projections, either at the level of the SN or at the mid-diencephalic level where 6-OHDA was infused into the MFB, does not cause a loss of PF thalamostriatal neurons.
Because Aymerich et al. did not indicate if animals were pretreated with a norepinephrine transporter antagonist to prevent accumulation of 6-OHDA by noradrenergic axons in the MFB, we reasoned that noradrenergic denervation of the PF might result in a decreased number of retrogradely-labeled PF cells. We therefore performed 6-OHDA lesions of the dorsal noradrenergic bundle at a level caudal to the SN, resulting in forebrain noradrenergic loss without concomitant dopaminergic involvement. These lesions decreased substantially the density of noradrenergic (DBH-ir) axons in the PF, although residual noradrenergic axons could be clearly seen (see Supplemental Fig. 2). The overall ANOVA did not reveal a significant lesion effect, and we were therefore precluded from making specific statistical determinations of the effects of any of the three lesion groups separately. However, it subjectively appeared that the DNAB lesion tended to decrease the number of ipsilateral PF neurons, although there was considerable variability in the DNAB-lesioned data. We therefore conducted a power analysis and found that we would need to use 29 animals per group to achieve 80% power to detect an effect significant at the .05 alpha level. One interpretation of these data is that lesions of noradrenergic axons during the 6-OHDA lesions of the MFB do not contribute to the loss of retrogradely-labeled PF cells reported by Aymerich et al. (2006). Alternatively, complete thalamic noradrenergic denervation may be required to elicit PF cell loss; future studies using DBH knockout mice may help in ruling out some NE contribution.
3.4. Functional changes in PF neurons and dopamine denervation
We focused our efforts on uncovering evidence of degeneration of PF neurons in response to neurotoxic lesions disrupting the forebrain dopamine innervations. We did not observe ongoing degeneration of PF neurons as reflected by FJC accumulation, nor did we uncover a decrease in the number of PF neurons after 6-OHDA lesions. However, it is possible that the function of PF neurons is compromised by dopamine depletion, although they retain their structural integrity. For example, Parr-Brownlie et al. (2009) found that although the firing rate of PF neurons was unchanged in response to 6-OHDA MFB lesions, the number of spontaneously active PF cells was decreased in response to 6-OHDA MFB lesions at 1 to 2 weeks postoperatively. Other groups have reported time dependent changes in the firing rate of PF neurons after nigrostriatal dopamine denervation, with changes observed relatively early after dopamine denervation but then returning to normal within several weeks (Ni et al., 2000; Yan et al., 2008); Yan et al. also noted a rebound to an increase in firing rate at five weeks postoperatively.
Because there is virtually no dopamine innervation of the rat PF, it is not clear how 6-OHDA lesions change the activity of PF neurons. It is possible that changes in the firing rate and pattern of PF neurons seen after dopamine depletion may not reflect a direct dopamine modulation of the PF but may arise in response to changes in GABAergic nigrothalamic neurons. Thus, a number of electrophysiological studies have reported changes in the firing rate and pattern of SNr neurons after 6-OHDA lesions of the SN (Breit et al., 2008; MacLeod et al., 1990; Sanderson et al., 1986; Wang et al., 2010a,b). The transient changes in the firing rate or pattern of PF neurons after disruption of the nigrostriatal dopamine innervation could also reflect complex transsynaptic (striato-fugal) effects on PF neurons (Parr-Brownlie et al., 2009; Tseng, 2009).
3.5. Summary
Our data do not support the hypothesis that lesions of nigrostriatal dopamine neurons result in degeneration or loss of neurons in the thalamic parafascicular nucleus, nor did we find any evidence of a significant dopamine innervation of the rat PF. Postmortem studies find clear evidence of an approximately 40% decrease in the numbers of PF-and CM neurons in idiopathic PD (Henderson et al., 2000). Our studies were conducted in the rat, as were earlier studies suggesting that nigrostriatal dopamine lesions lead to PF cell loss. Our data suggest that the loss of dopamine is not sufficient to cause PF cell loss. However, in light of the more extensive dopaminergic innervation of the PF in primate species, it will be important to assess the PF in primate models of PD.
Neuronal loss in the CM-PF complex in PD is not related to the duration of illness or the severity of disease (Henderson et al., 2000). Alpha-synuclein aggregation in neurons has been advanced as a means of staging progression of PD (Braak et al., 2002, 2003). Synuclein-positive neurites are found in the PF in stage 4 (Rub et al., 2002), after the appearance of synuclein-rich aggregates are seen in the SN (stage 3). However, given the long period during which dopamine loss is ongoing but does not reach the point at which motor symptoms appears, it is not possible to discern with any confidence if dopamine denervation precedes the loss of PF cells.
Recent studies have found that PF neurons, in addition to using glutamate as a transmitter, express certain other proteins, including cerebellin1 (Cbln1; Kusnoor et al., 2010), the leucine-rich repeat-containing G protein coupled receptor 8 (LGR8; Sedaghat et al., 2008), and frizzled5 (Fz5), a wnt receptor (Liu et al., 2008). Interestingly, conditional knockouts of Fz5 lead to loss of PF neurons (Liu et al., 2008), suggesting at least one mechanism independent of dopamine signaling that may contribute to degeneration of PF neurons in PD.
4. Experimental procedures
4.1. Animals
Adult male Sprague–Dawley rats (Harlan; Indianapolis, IN, USA) were group-housed with ad libitum access to food and water, and maintained on a 12 h light–dark cycle with lights on at 6 A.M. All experiments were conducted in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
4.2. Immunohistochemistry
Rats were perfused with phosphate-buffered saline followed by 4% paraformaldehyde in phosphate buffered saline. The brains were removed from the skull, postfixed in 4% paraformaldehyde, and then cryoprotected in 30% sucrose. Coronal 42 μm-thick sections were cut through the brain, and then processed using immunohistochemical methods following our previously-described procedures (Bubser et al., 2000). The following primary antibodies were used: mouse anti-TH (1:1000 or 1:3000; immunofluorescent or immunoperoxidase methods, respectively; ImmunoStar, Inc., Hudson, WI), sheep anti-TH (1:800 or 1:3000; Millipore, Billerica, MA), mouse anti-DBH (1:800 or 1:2000; Millipore), rabbit anti-FluoroGold (1:3000; immunoperoxidase; Millipore), mouse anti-NeuN (1:1000; immunoperoxidase; Millipore), and rabbit anti-dopamine (1:1000; immunoperoxidase; Millipore). In cases in which we examined the distribution of dopamine-ir axons in the thalamus, animals (N=4) were rapidly perfused at a high flow rate with 50 mL of 0.2% NaNO2 in 0.9% NaCl, followed by 400 mL of ice-cold 5% glutaraldehyde in 50 mM sodium acetate (pH 4.0) and 1.0% sodium metabisulfite; the metabisulfite was added to all subsequent steps (see McRae-Degueurce and Geffard, 1986). The brains were then removed and immersed in the same fixative for 1 h at 4 °C before cutting 50 μm-thick coronal sections through the thalamus and midbrain on a vibrating microtome. Sections were then washed extensively in Tris-metabisulfite [1% sodium metabisulfite (MBS) in 50 mM Tris–HCl, pH 7.2], blocked in Tris-MBS containing 0.5% Triton-X-100 and 4% normal horse serum (NHS), and then incubated overnight in the anti-dopamine antibody. This antibody recognizes dopaminergic but not noradrenergic neurons and processes, nor does it recognize trace amines (Chagnaud et al., 1987; Geffard et al., 1984a,b).
The following day, the sections were again washed in Tris-MBS, immersed for 2 h in donkey anti-rabbit serum (1:40 in the blocking solution), and rinsed in TBS. After incubating the sections for 90 min in rabbit peroxidase-anti-peroxidase (1:200 in TBS containing 4% NHS and 0.5% Triton-x-100 and metabisulfite), they were developed in 0.025% diaminobenzidine in TBS containing 1.5% nickel ammonium sulfate and 0.15% cobalt chloride, with 0.0009% H2O2. Sections were then mounted and coverslipped.
4.3. Anterograde labeling of the nigrothalamic projection
Animals were deeply anesthetized with isofluorane and placed in a stereotaxic frame. The anterograde tracer biotinylated dextran amine (BDA; 10,000 MW; Invitrogen, Carlsbad, CA) was iontophoretically deposited into the SN (N=3) through fiber-filled micropipettes (tip diameter 18–25 μm), using a pulsed (7 s on/off) positive current of 5 μA for 15–20 min. The animals were sacrificed 10 days later, and immunohistochemical methods (see above) used to reveal the presence of anterogradely-labeled BDA-positive axons in the thalamus.
4.4. Retrograde tract tracing-immunohistochemistry
In order to determine if there is a dopamine innervation of the PF or CM complex derived from the SN, rats (N=5 for each site) received iontophoretic deposits of the retrograde tracer Fluor-oGold (FG; 3% in 0.1 M cacodylate buffer; Fluorochrome, LLC, Denver, CO) into the lateral PF, using pulsed (7 s on/off) positive current of 1.0 μA, and were sacrificed 10 days later. Sections were then processed to reveal tyrosine hydroxylase in the SN, using a Cy3-congugated donkey anti-rabbit IgG after the primary anti-TH antibody.
4.5. 6-OHDA lesions of the SN, MFB, and DNAB
To lesion dopamine neurons in the SN of rats, we infused 1.5 μL of the catecholaminergic neurotoxin 6-OHDA-HBr (6 μg/μL in 0.02% ascorbic acid) at coordinates AP: −5.2, ML: 2.4, DV: −8.4 over 15 min; a second 1.0 μL injection of the toxin was made at AP: −5.3, ML: 1.5, DV: −8.4 over 10 min. For 6-OHDA lesions of the MFB, 1.5 μL of 6-OHDA-HBr (6 μg/μL free base in ice-cold 0.02% ascorbic acid) was infused at AP: −3.60; ML: +2.0; DV: −8.4. To lesion noradrenergic neurons, 0.75 μL of the 6-OHDA-HBr solution was injected into the dorsal noradrenergic bundle (AP: −6.3, ML: 0.9, DV: −6.3). Control animals received sham surgeries.
4.6. FluoroJade C accumulation
In order to determine if lesions of the nigrostriatal dopamine neurons cause degeneration of PF cells, animals were subjected to unilateral 6-OHDA SN lesions (N=10) or sham lesions (N=3) and sacrificed 2, 4, 7, or 42 days later. FluoroJade C staining was used to reveal degenerating neurons in the thalamus and midbrain, following the protocol of Schmued et al. (2005). The 6-OHDA SN lesions were verified by examining the presence of TH-ir neurons in the SN.
4.7. Retrograde tract tracing and the PF thalamostriatal projection
Lesions of the SN, MFB, or DNAB were performed as described above. Rats receiving 6-OHDA SN lesions were pretreated with the noradrenergic transporter blocker desipramine (20 mg/kg), and 6-OHDA-HBr was infused into the SN, MFB, or DNAB as described above. Approximately one month (26–33 days) later, FG (3% in 0.1 M cacodylate, pH 3.3; Fluorochrome, Denver, CO) was iontophoretically deposited into the dorsolateral striatum (AP: 0.2, ML: ±3.4, DV: −4.2) bilaterally, delivering 15 min pulsed (7 s on/off), +5.0 μA current to FG-loaded fiber-filled pipettes with tip diameters of 20–30 μm. The animals were sacrificed approximately 10 days thereafter. Striatal sections were prepared and then immunohistochemically stained for TH and FG. In the animals with DNAB lesions, immunohistochemistry was used to reveal DBH-ir axons in the PF and forebrain.
One set of thalamic sections from the animals was immunohistochemically processed using a rabbit anti-FG antibody in order to assess changes in the number of PF neurons that were retrogradely-labeled from the striatum. A second adjacent set of thalamic sections was stained with cresyl violet in order to count the number of PF neurons ipsilateral to the 6-OHDA lesion.
4.8. Data analysis
The software program Neurolucida (MBF Bioscience; Williston, VT) was used to chart BDA-ir and DA-ir fibers in the thalamus and TH-ir/FG-positive neurons in the SN.
Retrogradely-labeled (FG-ir) thalamostriatal neurons were charted using Neurolucida (MBF Bioscience). A t-test was used to compare the number of FG neurons in the thalamus ipsilateral to the 6-OHDA MFB lesion relative to the contralateral hemisphere. A one-way ANOVA was used to compare differences in the numbers of FG neurons ipsilateral to the lesion in 6-OHDA MFB (N=7), SN (N=6), and DNAB-lesioned animals (N=9) relative to the number of retrogradely-labeled PF cells in sham-operated control animals (N=10).
The number of neurons in the PF of sham-lesioned animals (N=8) and animals receiving 6-OHDA lesions of the SN (N=4), MFB (N=7), and DNAB (N=9) was determined by staining sections through the thalamus with cresyl violet, which were then counted using the optical dissector method, using the software program Stereo Investigator (MBF Bioscience). Changes in the number of Nissl-stained neurons were analyzed using a one-way ANOVA.
Supplementary Material
Acknowledgments
We are indebted to the National Parkinson Foundation (NPF) for support of this work, and to NINDS F31 NS061528 (SVK). In addition, we acknowledge NICHD Grant P30 HD15052 to the Vanderbilt Kennedy Center for Research on Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NPF or of the NIH or NINDS.
Abbreviations
- 6-OHDA
6-hydroxydopamine
- BDA
biotinylated dextran amine
- CeM
central medial thalamic nucleus
- CL
central lateral thalamic nucleus
- CM
centromedian thalamic nucleus
- DAT
dopamine transporter
- DBH
dopamine β-hydroxylase
- FG
FluoroGold
- FJC
FluoroJade C
- MD
mediodorsal thalamic nucleus
- MFB
median forebrain bundle
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NHS
normal horse serum
- PC
paracentral thalamic nucleus
- PD
Parkinson’s Disease
- PF
parafascicular nucleus
- SN
substantia nigra
- SNpc
substantia nigra, pars compacta
- SNpr
substantia nigra, pars reticulata
- TBS
Tris-buffered saline
- TH
tyrosine hydroxylase
- VGluT2
vesicular glutamate transporter 2
- VTA
ventral tegmental area
Footnotes
Supplementary materials related to this article can be found online at doi:10.1016/j.brainres.2012.01.040.
References
- Aymerich MS, Barroso-Chinea P, Perez-Manso M, Munoz-Patino AM, Moreno-Igoa M, Gonzalez-Hernandez T, Lanciego JL. Consequences of unilateral nigrostriatal denervation on the thalamostriatal pathway in rats. Eur J Neurosci. 2006;23:2099–2108. doi: 10.1111/j.1460-9568.2006.04741.x. [DOI] [PubMed] [Google Scholar]
- Bacci JJ, Kachidian P, Kerkerian-Le Goff L, Salin P. Intralaminar thalamic nuclei lesions: widespread impact on dopamine denervation-mediated cellular defects in the rat basal ganglia. J Neuropathol Exp Neurol. 2004;63:20–31. doi: 10.1093/jnen/63.1.20. [DOI] [PubMed] [Google Scholar]
- Beckstead RM, Domesick VB, Nauta WJH. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res. 1979;175:191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Groenewegen HJ. Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. J Comp Neurol. 1990;299:187–228. doi: 10.1002/cne.902990206. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rüb U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages) J Neurol. 2002;249(Suppl. 3):III/1–III/5. doi: 10.1007/s00415-002-1301-4. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Breit S, Martin A, Lessmann L, Cerkez D, Gasser T, Schulz J. Bilateral changes in neuronal activity of the basal ganglia in the unilateral 6-hydroxydopamine rat model. J Neurosci Res. 2008;86:1388–1396. doi: 10.1002/jnr.21588. [DOI] [PubMed] [Google Scholar]
- Bubser M, Scruggs JL, Young CD, Deutch AY. The distribution and origin of the calretinin-containing innervation of the nucleus accumbens of the rat. Eur J Neurosci. 2000;12:1591–1598. doi: 10.1046/j.1460-9568.2000.00052.x. [DOI] [PubMed] [Google Scholar]
- Cebrián C, Prensa L. Basal ganglia and thalamic input from neurons located within the ventral tier cell cluster region of the substantia nigra pars compacta in the rat. J Comp Neurol. 2010;518:1283–1300. doi: 10.1002/cne.22275. [DOI] [PubMed] [Google Scholar]
- Chagnaud JL, Mons N, Tuffet S, Grandier-Vazeilles X, Geffard M. Monoclonal antibodies against glutaraldehyde-conjugated dopamine. J Neurochem. 1987;49:487–494. doi: 10.1111/j.1471-4159.1987.tb02890.x. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Heilman C, Demchyshyn LL, Pristupa ZB, Ince E, Hersch SM, Niznik HB, Levey AI. The dopamine transporter: immunochemical characterization and localization in brain. J Neurosci. 1995;15:1714–1723. doi: 10.1523/JNEUROSCI.15-03-01714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschênes M, Bourassa J, Parent A. Two different types of thalamic fibers innervate the rat striatum. Brain Res. 1995;701:288–292. doi: 10.1016/0006-8993(95)01124-3. [DOI] [PubMed] [Google Scholar]
- Dubé L, Smith AD, Bolam JP. Identification of synaptic terminals of thalamic or cortical origin in contact with distinct medium-size spiny neurons in the rat neostriatum. J Comp Neurol. 1988;267:455–471. doi: 10.1002/cne.902670402. [DOI] [PubMed] [Google Scholar]
- François C, Tande D, Yelnik J, Hirsch EC. Distribution and morphology of nigral axons projecting to the thalamus in primates. J Comp Neurol. 2002;447:249–260. doi: 10.1002/cne.10227. [DOI] [PubMed] [Google Scholar]
- Freeman A, Ciliax B, Bakay R, Daley J, Miller RD, Keating G, Levey A, Rye D. Nigrostriatal collaterals to thalamus degenerate in parkinsonian animal models. Ann Neurol. 2001;50:321–329. doi: 10.1002/ana.1119. [DOI] [PubMed] [Google Scholar]
- Freyaldenhoven TE, Ali SF, Schmued LC. Systemic administration of MPTP induces thalamic neuronal degeneration in mice. Brain Res. 1997;759:9–17. doi: 10.1016/s0006-8993(97)00045-0. [DOI] [PubMed] [Google Scholar]
- García-Cabezas MA, Rico B, Sánchez-González MA, Cavada C. Distribution of the dopamine innervation in the macaque and human thalamus. NeuroImage. 2007;34:965–984. doi: 10.1016/j.neuroimage.2006.07.032. [DOI] [PubMed] [Google Scholar]
- García-Cabezas MA, Martínez-Sánchez P, Sánchez-González MA, Garzón M, Cavada C. Dopamine innervation in the thalamus: monkey versus rat. Cereb Cortex. 2009;19:424–434. doi: 10.1093/cercor/bhn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffard M, Buijs RM, Seguela P, Pool CW, Le Moal M. First demonstration of highly specific and sensitive antibodies against dopamine. Brain Res. 1984a;294:161–165. doi: 10.1016/0006-8993(84)91323-4. [DOI] [PubMed] [Google Scholar]
- Geffard M, Kah O, Onteniente B, Seguela P, Le Moal M, Delaage M. Antibodies to dopamine: radioimmunological study of specificity in relation to immunocytochemistry. J Neurochem. 1984b;42:1593–1599. doi: 10.1111/j.1471-4159.1984.tb12747.x. [DOI] [PubMed] [Google Scholar]
- Geffard M, Henrich-Rock AM, Dulluc J, Seguela P. Antisera against small neurotransmitter-like molecules. Neurochem Int. 1985;7:403–413. doi: 10.1016/0197-0186(85)90162-7. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ. Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience. 1988;24:379–431. doi: 10.1016/0306-4522(88)90339-9. [DOI] [PubMed] [Google Scholar]
- Halliday G, Lees A, Stern M. Milestones in Parkinson’s Disease—clinical and pathological features. Mov Disord. 2011;26:1015–1021. doi: 10.1002/mds.23669. [DOI] [PubMed] [Google Scholar]
- Henderson JM, Carpenter K, Cartwright H, Halliday GM. Degeneration of the centre median-parafascicular complex in Parkinson’s disease. Ann Neurol. 2000;47:345–352. [PubMed] [Google Scholar]
- Henderson JM, Schleimer SB, Allbutt H, Dabholkar V, Abela D, Jovic J, Quinlivan M. Behavioural effects of parafascicular thalamic lesions in an animal model of parkinsonism. Behav Brain Res. 2005;162:222–232. doi: 10.1016/j.bbr.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Kusnoor SV, Parris J, Muly EC, Morgan JI, Deutch AY. Extracerebellar role for Cerebellin1: modulation of dendritic spine density and synapses in striatal medium spiny neurons. J Comp Neurol. 2010;518:2525–2537. doi: 10.1002/cne.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciego JL, Rodríguez-Oroz MC, Blesa FJ, Alvarez-Erviti L, Guridi J, Barroso-Chinea P, Smith Y, Obeso JA. Lesion of the centromedian thalamic nucleus in MPTP-treated monkeys. Mov Disord. 2008;23:708–715. doi: 10.1002/mds.21906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapper SR, Bolam JP. Input from the frontal cortex and the parafascicular nucleus to cholinergic interneurons in the dorsal striatum of the rat. Neuroscience. 1992;51:533–545. doi: 10.1016/0306-4522(92)90293-b. [DOI] [PubMed] [Google Scholar]
- Liu C, Wang Y, Smallwood PM, Nathans J. An essential role for Frizzled5 in neuronal survival in the parafascicular nucleus of the thalamus. J Neurosci. 2008;28:5641–5653. doi: 10.1523/JNEUROSCI.1056-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod NK, Ryman A, Arbuthnott GW. Electrophysiological properties of nigrothalamic neurons after 6-hydroxydopamine lesions in the rat. Neuroscience. 1990;38:447–456. doi: 10.1016/0306-4522(90)90041-2. [DOI] [PubMed] [Google Scholar]
- McRae-Degueurce A, Geffard M. One perfusion mixture for immunocytochemical detection of noradrenaline, dopamine, serotonin and acetylcholine in the same rat brain. Brain Res. 1986;376:217–219. doi: 10.1016/0006-8993(86)90922-4. [DOI] [PubMed] [Google Scholar]
- Ni ZG, Gao DM, Benabid AL, Benazzouz A. Unilateral lesion of the nigrostriatal pathway induces a transient decrease of firing rate with no change in the firing pattern of neurons of the parafascicular nucleus in the rat. Neuroscience. 2000;101:993–999. doi: 10.1016/s0306-4522(00)00337-7. [DOI] [PubMed] [Google Scholar]
- Orieux G, François C, Féger J, Yelnik J, Vila M, Ruberg M, Agid Y, Hirsch EC. Metabolic activity of excitatory parafascicular and pedunculopontine inputs to the subthalamic nucleus in a rat model of Parkinson’s disease. Neuroscience. 2000;97:79–88. doi: 10.1016/s0306-4522(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Parr-Brownlie LC, Poloskey SL, Bergstrom DA, Walters JR. Parafascicular thalamic nucleus activity in a rat model of Parkinson’s disease. Exp Neurol. 2009;217:269–281. doi: 10.1016/j.expneurol.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rub U, Del Tredici K, Schultz C, Ghebremedhin E, de Vos RA, Jansen Steur E, Braak H. Parkinson’s disease: the thalamic components of the limbic loop are severely impaired by alpha-synuclein immunopositive inclusion body pathology. Neurobiol Aging. 2002;23:245–254. doi: 10.1016/s0197-4580(01)00269-x. [DOI] [PubMed] [Google Scholar]
- Sánchez-González MA, García-Cabezas MA, Rico B, Cavada C. The primate thalamus is a key target for brain dopamine. J Neurosci. 2005;25:6076–6083. doi: 10.1523/JNEUROSCI.0968-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson P, Mavoungou R, Albe-Fessard D. Changes in substantia nigra pars reticulata activity following lesions of the substantia nigra pars compacta. Neurosci Lett. 1986;67:25–30. doi: 10.1016/0304-3940(86)90202-8. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Albertson C, Slikker W., Jr Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Stowers CC, Scallet AC, Xu L. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res. 2005;1035:24–31. doi: 10.1016/j.brainres.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Sedaghat K, Finkelstein DI, Gundlach AL. Effect of unilateral lesion of the nigrostriatal dopamine pathway on survival and neurochemistry of parafascicular nucleus neurons in the rat—evaluation of time-course and LGR8 expression. Brain Res. 2009;1271:83–94. doi: 10.1016/j.brainres.2009.03.026. [DOI] [PubMed] [Google Scholar]
- Sedaghat K, Shen PJ, Finkelstein DI, Henderson JM, Gundlach AL. Leucine-rich repeat-containing G-protein-coupled receptor 8 in the rat brain: enrichment in thalamic neurons and their efferent projections. Neuroscience. 2008;156:319–333. doi: 10.1016/j.neuroscience.2008.07.029. [DOI] [PubMed] [Google Scholar]
- Séguéla P, Watkins KC, Geffard M, Descarries L. Noradrenaline axon terminals in adult rat neocortex: an immunocytochemical analysis in serial thin sections. Neuroscience. 1990;35:249–264. doi: 10.1016/0306-4522(90)90079-j. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Snyder CL, Lewis DA. Axon terminals immunolabeled for dopamine or tyrosine hydroxylase synapse on GABA-immunoreactive dendrites in rat and monkey cortex. J Comp Neurol. 1995;363:264–280. doi: 10.1002/cne.903630208. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidibé M, Paré JF, Smith Y. Nigral and pallidal inputs to functionally segregated thalamostriatal neurons in the centromedian/parafascicular intralaminar nuclear complex in monkey. J Comp Neurol. 2002;447:286–299. doi: 10.1002/cne.10247. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Williams SM, Szigeti K, Goldman-Rakic PS. Light and electron microscopic characterization of dopamine-immunoreactive axons in human cerebral cortex. J Comp Neurol. 1992;321:325–335. doi: 10.1002/cne.903210302. [DOI] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibé M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27:520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Tseng KY. Facing the lack of anti-phase oscillation in the parafascicular nucleus after dopamine depletion. Exp Neurol. 2009;219:62–65. doi: 10.1016/j.expneurol.2009.05.031. [DOI] [PubMed] [Google Scholar]
- Tsumori T, Yokota S, Ono K, Yasui Y. Synaptic organization of GABAergic projections from the substantia nigra pars reticulata and the reticular thalamic nucleus to the parafascicular thalamic nucleus in the rat. Brain Res. 2002;957:231–241. doi: 10.1016/s0006-8993(02)03554-0. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang QJ, Liu J, Ali U, Gui ZH, Hui YP, Chen L, Wang T. Changes in firing rate and pattern of GABAergic neurons in subregions of the substantia nigra pars reticulata in rat models of Parkinson’s disease. Brain Res. 2010a;1324:54–63. doi: 10.1016/j.brainres.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang QJ, Liu J, Ali U, Gui ZH, Hui YP, Chen L, Wu ZH, Li Q. Noradrenergic lesion of the locus coeruleus increases apomorphine-induced circling behavior and the firing activity of substantia nigra pars reticulata neurons in a rat model of Parkinson’s disease. Brain Res. 2010b;1310:189–199. doi: 10.1016/j.brainres.2009.10.070. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Characterization of the dopaminergic innervation of the primate frontal cortex using a dopamine-specific antibody. Cereb Cortex. 1993;3:199–222. doi: 10.1093/cercor/3.3.199. [DOI] [PubMed] [Google Scholar]
- Yan W, Zhang QJ, Liu J, Wang T, Wang S, Liu X, Chen L, Gui ZH. The neuronal activity of thalamic parafascicular nucleus is conversely regulated by nigrostriatal pathway and pedunculopontine nucleus in the rat. Brain Res. 2008;1240:204–212. doi: 10.1016/j.brainres.2008.09.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


