Abstract
Objectives
Whole-genome sequencing potentially represents a single, rapid and cost-effective approach to defining resistance mechanisms and predicting phenotype, and strain type, for both clinical and epidemiological purposes. This retrospective study aimed to determine the efficacy of whole genome-based antimicrobial resistance prediction in clinical isolates of Escherichia coli and Klebsiella pneumoniae.
Methods
Seventy-four E. coli and 69 K. pneumoniae bacteraemia isolates from Oxfordshire, UK, were sequenced (Illumina HiSeq 2000). Resistance phenotypes were predicted from genomic sequences using BLASTn-based comparisons of de novo-assembled contigs with a study database of >100 known resistance-associated loci, including plasmid-associated and chromosomal genes. Predictions were made for seven commonly used antimicrobials: amoxicillin, co-amoxiclav, ceftriaxone, ceftazidime, ciprofloxacin, gentamicin and meropenem. Comparisons were made with phenotypic results obtained in duplicate by broth dilution (BD Phoenix). Discrepancies, either between duplicate BD Phoenix results or between genotype and phenotype, were resolved with gradient diffusion analyses.
Results
A wide variety of antimicrobial resistance genes were identified, including blaCTX-M, blaLEN, blaOKP, blaOXA, blaSHV, blaTEM, aac(3′)-Ia, aac-(3′)-IId, aac-(3′)-IIe, aac(6′)-Ib-cr, aadA1a, aadA4, aadA5, aadA16, aph(6′)-Id, aph(3′)-Ia, qnrB and qnrS, as well as resistance-associated mutations in chromosomal gyrA and parC genes. The sensitivity of genome-based resistance prediction across all antibiotics for both species was 0.96 (95% CI: 0.94–0.98) and the specificity was 0.97 (95% CI: 0.95–0.98). Very major and major error rates were 1.2% and 2.1%, respectively.
Conclusions
Our method was as sensitive and specific as routinely deployed phenotypic methods. Validation against larger datasets and formal assessments of cost and turnaround time in a routine laboratory setting are warranted.
Keywords: antibiotic, phenotype, Gram-negative
Introduction
The advances in sequencing technology over the last decade promise a potential revolution in clinical microbiology, with the cost-effective use of pathogen whole-genome sequence data for species identification, antimicrobial susceptibility prediction and outbreak detection having been proposed as applications of bench-top sequencers, such as the MiSeq (Illumina, San Diego, CA, USA) or Ion Torrent (Life Technologies Corp., Carlsbad, CA, USA), in routine laboratories.1 Conceivably, this could enable a ‘one-stop’ approach to the microbiological analysis of cultured bacterial isolates with turnaround times of <1 day.
At present, routine antimicrobial susceptibility testing is undertaken using a variety of approaches, including disc diffusion, gradient diffusion and broth dilution methods, the latter being automated as part of commercial platforms such as BD Phoenix (BD, Franklin Lakes, NJ, USA) or Vitek 2 (bioMérieux, Marcy l'Etoile, France).2–4 Despite extensive efforts to standardize laboratory assays, problems with particular test methods for certain organism–antimicrobial combinations are well recognized and may relate to inherent properties of the organism or antimicrobial being tested.5,6 Other errors can arise in inoculum preparation, culture conditions or data entry.
Susceptibility phenotyping errors are typically classified as very major, resulting from a false-susceptible result, or major, resulting from a false-resistant result.2 The US FDA stipulates rates must be <1.5% for very major errors and <3% for major errors prior to authorizing marketing approval for new susceptibility testing devices; similar cut-offs have been proposed by others.2 In controlled research studies, overall error rates are 0%–8%,7 but in routine settings the actual error rates are not generally known.
Routine genotypic prediction of bacterial antimicrobial susceptibility is currently used only in limited contexts, typically with single gene targets known to be highly associated with resistance, such as mecA assays to determine methicillin resistance in Staphylococcus aureus. The prevailing view has been that genotypic assays would be too difficult to implement for complex patterns of antimicrobial resistance, e.g. those in major Gram-negative pathogens such as Escherichia coli or Klebsiella pneumoniae.2 However, recent data investigating whole-genome sequencing approaches to identifying susceptibility phenotypes of porcine Salmonella Typhimurium, E. coli, Enterococcus faecium and Enterococcus faecalis isolates for resistance surveillance purposes showed high concordance between phenotypic and predicted antimicrobial susceptibilities.8 Caveats to this acknowledged by the authors include the low complexity of the resistance genotypes in the bacterial populations studied (i.e. small numbers of resistance genes per isolate conferring resistance to the same antimicrobial class) and that no assessment of some important chromosomal markers of resistance, such as gyrA mutations for fluoroquinolones, was made.
E. coli and K. pneumoniae are the Gram-negative species most commonly identified in bacteraemic patients in the UK,9,10 with increases in incidence noted across Europe.11 As such, these organisms, in which multidrug resistance is increasingly recognized,12,13 represent species for which accurate and rapid antimicrobial susceptibility testing has the potential to deliver direct clinical benefit. Consequently, in this study we aimed to assess the feasibility of using whole-genome sequence data from human blood culture isolates of E. coli and K. pneumoniae representative of those seen in clinical practice to predict susceptibility phenotypes for antibiotics commonly used to manage infections caused by these organisms.
Materials and methods
Clinical isolate selection and in vitro antimicrobial susceptibility testing
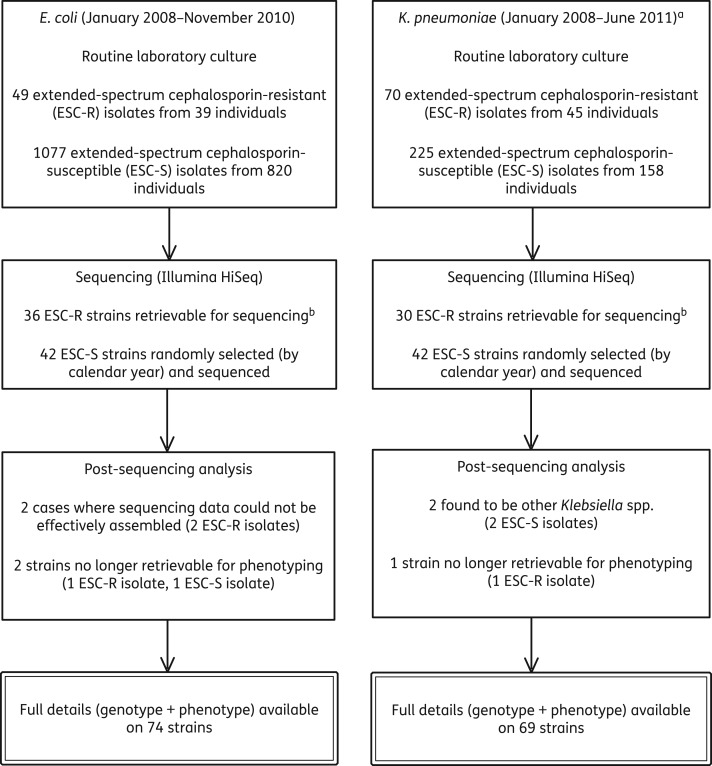
We selected all retrievable extended-spectrum cephalosporin-resistant (commonly representative of multidrug-resistant phenotypes)14 E. coli and K. pneumoniae blood culture isolates obtained from patients at the Oxford University Hospitals NHS Trust, Oxford, UK, between January 2008 and November 2010 (E. coli) or June 2011 (K. pneumoniae). Time-matched (by calendar year) susceptible control blood culture isolates were also selected at random and retrieved (Figure 1).
Figure 1.
Sampling frame and processing of isolates. aThe study time period for K. pneumoniae was extended to find similar numbers of organisms across both species groups. bLosses in retrieval rates were mostly due to the fact that repeat isolates from individuals were not routinely stored; other missing isolates could not be found in the routine laboratory freezer.
Isolates were recultured from frozen stocks (−80°C) and underwent automated susceptibility testing in duplicate with the BD Phoenix system using EUCAST breakpoints,15 to allow comparisons with genotypic data. Intermediate BD Phoenix susceptibilities were considered as resistant (Tables 1 and 2). In cases where duplicate BD Phoenix runs were concordant regarding an isolate's resistance category [susceptible/susceptible (S/S), resistant/resistant (R/R)], the consensus BD Phoenix phenotype was compared with the genotype. Discrepancies, defined as discordance between BD Phoenix runs (S/R), or between predicted genotypic susceptibility and concordant BD Phoenix phenotype (S + R/R, or R + S/S respectively), were further investigated using gradient diffusion testing (Etest, bioMérieux, Basingstoke, UK; M.I.C. Evaluator, Fisher Scientific UK, Loughborough, UK) on Iso-Sensitest agar in accordance with BSAC guidelines.16 In such cases, regardless of the nature of the discrepancy, the gradient diffusion result was adopted as the comparison standard phenotype; in all other cases, the concordant BD Phoenix phenotype was the comparison standard.
Table 1.
Analysis of discordance in phenotypic and/or genotypic resistance predictions for 74 E. coli bloodstream isolates
| Antibiotic | Discrepancies (n; % of total, 74 isolates) |
Agreement of gradient diffusion with genotype in all discrepancies (n/total discrepancies; %) | Agreement of gradient diffusion with genotype in BD Phoenix-concordant discrepancies (n/number of BD Phoenix-concordant discrepancies; %) | ||
|---|---|---|---|---|---|
| S/Ra discordant BD Phoenix | BD Phoenix S/S, genotype R | BD Phoenix R/R, genotype S | |||
| Amoxicillin | 0 (0) | 2 (3) | 0 (0) | 1/2 (50) | 1/2 (50) |
| Co-amoxiclav | 5 (7) | 0 (0) | 15 (20) | 20/20 (100) | 15/15 (100) |
| Gentamicin | 1 (1) | 0 (0) | 0 (0) | 1/1 (100) | NAb |
| Ciprofloxacin | 0 (0) | 0 (0) | 0 (0) | NA | NA |
| Ceftriaxone | 0 (0) | 1 (1) | 1 (1) | 0/2 (0) | 0/2 (0) |
| Ceftazidime | 1 (1) | 11 (15) | 1 (1) | 1/13 (8) | 1/12 (8) |
| Meropenem | 0 (0) | 0 (0) | 0 (0) | NA | NA |
| Total | 7/518 (1)c | 14/518 (3)c | 17/518 (3)c | 23/38 (61) | 17/31 (55) |
aS/R denotes susceptible/resistant category. Initial BD Phoenix intermediate results were counted as resistant—this occurred in one isolate with an S/R discrepancy for ceftazidime and one isolate with an S/R discrepancy for gentamicin.
bNA = not applicable.
cn/overall total of 518 antimicrobial susceptibility results (%).
Table 2.
Analysis of discordance in phenotypic and/or genotypic resistance predictions for 69 K. pneumoniae bloodstream isolates
| Antibiotic | Discrepancies (n; % of total, 69 isolates) |
Agreement of gradient diffusion with genotype in all discrepancies (n/total discrepancies; %) | Agreement of gradient diffusion with genotype in BD Phoenix-concordant discrepancies (n/number of BD Phoenix-concordant discrepancies; %) | ||
|---|---|---|---|---|---|
| S/Ra discordant BD Phoenix | BD Phoenix S/S, genotype R | BD Phoenix R/R, genotype S | |||
| Amoxicillin | 3 (4) | 3b (4) | 0 (0) | 3/6 (50) | 1/3 (33) |
| Co-amoxiclav | 2 (3) | 0 (0) | 6 (9) | 7/8 (88) | 6/6 (100) |
| Gentamicin | 1 (1) | 0 (0) | 1 (1) | 1/2 (50) | 0/1 (0) |
| Ciprofloxacin | 1 (1) | 2 (3) | 7 (10) | 4/10 (40) | 3/9 (33) |
| Ceftriaxone | 0 (0) | 1 (1) | 2 (3) | 0/3 (0) | 0/3 (0) |
| Ceftazidime | 0 (0) | 1 (1) | 2 (3) | 0/3 (0) | 0/3 (0) |
| Meropenem | 1 (1) | 0 (0) | 0 (0) | 0/1 (0) | NAc |
| Total | 8/483 (2)d | 7/483 (1)d | 18/483 (4)d | 15/33 (45) | 10/25 (40) |
aS/R denotes susceptible/resistant category. Initial BD Phoenix intermediate results were counted as resistant—this occurred in one isolate with an S/R discrepancy for ciprofloxacin and one isolate with an S/R discrepancy for meropenem.
bThis applies to an MIC-based assessment of BD Phoenix results, disregarding interpretative guidelines (which would suggest that K. pneumoniae be universally reported as amoxicillin resistant for clinical purposes, irrespective of the MIC).
cNA = not applicable.
dn/overall total of 483 antimicrobial susceptibility results (%).
Reference gene database
Genetic loci and sequence variants known to be associated with resistance to antimicrobial agents commonly used in our hospital to treat E. coli and K. pneumoniae infections were identified from published reviews and web-based resources and were compiled as a reference gene database.17–25 Chromosomal and plasmid-mediated loci conferring resistance to amoxicillin, co-amoxiclav, ciprofloxacin, gentamicin, ceftriaxone, ceftazidime and meropenem were included (full details of all mechanisms included in the algorithm are provided in Tables S1, S2 and S3, available as Supplementary data at JAC Online). An additional search of complete coding sequences annotated as being members of relevant bacterial (other than mycobacterial) resistance gene families deposited at the National Centre for Biotechnology Information was performed, using the following search terms: (i) ‘lactamase’, (ii) ‘carbapenemase’, (iii) ‘aminoglycoside’ + ‘resistance’ and (iv) ‘fluoroquinolone’ + ‘resistance’ (December 2012; see Supplementary data for additional references).
DNA extraction and whole-genome sequencing
DNA was extracted using a commercial kit (QuickGene DNA Tissue Kit S, Fujifilm, Japan) as per the manufacturer's instructions, with an additional mechanical lysis step (FastPrep, MP Biomedicals, USA) immediately following chemical lysis. A combination of standard Illumina and in-house protocols was used to produce multiplexed paired-end libraries of extracted DNA with an average insert size of ∼200 bp. Sequencing was performed on the Illumina HiSeq 2000, generating 100 bp paired-end reads. Reads were mapped against reference sequences [CFT073 for E. coli (RefSeq: NC_004431.1) and MGH78578 for K. pneumoniae (RefSeq: NC_009653)] using Stampy.26 De novo assembly, for the purposes of resistance locus identification, was performed using Velvet,27 with automated optimization of assembly parameters using VelvetOptimiser,28 including the selection of k-mer length (length of overlapping read fragments), expected coverage (which assists in minimizing the impact of repetitive regions on the assemblies) and coverage cut-off [which minimizes the impact of areas of low sequencing coverage and repetitive regions (areas of high coverage) on assemblies]. De novo assembly quality was ensured by requiring >4 megabases (Mb) to be assembled into contigs and contig n50 values of >30 000 bp (n50 is the longest contig length such that 50% of the assembled genome is represented in contigs of this length or longer). Sequencing data files have been deposited at the European nucleotide archive (ENA) and are available using the following URL: http://www.ebi.ac.uk/ena/data/view/ERP002642.
In silico prediction of antimicrobial susceptibility phenotypes
BLASTn was used to identify the presence of relevant resistance gene loci (from the reference database) in the de novo-assembled contigs for each clinical isolate, with a word length of 11 and an Expect value (E) cut-off of 1 × 10−4. All matches were visually inspected for confirmation. Matches with >80% identity at the nucleotide level and representing a match of >80% of the reference gene length were retained; this included partial matches with >80% sequence homology over 80% of the reference gene length, but distributed over several contigs. Overlapping fragments were then aligned in SeaView29 and combined to give a single sequence. Chromosomal resistance gene sequences were analysed to identify mutations, including those known to be associated with resistance.
Each isolate's susceptibility phenotype was predicted from the genetic data on the basis of published associations with phenotypic resistance for each locus, without reference to the BD Phoenix phenotype [details for susceptibility predictions for all profiles found are shown in Tables S4, S5 and S6 (E. coli) and S7, S8 and S9 (K. pneumoniae); Supplementary data available at JAC Online]. For any novel sequence variants identified, the genotypic susceptibility prediction mirrored that of the closest reference database variant. Discrepancies between the BD Phoenix phenotype and genotype were then investigated using gradient diffusion, as described above.
The sensitivity, specificity and rates of major and very major errors for genotypic susceptibility predictions were calculated for each antibiotic and species against the comparison standard (determined as above). Statistical analyses were performed using Stata 11.2 (StataCorp, College Station, TX, USA).
Results
Quality of whole-genome sequences
Two of the 76 candidate E. coli study isolates were excluded because of poor sequence assembly (n50 <1250 and <0.3 Mb assembled into contigs); two Klebsiella isolates were excluded because they were non-pneumoniae Klebsiella spp. on the basis of mapping (Figure 1). Assemblies for the 74 remaining E. coli isolates had a median of 394 contigs (range: 93–1052) and n50 of 110 187 bp (range: 32 391–189 171 bp). For the 69 K. pneumoniae study isolates, the corresponding medians were 255 contigs (range: 171–863) and n50 of 97 195 bp (range: 58 500–135 350 bp).
Investigation of discrepancies
Susceptibility phenotypes for seven antimicrobials were available for 143 study isolates (74 E. coli and 69 K. pneumoniae), giving 1001 total susceptibility results (518 E. coli and 483 K. pneumoniae) for comparison with the corresponding genotypic predictions. Gradient diffusion analysis was used to establish the phenotype for 71 antimicrobial–isolate combinations (involving 55 different isolates), including 7 (1%) E. coli results and 8 (2%) K. pneumoniae results with categorical (S/R) discordance in duplicate BD Phoenix testing and 31 (6%) E. coli and 25 (5%) K. pneumoniae results with discordance between the predicted genotypic susceptibility and the (concordant) BD Phoenix phenotype [Table 1 (E. coli) and Table 2 (K. pneumoniae)].
Genotypic prediction versus comparison standard phenotype
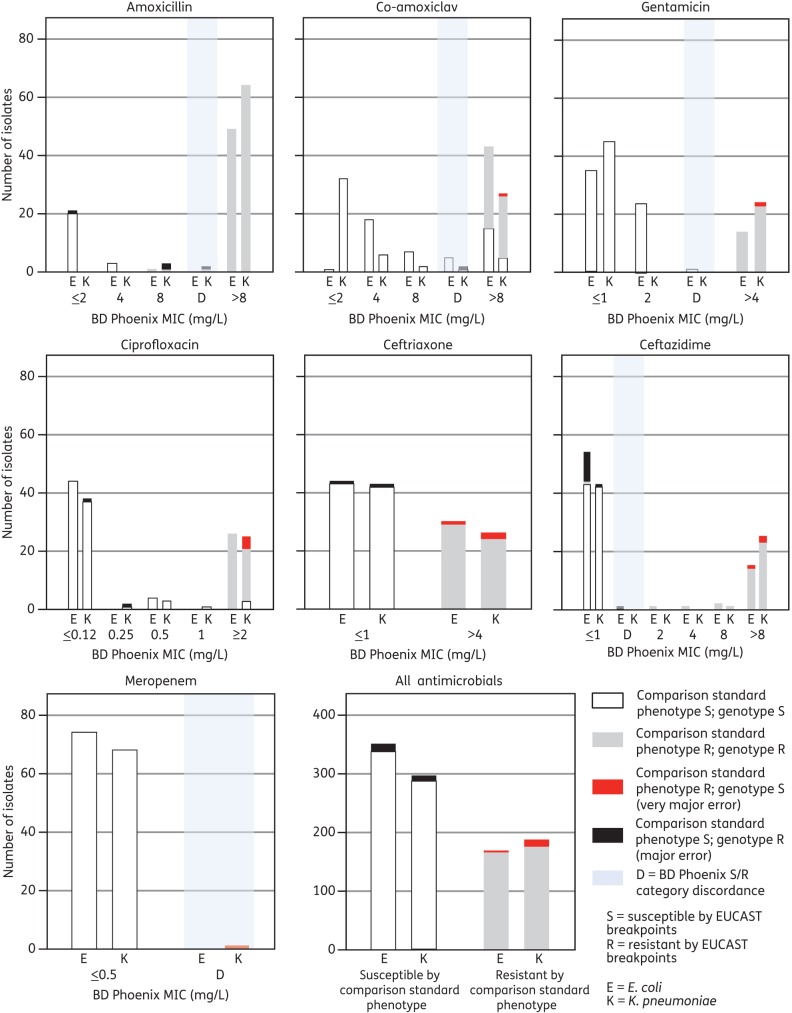
Overall, the sensitivity of genotype for predicting resistance across all antibiotics for both species was 0.96 (95% CI: 0.94–0.98) and the specificity was 0.97 (95% CI: 0.95–0.98) (Figure 2). Very major and major error rates, at 1.2% and 2.1%, respectively, were within the <1.5% and <3% FDA limits. For E. coli, the overall sensitivity was 0.99 (95% CI: 0.95–1.0) and the specificity was 0.96 (95% CI: 0.94–0.98) (Table 3); the major individual drug deficit being suboptimal specificity for ceftazidime (0.80; 95% CI: 0.66–0.89). Very major (0.3%) and major (3%) error rates were again within the FDA limits. For K. pneumoniae, the overall sensitivity was 0.95 (95% CI: 0.90–0.97) and the specificity was 0.97 (95% CI: 0.95–0.99), with a very major error rate (2%) just outside the 1.5% FDA limit, but an acceptable major error rate (2%, compared with <3% as per FDA) (Table 4).
Figure 2.
Comparisons of genotypic susceptibility prediction, BD Phoenix phenotype and results of gradient diffusion analyses for discrepancies in either (i) duplicate BD Phoenix testing or (ii) genotypic prediction and concordant BD Phoenix phenotype, for both species across all seven antimicrobials.
Table 3.
Sensitivity and specificity of genotypic resistance predictions versus comparison with standard phenotype results for 74 E. coli bloodstream isolates.
| Antibiotic | Susceptible by comparison standard phenotype |
Resistant by comparison standard phenotype |
Sensitivity (95% CI) | Specificity (95% CI) | ||
|---|---|---|---|---|---|---|
| susceptible by genotype (row %) | resistant by genotype (row %; major error) | susceptible by genotype (row %; very major error) | resistant by genotype (row %) | |||
| Amoxicillin | 23 (31) | 1 (1) | 0 (0) | 50 (68) | 1.00 (0.91–1.00) | 0.96 (0.77–1.00) |
| Co-amoxiclav | 46 (62) | 0 (0) | 0 (0) | 28 (38) | 1.00 (0.85–1.00) | 1.00 (0.90–1.00) |
| Gentamicin | 60 (81) | 0 (0) | 0 (0) | 14 (19) | 1.00 (0.73–1.00) | 1.00 (0.93–1.00) |
| Ciprofloxacin | 48 (65) | 0 (0) | 0 (0) | 26 (35) | 1.00 (0.84–1.00) | 1.00 (0.91–1.00) |
| Ceftriaxone | 43 (58) | 1 (1) | 1 (1) | 29 (39) | 0.97 (0.81–1.00) | 0.98 (0.87–1.00) |
| Ceftazidime | 43 (58) | 11 (15) | 1 (1) | 19 (26) | 0.95 (0.73–1.00) | 0.80 (0.66–0.89) |
| Meropenem | 74 (100) | 0 (0) | 0 (0) | 0 (0) | — | 1.00 (0.94–1.00) |
| Total | 337 (65) | 13 (3) | 2 (0.3) | 166 (32) | 0.99 (0.95–1.00) | 0.96 (0.94–0.98) |
Table 4.
Sensitivity and specificity of genotypic resistance predictions versus comparison standard phenotype results for 69 K. pneumoniae bloodstream isolates
| Antibiotic | Susceptible by comparison standard phenotype |
Resistant by comparison standard phenotype |
Sensitivity (95% CI) | Specificity (95% CI) | ||
|---|---|---|---|---|---|---|
| susceptible by genotype (row %) | resistant by genotype (row %; major error) | susceptible by genotype (row %; very major error) | resistant by genotype (row %) | |||
| Amoxicillin | 0 (0) | 3 (4) | 0 (0) | 66 (96) | 1.00 (0.93–1.00) | — |
| Co-amoxiclav | 47 (68) | 1 (1) | 0 (0) | 21 (30) | 1.00 (0.81–1.00) | 0.98 (0.88–1.00) |
| Gentamicin | 45 (65) | 0 (0) | 1 (1) | 23 (33) | 0.96 (0.77–0.98) | 1.00 (0.90–1.00) |
| Ciprofloxacin | 45 (65) | 2 (3) | 4 (6) | 18 (26) | 0.90 (0.67–0.98) | 0.92 (0.80–0.97) |
| Ceftriaxone | 42 (61) | 1 (1) | 2 (3) | 24 (35) | 0.92 (0.73–0.99) | 0.98 (0.86–1.00) |
| Ceftazidime | 42 (61) | 1 (1) | 2 (3) | 24 (35) | 0.92 (0.73–0.99) | 0.98 (0.86–1.00) |
| Meropenem | 68 (99) | 0 (0) | 1 (1) | 0 (0) | 0 (0–0.95) | 1.00 (0.93–1.00) |
| Total | 289 (60) | 8 (2) | 10 (2) | 176 (36) | 0.95 (0.90–0.97) | 0.97 (0.95–0.99) |
In E. coli, in 23 (61%) of the 38 isolate–antimicrobial combinations with a phenotype–genotype discrepancy according to BD Phoenix results, gradient diffusion analysis supported the genotypic prediction (Table 1). For the remaining 15 confirmed genotype–phenotype discrepancies, the results are summarized in Table 5. In 13 (87%) of these cases, a clear-cut genetic resistance mechanism was identified despite phenotypic susceptibility, although for 9 (69%) of these the gradient diffusion MIC was at the susceptibility breakpoint. The remaining 2 (13%) of the 15 discrepant genotype–phenotype cases had no identifiable genetic resistance mechanism, despite unequivocal phenotypic resistance.
Table 5.
List of relevant genotypic profiles for 13 E. coli and 15 K. pneumoniae isolates with genotype-gradient diffusion susceptibility discrepancies for one or more antimicrobials
| Species | Number of isolates | Antibiotic discrepancy | Genotypic prediction | Genotypic mechanism for resistance prediction | Phenotypic result | MIC (mg/L) on gradient diffusion (EUCAST susceptibility breakpoint) | Supplementary data Table no./complete genotypic profile number |
|---|---|---|---|---|---|---|---|
| E. coli | 1 | amoxicillin | R | P3 TEM-promoter and blaTEM-1 | S | 6 (8) | S4/3 |
| E. coli | 1 | ceftriaxonea | S | none | R | >32 (1) | S4/3 |
| ceftazidimea | S | R | 4 (1) | ||||
| E. coli | 1 | ceftriaxoneb | R | T-32A ampC promoter mutation | S | 0.38 (1) | S4/10 |
| ceftazidimeb | R | S | 1 (1) | ||||
| E. coli | 1 | ceftazidime | R | blaCTX-M-15 | S | 0.25 (0.25) | S4/2 |
| E. coli | 7 | ceftazidime | R | blaCTX-M-15 | S | 1 (1) | 6 × S4/4, 1 × S4/2 |
| E. coli | 1 | ceftazidime | R | blaCTX-M-14 | S | 0.5 (1) | S4/14 |
| E. coli | 1 | ceftazidime | R | blaCTX-M-1 | S | 1 (1) | S4/6 |
| K. pneumoniae | 2 | amoxicillin | R | blaLEN | S | 4, 8 (8) | S7/4 |
| K. pneumoniae | 1 | amoxicillin | R | blaSHV | S | 6 (8) | S7/3 |
| K. pneumoniae | 1 | co-amoxiclav | R | blaOXA-1 | S | 8 (8) | S7/19 |
| K. pneumoniae | 1 | ceftriaxonec | R | blaSHV-27 | S | 0.064 (1) | S7/11 |
| ceftazidimec | R | S | 0.25 (1) | ||||
| K. pneumoniae | 1 | ceftriaxoned | S | none | R | 8 (1) | S7/8 |
| ceftazidimed | S | R | 64 (1) | ||||
| K. pneumoniae | 1 | ceftriaxonee | S | none | R | >32 (1) | S7/1 |
| ceftazidimee | S | R | 8 (1) | ||||
| K. pneumoniae | 1 | meropenem | S | none | R | >32 (2) | S7/7 |
| K. pneumoniae | 1 | ciprofloxacin | R | 2 gyrA mutations (S83F + D87A) | S | 0.064 (0.5) | S8/5 |
| K. pneumoniae | 1 | ciprofloxacin | R | 2 gyrA mutations (S83I + D87N) | S | 0.047 (0.5) | S8/10 |
| K. pneumoniae | 2 | ciprofloxacin | S | 1 parC mutation (S80I) | R | 8, >32 (0.5) | S8/4 |
| K. pneumoniae | 1 | ciprofloxacin | S | 1 parC mutation (S80I) + aac(6′)-Ib-cr | R | 2 (0.5) | S8/12 |
| K. pneumoniae | 1 | ciprofloxacin | S | 1 parC mutation (E84K) + 1 parE mutation (S458T) | R | >32 (0.5) | S8/17 |
| K. pneumoniae | 1 | gentamicin | S | none | R | 16 (2) | S9/1 |
a–eMultiple genotype–phenotype discrepancies observed for several antibiotics for the same isolate.
In K. pneumoniae, in 15 (45%) of the 33 isolate–antimicrobial combinations with a phenotype–genotype discrepancy according to the BD Phoenix results, gradient diffusion analysis supported the genotypic prediction (Table 2). For the remaining 18 confirmed genotype–phenotype discrepancies, the results are summarized in Table 5. In 6/18 (33%) instances, a recognized resistance mechanism was identified in phenotypically susceptible isolates, although for two of these the MIC was at the susceptibility breakpoint. This group included two isolates predicted to be ciprofloxacin resistant based on chromosomal mutations (double gyrA amino acid replacements) that were phenotypically ciprofloxacin susceptible. In contrast, four isolates predicted to be ciprofloxacin susceptible [based on a single mutation in parC (three isolates with S80I) or combined mutations in parC (E84K) + parE (S458T)] were phenotypically resistant, suggesting the presence of unidentified resistance mechanisms. Similarly, six other cases with unequivocal phenotypic resistance to one or more agents from several antibiotic classes (ceftriaxone, ceftazidime, meropenem and gentamicin; eight total agent–isolate combinations) had no identifiable resistance mechanism.
Resistance gene profiles—E. coli
Genotypic resistance profiles in E. coli are summarized in Tables S4 (β-lactam resistance), S5 (fluoroquinolone resistance) and S6 (aminoglycoside resistance). There were 15 distinct profiles for β-lactam resistance mechanisms, 22 for ciprofloxacin-associated resistance mechanisms and 12 for aminoglycoside-associated resistance mechanisms.
β-Lactam resistance
Twelve (16%) isolates had blaTEM, blaOXA-1 and blaCTX-M conferring β-lactam resistance; 15 (20%) had two of these three mechanisms, 24 (32%) had one and 23 (31%) had none. Most blaTEM-containing isolates had blaTEM-1 (35/36), with five distinct nucleotide sequences (including the reference sequence) observed. In addition to the P3 and Pa/Pb blaTEM-1 promoters,19 two novel promoter sequences were identified [single nucleotide polymorphisms compared with promoter P3: C → T at position 75 (Sutcliffe numbering);30 G → A at position 175]. However, co-amoxiclav resistance was identified only in the presence of other explanatory mechanisms with these novel promoter sequences. All blaOXA variants were blaOXA-1 and most blaCTX-M variants were blaCTX-M-15 (25/29).
Only one isolate had a chromosomal ampC promoter mutation previously associated with significant resistance (T-32A).22 This isolate was resistant only to amoxicillin and co-amoxiclav, with no other mechanism identified to explain this, and was phenotypically susceptible to ceftriaxone and ceftazidime (on duplicate BD Phoenix testing and gradient diffusion analysis).
Quinolone resistance
Ciprofloxacin resistance was invariably associated with S83L/D87N mutations in gyrA; almost all (23/26; 88%) ciprofloxacin-resistant isolates also had S80I/E84V mutations in parC. The presence of aac-6′-Ib-cr was also common in ciprofloxacin-resistant isolates, although not universal (23/26; 88%); aac-6′-Ib-cr was also identified in one ciprofloxacin-susceptible isolate without any resistance-conferring chromosomal mutations. A single isolate had a gyrB quinolone resistance-determining region (QRDR) mutation (S463A) with a parE truncation; this isolate was phenotypically susceptible. No qnr variants or qepA or oqxAB loci were found.
Aminoglycoside resistance
Four (5%) isolates had four or five different aminoglycoside resistance-conferring elements; 15 (20%) had three, 12 (16%) had two, 11 (15%) had one and 32 (43%) had none. All gentamicin resistance was associated with the presence of aac(3′)-II-like enzymes, mostly aac(3′)-IIe variants (13/14), with one isolate containing aac(3′)-IId. Other aminoglycoside resistance loci included aac(6′)-Ib-cr (24 isolates), aadA1a (3), aadA4 (17), aadA5 (17), aph(6′)-Id (16), aph(6′)-Id-like loci (>80% but <95% sequence homology; 3 isolates) and aph(3′)-Ia (4).
Resistance gene profiles—K. pneumoniae
Genotypic profiles associated with resistance in K. pneumoniae are summarized in Tables S7 (β-lactam resistance, 24 profiles), S8 (fluoroquinolone resistance, 20 profiles) and S9 (aminoglycoside resistance, 17 profiles).
β-Lactam resistance
Twenty-one (30%) isolates had three or four β-lactam resistance-conferring elements; 8 (12%) had two, 29 (42%) had one and 11 (16%) had none. All blaTEM were blaTEM-1, with P3 (n = 24) or Pa/Pb (n = 3) promoters. All blaCTX-M were blaCTX-M-15 and all blaOXA were blaOXA-1 (only observed with blaCTX-M-15).
Most K. pneumoniae isolates (61/69; 88%) contained blaSHV genes encoding β-lactamases. Six contained blaLEN (two blaLEN-7, four novel variants), one blaOKP-B-6 and one blaLAP-2 in conjunction with blaSHV-11. The most common blaSHV β-lactamase variant was blaSHV-1 (n = 28), with additional variants in order of frequency as follows: blaSHV-11 (19), blaSHV-28 (4), blaSHV-33 (2), blaSHV-121 (2), blaSHV-27 (1), blaSHV-60 (1) and blaSHV-135 (1). Three novel amino acid blaSHV variants were identified (Y7F + S14F, Y7F + M211L and D101H; assigned allele numbers 169, 170 and 171, respectively, in the Lahey database).17 One of the 69 Klebsiella isolates contained none of these resistance loci; its β-lactam resistance was explained by the presence of blaTEM-1, blaOXA-1 and blaCTX-M-15.
Quinolone resistance
Isolates with wild-type amino acids or only single amino acid mutations in the QRDRs of gyrA, gyrB, parC and parE, and no more than one plasmid-mediated resistance mechanism (aac-6′-Ib-cr, qnr or qepA), were all ciprofloxacin susceptible (41 wild-type isolates, four single amino acid mutations). In contrast, isolates with both aac-6′-Ib-cr and qnrB1 (n = 15) were invariably resistant, irrespective of underlying chromosomal mutations. Likewise, isolates with single gyrA and parC mutations and a plasmid-mediated resistance mechanism (n = 2: S83I + S80I + aac-6′-Ib-c; S83T + S80I + qnrS1), or a double mutation in gyrA, a single mutation in parC and a plasmid-mediated resistance mechanism (n = 1: S83F + D87N + S80I + aac-6′-Ib-cr), were also resistant.
There were no observed mutations compared with wild-type in the QRDR of gyrB. All isolates contained oqxAB, which is commonly located chromosomally in K. pneumoniae, although its association with ciprofloxacin resistance in this context is unclear.31
Aminoglycoside resistance
One (1%) isolate had five different resistance-conferring elements; 16 (23%) had three, 9 (13%) had two, 7 (10%) had one and 36 (52%) had none. As in E. coli, gentamicin resistance in K. pneumoniae was typically associated with the presence of aac(3′)-II-like enzymes, mostly aac(3′)-II-e (19/23). Three isolates had an aac(3′)-IId enzyme and one an aac(3′)-Ia variant. Other aminoglycoside resistance loci included aph(6′)-Id (25 isolates), aph(3′)-Ia (4), aadA2 (4), aadA1 (2) and aadA16 (1).
Discussion
In this study, we determined the sensitivity and specificity of a genotypic prediction algorithm for the two most commonly isolated Gram-negative species, E. coli and K. pneumoniae, using whole-genome data from clinical isolates from bacteraemic patients with a wide range of resistance phenotypes. In our centre, the epidemiology of these organisms has been found to be similar to the wider national and European contexts.12,13 Using publicly available resources, we determined the presence/absence of published variants (including genes and resistance-determining mutations) in >100 resistance-associated gene families, with particular reference to those relevant to commonly used antimicrobials. Relative to a comparison standard phenotype based on BD Phoenix plus gradient diffusion testing, genotype-based resistance prediction yielded overall sensitivity and specificity values of 0.96 and 0.97, respectively, plus rates of very major errors (1.2%) and major errors (2.1%) below the corresponding FDA-specified thresholds of 1.5% and 3%.
Applying genetic ‘resistotyping’ to Gram-negative species is not new, with PCR-based methods having been widely used in the epidemiological assessment of both E. coli and K. pneumoniae collections. However, the number of resistance mechanisms involved is extremely large, limiting the use of comprehensive PCR methods in any real-time diagnostic capacity. One response to this challenge has been to develop microarray-based approaches to assess a much larger panel of resistance mechanisms than is feasible with PCR; this method, however, has issues with sensitivity and cannot easily identify numerous mutation-based mechanisms of resistance.32 In addition, microarrays are expensive to develop and difficult to upgrade flexibly in response to the evolution of resistance mechanisms. We have demonstrated that whole-genome sequencing provides a viable alternative approach.
This study has also demonstrated that novel variants of known resistance-associated loci can be easily identified using our approach. To expand on this, BLASTn-based cut-offs could be made less stringent to facilitate the discovery of putative, distantly related resistance genes or a tBLASTx-based approach could be used to identify protein homologues with different underlying coding sequences. Similar approaches have been used in the past, although in a limited manner.33
Our data highlight some known issues with the accuracy of some phenotypic methods commonly used in diagnostic microbiology—particularly with the assessment of β-lactam/β-lactamase inhibitor susceptibilities. Duplicate BD Phoenix tests gave discordant results in 7 (5%) of 143 co-amoxiclav tests performed; on gradient diffusion, 6/7 isolates had MICs more than one dilution away from the breakpoint. In 21 instances where the co-amoxiclav genotypic prediction disagreed with the BD Phoenix results (all involving genotypically susceptible isolates that were resistant by BD Phoenix), all isolates were susceptible according to gradient diffusion, suggesting that in 15% of tests automated phenotyping was overcalling resistance. Problems with the correct assessment of susceptibility by phenotyping for β-lactam/β-lactamase inhibitor combinations have been observed previously,34 particularly in the context of complex β-lactamase genotypes,35 which were disproportionately represented in our dataset.
For extended-spectrum cephalosporins and E. coli, we found certain genetic mechanisms known to be associated with resistance (such as the CTX-M enzyme family) in isolates considered susceptible. However, this was using the new, lower EUCAST breakpoints in the context of EUCAST's decision (mirrored by the CLSI) to report susceptibilities as observed without interpretative modifications for these drugs. This phenomenon has also been documented in other studies of CTX-M-producing organisms from China and New Zealand36,37 and highlights the controversy over whether an in vitro MIC or the presence of a genetic mechanism is more predictive of clinical outcomes38—whether this is a limitation or a strength of genotypic resistance prediction methods is therefore unclear. The large-scale clinical outcome data needed to resolve this quandary are currently lacking, but could be obtained by using integrated routine clinical, phenotyping and antibiotic-prescribing data, combined with whole genome-based, comprehensive assessments of resistance mechanisms.
Overall, among 1001 isolate–antimicrobial combinations tested, we found 12 instances of phenotypic resistance that were supported by gradient diffusion analysis without any resistance mechanism being identified, indicating deficits with our initial gene reference database and/or genotypic prediction algorithm. We have yet to systematically investigate potential contributions made by other known resistance mechanisms, such as porin genes or efflux pumps, in part because associations of the latter with phenotypic resistance are incompletely defined. Assessing the performance of our approach in determining all known mechanisms of resistance, including rare variants, is clearly important future work. For this study, however, we were particularly focused on characterizing the potential of genotypic resistance prediction for organisms typically isolated in our clinical practice. Of interest, given the absence of any initial mechanism identified for carbapenem resistance in the single meropenem-resistant K. pneumoniae isolate, we subsequently studied the ompK35 and ompK36 loci as possible candidate loci using our BLASTn-based approach and identified a 5 bp deletion in ompK36 leading to a truncation at position 227. Although we did not measure protein expression, porin deficiencies associated with prematurely truncated ompK36, coupled with the presence of blaCTX-M-15, have been associated previously with carbapenem resistance39 and could plausibly explain resistance in this isolate. This demonstrates that once an isolate's genome sequence is available, it can be reassessed rapidly for additional resistance gene mechanisms as necessary, without the need for further laboratory work.
There are several limitations to our approach as described. Establishing the sequencing and computational infrastructure required to process large volumes of sequencing data in real time involves a substantial initial investment in terms of time and money. Our study was a retrospective, proof-of-principle experiment and further work would be required to assess its performance and cost-effectiveness in a routine diagnostic setting on a larger dataset. In addition, it remains to be seen whether predictions would be equally successful for all antimicrobials currently incorporated in phenotypic susceptibility testing strategies. The bioinformatic strategy used does not determine plasmid copy number and therefore cannot quantify the possible contribution of multiple gene copies (e.g. of blaTEM), which might lead to hyperproduction of certain enzymes and phenotypic resistance by a gene dosage effect. Another limitation is that the phenotypic manifestations of certain allelic variants and promoter/attenuator mechanisms are not fully determined (e.g. for some of the blaSHV variants), precluding reliable predictions. Importantly, resistance mechanisms evolve; approaches based on genotypic prediction rely on a resistance locus reference database requiring regular updating based on a scheme incorporating ongoing phenotyping, albeit in a more limited number of samples, such as those isolated from treatment failures. Phenotyping would also be needed to validate any novel genetic resistance mutations/mechanisms. Finally, epigenetic and expression-associated mechanisms cannot be determined using our DNA-based analysis, thus highlighting the intrinsic limitation of approaches based on gene/mutation identification with no direct evidence of functional resistance. However, alternative sequencing-based methods could be explored to address this shortcoming, such as RNA-Seq, chromatin immunoprecipitation sequencing or methylation analysis.40
Despite these limitations, our approach achieved high sensitivity and specificity in proof-of-principle experiments using typical clinical isolates and its performance was comparable to that of some phenotyping methods currently in routine use. Whole-genome sequencing-based approaches may well become part of routine microbiology workflows in some settings within the next 5 years. This would afford the ability to undertake species identification, strain typing for epidemiological purposes or infection prevention and control, and prediction of antimicrobial susceptibilities reliably and quickly using a single method for ∼£40/isolate.1
Funding
This work was supported by the Wellcome Trust (doctoral fellowship award to N. S.). It was also supported by the National Institute for Health Research (NIHR) under its Oxford Biomedical Research Centre Infection Theme and the UKCRC Modernising Medical Microbiology Consortium, the latter funded under the UKCRC Translational Infection Research Initiative supported by the Medical Research Council, the Biotechnology and Biological Sciences Research Council and the National Institute for Health Research on behalf of the Department of Health [Grant G0800778] and the Wellcome Trust [Grant 087646/Z/08/Z]. D. W. C. and T. E. A. P. are NIHR Senior Investigators. D. W. E. is an NIHR Doctoral Research Fellow. This material is also based partly upon work supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, grant # 1 I01 CX000192 01 (J. R. J.).
Transparency declarations
J. R. J. has received research grants or contracts from Merck, Rochester Medical, ICET and Syntiron. The other authors report no financial conflicts of interest.
Disclaimer
The views expressed in this publication are those of the author(s) and not necessarily those of the National Health Service, the NIHR or the Department of Health.
Supplementary data
Tables S1–S9 are available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/).
Acknowledgements
We would like to acknowledge the help and support of staff of the John Radcliffe Hospital Department of Microbiology, the sequencing centre at the Wellcome Trust Centre for Human Genetics and the Modernising Medical Microbiology (MMM) bioinformatics team, all in Oxford, UK. We also thank Dr Michael Mulvey of the Antimicrobial Resistance and Nosocomial Infections section of the National Microbiology Laboratory, Winnipeg, Canada, for his review of the draft manuscript.
References
- 1.Didelot X, Bowden R, Wilson DJ, et al. Transforming clinical microbiology with bacterial genome sequencing. Nat Rev Genet. 2012;13:601–12. doi: 10.1038/nrg3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jorgensen JH, Ferraro MJ. Antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clin Infect Dis. 2009;49:1749–55. doi: 10.1086/647952. [DOI] [PubMed] [Google Scholar]
- 3.BSAC. BSAC Methods for Antimicrobial Susceptibility Testing; Version 11. http://bsac.org.uk/wp-content/uploads/2012/02/Version-11.1-2012-Final-.pdf. (19 March 2013, date last accessed)
- 4.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-second Informational Supplement M100-S22. Wayne, PA, USA: CLSI; 2012. [Google Scholar]
- 5.Andrews JM. The development of the BSAC standardized method of disc diffusion testing. J Antimicrob Chemother. 2001;48(Suppl 1):29–42. doi: 10.1093/jac/48.suppl_1.29. [DOI] [PubMed] [Google Scholar]
- 6.Bulik CC, Fauntleroy KA, Jenkins SG, et al. Comparison of meropenem MICs and susceptibilities for carbapenemase-producing Klebsiella pneumoniae isolates by various testing methods. J Clin Microbiol. 2010;48:2402–6. doi: 10.1128/JCM.00267-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snyder JW, Munier GK, Johnson CL. Direct comparison of the BD Phoenix system with the MicroScan WalkAway system for identification and antimicrobial susceptibility testing of Enterobacteriaceae and nonfermentative gram-negative organisms. J Clin Microbiol. 2008;46:2327–33. doi: 10.1128/JCM.00075-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zankari E, Hasman H, Kaas RS, et al. Genotyping using whole-genome sequencing is a realistic alternative to surveillance based on phenotypic antimicrobial susceptibility testing. J Antimicrob Chemother. 2013;68:771–7. doi: 10.1093/jac/dks496. [DOI] [PubMed] [Google Scholar]
- 9.HPA, UK. Escherichia coli Bacteraemia in England, Wales and Northern Ireland, 2007–2011. http://www.hpa.org.uk/hpr/archives/2012/hpr2212_ecoli_bctrm2011.pdf. (15 January 2013, date last accessed)
- 10.HPA, UK. Klebsiella, Enterobacter, Serratiaand Citrobacter spp. Bacteraemia, England, Wales and Northern Ireland: 2006–2010. http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1287142531496. (15 January 2013, date last accessed)
- 11.Gagliotti C, Balode A, Baquero F, et al. Escherichia coli and Staphylococcus aureus: bad news and good news from the European antimicrobial resistance surveillance network (EARS-Net, formerly EARSS), 2002–2009. Euro Surveill. 2011;16 doi: 10.2807/ese.16.11.19819-en. pii=19819. [DOI] [PubMed] [Google Scholar]
- 12.Schlackow I, Stoesser N, Walker AS, et al. Increasing incidence of Escherichia coli bacteraemia is driven by an increase in antibiotic-resistant isolates: electronic database study in Oxfordshire 1999–2011. J Antimicrob Chemother. 2012;67:1514–24. doi: 10.1093/jac/dks082. [DOI] [PubMed] [Google Scholar]
- 13.Webster DP, Young BC, Morton R, et al. Impact of a clonal outbreak of extended-spectrum β-lactamase-producing Klebsiella pneumoniae in the development and evolution of bloodstream infections by K. pneumoniae and Escherichia coli: an 11 year experience in Oxfordshire, UK. J Antimicrob Chemother. 2011;66:2126–35. doi: 10.1093/jac/dkr246. [DOI] [PubMed] [Google Scholar]
- 14.Paterson DL. Resistance in gram-negative bacteria: Enterobacteriaceae. Am J Med. 2006;119(Suppl 1):S20–8. doi: 10.1016/j.amjmed.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 15.EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters; Version 3.0 (1 January 2013). http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/EUCAST_Breakpoint_table_v_3.0.pdf. (11 February 2013, date last accessed)
- 16.BSAC. Use of Gradient Tests for Determination of MICs by BSAC Methodology. http://bsac.org.uk/wp-content/uploads/2012/02/Use-of-gradient-tests.pdf. (27 December 2012, date last accessed)
- 17.Jacoby G, Bush K. β-Lactamase Classification and Amino Acid Sequences for TEM, SHV and OXA Extended-spectrum and Inhibitor-resistant Enzymes. http://www.lahey.org/Studies/ (27 December 2012, date last accessed)
- 18.Ramirez MS, Tolmasky ME. Aminoglycoside modifying enzymes. Drug Resist Updat. 2010;13:151–71. doi: 10.1016/j.drup.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lartigue MF, Leflon-Guibout V, Poirel L, et al. Promoters P3, Pa/Pb, P4, and P5 upstream from blaTEM genes and their relationship to β-lactam resistance. Antimicrob Agents Chemother. 2002;46:4035–7. doi: 10.1128/AAC.46.12.4035-4037.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulvey MR, Bryce E, Boyd DA, et al. Molecular characterization of cefoxitin-resistant Escherichia coli from Canadian hospitals. Antimicrob Agents Chemother. 2005;49:358–65. doi: 10.1128/AAC.49.1.358-365.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caroff N, Espaze E, Bérard I, et al. Mutations in the ampC promoter of Escherichia coli isolates resistant to oxyiminocephalosporins without extended spectrum β-lactamase production. FEMS Microbiol Lett. 1999;173:459–65. doi: 10.1111/j.1574-6968.1999.tb13539.x. [DOI] [PubMed] [Google Scholar]
- 22.Caroff N, Espaze E, Gautreau D, et al. Analysis of the effects of −42 and −32 ampC promoter mutations in clinical isolates of Escherichia coli hyperproducing ampC. J Antimicrob Chemother. 2000;45:783–8. doi: 10.1093/jac/45.6.783. [DOI] [PubMed] [Google Scholar]
- 23.Jacoby GA. Mechanisms of resistance to quinolones. Clin Infect Dis. 2005;41(Suppl 2):S120–6. doi: 10.1086/428052. [DOI] [PubMed] [Google Scholar]
- 24.Cattoir V, Nordmann P. Plasmid-mediated quinolone resistance in gram-negative bacterial species: an update. Curr Med Chem. 2009;16:1028–46. doi: 10.2174/092986709787581879. [DOI] [PubMed] [Google Scholar]
- 25.Zankari E, Hasman H, Cosentino S, et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother. 2012;67:2640–4. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lunter G, Goodson M. Stampy: a statistical algorithm for sensitive and fast mapping of Illumina sequence reads. Genome Res. 2011;21:936–9. doi: 10.1101/gr.111120.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18:821–9. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gladman S, Seemann T. VelvetOptimiser. http://bioinformatics.net.au/software.velvetoptimiser.shtml. (27 December 2012, date last accessed)
- 29.Gouy M, Guindon S, Gascuel O. SeaView version 4: A multi-platform graphical user interface for sequence alignment and phylogenetic tree-building. Mol Biol Evol. 2010;27:221–4. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- 30.Sutcliffe JG. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci USA. 1978;75:3737–41. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan J, Xu X, Guo Q, et al. Prevalence of the oqxAB gene complex in Klebsiella pneumoniae and Escherichia coli clinical isolates. J Antimicrob Chemother. 2012;67:1655–9. doi: 10.1093/jac/dks086. [DOI] [PubMed] [Google Scholar]
- 32.Card R, Zhang J, Das P, et al. Evaluation of an expanded microarray for detecting antibiotic resistance genes in a broad range of Gram-negative bacterial pathogens. Antimicrob Agents Chemother. 2012;57:458–65. doi: 10.1128/AAC.01223-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis MA, Baker KN, Orfe LH, et al. Discovery of a gene conferring multiple-aminoglycoside resistance in Escherichia coli. Antimicrob Agents Chemother. 2010;54:2666–9. doi: 10.1128/AAC.01743-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bond A, Plumb H, Turner P. Susceptibility testing of Escherichia coli isolates from urines: are we at risk of reporting false antibiotic resistance to co-amoxiclav? J Antimicrob Chemother. 2012;67:1557–8. doi: 10.1093/jac/dks034. [DOI] [PubMed] [Google Scholar]
- 35.Cantón R, Loza E, Del Carmen Conejo M, et al. MENSURA Collaborative Group. Quality control for β-lactam susceptibility testing with a well-defined collection of Enterobacteriaceae and Pseudomonas aeruginosa strains in Spain. J Clin Microbiol. 2003;41:1912–8. doi: 10.1128/JCM.41.5.1912-1918.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang P, Hu F, Xiong Z, et al. Susceptibility of extended-spectrum-β-lactamase-producing Enterobacteriaceae according to the new CLSI breakpoints. J Clin Microbiol. 2011;49:3127–31. doi: 10.1128/JCM.00222-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williamson DA, Roberts SA, Smith M, et al. High rates of susceptibility to ceftazidime among globally prevalent CTX-M-producing Escherichia coli: potential clinical implications of the revised CLSI interpretive criteria. Eur J Clin Microbiol Infect Dis. 2012;31:821–4. doi: 10.1007/s10096-011-1380-1. [DOI] [PubMed] [Google Scholar]
- 38.Livermore DM, Andrews JM, Hawkey PM, et al. Are susceptibility tests enough, or should laboratories still seek ESBLs and carbapenemases directly? J Antimicrob Chemother. 2012;67:1569–77. doi: 10.1093/jac/dks088. [DOI] [PubMed] [Google Scholar]
- 39.Novais A, Rodrigues C, Branquinho R, et al. Spread of an OmpK36-modified ST15 Klebsiella pneumoniae variant during an outbreak involving multiple carbapenem-resistant Enterobacteriaceae species and clones. Eur J Clin Microbiol Infect Dis. 2012;31:3057–63. doi: 10.1007/s10096-012-1665-z. [DOI] [PubMed] [Google Scholar]
- 40.Güell M, Yus E, Lluch-Senar M, et al. Bacterial transcriptomics: what is beyond the RNA horiz-ome? Nat Rev Microbiol. 2011;9:658–69. doi: 10.1038/nrmicro2620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.