Abstract
Objectives
Very different labelling conventions are employed by different products of colistimethate (CMS), an inactive prodrug of colistin that is used as a last-line defence against Gram-negative ‘superbugs’. This study examined the chemical composition and pharmacokinetics in rats of four commercial parenteral products of CMS.
Methods
Contents per vial of four brands of CMS from three different continents were weighed (n = 3). Elemental analysis and HPLC examination were conducted. The pharmacokinetics of CMS and formed colistin were investigated for each product after intravenous administration in rats (28.1 mg/kg CMS; n = 4). Blood was collected over 180 min, and concentrations of CMS and colistin were measured followed by pharmacokinetic analysis.
Results
X-GEN, Paddock and Atlantic products, labelled with 150 mg ‘colistin base activity’, contained 366.8 ± 0.80, 340.6 ± 0.08 and 380.0 ± 5.97 mg CMS (sodium) per vial, respectively; while the Forest product (labelled with 2 000 000 IU) contained 159.3 ± 1.75 mg CMS (sodium). The elemental compositions of the four products were similar; however, the HPLC profile of the Atlantic CMS was different from those of the other three products. The pharmacokinetics of CMS were generally comparable across brands; however, the molar ratios (%) of the AUC0–180min of colistin to CMS (1.68% ± 0.35% to 3.29% ± 0.43%) were significantly different (P = 0.0157).
Conclusion
This is the first study to demonstrate that although different brands of CMS from various parts of the world have similar elemental compositions, they lead to different exposures to the microbiologically active formed colistin. The study has significant implications for the interpretation of pharmacological studies of CMS conducted in different parts of the world.
Keywords: elemental analysis, HPLC, intravenous administration, colistin base activity
Introduction
Over the last two decades there has been a remarkable increase in resistance to almost all current antibiotics.1 In particular, for the Gram-negative ‘superbugs’ identified by the Infectious Diseases Society of America (IDSA) (e.g. Pseudomonas aeruginosa, Acinetobacter baumannii and Klebsiella pneumoniae), there are few therapeutic options available; unfortunately, no new antibiotics active against these bacteria will be available for many years to come.1–3 Increasingly, polymyxins, mainly colistin (also known as polymyxin E), are often used as a last-line therapy.4–7
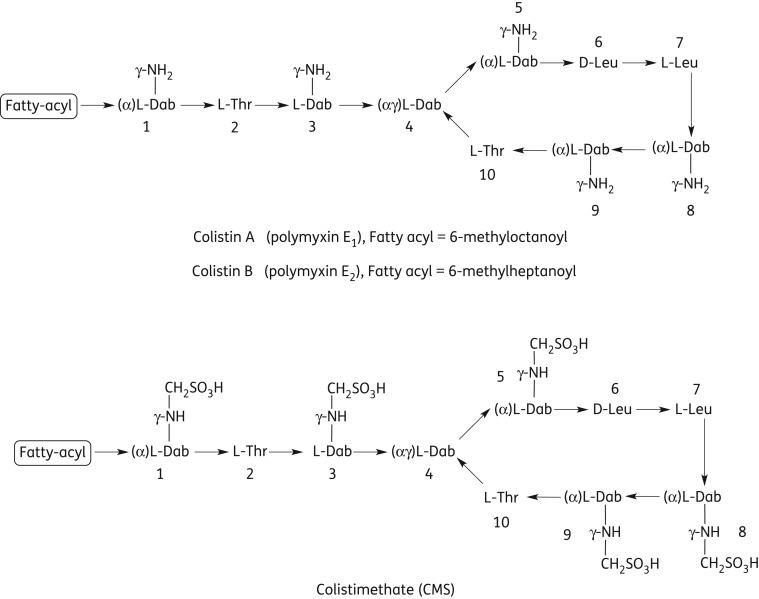
Colistin (Figure 1) is a mixture of multiple components with colistin A and B as the two major components, differing only in the structure of their N-terminal fatty acyl chains.8,9 Colistimethate (CMS; Figure 1; synonyms colistin methanesulphonate, colistin sulphomethate and sulphomethyl colistin), an inactive prodrug of colistin,10 is the only parenteral form used clinically for colistin.5 In CMS, the side chain amino groups of the diaminobutyric acid (Dab) residues of colistin are derivatized with methanesulphonate groups (Figure 1). Colistin is a polycation at physiological pH due to the five primary amine groups, while CMS is a polyanion due to the covalent addition of methanesulphonate moieties.5,9 CMS is generally not stable and converts to colistin in vitro11,12 and in vivo after administration in animals13,14 and humans.15–21 CMS is believed to be a rather complex mixture of various intermediates of methanesulphonate derivatives (i.e. different numbers and locations of methanesulphonate moieties on the colistin molecule).11 It is still unknown whether all five of the primary amine groups of colistin are methanesulphonated in CMS.5,11 Even for a single colistin component (e.g. colistin A), there can be 32 (i.e. 25) different chemical entities in CMS, including colistin, depending on the number and location of methanesulphonate groups attached. CMS has been off patent for many years and currently there are at least four commercially available parenteral products of CMS worldwide. These products employ very different dosage definitions.5,22 In North America, Australia, Singapore and Thailand, the labelling convention of CMS products [i.e. colistin base activity (CBA) per vial] is based upon in vitro standardization of microbiological activity relative to that of colistin base, while in the British Pharmacopeia and European Pharmacopeia CMS products are labelled with international units per vial. Therefore, there is great potential for confusion among clinicians wishing to administer CMS to patients and in comparing pharmacological data from studies conducted in various parts of the world. This significant labelling inconsistency was first noted in our review5 and highlighted again by a recent fatal case due to the confusion associated with the dose definitions.23 To complicate the issue even further, currently very little is known about the chemical compositions of the different brands of CMS products. The aim of this study was to examine the chemical composition and pharmacokinetics in rats of the four commercial parenteral products of CMS.
Figure 1.
Chemical structures of colistin and colistimethate (CMS). Thr, threonine; Leu, leucine; Dab, α,γ-diaminobutyric acid (α and γ indicate the respective amino group involved in the peptide linkage).
Materials and methods
Antibiotics and reagents
Colistate 150 (Atlantic Laboratories Corp Ltd, Bangkok, Thailand) and Colomycin Injection (Forest Laboratories, Kent, UK) were kindly provided by the respective companies, while Colistimethate for Injection USP (X-GEN Pharmaceuticals, Inc., NY, USA) and Colistimethate for Injection (Paddock Laboratories, Inc., MN, USA) were purchased from these companies. All four parenteral products are presented as lyophilized powders for reconstitution prior to administration. Colistin (sulphate) and CMS (sodium) were obtained from the U.S. Pharmacopeia (Rockville, MD, USA). The derivatizing reagent 9-fluorenylmethyl chloroformate (FMOC-Cl) was from Sigma-Aldrich (Sydney, NSW, Australia). Acetonitrile, acetone, methanol (Biolab, Scoresby, VIC, Australia) and tetrahydrofuran (Science Supply, Mitcham, VIC, Australia) were HPLC grade. All other reagents were of analytical grade. Water was purified by a Milli-Q system (Millipore, Billerica, MA, USA). All solutions were stored at 4°C.
Characterization of four different brands of CMS by elemental analysis and HPLC
The contents per vial of the four different brands of CMS were weighed (n = 3) and elemental analysis (C, H, N, S, O) was conducted by CMAS (Chemical & MicroAnalytical Services Pty Ltd, Highton, VIC, Australia). Briefly, samples were burned in the presence of oxygen and injected into a helium carrier gas flow. Combustion was completed over copper oxide, then excess oxygen was removed. Nitrogen oxides were reduced to nitrogen and sulphur trioxide to sulphur dioxide in a layer of metallic copper. The remaining combustion gases (nitrogen, carbon dioxide, water and sulphur dioxide) were separated by gas chromatography and measured using a hot-wire detector.24 The measured elemental contributions were compared with the average theoretical molecular weight (mol. wt) of CMS sodium (C57.5H105N16O28S5Na5, mol. wt = 1743.8 Da), which was calculated from the mol. wt of CMS A sodium (C58H106N16O28S5Na5, mol. wt = 1750.8 Da) and CMS B sodium (C57H104N16O28S5Na5, mol. wt = 1736.8 Da).
Liquid chromatography–mass spectrometry (LC-MS) analysis was conducted using a Shimadzu LCMS 2010 EV quadrupole mass spectrometer equipped with an electrospray ionization (ESI) source coupled to a Shimadzu Prominence chromatography system (Kyoto, Japan). Reversed-phase (RP) HPLC analysis was conducted with a Phenosphere-NEXT C18 column (5 μm, 150 × 4.6 mm). Solutions of colistin (U.S. Pharmacopeia) and the four different brands of CMS products were prepared in Milli-Q water at 5 mg/mL, and Milli-Q water was used as the control. An aliquot (100 μL) of each solution was injected into the RP-HPLC system. A linear gradient was employed: 0%–100% mobile phase B over 12.0 min at a flow rate of 1.5 mL/min (mobile phase A: 0.05% [v/v] trifluoroacetic acid (TFA) in water; mobile phase B: 0.05% [v/v] TFA in acetonitrile). The eluent was infused directly into the ESI source. Mass spectra were acquired in the positive ion mode over 50 min with a scan range of 200–1800 m/z. The chromatographic system also included a photodiode array detector that was set at 214 nm.
Pharmacokinetic study
All animal experiments were approved by the Monash Institute of Pharmaceutical Sciences Animal Ethics Committee, Monash University, and were complied using the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. The pharmacokinetic study followed our previous method with minor modifications.13 Briefly, Sprague-Dawley rats (male, body weight 263–326 g) were anaesthetized using isoflurane by inhalation and a polyethylene cannula was inserted into the jugular vein. Each rat was placed into a metabolic cage and allowed unrestricted access to food and water. Following overnight recovery, CMS (28.1 mg/kg, n = 4 per product) was administered as a bolus (in 200 μL of sterile saline) through the jugular vein cannula followed by flushing with 1 mL of heparinized saline. Blood was collected through the cannula before the dose and at 5, 10, 20, 30, 60, 90, 120 and 180 min after administration of CMS. Blood samples were immediately placed into pre-heparinized tubes on ice and centrifuged at 10 000 g for 5 min. Plasma samples were immediately stored at −80°C and analysed for colistin and CMS within 4 weeks.25
Quantification of CMS and colistin in plasma by HPLC
Concentrations of CMS and formed colistin in plasma were determined by HPLC with minor modifications.26–28 The injection volume was 30 μL and the mobile phase was acetonitrile–tetrahydrofuran–water (50 : 30 : 20, v/v). Calibration curves for CMS (U.S. Pharmacopeia) and colistin (U.S. Pharmacopeia) ranged from 0.31 to 30.0 mg/L and from 0.13 to 1.50 mg/L, respectively. The low limits of quantification were 0.31 mg/L for CMS and 0.13 mg/L for colistin (n = 4), with accuracy and reproducibility within 15.0% for both entities. Analysis of independently prepared quality control plasma samples (1.00, 10.0 and 30.0 mg/L for CMS; 0.25, 1.00 and 3.00 mg/L for colistin) indicated good reproducibility (coefficients of variation ≤15.0%) and accuracy (measured concentrations ≤14.9% from respective target concentrations).
Data analysis
Group data are presented as mean ± standard deviation (SD). Non-compartmental analysis of pharmacokinetics of CMS and formed colistin was performed using WinNonlin (version 5.2.1; Pharsight Corp., Cary, NC, USA).13 For the four different brands of CMS, the differences in the elemental composition, total body clearance and volume of distribution of CMS, the AUC0–180min of CMS and formed colistin and their molar ratios were evaluated using analysis of variance (ANOVA). A P value <0.05 was considered significant.
Results
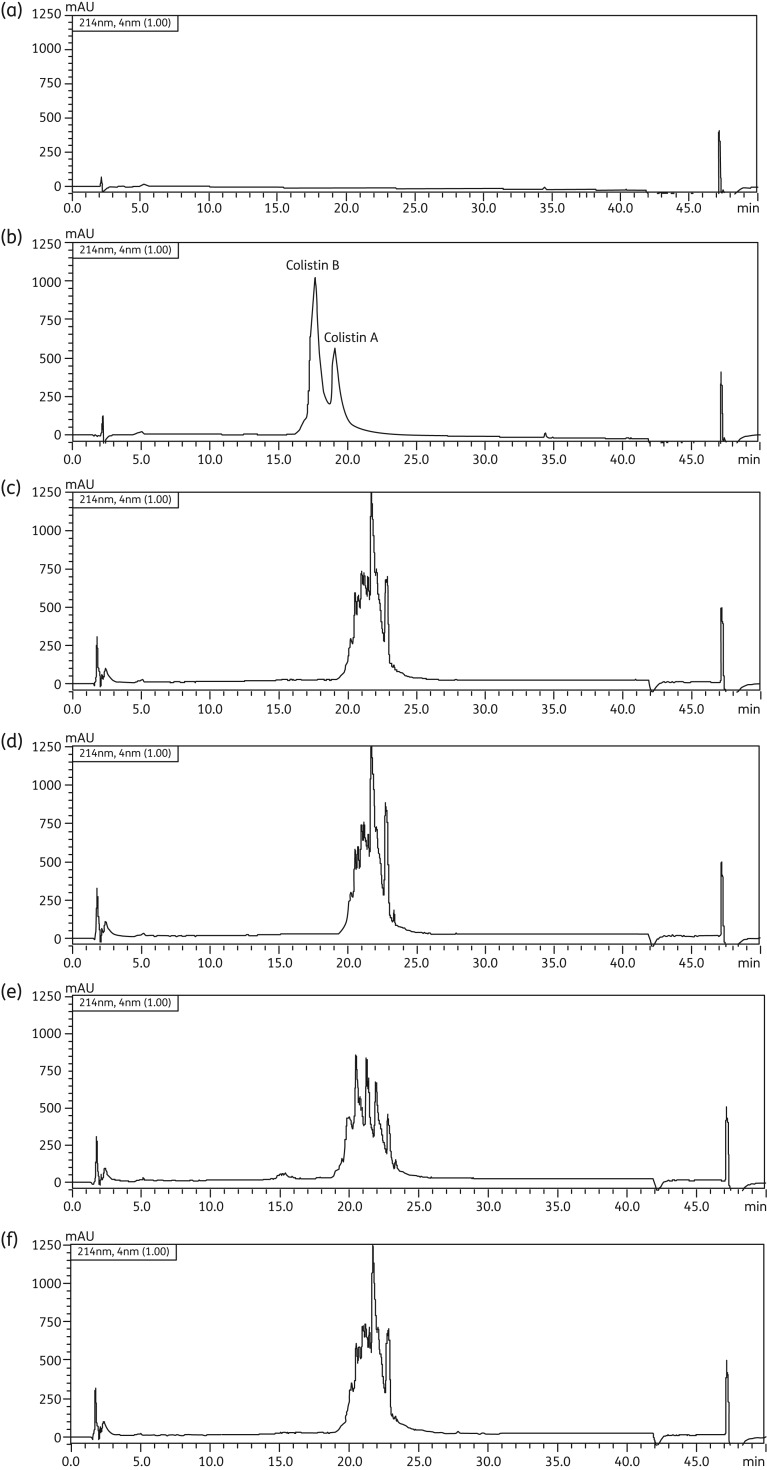
X-GEN, Paddock and Atlantic products, labelled with 150 mg ‘colistin base activity’, contained ∼360 mg CMS (sodium) per vial, while the Forest product (labelled with 2 000 000 IU) contained ∼160 mg CMS (sodium) (Table 1). The elemental composition of vial contents was similar for all four brands (P = 0.20). However, the nitrogen and carbon contents were >10% lower than the theoretical values expected for colistin penta-methanesulphonate, while the oxygen content was >33% higher than the theoretical value (Table 1). Based upon the RP-HPLC analysis of the four brands, the chromatographic profile of the Atlantic CMS was distinct from those of the other three CMS products (Figure 2). None of the four brands had evidence of the presence of detectable colistin (Figure 2).
Table 1.
CMS (sodium) contents per vial (n = 3) of four different brands and elemental analysis
| Parameter | 150 mg colistin base activity (CBA) |
Forest (UK) | Theoretical values | ||
|---|---|---|---|---|---|
| X-GEN (USA) | Paddock (USA) | Atlantic (Thailand) | 2 000 000 IU | ||
| Weight (mg/vial) | 366.8 ± 0.80 | 340.6 ± 0.08 | 380.0 ± 5.97 | 159.3 ± 1.75 | — |
| Carbon (%) | 34.56 | 34.91 | 34.41 | 34.67 | 39.60 |
| Hydrogen (%) | 5.87 | 5.86 | 5.80 | 5.95 | 6.07 |
| Nitrogen (%) | 10.95 | 11.42 | 11.22 | 11.22 | 12.85 |
| Oxygen (%) | 34.26 | 36.83 | 34.46 | 34.91 | 25.69 |
| Sulphur (%) | 10.35 | 9.76 | 8.71 | 8.80 | 9.19 |
Figure 2.
RP-HPLC profiles at 214 nm for (a) blank control, (b) colistin (U.S. Pharmacopeia) and the commercially available products of CMS: (c) X-GEN, (d) Paddock, (e) Atlantic and (f) Forest. The concentrations of colistin (b) and CMS (c–f) were 5 mg/mL.
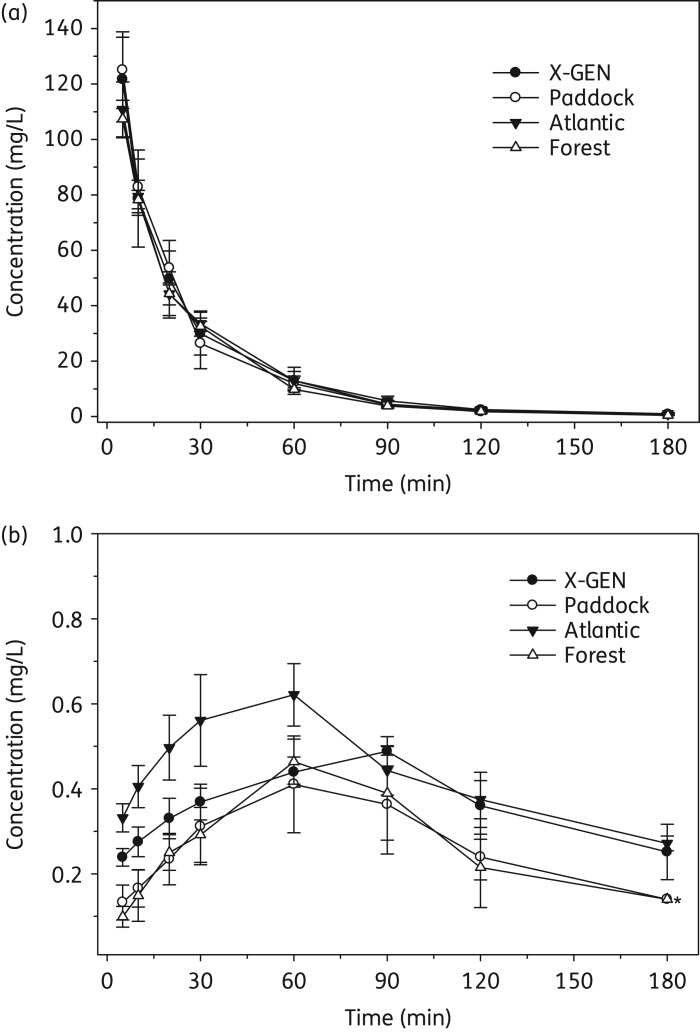
Figure 3 shows the mean (± SD) plasma concentration–time profiles of CMS and formed colistin in rats after administration of each brand (28.1 mg/kg). The profiles of CMS were very similar across all products. According to the peak areas in HPLC analysis, the ratios of CMS B to CMS A of the X-GEN, Paddock and Forest products in rat plasma over 180 min after CMS administration were 0.15 ± 0.054, 0.15 ± 0.044 and 0.17 ± 0.059, respectively, while that for the Atlantic product was 0.98 ± 0.20. Clearly, for a given product the ratios were generally consistent over the experimental period but differed in the Atlantic product. The pharmacokinetic parameters of CMS and formed colistin, AUC0-180min and their molar ratios for different brands of CMS products are presented in Table 2. There was no significant difference in the values of total body clearance (CL, P = 0.62), volume of distribution (Vz, P = 0.42) and AUC0-180min of CMS (P = 0.58) among the different CMS products (Table 2). However, for formed colistin, significant differences were observed across the four different brands of CMS in the AUC0-180min (P = 0.0121) and the ratio of the AUC0-180min of colistin to that of CMS (in molar terms; P = 0.0157).
Figure 3.
Mean (± SD) plasma concentration–time profiles of (a) CMS and (b) formed colistin in rats (n = 4) following an intravenous dose of CMS (28.1 mg/kg). An asterisk indicates concentrations in three out of four rats were below the limit of quantification.
Table 2.
Pharmacokinetic parameters of CMS and formed colistin in rats (n = 4)
| Parameters | X-GEN (USA) | Paddock (USA) | Atlantic (Thailand) | Forest (UK) |
|---|---|---|---|---|
| CMS | ||||
| CL (mL/min/kg) | 8.30 ± 1.50 | 8.35 ± 1.05 | 8.33 ± 0.75 | 9.13 ± 0.49 |
| Vz (L/kg) | 0.36 ± 0.11 | 0.31 ± 0.014 | 0.34 ± 0.046 | 0.29 ± 0.010 |
| t1/2 (min) | 29.2 ± 4.24 | 25.9 ± 2.45 | 28.4 ± 4.75 | 21.9 ± 1.02 |
| AUC0–180min (mg·min/L) | 3429 ± 642 | 3371 ± 375 | 3336 ± 293 | 3026 ± 170 |
| Formed colistin | ||||
| t1/2 (min)a | 108.0 ± 57.2 | 68.9 ± 12.0 | 107.2 ± 13.5 | 45.3 ± 10.0 |
| Cmax (mg/L) | 0.49 ± 0.035 | 0.44 ± 0.10 | 0.62 ± 0.075 | 0.47 ± 0.053 |
| AUC0–180min (mg·min/L) | 65.4 ± 6.81 | 40.5 ± 10.6 | 77.8 ± 9.54 | 42.4 ± 12.0 |
| ratio of AUC0–180min of colistin to CMS (%)b | 2.73 ± 0.41 | 1.68 ± 0.35 | 3.29 ± 0.43 | 1.98 ± 0.58 |
at1/2 of formed colistin was calculated based on the last three timepoints.
bIn molar terms.
Discussion
Considering CMS is an inactive prodrug,10 the use of microbiological assays to standardize antibacterial activity in vitro may not necessarily reflect the exposure to formed colistin in vivo. In order to optimize the clinical use of CMS/colistin, it is important to investigate the compositions of different CMS products and determine their pharmacokinetics in animals. Such a study will greatly help clinicians compare pharmacokinetic and pharmacodynamic data from studies conducted in different parts of the world.
As colistin is a generic drug, it is difficult to identify the manufacturers of the CMS raw material used in all four brands of parenteral products investigated here. Colistin contents in all four products of CMS were negligible (Figure 2). For the vial contents of each brand, our quantitative elemental analysis did not reveal any substantial differences in the content of carbon, hydrogen, nitrogen, oxygen and sulphur. This suggests that the different CMS products have similar elemental compositions and peptide content. The observed deviations from the theoretical values (the low content of carbon and nitrogen and higher content of oxygen) and non-deviations (hydrogen and sulphur) (Table 1) showed that the peptide content of the samples was ∼88% based upon the carbon and nitrogen content, with the remaining ∼12% most likely consisting of sodium counter ions and water, as well as bisulphate salts. These results also suggest that the CMS content in the four brands is not exclusively the penta-methanesulphonate form.
The RP-HPLC analysis revealed that three of the four brands had very similar chromatographic profiles (Figure 2). Furthermore, the multiplicity of peaks observed in the chromatograms for all four products supports previous observations that CMS is a mixture of a number of different methanesulphonate derivatives11 rather than exclusively the penta-methanesulphonate form as suggested by the elemental analysis. Attempts were made to further separate the peaks and identify via MS analysis the individual peaks in the HPLC profiles for all four brands (data not shown); however, no molecular ions corresponding to the expected methanesulphonate derivatives were observed. It is very likely that this was due to fragmentation of the methanesulphonate groups in the ionization source during MS analysis.
The plasma concentration–time profiles of CMS were generally consistent among all four products after intravenous administration (28.1 mg/kg; Figure 3a). As discussed in our previous study13 and above in relation to the chromatographic profiles in Figure 2, the pharmacokinetic parameters for CMS should be considered as hybrid parameters for CMS and the partially sulphomethylated derivatives present initially in the product and formed during the in vivo conversion of CMS to colistin. For all CMS products, formed colistin appeared in the plasma of all rats within 5 min after administration of CMS and achieved Cmax in 1–1.5 h (Figure 3b). The pharmacokinetic parameters of CMS and formed colistin estimated in the present study are generally consistent with those previously reported for rats.13,14,29 The terminal half-life of formed colistin was longer than that of CMS (Table 2), indicating that the disposition of colistin was not rate limited by its formation from CMS. The AUC0-180min of formed colistin was significantly lower for Paddock and Forest products (P = 0.0121) than for the other two products, most likely due to differences in the conversion of CMS to colistin. The ratio of AUC0-180min of formed colistin to that of CMS was very low (<3.5%) across all four brands, consistent with only a very small percentage of CMS having been converted to colistin systemically after intravenous administration, as reported previously.13,14 It is noteworthy, however, that there were clear differences across the CMS products in the time course of plasma concentrations of formed colistin (Figure 3b), and the molar ratio (%) of the AUC0-180min of colistin to that of CMS differed almost 2-fold (1.68% ± 0.35% to 3.29% ± 0.43%; Table 2). The chemical differences observed chromatographically (Figure 2) may have led to the different plasma concentration–time profiles of formed colistin in rat plasma after intravenous administration of the various CMS products (Figure 3). While all products had been standardized microbiologically in vitro, the exposure to formed colistin in vivo differed. Considering that scientifically based dosing recommendations for intravenous CMS should be based upon the exposure to formed colistin in patients,16,19,21 clinical investigations are needed on the pharmacokinetics of formed colistin across different brands of CMS.
In conclusion, this is the first study to demonstrate that different brands of CMS from various countries had similar elemental compositions and comparable pharmacokinetics to CMS in rats but generated different exposure to colistin in vivo. The study has significant implications for the interpretation of pharmacokinetic, pharmacodynamic and toxicodynamic studies of CMS conducted in different parts of the world.
Funding
The project described was supported by award numbers R01AI098771 (to J. L., T. V., R. L. N., P. E. T. and K. R.) and R01AI070896 (to R. L. N., J. L. and B. T. T.) from the National Institute of Allergy and Infectious Diseases. J.-C. L. was supported by award number 31272613 from the National Natural Science Foundation of China. T. V. is an Australian National Health and Medical Research Council Career Development Award Industry Fellow. J. L. is an Australian National Health and Medical Research Council Senior Research Fellow.
Transparency declarations
None to declare.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
References
- 1.Infectious Diseases Society of America. The 10 × ‘20 Initiative: Pursuing a global commitment to develop 10 new antibacterial drugs by 2020. Clin Infect Dis. 2010;50:1081–3. doi: 10.1086/652237. [DOI] [PubMed] [Google Scholar]
- 2.Payne DJ, Gwynn MN, Holmes DJ, et al. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov. 2007;6:29–40. doi: 10.1038/nrd2201. [DOI] [PubMed] [Google Scholar]
- 3.Talbot GH, Bradley J, Edwards JE, Jr, et al. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis. 2006;42:657–68. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- 4.Landman D, Georgescu C, Martin DA, et al. Polymyxins revisited. Clin Microbiol Rev. 2008;21:449–65. doi: 10.1128/CMR.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Nation RL, Turnidge JD, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis. 2006;6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 6.Bergen PJ, Landersdorfer CB, Zhang J, et al. Pharmacokinetics and pharmacodynamics of ‘old’ polymyxins: what is new? Diagn Microbiol Infect Dis. 2012;74:213–23. doi: 10.1016/j.diagmicrobio.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nation RL, Li J. Colistin in the 21st century. Curr Opin Infect Dis. 2009;22:535–43. doi: 10.1097/QCO.0b013e328332e672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decolin D, Leroy P, Nicolas A, et al. Hyphenated liquid chromatographic method for the determination of colistin residues in bovine tissues. J Chromatogr Sci. 1997;35:557–64. doi: 10.1093/chromsci/35.12.557. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Nation RL, Milne RW, et al. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int J Antimicrob Agents. 2005;25:11–25. doi: 10.1016/j.ijantimicag.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Bergen PJ, Li J, Rayner CR, et al. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2006;50:1953–8. doi: 10.1128/AAC.00035-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Milne RW, Nation RL, et al. Stability of colistin and colistin methanesulfonate in aqueous media and plasma studied by high-performance liquid chromatography. Antimicrob Agents Chemother. 2003;47:1364–70. doi: 10.1128/AAC.47.4.1364-1370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace SJ, Li J, Rayner CR, et al. Stability of colistin methanesulfonate in pharmaceutical products and solutions for administration to patients. Antimicrob Agents Chemother. 2008;52:3047–51. doi: 10.1128/AAC.00103-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Milne RW, Nation RL, et al. Pharmacokinetics of colistin methanesulphonate and colistin in rats following an intravenous dose of colistin methanesulphonate. J Antimicrob Chemother. 2004;53:837–40. doi: 10.1093/jac/dkh167. [DOI] [PubMed] [Google Scholar]
- 14.Marchand S, Lamarche I, Gobin P, et al. Dose-ranging pharmacokinetics of colistin methanesulphonate (CMS) and colistin in rats following single intravenous CMS doses. J Antimicrob Chemother. 2010;65:1753–8. doi: 10.1093/jac/dkq183. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Coulthard K, Milne R, et al. Steady-state pharmacokinetics of intravenous colistin methanesulphonate in patients with cystic fibrosis. J Antimicrob Chemother. 2003;52:987–92. doi: 10.1093/jac/dkg468. [DOI] [PubMed] [Google Scholar]
- 16.Plachouras D, Karvanen M, Friberg LE, et al. Population pharmacokinetic analysis of colistin methanesulphonate and colistin after intravenous administration in critically ill patients with Gram-negative bacterial infections. Antimicrob Agents Chemother. 2009;53:3430–6. doi: 10.1128/AAC.01361-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Couet W, Gregoire N, Gobin P, et al. Pharmacokinetics of colistin and colistimethate sodium after a single 80-mg intravenous dose of CMS in young healthy volunteers. Clin Pharmacol Ther. 2011;89:875–9. doi: 10.1038/clpt.2011.48. [DOI] [PubMed] [Google Scholar]
- 18.Marchand S, Frat JP, Petitpas F, et al. Removal of colistin during intermittent haemodialysis in two critically ill patients. J Antimicrob Chemother. 2010;65:1836–7. doi: 10.1093/jac/dkq185. [DOI] [PubMed] [Google Scholar]
- 19.Mohamed AF, Karaiskos I, Plachouras D, et al. Application of a loading dose of colistin methanesulfonate in critically ill patients: population pharmacokinetics, protein binding, and prediction of bacterial kill. Antimicrob Agents Chemother. 2012;56:4241–9. doi: 10.1128/AAC.06426-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karvanen M, Plachouras D, Friberg LE, et al. Colistin methanesulphonate and colistin pharmacokinetics in critically ill patients receiving continuous veno-venous hemodiafiltration (CVVHDF) Antimicrob Agents Chemother. 2013;57:668–71. doi: 10.1128/AAC.00985-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garonzik SM, Li J, Thamlikitkul V, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother. 2011;55:3284–94. doi: 10.1128/AAC.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Nation RL, Turnidge JD. Defining the dosage units for colistin methanesulfonate: urgent need for international harmonization. Antimicrob Agents Chemother. 2006;50:4231. doi: 10.1128/AAC.00756-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Institute for Safe Medication Practices. Warning! Dosing confusion with colistimethate for injection. http://www.ashp.org/DocLibrary/Policy/PatientSafety/NANAlert-Colistimethatesodium.aspx. (24 April 2013, date last accessed)
- 24.Kirsten WJ. Organic Elemental Analysis. New York: Academic Press; 1983. [Google Scholar]
- 25.Dudhani RV, Nation RL, Li J. Evaluating the stability of colistin and colistin methanesulphonate in human plasma under different conditions of storage. J Antimicrob Chemother. 2010;65:1412–5. doi: 10.1093/jac/dkq134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Milne RW, Nation RL, et al. A simple method for the assay of colistin in human plasma, using pre-column derivatization with 9-fluorenylmethyl chloroformate in solid-phase extraction cartridges and reversed-phase high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 2001;761:167–75. doi: 10.1016/s0378-4347(01)00326-7. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Milne RW, Nation RL, et al. Simple method for assaying colistin methanesulfonate in plasma and urine using high-performance liquid chromatography. Antimicrob Agents Chemother. 2002;46:3304–7. doi: 10.1128/AAC.46.10.3304-3307.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao G, Ali FE, Chiu F, et al. Development and validation of a reversed-phase high-performance liquid chromatography assay for polymyxin B in human plasma. J Antimicrob Chemother. 2008;62:1009–14. doi: 10.1093/jac/dkn343. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Milne RW, Nation RL, et al. Use of high-performance liquid chromatography to study the pharmacokinetics of colistin sulfate in rats following intravenous administration. Antimicrob Agents Chemother. 2003;47:1766–70. doi: 10.1128/AAC.47.5.1766-1770.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]