Abstract
G-protein coupled receptors (GPCRs) are a superfamily of cell signaling membrane proteins that include >750 members in the human genome alone. They are the largest family of drug targets. The vast diversity and relevance of GPCRs contrasts with the paucity of structures available: only 21 unique GPCR structures have been experimentally determined as of the beginning of 2013. User-friendly modeling and small molecule docking tools are thus in great demand. While both GPCR structural predictions and docking servers exist separately, with GOMoDo (GPCR Online M odeling and D ocking), we provide a web server to seamlessly model GPCR structures and dock ligands to the models in a single consistent pipeline. GOMoDo can automatically perform template choice, homology modeling and either blind or information-driven docking by combining together proven, state of the art bioinformatic tools. The web server gives the user the possibility of guiding the whole procedure. The GOMoDo server is freely accessible at http://molsim.sci.univr.it/gomodo.
Introduction
GPCRs are a vast superfamily of eukaryotic transmembrane receptors which act as ubiquitously expressed key regulatory elements and constitute more than 30% of current drug targets [1]. Solving GPCR structures is notoriously technically daunting: as of June 2013, structures for only 21 currently unique GPCRs were available, less than 3% of the GPCR diversity of the human genome. Thus, computational tools are needed to obtain structural information for most GPCR-targeting drug design and/or biophysical studies of receptor/ligand interactions.
All GPCRs share a 7-helix membrane spanning architecture; however the average sequence identity between members of the superfamily is often below 20% [2], making target selection and alignment required for homology modeling far from trivial. Nonetheless, homology modeling has succeeded in predicting ligand-target interactions information for several different GPCRs [3–6]. In many cases, bioinformatic tools aided by experimental validation have been crucial for accurate structural characterization [7–11].
Both GPCR modeling servers/databases (GPCR-SSFE [12], GPCR-ITASSER [13], and GPCR-ModSim [14]) and small ligand docking servers exist (MEDock [15], PatchDock [16], and SwissDock [17]). However, there is a lack of tools that allow users to go seamlessly from sequence to docking, as well as letting users guide the procedure with experimental data. An additional hindrance is that a robust in silico approach to drug design and ligand-receptor investigation requires mastering a wide array of software tools, from alignment to modeling to docking and structural refinement. Groups interested in ligand-GPCR structural information do not necessarily have this breadth of structural bioinformatics expertise; furthermore, even users familiar with modeling and docking software may find a quicker and reproducible method very useful. GOMoDo is meant to allow both expert and non-expert users to obtain readily, with minimum effort, biologically and pharmacologically relevant results. GOMoDo, by itself, does not use any novel or untested method, but simply puts together state-of-the-art, proven bioinformatic tools in an easy user interface. The procedure herein automated has been already successfully tested multiple times [8,11,44].
The GOMoDo Pipeline
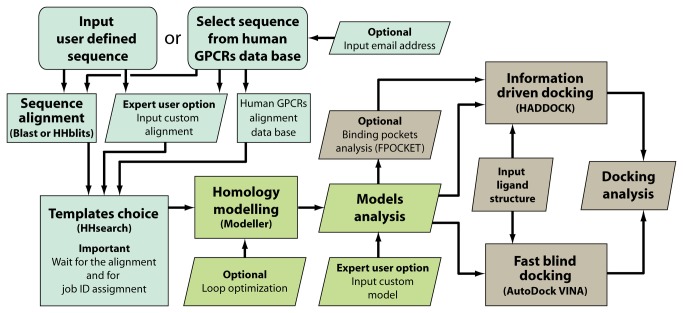
The GOMoDo pipeline is briefly resumed in Figure 1. All the programs used in the pipeline make use of the standard parameters, unless noted.
Figure 1. GOMoDo flow chart colored accordingly to the pipeline section.
Alignment and template choice, homology modeling and model assessment, and docking sections are reported as light blue, light green and gray, respectively.
Alignment and template choice
The server follows two possible routes (Fig. 1). Users can upload a target sequence or can select one of the human GPCRs available in a local database by using the name of the receptor. A standard HHsearch protocol [18,19] is then used to find structurally related templates from remotely homologous sequences. Given that in some cases heuristic algorithms are not accurate enough to obtain a reliable HMM for the target [20], a good alignment is recommended for the generation of a robust HMM profile. For the generation of the HMM, the users can (i) choose to input an alignment of the target with relevant sequences from the same subfamily, (ii) let the server calculate such alignment, or (iii) in the case of human GPCRs, use one of the pre-generated alignment present in the server. For the latter case, sequences of the different GPCRs subclasses, as obtained in the work by Almén and collaborators [21], were aligned by us. We have used the program PROMALS [22], following the same methodology as in refs. 8,11. If a user- or database-provided alignment is not available, the initial multiple sequence alignment is automatically generated by a search of similar sequences, using either classic BLAST [23] or the HMM-based search with HHblits [24]. The user can choose the number of rounds of sequence search in either case. Secondary structure information is added to the alignment using PSI-PRED [25]. A hidden Markov model (HMM) is then built and calibrated from the multiple alignment using HHmake [19], matched to suitable templates of known structure with HHsearch [19] using the database of HMMs available at ftp://toolkit.lmb.uni-muenchen.de/pub/HHsearch/databases/. Suitable templates are chosen from a complete set of GPCRs three-dimensional structures (see Table S1 for a list of PDB codes of available templates as of manuscript submission). Templates and the corresponding HMM database are updated every 1-2 months. Finally a structural alignment is produced for each template using HHmakemodel. Before proceeding to modeling, we allow the users to check the correctness of the alignment with JALVIEW [26]. This procedure has been shown previously to obtain template/target alignments useful for homology modeling whenever the sequence similarity is low but the overall fold similarity is high, as in the case of GPCRs [8,11].
Homology modeling and model quality assessment
A user-chosen number of models is made for each template using MODELLER9v10 [27] with standard single-template parameters (Figure 1, users must have their own MODELLER key). The user can also choose for automatic loop refinements to be performed for each model by means of the standard loopmodel class. For each model, GOMoDo outputs the template PDB code, DOPE score (full and normalized) [28], MOLPDF score [27] and GA341 score [29] as well as other information A scatter plot of the GA341 vs. normalized DOPE score for all models is available. Furthermore, models can be directly submitted to the VADAR model quality assessment server [30]. By using VADAR users can check more than 30 model quality indicators, which include structural descriptors calculated by DSSP [31], WHATIF [32] or PROCHECK [33] like the Ramachandran plot, fractional accessible surface area, fractional volume, 3D quality index and stereo/packing quality index. Upon completion of the modeling job, the user is notified by an automatic e-mail sent by the server. Alternatively, the modeling results can be retrieved directly from the webserver up to 60 days later, by inserting the modeling job ID and/or e-mail address. Alignment and modeling together can take from several tens of minutes to several hours, depending on server load and the number of requested homology models.
Advanced users may prefer to refine or modify the GOMoDo-obtained models by themselves before proceeding to docking. We allow users to upload custom models along with the ones obtained in the output, and to compare them before proceeding. In order to facilitate the user, we include a page with the web links of other GPCRs modeling online tools.
Docking
GOMoDO can dock small molecules and peptides (i) blindly (using AutoDock VINA [34], herein referred as VINA) or (ii) using experimental information (using HADDOCK [35,36]) as reported in Figure 1.
(i) VINA [34] is a fast, multithreaded and accurate rewriting of AutoDock that often outperforms classical AutoDock both in speed and quality [37,38]. A three-dimensional SDF or PDB file of the ligand is all the input required. We also provide a library of olfaction-related compounds from the OlfactionDB database [39]. On the GOMoDo server, we can return ten VINA-docked structures in less than a minute. VINA requires knowledge of the location search space for conformations of the ligand --- in other words, the generic location of the binding site. The server already contains such information for all templates with respect to the template PDB coordinate system; therefore before docking, the model is structurally aligned with the templates using LOVOALIGN [40] to guarantee that the search space box is in the correct position with respect to the model coordinate system. In simple cases (small ligands and targets close in sequence to a modeling template) GOMoDo with VINA can yield quick and reasonable results.
(ii) Available experimental information on the residues involved in ligand binding such as NMR titration experiments or mutagenesis data can be used to guide docking. This can be of crucial importance when docking is based on non-trivial homology models, as is often the case for GPCRs. We therefore offer an interface to the HADDOCK software [35,36], where the user can indicate explicitly the protein residues involved in the receptor-ligand interaction. HADDOCK performs a slower but more refined docking than VINA. In particular, HADDOCK includes a final refinement step with molecular dynamics in explicit water which allows for flexibility of specified residues. This feature has the further positive side effect of including side-chain optimization of the binding cavity. While advanced users can take advantage of the online official HADDOCK server [41] for this (which gives full control over all docking options), we also offer a simplified in-house interface to the HADDOCK software that allows the docking of ligands, peptides or interacting proteins. If the ligand is a small non-protein molecule, HADDOCK requires parameter files with partial charges for the ligand, as well as CNS parameter files. Users can obtain them from PRODRG [42] or produce them using their own calculations. GOMoDo allows downloading the entire HADDOCK output either as a compressed archive, individual clusters or single output structures. HADDOCK advanced refinement is also attractive when experimental information is missing. To use HADDOCK for blind docking in GOMoDo, the user can analyze the model on the server with FPOCKET [43] and obtain in a few seconds predictions of plausible binding pockets; in the Supplementary Information, we give two successful examples of this usage (Figure S1). FPOCKET is also useful for obtaining accessory information for experiment-guided docking.
Application Cases
The methodology automatized in GOMoDo has been already used to structurally characterize ligand-GPCRs adducts [8,11,44]. To assess the server, we tested GOMoDo by reproducing selected examples of known GPCR-ligand complexes present in the PDB (targets). In every case we mimicked what a standard user would do, as advised by the manual. For the selected PDB complexes we took the full protein sequence from UniProt and generated 30 models per template using the default BLAST alignment for HMM generation. We then picked up the best model according to DOPE and GA341 scores, obviously excluding models built on the structure of the target. The ligands present in the crystal structure were then docked to the model using VINA or HADDOCK programs.
Here we describe three examples of applications (Figure 2): the first example is a simple application using the human beta-2 adrenergic receptor (hβ2AR) that has been previously used by some of us as a test case [44]. The second example is the modeling of human dopamine D3 receptor (hD3R), a slightly more complex case because of the low sequence identity between the receptor and the templates. Finally, the third example deals with the human A2A adenosine receptor (hA 2AR) in complex with the antagonist ligand ZM241385 (ZMA). In this case, some difficulties can be expected because the antagonist is a rather bulky molecule. Other application examples, which can be found in Figure S1, deal with the human histamine H1 receptor and human kappa-opioid receptor.
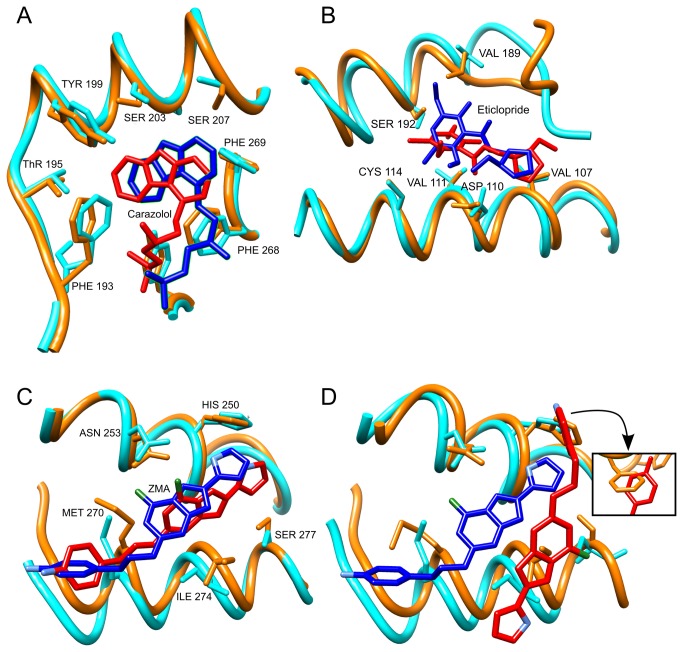
Figure 2. Reproducing GPCR-ligand complexes with GOMoDo.
hβ2AR (A), hD3R (B) and hA 2AR (C,D) binding sites in the immediate neighbourhood of ligands for crystal structures and models obtained with GOMoDo. The homology model and the docked ligand are orange and red, respectively. The experimental structure and ligand are cyan and blue, respectively. (A) VINA docking of carazolol to the hβ2AR model and compared to the crystal structure (PDB: 2RH1). (B) VINA docking of eticlopride to the hD3R model and compared to the crystal structure (PDB: 3PBL). HADDOCK (C) and VINA (D) docking of ZMA to the hA 2AR compared with the crystal structure (PDB: 3EML). Insert in D shows the unphysical artefacts generated by the VINA docking.
hβ2AR
The structure of the hβ2AR (UniProt ID: P07550) was modeled using as a template the structure of the turkey beta-1 adrenergic (tβ1AR) receptor (PDB entry: 2Y00 [45], UniProt ID: P07700). The sequence identity between the two receptors is about 46.1% and thus the model is indeed of relatively good quality [Cα root mean square deviation (rmsd) = 1.3 Å]. Furthermore, the ligand is relatively small and thus it probably does not require significant conformational changes of the side chains to fit in the binding cavity. We docked the ligand carazolol to the hβ2AR model with fast VINA and compared our results against the experimentally determined X-ray structure (PDB code: 2RH1 [46]). The resulting structure of the ligand-receptor complex is in fair agreement with the crystal structure, in terms of both the ligand pose and the side-chains orientation (Figure 2A, rmsd calculated on heavy atoms = 2.9 Å).
hD3R
VINA can also give reasonable results when used with distant homology models. We modelled hD3R (UniProt ID: P35462) in complex with the antagonist eticlopride, and compared the results with the corresponding crystal structure (PDB code: 3PBL [47]). The best template in this case was human sphingosine-1 phosphate receptor (PDB code: 3V2Y [48], UniProt ID: P21453), with a sequence identity of 22.1%. Despite the relatively poor modelling of loops in the binding site, the orientation of the binding pose is correct (Figure 2B, rmsd = 4.0 Å).
hA2AR
An example of a harder task is reproducing the structure of hA 2AR in complex with ZMA, whose crystal structure is available as PDB code 3EML [49] (UniProt ID: P29274). Here, the best template, which happens to be again the tβ1AR (PDB: 2Y00) structure, shares only 28.1% of sequence identity with the target. Hence, the model can be expected to be less accurate (model/target Cα rmsd = 4.0 Å) than the one previously described. In this case, the antagonist is also significantly bulkier. We therefore used HADDOCK [35,36] and we set inter-molecular interactions between the ligand and residues Asn253, Met270, Ile274, Ser277, and His250 as active restraints. We also used the best FPOCKET predicted binding pocket - which encompassed the binding residues - as passive restraints (that is, the residues that HADDOCK is given permission to move to accommodate the ligand). Default options of HADDOCK and default PRODRG settings for ligand were used. The best structure of the most populated HADDOCK-derived cluster, (Figure 2C) is very similar to that of the X-ray structure (rmsd = 2.1 Å) [49].
The third case (the modeling of hA 2AR) shows that advanced docking methods are often required to obtain reasonable structures. Docking of ZMA to the hA 2AR model structure with VINA fails: the ligand is shifted from the correct binding site and flipped by 180 degrees on two axes (Figure 2D, rmsd = 10.5 Å). Moreover, even the VINA best structure in this case shows gross clashes between the ligand and protein side chains due to the difficulty in accommodating the ligand while treating the protein as rigid (Figure 2D, insert). Notice that even if the starting model is the same for VINA and HADDOCK, the backbone and side chain orientation of models is slightly different and often closer to the experimental structure in the latter. This is due to the flexible refinement of HADDOCK performed in explicit solvent, while VINA treats the model as rigid. In Figures S1A and S1B, we show how HADDOCK can also be useful in the absence of experimental information by using FPOCKET to obtain restrains.
Conclusions
GOMoDo is intended to be a user friendly pipeline that puts together several state-of-the-art tools and allows experienced and inexperienced users to obtain GPCR’s homology models together with predictions of ligand binding poses. GOMoDo was developed to be flexible and adaptable to the user’s demands. For this reason, every step of the GOMoDo pipeline allows user intervention (if needed) to i) insert alignments, ii) use homology models generated by other methods, iii) predict binding cavities and iv) include experimental restraints for performing knowledge-based virtual docking experiments. The combination of all these tools in a single publicly available web server is GOMoDo’s novelty. In particular, the possibility of interacting with the server all along the pipeline allows the user to include experimental information from molecular biology experiments into the process, preventing it from becoming a fully-automatic black-box.
Supporting Information
GPCR structures available as templates in GOMoDo as of June 2013. Note that the template database is regularly updated every 1-2 months.
(DOCX)
Further examples of GOMODO in action. Receptor binding sites in the immediate neighbourhood of ligands for crystal structures and models obtained with GOMoDo. The homology model and the docked ligand are orange and red, respectively. The experimental structure and ligand are cyan and blue, respectively.
Here we show examples of successful blind HADDOCK docking, exploiting FPOCKET to guess residues involved in the binding cavity. All the residues corresponding to the best FPOCKET-calculated binding cavity were used as both active and passive restraints. (A) Human histamine H1 receptor (UniProt ID: P35367) in complex with trans-doxepin: model and docking compared with crystal structure (PDB code: 3RZE). Model template is human M2 muscarinic acetylcholine receptor (PDB code: 3UON, UniProd ID: P08172). (B) Human kappa-opioid receptor (UniProt ID: P41145) in complex with the bulky and flexible ligand JDTic: model and docking compared with crystal structure (PDB code: 4DJH). Model template is the mouse μ-opioid receptor (PDB structure: 4DKL, UniProt ID: P42866). In this case the pose is slightly shifted with respect to the crystal structure and rotameric state is different; however position and global orientation are correct. Here nitrogen atoms are in dark green and oxygen atoms in cornflower blue.
(TIF)
Acknowledgments
Faculty of Science, Utrecht University, The Netherlands) For kindly allowing us to include HADDOCK in GOMoDo, Sergio Marin Vargas (University of Verona, Italy) for technical help, and Alexander Peyser (German Research School for Simulation Sciences GmbH, Jülich, Germany) for comments on the manuscript.
Funding Statement
This work has been funded by a grant from the Deutsche Forschungsgemeinschaft (CA 973/6-1) and a grant from Vietnam National Foundation for Science and Technology Development (DFG.2011.01). MS benefited of a postdoctoral fellowship from UniVR. FM is funded by Programma Operativo del Fondo Sociale Europeo 2007/2013 of Friuli Venezia Giulia Region. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Overington JP, Al-Lazikani B, Hopkins AL (2006) How many drug targets are there? Nat Rev Drug Discov 5: 993-996. doi:10.1038/nrd2199. PubMed: 17139284. [DOI] [PubMed] [Google Scholar]
- 2. Rayan A (2010) New vistas in GPCR 3D structure prediction. J Mol Model 16: 183-191. doi:10.1007/s00894-009-0533-y. PubMed: 19551412. [DOI] [PubMed] [Google Scholar]
- 3. Giorgetti A, Raimondo D, Miele AE, Tramontano A (2005) Evaluating the usefulness of protein structure models for molecular replacement. Bioinformatics 21 Suppl 2: ii72-ii76. doi:10.1093/bioinformatics/bti1112. PubMed: 16204129. [DOI] [PubMed] [Google Scholar]
- 4. Lupieri P, Nguyen CH, Bafghi ZG, Giorgetti A, Carloni P (2009) Computational molecular biology approaches to ligand-target interactions. Hfsp J 3: 228-239. doi:10.2976/1.3092784. PubMed: 20119480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tramontano A, Cozzetto D, Giorgetti A, Raimondo D (2008) The assessment of methods for protein structure prediction. Methods Mol Biol 413: 43-57. PubMed: 18075161. [DOI] [PubMed] [Google Scholar]
- 6. Kufareva I, Rueda M, Katritch V, Stevens RC, Abagyan R (2011) Status of GPCR modeling and docking as reflected by community-wide GPCR Dock 2010 assessment. Structure 19: 1108-1126. doi:10.1016/j.str.2011.05.012. PubMed: 21827947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khafizov K, Anselmi C, Menini A, Carloni P (2007) Ligand specificity of odorant receptors. J Mol Model 13: 401-409. doi:10.1007/s00894-006-0160-9. PubMed: 17120078. [DOI] [PubMed] [Google Scholar]
- 8. Biarnés X, Marchiori A, Giorgetti A, Lanzara C, Gasparini P et al. (2010) Insights into the binding of Phenyltiocarbamide (PTC) agonist to its target human TAS2R38 bitter receptor. PLOS ONE 5: e12394. doi:10.1371/journal.pone.0012394. PubMed: 20811630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carlsson J, Coleman RG, Setola V, Irwin JJ, Fan H et al. (2011) Ligand discovery from a dopamine D3 receptor homology model and crystal structure. Nat Chem Biol 7: 769-778. doi:10.1038/nchembio.662. PubMed: 21926995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levit A, Barak D, Behrens M, Meyerhof W, Niv MY (2012) Homology model-assisted elucidation of binding sites in GPCRs. Methods Mol Biol 914: 179-205. PubMed: 22976029. [DOI] [PubMed] [Google Scholar]
- 11. Marchiori A, Capece L, Giorgetti A, Gasparini P, Behrens M et al. (2013) Coarse-grained/molecular mechanics of the TAS2R38 bitter taste receptor: experimentally-validated detailed structural prediction of agonist binding. PLOS ONE 8: e64675. doi:10.1371/journal.pone.0064675. PubMed: 23741366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Worth CL, Kleinau G, Krause G (2009) Comparative Sequence and Structural Analyses of G-Protein-Coupled Receptor Crystal Structures and Implications for Molecular Models. PLOS ONE 4: e7011. doi:10.1371/journal.pone.0007011. PubMed: 19756152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang J, Zhang Y (2010) GPCRRD: G protein-coupled receptor spatial restraint database for 3D structure modeling and function annotation. Bioinformatics 26: 3004-3005. doi:10.1093/bioinformatics/btq563. PubMed: 20926423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodríguez D, Bello X, Gutiérrez-de-Terán H (2012) Molecular Modelling of G Protein-Coupled Receptors Through the Web. Molecular Informatics 31: 334-341. doi:10.1002/minf.201100162. [DOI] [PubMed] [Google Scholar]
- 15. Chang DT, Oyang YJ, Lin JH (2005) MEDock: a web server for efficient prediction of ligand binding sites based on a novel optimization algorithm. Nucleic Acids Res 33: W233-W238. doi:10.1093/nar/gki586. PubMed: 15991337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schneidman-Duhovny D, Inbar Y, Nussinov R, Wolfson HJ (2005) PatchDock and SymmDock: servers for rigid and symmetric docking. Nucleic Acids Res 33: W363-W367. doi:10.1093/nar/gki481. PubMed: 15980490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grosdidier A, Zoete V, Michielin O (2011) SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res 39: W270-W277. doi:10.1093/nar/gkr366. PubMed: 21624888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Söding J (2005) Protein homology detection by HMM-HMM comparison. Bioinformatics 21: 951-960. doi:10.1093/bioinformatics/bti125. PubMed: 15531603. [DOI] [PubMed] [Google Scholar]
- 19. Hildebrand A, Remmert M, Biegert A, Söding J (2009) Fast and accurate automatic structure prediction with HHpred. Proteins 77 Suppl 9: 128-132. doi:10.1002/prot.22499. PubMed: 19626712. [DOI] [PubMed] [Google Scholar]
- 20. Venclovas C (2012) Methods for sequence-structure alignment. Methods Mol Biol 857: 55-82. PubMed: 22323217. [DOI] [PubMed] [Google Scholar]
- 21. Almén MS, Nordström KJ, Fredriksson R, Schiöth HB (2009) Mapping the human membrane proteome: a majority of the human membrane proteins can be classified according to function and evolutionary origin. BMC Biol 7: 50. doi:10.1186/1741-7007-7-50. PubMed: 19678920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pei J, Grishin NV (2007) PROMALS: towards accurate multiple sequence alignments of distantly related proteins. Bioinformatics 23: 802-808. doi:10.1093/bioinformatics/btm017. PubMed: 17267437. [DOI] [PubMed] [Google Scholar]
- 23. Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z et al. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389-3402. doi:10.1093/nar/25.17.3389. PubMed: 9254694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Remmert M, Biegert A, Hauser A, Söding J (2012) HHblits: lightning-fast iterative protein sequence searching by HMM-HMM alignment. Nat Methods 9: 173-175. PubMed: 22198341. [DOI] [PubMed] [Google Scholar]
- 25. Bryson K, McGuffin LJ, Marsden RL, Ward JJ, Sodhi JS et al. (2005) Protein structure prediction servers at University College London. Nucleic Acids Res 33: W36-W38. doi:10.1093/nar/gni035. PubMed: 15980489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ (2009) Jalview Version 2--a multiple sequence alignment editor and analysis workbench. Bioinformatics 25: 1189-1191 [DOI] [PMC free article] [PubMed]
- 27. Martí-Renom MA, Stuart AC, Fiser A, Sánchez R, Melo F et al. (2000) Comparative protein structure modeling of genes and genomes. Annu Rev Biophys Biomol Struct 29: 291-325. doi:10.1146/annurev.biophys.29.1.291. PubMed: 10940251. [DOI] [PubMed] [Google Scholar]
- 28. Shen MY, Sali A (2006) Statistical potential for assessment and prediction of protein structures. Protein Sci 15: 2507-2524. doi:10.1110/ps.062416606. PubMed: 17075131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Melo F, Sánchez R, Sali A (2002) Statistical potentials for fold assessment. Protein Sci 11: 430-448. PubMed: 11790853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Willard L, Ranjan A, Zhang H, Monzavi H, Boyko RF et al. (2003) VADAR: a web server for quantitative evaluation of protein structure quality. Nucleic Acids Res 31: 3316-3319. doi:10.1093/nar/gkg565. PubMed: 12824316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kabsch W, Sander C (1983) Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22: 2577-2637. doi:10.1002/bip.360221211. PubMed: 6667333. [DOI] [PubMed] [Google Scholar]
- 32. Vriend G (1990) WHAT IF: a molecular modelling and drug design program. J Mol Graph 8: 52-56. doi:10.1016/0263-7855(90)80070-V. PubMed: 2268628. [DOI] [PubMed] [Google Scholar]
- 33. Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26: 283–291. doi:10.1107/S0021889892009944. [Google Scholar]
- 34. Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31: 455-461. PubMed: 19499576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dominguez C, Boelens R, Bonvin AM (2003) HADDOCK: a protein-protein docking approach based on biochemical or biophysical information. J Am Chem Soc 125: 1731-1737. doi:10.1021/ja026939x. PubMed: 12580598. [DOI] [PubMed] [Google Scholar]
- 36. de Vries SJ, van Dijk AD, Krzeminski M, van Dijk M, Thureau A et al. (2007) HADDOCK versus HADDOCK: new features and performance of HADDOCK2.0 on the CAPRI targets. Proteins 69: 726-733. doi:10.1002/prot.21723. PubMed: 17803234. [DOI] [PubMed] [Google Scholar]
- 37. Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE et al. (1998) Automated Docking Using a Lamarckian Genetic Algorithm and and Empirical Binding Free Energy Function. J Comput Chem 19: 1639-1662. doi:10.1002/(SICI)1096-987X(19981115)19:14. [Google Scholar]
- 38. Huey R, Morris GM, Olson AJ, Goodsell DS (2007) A semiempirical free energy force field with charge-based desolvation. J Comput Chem 28: 1145-1152. doi:10.1002/jcc.20634. PubMed: 17274016. [DOI] [PubMed] [Google Scholar]
- 39. Modena D, Trentini M, Corsini M, Bombaci A, Giorgetti A (2011) OlfactionDB: A Database of Olfactory Receptors and Their Ligands. Adv Lif Sci 1: 1-5. [Google Scholar]
- 40. Martínez L, Andreani R, Martínez JM (2007) Convergent algorithms for protein structural alignment. BMC Bioinformatics 8: 306. doi:10.1186/1471-2105-8-306. PubMed: 17714583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Vries SJ, van Dijk M, Bonvin AM (2010) The HADDOCK web server for data-driven biomolecular docking. Nat Protoc 5: 883-897. doi:10.1038/nprot.2010.32. PubMed: 20431534. [DOI] [PubMed] [Google Scholar]
- 42. Schüttelkopf AW, van Aalten DM (2004) PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr D Biol Crystallogr 60: 1355-1363. doi:10.1107/S0907444904011679. PubMed: 15272157. [DOI] [PubMed] [Google Scholar]
- 43. Le Guilloux V, Schmidtke P, Tuffery P (2009) Fpocket: an open source platform for ligand pocket detection. BMC Bioinformatics 10: 168. doi:10.1186/1471-2105-10-168. PubMed: 19486540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leguèbe M, Nguyen C, Capece L, Hoang Z, Giorgetti A et al. (2012) Hybrid molecular mechanics/coarse-grained simulations for structural prediction of G-protein coupled receptor/ligand complexes. PLOS ONE 7: e47332. doi:10.1371/journal.pone.0047332. PubMed: 23094046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Warne T, Moukhametzianov R, Baker JG, Nehmé R, Edwards PC et al. (2011) The structural basis for agonist and partial agonist action on a β1-adrenergic receptor. Nature 469: 241-244. doi:10.1038/nature09746. PubMed: 21228877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS et al. (2007) High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science 318: 1258-1265. doi:10.1126/science.1150577. PubMed: 17962520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chien EY, Liu W, Zhao Q, Katritch V, Han GW et al. (2010) Structure of the human dopamine D3 receptor in complex with a D2/D3 selective antagonist. Science 330: 1091-1095. doi:10.1126/science.1197410. PubMed: 21097933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hanson MA, Roth CB, Jo E, Griffith MT, Scott FL et al. (2012) Crystal structure of a lipid G protein-coupled receptor. Science 335: 851-855. doi:10.1126/science.1215904. PubMed: 22344443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jaakola VP, Griffith MT, Hanson MA, Cherezov V, Chien EY et al. (2008) The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science 322: 1211-1217. doi:10.1126/science.1164772. PubMed: 18832607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GPCR structures available as templates in GOMoDo as of June 2013. Note that the template database is regularly updated every 1-2 months.
(DOCX)
Further examples of GOMODO in action. Receptor binding sites in the immediate neighbourhood of ligands for crystal structures and models obtained with GOMoDo. The homology model and the docked ligand are orange and red, respectively. The experimental structure and ligand are cyan and blue, respectively.
Here we show examples of successful blind HADDOCK docking, exploiting FPOCKET to guess residues involved in the binding cavity. All the residues corresponding to the best FPOCKET-calculated binding cavity were used as both active and passive restraints. (A) Human histamine H1 receptor (UniProt ID: P35367) in complex with trans-doxepin: model and docking compared with crystal structure (PDB code: 3RZE). Model template is human M2 muscarinic acetylcholine receptor (PDB code: 3UON, UniProd ID: P08172). (B) Human kappa-opioid receptor (UniProt ID: P41145) in complex with the bulky and flexible ligand JDTic: model and docking compared with crystal structure (PDB code: 4DJH). Model template is the mouse μ-opioid receptor (PDB structure: 4DKL, UniProt ID: P42866). In this case the pose is slightly shifted with respect to the crystal structure and rotameric state is different; however position and global orientation are correct. Here nitrogen atoms are in dark green and oxygen atoms in cornflower blue.
(TIF)