Abstract
The Hawaiian Diptera offer an opportunity to compare patterns of diversification across large and small endemic radiations with varying species richness and levels of single island endemism. The craneflies (Limoniidae: Dicranomyia) represent a small radiation of 13 described species that have diversified within the Hawaiian Islands. We used Bayesian and maximum likelihood approaches to generate a molecular phylogeny of the Hawaiian Dicranomyia using a combination of nuclear and mitochondrial loci, estimated divergence times and reconstructed ancestral ranges. Divergence time estimation and ancestral range reconstruction suggest that the colonization that led to most of the diversity within the craneflies arrived prior to the formation of Kauai and demonstrates that the two major clades within that radiation contrast sharply in their patterns of diversification.
Introduction
The Hawaiian Islands contain a large number of evolutionary radiations across a diversity of plant and animal groups (e.g. [1], [2], [3], [4], [5], [6], [7]), making it an “unparalleled scientific laboratory for studying processes of evolution” [8]. Arthropods contain some of the most spectacular of these radiations, with some lineages numbering in the dozens or even hundreds of species and single island endemism rates approaching 99% [9]. These radiations, each arising from independent colonization events, offer an opportunity to compare patterns of diversification across lineages with different numbers of species, ecological roles, and ages of colonization. Diptera are particularly interesting with 24 families containing endemic taxa in Hawaii, eight of which contain radiations with more than ten species (Table 1).
Table 1. Hawaiian Diptera lineages with more than 10 endemic species.
| Family | Genus/complex | # of species* |
| Limoniidae | Dicranomyia [25] | 13 |
| Ephydridae | Scatella [25] | 18 |
| Calliphoridae | Dyscritiomyia [25] | 25 |
| Tephritidae | Trupanea [25] , [78] | 25 |
| Pipunculidae | Cephalops [25] | 26 |
| Dolichopodidae | Eurynogaster Complex[79] | 70+ |
| Muscidae | Lispocephala [25] | 102 |
| Drosophilidae | Scaptomyza [25] | 155+ |
| Dolichopodidae | Campsicnemus [79] | 242 |
| Drosophilidae | Drosophila [25] | 403+ |
+ indicates undescribed species in the group.
Geological heterogeneity has been proposed as a potential factor driving diversification in Hawaiian lineages and the unique geology of the Hawaiian Islands may contribute to rapid species formation ([10] and references therein). The Hawaiian – Emperor chain is an archipelago formed by the motion of the Pacific plate over a stationary hotspot [11]. This has generated an archipelago that has formed sequentially in the central Pacific Ocean over the past approximately 80 million years [12], [13], [14]. The eight contemporary high Hawaiian Islands (in chronological sequence from oldest to youngest: Niihau, Kauai, Oahu, Molokai, Lanai, Maui, Kahoolawe and Hawaii) that comprise the south-eastern corner of this chain have been forming over the past approximately five million years [14], [15]. Each island is physically isolated from the others by open ocean, although during historical periods of lower sea levels, the islands comprising the Maui Nui complex (Molokai, Lanai, Maui, Kahoolawe) were connected during much of their histories [16]. Each island is further subdivided into habitat patches generated by the distinctive within-island microclimates and the active volcanic and erosional processes that give them their characteristically rugged profiles [17]. These conditions combine to allow for both between and within island diversification of many of the taxa inhabiting them.
The largest endemic Hawaiian lineage is the Drosophilidae [18], [19], [20], a clade estimated to contain over 1000 species [21]. Hawaiian Drosophilidae tend to have small ranges, with approximately 90% of species endemic to a single island and a high number of taxa endemic to single volcanoes [22], [23]. Drosophilidae are also known to exploit a wide range of host plants within high elevation rainforests and have been reared from 34 of the 87 endemic Hawaiian plant genera [14]. Diversification of Hawaiian Drosophilidae may be driven by a combination of geographic and ecological forces, mediated by host plant specialization and microbial community [24]. While Drosophilidae constitute a spectacular example of an adaptive radiation, the Hawaiian Islands are also home to several smaller dipteran radiations [9], [25], [26], each derived from one or more independent colonization events and comprised of species whose geographic ranges, dispersal abilities, and other life history traits provide a contrast to the Drosophilidae.
One smaller radiation of Hawaiian Diptera is the Dicranomyia craneflies in the family Limoniidae. Larvae in this family are known to use a wide diversity of habitats, including a spectrum of aquatic habitats such as running and stagnant freshwater, brackish pools, algae and other plant material present in the intertidal zone and the leaves of terrestrial plants [27], [28]. Within the Hawaiian islands, however, very little is known about Dicranomyia biology or ecology. Their larvae are aquatic or semi-aquatic [29]. Little else is known about the immature stages in these Hawaiian taxa, though there is at least one leaf-mining species [30], and others have been observed in dripping wet banks, algal growth on rocks in mountain streams, and tree holes or leaf axils filled with water [31]. Dicranomyia grimshawi larvae have been observed feeding on the pupae of D. jacobus [31], though their primary food source appears to be decaying plant tissue. Adults are typically collected in moist, dark places like shady spots near streams, in dense mountain vegetation and entrances to caves [29]. They are detritivores, feeding on decomposing plant material and associated microbes, although some are known to feed directly on mosses and liverworts [27]. It is possible that, while adults appear to share similar ecological roles in the Hawaiian Islands, larval ecology may be quite distinct among species.
Thirteen Dicranomyia species are known from the Hawaiian Islands [29], [32], [33], [34]. The Hawaiian Dicranomyia were treated by Hardy [29], with subsequent work by Byers [32], [33], [34]. Beyond the basic alpha taxonomy, there has been very little ecological or evolutionary work done on this group. Seven species are present on all of the current high islands (Kauai, Oahu, Molokai, Maui, Lanai, Hawaii), three are found on more than one high island, and three have distributions restricted to single islands. Although their diversity on Hawaii is small, they belong to the largest genus in the largest and one of the oldest families of Diptera [35], [36]. Craneflies in the family Limoniidae contain 10,541 currently recognized species [37] with fossils that date to the upper Triassic approximately 208 million years ago [38]. Dicranomyia is the largest genus within the Limoniidae, containing 1,086 species distributed worldwide. Of the 148 Limoniidae genera, only one other genus is comparably large, containing more than 1,000 species (Molophilus) while two additional genera contain more than 500 species (Gonomyia and Hexatoma) [37]. Based on a recent study of 88 morphological characters from 104 species within Limoniidae, Dicranomyia appears to be a relatively derived genus within the family [39]. Oosterbroek [37] reports 236 Dicranomyia species from the Oceanic region, including Australia and New Zealand. The colonization pathway to Hawaii is presently unknown.
Nitta and O'Grady [40] examined four mitochondrial loci from eight of the 13 endemic Hawaiian Dicranomyia, and found that species with populations distributed on multiple islands had complex histories that didn't conform to patterns seen in other Hawaiian radiations [41]. Their data also suggested that species in this group may be derived from two separate colonization events into the Hawaiian archipelago, one that led to the majority of Hawaiian diversity, and another represented by a single species, D. iniquispina. In this study, we build on previous phylogenetic work [40] by expanding species, geographic, and gene sampling. We reconstruct phylogenetic relationships for this group, estimate divergence dates, and reconstruct ancestral ranges to analyze the biogeographic history of Dicranomyia in Hawaii.
Methods
Taxonomic sampling
Specimens were collected from sites across the Hawaiian Islands and French Polynesia (Appendix S1 in File S1) by general sweeping in moist areas such as streams and seeps. All material was preserved in 95% ethanol. Collecting permits for public land in Hawaii were issued by the State of Hawaii's Department of Land and Natural Resources and the National Park Service, and permission to collect on private land was granted by Maui Land and Pineapple, East Maui Irrigation, and Parker Ranch. Collections in French Polynesia were made from public land and permits were obtained from the Delegation a la Recherche. No protected species were sampled as a part of this work.
Hardy's [29] key was used to identify Hawaiian material. A number of resources were employed to identify taxa from French Polynesia [42], [43], [44], [45], [46], [47], [48], [49], [50]. Wings and mounted genitalia were preserved as vouchers for all DNA accessions used in this study. Whenever possible, a series of conspecifics from the same collection site and date were also preserved in 95% ethanol. Voucher material has been deposited in the Bernice P. Bishop Museum (Honolulu, HI) and the Essig Museum of Entomology (U.C. Berkeley).
Nine of the 13 species of Hawaiian Dicranomyia were sampled for this study. Three of the unsampled species are flightless single mountaintop endemics [32], [33], [34] that are very rare and were not possible to collect. One additional species (D. nigropolita) was not collected. All Dicranomyia species included in this study are known to have populations on multiple islands, and every effort was made to sample as widely as possible from throughout their known geographic ranges. The ingroup includes geographic representation from as much of the known range of each species as possible. Five outgroup species thought to be closely related to the Hawaiian Dicranomyia were included: the congeneric species D. tahitiensis from French Polynesia, and three species from within the same subfamily as Dicranomyia, Limoniinae: Geronomyia advena, Libnotes orofenaae and Libnotes perkinsi from Hawaii and French Polynesia. The fifth outgroup taxon was Styringomyia didyma, a species in the subfamily Chioneinae (Appendix S1 in File S1).
DNA extraction, amplification and sequencing
Genomic DNA was extracted from individuals using a Qiagen DNeasy® DNA extraction kit (Qiagen Inc.), following the manufacturer's protocol. Four mitochondrial (COI, COII, ND2, 16S) and two nuclear loci (SNF, CAD) were then amplified and sequenced to estimate phylogenetic relationships within this group (see primer information in Appendix S2 in File S1). PCR reactions were performed using standard master mixes of 25 μL final volumes including: 1.5–3 μL DNA, 2.5 μL of 10X PCR Buffer (BioRad), 0.5 μL of 10 mM dNTPs (New England BioLabs), 1.25–2 μL of each primer (1:9 dilution), 0.75–2 μL of 50 mM MgCl2 (BioRad), 0.125 μL of 5 U/μL iTaq® (BioRad) and 14.175–15.62 μL ddH2O. Thermal cycling involved either a simple protocol for the mitochondrial genes (described in [40]), a nested reaction for CAD (described in [51]), or a simple protocol for SNF, which began with an initial denaturing step at 95°C for 4 minutes, 30 cycles of 90°C for 30 s, 54°C–58°C for 30 s, 72°C for 60 s and a final extension for 5–10 minutes 72°C. PCR products were purified using ExoSAP-IT (USB Corporation, Cleveland, OH) following standard protocols, and the products were sent to the UC Berkeley DNA Sequencing Center for sequencing in both directions on an ABI 3730 capillary sequencer.
Sequence Editing and Alignment
Contigs were assembled from raw forward and reverse sequence reads and edited using Geneious Pro 5.4.6 (Biomatters). The ClustalW Alignment plugin in Geneious was used to create an aligned data matrix. Alignments for each gene were imported into MacClade 4.08 [52] in order to calculate codon positions using the conceptual translation and comparison to a Drosophila yakuba reference sequence. The 16S locus is non-coding and was adjusted manually.
Phylogenetic Analysis
Analyses were performed on each gene individually and on the combined dataset, using both maximum likelihood (ML) and Bayesian inference (BI) optimality criteria. Individual ML analyses were performed on each gene partition using PhyML [53] in Geneious Pro 5.4.6 (Biomatters) under a general time-reversible (GTR+GAMMA) model [54] with 200 bootstrap replicates. PartitionFinder [55] was used to determine the optimum partitioning scheme and the best fit nucleotide models for each partition for the individual genes and combined data sets, selected using Bayesian Information Criterion (BIC). These partitions and models (Appendix S3 in File S1) were applied to the individual BI analyses, which were run for 5,000,000 generations with 2 independent runs using MrBayes 3.1.2 [56] on CIPRES [57].
The concatenated data set consisted of 45 individuals and 6 loci (3880 bp). The partition and model selection procedure yielded nine partitions for the final analyses, each presented with its best-fit model in Appendix S3 in File S1. ML analysis was performed on the concatenated data set in RAxML 3.7.2 [58] on CIPRES [57] under the GTR+GAMMA model with 1,000 bootstrap replicates and a final search for the best tree. Concatenated BI analyses were performed using MrBayes 3.1.2 [56] on CIPRES [57], with the analysis run for 15,000,000 generations with 4 independent runs each. Stationarity in BI runs was assessed using several complimentary approaches: (1) convergence metrics provided by MrBayes 3.1.2 were checked (Huelsenbeck and Ronquist, 2001) to ensure that the maximum standard deviation of split frequencies of any of the runs was under 0.05 and that the potential scale reduction factor for all parameters approached 1.0, and (2) the log-likelihood values for each run were plotted, the effective sample sizes were checked to ensure there were an adequate number of independent samples, and the posterior distributions of all parameters were examined using Tracer v.1.7.2 [59]. Tracer v.1.7.2 was also used to determine the burn-in phase by assessing each run's plot of log-likelihood values over generations; stationarity was assumed to have been reached when the log likelihood values reached a stable plateau (i.e. bounced around a mean rather than rising). Finally, a 50% majority rule consensus tree was created from the resulting post burn-in trees.
Divergence Time Estimation
Divergence time estimation was performed using a Bayesian relaxed-clock method implemented in Beast 1.7.4 [60] on CIPRES [57]. Molecular clocks can be calibrated using fossils or biogeography, or they can be set using approximations of known molecular rates. While fossils do exist in the genus Dicranomyia, they are quite old (∼200 million years) and are very distantly related to the Hawaiian members of this genus. Taxon sampling in this project focused on the taxa endemic to the Hawaiian Islands and was not intended to represent genus-wide diversity within Dicranomyia. Therefore, it was inappropriate to apply the known fossils as calibrations in this analysis [61]. Instead, we estimated dates using island calibrations and divergence rates. Because each approach has its own set of limitations (discussed below), in order to fully explore dating parameter space, we performed two calibration-based estimates and two rates-based estimates.
Island calibrations
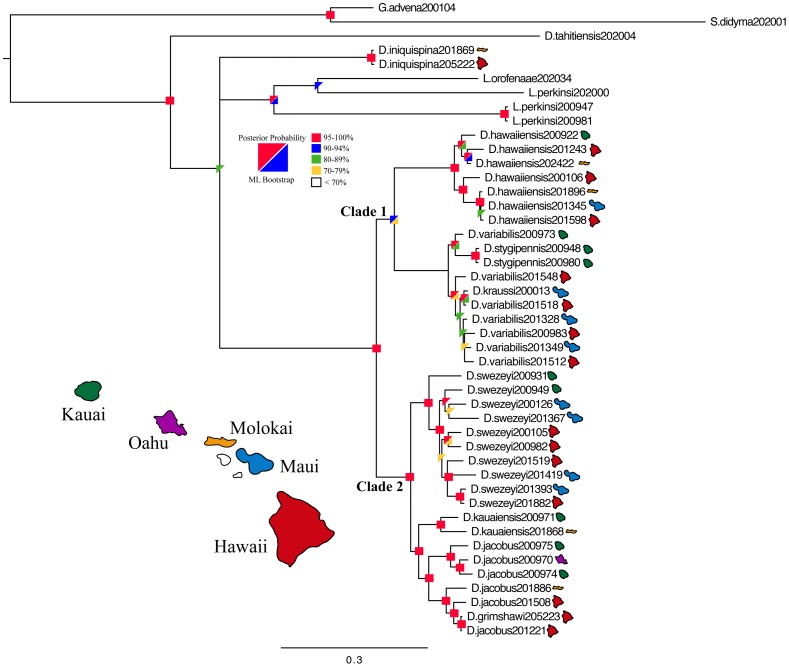
The application of island calibrations rests on the assumption that a taxon colonized a new island from an older island after it emerged and thus the age of that biogeographic event can then be used to date the most recent common ancestor (MRCA) of that group. Thus, in order to apply calibrations, pairs of taxa must be used in which one group is restricted to an older island (or islands) and the other group is restricted to a younger island (or islands) [62]. For this reason, applying island calibrations to species that predominantly have widespread ranges can be difficult because such patterns are not always apparent. However, the Hawaiian Dicranomyia do have two groups that show clear and well-supported splits between old and young island taxa (D. jacobus/grimshawi and D. variabilis/kraussi/stygipennis: Figure 1).
Figure 1. Majority rule consensus tree summarizing Bayesian analysis of Dicranomyia.
Bayesian posterior probabilities and bootstrap supports from the maximum likelihood analysis are displayed as colored boxes. Islands that each specimen was collected from are shown next to each tip.
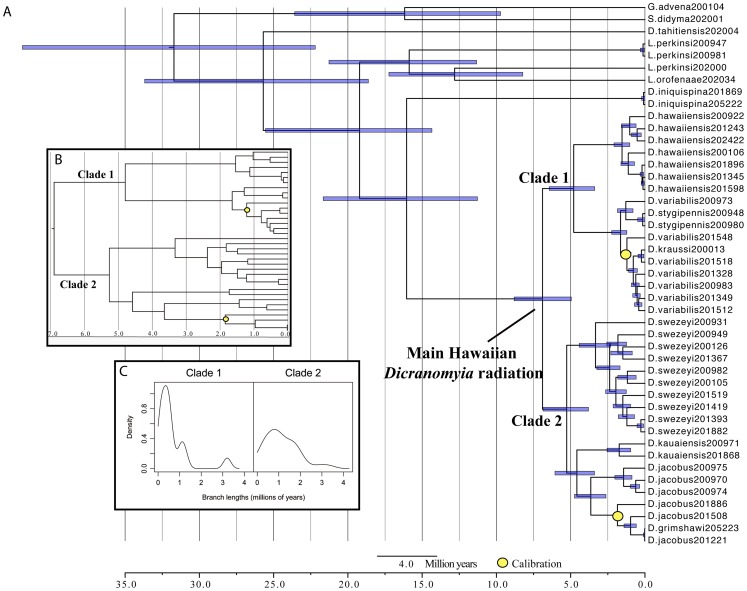
One node from each of these groups was calibrated (shown on Figure 2), using normally distributed priors with the means set on the age thought to be most relevant for biota – the end of shield building [63], and standard deviations set to accommodate uncertainty as to when the taxa colonized the islands. The island calibration for each node was selected using the ancestral range reconstruction method described in the following section (unconstrained model). In the case of D. variabilis, the ancestral range was reconstructed most strongly as Hawaii, but ranges of Maui or Maui and Hawaii were also assigned significant weight (Table 2). In the case of D. jacobus, the reconstruction was Molokai or Molokai and Hawaii (Table 2). In order to accommodate uncertainty in the reconstruction of the D. variabilis node, two divergence time analyses were run: Island Calibrations I used Hawaii's age for D. variabilis (0.5 my, SD = 0.15), Island Calibrations II used Maui's age (1.3 my, SD = 0.15). Both analyses used the age of Molokai for the D. jacobus node (1.9 my, SD = 0.15).
Figure 2. Time tree with error bars, Island Calibrations II.
A. main figure: purple bars are 95%HPD of node age estimates. D. variabilis calibration = (1.3 my SD = 0.15), D. jacobus calibration = (1.9 my, SD = 0.15); B. Inset: figure is a closeup of the ingroup, showing the disparity in branch lengths among Clade 1 and Clade 2; C. Inset: a comparison of branch length distributions between Clade 1 and Clade 2.
Table 2. Divergence times and biogeography.
| Node | Divergence time estimates: (95% HPD) | Ancestral range reconstruction Unconstrained Model (U) | |||
| Calibration based estimates | Rates based estimates | ||||
| Island Calibrations I | Island Calibrations II | Rates I 5.2%/my | Rates II 2.3%/my | ||
| D. iniquispina/main Hawaiian radiation | 12.58 | 16.05 | 11.63 | 16.63 | |
| (8.47–17.07) | (11.28–21.66) | (6.32–22.27) | (8.05–29.98) | ||
| MRCA of main Hawaiian radiation | 5.37 | 6.90 | 5.25 | 7.73 | 0.45 widespread, 0.11 Kauai, 0.09 Oahu, 0.10 Molokai, |
| (3.87–7.28) | (4.96–8.8) | (2.93–9.28) | (3.93–13.40) | 0.08 Maui, 0.13 Hawaii | |
| Clade 1 | |||||
| D. variabilis/D. hawaiiensis | 3.76 | 4.80 | 3.83 | 5.6 | 0.33 Hawaii, 0.24 widespread, 0.21 Kauai, 0.07 Oahu, |
| (2.45–5.30) | (3.39–6.44) | (2.05–7.05) | (2.79–10.04) | 0.06 Molokai, 0.04 Maui | |
| D. variabilis (with D. stygipennis and D. Kraussi) | 1.21 | 1.64 | 1.26 | 1.78 | 0.32 Hawaii, 0.30 widespread, 0.20 Kauai, 0.08 Oahu, |
| (0.79–1.77) | (1.22–2.24) | (0.57–2.28) | (0.88–3.12) | 0.02 Molokai, 0.04 Maui | |
| D. variabilis (with D. stygipennis) | 0.95 | 1.29 | 1.00 | 1.35 | 0.65 widespread, 0.16 Kauai, 0.14 Oahu, 0.007 Hawaii |
| (0.59–1.41) | (0.80–1.85) | (0.43–1.91) | (0.62–2.61) | ||
| D. variabilis (with D. kraussi)* | 0.53 | 1.21 | 0.74 | 1.10 | 0.66 Hawaii, 0.17 Maui/Hawaii, 0.12 Maui |
| (0.44–0.85) | (0.94–1.49) | (0.34–1.42) | (0.45–2.02) | ||
| D. hawaiiensis | 1.23 | 1.54 | 1.21 | 1.75 | 0.40 Hawaii, 0.23 widespread, 0.20 Kauai, 0.02 Oahu, |
| (1.75–1.72) | (1.02–2.08) | (0.61–2.44) | (0.87–3.35) | 0.08 Molokai, 0.03 Maui | |
| Clade 2 | |||||
| D. swezeyi/D. kauaiensis/ D. jacobus | 4.19 | 5.27 | 4.25 | 6.21 | 0.62 widespread, 0.10 Kauai, 0.09 Oahu, 0.06 Molokai, |
| (2.97–5.55) | (3.80–6.86) | (2.19–7.35) | (3.23–10.82) | 0.02 Maui, 0.06 Hawaii | |
| D. swezeyi | 2.67 | 3.33 | 2.65 | 3.88 | 0.49 widespread, 0.16 Kauai, 0.08 Oahu, 0.03 Molokai, |
| (1.71–3.65) | (2.31–4.42) | (1.44–4.68) | (1.89–6.68) | 0.10 Maui, 0.09 Hawaii | |
| D. kauaiensis/D. jacobus | 3.69 | 4.58 | 3.56 | 5.20 | 0.32 Molokai, 0.26 widespread, 0.21 Oahu, 0.10 Kauai, |
| (2.65–4.89) | (3.40–6.06) | (2.00–6.33) | (2.70–8.91) | 0.004 Maui, 0.06 Hawaii | |
Node age estimates from Beast analyses are shown; estimates based on island calibrations are presented in columns 2 and 3, estimates based on a divergence rates are presented in columns 4 and 5. Ancestral range reconstructions from are shown on the far right in column 6. We have simplified the Lagrange results by summarizing the probabilities for reconstructions that occurred across multiple islands, naming them “widespread”. Results with the highest probabilities are underlined. Asterisk indicates that the node was used as a calibration in the calibration based analyses.
While island calibrations have been widely used in Hawaiian lineages (e.g., [1], [64]) there are several caveats to their application that should be considered. For example, it is plausible that divergence among populations occurred prior to island emergence and was thus unrelated [65]. Furthermore, among species with such widespread ranges as Dicranomyia, it is conceivable that present and past movement among islands has obscured the true biogeographic history. In that case, the distribution patterns and ancestral state reconstructions would be skewed. For this reason, we sought to use an alternative method to estimate divergence dates in order to provide a comparison.
Divergence rate
An alternative approach to divergence date estimation is to use locus-specific rates. We performed two analyses using COI rates from the literature, applying the rates as a prior to the COI partition and allowing rates of the other partitions to vary. The mitochondrial gene COI has been used extensively to estimate divergence times among arthropod taxa (e.g.: [66], [67], [68]) and researchers are accustomed to thinking about molecular rates based on this gene. However, the difficulty in applying divergence rates is in selecting which rates to use, since this parameter is unknown for most species.
In the first analysis (Rates I), a COI divergence rate derived from Hawaiian arthropods, 5.2%/million years, was applied as a normal prior to the ucld.mean parameter of the COI partition (ucld.mean, x = 0.026, SD = 0.007). This rate was based on divergence among pairs of taxa that are situated with one taxon on Maui and the other on Hawaii, and includes a wide variety of arthropods including moths, beetles, flies and spiders (data is from Table S3 within reference [69]). The uncorrected pairwise sequence divergence was calculated for each of the pairs of taxa, outliers were excluded, and an average was taken. This average was divided by the age when Hawaii reached its maximum height, approximately 0.5 million years ago [63], which tends to be considered the most biologically plausible scenario for most taxa that rely on having mature habitats in place before being able to establish. In the second analysis (Rates II), a commonly applied divergence rate for the COI locus in arthropods (2.3%/million years [68]) was applied as a normal prior to the ucld.mean parameter of the COI partition (ucld.mean, x = 0.0115, SD = 0.0068).
The same 5 gene concatenated data set (COI, COII, ND2, 16s and CAD) including all individuals was analyzed in each of the four analyses (Island Calibrations I and II; Rates I and II). Partitions and the best fit models of evolution for each partition were selected using BIC in PartitionFinder [55]. Partitioning in the divergence rate analysis differed only slightly from the island calibration analysis in that COI was assigned its own partition (Appendix S3 in File S1). Site and clock models were unlinked and all partitions were analyzed using the uncorrelated lognormal relaxed clock except for the partitions including 16S, for which a strict clock could not be rejected and was thus applied. The tree-shape prior was linked across partitions and specified as a Yule Process. Base frequencies were estimated from the data and a starting tree was generated in RAxML [60]. Six independent MCMC searches were conducted, each running for 100 million generations and sampled every 1000 generations. The number of generations were selected to generate Effective Sample Sizes (ESS) values greater than 200 for each of the parameters [70]. Convergence was assessed using Tracer v. 1.7.2 and trees were summarized using Tree Annotator v. 1.7.2 after removing trees from the burn-in phase.
Pairwise distances were calculated on the time-calibrated tree using the cophenetic.phylo function in APE [71], performed in R [72]. Differences in branch length distributions among Clades 1 and 2 were assessed by testing for differences in the skewness of each clade. We performed 1000 permutations of the branch length distributions of each clade to create a null distribution of differences in skewness and tested the observed difference in skewness to the 95% quantile of this permutational null distribution.
Ancestral Range Reconstruction
The historical biogeographic ranges of the main Hawaiian Dicranomyia radiation were estimated using the maximum likelihood Dispersal-Extinction-Cladogenesis ancestral range reconstruction method in Lagrange v.2 [73]. This method was selected because it allows for the evaluation of changing geology over time and generates estimates of uncertainty in ancestral ranges. Furthermore, it models peripheral isolate speciation [73], which has been suggested to be important to the evolution of Hawaiian taxa [74]. Reconstructions were conditioned in absolute time with the dated phylogeny from Beast (Island Calibrations II). A five-state model was used, including Kauai, Oahu, Molokai, Maui and Hawaii Island. Dicranomyia iniquispina, was used as the outgroup. Two ancestral range models were tested: 1) unconstrained and 2) time-stratified. Because there is uncertainty between the divergence time analyses, the unconstrained model was used in order to assess the strength of the biogeographic signal in the data without any constraints. The time-stratified model did not allow lineages to colonize islands before their emergence [63]. In all models, transitions rates between coded regions were set as equal. Model performance was assessed by selecting the reconstruction method that yielded the highest log-likelihood value (ensuring that at least 2 log-likelihood units separated the top model from the others), while considering the results in the geologic context [75].
Results
Phylogenetic Relationships
Tree topologies generated by analyses of different individual loci or by different optimality criteria for each locus were very similar, although levels of support and resolution varied based on the size of the data set and numbers of informative characters (Appendices S4a-f in File S1). Likewise, tree topologies generated using ML and BI approaches of the concatenated data set were very similar and, at well-supported nodes, they were identical. Figure 1 is the BI consensus tree showing both ML and BI support values (see Appendix S5 in File S1 for the ML topology). Basal nodes are generally well supported (Figure 1). However, the BI and ML analyses disagree with respect to the placement of Libnotes and D. iniquispina. While the BI analysis resulted in a polytomy containing Libnotes, D. iniquispina and the rest of the Hawaiian Dicranomyia (Figure 1), the ML analysis showed some support for a sister relationship of D. iniquispina with the rest of the Hawaiian Dicranomyia (BS = 73: Appendix S5 in File S1). There is strong support for a radiation of Dicranomyia within Hawaii (BS = 100, PP = 100) exclusive of D. iniquispina and we will focus on this lineage in the present paper.
The radiation within Hawaii is split into two major clades: (1) D. hawaiiensis + D.variabilis + D. krausii + D. stygipennis (BS = 100, PP = 100) and (2) D. sweyzeyi + D. kauaiensis + D. jacobus + D. grimshawii (BS = 100, PP = 100: Figure 1). Within these clades, there is good support for the monophyly of D. hawaiiensis, D. stygipennis, D. swezeyi and D. kauaiensis. D. variabilis is not monophyletic; however, together D. variabilis, D. kraussi and D. stygipennis form a well-supported monophyletic group. D. grimshawi is nested within D. jacobus, but there is strong support for a monophyletic grouping of the two (Figure 1).
Divergence Time Estimation
The topologies generated from the divergence time analyses are identical to the topology in Figure 1 with one exception: all four analyses show strong support for a sister relationship between D. iniquispina and the rest of the Hawaiian Dicranomyia (PP = 98–100). The chronograms are shown in Figure 2 and Appendices S6a-c in File S1.
The Island Calibrations I and Rates I analyses resulted in similar node age estimates; the median age for the MRCA of the main Hawaiian radiation was estimated in both analyses at approximately 5 million years, while the estimate for the age of the MRCA of D. iniquispina and the main Hawaiian radiation of Dicranomyia was approximately 12 million years (Table 2). The Island Calibrations II and Rates II analyses also yielded very similar estimates; the median age for the MRCA of the main Hawaiian radiation was estimated as approximately 7 million years, while the estimate for the age of the MRCA of D. iniquispina and the main Hawaiian radiation of Dicranomyia was approximately 12 million years (Table 2). In all cases, the 95% HPDs are wider for the rates-based analyses than the calibration-based analyses. Estimates for all main nodes are presented in Table 2, Figure 2 and Appendices S6a-c.
The difference between Islands Calibrations I and Islands Calibrations II is that the former used Hawaii's age as the calibration for D. variabilis + D. kraussi, while the latter used Maui's age. There is support for either Hawaii (prob = 0.66) or Maui and Maui + Hawaii (prob = 0.29) as the ancestral range for the calibrated node (Table 2). While Hawaii has a higher probability of being the ancestral range given this reconstruction, this could simply be an effect of sampling, and because of this, we ran the analysis both ways. Where both calibrations are possibilities, assuming a MRCA on Maui is more conservative because it results in the upper bounds of possible age estimates. Likewise, the COI rate of 2.3% per million years used in the Rates II analysis is the more conservative rates-based estimate, and Island Calibrations II and Rates II corroborate one another well. Because of this, the results from Island Calibrations II are presented in Figure 2 and discussed below. The median colonization time for the main radiation of Dicranomyia is estimated at 6.9 million years ago (4.96–8.8 95% HPD, Island Calibrations II, Table 2), prior to the emergence of Kauai. However, the 95% HPD interval very slightly overlaps the age of Kauai, so the possibility that the ancestor arrived to Kauai cannot be excluded. Together, Island Calibrations I and Rates I also corroborate one another well and provide the younger bounds for Dicranomyia in the Hawaiian Islands (Table 2, Appendices S6a and S6b).
Although the crown groups within Clade 1 and Clade 2 both originate in the same time period (4.5–5.5 million years ago), species within Clade 1 are much younger. Pairwise distances in units of million years in the two main clades and within species are as follows: Clade 1: 0.31–9.59; D. variabilis (+ D. krausii + D. sygipennis): 0.31–3.29; D. hawaiiensis: 0.39–3.08; Clade 2: 0.04–10.53; D. kauaiensis: 3.46; D. swezeyi: 0.55–6.65; and D. jacobus (+D. grimshawi): 0.04–7.29. The distribution of branch lengths within Clade 1 is significantly more skewed toward the present relative to Clade 2 (observed value = 1.59; null distribution 95% confidence interval = [–1.177, 1.11]; Figure 2c).
Ancestral Range Reconstruction
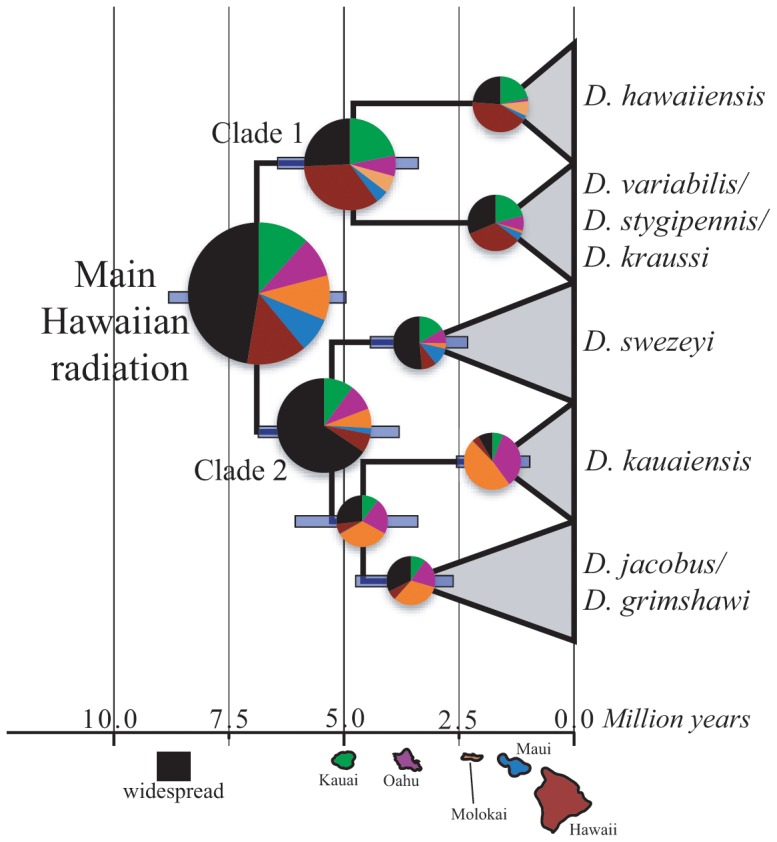
Overall likelihood scores (–lnL) for each model were as follows: U = –94.41 and TS- = –119.7. The unconstrained model clearly outperformed the time stratified model, and its reconstructions are presented in Table 2 and Figure 3. However, the unconstrained model results must be interpreted carefully because of the timing of the appearance of the islands. Although the timing of the islands was explicitly considered in the time stratified model, the very large difference in likelihood scores between this model and the unconstrained model indicates a poor fit to the data. Thus, we present the unconstrained model results here.
Figure 3. Results of divergence dating analysis and ancestral range reconstruction.
The Maximum Clade Credibility tree is shown compared to a timescale bar, with populations collapsed into species. Outgroup has been trimmed.
As discussed in the introduction, the Dicranomyia are characterized by a low rate of single island endemism relative to the Hawaiian Drosophila and some other endemic Hawaiian radiations (30% vs. >90%, [25], [26]). The presence of single species on multiple islands yields ancestral reconstructions that span multiple islands. For simplicity, we summarize all reconstructions that are spread over multiple islands as widespread, except in cases where one particular multi-island range has a large probability assigned to it.
The ancestral reconstruction for the root of the Dicranomyia radiation on Hawaii was reconstructed as widespread (prob = 0.45), consisting of 26 different multi-island range configurations, with the remaining probability fairly evenly split among all of the islands (0.08–0.13). The ancestral range for the most recent common ancestor (MRCA) of Clade 1 was equivocal between Kauai, Hawaii and widespread (prob = 0.21, 0.33 and 0.24), as was D. hawaiiensis (prob = 0.21, 0.40, 0.23) and D. variabilis (+D. krausi + D. stygipennis) (prob = 0.20, 0.32, 0.30). The ancestral range for D. variabilis + D. kraussi was reconstructed as Hawaii or Maui and Hawaii (prob = 0.66, 0.29). The ancestral range for Clade 2 was reconstructed as widespread (prob = 0.62), as was the range for D. swezeyi (prob = 0.49). The MRCA of D. kauaiensis and of D. jacobus was reconstructed as Molokai and widespread (prob = 0.32, 0.26, respectively). The MRCA of D. kauaiensis was reconstructed as Molokai or Oahu (prob = 0.45, 0.32) and of D. jacobus was reconstructed as Molokai and widespread (prob = 0.30, 0.31, respectively). Finally, the MRCA for the young island D. jacobus was reconstructed as Molokai or Molokai and Hawaii (prob = 0.60) (Table 2, Figure 3). All of these same nodes had ancestral ranges reconstructed as Kauai under the time-stratified model.
Discussion
Taxonomic implications
Results from this study indicate the need for further taxonomic study of the Hawaiian Dicranomyia. While most nodes in the tree are very well supported and several of the original species appear to be good species and are monophyletic, there are two species clusters that will require additional taxonomic study. In each case, voucher specimens were re-examined and were found to have been accurately identified according to their morphological descriptions. First, our analyses place D. grimshawi within D. jacobus, (BS = 100, P = 100: Fig. 1), rendering the latter taxon paraphyletic, in spite of the fact that these taxa are morphologically distinct. Another species, D. variabilis, is paraphyletic with respect to D. kraussi (BS = 97, PP = 100: Fig. 1) and D. stygipennis (BS = 69, PP = 100: Fig. 1). These three species are closely related morphologically and the discordance in the molecular data may be the result of an incomplete lineage sorting event or current or past gene flow. Future morphological and genetic work should focus on examining multiple individuals of D. grimshawi to better understand its relationship to D. jacobus and on clarifying the relationship between D. variabilis, D. kraussi and D. stygipennis.
Biogeography
Nitta and O'Grady (2008) suggested that D. iniquispina may be the result of a separate colonization to the Hawaiian Islands from the rest of the Hawaiian Dicranomyia. While its position relative to the rest of the Hawaiian Dicranomyia is equivocal in our BI analysis (Figure 1), both the ML analysis and the Bayesian divergence time analyses recover topologies that show support for a sister relationship for D. iniquispina with the main Hawaiian radiation, suggesting a single colonization event. However, it is distinct, both in terms of its genital morphology and genetics. Hardy [29] considered D. iniquispina a relative of D. grimshawi, differing mainly in the number of strong spines on the ventral prolongation of the ventral dististylus. He also stated that the “differences in the shapes of the ventromesal lobes of the basistyli and the posterior margin of the ninth tergum. are also significant.” It is clear from our analyses that D. iniquispina is quite distantly related to D. grimshawi and the other Hawaiiana craneflies. Furthermore, D. iniquispina resides on a very long branch and was equally divergent in the BI analyses (Fig. 1) from the other Hawaiian endemic taxa as was the genus Libnotes. The median age estimate for the MRCA of D. iniquispina and the main Hawaiian radiation range between approximately 11.5 and 16.5 million years in the four divergence time analyses (Table 2), a significant increase over the age of the other Hawaiian taxa in clades 1 and 2 (min = 5.37, max = 7.73 million years). It is possible that they are derived from the same colonization event – this would have involved colonization to an older, now eroded island followed by colonization to the current high Hawaiian Islands 5–8 million years later (Table 2), possibly with several extinction events in intervening taxa to generate the long branches we observe in the extant species. Alternatively, it is plausible that the archipelago was colonized two times by the genus Dicranomyia, once by D. iniquispina and again by the ancestor of the other Dicranoymia species. The current data do not allow us to differentiate between these two hypotheses and further research, sampling this genus across the Pacific, is needed to resolve this question.
The median colonization time for the main radiation of Dicranomyia is estimated at 6.9 million years ago (4.96–8.8 95% HPD, Table 2: Island Calibrations II). This is prior to the emergence of Kauai, about the time when Nihoa, one of the now-eroded Northwest Hawaiian Islands, was at its maximum height of 1300 m [76], 7.2 million years ago [63]. Unfortunately, it is not possible to include contemporary taxa from Nihoa or any of the other formerly high islands as these highly eroded islands no longer contain suitable habitat for Dicranomyia and no species from this genus have been recorded from islands older than Kauai [25].
The range reconstruction for the MRCA of the main Hawaiian Dicranomyia radiation is widespread (Table 2, Figure 3), which is not surprising given that many of the modern Hawaiian Dicranomyia are found on one or more islands. However, the reconstruction for this node is widespread across the contemporary high islands, which would not be possible given the divergence time estimate and emergence dates of the high Hawaiian islands. In the Lagrange analysis, this issue was handled by comparing the unconstrained model to a time-stratified model that did not allow the taxa to colonize each island prior to its formation. The two models returned results at almost opposite ends of the possible spectrum. The time stratified model reconstructed most of the ancestral ranges at the species level and above as Kauai. The unconstrained model, on the other hand, reconstructed many of these same nodes as occurring on multiple islands. There is more certainty in the reconstructions towards the tips of the tree in the unconstrained model (e.g., within D. jacobus and D. variabilis), but the biogeographic signal decays rapidly as you move back in time, most likely due to past and present movement among islands.
What do the ancestral range reconstructions and divergence time estimates tell us about the biogeography of Dicranomyia in the context of the dynamic Hawaiian Islands? The divergence time estimates indicate that diversification has been occurring in the Dicranomyia more or less continuously since the colonization of the islands by the MRCA of the main radiation (Figure 2). It is difficult to imagine that all of this diversification occurred on Kauai, as is indicated by the time-stratified model, given the wide distributions of the contemporary taxa. However, the better-performing unconstrained model has generated range estimates on islands that did not exist at the time of the diversification event, which seems equally unlikely. We propose, based on the divergence time estimates and biogeographic analyses, that once new species of Dicranomyia formed they rapidly moved between whatever islands existed at the time. This effectively obscured any biogeographic signal. Therefore, at least in Dicranomyia, ancestral range reconstructions deeper than the tips of the tree are likely to show little of the biogeographic history of this group.
Although the crown groups within Clade 1 and Clade 2 originate in roughly the same timeframe 4.5–5.5 million years ago, a clear difference is apparent between them in their distributions of branch lengths (inset, Figure 2b,c) and pairwise phylogenetic distances. Species in Clade1 are much younger, and the topology within this clade is characterized by long branches leading to a near-simultaneous burst of diversification around 1.5 million years ago in its two separate lineages, in the timeframe of Maui Nui [63] (Figure 2b). In contrast, the diversity within Clade 2 is much older and it appears that diversification has been occurring more evenly over time since it originated (Figure 2b). Why the two are so different is not clear, and may be a result of differing speciation or extinction rates among the two clades.
Apart from the MRCA of the main radiation in the islands, the remaining node estimates fall comfortably within the timeframe of the contemporary high islands (Table 2). The natural history of the group in Hawaii supports these timing results demonstrating that substantial diversification has occurred within the context of the current high islands. There are three species of endemic flightless Dicranomyia known from the high elevation terrain of Kauai and Oahu (D. sabroskyana Byers 1982 [34] from Kauai, D. hardyana Byers 1985 [33] from Oahu and D. gloria Byers 1994 [32] from Oahu). Although they were not included in this study, they are still interesting because a transition to flightlessness would, by definition, restrict dispersal and is typically thought of as an island syndrome that evolves in situ [77].
Several present-day Dicranomyia have range distributions span multiple islands, indicating that dispersal between neighboring islands is fairly easy. But if they are able to disperse so well, what would cause speciation in this group? A history in the archipelago that extends back prior to the emergence of Kauai would have involved numerous inter-island colonization events as the ancestral taxa navigated the repeated appearance and disappearance of islands over time, and would also have involved stochastic extinction as some lineages died out with disappearing habitat. This would have pruned out some of the diversity in the group, and potentially is what gave rise to the long branches observed in Clade 1. Certainly divergence in allopatry, most likely induced when ranges became disjunct via dispersal and extinction, played a role in divergence of this group. However, because it is known that larval Dicranomyia make use of a diverse range of habitats, future work that focuses on the ecological requirements of the immature stages of each species would be an interesting complement to the biogeographic and temporal patterns established here and may provide insight into the role of ecological diversification in this group.
Supporting Information
Appendix S1, Collection Information and Genbank Accessions of Species Included in the Present Study. Appendix S2, Primer names and references. Appendix S3, Summary of data partitions and the nucleotide models determined for each partition. Appendix S4, Individual Bayesian Gene Trees. Appendix S5, Combined maximum likelihood analysis of all genes. Appendix S6, Alternate BEAST Trees.
(DOCX)
Acknowledgments
We thank Crystal Teng and Jessica Leu for help with laboratory work and Andrew Rominger and Gordon Bennett for help with analyses. Gordon Bennett, Neal Evenhuis, David Foote, Richard Lapoint, Karl Magnacca, Brian Ort, Norma Pantoja, and Sue Wang helped with collecting and specimens. We also thank the following institutions and individuals for access and permission to collect: the State of Hawaii Department of Land and Natural Resources (Cynthia King) and Natural Area Reserves (Betsy Gagne), the National Park Service, the Nature Conservancy Molokai, Maui Land and Pineapple (Randy Bartlett), East Maui Irrigation (Mark Vaught), Parker Ranch and the Delegation a la Recherche in French Polynesia. Finally, we thank two anonymous reviewers for their thoughtful feedback.
Funding Statement
This work was funded by an NSF DEB award 0842348 to PMO (http://www.nsf.gov/div/index.jsp?div=DEB).The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rubinoff D, Schmitz P (2010) Multiple aquatic invations by an endemic, terrestrial hawaiian moth radiation. Proceedings of the National Academy of Sciences 107: 5903–5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pratt HD (2005) The Hawaiian Honeycreepers. New York: Oxford University Press.
- 3. Magnacca KN, Danforth BN (2006) Evolution and biogeography of native Hawaiian Hylaeus bees (Hymenoptera: Colletidae). Cladistics 22: 393–411. [Google Scholar]
- 4. Gillespie RG (2004) Community assembly through adaptive radiation in Hawaiian spiders. Science 303: 356–359. [DOI] [PubMed] [Google Scholar]
- 5. Carr G (1985) Monograph of the Hawaiian Madiinae (Asteraceae): Argyroxiphium, Dubautia, and Wilkesia . Allertonia 4: 1–123. [Google Scholar]
- 6. Bennett GM, O'Grady PM (2012) Host plants shape insect diversity: origins, diversity, and host plant associations of the native Hawaiian leafhoppers (Cicadellidae: Nesophrosyne). Molecular Phylogenetics and Evolution 65: 705–717. [DOI] [PubMed] [Google Scholar]
- 7. Baldwin BG (2006) Contrasting patterns and processes of evolutionary change in the tarweed – silversword lineage: revisiting Clausen, Keck and Hiesey's findings. Annals of the Missouri Botanical Garden 93: 66–96. [Google Scholar]
- 8. Roderick GK, Gillespie RG (1998) Speciation and phylogeography of Hawaiian terestrial arthropods. Molecular Ecology 7: 519–531. [DOI] [PubMed] [Google Scholar]
- 9.Liebherr JK, Zimmerman EC (2000) Hawaiian Carabidae (Coleoptera) Part I: Introduction and Tribe Platynini. Honolulu: University of Hawaii Press.
- 10.Wagner W, Funk V (1995) Hawaiian Biogeography: Evolution on a Hot Spot Archipelago. Washington DC: Smithsonian Institution Press. 467 p. [Google Scholar]
- 11. Wilson JT (1963) A Possible Origin of the Hawaiian Islands. Canadian Journal of Physics 41: 863–870. [Google Scholar]
- 12. Sharp WD, Clague DA (2006) 50-Ma Initiation of Hawaiian-Emperor Bend Records Major Change in Pacific Plate Motion. Science 313: 1281–1284. [DOI] [PubMed] [Google Scholar]
- 13. Duncan RA, Keller RA (2004) Radiometric ages for basement rocks from the Emperor Seamounts, ODP Leg 197. Geochemistry Geophysics Geosystems 5: Q08L03 doi:10.1029/2004GC000704 [Google Scholar]
- 14. Clague DA, Dalrymple GB (1987) The Hawaiian-Emperor volcanic chain: part 1. Geologic evolution. United States Geological Survey Professional Paper 1350: 5–54. [Google Scholar]
- 15.Clague DA (1996) The growth and subsidence of the Hawaiian-Emporer volcanic chain. In: Keast A, Miller SE, editors. The origin and evolution of Pacific island biotas, New Guinea to eastern Polynesia: patterns and processes. Amsterdam: SPB Academic Publishing pp. 35–50.
- 16. Price JP, Elliot-Fisk D (2004) Topographic History of the Maui Nui Complex, Hawaii, and Its Implications for Biogeography. Pacific Science 58: 27–45. [Google Scholar]
- 17.Juvik JO (1998) Biogeography. In: Juvik SP, Juvik JO, editors. Atlas of Hawaii. Honolulu, HI: University of Hawaii Press. pp. 103–106.
- 18. O'Grady PM, Lapoint RT, Bonacum J, Lasola J, Owen E, et al. (2011) Phylogenetic and ecological relationships of the Hawaiian Drosophila inferred by mitochondrial DNA analysis. Molecular Phylogenetics and Evolution 58: 244–256. [DOI] [PubMed] [Google Scholar]
- 19. Kambysellis M, Ho KF, Craddock EM, Piano F, Parisi M, et al. (1995) Pattern of ecological shifts in the diversification of Hawaiian Drosophila inferred from a molecular phylogeny. Current Biology 5: 1129–1139. [DOI] [PubMed] [Google Scholar]
- 20. Carson HL, Kaneshiro KY (1976) Drosophila of Hawaii – systematics and ecological genetics. Annual Review of Ecology and Systematics 7: 311–345. [Google Scholar]
- 21. Kaneshiro KY (1997) R.C.L. Perkins' legacy to evolutionary research on Hawaiian Drosophilidae, Diptera. Pacific Science 51: 450–461. [Google Scholar]
- 22.O'Grady PM, Markow TA (2012) Rapid morphological, behavioral, and ecological evolution in Drosophila: Comparisons between the endemic Hawaiian Drosophila and the catophilic repleta species group. In: Singh RS, Kulanthial RJ, editors. Rapidly Evolving Genes and Genetic Systems: Oxford University Press. pp. 176–186.
- 23.O'Grady PM, Magnacca KN, Lapoint RT (2009) Drosophila. In: Gillespie RG, Clague DA, editors. Encyclopedia of Islands. Berkeley, CA: University of California Press. pp. 232–235.
- 24.Ort BS, Bantay RM, Pantoja NA, O'Grady PM (2012) Fungal diversity associated with Hawaiian Drosophila host plants. PLoS One 10.1371/journal.pone.0040550. [DOI] [PMC free article] [PubMed]
- 25.Nishida GN (2002) Hawaiian Terrestrial Arthropod Checklist, Fourth Edition. Honolulu: B.P. Bishop Museum.
- 26. Eldredge LG, Evenhuis NL (2003) Hawaii's biodiversity: a detailed assessment of the numbers of species in the Hawaiian Islands. Bishop Museum Occasional Papers 75: 34p. [Google Scholar]
- 27. Pritchard G (1983) Biology of Tipulidae. Annual Review of Entomology 28: 1–22. [Google Scholar]
- 28. Alexander CP (1920) The crane-flies of New York. Pt. 2. Biology and phylogeny. Cornell University Agricultural Experiment Station Memoirs 38: 693–1133. [Google Scholar]
- 29. Hardy DE (1960) Diptera, Nematocera-Brachycera. Insects of Hawaii 10: 1–368. [Google Scholar]
- 30. Swezey OH (1915) A Leaf-Mining Cranefly in Hawaii. Proceedings of the Hawaiian Entomological Society 3: 87–89. [Google Scholar]
- 31. Williams FX (1943) Biological Studies in Hawaiian Water-Loving Insects. Part III, Diptera or Flies – C, Tipulidae and Psychodidae. Proceedings of the Hawaiian Entomological Society 6: 313–350. [Google Scholar]
- 32. Byers GW (1994) A 3rd subapterous crane-fly from Hawaii (Diptera, Tipulidae). Proceedings of the Entomological Society of Washington 96: 496. [Google Scholar]
- 33. Byers GW (1985) A second flightless crane fly from Hawaii (Diptera, Tipulidae). International Journal of Entomology 27: 239–245. [Google Scholar]
- 34. Byers GW (1982) A subapterous crane fly from Hawaii (Diptera, Tipulidae). Memoirs of the Entomological Society of Washington 10: 37–41. [Google Scholar]
- 35.Evenhuis NL, Pape T, Pont AC, Thompson FC (2009) Appendix. Species of Diptera per family for all regions. In: Pape T, Bickel D, Meier R, editors. Diptera diversity: status, challenges and tools. Leiden & Boston: Brill. pp. 459.
- 36.Evenhuis NL (2004) Catalogue of the fossil flies of the world (Insecta: Diptera).
- 37.Oosterbroek P (2011) Catalogue of the Craneflies of the World.
- 38. Krzeminski W (2001) New fossil Tipuloidea (Diptera) from the Fur Formation of Denmark in the collection of the Natural History Museum in London. Polish Journal of Entomology 70: 333–339. [Google Scholar]
- 39. Ribeiro GC (2008) Phylogeny of the Limnophilinae (Limoniidae) and early evolution of the Tipulomorpha (Diptera). Invertebrate Systematics 22: 627–694. [Google Scholar]
- 40. Nitta JH, O'Grady PM (2008) Mitochondrial phylogeny of the endemic Hawaiian craneflies (Diptera, Limoniidae, Dicranomyia): Implications for biogeography and species formation. Molecular Phylogenetics and Evolution 46: 1182–1190. [DOI] [PubMed] [Google Scholar]
- 41.Funk VA, Wagner WL (1995) Biogeographic patterns in the Hawaiian islands; Funk VA, Wagner WL, editors. Washington: Smithsonian Institution.
- 42. Edwards FW (1928) Nematocera. Insects of Samoa 6: 23–102. [Google Scholar]
- 43. Edwards FW (1927) Diptera Nematocera from the South Pacific collected by the St. George expedition, 1925. Annals and Magazine of Natural History 20: 236–244. [Google Scholar]
- 44. Brunetti E (1912) Diptera Nematocera (excluding Chironomidae and Culicidae). Fauna of British India, Including Ceylon and Burma 1: 1–581. [Google Scholar]
- 45. Alexander CP (1947) Tipulidae of the Southeastern Pacific (Diptera). Occasional Papers of the Bernice P Bishop Museum 18: 337–347. [Google Scholar]
- 46. Alexander CP (1935) A new species of Tipulidae from the Marquesas. Bernice P Bishop Museum Bulletin 142: 123–124. [Google Scholar]
- 47. Alexander CP (1933) New Tipulidae from the Society Islands. Bernice P Bishop Museum Bulletin 113: 53–56. [Google Scholar]
- 48. Alexander CP (1932) Check list of Tipulidae of Oceania. Occasional Papers of the Bernice P Bishop Museum 9: 1–12. [Google Scholar]
- 49. Alexander CP (1921) Undescribed or little-known crane-flies from the Pacific islands (Tipulidae, Diptera). Bulletin of the Brooklyn Entomological Society 16: 9–13. [Google Scholar]
- 50. Alexander CP (1914) On a collection of crane-flies (Tipulidae, Diptera) from the Fiji Islands. Annals of the Entomological Society of America 7: 239–244. [Google Scholar]
- 51. Moulton JK, Weigmann BM (2004) Evolution and phylogenetic utility of cad (rudimentary) amond Mesozoic-aged Eremoneuran Diptera (Insecta). Molecular Phylogenetics and Evolution 31: 363–378. [DOI] [PubMed] [Google Scholar]
- 52.Maddison WP, Maddison DR (2000) MacClade version 4: Analysis of phylogeny and character evolution. Sunderland, Massachusetts: Sinauer Associates.
- 53. Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52: 696–704. [DOI] [PubMed] [Google Scholar]
- 54.Tavare S (1986) Some Probabilistic and Statistical Problems in the Analysis of DNA Sequences. In: Miura RM, editor; New York, New York. The American Mathematical Society. pp. 57–86.
- 55. Lanfear R, Calcott B, Ho SYW, Guindon S (2012) PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution 29: 1695–1701. [DOI] [PubMed] [Google Scholar]
- 56. Huelsenbeck JP, Ronquist F (2001) MrBayes: Bayesian inference of phylogenetic trees. 17: 754–755. [DOI] [PubMed] [Google Scholar]
- 57.Miller MA, Pfeiffer W, Schwartz T (2010) “Creating the CIPRES Science Gateway for inference of large phylogenetic trees” New Orleans, LA. pp. 1–8.
- 58. Stamatakis A (2006) RAxML-VI-HPC: Maximum Likelihood-based Phylogenetic Analyses with Thousands of Taxa and Mixed Models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- 59.Rambaut A, Drummond AJ (2012) Tracer v1.7.2, obtained from the “Workshop on Molecular Evolution”, Aug 2011. Tracer v1.5. Available: http://beastbioedacuk/Tracer.
- 60. Drummond AJ, Suchard MA, Xie D, Rambaut A (2012) Bayesian phylogenetics with BEAUTi and the BEAST 1.7. Molecular Biology and Evolution 29: 1969–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ho SYW, Saarma U, Barnett R, Haile J, Shapiro B (2008) The effect of inappropriate calibration: three case studies in molecular ecology. PLoS One 3: e1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fleischer RC, McIntosh CE, Tarr CL (1998) Evolution on a volcanic conveyor belt: using phylogeographic reconstructions and K-Ar based ages of the Hawaiian islands to estimate molecular evolutionary rates. Molecular Ecology 7: 533–545. [DOI] [PubMed] [Google Scholar]
- 63.Carson HL, Clague DA (1995) Geology and biogeography of the Hawaiian Islands. In: Wagner WL, Funk VA, editors. Hawaiian biogeography: evolution on a hotspot archipelago. Washington, D.C.: Smithsonian Institution Press. pp. 14–29.
- 64. Lerner HRL, Meyer M, James HF, Hofreiter M, Fleischer RC (2011) Multilocus Resolution of Phylogeny and Timescale in the Extant Adaptive Radiation of Hawaiian Honeycreepers. Current Biology 21: 1–7. [DOI] [PubMed] [Google Scholar]
- 65. Heads M (2005) Dating nodes on molecular phylogenies: a critique of molecular biogeography. Cladistics 21: 62–78. [DOI] [PubMed] [Google Scholar]
- 66. Quek SP, Davies SJ, Itino T, Pierce NE (2004) Codiversification in an ant-plant mutualism: Stem texture and the evolution of host use in Crematogaster (Formicidae: Myrmicinae) inhabitants of Macaranga (Euphorbiaceae). Evolution 58: 554–570. [PubMed] [Google Scholar]
- 67. Papadopoulou A, Anastasiou I, Vogler AP (2010) Revisiting the insect mitochondrial molecular clock: the mid-Aegean trench calibration. Molecular Biology and Evolution 27: 1659–1672. [DOI] [PubMed] [Google Scholar]
- 68. Brower AVZ (1994) Rapid morphological radiation and convergence among races of the butterfly Heliconius erato inferred from patterns of mitochondrial DNA evolution. Proceedings of the National Academy of Sciences 91: 6491–6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Goodman KR, Welter SC, Roderick GK (2012) Genetic divergence is decoupled from ecological diversification in the Hawaiian Nesosydne planthoppers. Evolution 66: 2798–2813. [DOI] [PubMed] [Google Scholar]
- 70.Drummond AJ, Ho SYW, Rawlence N, Rambaut A (2007) A Rough Guide to BEAST 1.4 (Program Manual).
- 71. Paradis E, Claude J, Strimmer K (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20: 289–290. [DOI] [PubMed] [Google Scholar]
- 72.R Core Team (2012) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. ISBN 3-9000-51-07-0, Available: http://www.R-project.org, Accessed 2013 Mar.
- 73. Ree RH, Smith SA (2008) Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Systematic Biology 57: 4–14. [DOI] [PubMed] [Google Scholar]
- 74.Coyne JA, Orr HA (2004) Speciation. Massachusetts: Sinauer Associates, Inc. 545 p. [Google Scholar]
- 75. Ree RH, Smith SA (2008) Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Systematic Biology 57: 4–14. [DOI] [PubMed] [Google Scholar]
- 76. Moore JG, Clague DA (1992) Volcano growth and evolution of the island of Hawaii. Geological Society of America Bulletin 104: 1471–1484. [Google Scholar]
- 77.Carlquist S (1965) Island Life. Garden CityNew York: The Natural History Press. 451 p. [Google Scholar]
- 78. Brown JM, Todd-Thompson M, McCord A, O'Brien A, O'Fallon B (2006) Phylogeny, host association, and wing pattern variation in the endemic Hawaiian fruit flies (Diptera, Tephritidae). Instrumenta Biodiversitatis 7: 1–16. [Google Scholar]
- 79.Evenhuis NL (2013) Personal communication to Goodman, K.R. Honolulu, HI.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1, Collection Information and Genbank Accessions of Species Included in the Present Study. Appendix S2, Primer names and references. Appendix S3, Summary of data partitions and the nucleotide models determined for each partition. Appendix S4, Individual Bayesian Gene Trees. Appendix S5, Combined maximum likelihood analysis of all genes. Appendix S6, Alternate BEAST Trees.
(DOCX)