Abstract
After more than 30 years of iterations of surgical debulking plus chemotherapy, the need for complementary ovarian cancer treatments has become clear. In the ovarian cancer microenvironment, myeloid immunosuppressive leukocytes, lymphocytes, fibroblasts and endothelial cells, as well as their secreted products, surface molecules and paracrine survival factors, all provide opportunities for novel interventions. The potential of targeting microenvironmental elements in ovarian cancer patients is underscored by recently successful anti-angiogenic therapies. The compartmentalized nature of ovarian cancer, its immunogenicity and its accessibility make it an ideal disease for targeting non-tumor host cells. This review discusses the ‘state-of-the-art’ of the field, with an emphasis on the potential of modulating the activity of abundant microenvironmental immune cells, which govern both angiogenesis and immunosuppression.
Keywords: Ovarian cancer, tumor microenvironment, immunotherapy, tumor immunology, dendritic cell, fibroblast, angiogenesis
Ovarian cancer is the fifth most common cancer among women. With over 120,000 women worldwide dying each year from the disease, it has the highest fatality-to-incidence ratio of all gynecologic cancers [1]. In the United States, ovarian cancer causes even more deaths than any other type of female reproductive cancer, melanoma or brain tumors [2]. The major clinical challenge for this disease is that patients are typically presented with symptoms only after the cancer has metastasized, leading most diagnosis to take place at advanced stages [3].
Based on converging genomic and clinical information, the divergent hypothesis emerging now in the field is that only a small fraction of ovarian cancers (designated type I) persist as stable masses for extended periods [4]. In contrast, the type of ovarian carcinomas that are responsible for 90% of deaths (designated type II) [5] could evolve aggressively without an identifiable localized precursor macroscopic mass [4]. Additionally, the lack of effective treatments demands urgent alternative interventions against advanced tumors.
Treatments have evolved very little, and are still primarily restricted to surgical debulking plus cyclic iterations (i.e., IV vs. IP) of untargeted chemotherapies, focused on the tumor cell cycle. Only very recently, complementary interventions targeting elements of the tumor microenvironment (TME) have started undergoing clinical testing. Currently, the only drug in clinical use that targets the TME is bevacizumab, which blocks vascular endothelial growth factor to inhibit angiogenesis [6]. Last December, results from two positive phase III trials illustrated for the first time the potential of targeting the TME through this drug [7,8]. Thus to increase the number of targets within the TME, a rigorous understanding of the ovarian cancer microenvironment is necessary and will open both new avenues for effective therapies.
This review will emphasize the areas that the authors consider to be the most promising targets for the design of new clinical interventions in the near future, which also represent the most critical aspects that are currently moving the field forward; namely, the immunobiology of ovarian tumors and their vascularization, which utilize closely related mechanisms. A detailed overview of all recent papers in the broad area of the ovarian cancer microenvironment is beyond the scope of this review. We acknowledge that the view of other authors could be different, and also that new developments in the field could change this focus.
The ovarian cancer microenvironment
Ovarian cancer is a peculiar disease in that multiple cellular types and molecules become accessible to both primary and metastatic masses typically as they disseminate throughout the peritoneal cavity. For tumor cells, ascites provides an ideal milieu for them to detach and seed distally. Furthermore, crucial microenvironmental differences that define metastatic spreading in other tumors are not necessarily found in ovarian carcinoma. For instance, we found identical leukocyte infiltrates in matching solid metastatic and primary tumors in virtually every patient analyzed, as well as comparable extracellular structures and cell types [9–11]. In contrast, metastatic masses in most cancers are very different from the primary tumor. However, the inflammatory component of matching ascites and dissociated solid tumors is typically very different in patient samples. Ascites contains a predominant population of canonical CD45+CD14+CD11b+ macrophages, as well as CD31−CD45−FAP− tumor and CD31+CD45− endothelial cells. It also includes an abundant mix of T cells, including Th1, Treg, CD8+ and, to a lesser extent, Th17 lymphocytes. However, unlike other tumors [12], very little Th2 cells are found in the ovarian cancer microenvironment. The same cell types are also represented in corresponding solid tumors, but FAP+ fibroblasts are obviously much more abundant, and the inflammatory microenvironment is much more complex. Thus, solid tumors mobilize a heterogeneous population of myeloid cells different from classical macrophages [13]. Over the last years, we have demonstrated that the most abundant leukocyte subset in solid tumors expresses determinants of bona fide DCs, including CD11c, DEC205, CD86 and relatively high levels of MHC-II [9,10,14–18]. In at least a third of specimens, these cells lack the expression of the macrophage marker CD11b. In other patients, conclusive categorization is more complicated by phenotypic overlap with macrophages and myeloid-derived suppressor cells (MDSCs) [19]. However, irrespective of nomenclature, these cells respond to immunostimulatory signals by up-regulating co-stimulatory molecules and, at least in mouse models, by up-taking, processing and presenting antigens [10,11,16]. Most importantly, these leukocytes are crucial for both the generation and the maintenance of tumor vasculature in ovarian tumors [9]. In addition, they secrete growth factors and proteases that promote tumor growth. As we will discuss later, they are also critical promoters of immunosuppression [9–11,16,17,20–25]. Correspondingly, their depletion in preclinical models delays tumor progression and boosts anti-tumor immunity [9], underscoring the potential of targeting this major microenvironmental compartment.
Accumulating evidence suggests that chronic inflammation in ovarian cancer plays a role in the development of the disease [26–28]. Ovulation induces insult to the ovarian surface, which triggers an influx of leukocytes to facilitate repair. Over time, the process of continuous damage and repair of the epithelial cells (‘the incessant ovulation hypothesis’) increases the chances of genetic error [29]. The initial inflammatory trigger, in addition to other environmental and genetic cues, is thought to create the foundation for chronic inflammation in ovarian cancer. This has been demonstrated in ovarian epithelial cells, where induced inflammation controlled the production of keratinocyte chemoattractant (KC/IL-8) and growth regulated oncogene (GRO1/2) with a slightly lower induction of CCL20, IP-10 and CCL7, which were collectively involved in the recruitment of inflammatory neutrophils, lymphocytes and dendritic cells [26]. Furthermore, the expression of IL-6, TNF and CXCR4 have been found in high-grade serous tumors, where play a crucial role in promoting angiogenesis and the recruitment of myeloid infiltrates [30].
Exploiting spontaneous anti-tumor immunity in the TME as a therapeutic goal
Not all microenvironmental leukocytes, however, promote tumor progression. Among all non-tumor cells in the ovarian cancer microenvironment, T lymphocytes represent the only element that spontaneously exerts confirmed clinically relevant, although obviously non-curative, immune pressure against disease advancement [31–33]. Direct recognition of specific tumor antigens by these cells has now been conclusively demonstrated by independent groups, and their infiltration patterns clearly predict the patient’s outcome. For instance, in multiple independent studies, the narrow set of long-term (>10y) survivors, consistently shows significantly stronger T cell infiltrates in their tumor samples [31,34,35]. Whether their protective effect can be attributed to both CD4+ and CD8+ subsets [31,36], or only to the latter [34,35], is debatable, because CD4+ T cells include a subset of Treg that clearly promotes immunosuppression and is associated with accelerated cancer progression [37]. Nevertheless, it is clear that boosting T cell-mediated protective activity represents a major opportunity for the design of novel therapeutic interventions against this devastating disease. Dendritic cell-based vaccines, however, have shown limited clinical success, due to the difficulty to overcome tumor-induced immunosuppression. Nevertheless, enhanced immunogenicity was demonstrated in two independent studies upon vaccination with either NY-ESO-1b or a heptavalent-keyhole limpet hemocyanin (KLH) construct [38] [39], highlighting the potential for an ovarian cancer vaccine.
Currently, the most promising immunotherapy is based on engineering T cells to express chimeric receptors targeting specific tumor surface markers. This approach has produced impressive clinical results against other tumors. Among multiple candidates for targeting on the surface of ovarian cancer cells, mesothelin appears to be a relatively safe goal, for which clinical reagents are available [40–42]. Other obvious possibilities include Her2/neu and MUC1, which are shared by other tumors. Recognizing the tremendous potential of this approach, however, our view is that engineering chimeric T cells involves a high degree of sophisticated expertise, patient-specific preparations and technological resources. It is therefore unlikely to generate broad pharmaceutical interest. Consequently, it is likely that these promising (although also complicated and expensive) procedures will be limited to select institutions in the future. Based on previous T cell-based approaches, it is also possible that adoptive transfer of chimeric T cells will work in some patients and not in others.
Immunosuppressive elements of the TME as novel therapeutic targets
In contrast to T cells, virtually any other leukocyte in the TME contributes to tumor growth and, paradoxically, immunosuppression. The most abundant hematopoietic subset myeloid leukocytes with attributes of dendritic cells (FIGURE 1) are particularly immunosuppressive in solid tumors. From their perivascular location, these cells abrogate the activity of anti-tumor T cells extravasating from blood vessels. Their suppressive activity involves multiple complementary mechanisms that include the production of Arginase (FIGURE 1) and the expression of various surface immunosuppressive ligands [9,10,16,21]. Therefore, any vaccination or T cell-based strategy will likely require concurrent targeting of this abrasive compartment for success.
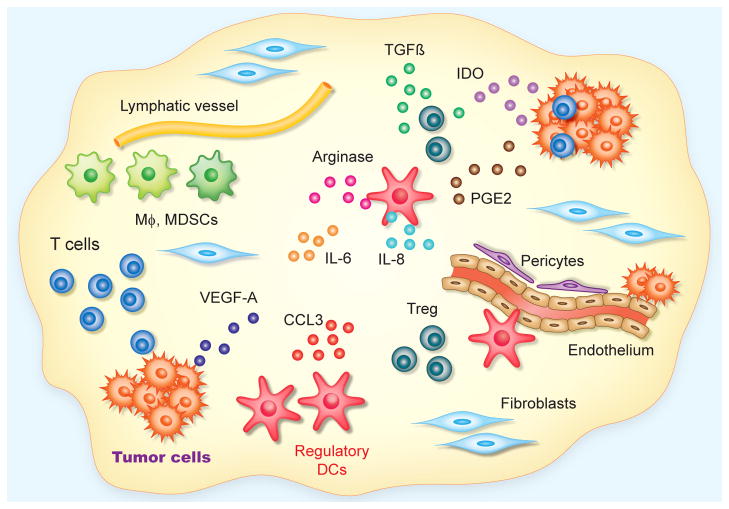
Figure 1. Targetable elements in the ovarian cancer microenvironment.
In solid ovarian tumors, infiltration of tumor islets by anti-tumor T cells is associated with improved outcomes. The protective function of T cells is eventually abrogated, among other factors, by signals produced by tumor cells (e.g., TGFβ, PGE2 and IDO); by Treg; and by a very abundant and heterogeneous population of immunosuppressive/proangiogenic myeloid leukocytes with predominant attributes of dendritic cells (through Arginase production and up-regulation of a variety of inhibitory surface molecules). Fibroblasts and collagen constitute the main elements of the stroma, in which anarchically distributed vessels, covered by few pericytes, provide nutrients for tumor growth. Abundant inflammatory cytokines (e.g., IL-6, IL-8 or CCL3), primarily produced by infiltrating leukocytes, also impact the functions of other microenvironmental compartments. Finally, fibroblasts inhibit anti-tumor immunity through unknown mechanisms.
The crucial role of myeloid leukocytes in ovarian cancer progression has been recently illustrated by our studies in a preclinical model of sarcomatoid carcinoma in immunocompetent and previously healthy hosts [11]. As in patients, we detected measurable anti-tumor immunity, which was initiated by dendritic cells at very early stages. Notably, these responses were able to put tumors in check for relatively prolonged periods. However after this latency period, tumors started to grow very rapidly. We found that the key to this switch in progression is a gradual phenotypic and numerical change in tumor-infiltrating dendritic cells, whereby they are transformed from an immunostimulatory to an immunosuppressive cell type. Correspondingly, depleting dendritic cells at early stages accelerates tumor initiation, but at later stages prevents exponential growth, in the absence of any direct targeting of tumor cells. Promotion of immunostimulatory dendritic cells at the macroscopic stage may therefore improve patient outcomes. Our results hence support that microenvironmental myeloid leukocytes drive both inhibition of tumor growth (first) and aggressive malignant expansion (later). These data also support that the time ovarian tumors take to progress from macroscopically detectable tumors to terminal disease is very short, which may hinder the implementation of effective early diagnostic strategies. Of course the applicability of this model to the human disease can be only assumed, but it is important to emphasize that the inflammatory microenvironment of advanced tumors in our system faithfully recapitulated the molecular and cellular components of leukocytes in human solid tumors [11].
Although the effectiveness of this approach need to be tested in ovarian cancer patients, the potential of targeting immunosuppression in cancer patients is best illustrated by the recent success of antibodies blocking common checkpoints in T cells, such as CTLA4 and, especially, PD-1 [43]. Regulatory myeloid leukocytes in ovarian cancer utilize both mechanisms, and many tumor antigens have been identified in advanced ovarian tumor cells. Tumor-associated myeloid leukocytes therefore emerge as promising direct or indirect targets to unleash the spontaneous activity of anti-tumor T cells and induce the regression of established tumors.
In situ re-programming tumor-associated leukocytes
Despite its grim prognosis, ovarian cancer offers significant advantages for the design of interventions targeting the TME, and in particular tumor-associated leukocytes: Firstly, even at a metastatic stage, ovarian cancer is most frequently restricted to the peritoneal cavity, so that treatments do not need to be systemically administered. Secondly, its peritoneal nature also makes ovarian tumors accessible, so that therapies can be directly administered where the targets are. Thirdly, pro-angiogenic/immunosuppressive myeloid leukocytes are extremely abundant and predominantly accumulate at the growing edge of tumor masses. They also show enhanced endocytic activity [9,16], therefore they are ideal targets for selective take-up of particulate nanomaterials delivered intraperitoneally. Taking advantage of these peculiarities, we have successfully depleted dendritic cells from ovarian cancer locations, which results in significant therapeutic effects in the absence of any direct targeting of tumor cells [9]. This could be clinically achieved using immunotoxins or various nanoparticles that are spontaneously and rabidly engulfed by these cells in vivo, resulting in significant immunogenic effects [16,17].
Most importantly, as tumor-associated myeloid leukocytes spontaneously take up tumor materials [10], promoting their capacity to present these antigens in vivo [14] represents a major opportunity for complementary interventions. We have demonstrated both the feasibility and the potential of this strategy through multiple approaches in preclinical models. For instance, we have shown that CD40 and Toll-Like Receptor (TLR) agonists synergize to transforms tumor-associated dendritic cells from an immunosuppressive to an immunostimulatory cell type when administered intraperitoneally. Because both agonists have been tested in clinical trials against different tumors [44,45], and synergistic activity has been demonstrated in various settings [46,47], combinatorial testing in ovarian cancer patients is only a matter of industrial interest.
Alternatively, the enhanced endocytic activity of these phagocytes can be exploited to selectively deliver, in vivo and in situ, polyplexes in the nanometer range carrying functional oligonucleotides [16,48]. Although excellent groups are trying to optimize the delivery of functional siRNA to ovarian cancer cells [49], preventing phagocytic uptake in vivo in the peritoneal cavity has been extremely challenging in our hands. In contrast, nanocomplexes made of biocompatible polymers and stabilized double-stranded RNA, are avidly engulfed by immunosuppressive phagocytes at tumor locations, without any targeting motif. These nanocomplexes, besides silencing their targeted mRNAs, activate multiple TLRs, thus promoting the immunostimulatory potential of otherwise tolerogenic myeloid leukocytes. In addition, longer, Dicer-mediated cleavage-dependent oligonucleotides mimicking the sequence and the structure of endogenous miRNAs can be used to recapitulate the broad range of silencing activities of endogenous immunoactivating miRNAs [48].
Finally, the pathways driving the tolerization of initially immunocompetent dendritic cells in the TME can be locally blocked with neutralizing antibodies or antagonists. Two signals that appear to be critical for the phenotypic switch in leukocytes that drives aggressive malignant expansion in ovarian cancer are TGFβ and PGE2 [11], for which reagents (e.g., COX2 inhibitors) are available.
Targeting the cross-talk between immune and non-immune host cells in the TME
Besides immune cells and angiogenic cytokines, the TME obviously provides many other compartments for therapeutic interventions. Importantly, intervening on a particular element may dramatically affect other microenvironmental events. For instance, overexpression of endothelin-B in tumor endothelium has been reported to prevent T-cell adhesion and subsequent homing to tumors [50]. Targeting tumor vasculature, therefore, could also have a profound impact on anti-tumor immunity. Not mutually exclusive, targeting immune cells that are crucial for neovascularization [14], could in turn abrogate angiogenesis, which is intimately associated with immunosuppression [51].
Finally, another prime opportunity to interrupt synergistic interactions between different cellular compartments in the TME is by targeting (as an individual or combinatorial intervention), cancer-associated fibroblasts (CAFs). CAFs provide structural and secretory support for tumor growth and dissemination (FIGURE 1), and therefore are targets on their own right. However, recent evidence indicates that depletion of FAP+ cells, primarily expressed by CAFs, results in immunological control of established non-ovarian tumors of different histological origins [52]. Tumor regression appears to be mediated by TNF-α and INF-γ, which are primarily produced by immune cells. Therefore, CAFs also dampen anti-tumor immunity by abrogating the activity of INF-γ–producing immune cells (primarily lymphocytes). The precise pathways whereby CAFs impair the protective activity of microenvironmental T cells remain completely unknown, but are the subject of intense investigation in various labs. Most importantly, FAP is a relatively specific surface marker in CAFs, as normal fibroblasts only express marginal levels. Therefore, FAP can be targeted with antibodies, vaccines and even chimeric T cells.
Expert commentary
A better understanding of the interactions between tumor and non-tumor host cells, extracellular matrix and secreted molecules is revolutionizing our general views on tumor initiation and malignant expansion. Tumors, including ovarian cancer, are now seen as organ-like structures where a dynamic cross-talk between tumor and the predominant non-tumor compartments is required for progression. However, preclinical optimization of targeting elements of the TME is very challenging in terms of high-throughput screening. Unlike classical screening approaches for drugs targeting tumor cells, testing the effect of interventions on the TME requires in vivo models that recapitulate the human disease, because the interactions between these complex networks affect multiple compartments that cannot be frequently mimicked in a Petri dish. The recent availability of oncogene-driven genetic models of cancer is opening the field for the design of alternative interventions against multiple tumors, including ovarian cancer.
Five year view
After more than 40 years of therapeutic approaches restricted to eliminate tumor cells, the need for new complementary therapeutic targets has become urgent. The ovarian cancer microenvironment offers many cell types and molecular elements for mutually exclusive interventions (FIGURE 1). Although the field is only in its infancy, emerging results from targeting vascular compartments illustrate the potential of targeting the TME. Myeloid leukocytes, lymphocytes, fibroblasts and endothelial cells, as well as their secreted products, surface molecules and paracrine survival factors, provide a fertile ground for novel therapies. The compartmentalized nature of ovarian cancer and the accessibility of its microenvironment offer extra openings for future clinical testing. We envision that the next 5 years will see a consolidation of several anti-angiogenic drugs as first-line therapies. Available immunostimulatory drugs (such as combined CD40 and TLR agonists), alone or in combination with antibodies blocking crucial tolerogenic pathways (e.g., PD1), should be incorporated to the therapeutic arsenal in the near future. In addition, the adoptive transfer of tumor-reactive T cells is expected to produce the most impressive clinical results in selected patients. However, our view is that it is unlikely that engineered T cells will be routinely applied outside specialized institutions. We finally anticipate that an area of accelerated achievements in the next years will be the identification of molecular and immune signatures that predict big cohorts of patients that can benefit from specific treatments (e.g., immunotherapy). Strides towards personalized medicine and microenvironmental targeting should thus progressively reverse the dismaying prognosis of this terrible disease.
Key issues.
Last December, results from 2 positive phase III trials illustrated for the first time the potential of targeting the ovarian cancer microenvironment with bevacizumab
The compartmentalized nature of ovarian cancer, its immunogenicity, abundance of inflammatory cells, aggressiveness and the accessibility of its microenvironment make it an ideal disease for new microenvironmental interventions and offer extra opportunities for future clinical testing
T cells engineered to express chimeric receptors targeting specific tumor markers should produce impressive clinical results in a particular group of patients treated at selected institutions in the near future. However, routine implementation of this approach may be limited to a few hospitals with the required technical expertise and sophisticated facilities
In contrast, immunostimulatory adjuvants such as CD40 and TLR agonists are already approved for clinical testing and could synergize in vivo at activating ovarian cancer microenvironmental leukocytes
Similarly, antibody-based neutralization of common immunosuppressive pathways in the TME (primarily, PD1), may prove successful in current clinical testing
Combinatorial targeting of different compartments of the TME, including endothelial cells, myeloid leukocytes and fibroblasts, is expected to result in synergistic effects by breaking their cross-talk
Molecular signatures that predict sets of patients to benefit from specific treatments targeting the TME (thus advancing towards the goal of personalized medicine) are expected to revolutionize the future management of ovarian cancer
Acknowledgments
This study was supported by NCI Grants CA157664 and CA124515, and by DoD grant OC100059. UKS was supported by the National Research Service Award F31CA134188.
Footnotes
FINANCIAL DISCLOSURES
The authors declare no conflict of interest.
References
- 1.Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12(1):20–37. doi: 10.1634/theoncologist.12-1-20. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Yancik R. Ovarian cancer. Age contrasts in incidence, histology, disease stage at diagnosis, and mortality. Cancer. 1993;71(2 Suppl):517–523. doi: 10.1002/cncr.2820710205. [DOI] [PubMed] [Google Scholar]
- 4.Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34(3):433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurman RJ, Shih Ie M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol. 2011;42(7):918–931. doi: 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Presta LG, Chen H, O’Connor SJ, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57(20):4593–4599. [PubMed] [Google Scholar]
- 7**.Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473–2483. doi: 10.1056/NEJMoa1104390. The first of two recent trials demonstrating the potential of targeting angiogenesis as a first-line intervention against ovarian cancer. [DOI] [PubMed] [Google Scholar]
- 8*.Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365(26):2484–2496. doi: 10.1056/NEJMoa1103799. The other trial underscoring the effectiveness of bevazuzimab, published in the same issue. [DOI] [PubMed] [Google Scholar]
- 9.Huarte E, Cubillos-Ruiz JR, Nesbeth YC, et al. Depletion of dendritic cells delays ovarian cancer progression by boosting antitumor immunity. Cancer Res. 2008;68(18):7684–7691. doi: 10.1158/0008-5472.CAN-08-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scarlett UK, Cubillos-Ruiz JR, Nesbeth YC, et al. In situ Stimulation of CD40 and Toll-like Receptor 3 Transforms Ovarian Cancer-Infiltrating Dendritic Cells from Immunosuppressive to Immunostimulatory Cells. Cancer Res. 2009;69(18):7329–7337. doi: 10.1158/0008-5472.CAN-09-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11*.Scarlett UK, Rutkowski MR, Rauwerdink AM, et al. Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J Exp Med. 2012;209(3):495–506. doi: 10.1084/jem.20111413. Studies in preclinical models show for the first time how immunosuppressive leukocytes in the TME govern ovarian cancer progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pedroza-Gonzalez A, Xu K, Wu TC, et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J Exp Med. 2011;208(3):479–490. doi: 10.1084/jem.20102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marigo I, Bosio E, Solito S, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 32(6):790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Conejo-Garcia JR, Benencia F, Courreges MC, et al. Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med. 2004;10(9):950–958. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- 15.Conejo-Garcia JR, Buckanovich RJ, Benencia F, et al. Vascular leukocytes contribute to tumor vascularization. Blood. 2005;105(2):679–681. doi: 10.1182/blood-2004-05-1906. [DOI] [PubMed] [Google Scholar]
- 16.Cubillos-Ruiz JR, Engle X, Scarlett UK, et al. Polyethylenimine-based siRNA nanocomplexes reprogram tumor-associated dendritic cells via TLR5 to elicit therapeutic antitumor immunity. J Clin Invest. 2009;119(8):2231–2244. doi: 10.1172/JCI37716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cubillos-Ruiz JR, Fiering S, Conejo-Garcia JR. Nanomolecular targeting of dendritic cells for ovarian cancer therapy. Future Oncol. 2009;5(8):1189–1192. doi: 10.2217/fon.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cubillos-Ruiz JR, Rutkowski M, Conejo-Garcia JR. Blocking ovarian cancer progression by targeting tumor microenvironmental leukocytes. Cell Cycle. 9(2):260–268. doi: 10.4161/cc.9.2.10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells in human cancer. Cancer J. 2010;16(4):348–353. doi: 10.1097/PPO.0b013e3181eb3358. [DOI] [PubMed] [Google Scholar]
- 20.Cubillos-Ruiz JR, Conejo-Garcia JR. It never rains but it pours: Potential role of butyrophilins in inhibiting anti-tumor immune responses. Cell Cycle. 2011;10(3) doi: 10.4161/cc.10.3.14565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cubillos-Ruiz JR, Martinez D, Scarlett UK, et al. CD277 is a Negative Co-stimulatory Molecule Universally Expressed by Ovarian Cancer Microenvironmental Cells. Oncotarget. 2010;1(5):329–328. doi: 10.18632/oncotarget.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cubillos-Ruiz JR, Rutkowski M, Conejo-Garcia JR. Blocking ovarian cancer progression by targeting tumor microenvironmental leukocytes. Cell Cycle. 2010;9(2):260–268. doi: 10.4161/cc.9.2.10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nesbeth Y, Conejo-Garcia JR. Harnessing the effect of adoptively transferred tumor-reactive T cells on endogenous (host-derived) antitumor immunity. Clin Dev Immunol. 2010;2010:139304. doi: 10.1155/2010/139304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nesbeth Y, Scarlett U, Cubillos-Ruiz J, et al. CCL5-mediated endogenous antitumor immunity elicited by adoptively transferred lymphocytes and dendritic cell depletion. Cancer Res. 2009;69(15):6331–6338. doi: 10.1158/0008-5472.CAN-08-4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nesbeth YC, Martinez DG, Toraya S, et al. CD4+ T cells elicit host immune responses to MHC class II- ovarian cancer through CCL5 secretion and CD40-mediated licensing of dendritic cells. J Immunol. 2010;184(10):5654–5662. doi: 10.4049/jimmunol.0903247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Son DS, Parl AK, Rice VM, Khabele D. Keratinocyte chemoattractant (KC)/human growth-regulated oncogene (GRO) chemokines and pro-inflammatory chemokine networks in mouse and human ovarian epithelial cancer cells. Cancer Biol Ther. 2007;6(8):1302–1312. doi: 10.4161/cbt.6.8.4506. [DOI] [PubMed] [Google Scholar]
- 27.Sangaletti S, Tripodo C, Ratti C, et al. Oncogene-driven intrinsic inflammation induces leukocyte production of tumor necrosis factor that critically contributes to mammary carcinogenesis. Cancer Res. 2010;70(20):7764–7775. doi: 10.1158/0008-5472.CAN-10-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burke F, Relf M, Negus R, Balkwill F. A cytokine profile of normal and malignant ovary. Cytokine. 1996;8(7):578–585. doi: 10.1006/cyto.1996.0077. [DOI] [PubMed] [Google Scholar]
- 29.Fleming JS, Beaugie CR, Haviv I, Chenevix-Trench G, Tan OL. Incessant ovulation, inflammation and epithelial ovarian carcinogenesis: revisiting old hypotheses. Mol Cell Endocrinol. 2006;247(1–2):4–21. doi: 10.1016/j.mce.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Kulbe H, Chakravarty P, Leinster DA, et al. A dynamic inflammatory cytokine network in the human ovarian cancer microenvironment. Cancer Res. 2012;72(1):66–75. doi: 10.1158/0008-5472.CAN-11-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31**.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–213. doi: 10.1056/NEJMoa020177. Seminal demonstration of the immunogenicity of ovarian cancer. T cell infiltrating tumor islets are shown for the first time to determine the patient’s outcome. [DOI] [PubMed] [Google Scholar]
- 32.Coukos G, Conejo-Garcia JR, Roden RB, Wu TC. Immunotherapy for gynaecological malignancies. Expert Opin Biol Ther. 2005;5(9):1193–1210. doi: 10.1517/14712598.5.9.1193. [DOI] [PubMed] [Google Scholar]
- 33.Conejo-Garcia JR, Benencia F, Courreges MC, et al. Ovarian carcinoma expresses the NKG2D ligand Letal and promotes the survival and expansion of CD28- antitumor T cells. Cancer Res. 2004;64(6):2175–2182. doi: 10.1158/0008-5472.can-03-2194. [DOI] [PubMed] [Google Scholar]
- 34.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104(9):3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102(51):18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kryczek I, Banerjee M, Cheng P, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114(6):1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37**.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. First conclusive demonstration of the crucial role of Treg in the pathophysiology of any human tumor, and specifically in ovarian cancer. [DOI] [PubMed] [Google Scholar]
- 38.Diefenbach CS, Gnjatic S, Sabbatini P, et al. Safety and immunogenicity study of NY-ESO-1b peptide and montanide ISA-51 vaccination of patients with epithelial ovarian cancer in high-risk first remission. Clin Cancer Res. 2008;14(9):2740–2748. doi: 10.1158/1078-0432.CCR-07-4619. [DOI] [PubMed] [Google Scholar]
- 39.Sabbatini PJ, Ragupathi G, Hood C, et al. Pilot study of a heptavalent vaccine-keyhole limpet hemocyanin conjugate plus QS21 in patients with epithelial ovarian, fallopian tube, or peritoneal cancer. Clin Cancer Res. 2007;13(14):4170–4177. doi: 10.1158/1078-0432.CCR-06-2949. [DOI] [PubMed] [Google Scholar]
- 40.Kelly RJ, Sharon E, Pastan I, Hassan R. Mesothelin-targeted agents in clinical trials and in preclinical development. Mol Cancer Ther. 2012;11(3):517–525. doi: 10.1158/1535-7163.MCT-11-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carpenito C, Milone MC, Hassan R, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106(9):3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tchou J, Wang LC, Selven B, et al. Mesothelin, a novel immunotherapy target for triple negative breast cancer. Breast Cancer Res Treat. 2012;133:799–804. doi: 10.1007/s10549-012-2018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43**.Pardoll D, Drake C. Immunotherapy earns its spot in the ranks of cancer therapy. J Exp Med. 2012;209(2):201–209. doi: 10.1084/jem.20112275. Visionary perspective about the potential of anti-cancer immunotherapies in the near future. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44*.Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331(6024):1612–1616. doi: 10.1126/science.1198443. First conclusive clinical demonstration of the effectiveness of CD40 agonists in a different lethal tumor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grossman SA, Ye X, Piantadosi S, et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res. 2010;16(8):2443–2449. doi: 10.1158/1078-0432.CCR-09-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahonen CL, Wasiuk A, Fuse S, et al. Enhanced efficacy and reduced toxicity of multifactorial adjuvants compared with unitary adjuvants as cancer vaccines. Blood. 2008;111(6):3116–3125. doi: 10.1182/blood-2007-09-114371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahonen CL, Doxsee CL, McGurran SM, et al. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J Exp Med. 2004;199(6):775–784. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48**.Cubillos-Ruiz JR, Baird JR, Tesone AJ, et al. Reprogramming tumor-associated dendritic cells in vivo using microRNA mimetics triggers protective immunity against ovarian cancer. Cancer Res. 2012;72:1683–1693. doi: 10.1158/0008-5472.CAN-11-3160. First optimization and implementation of non-viral miRNA mimetics as a new cancer intervention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nick AM, Stone RL, Armaiz-Pena G, et al. Silencing of p130cas in ovarian carcinoma: a novel mechanism for tumor cell death. J Natl Cancer Inst. 2011;103(21):1596–1612. doi: 10.1093/jnci/djr372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buckanovich RJ, Facciabene A, Kim S, et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14(1):28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- 51.Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol. 2011;11(10):702–711. doi: 10.1038/nri3064. [DOI] [PubMed] [Google Scholar]
- 52.Kraman M, Bambrough PJ, Arnold JN, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330(6005):827–830. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]