SUMMARY
Reprogramming somatic cells into pluripotent embryonic stem cells (ESCs) by somatic cell nuclear transfer (SCNT) has been envisioned as an approach for generating patient-matched nuclear transfer (NT)-ESCs for studies of disease mechanisms and for developing specific therapies. Past attempts to produce human NT-ESCs have failed secondary to early embryonic arrest of SCNT embryos. Here, we identified premature exit from meiosis in human oocytes and suboptimal activation as key factors that are responsible for these outcomes. Optimized SCNT approaches designed to circumvent these limitations allowed derivation of human NT-ESCs. When applied to premium quality human oocytes, NT-ESC lines were derived from as few as two oocytes. NT-ESCs displayed normal diploid karyotypes and inherited their nuclear genome exclusively from parental somatic cells. Gene expression and differentiation profiles in human NT-ESCs were similar to embryo-derived ESCs, suggesting efficient reprogramming of somatic cells to a pluripotent state.
INTRODUCTION
Cytoplasmic factors present in mature, metaphase II (MII)-arrested oocytes have a unique ability to reset the identity of transplanted somatic cell nuclei to the embryonic state. Since the initial discovery in amphibians (Gurdon, 1962), somatic cell nuclear transfer (SCNT) success in a range of different mammalian species has demonstrated that such reprogramming activity in enucleated or spindle-free oocytes (cytoplasts) is universal (Campbell et al., 1996; Solter, 2000; Wilmut et al., 1997, 2002). However, despite numerous applications of SCNT for animal cloning, the nature of reprogramming oocyte factors and their mechanism of action remain largely unknown.
In humans, SCNT was envisioned as a means of generating personalized embryonic stem cells from patients’ somatic cells, which could be used to study disease mechanisms and ultimately for cell-based therapies (Lanza et al., 1999; Yang et al., 2007). However, the derivation of human nuclear transfer-embryonic stem cells (NT-ESCs) has not been achieved despite numerous attempts during the past decade. The roadblock responsible for failure is early embryonic arrest of human SCNT embryos precluding derivation of stable NT-ESCs. Typically, SCNT embryos fail to progress beyond the eight-cell stage, presumably due to an inability to activate critical embryonic genes from the somatic donor cell nucleus (Egli et al., 2011; Noggle et al., 2011). In a few cases, when SCNT embryos did reach the blastocyst stage, either stable ESCs were not recovered or derivation was not attempted (Fan et al., 2011; French et al., 2008). Though the underlying cause of early developmental arrest remains unclear, most of these studies involving human oocytes applied SCNT protocols developed for nonprimate species. Previously, we demonstrated that SCNT procedures, when adapted to primates, succeeded in reprogramming rhesus macaque adult skin fibroblasts into NT-ESCs (Byrne et al., 2007; Sparman et al., 2009). Therefore, we reasoned that, similar to other mammals, human MII oocytes must contain reprogramming activity.
Several recent observations are relevant. Removal of human oocytes’ nuclear genetic material (chromosomes) negatively impacts the cytoplast’s subsequent ability to induce reprogramming (Noggle et al., 2011). However, when a somatic cell nucleus is transplanted into an intact oocyte containing its own chromosomes, the resulting polyploid embryos are able to develop to blastocysts and support ESC derivation. One possible explanation for these observations is that critical reprogramming factors in human MII oocytes are physically associated with the chromosomes or spindle apparatus and are depleted or critically diminished upon enucleation. Alternatively, it is possible that one or more of the steps in SCNT—namely, oocyte enucleation, donor cell nucleus introduction, or cytoplast activation—negatively impact cytoplast quality, rendering it incapable of inducing sufficient reprogramming.
In considering distinct biological features of human oocytes that could be involved, we focused on our recent observation that meiotic arrest in human MII oocytes is unstable and can be easily perturbed by mechanical manipulations (Tachibana et al., 2013). Earlier, we suggested that retention of meiosis-specific activities in the cytoplast is critical for nuclear remodeling after transplantation of an interphase-stage somatic cell nucleus (Mitalipov et al., 2007). This remodeling is positively correlated with onward development of SCNT embryos after activation. Therefore, we systematically evaluated modifications in oocyte enucleation and donor cell introduction that might work to retain meiosis factors in human cytoplasts. We also determined that routine cytoplast activation treatments were insufficient to support subsequent human SCNT embryo development. We initially used rhesus macaque oocytes to evaluate factors affecting successful SCNT reprogramming in a primate system. Subsequently, we refined SCNT approaches with high-quality human oocytes donated by healthy volunteers and demonstrated that methodological alterations significantly improve blastocyst formation from human SCNT embryos. Moreover, we derived several human NT-ESC lines from these embryos and validated that their nuclear DNA is an exclusive match to parental somatic cells, whereas mitochondrial DNA originated almost exclusively from oocytes. We also conducted extensive pluripotency assays on human NT-ESCs to verify reprogramming.
RESULTS
SCNT Protocol Optimization in a Nonhuman Primate Model
Our recent studies demonstrated human MII oocyte sensitivity to premature activation induced by removal and reintroduction of meiotic spindles (Tachibana et al., 2013) and to the use of electrofusion in the context of cytoplast activation (Tachibana et al., 2009). Consequently, our present investigation began with optimizing the use of envelope from inactivated hemagglutinating virus of Japan (HVJ-E) to fuse nuclear donor cells with enucleated MII oocytes while maintaining cytoplasts in meiosis (Tachibana et al., 2009). Because of limited oocyte availability, we first tested both the feasibility and efficacy of HVJ-E-based cell fusion on the development of rhesus macaque SCNT embryos.
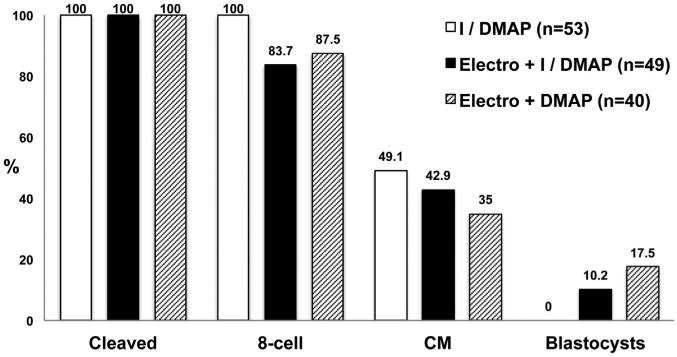
The fusion rate of adult fibroblasts with cytoplasts was 100% after HVJ-E treatment; however, and unexpectedly, the SCNT embryos generated by HVJ-E fusion failed to progress beyond the compact morula (CM) stage following standard ionomycin/DMAP (I/DMAP) activation. We previously demonstrated that monkey SCNT embryos produced by electrofusion developed into blastocysts (Byrne et al., 2007; Sparman et al., 2009). Therefore, we postulated that exposure of the cytoplast to an electropulse (electroporation) could be beneficial for SCNT reprogramming, perhaps as a supplemental activation stimulus. To test this possibility, we exposed HVJ-E-fused SCNT embryos to electroporation before the standard I/DMAP activation treatment. Ten percent of SCNT embryos were capable of reaching the blastocyst stage (Figure 1). Interestingly, this SCNT blastocyst formation rate was unaffected even when exposure to ionomycin was omitted and SCNT embryos were activated with electroporation followed by DMAP treatment (Figure 1). Together, these results indicate that, although an electroporation stimulus is not required for cell fusion, it is supportive of proper cytoplast activation following SCNT.
Figure 1. Development of Monkey SCNT Embryos Reconstructed with Optimized Protocols.
Although HVJ-E fusion was efficient, SCNT constructs required activation by electroporation for blastocyst formation. Elimination of ionomycin from the activation treatment further improved blastocyst development. I, ionomycin; DMAP, 6-DMAP; CM, compact morula. See also Tables S1 and S2.
Histone deacetylase inhibitors, such as trichostatin A (TSA), have been associated with improved SCNT reprogramming in several mammalian species (Ding et al., 2008; Kishigami et al., 2006; Li et al., 2008). We previously demonstrated enhanced development of monkey SCNT embryos treated with 37.5 nM TSA (from 4% up to 18% blastocyst development rate [Sparman et al., 2010]). However, blastocyst quality and potential to give rise to stable ESCs remained unknown. Here, we plated 16 monkey SCNT blastocysts produced during TSA treatment on mitotically inactivated mouse embryonic fibroblast (mEF) feeders, but none resulted in NT-ESC line isolation (Table S1). We reasoned that, although TSA treatment was promoting blastocyst formation, high TSA concentrations may negatively affect blastocyst quality and epiblast lineage integrity. Therefore, we tested several lower TSA concentrations, as well as shorter exposure times, on monkey SCNT blastocyst development and ESC isolation. Reducing the TSA concentration to 10 nM or shortening the TSA exposure time from 24 to 12 hr did not affect blastocyst rates (Table S2). However, only SCNT blastocysts produced with 10 nM TSA supported derivation of stable monkey NT-ESC lines, though the number of plated blastocysts was small (Table S1). We concluded that these optimized protocols in a nonhuman primate model were adequate to serve as a starting point for further testing with human oocytes.
Producing Human SCNT Blastocysts and NT-ESC Lines
Initially, human MII oocytes from healthy volunteers were exposed to the SCNT approach that produced the best results in the nonhuman primate. Oocytes were retrieved following standard ovarian stimulation protocols and transvaginal follicular aspirations. Human dermal fibroblasts of fetal origin (HDF-f) synchronized in G0/G1 cell-cycle phase were used as nuclear donors (Figure 2A). Spindle removal and HVJ-E-assisted donor cell fusion were carried out within 60 min of oocyte retrieval.
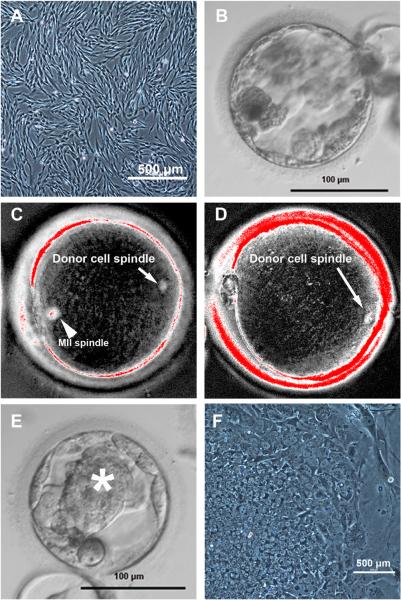
Figure 2. SCNT Blastocyst Development Is Affected by Premature Cytoplast Activation.
(A) Morphology of nuclear donor fetal fibroblasts before SCNT. (B) Poor-quality human SCNT blastocyst without distinct ICM produced with suboptimal protocols; note the presence of excluded cells.
(C) Spindle-like structures detected when donor nuclei were introduced into intact MII oocytes, but not when introduction was conducted after enucleation. Arrowhead and arrow point at the maternal MII spindle and somatic cell spindle, respectively.
(D) Somatic nuclear cell spindles were formed in cytoplasts when oocyte enucleation and fusion were conducted in the presence of caffeine.
(E) Human SCNT blastocyst with prominent ICM (asterisk) produced after caffeine treatment.
(F) NT-ESC colony with typical morphology derived from a caffeine-treated SCNT human blastocyst.
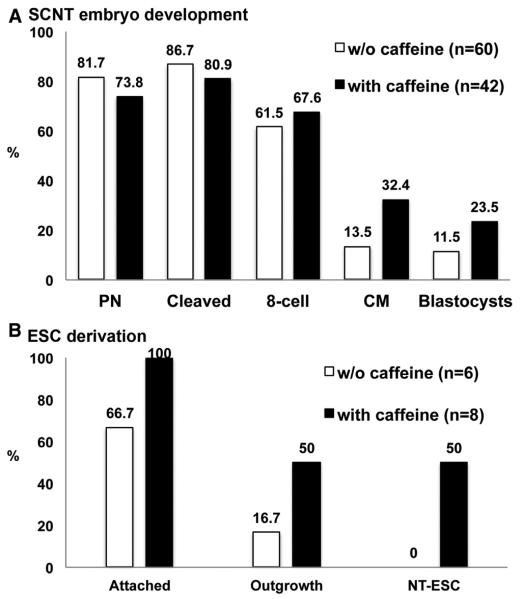
Most oocytes (95.2% [60 out of 63]) survived MII spindle removal conducted under polarized microscopy (Oosight) (Byrne et al., 2007; Sparman et al., 2009), and nuclear donor fibroblasts were introduced with 100% efficacy using HVJ-E based fusion. Somatic cell nuclei did not form spindle-like structures that were detectable by noninvasive examination under polarized microscopy. Immediately after confirmation of fusion, oocytes were activated with electroporation/DMAP (4 hr) and exposed to 10 nM TSA for 12 hr. Most embryos (81.7% [49 out of 60]; Figure 3A, without caffeine group) formed one or two pronuclei at the time of removal from TSA, whereas a slightly higher portion of embryos cleaved (86.7% [52 out of 60]), suggesting that some SCNT embryos did not exhibit visible pronuclei at the time of examination (Figure 3A). Most cleaved embryos developed to the eight-cell stage (61.5% [32 out of 52]), but few progressed to compact morula (13.5% [7 out of 52]) and blastocyst (11.5% [6 out of 52]) stages (Figure 3A). Activation of embryonic genes and transcription from the transplanted somatic cell nucleus are required for development of SCNT embryos beyond the eight-cell stage (Egli et al., 2011; Noggle et al., 2011). Therefore, these results are consistent with the premise that our modified SCNT protocol supports reprogramming of human somatic cells to the embryonic state.
Figure 3. Development of Human SCNT Embryos and NT-ESC Derivation after Caffeine Treatment.
(A) Improved blastocyst development of human SCNT embryos treated with caffeine. A total of 63 (five cycles) and 43 (three cycles) oocytes were utilized for SCNT without or with caffeine, respectively. Sixty (95.2%) and 42 (97.7%) oocytes survived after SCNT micromanipulations.
(B) NT-ESCs were derived only from blastocysts produced with caffeine. See also Figures S1, S2 and S3.
Of note, human SCNT blastocysts exhibited poorly organized trophectoderm and small or undetectable inner cell masses (ICMs). In addition, large blastomere-like cells were often excluded (Figure 2B). Nevertheless, we plated six SCNT blastocysts onto feeder layers to examine their ability to support ESC derivation. Though four blastocysts attached to mEFs, only one gave an outgrowth that, after further passaging, failed to produce stable ESC-like cells (Figure 3B).
Though the observed SCNT blastocyst development rate was encouraging, further optimization of the human protocol focused on improvements in embryo quality. To assess whether spindle removal in human oocytes could cause spontaneous exit from meiosis, we introduced somatic nuclei into intact MII oocytes and examined their birefringence properties under polarized microscopy. All introduced somatic cell nuclei efficiently formed spindle-like structures that were visible within 30 min of fusion (17 out of 17) (Figure 2C). Again, spindle formation was not observed when somatic cell nuclei were fused with manipulated, spindle-free oocytes (0 out of 3). These observations are consistent with recent conclusions that human MII oocytes undergo premature activation secondary to spindle removal (Tachibana et al., 2013). Assuming that meiosis-specific factors are retained during spindle removal but decline due to spontaneous activation, we focused on noninvasive treatments to maintain meiotic arrest during manipulation.
We reported previously that the exposure of monkey oocytes to caffeine, a protein phosphatase inhibitor, was effective in protecting the cytoplast from premature activation and improved development of SCNT embryos (Mitalipov et al., 2007). Therefore, human oocytes were maintained in 1.25 mM caffeine during spindle removal and somatic cell fusion. As expected, somatic cell nuclei introduced into cytoplasts under these conditions efficiently formed spindle-like structures that were detectable under birefringence microscopy (83.3% [10 out of 12]) (Figure 2D). More importantly, the blastocyst development rate of caffeine-treated embryos was notably enhanced (23.5%) compared to the standard SCNT group (Figure 3A, with caffeine group), and blastocysts were characterized by visible and prominent ICMs, similar to those observed for IVF-produced embryos (Figure 2E).
Remarkably, when eight SCNT blastocysts produced with caffeine exposure were utilized for ESCs isolation, all attached to mEFs and four formed ICM outgrowths (Figure 3B), which gave rise to ESC-like colonies upon manual splitting onto fresh mEF plates (Figure 2F). Subsequent passaging resulted in the propagation of stable ESC colonies with typical morphology and growth characteristics. This surprisingly high ESC derivation rate was similar to that reported in our previous study with human IVF-derived blastocysts (50%) and was even higher than in manipulated spindle transfer embryos (38%) (Figure 3B) (Tachibana et al., 2013).
Collectively, our findings indicate that a protocol developed in the monkey model supported blastocyst development for human SCNT embryos. However, poor SCNT blastocyst quality precluded ESC isolation. Subsequent incorporation of caffeine during enucleation and fusion allowed improved blastocyst development and ESC line derivation.
Reproducibility of Human SCNT Results
Interestingly, all four human NT-ESC lines were derived from oocytes retrieved from one egg donor (egg donor A). Eight mature MII oocytes were recovered after a single stimulation cycle. Using fetal dermal fibroblasts as nuclear donor cells and following our caffeine-incorporated SCNT protocol, five blastocysts were produced (62.5%) that gave rise to four NT-ESC lines (80%) (Figure S1 available online). In the context of generating patient-specific pluripotent stem cells, reproducible results with various patient-derived somatic cells and with different egg donors are a necessity.
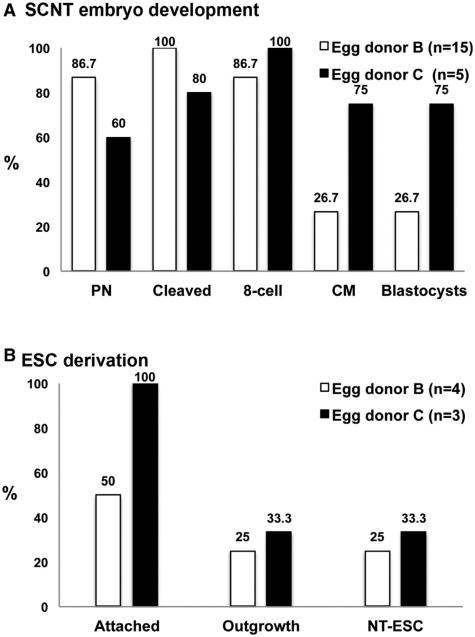
We therefore acquired a skin fibroblast culture from a patient with Leigh syndrome. A total of 15 and 5 MII oocytes were collected from two unrelated egg donor volunteers (B and C) and were used for SCNT with these fibroblasts. All oocytes survived spindle removal and successfully fused with nuclear donor cells. Following activation and culture, four (27%, [4 out of 15]) and three (60% [3 out of 5]) blastocysts were produced from these egg donors (Figure 4A). After plating on mEFs and manual passaging, we established two stable NT-ESC lines—one from each oocyte cohort (Figure 4B). Thus, these outcomes confirm the reproducibility of our human SCNT protocols.
Figure 4. Validation of Human SCNT with Nuclear Donor Cells Derived from a Leigh’s Disease Patient.
(A) In vitro development of SCNT embryos produced with skin fibroblast cells from a Leigh’s disease patient and two different egg donors (egg donors B and C). Fifteen MII oocytes were retrieved from egg donor B, whereas only five oocytes were collected from donor C. SCNT blastocysts were generated from both oocyte cohorts.
(B) NT-ESC derivation efficiency allowed isolation of one cell line per egg donor cycle.
See also Figures S2 and S3.
Retrospective Analysis of Factors Affecting the Success of Human SCNT
Whereas SCNT manipulations and treatments were strictly controlled, the quality and quantity of human oocytes retrieved from different egg donors varied significantly. High oocyte numbers retrieved from an ovarian stimulation cycle are generally associated with poor clinical IVF outcomes (Pellicer et al., 1989; Santos et al., 2010; van der Gaast et al., 2006).
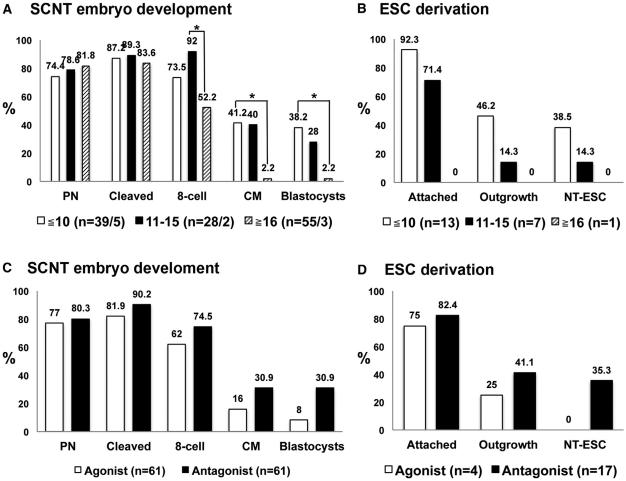
Therefore, we conducted a retrospective analysis of ovarian stimulation procedures and the number of oocytes retrieved per cycle versus SCNT embryo development and NT-ESC derivation outcomes. We divided oocyte donation cycles into three groups based on the range of collected mature MII oocytes— specifically, 10 or fewer oocytes per cycle (five donors), between 11 and 15 oocytes (two donors), and more than 16 oocytes per cycle (three donors). Although survival after spindle removal, fusion, pronuclear formation, and cleavage of SCNT embryos was similar between these groups, more embryos derived from the ≥ 16 MII oocytes/cycle group arrested after the eight-cell stage compared to the other two groups (Figure 5A). In addition, the quality of recovered SCNT blastocysts correlated inversely with the number of collected oocytes per cycle. Whereas five NT-ESC lines were derived from donors producing ≤ 10 oocytes/cycle, only one was recovered from the 11–15 group, and no cell line was established from cycles with ≥ 16 oocytes (Figure 5B). The peak systemic estradiol (E2) level in egg donors prior to hCG priming positively correlated with the subsequent yield of oocytes (Figure S2). Thus, these observations imply that high numbers of oocytes collected from ovarian stimulation protocols are associated with poor oocyte quality and reduced reprogramming ability in the context of SCNT.
Figure 5. Ovarian Stimulation and Human SCNT Outcomes.
(A) Human SCNT development varied with the number of oocytes collected from each ovarian stimulation cycle. Cycles producing ten or fewer oocytes were associated with improved development of SCNT embryos.
(B) The efficacy of NT-ESC derivation also positively correlated with fewer numbers of oocytes collected in the ovarian stimulation cycle.
(C and D) SCNT embryo development from cycles treated with GnRH agonists or antagonists. Blastocyst development was higher for oocytes recovered from donors receiving a GnRH antagonist. NT-ESCs were derived only from oocytes recovered from donors receiving GnRH antagonist.
See also Figures S2 and S3 and Table S3.
In an effort to define optimal stimulation protocols that are compatible with high-quality oocytes for SCNT, we analyzed the impact of GnRH agonist and antagonist used to suppress pituitary function in egg donors. Prior to ovarian stimulation, antimullerian hormone (AMH) levels and antral follicle counts (AFC) were measured for each egg donor (Table S3). Donors with higher AMH and AFC profiles indicative of high ovarian reserve received GnRH agonist (Lupron, four cycles), and the remaining donors received GnRH antagonist (Ganirelix, six cycles) (Table S3). The average number of MII oocytes (mean ± SD) collected per cycle was not statistically different between the two groups (11.7 ± 5.6 and 20.5 ± 11.9, respectively). However, SCNT embryo development beyond the eight-cell stage was suboptimal for oocytes produced following GnRH agonist treatment (Figure 5C). Moreover, all six NT-ESC lines were derived exclusively from oocytes collected from GnRH antagonist-treated cycles (Figure 5D). Based on these observations, it is possible that pituitary suppression with GnRH antagonist during ovarian stimulation may positively impact oocyte reprogramming capability, making them compatible with SCNT blastocyst development and ESC isolation.
Lastly, we looked at whether pronuclear formation can be used as a predictive marker for SCNT outcomes. Most SCNT embryos formed a single pronucleus the day after nuclear transfer (56% [68 out of 122]), whereas a small portion (20% [24 out of 122]) displayed two pronuclei (Figure S3). As indicated above, pronuclear formation was not observed in some one-cell SCNT embryos (20% [24 out of 122]), whereas a minority (5% [6 out of 122]) were already at the two-cell stage by the time of examination (Figure S3). After separate culture, we determined that cleavage and early preimplantation development were similar among these groups, and although the blastocyst rate was higher in SCNT embryos with two pronuclei (39%), stable NT-ESC lines were produced from all four embryo types (Figure S3). Thus, despite the small number of SCNT embryos analyzed, it is reasonable to conclude that visible pronuclear formation as conducted in these studies does not directly correlate with NT-ESC derivation.
Analysis of Human NT-ESCs
To confirm SCNT origin and define the degree of reprogramming, we expanded and extensively analyzed the four NT-ESC lines derived from HDF-f fetal fibroblasts (designated as hESO-NT1, hESO-NT2, hESO-NT3, and hESO-NT4).
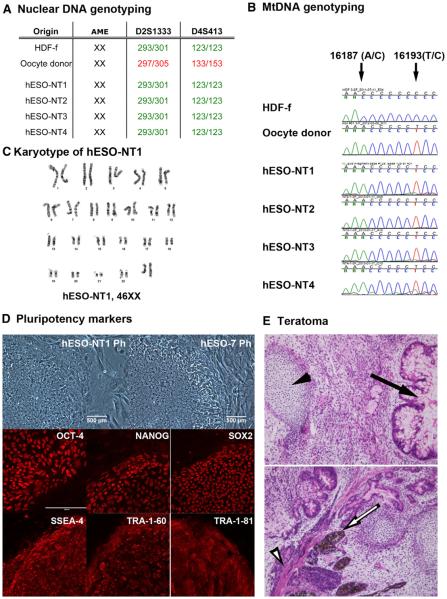
Initially, we employed microsatellite typing for 23 markers mapping 22 human autosomal loci and one X-linked locus for nuclear genome genotyping (Tachibana et al., 2013). The results unequivocally matched all four NT-ESC lines to the nuclear donor fetal fibroblasts, with no detectable contribution of oocyte alleles (Figure 6A and Table S4). The defining feature of SCNT is that the mitochondrial genome (mtDNA) in SCNT embryos and in NT-ESCs is largely contributed by the oocyte. As expected, analysis of mtDNA sequences within the displacement loop (D loop) containing the hypervariable segment (HSV) confirmed that NT-ESC lines inherited mainly oocyte mtDNA (Figure 6B). During fusion of cytoplasts with nuclear donor fibroblasts, a small amount of somatic cell mtDNA is cotransferred into the resultant embryos that may result in heteroplasmy. We employed sensitive ARMS-qPCR (amplification refractory mutation system-quantitative polymerase chain reaction) and detected a low level of somatic cell mtDNA in all four NT-ESC lines (3.4% ± 1.7%; range 1.2%–4.9%) (Table S5). Interestingly, this carryover was higher than what we had previously observed in ST-ESC lines (0.6% ± 0.9%) derived after spindle transfer between oocytes (Tachibana et al., 2013). Cytogenetic analysis by G-banding analysis indicated that all four NT-ESC lines contained a normal euploid female karyotype (46XX) with no numerical or structural abnormalities (Figures 6C and S4).
Figure 6. Genetic, Cytogenetic, and Pluripotency Analysis of Human NT-ESCs.
(A) Nuclear DNA genotyping from four human NT-ESC lines (hESO-NT1, hESO-NT2, hESO-NT3, and hESO-NT4) determined by microsatellite parentage analysis. A total of 24 microsatellite markers were used for each analysis. The representative markers for D2S1333 and D4S413 loci demonstrate that the nuclear DNA in these cell lines was exclusively derived from the somatic HDF-f cell line. No contribution of oocyte nuclear DNA was detected.
(B) mtDNA genotyping by Sanger sequencing demonstrated that all NT-ESC lines contain oocyte mtDNA.
(C) Cytogenetic G-banding analysis confirmed that all NT-ESCs exhibited a normal 46XX karyotype (hESO-NT1 result is representative).
(D) Human NT-ESCs expressed standard pluripotency markers detected by immunocytochemistry for antibodies against OCT4, NANOG, SOX2, SSEA-4, TRA-1–60, and TRA-1–81. Original magnification, ×200; Ph, phase contrast. Note that the upper-left image for hESO-NT1 is the same shown in Figure 2F. The upper-right image for hESO-7 is the same shown in Figure S5 (upper-right).
(E) Histological analysis of teratoma tumors produced after injection of human NT-ESCs into SCID mice. An arrow and arrowhead in the top panel indicate intestinal-type epithelium with goblet cells (endoderm) and cartilage (mesodermal), respectively. An arrow and arrowhead in the lower panel depict neuroecto-dermal (ectoderm) and muscle (mesoderm) tissues, respectively. Original magnification, ×200.
See also Figures S4 and S5 and Tables S4 and S5.
To assess pluripotency in NT-ESC lines, we initially examined expression of generic stem cell markers by immunocytochemistry (ICC) and compared the results to two IVF-derived ESC lines (hESO-7 and -8). These control ESC lines and the four NT-ESC lines were established from oocytes donated by the same donor (egg donor A), and thus they carried identical mtDNA (Tachibana et al., 2013). Similar to controls, all NT-ESC lines expressed OCT-4, NANOG, SOX2, SSEA-4, TRA-1-60, and TRA-1-81 (Figures 6D and S5). Moreover, when injected into immunodeficient SCID mice, all NT-ESC lines produced tumors containing tissue and cell types representing all three germ layers (Figure 6E). An in vitro differentiation assay demonstrated efficient formation of embryoid bodies in suspension culture that, after attachment, formed spontaneously contracting cardiomyocytes (Movie S1).
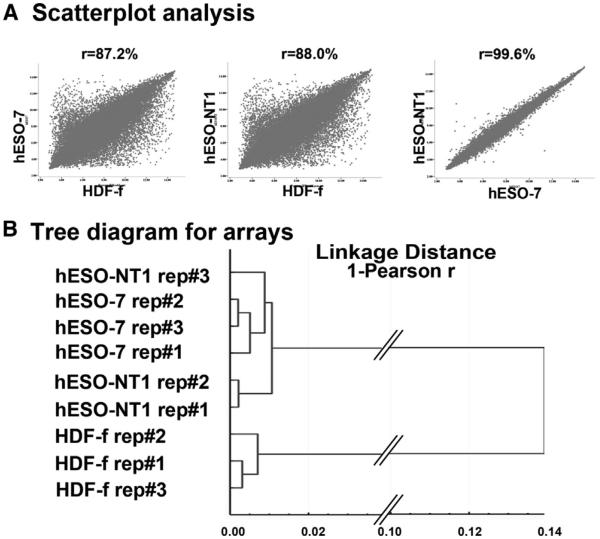
Lastly, we conducted microarray expression analysis of the hESO-NT1 cell line and compared results to the IVF control, hESO-7, and parental somatic cells HDF-f using the Affymetrix PrimeView platform. Initially, three biological replicates within each sample were compared against each other. For comparisons, the detected signal for each probe set was plotted in a scattergraph, and the correlation value was calculated. This assay demonstrated 99% transcriptional correlation within each cell type, suggesting that minimal variations existed between biological replicates collected from different culture plates (Figure S6). Each NT-ESC and IVF-ESC type was compared against each other and against somatic cells (HDF-f). As expected, both stem cell types displayed low transcriptional correlation to fibroblasts (Figures 7A and 7B). Among 50 genes with the highest fold change, many known pluripotency genes were observed, including LIN28, POU5F1, NANOG, and SOX2 (Table S6). In contrast, ESCs derived by IVF and SCNT were similar to each other (Figures 7A and 7B). Some transcriptional differences between human NT-ESCs and IVF-ESCs were observed. However no known pluripotency genes were included in this list (Tables S7 and S8). Interestingly, the HLA-C major histocompatibility gene was highly downregulated in hESO-NT1 compared with hESO-7 (79-fold) (Table S8).
Figure 7. Microarray Expression Analysis of Human NT-ESCs.
(A) Scatterplot analysis comparing expression profiles of human NT-ESCs (hESO-NT1) with IVF-derived ESC controls (hESO-7) and parental skin fibroblasts (HDF-f). Both IVF-ESCs and NT-ESCs displayed low transcriptional correlation to fibroblasts (left and middle) but were similar to each other (right).
(B) Tree diagram analysis linking NT-ESCs to IVF-ESCs. See also Figure S6 and Tables S6, S7, and S8.
DISCUSSION
We demonstrate here for the first time the successful reprogramming of human somatic cells into ESCs following SCNT. By systematic analysis of SCNT procedures, in some cases informed by studies in the rhesus monkey, we identified several steps, including spindle removal, donor cell fusion, and cytoplast activation, that are critical for cellular reprogramming and SCNT blastocyst development. Previous studies have indicated that meiotic arrest in human MII oocytes is unstable such that intrusive manipulations can induce rapid exit from the metaphase stage (Tachibana et al., 2013). However, successful integration into and reprogramming of interphase (G0/G1) somatic cell nuclei in MII cytoplasts are critically important and dependent on nuclear remodeling events associated with high meiotic kinase activities present in cytoplasts (Choi and Campbell, 2010; Egli et al., 2008; Mitalipov et al., 2007). Meiotic cytoplasts induce rapid nuclear envelope breakdown (NEBD) and premature chromosome condensation (PCC) in transplanted interphase nuclei and convert them to spindle-like structures. It has been proposed that NEBD and PCC are required for precise cell-cycle synchronization between the incoming interphase donor nucleus and the recipient mitotic cytoplast (Egli et al., 2008). Such drastic chromatin transformations are associated with efficient reprogramming and improved development of SCNT embryos, whereas lack of or incomplete nuclear remodeling leads to early developmental arrest (Mitalipov et al., 2007; Nakajima et al., 2008; Wakayama et al., 1998; Wu et al., 2007).
Another issue is that somatic cell-specific transcription and epigenetic factors maintaining cellular identity become dissociated from the chromatin during NEBD and PCC and are more actively replaced by oocyte-specific programs (Egli et al., 2008). Here, we suggest that retention of meiotic activity in human MII cytoplasts aided by enucleation in the presence of caffeine and HVJ-E-based fusion enhances conversion of somatic cell nuclei to spindle-like structures. Moreover, human SCNT embryos generated with our approach developed to blastocysts and NT-ESCs.
Another important finding in our study is that standard activation treatments involving exposure to ionomycin and 6-DMAP are not sufficient for supporting development of human SCNT embryos. During normal fertilization, sperm entry triggers oocyte activation that is critical for completion of meiosis and for the initiation of mitotic divisions. Activation is also critical for the oocyte’s cytoplasm to acquire the reprogramming and metabolic activity that is necessary to support subsequent development (Susko-Parrish et al., 1994). Presently, the efficacy of artificial activation protocols is commonly measured by induction of parthenogenetic development in intact MII oocytes (Mitalipov et al., 2001), and it is generally accepted that, if artificial activation supports parthenogenetic embryo development to blastocysts and ESCs, such treatments should be sufficient to induce similar outcomes with reconstructed SCNT cytoplasts. However, in the present studies, we found that supplemental electroporation treatment was critical for cytoplast activation and subsequent reprogramming was compatible with improved SCNT development and ESC derivation. Thus, it is reasonable to speculate that the requirements for oocyte or cytoplast activation in SCNT and parthenogenetic systems are different.
We also show that human SCNT reprogramming is dependent on human oocyte quality. Particularly, larger numbers of oocytes retrieved following ovarian stimulation protocols with agonist were negatively correlated with human SCNT blastocyst development. Thus, it is speculated that ovarian stimulation protocols routinely utilized for IVF treatments might be altered somewhat if the goal is obtaining ESC derivation by SCNT. Conversely, suboptimal quality oocytes derived by in vitro maturation or other sources are likely to be unsuitable for SCNT (S.M., unpublished data). Clearly, further studies addressing gonadotropin dosage and pituitary suppression regimens should be evaluated in the context of recovering human oocytes suitable for SCNT.
It is also important to note that the oocyte quality is ultimately linked to the genetic constitution of individual egg donors. Indeed, some oocyte cohorts did not support SCNT blastocyst development with the present protocol, whereas efficient blastocyst formation (23% [5 out of 21]) has been reported using different protocols involving electrofusion followed by activation with calcium ionophore and DMAP or DMAP/cytochalasin D (French et al., 2008). Nevertheless, reflecting what we presumed to be exceptional oocyte quality from one donor, five blastocysts were produced from just eight oocytes, which subsequently resulted in the derivation of four NT-ESC lines. Interestingly, prior egg donation from this donor was also associated with exceptional outcomes that supported derivation of four ESC lines following ST procedures (Tachibana et al., 2013). Although the underlying genetic factors contributing to oocyte quality remain unknown, certain FMR-1 alleles, defined by the CGG nucleotide repeats, correlate with improved oocyte quality and IVF success (Gleicher et al., 2011). Clearly, further studies are warranted to elucidate the genetic and clinical parameters associated with optimal oocyte quality for human SCNT.
Given past difficulties in achieving the ultimate goal of producing human NT-ESCs, it was generally assumed that derivation of ESCs via SCNT would require an inordinate number of oocytes and thus could not be scaled up for widespread therapeutic use (Daley and Solbakk, 2011). However, here, our revamped SCNT protocols allowed derivation of at least one ESC line from each oocyte donation cycle.
A battery of pluripotency tests performed on human NT-ESCs demonstrated their similarities to genuine IVF-derived ESCs. Transcriptional interrogation indicated that NT-ESCs departed from their parental somatic cell gene expression pattern with up-regulation of pluripotency associated genes. In addition, NT-ESCs demonstrated the ability to differentiate into a variety of other cell types in teratoma tumors. In-vitro-directed differentiation induced formation of contracting cardiomyocytes, demonstrating their potential for regenerative medicine. Genetic analyses showed that all four NT-ESC lines tested to date contained normal diploid karyotypes, with no detectable gross chromosomal abnormalities or contribution from the oocyte genome apart from mtDNA.
An approach to patient-specific pluripotent stem cell derivation that precludes the use of embryos is based on somatic cell reprogramming by induced expression of a few critical transcription factors (referred as induced pluripotent stem cells [iPSCs]) (Takahashi et al., 2007; Takahashi and Yamanaka, 2006; Yu et al., 2007). Recent studies have concluded that human iPSCs are characterized by high frequencies of subchromosomal copy number alterations compared to IVF-derived ESCs (Laurent et al., 2011). Some of these genetic changes were associated with the reprogramming process itself, whereas others could have been inherited from the parental somatic cells (Laurent et al., 2011). In addition, iPSC-specific methylation and transcriptional abnormalities in imprinted regions and X chromosomes were also described (Nazor et al., 2012). Direct comparisons between iPSCs and NT-ESCs in the mouse indicated that such abnormalities are less frequent in the latter case, concluding that SCNT-based reprogramming is more efficient in resetting the epigenetic identity of parental somatic cells (Kim et al., 2010). Further comparisons of the genetic, epigenetic, and transcriptional characteristics of human NT-ESCs, IVF-ESCs, and iPSCs are clearly justified.
Lastly, one of the fundamental differences of SCNT-based reprogramming is that NT-ESCs contain mtDNA almost exclusively originating from the oocyte. This fact is generally underappreciated but may represent an advantage over iPSC derivation because it ensures that NT-ESCs acquire the potential to produce metabolically functional cells and tissues for cell therapies, irrespective of the nuclear donor cell mtDNA. Thus, SCNT offers a strategy for correcting of mtDNA mutations and rescuing the metabolic function of pluripotent cells from patients with inherited or acquired mtDNA diseases.
EXPERIMENTAL PROCEDURES
Rhesus Macaque SCNT
All animal procedures were approved by the Institutional Animal Care and Use Committee at the Oregon National Primate Research Center. Oocyte collections, SCNT, embryo culture, and NT-ESC isolation procedures were performed as previously described (Byrne et al., 2007; Sparman et al., 2009, 2010).
Human Oocyte Donations
The study protocols were approved by both the OHSU Embryonic Stem Cell Research Oversight Committee and the Institutional Review Board. Anonymous egg donors of ages 23–33 were recruited through the OHSU Women’s Health Research Unit via print and web-based advertising. Responding women were screened with respect to their reproductive, medical, and psychosocial health. Healthy nonobese (BMI < 28 kg/m2) women who passed the initial medical and psychological evaluations were invited to participate in a research egg donation program. Egg donors were financially compensated for the time, effort, discomfort, and inconvenience associated with the donation process.
Ovarian stimulation protocols followed established clinical IVF guidelines as described previously (Tachibana et al., 2013). In brief, a combination of recombinant human-follicle-stimulating hormone (rFSH) and human menopausal gonadotropins (hMG) and either GnRH agonist (Lupron, Tap Pharmaceutical Products) or antagonist (Ganirelix, Merck) were given. Human chorionic gonadotropin (hCG) was prescribed to trigger oocyte maturation. Self-administration of injectable rFSH (sc, Follistim, Merck) commenced on cycle day 2 or 3 and continued for ~8–12 days. The starting gonadotropin dose was 75–125 IU/day and 1–2 A hMG (sc, Menopur, Ferring Pharmaceuticals). The dose was adjusted per individual response using an established stepdown regimen until the day of hCG injection. Ovarian response and follicular growth were monitored by transvaginal ultrasound and measurement of serum estradiol levels. When two or more follicles reached ≥ 18 mm in diameter, subjects received hCG (104 IU, sc, Ovidrel, EMDSerono) to trigger follicle and oocyte maturation. Thirty-six hours following hCG injection, subjects underwent oocyte retrieval via transvaginal follicular aspiration.
Cumulus-oocyte complexes (COCs) were collected from aspirates and placed in HTF w/HEPES medium (LifeGlobal, IVFonline) supplemented with 10% serum substitute supplement (SSS; Quinns Advantage Serum, CooperSurgical) (HTF w/HEPES 10%) at 37°C. COCs were treated with hyaluronidase to disaggregate cumulus and granulosa cells. Oocytes were isolated and classified as germinal vesicle (GV), meiotic metaphase I (MI), and mature metaphase II (MII) stage and were then placed in Global medium (LifeGlobal, IVFonline) supplemented with 10% SSS (Global 10%) at 37°C in 5% CO2 and covered with tissue culture oil (Sage IVF, Cooper Surgical).
Nuclear Donor Cell Preparations
Commercially available female dermal fibroblasts of fetal origin (HDF-f) were obtained from ScienCell Research Laboratories, and Leigh syndrome patient cells were acquired from the Coriell Cell Repositories. Cells were expanded in 75 cm3 cell culture flasks (Corning) containing DMEM/F12 supplemented with 100 IU ml−1 penicillin, 100 μg ml−1 streptomycin (Invitrogen), 10% FBS at 37° C in 5% CO2. Fibroblasts were then disaggregated with trypsin treatment and were frozen down in aliquots of 3 × 105 cells in medium containing 10% dimethyl sulphoxide (DMSO, Sigma). Cells were subsequently thawed prior to SCNT and cultured in four-well dishes (Nunc) under standard conditions until they reached confluency. Confluent cells were synchronized in the G0/G1 phase of the cell cycle by culture in DMEM/F12 medium with 0.5% FBS for 2–4 days before SCNT.
Human SCNT Procedure and Embryo Culture
Enucleations were performed as described previously (Tachibana et al., 2013). Oocytes were placed into a 50 μl manipulation droplet of HTF w/HEPES 10% medium containing 5 μg/ml cytochalasin B and 1.25 mM caffeine in a glass-bottom dish. The droplet was covered with tissue culture oil, and oocytes were maintained at 37°C for 10–15 min before spindle removal. The dish was then mounted on the stage of an inverted microscope (Olympus IX71) equipped with a stage warmer (http://www.tokaihit.com), Narishige micromanipulators, Oosight Imaging System (http://www.cri-inc.com), and a laser objective (http://www.hamiltonthorne.com). An oocyte was positioned using a holding pipette so that the spindle was situated close to the 2 to 4 o’clock position. The zona pellucida next to the spindle was drilled with laser pulses, and an enucleation pipette was inserted through the opening. A small amount of cytoplasm surrounded by plasma membrane and contacting the spindle was aspirated into the pipette. Next, a disaggregated fibroblast was aspirated into a micropipette and was briefly transferred to the drop containing HVJ-E extract (Ishihara Sangyo Kaisha). The cell was then placed into the perivitelline space of the cytoplast on the side opposite the first polar body. This construct was rinsed with HTF w/HEPES 10%, transferred to global 10% medium, and incubated at 37°C in 5% CO2 for 30 min until fusion occurred as confirmed visually by the disappearance of the donor cell from the perivitelline space. Constructs were then subjected to artificial activation consisting of electroporation pulses (two 50 μs DC pulses of 2.7 kV cm−1 ) (Electro Square Porator T-820, BTX) in 0.25 M d-sorbitol buffer containing 0.1 mM calcium acetate, 0.5 mM magnesium acetate, 0.5 mM HEPES, and 1 mg ml−1 fatty-acid-free BSA. Activated SCNT constructs were then incubated in Global medium (without serum) containing 2 mM DMAP at 37°C in 5% CO2 for 4 hr. After DMAP, SCNT embryos were rinsed with HTF w/HEPES 10% and transferred into four-well dishes containing Global medium supplemented with 10% FBS, 12 μM β-mercaptoethanol (BME), and 10 nM Trichostatin A (TSA, Sigma) and cultured at 37°C in 5% CO2, 5% O2, and 90% N2 for 12 hr. Embryos were then rinsed, checked for pronuclear formation, and cultured in Global medium supplemented with 10% FBS and 12 μM β-mercaptoethanol (BME) at 37°C in 5% CO2, 5% O2, and 90% N2 for a maximum of 7 days. The medium was changed only once, at day 3 of culture.
Isolation, Culture, and Characterization of Human NT-ESCs
After zona pellucida removal via brief exposure to 0.5% protease (Sigma), SCNT blastocysts were plated onto confluent feeder layers of mitomycin-C-inactivated mouse embryonic fibroblasts (mEFs) and were cultured for 5–7 days at 37°C, 3% CO2, 5% O2, and 92% N2 in ESC derivation medium consisting of DMEM/F12 (Invitrogen) supplemented with 0.1 mM nonessential amino acids, 1 mM l-glutamine, 0.1 mM β-mercaptoethanol, 5 ng/ml basic fibroblast growth factor, 10 mM ROCK inhibitor (Sigma), 10% FBS, and 10% knockout serum replacement (KSR; Invitrogen). Before use, fresh ESC derivation medium was mixed (50%:50%, v/v) with derivation medium conditioned in a 24 hr culture with growing human ESCs. Outgrowths of the inner cell mass (ICMs) were manually dissociated into small clumps with a microscalpel and were replated on fresh mEF plates. After the first passage of ICM outgrowths, FBS and ROCK inhibitor were omitted, and KSR was increased to 20%. Colonies with ESC-like morphologies were selected for further propagation, characterization, and cytogenetic analyses.
Immunocytochemistry, in vivo and in vitro differentiation, and microarray analyses were performed as described (Byrne et al., 2007; Mitalipov et al., 2007; Tachibana et al., 2013). Detailed protocols are also available in the Extended Experimental Procedures.
Nuclear DNA Genotyping and Cytogenetic Analyses
Genotyping of NT-ESCs was performed by microsatellite typing using 23 markers representing 22 human autosomal loci and one X-linked locus, as previously described (Tachibana et al., 2013). Karyotyping was performed by GWT banding on 20 metaphase cells from each human NT-ESC line at the Human Genetics Laboratory, University of Nebraska Medical Center, as previously described (Tachibana et al., 2013).
Mitochondrial DNA Genotyping
Genotyping of mtDNA was performed as previously described (Tachibana et al., 2013). The human mitochondrial displacement loop region (D loop) harboring the hypervariable segment 1(HSV-1) was amplified using published primers (Danan et al., 1999). PCR products were sequenced, and the informative single nucleotide polymorphic (SNP) sites were identified using Sequencher v. 4.7 software (GeneCodes). Quantitative mtDNA analysis was performed by ARMS-qPCR as described (Tachibana et al., 2013). The detailed protocol is also available in Extended Experimental Procedures.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to acknowledge the OHSU Embryonic Stem Cell Research Oversight Committee and the Institutional Review Board for providing oversight and guidance with human embryo and ESC studies. We thank egg donor volunteers and the staff at the Women’s Health Research Unit at the Center for Women’s Health, the Division of Reproductive Endocrinology & Infertility of the Department of Obstetrics & Gynecology of Oregon Health & Science University for their support and procurement of human gametes. The Division of Animal Resources, Surgery Team, Assisted Reproductive Technology & Embryonic Stem Cell Core, Endocrine Technology Core, and Imaging & Morphology Core at the Oregon National Primate Research Center provided expertise and services for the nonhuman primate studies. Hamilton Thorne donated the XYClone laser system for this study. We are grateful to Dr. Warren Sanger and Dianna Zaleski for karyotyping services, Dr. Cecilia Penedo for microsatellite analysis, and Dr. Cary Harding for assistance with nuclear donor cells. We are also indebted to Vanessa Domush, Elizabeth Smolens, Cathy Ramsey, Ying Li, Riffat Ahmed, Brittany Daughtry, and Erin Wolff for their technical support. The human oocyte/embryo research was supported by OHSU institutional funds and by the grant from Leducq Foundation. The nonhuman primate studies were supported by grants from the National Institutes of Health HD063276, HD057121, HD059946, EY021214, and 8P51OD011092.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Extended Experimental Procedures, six figures, eight tables, and one movie and can be found with this article online at http://dx.doi.org/10.1016/j.cell.2013.05.006.
REFERENCES
- Byrne JA, Pedersen DA, Clepper LL, Nelson M, Sanger WG, Gokhale S, Wolf DP, Mitalipov SM. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature. 2007;450:497–502. doi: 10.1038/nature06357. [DOI] [PubMed] [Google Scholar]
- Campbell KH, McWhir J, Ritchie WA, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996;380:64–66. doi: 10.1038/380064a0. [DOI] [PubMed] [Google Scholar]
- Choi I, Campbell KH. Treatment of ovine oocytes with caffeine increases the accessibility of DNase I to the donor chromatin and reduces apoptosis in somatic cell nuclear transfer embryos. Reprod. Fertil. Dev. 2010;22:1000–1014. doi: 10.1071/RD09144. [DOI] [PubMed] [Google Scholar]
- Daley GQ, Solbakk JH. Stem cells: Triple genomes go far. Nature. 2011;478:40–41. doi: 10.1038/478040a. [DOI] [PubMed] [Google Scholar]
- Danan C, Sternberg D, Van Steirteghem A, Cazeneuve C, Duquesnoy P, Besmond C, Goossens M, Lissens W, Amselem S. Evaluation of parental mitochondrial inheritance in neonates born after intracytoplasmic sperm injection. Am. J. Hum. Genet. 1999;65:463–473. doi: 10.1086/302484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Wang Y, Zhang D, Wang Y, Guo Z, Zhang Y. Increased pre-implantation development of cloned bovine embryos treated with 5-aza-2′-deoxycytidine and trichostatin A. Theriogenology. 2008;70:622–630. doi: 10.1016/j.theriogenology.2008.04.042. [DOI] [PubMed] [Google Scholar]
- Egli D, Birkhoff G, Eggan K. Mediators of reprogramming: transcription factors and transitions through mitosis. Nat. Rev. Mol. Cell Biol. 2008;9:505–516. doi: 10.1038/nrm2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli D, Chen AE, Saphier G, Ichida J, Fitzgerald C, Go KJ, Acevedo N, Patel J, Baetscher M, Kearns WG, et al. Reprogramming within hours following nuclear transfer into mouse but not human zygotes. Nat. Commun. 2011;2:488. doi: 10.1038/ncomms1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Jiang Y, Chen X, Ou Z, Yin Y, Huang S, Kou Z, Li Q, Long X, Liu J, et al. Derivation of cloned human blastocysts by histone deacetylase inhibitor treatment after somatic cell nuclear transfer with β-thalassemia fibroblasts. Stem Cells Dev. 2011;20:1951–1959. doi: 10.1089/scd.2010.0451. [DOI] [PubMed] [Google Scholar]
- French AJ, Adams CA, Anderson LS, Kitchen JR, Hughes MR, Wood SH. Development of human cloned blastocysts following somatic cell nuclear transfer with adult fibroblasts. Stem Cells. 2008;26:485–493. doi: 10.1634/stemcells.2007-0252. [DOI] [PubMed] [Google Scholar]
- Gleicher N, Weghofer A, Lee IH, Barad DH. Association of FMR1 genotypes with in vitro fertilization (IVF) outcomes based on ethnicity/ race. PLoS ONE. 2011;6:e18781. doi: 10.1371/journal.pone.0018781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon JB. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J. Embryol. Exp. Morphol. 1962;10:622–640. [PubMed] [Google Scholar]
- Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishigami S, Mizutani E, Ohta H, Hikichi T, Thuan NV, Wakayama S, Bui HT, Wakayama T. Significant improvement of mouse cloning technique by treatment with trichostatin A after somatic nuclear transfer. Biochem. Biophys. Res. Commun. 2006;340:183–189. doi: 10.1016/j.bbrc.2005.11.164. [DOI] [PubMed] [Google Scholar]
- Lanza RP, Cibelli JB, West MD. Prospects for the use of nuclear transfer in human transplantation. Nat. Biotechnol. 1999;17:1171–1174. doi: 10.1038/70709. [DOI] [PubMed] [Google Scholar]
- Laurent LC, Ulitsky I, Slavin I, Tran H, Schork A, Morey R, Lynch C, Harness JV, Lee S, Barrero MJ, et al. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell. 2011;8:106–118. doi: 10.1016/j.stem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Svarcova O, Villemoes K, Kragh PM, Schmidt M, Bøgh IB, Zhang Y, Du Y, Lin L, Purup S, et al. High in vitro development after somatic cell nuclear transfer and trichostatin A treatment of reconstructed porcine embryos. Theriogenology. 2008;70:800–808. doi: 10.1016/j.theriogenology.2008.05.046. [DOI] [PubMed] [Google Scholar]
- Mitalipov SM, Nusser KD, Wolf DP. Parthenogenetic activation of rhesus monkey oocytes and reconstructed embryos. Biol. Reprod. 2001;65:253–259. doi: 10.1095/biolreprod65.1.253. [DOI] [PubMed] [Google Scholar]
- Mitalipov SM, Zhou Q, Byrne JA, Ji WZ, Norgren RB, Wolf DP. Reprogramming following somatic cell nuclear transfer in primates is dependent upon nuclear remodeling. Hum. Reprod. 2007;22:2232–2242. doi: 10.1093/humrep/dem136. [DOI] [PubMed] [Google Scholar]
- Nakajima N, Inomata T, Ito J, Kashiwazaki N. Treatment with proteasome inhibitor MG132 during cloning improves survival and pronuclear number of reconstructed rat embryos. Cloning Stem Cells. 2008;10:461–468. doi: 10.1089/clo.2008.0038. [DOI] [PubMed] [Google Scholar]
- Nazor KL, Altun G, Lynch C, Tran H, Harness JV, Slavin I, Garitaonandia I, Müller FJ, Wang YC, Boscolo FS, et al. Recurrent variations in DNA methylation in human pluripotent stem cells and their differentiated derivatives. Cell Stem Cell. 2012;10:620–634. doi: 10.1016/j.stem.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noggle S, Fung HL, Gore A, Martinez H, Satriani KC, Prosser R, Oum K, Paull D, Druckenmiller S, Freeby M, et al. Human oocytes reprogram somatic cells to a pluripotent state. Nature. 2011;478:70–75. doi: 10.1038/nature10397. [DOI] [PubMed] [Google Scholar]
- Pellicer A, Ruiz A, Castellvi RM, Calatayud C, Ruiz M, Tarin JJ, Miró F, Bonilla-Musoles F. Is the retrieval of high numbers of oocytes desirable in patients treated with gonadotrophin-releasing hormone analogues (GnRHa) and gonadotrophins? Hum. Reprod. 1989;4:536–540. doi: 10.1093/oxfordjournals.humrep.a136940. [DOI] [PubMed] [Google Scholar]
- Santos MA, Kuijk EW, Macklon NS. The impact of ovarian stimulation for IVF on the developing embryo. Reproduction. 2010;139:23–34. doi: 10.1530/REP-09-0187. [DOI] [PubMed] [Google Scholar]
- Solter D. Mammalian cloning: advances and limitations. Nat. Rev. Genet. 2000;1:199–207. doi: 10.1038/35042066. [DOI] [PubMed] [Google Scholar]
- Sparman M, Dighe V, Sritanaudomchai H, Ma H, Ramsey C, Pedersen D, Clepper L, Nighot P, Wolf D, Hennebold J, Mitalipov S. Epigenetic reprogramming by somatic cell nuclear transfer in primates. Stem Cells. 2009;27:1255–1264. doi: 10.1002/stem.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparman ML, Tachibana M, Mitalipov SM. Cloning of non-human primates: theroad ‘‘less traveled by’’. Int. J. Dev. Biol. 2010;54:1671–1678. doi: 10.1387/ijdb.103196ms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susko-Parrish JL, Leibfried-Rutledge ML, Northey DL, Schutzkus V, First NL. Inhibition of protein kinases after an induced calcium transient causes transition of bovine oocytes to embryonic cycles without meiotic completion. Dev. Biol. 1994;166:729–739. doi: 10.1006/dbio.1994.1351. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Sparman M, Sritanaudomchai H, Ma H, Clepper L, Woodward J, Li Y, Ramsey C, Kolotushkina O, Mitalipov S. Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature. 2009;461:367–372. doi: 10.1038/nature08368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Amato P, Sparman M, Woodward J, Sanchis DM, Ma H, Gutierrez NM, Tippner-Hedges R, Kang E, Lee HS, et al. Towards germline gene therapy of inherited mitochondrial diseases. Nature. 2013;493:627–631. doi: 10.1038/nature11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- van der Gaast MH, Eijkemans MJ, van der Net JB, de Boer EJ, Burger CW, van Leeuwen FE, Fauser BC, Macklon NS. Optimum number of oocytes for a successful first IVF treatment cycle. Reprod. Biomed. Online. 2006;13:476–480. doi: 10.1016/s1472-6483(10)60633-5. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Perry AC, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998;394:369–374. doi: 10.1038/28615. [DOI] [PubMed] [Google Scholar]
- Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- Wilmut I, Beaujean N, de Sousa PA, Dinnyes A, King TJ, Paterson LA, Wells DN, Young LE. Somatic cell nuclear transfer. Nature. 2002;419:583–586. doi: 10.1038/nature01079. [DOI] [PubMed] [Google Scholar]
- Wu YG, Zhou P, Lan GC, Wang G, Luo MJ, Tan JH. The effects of delayed activation and MG132 treatment on nuclear remodeling and preimplantation development of embryos cloned by electrofusion are correlated with the age of recipient cytoplasts. Cloning Stem Cells. 2007;9:417–431. doi: 10.1089/clo.2006.0023. [DOI] [PubMed] [Google Scholar]
- Yang X, Smith SL, Tian XC, Lewin HA, Renard JP, Wakayama T. Nuclear reprogramming of cloned embryos and its implications for therapeutic cloning. Nat. Genet. 2007;39:295–302. doi: 10.1038/ng1973. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.