Abstract
Objective:
To test the hypothesis that cognitive activity across the life span is related to late-life cognitive decline not linked to common neuropathologic disorders.
Methods:
On enrollment, older participants in a longitudinal clinical-pathologic cohort study rated late-life (i.e., current) and early-life participation in cognitively stimulating activities. After a mean of 5.8 years of annual cognitive function testing, 294 individuals had died and undergone neuropathologic examination. Chronic gross infarcts, chronic microscopic infarcts, and neocortical Lewy bodies were identified, and measures of β-amyloid burden and tau-positive tangle density in multiple brain regions were derived.
Results:
In a mixed-effects model adjusted for age at death, sex, education, gross and microscopic infarction, neocortical Lewy bodies, amyloid burden, and tangle density, more frequent late-life cognitive activity (estimate = 0.028, standard error [SE] = 0.008, p < 0.001) and early-life cognitive activity (estimate = 0.034, SE = 0.013, p = 0.008) were each associated with slower cognitive decline. The 2 measures together accounted for 14% of the residual variability in cognitive decline not related to neuropathologic burden. The early-life–activity association was attributable to cognitive activity in childhood (estimate = 0.027, SE = 0.012, p = 0.026) and middle age (estimate = 0.029, SE = 0.013, p = 0.025) but not young adulthood (estimate = −0.020, SE = 0.014, p = 0.163).
Conclusions:
More frequent cognitive activity across the life span has an association with slower late-life cognitive decline that is independent of common neuropathologic conditions, consistent with the cognitive reserve hypothesis.
Participation in cognitively stimulating activities has been associated with reduced late-life cognitive decline in most1–5 although not all6 studies, but the mechanisms underlying the association are not well understood. One idea is that cognitive activity somehow helps delay the cognitive consequences of neuropathologic lesions (cognitive reserve hypothesis7–9), possibly because of activity-dependent changes in key cognitive systems in the brain.10,11 Alternatively, cognitive inactivity may be a consequence of neuropathologic lesions rather than a risk factor (reverse causality hypothesis1,2,12). Establishing the direction of the association between change in cognitive activity and cognitive function over time could differentiate the hypotheses, but this has been difficult to do in observational studies.1,2,6,13 Determining whether the association between cognitive activity and cognitive decline is attributable to (reverse causality hypothesis) or independent of (cognitive reserve hypothesis) neuropathologic conditions could also differentiate the hypotheses, but little relevant data have been published.5
We used data from participants in the Rush Memory and Aging Project to test the hypothesis that cognitive activity has an association with cognitive decline that is independent of common neuropathologic conditions. Participants (n = 294) rated early- and late-life participation in cognitive activities, completed annual cognitive testing (mean = 5.8 years), died, and underwent a uniform neuropathologic examination from which measures of 5 common lesions were derived. We previously found no association between cognitive activity and neuropathologic markers.5 In this study, we tested whether cognitive activity in late life or at earlier points in the life span can account for variability in cognitive decline not linked to neuropathologic markers.
METHODS
Participants.
All participants were from the Rush Memory and Aging Project. Eligibility required that participants be older than 55 years and agree to annual clinical evaluations (begun in 1997) and brain autopsy at death.14 Persons from the Chicago area were recruited from retirement communities, churches, social service agencies, and subsidized housing facilities.
As of October 2012, 568 of 1,651 participants had died, 456 (80.3%) had undergone a brain autopsy, and the neuropathologic examination had been completed on the first consecutive 427 individuals. We excluded 27 persons who had dementia at baseline, 83 with missing cognitive activity data (added to the study in 2001), and 18 who died before the first follow-up evaluation. Longitudinal cognitive function data were available on 294 of the remaining 299 persons (98.3%), and analyses are based on this group. They had a mean age at death of 89.3 years (SD = 5.9), a mean of 14.4 years of education (SD = 2.7), 199 (67.7%) were women, and 109 (37.1%) had mild cognitive impairment (MCI) at baseline. During a mean of 5.8 years (SD = 2.7) of annual follow-up, participants completed 1,695 of a possible 1,725 cognitive assessments (98.4%).
Standard protocol approvals, registrations, and patient consents.
After a presentation, interested individuals had further discussions with staff who obtained written informed consent. The project was approved by the institutional review board of Rush University Medical Center.
Assessment of cognitive activity.
At baseline, individuals completed a 37-item cognitive activity questionnaire.5,15–17 Frequency of participation was rated from 1 (once a year or less) to 5 (every day or about every day). The activities, which included reading books, visiting a library, and writing letters, involved seeking or processing information, had few barriers to participation, and were relatively common. There were 7 late-life (i.e., at baseline) activities and 30 early-life activities including 11 about childhood (age 6–12 years), 10 about young adulthood (age 18 years), and 9 about middle age (age 40 years). Item scores were averaged to yield separate early- and late-life activity measures. In secondary analyses, the early-life measure was divided into childhood, young-adulthood, and middle-age subscores.17
Assessment of cognitive function.
Nineteen cognitive performance tests were administered annually. There were 7 measures of episodic memory, 3 measures of semantic memory, 3 measures of working memory, 4 measures of perceptual speed, and 2 measures of visuospatial ability. Composites of multiple tests were used to reduce measurement error. A measure of global cognition based on all 19 tests and measures of episodic memory (7 tests), semantic memory (3 tests), working memory (3 tests), perceptual speed (4 tests), and visuospatial ability (2 tests) were created by converting raw scores to z scores, using the baseline mean and SD, and averaging the z scores. Further information on the individual tests and composite measures is published elsewhere.15,16
Clinical evaluation.
The annual evaluations also included a medical history, neurologic examination, and clinical classification.14 The diagnosis of dementia required a history of cognitive decline and impairment in at least 2 cognitive domains, and MCI required impairment in one or more cognitive domains in the absence of dementia.5,14
Neuropathologic examination.
Individuals died a median of 7.7 months after the last clinical evaluation (interquartile range = 7.4) and the brain was removed a median of 6.2 hours after death (interquartile range = 3.6). Examiners blinded to all clinical data followed a standard protocol for tissue preservation, tissue sectioning, and quantification of pathologic findings.18,19 The cerebral hemispheres were coronally cut into 1-cm slabs, the cerebellar hemispheres were sagittally cut into 1-cm slabs, and the brainstem was removed at the level of the mamillary bodies and bisected at the mid pons level.
Slabs were visually inspected for gross infarcts. Slabs from one cerebral hemisphere and one cerebellar hemisphere and all slabs with suspected infarcts were fixed for at least 3 days in 4% paraformaldehyde. Suspected infarcts were processed for histologic confirmation20,21 and the age (acute, subacute, chronic) was noted. One hemisphere was examined for microinfarcts in 6 cortical regions, 2 subcortical regions, and the midbrain using 6-μm paraffin-embedded sections stained with hematoxylin & eosin. Chronic microinfarcts included cavitated lesions with few macrophages and fibrillary gliosis or incomplete infarcts.21 Gross infarcts and microinfarcts were each treated as present or absent in analyses.
We quantified the percent area occupied by β-amyloid–immunoreactive plaques and the density of tau-immunoreactive tangles in 8 brain regions: anterior cingulate cortex, dorsal lateral prefrontal cortex, superior frontal cortex, inferior temporal cortex, hippocampus (CA1/subculum), entorhinal cortex, angular/supramarginal gyrus, and primary visual cortex. In the cortical regions, tissue blocks from adjacent slabs were embedded in paraffin and cut into 20-μm sections. In the hippocampus, 0.5-cm blocks were dissected from consecutive 1-cm slabs, embedded in paraffin, and cut into 20-μm sections.
The β-amyloid burden was assessed using an N-terminus–directed monoclonal antibody (1:1,000, 10D5; Elan Pharmaceuticals, Dublin, Ireland) and diaminobenzidine as the reporter, with 2.5% nickel sulfate to enhance immunoreaction product contrast, and a computer-assisted sampling procedure. The percent area occupied by β-amyloid–immunoreactive pixels in each region was calculated and the regional values were averaged to yield a composite measure of β-amyloid burden.22
The density of tau-immunoreactive tangles was quantified with an antipaired helical filaments-tau antibody clone AT8 (1:2000; ThermoScientific, Rockford, IL) and computer-assisted sampling. Tangle density/mm2 in each region was standardized and the mean of the standard scores was used as a composite measure of tangle density.22
Six regions (substantia nigra, anterior cingulate cortex, entorhinal cortex, midfrontal cortex, superior or middle temporal cortex, inferior parietal cortex) were assessed for Lewy bodies using a monoclonal phosphorylated antibody to α-synuclein (1:20,000; Wako Chemical USA Inc., Richmond, VA) with alkaline phosphatase as the chromogen.18 Lewy bodies were treated as present or absent in analyses.
Statistical analysis.
We used mixed-effects models to characterize trajectories of cognitive function over time and to estimate the association of covariates with both level of cognitive function proximate to death and rate of cognitive change. Each model included a term for time from death (in years), which indicates the mean annual change in the cognitive outcome measure; 8 covariates (3 demographic, 5 pathologic) to assess the relation of each covariate to cognitive level proximate to death; and the interaction of each covariate with time, which indicates the relation of the covariate to annual rate of change in cognitive function. We then added terms for different activity measures and their interactions with time. The cognitive activity × time interactions test whether activity is related to residual variation in cognitive decline after adjustment for pathology and demographics. In separate analyses, we tested for interactions between activity and each pathologic measure and between MCI and activity. The primary outcome was a composite measure of global cognition. The core model was repeated with measures of specific cognitive functions.
RESULTS
At baseline, ratings of late-life (mean = 3.18, SD = 0.83, skewness = −0.57) and early-life (mean = 3.11, SD = 0.59, skewness = −0.28) participation in cognitively stimulating activities had approximately normal distributions. Older age at baseline was associated with less frequent late-life activity (r = −0.19, p = 0.001) but was unrelated to early-life activity (r = 0.01, p = 0.863). Neither age at death nor sex was related to either activity measure. Higher education was associated with more frequent late-life (r = 0.23, p < 0.001) and early-life (r = 0.40, p < 0.001) activity. Early- and late-life activity were moderately correlated (r = 0.36, p < 0.001).
Baseline scores on the composite measure of global cognitive function ranged from −1.36 to 1.00 (mean = −0.06, SD = 0.50, skewness = −0.31), with higher values indicating better performance. During follow-up, 102 individuals developed dementia (60 with MCI at baseline) and 51 developed MCI, indicating that substantial cognitive decline occurred. On neuropathologic examination, the measures of amyloid burden (mean = 4.89, SD = 5.05, skewness = 0.99) and tangle density (mean = 5.59, SD = 6.12, skewness = 2.26) were positively skewed, 33.7% had one or more chronic gross cerebral infarcts, 23.8% had one or more chronic microscopic cerebral infracts, and 10.2% had neocortical Lewy bodies.
We constructed a series of mixed-effects models to assess change in cognitive function. Each model included terms to account for the association of age at death, sex, education, gross and microscopic infarction, neocortical Lewy bodies, amyloid burden, and tangle density with rate of cognitive decline. These demographic and neuropathologic measures accounted for 33% of the variability in rates of cognitive decline. We then added terms for cognitive activity to test for its hypothesized association with cognitive decline. In the initial analysis (table 1, model A), higher frequency of late-life cognitive activity was associated with higher level of cognitive function, as shown by the term for late-life activity, and slower rate of cognitive decline, as shown by the interaction term. In this analysis, activity accounted for 10% of the residual variance (7% of total variance) in rate of cognitive decline not associated with neuropathologic burden. Figure 1A shows that compared to those with average late-life activity (50th percentile, blue line), decline increased by 48.4% in those with infrequent activity level (10th percentile, red line) and was reduced by 32.3% in those with frequent late-life activity (90th percentile, green line).
Table 1.
Relation of cognitive activity to global cognitive decline after adjustment for neuropathologic burdena
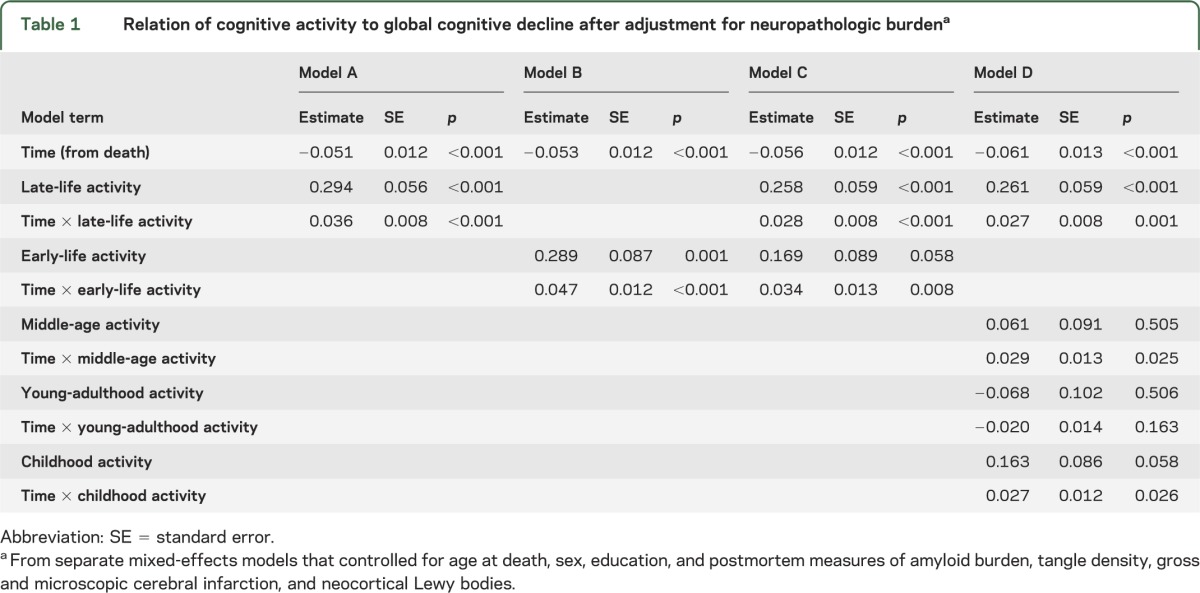
Figure 1. Relation of early- and late-life cognitive activity to cognitive decline.
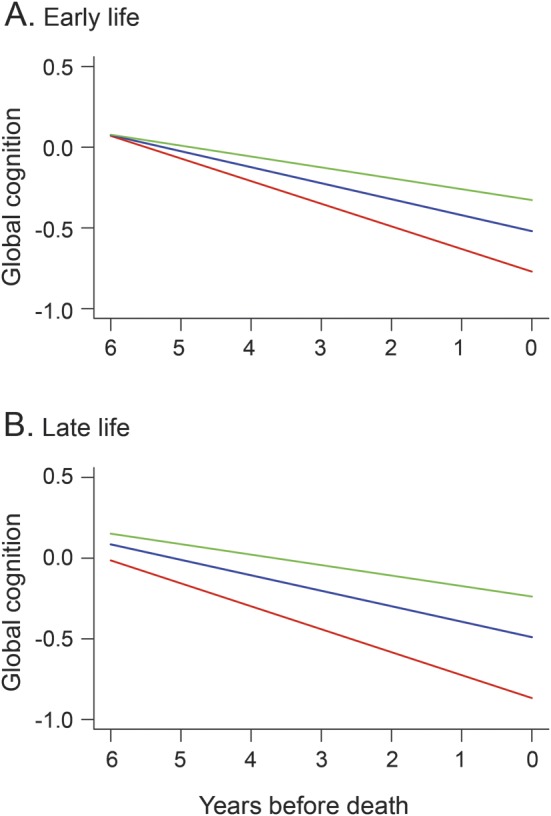
Predicted paths of change in global cognition during the last 6 years of life for persons with high (green line, 90th percentile), middle (blue line, 50th percentile), and low (red line, 10th percentile) cognitive activity in early life (A) and late life (B), adjusted for age at death, sex, education, plaques, tangles, infarction, and Lewy bodies.
Results were similar for the early-life cognitive activity measure (table 1, model B), with activity accounting for 9% of residual variability (6% of total variance) in cognitive decline not related to neuropathologic burden. Thus, as shown in figure 1B, compared with those with average early-life cognitive activity (50th percentile, blue line), cognition declined 41.5% faster in those with infrequent activity (10th percentile, red line) and 31.9% slower in those with frequent activity (90th percentile, green line). When early- and late-life activity were modeled together (table 1, model C), late-life cognitive activity was related to level of cognitive function but early-life cognitive activity was not. However, both activity measures were related to change in cognition, accounting for 14% of residual variability in cognitive decline (10% of total variance).
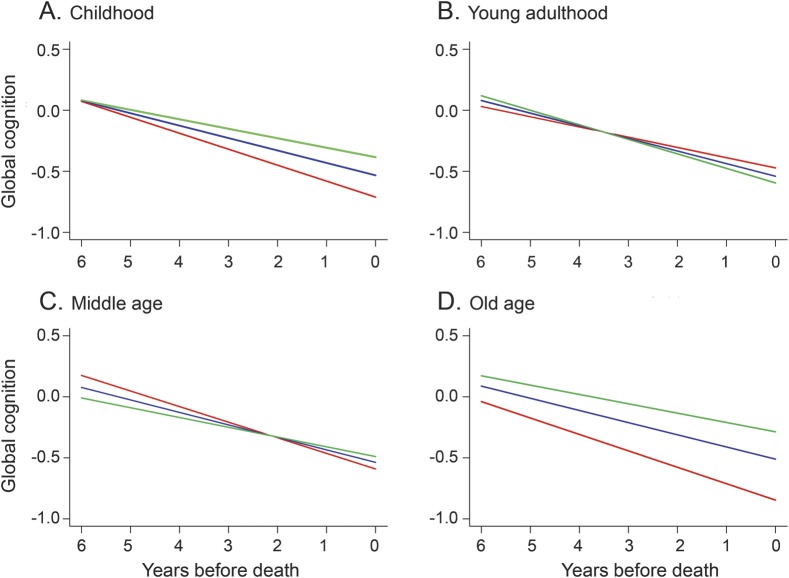
The early-life cognitive activity measure included questions about childhood (i.e., ages 6 and 12; mean = 2.97, SD = 0.73, skewness = −0.32), young adulthood (i.e., age 18; mean = 3.06, SD = 0.70, skewness = −0.37), and middle age (i.e., age 40; mean = 3.33, SD = 0.65, skewness = −0.33). These subscales had similar levels of internal consistency (Cronbach coefficient α 0.77 for childhood, 0.76 for young adulthood, and 0.70 for middle age) and were moderately intercorrelated (range of correlations: 0.28–0.69). To determine whether cognitive activity at these points was differentially related to cognitive decline, we repeated the previous analysis with activity measures from each period in place of the early-life activity measure (table 1, model D). As shown in figure 2, more frequent cognitive activity in childhood (A), middle age (C), and old age (D) was related to slower cognitive decline in each, with no effect for activity during young adulthood (B), possibly because it was strongly correlated with activity levels in childhood (r = 0.69, p < 0.001) and middle age (r = 0.60, p < 0.001).
Figure 2. Relation of life-span cognitive activity to cognitive decline.
Predicted paths of change in global cognition during the last 6 years of life for persons with high (green line, 90th percentile), middle (blue line, 50th percentile), and low (red line, 10th percentile) cognitive activity in childhood (A), young adulthood (B), middle age (C), and old age (D), adjusted for age at death, sex, education, plaques, tangles, infarction, and Lewy bodies.
To determine whether cognitive activity modified the association of pathology with level of cognition proximate to death or rate of cognitive decline, we repeated the initial analysis (model A in table 1) with terms to test for interactions between late-life activity and each pathologic measure. There were no interactions. In a similar repetition of model B, there was no evidence of an interaction between early-life activity and the pathologic measures.
The cognitive activity questionnaire was completed at the baseline evaluation when 109 participants had MCI. Therefore, we repeated model C with terms for baseline MCI and its interaction with time, and both late-life (estimate = 0.026, SE = 0.008, p = 0.002) and early-life (estimate = 0.034, SE = 0.013, p = 0.007) activity continued to be associated with cognitive decline. In a subsequent analysis, there was no evidence that MCI modified the association of either late-life (estimate = −0.007, SE = 0.017, p = 0.672) or early-life (estimate = 0.015, SE = 0.024, p = 0.527) activity with cognitive decline.
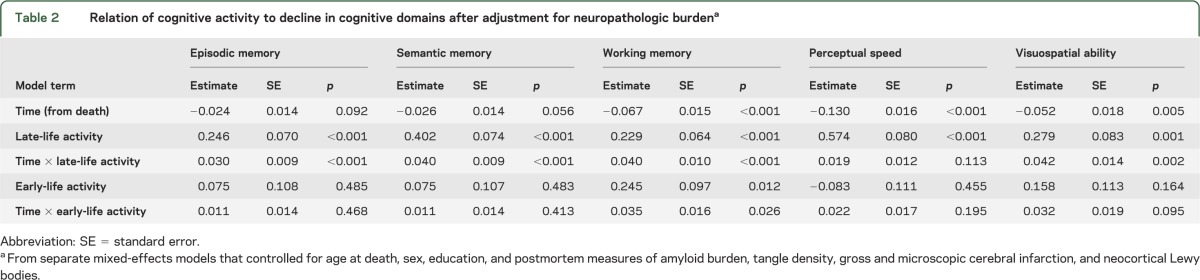
To determine whether cognitive activity was related to residual decline in some cognitive domains but not others, we repeated the core analysis (model C in table 1) using measures of specific cognitive functions instead of the measure of global cognition. In these analyses (table 2), late-life activity was related to change in all domains except perceptual speed, whereas early-life activity was only related to change in working memory.
Table 2.
Relation of cognitive activity to decline in cognitive domains after adjustment for neuropathologic burdena
DISCUSSION
Older persons in a longitudinal clinical-pathologic study rated current and past frequency of participation in cognitively stimulating activities and then underwent annual cognitive function testing. During a mean of 5.8 years of follow-up, nearly 300 individuals died and underwent neuropathologic examination. After adjustment for plaques, tangles, infarcts, and Lewy bodies, higher levels of cognitive activity in childhood, middle age, and old age were associated with slower rate of cognitive decline, together accounting for nearly 15% of variability in cognitive decline not attributable to neuropathologic burden.
The finding that more frequent cognitive activity predicts reduced cognitive decline is consistent with most prior research.1–5 These results add to knowledge by showing that cognitive activity was related to residual decline in cognitive function after adjustment for the association of neuropathologic burden with cognitive decline. The fact that cognitive activity has an association with cognitive decline that is independent of neuropathologic burden shows that more frequent cognitive activity can counterbalance the cognitive loss associated with neuropathologic conditions. This finding supports the cognitive reserve hypothesis and suggests that the association of cognitive activity with loss of cognition is not the result of reverse causality.
Retrospective ratings of cognitive activity in childhood and middle age were also related to late-life cognitive change, particularly working memory, even after accounting for late-life cognitive activity and neuropathologic burden. This suggests that cognitive experiences across the life span may influence cognitive reserve in old age.11,23 We are aware of previous studies linking cognitive activity24 and occupational complexity25,26 during middle age to late-life cognitive change but not studies linking childhood cognitive activity to late-life cognitive change.
Neuroimaging research suggests that cognitive activity can lead to changes in brain structure and function that might enhance cognitive reserve. Thus, occupations (e.g., professional musician,27 London taxi driver28) and leisure activities (e.g., playing Baduk29) that challenge particular cognitive functions are associated with differences in the gray and white matter of brain regions that support the cognitive functions. Importantly, longitudinal studies have documented regional increases in gray matter volume and white matter microstructural integrity over temporal intervals ranging from a few hours30 to several years11 in persons engaged in diverse cognitive activities, including studying for a test of medical knowledge,10 apprenticing as a London taxi driver,11 reading mirrored words,31 deciphering Morse code,32 learning novel color names,30 and performing cognitive exercises.33–36
The neurobiological bases of these structural MRI changes are uncertain, but an array of experience-dependent neuroplastic changes have been documented in developing and mature animals exposed to enriched environments. These include dendritic branching, growth of new dendritic spines, axonal remodeling, and increased myelination in brain regions involved in adapting to the environmental contingencies.37–39 To the extent that such neurobiological changes occur in conjunction with cognitive activity in humans, habitual participation in cognitively stimulating pursuits over a lifetime might substantially increase the efficiency of some cognitive systems so that a relatively greater neuropathologic burden would be required to impair functioning.
Study strengths and limitations should be noted. Rates of participation in clinical evaluations and autopsy were high, minimizing bias caused by selective attrition. Cognitive activity and cognitive function were assessed with psychometrically established scales, minimizing measurement error. Cognition was assessed at multiple evenly spaced intervals proximate to death, enhancing our ability to reliably assess individual paths of change and their association with postmortem pathologic and antemortem experiential measures. An important limitation is that the cohort is selected, so the generalizability of the findings will need to be demonstrated. In addition, assessment of early-life cognitive activity was based on retrospective report, which may have biased results and limited our ability to capture activity differences between early-life epochs. We had too few participants and insufficient follow-up to test whether the association of cognitive activity with rate of cognitive change shifts during the development of dementia, as predicted by the cognitive reserve hypothesis.40
Supplementary Material
ACKNOWLEDGMENT
The authors thank the Illinois residents who participated in the Rush Memory and Aging Project; Traci Colvin, MPH, and Karen Skish, MS, for study coordination; John Gibbons, MS, and Greg Klein, MS, for data management; and Woojeong Bang, MS, for statistical programming.
GLOSSARY
- MCI
mild cognitive impairment
- SE
standard error
Footnotes
Editorial, page >308
AUTHOR CONTRIBUTIONS
Drafting/revising the manuscript for content: Dr. Wilson, Dr. Boyle, Dr. Yu, Dr. Barnes, Dr. Schneider, Dr. Bennett. Study concept or design: Dr. Wilson, Dr. Boyle, Dr. Barnes, Dr. Schneider, Dr. Bennett. Analysis or interpretation of the data: Dr. Wilson, Dr. Boyle, Dr. Yu, Dr. Barnes, Dr. Schneider, Dr. Bennett. Acquisition of data: Dr. Bennett. Statistical analysis: Dr. Yu. Study supervision or coordination: Dr. Bennett. Obtaining funding: Dr. Boyle, Dr. Bennett.
STUDY FUNDING
This research was supported by the National Institute on Aging (R01AG17917, R01AG15819, R01AG33678, R01AG34374) and the Illinois Department of Public Health. The funding organizations had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
DISCLOSURE
R. Wilson serves as a Consulting Editor for Aging, Neuropsychology, and Cognition and Psychology and Aging; has served as a consultant for Pain Therapeutics, Inc.; and receives research support from NIH (R01AG024871 [principal investigator], P30AG10161 [coinvestigator], R01AG11101 [coinvestigator], R01AG15819 [coinvestigator], U24AG026395 [coinvestigator], R01AG017917 [coinvestigator], R01AG009966 [coinvestigator], R01AG034374 [coinvestigator], and RC2AG036547 [coinvestigator]). P. Boyle receives research support from the NIH (R01AG034374 [principal investigator], R01AF034119 [coinvestigator], and R01AG033678 [principal investigator]). L. Yu receives research support from NIH (R01AG033678 [coinvestigator], R01AG024871 [coinvestigator], R01AG024871 [coinvestigator], P30AG010161 [coinvestigator], R01AG015819, R01HL096944 [coinvestigator], R01AG036042 [coinvestigator], R01AG038651 [coinvestigator], R01AG040039 [coinvestigator], R01AG034374 [coinvestigator], U01AG032984 [coinvestigator]). L. Barnes serves as an editorial board member of the Journal of Aging and Health and is funded by NIH (grants R01AG022018 [principal investigator], P30AG010161 [coinvestigator], R01AG031553 [coinvestigator], R01AG032247 [coinvestigator], R01NR009543 [coinvestigator], and P20MD006886 [coinvestigator]). J. Schneider receives research support from NIH (R01AG042210 [principal Investigator], P30 AG010161 [coinvestigator], R01HL096944 [coinvestigator], R01AG039478 [coinvestigator], R01 AG017917 [coinvestigator], R01 AG015819 [coinvestigator], R01 AG022018 [coinvestigator], R01AG036042 [coinvestigator], R01AG040039 [coinvestigator], R01AG036836 [coinvestigator], R01AG034374 [coinvestigator], contract no. 23282007 [IDPH] [coinvestigator], R01 AG031553 [coinvestigator], P01 AG014449 [coinvestigator]). D. Bennett serves on the editorial board of Neurology®; has served as a consultant to Schering-Plough Corp., Medivation, Inc., and the Gerson Lehrman Group; and receives research support from Danone Inc., the NIH (R01AG017917 [principal investigator], R01AG015819 [principal investigator], R01AG036042 [principal investigator], RC2AG036547 [principal investigator], U01AG032984 [coprincipal investigator, leader of epidemiologic cohort studies], R01AG024480 [coinvestigator], R01AG024871 [coinvestigator], P01AG009466 [coinvestigator], U24AG026395 [coinvestigator], R01AG030142 [coinvestigator], P01AG014449 [coinvestigator], R01HL096944 [coinvestigator], R01AG033678 [coinvestigator], R01AG034374 [coinvestigator], R01AG032755 [coinvestigator], R01AG022018 [coinvestigator], R01AG034119 [coinvestigator], R01AG027040 [coinvestigator], R01AG026147 [coinvestigator], R01AG031553 [coinvestigator], and P30AG010161 [principal investigator–administrative core leader, Religious Orders Study core leader]), and the Illinois Department of Public Health. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Bosma H, van Boxtel MP, Ponds RW, et al. Engaged lifestyle and cognitive function in middle and old-aged, non-demented persons: a reciprocal association? Z Gerontol Geriatr 2002;35:575–581 [DOI] [PubMed] [Google Scholar]
- 2.Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: engaged lifestyle as a buffer of cognitive decline in aging? Psychol Aging 1999;14:245–263 [DOI] [PubMed] [Google Scholar]
- 3.Wilson RS, Mendes de Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA 2002;287:742–748 [DOI] [PubMed] [Google Scholar]
- 4.Wilson RS, Bennett DA, Bienias JL, Mendes de Leon CF, Morris MC, Evans DA. Cognitive activity and cognitive decline in a biracial community population. Neurology 2003;61:812–816 [DOI] [PubMed] [Google Scholar]
- 5.Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. The relation of cognitive activity to risk of developing Alzheimer disease. Neurology 2007;69:1911–1920 [DOI] [PubMed] [Google Scholar]
- 6.Aartsen MJ, Smits CH, van Tilburg T, Knipscheer KC, Deeg DJ. Activity in older adults: cause or consequence of cognitive functioning? A longitudinal study on everyday activities and cognitive performance in older adults. J Gerontol B Psychol Sci Soc Sci 2002;57:P153–P162 [DOI] [PubMed] [Google Scholar]
- 7.Wilson RS, Bennett DA. Cognitive activity and risk of Alzheimer’s disease. Curr Dir Psychol Sci 2003;12:87–91 [Google Scholar]
- 8.Hertzog C, Kramer AF, Wilson RS, Lindenberger U. Enrichment effects on adult cognitive development: can the functional capacity of older adults be preserved and enhanced? Psychol Sci Pub Int 2009;9:1–65 [DOI] [PubMed] [Google Scholar]
- 9.Richards M, Deary IJ. A life course approach to cognitive reserve: a model for cognitive aging and development? Ann Neurol 2005;58:617–622 [DOI] [PubMed] [Google Scholar]
- 10.Draganski B, Gaser C, Kempermann G, et al. Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci 2006;26:6314–6317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woollett K, Maguire EA. Acquiring “the knowledge” of London’s layout drives structural brain changes. Curr Biol 2011;21:2109–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jagust WJ, Mormino EC. Lifespan brain activity, β-amyloid, and Alzheimer’s disease. Trends Cogn Sci 2011;15:520–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson RS, Segawa E, Boyle PA, Bennett DA. The influence of late-life cognitive activity on cognitive health. Neurology 2012;78:1123–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Curr Alzheimer Res 2012;9:646–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson RS, Barnes LL, Bennett DA. Assessment of lifetime participation in cognitively stimulating activities. J Clin Exp Neuropsychol 2003;25:634–642 [DOI] [PubMed] [Google Scholar]
- 16.Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc 2005;11:400–407 [PubMed] [Google Scholar]
- 17.Marquine MJ, Segawa E, Wilson RS, Bennett DA, Barnes LL. Association between cognitive activity and cognitive function in older Hispanics. J Int Neuropsychol Soc 2012;18:1041–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider JA, Li JL, Li Y, Wilson RS, Kordower JH, Bennett DA. Substantia nigra tangles are related to gait impairment in older persons. Ann Neurol 2006;59:166–173 [DOI] [PubMed] [Google Scholar]
- 19.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 2006;66:1837–1844 [DOI] [PubMed] [Google Scholar]
- 20.Schneider JA, Wilson RS, Cochran EJ, et al. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology 2003;60:1082–1088 [DOI] [PubMed] [Google Scholar]
- 21.Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke 2011;42:722–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol 2004;61:378–384 [DOI] [PubMed] [Google Scholar]
- 23.Gold BT, Kim C, Johnson NF, Kryscio RJ, Smith CD. Lifelong bilingualism maintains neural efficiency for cognitive control in aging. J Neurosci 2013;33:387–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crowe M, Andel R, Pedersen NL, Johansson B, Gatz M. Does participation in leisure activities lead to reduced risk of Alzheimer’s disease? A prospective study of Swedish twins. J Gerontol B Psychol Sci Soc Sci 2003;58:P249–P255 [DOI] [PubMed] [Google Scholar]
- 25.Finkel D, Andel R, Gatz M, Pedersen NL. The role of occupational complexity in trajectories of cognitive aging before and after retirement. Psychol Aging 2009;24:563–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marquie JC, Duarte LR, Bessier̀es P, Dalm C, Gentil C, Ruidavets JB. Higher mental stimulation at work is associated with improved cognitive functioning in both young and older workers. Ergonomics 2010;53:1287–1301 [DOI] [PubMed] [Google Scholar]
- 27.Gaser C, Schlaug G. Brain structures differ between musicians and non-musicians. J Neurosci 2003;23:9240–9245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maguire EA, Gadian DG, Johnsrude IS, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA 2000;97:4398–4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee B, Park JY, Jung WH, et al. White matter neuroplastic changes in long-term trained players of the game of “Baduk” (GO): a voxel-based diffusion-tensor imaging study. Neuroimage 2010;52:9–19 [DOI] [PubMed] [Google Scholar]
- 30.Kwok V, Niu Z, Kay P, et al. Learning new color names produces rapid increase in gray matter in the intact adult human cortex. Proc Natl Acad Sci USA 2011;108:6686–6688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ilg R, Wohlschlager AM, Gaser C, et al. Gray matter increase induced by practice correlates with task-specific activation: a combined functional and morphometric magnetic resonance imaging study. J Neurosci 2008;28:4210–4215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt-Wilcke T, Rosengarth K, Luerding R, Bogdahn U, Greenlee MW. Distinct patterns of functional and structural neuroplasticity associated with learning Morse code. Neuroimage 2010;51:1234–1241 [DOI] [PubMed] [Google Scholar]
- 33.Takeuchi H, Sekiguchi A, Taki Y, et al. Training of working memory impacts structural connectivity. J Neurosci 2010;30:3297–3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lövdén M, Bodammer NC, Kühn S, et al. Experience-dependent plasticity of white-matter microstructure extends into old age. Neuropsychologia 2010;48:3878–3883 [DOI] [PubMed] [Google Scholar]
- 35.Engvig A, Fjell AM, Westlye LT, et al. Effects of memory training on cortical thickness in the elderly. Neuroimage 2010;52:1667–1676 [DOI] [PubMed] [Google Scholar]
- 36.Engvig A, Fjell AM, Westlye LT, et al. Memory training impacts short-term changes in aging white matter: a longitudinal diffusion tensor imaging study. Hum Brain Mapp 2012;33:2390–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marham JA, Greenough WT. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biol 2004;1:351–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnes SJ, Finnerty GT. Sensory experience and cortical rewiring. Neuroscientist 2010;16:186–198 [DOI] [PubMed] [Google Scholar]
- 39.Qui X, Huang CX, Lu W, et al. Effects of a 4 month enriched environment on the hippocampus and the myelinated fibers in the hippocampus of middle-aged rats. Brain Res 2012;1465:26–33 [DOI] [PubMed] [Google Scholar]
- 40.Wilson RS, Barnes LL, Aggarwal NT, et al. Cognitive activity and the cognitive morbidity of Alzheimer disease. Neurology 2010;75:990–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.