Abstract
Background
The objective of this study was to determine the prognostic significance of viable tumor in post-chemoradiation neck dissection specimens in patients with squamous cell carcinoma of the laryngopharynx.
Methods
Retrospective analysis identified 181 patients treated with primary concurrent chemoradiation for carcinoma of the laryngopharynx at Memorial Sloan-Kettering Cancer Center between the years 1995 and 2005. Of these, 56 patients had a comprehensive neck dissection either as a planned or salvage procedure. Neck dissection specimens were analyzed by a single pathologist for the presence of viable tumor. The presence of viable tumor was correlated to the timing of neck dissection after chemoradiation and to tumor response. Overall survival (OS), disease-specific survival (DSS), and recurrence-free survival (RFS) were determined by the Kaplan–Meier method, and correlation to tumor viability was determined with the log-rank test.
Results
Nineteen (33%) patients had viable tumor in their neck dissection specimens. Viable tumor was higher in patients who had a less-than-complete response to chemoradiation compared with those who had a complete response (42% vs 25%, p = .1). There was no correlation to timing of neck dissection. The 5-year OS, DSS, and RFS were significantly lower in patients who had viable tumor in their neck dissection specimens (OS 49% vs 93%, p = .0005; DSS 56% versus 93%, p = .003; RFS 40% vs 75%, p = .004).
Conclusions
Patients with viable tumor in postchemoradiation neck dissection specimens had a poorer outcome compared with patients with no viable tumor.
Keywords: viable tumor, chemoradiation, neck dissection, prognosis
Management of the neck in patients treated with primary chemoradiation for cancer of the laryngopharynx with a clinically positive neck remains an area of controversy. The neck may be managed in 1 of 3 ways: by observation, by planned neck dissection, or by salvage neck dissection. Observation of the neck can be done in patients who have a complete or near-complete response to treatment and have a negative positron emission tomography (PET) scan result. Evidence for this approach comes from recent studies that have reported low regional recurrence rates.1–6
Planned neck dissection was carried out in the past in patients with N2 and N3 neck disease irrespective of the response to chemoradiation. The neck dissection was carried out 6 weeks after completion of chemoradiation; this approach had the advantage of carrying out the neck dissection before the onset of fibrosis, thereby making the neck dissection technically similar in complexity to a nonchemoradiation neck dissection. Evidence for a policy of planned neck dissection comes from studies that have shown the presence of tumor in neck dissection specimens in patients who have complete or near-complete radiologic and clinical response in the neck.4,7–10 Before the introduction of fluorodeoxyglucose–positron emission tomography (FDG-PET) scanning, planned neck dissection was a common method for managing the neck after chemoradiation. Some groups still practice this policy because of limitations or unavailability of PET. However, in general, most centers now reserve neck dissection for salvage for residual or recurrent neck disease.
Salvage neck dissection is normally carried out if there is clinical or radiologic evidence of neck disease after completion of chemoradiation. Before 2005, our policy was to assess response by clinical examination and CT imaging at 6 to 12 weeks after completion of chemoradiation. However, since 2005, we have been using 18FDG-PET for the assessment of tumor response. The 18FDG-PET is usually carried out at 12 weeks after completion of chemoradiation to minimize any false-positive results from residual inflammation from chemoradiation. Salvage neck dissection in patients who have neck disease as detected by PET is therefore carried out 12 weeks after chemoradiation. This has the disadvantage of making the operation technically more difficult because of the onset of fibrosis. As a consequence, some have advocated the use of selective and superselective salvage neck dissections in these patients.11–14
Clearly there is evidence in the literature to support the practice of planned, as well as salvage neck dissection. However, there is little in the literature that describes what the impact of viable tumor in such neck dissection specimen has on prognosis. In this study, we have examined our own experience of patients undergoing comprehensive neck dissection either as a planned procedure or salvage procedure in the time period before our routine use of PET. The objective was to determine whether viable tumor had a negative impact on prognosis, even after removal of residual tumor by neck dissection.
PATIENTS AND METHODS
Three hundred eighteen patients treated by primary chemoradiation for squamous cell carcinoma of the larynx or pharynx were identified by retrospective review of our database at Memorial Sloan-Kettering Cancer Center (MSKCC) from 1995 to 2005. The study had the approval of MSKCC institutional review board. Patients who did not receive their entire treatment including radiation and chemotherapy at MSKCC (n = 108), were not treated with concurrent chemoradiation (n = 13), and those who had less than a comprehensive neck dissection (n = 16) were excluded. The concurrent chemoradiation regime used consisted of cisplatin on days 1, 22, and 43 and a definitive dose of radiotherapy (once-daily fractions, 66–70 Gy). This left 181 evaluable patients; of these, 56 patients had a post-chemoradiation comprehensive neck dissection, and 125 patients had neck observation. The 56 patients who had a comprehensive neck dissection represent the patient population for this study. This population included patients who had a comprehensive neck dissection either as a planned or salvage procedure.
Table 1 shows the patient and tumor characteristics. The median age was 57 (range, 36–81) and 79% were men. The main primary site was the oropharynx (71%), and 89% of patients had either N2 or N3 neck disease at presentation. Complete response (CR) in the neck to chemoradiation was defined as the absence of abnormality on clinical and radiologic (CT/MRI) examination after chemoradiation. Less than CR was defined as suspicion for residual nodal disease on clinical or radiologic examination. Residual thickening was defined as vague palpable abnormality at the site of previous nodal disease with no discrete radiographic lymph node. Most patients had posttreatment CT scans to assess response to treatment except for 2 patients who had a planned neck dissection. FDG-PET was not routinely used during this study period (1995–2005) and was not used to evaluate response. Of the 56 patients, 12 patients (21%) had a CR, 38 (68%) less than CR, and 6 (11%) residual thickening. The timing of neck dissection ranged from 2 to 18 weeks; neck dissection was carried out at <6 weeks in 4 patients (7%), 6 to 12 weeks in 30 patients (54%), and >12 weeks in 22 patients (39%).
Table 1.
Patient and tumor characteristics
| Characteristic | No. of patients (%) (n = 56) |
|---|---|
| Age group | |
| <60, y | 35 (62%) |
| ≥60, y | 21 (38%) |
| Sex | |
| Female | 12 (21%) |
| Male | 44 (79%) |
| Site of primary | |
| Oropharynx | 40 (71%) |
| Larynx | 11 (20%) |
| Hypopharynx | 5 (9%) |
| Clinical T classification (before chemoradiation) |
|
| T1 | 6 (11%) |
| T2 | 16 (29%) |
| T3 | 19 (33%) |
| T4 | 15 (27%) |
| Clinical neck classification (before chemoradiation) |
|
| N1 | 6 (11%) |
| N2a | 9 (16%) |
| N2b | 26 (46%) |
| N2c | 9 (16%) |
| N3 | 6 (11%) |
| Neck response | |
| Complete response | 12 (21%) |
| Less than complete response | 38 (68%) |
| Residual thickening | 6 (11%) |
| Timing of neck dissection | |
| <6 wk | 4 (7%) |
| 6–12 wk | 30 (54%) |
| >12 wk | 22 (39%) |
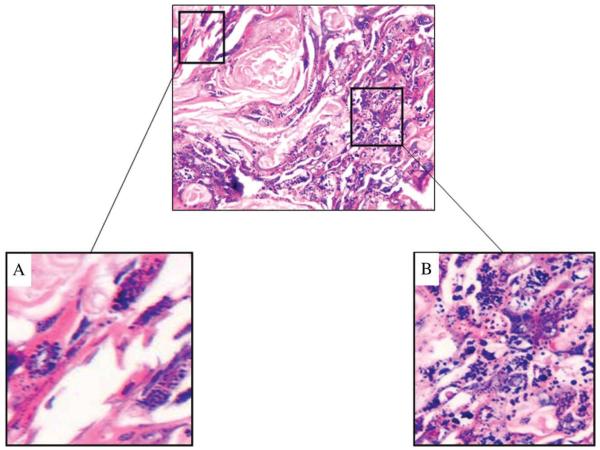
All neck dissection specimens were reviewed by a single pathologist to determine the presence of viable tumor with only hematoxylin and eosin staining. Viable tumor was defined as the presence of epithelial cells within a lymph node, adjacent fibroadipose tissue or muscle, which was still morphologically recognizable as squamous and with intact nuclear chromatin and cell structure. Nonviable tumor was defined as tumor cells showing signs of irreversible cell injury such as karyolysis (Figure 1A) and karyorrhexis (Figure 1B). The presence of viable tumor in the neck dissection specimen was compared to the timing of neck dissection and to the clinical and radiologic neck response to chemoradiation. The prognostic significance of viable tumor for overall survival (OS), disease-specific survival (DSS), and recurrence-free survival (RFS) was determined by the Kaplan–Meier method, and comparisons were performed with the log-rank test. A p value ≤.05 was considered statistically significant. Contingency table comparisons were performed with the Fisher’s exact or Pearson’s chi-square test. Disease-specific survival was defined as death with disease determined at the date of last follow-up or date of death; all patients who died of disease were considered as events, and all patients who died of other causes or were alive with or without disease were censored. Recurrence-free survival was defined as recurrence after treatment determined at the date of first recurrence; all patients who had recurrence after treatment were considered as events, and all patients who did not have recurrence up to the last follow-up or death were censored.
FIGURE 1.
Nonviable tumor was defined as tumor showing signs of irreversible cell injury such as karyolysis (A) and karyorrhexis (B).
RESULTS
The median follow-up time was 60 months (range, 6–130 months). The 5-year OS and DSS were 69% and 79%, respectively. Overall, 19 (33%) patients had viable tumor in their neck dissection specimens. The incidence of viable tumor in the neck dissection specimens stratified by response in the neck to chemoradiation is shown in Figure 2. As may be predicted, viable tumor was more likely in patients with less than a CR (42% of patients with a less than CR vs 25% of patients with a CR), but this was not statistically significant because of the small number of patients. There was no statistically significant correlation between the timing of neck dissection or the pretreatment neck stage with response to chemoradiation (Table 2)
FIGURE 2.
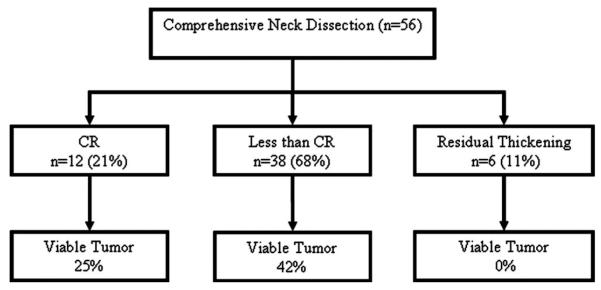
Percentage viable tumor in neck dissection specimens stratified by response to chemoradiation.
Table 2.
Correlation of viable tumor to clinical response, timing of neck dissection and pretreatment neck stage
| Nonviable tumor (n = 37) |
Viable tumor (n = 19) |
p value | |
|---|---|---|---|
| Response | .1 | ||
| Complete response | 9 (75%) | 3 (25%) | |
| Less than complete response | 22 (58%) | 16 (42%) | |
| Residual thickening | 6 (100%) | 0 (0%) | |
| Timing of neck dissection | .9 | ||
| <6 wk | 3 (75%) | 1 (25%) | |
| 6–12 wk | 20 (67%) | 10 (33%) | |
| >12 wk | 14 (64%) | 8 (36%) | |
| Pretreatment clinical neck classification |
.9 | ||
| 1 | 3 (60%) | 2 (40%) | |
| 2 | 29 (67%) | 14 (33%) | |
| 3 | 5 (62%) | 3 (38%) |
The 5-year OS (Figure 3), DSS (Figure 4), and RFS (Figure 5) were significantly lower in those patients who had viable tumor in their neck dissection specimens (OS 49% vs 93%, p = .0005; DSS 56% vs 93%, p = .003; RFS 40% vs 75%, p = .004). In the group with viable tumor (n = 19), recurrence occurred in 12 (63%) patients: 2 local, 1 regional, 5 distant, 2 local/distant, 1 locoregional, 1 regional/distant. In the group with no viable tumor (n = 37), recurrence occurred in 6 (16%) patients: 2 local, 2 distant, 1 locoregional, 1 local/distant.
FIGURE 3.
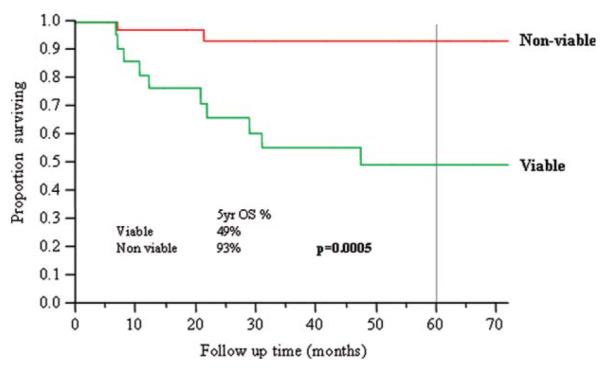
Overall survival and viability of tumor in neck dissection specimens. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
FIGURE 4.
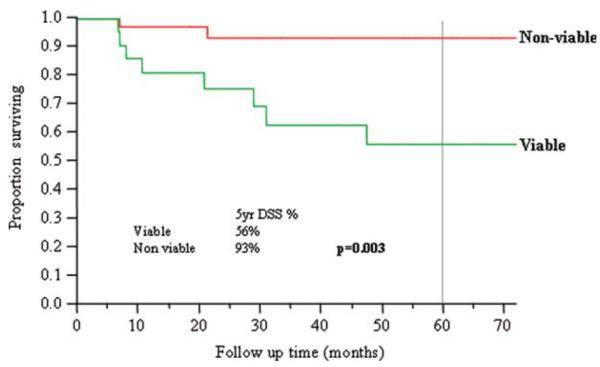
Disease-specific survival and viability of tumor in neck dissection specimens. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
FIGURE 5.
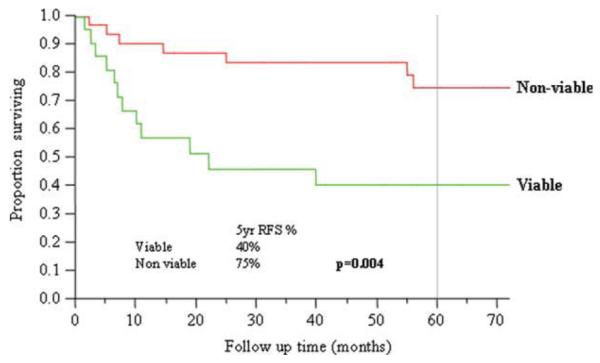
Recurrence-free survival and viability of tumor in neck dissection specimens. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
In this article, we report that patients with viable tumor in neck dissection specimens after chemoradiation had a poorer outcome compared with patients without viable tumor even though viable tumor was removed with neck dissection. Twelve (63%) of the 19 patients with viable tumor had development of further recurrence, of whom 8 had development of distant disease. This indicates that the presence of viable tumor is an indicator for poor outcome. Similar findings have recently been reported by Lango et al,16 who reported a RFS of 85% for tumor-free specimens but 37% for positive pathology. Despite this, however, it is still possible to cure up to one third of patients who have viable tumor in the neck dissection specimen, indicating that neck dissection does benefit select patients. Failure of chemoradiation to sterilize the neck reflects the biologic aggressiveness of the tumor and predicts for failure elsewhere. It is the identification of patients who do not get benefit that remains the challenge.
Limitations of defining viable tumor are severalfold. There is no established, standardized proven method that can objectively measure viable tumor cells remaining in a treated lymph node. The quantification of viable cells and the number necessary for the tumor to continue growth is not firmly established. We can only correlate the presence of viable cells with outcome, in an attempt to quantitate and extrapolate. Perhaps an in vivo murine model could further elucidate survival of malignant squamous cell in a non–growth-conducive environment, that is, a necrotic lymph node, lacking adequate blood supply, and, hence, nutrients. It is also not clear whether the pathologic determination of viable tumor cells correlates with timing of neck dissection. One may expect the number of viable tumor cells may be higher in neck dissection specimens carried out early after completion of chemoradiation. However, it is possible that even these viable cells may be in the process of dying at a molecular level, but the visible hallmarks of cell death are not present. The presence of viable cells may in fact be better assessed in neck dissection specimens carried out several weeks after completion of chemoradiation because the process of cell death will be complete, and any remaining viable cells will have had the chance to grow making detection more feasible. In this study, the detection of viable cells and its relationship to neck dissection timing must be viewed as an uncontrolled variable.
Our study also has several limitations in terms of the heterogenous patient population. Most of our patients had oropharyngeal primary tumors (71%), with a smaller number of patients having laryngeal (20%) and hypopharyngeal (9%) cancer. It is now widely recognized that the response rate to organ-preserving chemoradiation is superior in patients with oropharyngeal cancer than laryngeal and hypopharyngeal cancer and that this is due to the changing epidemiology of the cause of oropharynx cancer from a smoking- and alcohol-related cancer to a cancer caused by human papillomavirus (HPV). Over the past 2 decades, the incidence of HPV in patients with oropharyngeal cancer has increased from 25% to 80%. Nasman et al17 have reported data from Stockholm, in which the proportion of tonsil SCC with HPV positivity on polymerase chain reaction increased from 23% in the 1970s to 28% in the 1980s, 57% in the 1990s, and 79% in 2000–2007. In the most recent data from the University of Michigan, 90% of patients with oropharyngeal squamous cell carcinoma enrolled in the UMCC-0221 trial between 2002–2007 were found to have HPV-positive tumors on polymerase chain reaction and mass spectroscopy.18 The response rates of patients with HPV-related oropharynx cancer have been reported to be better than oropharynx cancer caused by smoking and alcohol.19–20 It is therefore possible that the patients in our study with viable tumor may have primary tumors that were HPV negative. The cell cycle tumor suppressor protein p16 has been reported to be a surrogate marker for HPV through the interaction of the HPV proteins E6 and E7 with the p16/Rb pathway. Retrospective analysis of the HPV status and p16 status of the primary tumors and correlation with patients with viable tumor would provide some insight into this. Additionally, assessment of the HPV and p16 status of neck dissection specimens with viable tumor would be useful because one may find such tumor cells are HPV negative and p16 negative.
We found that patients who had a less-than-complete response were more likely to harbor residual tumor, although this was not significant, most likely because of the small numbers. In our study, 25% of patients who had a complete response had viable tumor in the neck dissection specimen. Other studies have also reported an incidence of residual tumor in 22% to 29% of patients who have a complete response.7–9 This has led to the recommendation from some investigators that neck dissection should be carried out regardless of response to chemoradiation. However, there are several other reports in the literature that report results of neck observation in patients who have had a complete response in the neck.1–6,10 In these studies, the incidence of neck recurrence ranged from 0% to 14%. For example Corry et al,6 reported no regional failures with a median follow-up time of 3 years in 43 patients who had neck observation. Rengen et al3 reported a 14% regional failure rate with 9 of 65 patients having development of neck recurrence. These figures are likely to be a more accurate reflection of the true incidence of viable tumor in complete responders rather than those reported in this study. A policy of neck observation can be justified in these patients, but continued neck surveillance is essential in these patients to detect early the small percentage of patients who will have recurrence.
In patients who had less than a complete response, we found that 42% patients had viable tumor. These figures are also similar to those reported by other authors ranging from 47% to 76%.4,7 For example, Lavertu et al7 reported on 53 patients with stage III and IV head and neck cancer with N2 or N3 disease and who had planned neck dissection 4 to 6 weeks after concurrent chemoradiation. In patients with a less-than-complete response in the neck, 47% had tumor in the nodal specimen. Moreover, this pathologic positivity was demonstrated to negatively impact on disease-specific survival, an observation also found in our study. It is therefore widely accepted that patients without a complete response in the neck should proceed to neck dissection.
Because the presence of viable tumor is an indicator for poor outcome, the early identification of patients who are likely to harbor residual viable tumor, particularly those with the propensity for distant recurrence, is important. If this was feasible, it may be possible to alter the treatment of these patients, possibly by altering the chemoradiation regimen to target distant metastatic disease. Alternatively, it may be possible that using neck dissection as the primary treatment modality for the neck may prevent the formation of distant recurrence, although there is no reports in the literature that have investigated this. The problem is how to predict for the presence of viable tumor. Functional imaging such as 18FDG-PET scan may help. In our patient population, 18FDG-PET was not in routine use for the study period 1995–2005, and so we did not include it in our analysis as a measure of response. However, there are now data that support its ability to predict viable tumor in the neck and primary site after completion of chemoradiation. We have recently reported that 18FDG-PET/CT was able to exclude residual locoregional disease with a high negative predictive value in a cohort of 65 patients with 84 hemi-necks.21 This has therefore resulted in a reduction in the number of unnecessary neck dissections, thus sparing patients the associated morbidity of shoulder dysfunction, wound complications, and reduced quality of life that has been reported to occur.22–26 In addition, 18FDG-PET has also been reported to correlate clinical response to concurrent chemoradiation during therapy.27 However, the negative predictive value of 18FDG-PET increases only with time after chemoradiation, and its value is limited during therapy because of high false-positive results. Other new radiotracers, such as (18F) fluorothymidine, may be more useful than (18F) fluorodeoxyglucose. (18F) fluorothymidine has activity that correlates with cell proliferation through the cytosolic enzyme thymidine kinase.28–31 This is in contrast to (18F) fluorodeoxyglucose, which only provides information about expression of glucose transporters and hexokinase activity. The (18F) fluoromisonidazole is another radiotracer that may be useful. This radiotracer evaluates hypoxia within tumors. Because hypoxia is strongly associated with treatment failure in head and neck cancer,32 the identification of tumors with increased hypoxia before or early on in treatment may allow for alteration in treatment by the addition of radiation sensitizers or by altering treatment to surgery.33 Other noninvasive imaging techniques, such as DCI-MRI and 1H magnetic resonance spectroscopy, may also help in this area. DCI-MRI assesses alterations in tumor blood volume and blood flow during chemoradiation. A recent publication by Cao et al34 reported that increase in blood volume in the primary tumor after 2 weeks of chemoradiation correlated with superior local control rates. The technique could therefore be used to predict response to chemoradiation early on in treatment. Recent studies on brain and cervical tumors have also shown a role for 1H magnetic resonance spectroscopy to detect apoptotic cells in vivo, thus allowing the viable fraction of tumor to be determined.35 These types of approaches may allow clinicians to alter treatment early during chemoradiation and allow for surgical intervention 10 to 14 days into chemoradiation.
In conclusion, our study shows that even when viable tumor is removed with a neck dissection, patients with viable tumor still have a worse outcome, suggesting that viable tumor is an indicator for poor outcome. What we need is a tool that can predict chemoradiosensitivity before treatment is started so that we can select patients who are less likely to respond to chemoradiation and treat instead with primary surgical therapy to the neck followed by radiotherapy or chemoradiation. Alternatively, we may have to rely on methods that are able to predict response early during chemoradiation, such as DCI-MRI, and change course of treatment early to avoid the dilemma of “residual disease” in the neck.
REFERENCES
- 1.Corry J, Peters L, Fisher R, et al. N2-N3 neck nodal control without planned neck dissection for clinical/radiologic complete responders—results of Trans Tasman Radiation Oncology Group Study 98.02. Head Neck. 2008;30:737–742. doi: 10.1002/hed.20769. [DOI] [PubMed] [Google Scholar]
- 2.Lau H, Phan T, Mackinnon J, Matthews TW. Absence of planned neck dissection for the N2-N3 neck after chemoradiation for locally advanced squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck. 2008;134:257–261. doi: 10.1001/archoto.2007.49. [DOI] [PubMed] [Google Scholar]
- 3.Rengan R, Pfister DG, Lee NY, et al. Long term neck control rates after complete response to chemoradiation in patients with advanced head and neck cancer. Am J Clin Oncol. 2008;31:465–469. doi: 10.1097/COC.0b013e31816a6208. [DOI] [PubMed] [Google Scholar]
- 4.Clayman GL, Johnson CJ, Morrison W, Ginsberg L, Lippman SM. The role of neck dissection after chemoradiotherapy for oropharyngeal cancer with advanced nodal disease. Arch Otolaryngol Head Neck. 2001;127:135–139. doi: 10.1001/archotol.127.2.135. [DOI] [PubMed] [Google Scholar]
- 5.Argiris A, Stenson KM, Brockstein BE, et al. Neck dissection in the combined modality therapy of patients with locoregionally advanced head and neck cancer. Head Neck. 2004;26:447–455. doi: 10.1002/hed.10394. [DOI] [PubMed] [Google Scholar]
- 6.Corry J, Rischin, Smith JG, et al. Radiation with concurrent late chemotherapy intensification (“chemoboost”) for locally advanced head and neck cancer. Radiother Oncol. 2000;54:123–127. doi: 10.1016/s0167-8140(99)00182-6. [DOI] [PubMed] [Google Scholar]
- 7.Lavertu P, Adelstein DJ, Saxton JP, et al. Management of the neck in a randomized trial comparing concurrent chemotherapy and radiotherapy with radiotherapy alone in resectable stage III and IV squamous cell head and neck cancer. Head Neck. 1997;19:559–566. doi: 10.1002/(sici)1097-0347(199710)19:7<559::aid-hed1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Sewall GK, Palazzi-Churas KL, Richards GM, Hartig GK, Harari PM. Planned postradiotherapy neck dissection: Rationale and clinical outcomes. Laryngoscope. 2007;117:121–128. doi: 10.1097/01.mlg.0000246709.93530.72. [DOI] [PubMed] [Google Scholar]
- 9.Gourin CG, Williams HT, Seabolt WN, Herdman AV, Howington JW, Terris DJ. Utility of positron emission tomography-computed tomography in identification of residual nodal disease after chemoradiation for advanced head and neck cancer. Laryngoscope. 2006;116:705–710. doi: 10.1097/01.MLG.0000215176.98582.A9. [DOI] [PubMed] [Google Scholar]
- 10.Brizel DM, Prosnitz RG, Hunter S, et al. Necessity for adjuvant neck dissection in setting of concurrent chemoradiation for advanced head and neck cancer. Int J Radiat Oncol Biol Physiol. 2004;58:1418–1423. doi: 10.1016/j.ijrobp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Stenson KM, Haraf DJ, Pelzer H, et al. The role of cervical lymph-adenectomy after aggressive concomitant chemoradiotherapy: the feasibility of selective neck dissection. Arch Otolaryngol Head Surg. 2000;126:950–956. doi: 10.1001/archotol.126.8.950. [DOI] [PubMed] [Google Scholar]
- 12.Mukhija V, Gupta S, Jacobson AS, Anderson Eloy J, Genden EM. Selective neck dissection following adjuvant therapy for advanced head and neck cancer. Head Neck. 2009;31:183–188. doi: 10.1002/hed.20944. [DOI] [PubMed] [Google Scholar]
- 13.Sandhu A, Rao N, Giri S, et al. Role and extent of neck dissection for persistent nodal disease following chemoradiation for locally advanced head and neck cancer: how much is enough. Acta Oncol. 2008;47:948–953. doi: 10.1080/02841860701644060. [DOI] [PubMed] [Google Scholar]
- 14.Yeung AR, Liauw SL, Amdur RJ, et al. Lymph node positive head and neck cancer treated with definitive radiotherapy. Cancer. 2008;112:1076–1082. doi: 10.1002/cncr.23279. [DOI] [PubMed] [Google Scholar]
- 15.Robbins KT, Shannon K, Vieira F. Superselective neck dissection after chemoradiation: feasibility based on clinical and pathologic comparisons. Arch Otolaryngol Head Neck. 2007;133:486–489. doi: 10.1001/archotol.133.5.486. [DOI] [PubMed] [Google Scholar]
- 16.Lango MN, Andrews GA, Ahmad S, et al. Postradiotherapy neck dissection for head and neck squamous cell carcinoma: pattern of pathologic residual carcinoma and prognosis. Head Neck. 2009;31:328–337. doi: 10.1002/hed.20976. [DOI] [PubMed] [Google Scholar]
- 17.Nasman A, Attner P, Hammarstedt L, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer. 2009;125:362–366. doi: 10.1002/ijc.24339. [DOI] [PubMed] [Google Scholar]
- 18.Maxwell JH, Kumar B, Feng FY, et al. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010;16:1226–1235. doi: 10.1158/1078-0432.CCR-09-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 20.Lindquist D, Romanitan M, Hammarstedt L, et al. Human papillomavirus is a favourable prognostic factor in tonsillar cancer and its oncogenic role is supported by the expression of E6 and E7. Mol Oncol. 2007;1:350–355. doi: 10.1016/j.molonc.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ong SC, Schöder H, Lee NY, et al. Clinical utility of 18F-FDG PET/CT in assessing the neck after concurrent chemoradiotherapy for locoregional advanced head and neck cancer. J Nucl Med. 2008;49:532–540. doi: 10.2967/jnumed.107.044792. [DOI] [PubMed] [Google Scholar]
- 22.Sasslet AM, Esclamado RM, Wolf GT. Surgery after organ preservation therapy. Analysis of wound complications. Arch Otolaryngol Head Neck Surg. 1995;121:162–165. doi: 10.1001/archotol.1995.01890020024006. [DOI] [PubMed] [Google Scholar]
- 23.Lavertu P, Bonafede JP, Adelstein DJ, et al. Comparison of surgical complications after organ preservation therapy in patients with stage III and IV squamous cell head and neck cancer. Arch Otolaryngol Head Neck Surg. 1998;124:401–406. doi: 10.1001/archotol.124.4.401. [DOI] [PubMed] [Google Scholar]
- 24.Davidson BJ, Newlark KA, Harter KW, et al. Complications from planned, posttreatment neck dissections. Arch Otolaryngol Head Neck Surg. 1999;125:401–405. doi: 10.1001/archotol.125.4.401. [DOI] [PubMed] [Google Scholar]
- 25.Morgan JE, Breau RL, Suen JY, et al. Surgical wound complications after intensive chemoradiotherapy for advanced squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2007;133:10–14. doi: 10.1001/archotol.133.1.10. [DOI] [PubMed] [Google Scholar]
- 26.Donatelli-Lassig AA, Duffy SA, Fowler KE, Ronis DL, Chepeha DB, Terrell JE. The effect of neck dissection on quality of life after chemoradiation. Otolaryngol Head Neck Surg. 2008;139:511–518. doi: 10.1016/j.otohns.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berlangieri SU, Brizel DM, Scher RL, et al. Pilot study of positron emission tomography in patients with advanced head and neck cancer receiving radiotherapy and chemotherapy. Head Neck. 1994;16:340–346. doi: 10.1002/hed.2880160408. [DOI] [PubMed] [Google Scholar]
- 28.Buck AK, Herrmann K, Shen C, Dechow T, Schwaiger M, Wester HJ. Molecular imaging of proliferation in vivo: positron emission tomography with (18F) fluorothymidine. Methods. 2009;48:205–215. doi: 10.1016/j.ymeth.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Mankoff DA, Eary JF. Proliferation imaging to measure early cancer response to targeted therapy. Clin Cancer Res. 2008;14:7159–7160. doi: 10.1158/1078-0432.CCR-08-2233. [DOI] [PubMed] [Google Scholar]
- 30.Bading JR, Shields AF. Imaging of cell proliferation: status and prospects. J Nucl Med. 2008;49(Suppl):64S–80S. doi: 10.2967/jnumed.107.046391. [DOI] [PubMed] [Google Scholar]
- 31.Garcia C, Flamen P. Role of positron emission tomography in the management of head and neck cancer in the molecular therapy era. Curr Opin Oncol. 2008;20:275–279. doi: 10.1097/CCO.0b013e3282faa0cb. [DOI] [PubMed] [Google Scholar]
- 32.Nordsmark M, Bentzen SM, Rudat V, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy: an international multi-center study. Radiother Oncol. 2005;77:18–24. doi: 10.1016/j.radonc.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 33.Schoder H, Fury M, Lee N, Kraus D. PET monitoring of therapy response in head and neck squamous cell carcinoma. J Nucl Med. 2009;50:74–88. doi: 10.2967/jnumed.108.057208. [DOI] [PubMed] [Google Scholar]
- 34.Cao Y, Popovtzer A, Li D, et al. Early prediction of outcome in advanced head and neck cancer based on tumor blood volume alterations during therapy: a prospective study. Int J Radiation Oncology Biol Physiol. 2008;72:1287–1290. doi: 10.1016/j.ijrobp.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palambo B. Brain tumor recurrence: brain single-photon emission computed tomography, PET and proton magnetic resonance spectroscopy. Nucl Med Commun. 2008;29:730–735. doi: 10.1097/MNM.0b013e3283000049. [DOI] [PubMed] [Google Scholar]