Abstract
Objective:
We assessed whether clinical and imaging features of subjects with apraxia of speech (AOS) more severe than aphasia (dominant AOS) are more similar to agrammatic primary progressive aphasia (agPPA) or to primary progressive AOS (PPAOS).
Methods:
Sixty-seven subjects (PPAOS = 18, dominant AOS = 10, agPPA = 9, age-matched controls = 30) who all had volumetric MRI, diffusion tensor imaging, F18-fluorodeoxyglucose and C11-labeled Pittsburgh compound B (PiB)-PET scanning, as well as neurologic and speech and language assessments, were included in this case-control study. AOS was classified as either type 1, predominated by sound distortions and distorted sound substitutions, or type 2, predominated by syllabically segmented prosodic speech patterns.
Results:
The dominant AOS subjects most often had AOS type 2, similar to PPAOS. In contrast, agPPA subjects most often had type 1 (p = 0.01). Both dominant AOS and PPAOS showed focal imaging abnormalities in premotor cortex, whereas agPPA showed widespread involvement affecting premotor, prefrontal, temporal and parietal lobes, caudate, and insula. Only the dominant AOS and PPAOS groups showed midbrain atrophy compared with controls. No differences were observed in PiB binding across all 3 groups, with the majority being PiB negative.
Conclusion:
These results suggest that dominant AOS is more similar to PPAOS than agPPA, with dominant AOS and PPAOS exhibiting a clinically distinguishable subtype of progressive AOS compared with agPPA.
The term apraxia of speech (AOS) is used to denote a motor speech disorder in which abnormalities reflect deficits in the programming of movements for speech production.1,2 Although usually caused by left hemisphere stroke, AOS can also be associated with neurodegenerative disease, and when it is the only sign or symptom, it is labeled primary progressive AOS (PPAOS).3,4 Unlike PPAOS, the term primary progressive aphasia (PPA) is reserved for a neurodegenerative disorder in which the most salient feature is language dysfunction.5–7 Agrammatic PPA (agPPA) is one subtype of PPA, characterized by specific abnormalities that affect grammar and syntax in verbal or written expression.8,9 Although it is recognized that many subjects with agPPA may have AOS, the most salient feature of agPPA is aphasia. In fact, to be diagnosed with any variant of PPA,8 language difficulty must be the most prominent clinical feature at symptom onset and for the initial phases of the disease; deficits cannot be due to “disruption of the formation of words.”7
Over the past 2½ years, we have encountered subjects with AOS and agrammatic aphasia in whom AOS is the dominant feature. Such cases are difficult to classify. Some would classify them as agPPA, whereas others would disagree because the AOS, rather than language impairment, is the dominant feature. In addition, we have noticed different specific patterns of AOS characteristics in many of these subjects. To help clarify this diagnostic uncertainty, we assessed whether these difficult-to-classify cases are more similar to those with PPAOS or to those with agPPA.
METHODS
Recruitment.
Between July 1, 2010, and June 30, 2012, we recruited all patients presenting to the Mayo Clinic Department of Neurology with any combination of AOS and agrammatic aphasia secondary to a degenerative process (n = 37). Subjects with concurrent illnesses that could account for language deficits, or meeting criteria for another neurodegenerative syndrome, for example, progressive supranuclear palsy syndrome (PSPS)10 or corticobasal syndrome,11 were excluded. Subjects underwent detailed speech and language examination, neurologic evaluation, neuropsychological testing, and neuroimaging over a span of 2 to 3 days, as previously described.4
Standard protocol approvals and patient consents.
This study was approved by the Mayo Clinic Institutional Review Board. All subjects provided written informed consent. Authorization was obtained from both subjects shown in the videos.
Language, speech, and oral praxis assessments.
The language assessment, previously described in detail,4 included the Western Aphasia Battery (WAB)–revised,12 Part 1; a 22-item version of Part V of DeRenzi and Vignolo's Token Test13; a 15-item version of the Boston Naming Test14; Action (verb) Fluency15 and Letter (FAS) Fluency16 tasks. Several parameters of reading and writing were also assessed from the WAB, Part 2, and although not reported here, these were used in establishing classification.
An AOS Rating Scale (ASRS)4 was used to assess severity of AOS characteristics. The ASRS is not yet completely standardized, but its validity is supported by a strong correlation (r = 0.89) with independent consensus judgments about the presence and general severity of AOS for all 37 subjects. A designation of AOS type 1 was made if distorted sound substitutions or additions (often increasing in prominence with increased utterance length or syllable or word complexity) was judged to clearly dominate the speech pattern (see video 1 on the Neurology® Web site at www.neurology.org). A designation of AOS type 2 was made if syllable segmentation within multisyllabic words or across words in phrases and lengthened intersegment durations between syllables, words, or phrases were judged to clearly dominate the speech pattern (see video 2). If there was no clear predominance of type 1 or type 2 features, a designation of AOS-NOS (not otherwise specified) was made. There was 95% agreement on AOS subtype (35/37), with consensus achieved by both speech-language pathologists (J.R.D., E.A.S.) after further discussion on the remaining 2 subjects (both classified as AOS-NOS). The same speech tasks were also used to judge presence/absence of dysarthria (and dysarthria type), rated on a 0–4 severity scale. Nonverbal oral apraxia was measured using an 8-item scale (e.g., blow, click tongue, cough)4 and a qualitative judgment about its presence/absence.
Clinical diagnostic classification.
To be included in this study, subjects must have had progressive AOS, agrammatic aphasia, or both. Quantitative scores and video recordings of language tests, independent of neurologic, neuropsychological, and neuroimaging results, were reviewed by both speech-language pathologists who made independent judgments about the presence or absence of agrammatic aphasia and AOS. The presence of agrammatism was based on function word omissions or grammatic or syntactic errors during the WAB Picture Description task, general conversation, or the narrative Writing Output subtest of the WAB, Part 2. Examples of spoken or written utterances that led to the agrammatic diagnosis include: “This home is have cars,” “We have fish pond too,” and “Their son was try hard.” The writing measure was relied on particularly when speech intelligibility was compromised by AOS. Subjects with aphasia not characterized by agrammatic spoken or written language expression (e.g., semantic or logopenic variant of PPA) were excluded (n = 45).
Subjects were classified as PPAOS according to published criteria.4 The remaining subjects with AOS and agrammatic aphasia were subdivided into 2 groups: agPPA and dominant AOS. The WAB Aphasia Quotient, Token Test, and ASRS scores were used to quantitatively determine whether AOS severity was greater than aphasia severity (dominant AOS) or agrammatic aphasia severity was greater than AOS severity (agPPA). Token Test scores were transformed to a 100-point scale to match the WAB Aphasia Quotient scale and both were averaged to create a composite aphasia severity score. Composite aphasia and ASRS scores were z-transformed based on scores from all study subjects. Subjects with ASRS z score > composite aphasia z score were categorized as dominant AOS. Subjects with composite aphasia z score > ASRS z score were categorized as agPPA. No subject had equal ASRS and composite aphasia z scores. Both speech-language pathologists were also asked to come to consensus, using clinical judgment, regarding whether AOS severity was more or less than aphasia severity. Importantly, the quantitative and consensus classifications were identical, except for 2 subjects with agPPA who were judged clinically to have equivalent AOS and aphasia severity.
Neuroimaging methods.
All subjects had identical neuroimaging sequences, including volumetric head MRI, diffusion tensor imaging (DTI), F18-fluorodeoxyglucose (FDG)-PET, and C11-labeled Pittsburgh compound B (PiB)-PET. Details of the acquisition parameters are provided in appendix e-1.
Voxel-level comparisons of gray and white matter volumes and FDG-PET metabolism were performed with SPM5 software. Preprocessing steps have been previously described.4 Statistical comparisons between each disease group and 30 age-matched controls were assessed using false discovery rate correction (p < 0.005) and uncorrected for multiple comparisons (p < 0.001). Direct comparisons were performed between disease groups uncorrected for multiple comparisons (p < 0.001). Voxel-wise statistical analysis of DTI data was performed using Tract-Based Spatial Statistics17 (http://www.fmrib.ox.ac.uk/fsl), as previously described.4 Fractional anisotropy (FA) and mean diffusivity (MD) were assessed after correction for multiple comparisons (p < 0.05).
Measurements of midbrain area were performed on volumetric MRI because midbrain atrophy is a feature of PSPS,18–20 a tauopathy associated with AOS.21,22 Measurements were performed manually by one rater (J.L.W.) using Analyze software (Biomedical Imaging Resource; Mayo Clinic, Rochester, MN), based on previously published criteria.4,18
PiB-PET scans were classified as positive or negative according to previously published criteria.4 Global PiB ratios were calculated.23
Statistical methods.
Group differences for categorical variables were assessed with the χ2 test and Fisher exact test for small numbers. Differences in continuous variables were assessed using the Kruskal-Wallis test. Significance was set at p < 0.05. Mann-Whitney U post hoc testing was performed if Kruskal-Wallis testing was significant. Statistical analyses were performed using JMP software (version 8.0.0; SAS Institute Inc., Cary, NC). Results are reported as median (interquartile range).
RESULTS
Clinical results.
Of the 37 subjects who met inclusion criteria, 18 were diagnosed with PPAOS, 9 with agPPA, and 10 with dominant AOS. The difference subtracting the aphasia composite z score from the ASRS z score was −1.0 (−0.5, −1.5) for dominant AOS and 1.2 (0.5, 1.5) for agPPA.
There were no significant demographic differences among groups, with the exception of sex (table 1). No subject had a family history of neurodegenerative disease. Neurologic and neuropsychological test scores did not show striking differences between dominant AOS and the other 2 groups in any one direction (table 1). In fact, in the majority of tests there were no differences across groups.
Table 1.
Summary of demographic, neurologic, and neuropsychological dataa
As expected, dominant AOS subjects showed poorer performance on measures of language compared with PPAOS subjects, including WAB fluency ratings, which reflect a crude index of agrammatic severity (table 2). Dominant AOS subjects performed better on the auditory verbal comprehension subtest of the WAB and on the Token Test than agPPA subjects. Dominant AOS had the poorest motor speech ratings, including higher scores on the ASRS, than both PPAOS and agPPA (table 2). A difference was observed in AOS subtypes, with dominant AOS showing a higher proportion of type 2 compared with agPPA (p = 0.046). In fact, no subject with agPPA had AOS type 2. No differences in AOS subtype were observed between dominant AOS and PPAOS (p = 0.20). The severity distribution of nonverbal oral apraxia differed across all groups, with greatest impairment in dominant AOS and least impairment in PPAOS.
Table 2.
Summary of speech, language, and oral praxis dataa
Neuroimaging results.
MRI and FDG results.
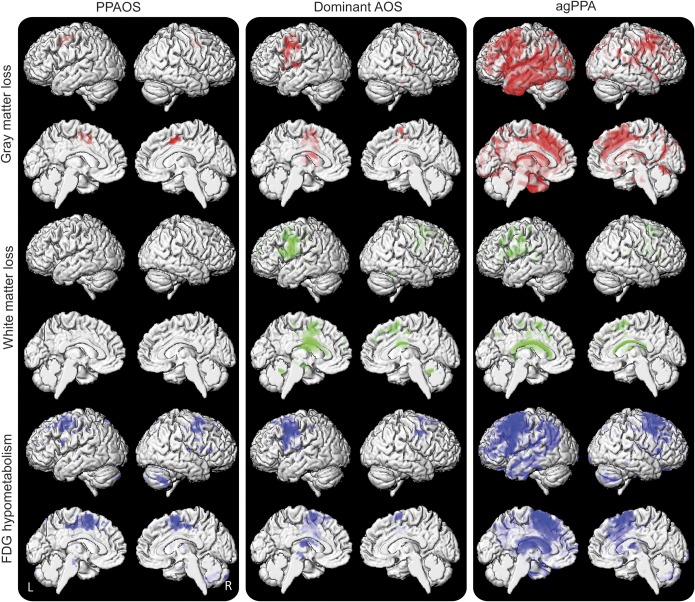
Imaging abnormalities in PPAOS were similar to those that we previously reported for a smaller subset of PPAOS subjects,4 with focal changes observed predominantly in superior lateral premotor cortex and bilateral supplementary motor area (figures 1 and e-1). Similar patterns were observed in dominant AOS, with changes relatively restricted to premotor cortex, although with greater extension into inferior and middle frontal lobes (figure 1). In contrast to PPAOS and dominant AOS, agPPA showed widespread abnormalities involving premotor cortex, as well as prefrontal, temporal, and parietal lobes (figure 1). Cortical abnormalities were greater in the left hemisphere in dominant AOS and agPPA.
Figure 1. Three-dimensional renderings showing patterns of gray (in red) and white matter (in green) loss and FDG-PET hypometabolism (in blue) in each group compared with controls.
Gray and white matter results are shown after correction for multiple comparisons using the FDR at p < 0.005. No regions of FDG-PET hypometabolism survived correction at FDR p < 0.005 for PPAOS and dominant AOS, and therefore FDG-PET results are shown uncorrected for multiple comparisons at p < 0.001. agPPA = agrammatic primary progressive aphasia; AOS = apraxia of speech; FDG = F18-fluorodeoxyglucose; FDR = false discovery rate; PPAOS = primary progressive AOS.
On direct comparison, dominant AOS showed greater abnormalities than PPAOS in a relatively restricted area in left inferior and middle frontal lobe, insula, and body of the corpus callosum (figure e-2). On the contrary, agPPA showed a more widespread pattern of greater abnormalities than dominant AOS, involving left inferior temporal lobe, prefrontal cortex, and parietal lobe (figure e-2). The agPPA subjects also showed greater abnormalities in left temporal and frontal lobes and corpus callosum compared with PPAOS subjects (figure e-2).
Midbrain areas were smaller in PPAOS (119.7, 107.3–161.9 mm2; p = 0.008) and dominant AOS (133.1, 122.5–146.7 mm2; p = 0.004), but not in agPPA subjects (149.56, 129.4–161.4 mm2; p = 0.52), compared with controls (158.3, 143.6–174.3 mm2). After adjusting for total gray matter volume, midbrain area was smaller in dominant AOS compared with agPPA (p = 0.04), with no difference observed between dominant AOS and PPAOS.
DTI results.
All 3 groups showed reduced FA in the body of the corpus callosum and premotor aspects of superior longitudinal fasciculus, involving tracts in inferior, middle, and superior frontal gyri, compared with controls (figure 2). However, agPPA also showed reduced FA in the splenium of the corpus callosum, as well as prefrontal, inferior parietal, and superior-posterior temporal aspects of superior longitudinal fasciculus. Increased MD was observed in the same regions in PPAOS and dominant AOS. No differences in FA or MD were observed on direct comparison across groups.
Figure 2. Results of the TBSS analysis of FA.
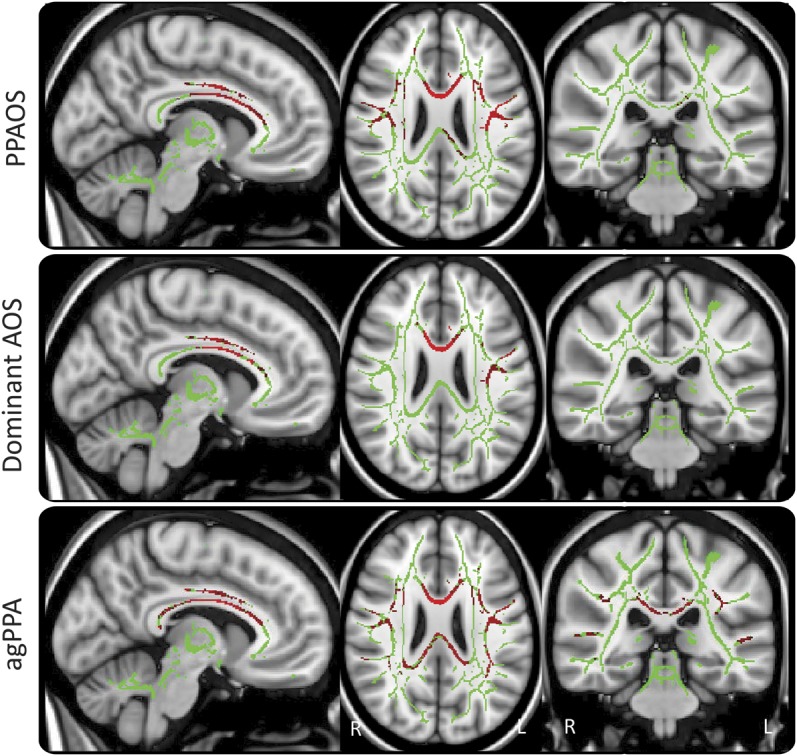
The mean FA skeleton is shown in green with red representing regions of reduced FA in PPAOS, dominant AOS, and agPPA compared with controls. Results were corrected for multiple comparisons at p < 0.05. agPPA = agrammatic primary progressive aphasia; AOS = apraxia of speech; FA = fractional anisotropy; PPAOS = primary progressive AOS; TBSS = Tract-Based Spatial Statistics.
PiB results.
Two PPAOS subjects (11%), 2 dominant AOS subjects (20%), and no agPPA subjects had positive PiB-PET scans. There were no differences in PiB ratios among the PPAOS (1.23, 1.21–1.29), dominant AOS (1.26, 1.21–1.42), and agPPA (1.22, 1.15–1.31) subjects.
DISCUSSION
In this study, we identified clinical, neuroanatomical, and functional imaging evidence suggesting that dominant AOS is more similar to PPAOS than to agPPA. Specifically, AOS characteristics (AOS subtypes) and neuroimaging features were more similar between PPAOS and dominant AOS.
One of the most compelling findings of this study was the difference in AOS subtypes among groups. The dominant AOS and PPAOS groups were both associated with AOS type 2. In contrast, agPPA was associated with AOS type 1. Our findings suggest that the 2 primary deficits in the broad characterizations of AOS—articulation and prosody—can be relatively, although not completely, dissociated in some cases. The severity of AOS cannot explain this distinction because there were no differences in clinical severity ratings between agPPA and PPAOS.
Discussions of stroke-induced AOS have included speculation about the existence of AOS subtypes,24 but consensus about distinguishing clinical characteristics of subtypes is not evident in the literature. Because motor speech planning and programming probably involve several processes that occur within a complex functional neuroanatomical network, and because it has been hypothesized that speech programming may be accomplished through more than one processing route,24,25 it is reasonable to hypothesize that different AOS patterns may exist. Pattern differences might be related to breakdowns in a) different stages or aspects of the programming process; b) varying degrees of reduced processing buffer capacity; c) different programming routes; or d) different neuroanatomical loci within the speech programming network.24,26,27 The characteristics of AOS type 1 generally seem to be emphasized in the AOS diagnosis. In contrast, the segmented, one syllable produced at a time, and associated prosodic characteristics of AOS type 2 appear to be underemphasized or not recognized, perhaps because the characteristics are more subtle or are considered to reflect dysarthria. These differences may explain why PPAOS and dominant AOS typically are embedded within agPPA.
The neuroanatomical correlate of AOS type 2 is likely the superior premotor cortex including supplementary motor cortex because this was the only region involved in PPAOS. The neuroanatomical correlate of AOS type 1 is, however, unclear, requiring further investigation. In general, our localization is not incompatible with results regarding the crude localization of AOS in the stroke literature,28 although it is noteworthy that the superior premotor cortex localization for AOS type 2 is not generally associated with reports of stroke-induced AOS. This suggests that superior premotor cortex (including supplementary motor area) may have some special role in planning and programming speech, perhaps one not generally recognized through studies of stroke-induced AOS.
Imaging findings also support the notion that dominant AOS is more similar to PPAOS. Both groups had atrophy and hypometabolism limited to premotor cortex, with the only difference being greater involvement of premotor inferior and middle frontal lobes in dominant AOS. This inferior frontal involvement is not unexpected given the presence of agrammatic aphasia in this group and the known association between Broca area and agrammatic aphasia.29,30 Similarities were also noted with the DTI analysis. In contrast to PPAOS and dominant AOS, the agPPA group showed widespread abnormalities for all imaging modalities. It is difficult to compare our results with previous imaging studies of agPPA because those studies included subjects with dominant AOS, and perhaps PPAOS, within the category of agPPA. Nevertheless, previous studies of agPPA have shown involvement of inferior premotor cortex,30–32 and often widespread atrophy patterns,33 likely driven by the subset of subjects that appropriately meet criteria for agPPA.
Previous clinicopathologic studies have shown that AOS is associated with tau pathologies,21,22 whereas other studies with agrammatic aphasia subjects report pathology characterized by TAR DNA binding protein of 43 kDa (TDP-43).34 Although a limitation of our study is the absence of pathology, our imaging findings may help shed light on the underlying pathologies. First, we observed midbrain atrophy in dominant AOS and PPAOS. Midbrain atrophy is a characteristic feature of PSPS,18–20 which is strongly associated with tau pathology, suggesting that PPAOS and dominant AOS subjects are eventually more likely to develop clinical features of PSPS and hence have tau pathology. Second, agPPA showed a widespread pattern of cortical and subcortical atrophy including frontal, temporal, and parietal lobes, without midbrain atrophy, more suggestive of TDP-43.35,36 Temporoparietal atrophy with sparing of midbrain can also be seen with Alzheimer disease. However, Alzheimer disease is unlikely in agPPA because no subject showed positive PiB-PET.37 The tau pathologies of corticobasal degeneration and Pick disease that can involve frontal, temporal, and parietal lobes, without midbrain atrophy, are also likely.
Unlike AOS type and imaging findings, neurologic and neuropsychological findings were not particularly helpful in differentiating these groups. This is not surprising given that these entities are, by definition, characterized by speech and language impairment. There was some suggestion that subjects with agPPA had memory deficits that could be explained by the presence of temporal lobe atrophy. The absence of quantification of agrammatism severity is another limitation, although the WAB fluency ratings and Writing Output scores, reflecting a crude index of such severity, were consistent with our group designations. Nonverbal oral apraxia was present in all groups, which is not surprising because it has been linked to premotor cortex and supplementary motor cortices.38
We recognize that some investigators do not support separating PPAOS from agPPA, either because AOS is “adequately captured under the heading progressive nonfluent aphasia” or because subjects with PPAOS will eventually develop agrammatism.8,39,40 Seventeen subjects from our PPAOS cohort were seen on more than one occasion, either before or after participation in the current study. Those seen before maintained a stable diagnosis of PPAOS. Of 9 subjects re-evaluated 4 to 21 months after study participation, one subject, seen 21 months later, developed aphasia less evident than the AOS, 2 subjects, seen 9 and 17 months later, had equivocal aphasia, and the remaining 6 subjects had no aphasia. Perhaps more important than the established fact that PPAOS may remain the predominant deficit for a prolonged period of time is the inconsistency of arguments that PPAOS need not be separated from aphasia because affected patients may eventually become aphasic. That is, it is generally accepted that many patients with PPA eventually develop motor or nonlanguage cognitive deficits consistent with, for example, PSPS, corticobasal syndrome, or Alzheimer dementia, but it is rarely argued that the concept and label of PPA should be abandoned and simply subsumed under the probabilistic, most likely, eventual clinical (or pathologic) diagnosis. In our view, there is nothing inherent in PPAOS or dominant AOS that requires different reasoning at this time. Our findings should generalize to subjects presenting with AOS, agrammatic aphasia, or both.
Our findings demonstrate the importance of not combining all subjects with progressive AOS and agrammatic aphasia under a single diagnostic category of agPPA. Instead, the results support a Level 1 separation of those with a pure or dominant AOS from those with PPA (figure 3). A Level 2 separation can then be made as depicted in figure 3. This classification scheme could have important prognostic value and be relevant to future genetic studies.
Figure 3. Flow chart illustrating the proposed 2-level classification scheme for subjects with AOS, agrammatic aphasia, or both.
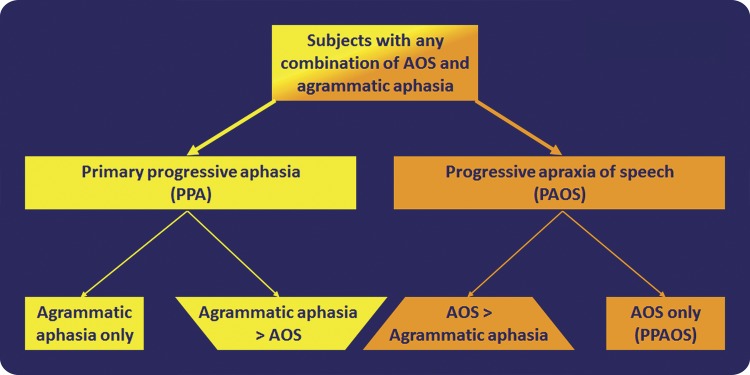
Subjects with agrammatic aphasia, AOS, or both should be first separated into those with PPA and those with PAOS. The diagnosis of PPA (specifically agPPA) should be made in those with either isolated agrammatic aphasia or dominant agrammatic aphasia, i.e., if AOS is present, it must be less severe than the aphasia. Similarly, the diagnosis of PAOS should be made in those with either isolated AOS or dominant AOS, i.e., if aphasia is present, it must be less severe than the AOS. agPPA and PAOS can then be further subdivided. Specifically, for agPPA, those with isolated agrammatic aphasia can be separated from those in which the aphasia is more severe than the AOS, and for PAOS, those with isolated AOS (PPAOS) can be separated from those in which the AOS is more severe than the aphasia (dominant AOS). agPPA = agrammatic PPA; AOS = apraxia of speech; PPAOS = primary progressive AOS.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Drs. Aksamit, Ahlskog, Boeve, Drubach, Knopman, and Petersen for subject referrals, and Miss Sarah Papenfuss for performing the neuropsychometric testing and organizing all subjects’ test schedules.
GLOSSARY
- agPPA
agrammatic primary progressive aphasia
- AOS
apraxia of speech
- ASRS
Apraxia of Speech Rating Scale
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- FDG
F18-fluorodeoxyglucose
- MD
mean diffusivity
- NOS
not otherwise specified
- PiB
Pittsburgh compound B
- PPA
primary progressive aphasia
- PPAOS
primary progressive apraxia of speech
- PSPS
progressive supranuclear palsy syndrome
- TDP-43
TAR DNA binding protein of 43 kDa
- WAB
Western Aphasia Battery
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Josephs: drafting/revising the manuscript for content, study concept or design, analysis or interpretation of the data, acquisition of data, statistical analysis, study supervision, obtaining funding. Dr. Duffy and Dr. Strand: drafting/revising the manuscript for content, study concept or design, analysis or interpretation of the data, acquisition of data. Dr. Machulda: drafting/revising the manuscript for content, analysis or interpretation of the data, acquisition of data. Dr. Senjem, Dr. Lowe, and Dr. Jack: drafting/revising the manuscript for content, acquisition of data. Dr. Whitwell: drafting/revising the manuscript for content, study concept or design, analysis or interpretation of the data, acquisition of data.
STUDY FUNDING
Supported by NIH grant R01-DC010367.
DISCLOSURE
K. Josephs receives research support from the NIH (NIDCD and NIA) and the Dana Foundation. J. Duffy, E. Strand, and M. Machulda receive research support from the NIH (NIDCD). M. Senjem has no disclosures. V. Lowe serves as a consultant for Bayer Schering Pharma and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals, the NIH (NIA, NCI), the MN Partnership for Biotechnology and Medical Genomics, and the Leukemia & Lymphoma Society. C. Jack serves as a consultant for Janssen, Bristol-Myers Squibb, General Electric, Siemens, and Johnson & Johnson and is involved in clinical trials sponsored by Allon and Baxter, Inc. He receives research funding from the NIH and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation. J. Whitwell receives research support from the NIH (NIDCD and NIA) and the Dana Foundation, and has served as a consultant for Bristol-Myers Squibb. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Duffy JR. Motor Speech Disorders: Substrates, Differential Diagnosis, and Management, 3rd ed St. Louis: Mosby; 2013 [Google Scholar]
- 2.McNeil MR, Robin DA, Schmidt RA. Apraxia of speech: definition and differential diagnosis. In: McNeil MR, editor. Clinical Management of Sensorimotor Speech Disorders. New York: Thieme; 2009:249–268 [Google Scholar]
- 3.Duffy J. Apraxia of speech in degenerative neurologic disease. Aphasiology 2006;20:511–527 [Google Scholar]
- 4.Josephs KA, Duffy JR, Strand EA, et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain 2012;135:1522–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol 1982;11:592–598 [DOI] [PubMed] [Google Scholar]
- 6.Mesulam MM. Primary progressive aphasia. Ann Neurol 2001;49:425–432 [PubMed] [Google Scholar]
- 7.Mesulam MM. Primary progressive aphasia: a language-based dementia. N Engl J Med 2003;349:1535–1542 [DOI] [PubMed] [Google Scholar]
- 8.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76:1006–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grossman M. The non-fluent/agrammatic variant of primary progressive aphasia. Lancet Neurol 2012;11:545–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Josephs KA, Petersen RC, Knopman DS, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology 2006;66:41–48 [DOI] [PubMed] [Google Scholar]
- 11.Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol 2003;54(suppl 5):S15–S19 [DOI] [PubMed] [Google Scholar]
- 12.Kertesz A. Western Aphasia Battery–Revised. San Antonio: PsychCorp; 2007 [Google Scholar]
- 13.Wertz RT, Keith RL, Custer DD. Normal and aphasic behavior on a measure of auditory input and a measure of verbal output. Presented at the Annual Convention of the American Speech and Hearing Association; 1971; Chicago
- 14.Lansing AE, Ivnik RJ, Cullum CM, Randolph C. An empirically derived short form of the Boston Naming Test. Arch Clin Neuropsychol 1999;14:481–487 [PubMed] [Google Scholar]
- 15.Woods SP, Scott JC, Sires DA, Grant I, Heaton RK, Troster AI. Action (verb) fluency: test-retest reliability, normative standards, and construct validity. J Int Neuropsychol Soc 2005;11:408–415 [PubMed] [Google Scholar]
- 16.Loonstra AS, Tarlow AR, Sellers AH. COWAT metanorms across age, education, and gender. Appl Neuropsychol 2001;8:161–166 [DOI] [PubMed] [Google Scholar]
- 17.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006;31:1487–1505 [DOI] [PubMed] [Google Scholar]
- 18.Cosottini M, Ceravolo R, Faggioni L, et al. Assessment of midbrain atrophy in patients with progressive supranuclear palsy with routine magnetic resonance imaging. Acta Neurol Scand 2007;116:37–42 [DOI] [PubMed] [Google Scholar]
- 19.Paviour DC, Price SL, Jahanshahi M, Lees AJ, Fox NC. Longitudinal MRI in progressive supranuclear palsy and multiple system atrophy: rates and regions of atrophy. Brain 2006;129:1040–1049 [DOI] [PubMed] [Google Scholar]
- 20.Whitwell JL, Xu J, Mandrekar JN, Gunter JL, Jack CR, Jr, Josephs KA. Rates of brain atrophy and clinical decline over 6- and 12-month intervals in PSP: determining sample size for treatment trials. Parkinsonism Relat Disord 2012;18:252–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deramecourt V, Lebert F, Debachy B, et al. Prediction of pathology in primary progressive language and speech disorders. Neurology 2010;74:42–49 [DOI] [PubMed] [Google Scholar]
- 22.Josephs KA, Duffy JR, Strand EA, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain 2006;129:1385–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jack CR, Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain 2008;131:665–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varley R, Whiteside SP. Exploring the enigma. Aphasiology 2001;15:78–84 [Google Scholar]
- 25.Hickok G. Computational neuroanatomy of speech production. Nat Rev Neurosci 2012;13:135–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maas E, Mailend ML. Speech planning happens before speech execution: online reaction time methods in the study of apraxia of speech. J Speech Lang Hear Res 2012;55:S1523–S1534 [DOI] [PubMed] [Google Scholar]
- 27.Rogers MA, Storkel HL. Planning speech one syllable at a time: the reduced buffer capacity hypothesis in apraxia of speech. Aphasiology 1999;13:9–11 [Google Scholar]
- 28.Ogar J, Slama H, Dronkers N, Amici S, Gorno-Tempini ML. Apraxia of speech: an overview. Neurocase 2005;11:427–432 [DOI] [PubMed] [Google Scholar]
- 29.Amici S, Brambati SM, Wilkins DP, et al. Anatomical correlates of sentence comprehension and verbal working memory in neurodegenerative disease. J Neurosci 2007;27:6282–6290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peelle JE, Troiani V, Gee J, et al. Sentence comprehension and voxel-based morphometry in progressive nonfluent aphasia, semantic dementia, and nonaphasic frontotemporal dementia. J Neurolinguistics 2008;21:418–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 2004;55:335–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonty SP, Mesulam MM, Thompson CK, et al. Primary progressive aphasia: PPA and the language network. Ann Neurol 2003;53:35–49 [DOI] [PubMed] [Google Scholar]
- 33.Rogalski E, Cobia D, Harrison TM, Wieneke C, Weintraub S, Mesulam MM. Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology 2011;76:1804–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006;314:130–133 [DOI] [PubMed] [Google Scholar]
- 35.Whitwell JL, Jack CR, Jr, Parisi JE, et al. Does TDP-43 type confer a distinct pattern of atrophy in frontotemporal lobar degeneration? Neurology 2010;75:2212–2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rohrer JD, Geser F, Zhou J, et al. TDP-43 subtypes are associated with distinct atrophy patterns in frontotemporal dementia. Neurology 2010;75:2204–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–259 [DOI] [PubMed] [Google Scholar]
- 38.Rohrer JD, Rossor MN, Warren JD. Apraxia in progressive nonfluent aphasia. J Neurol 2010;257:569–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amici S, Gorno-Tempini ML, Ogar JM, Dronkers NF, Miller BL. An overview on primary progressive aphasia and its variants. Behav Neurol 2006;17:77–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sajjadi SA, Patterson K, Arnold RJ, Watson PC, Nestor PJ. Primary progressive aphasia: a tale of two syndromes and the rest. Neurology 2012;78:1670–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.