Abstract
Objectives
To conduct a meta-analysis of randomized controlled trials (RCTs) to assess the therapeutic outcome of fluorescence cystoscopy (FC) guided transurethral resection (TUR) in non-muscle invasive bladder cancer (NMIBC).
Materials and Methods
Relevant RCTs were identified from electronic database (MEDLINE, Embase and the Cochrane Library). The proceedings of relevant congress were also searched. The primary parameters were recurrence rate, the time to fist recurrence, recurrence free survival rate (RFS) and progression rate.
Results
12 RCTs including 2258 patients, which were identified for analysis in our study. Our study showed that the FC group have lower recurrence rate than the white light cystoscopy (WLC) group with statistically significant difference (OR: 0.5; p<0.00001). The time of the FC group first recurrence delayed significantly 7.39 weeks than WLC group (MD: 7.39 weeks; p<0.0001). There was a statistically significant difference in favor of FC in RFS at 1 yr (HR: 0.69; p<0.00001) and 2 yrs (HR: 0.65; p=0.0004). However, the FC group cannot significantly reduce the rate of progression into muscle invasive bladder cancer compared with the WLC group (OR: 0.85; p=0.39).
Conclusions
FC guided TUR was demonstrated to be an effective procedure for delaying recurrence of NMIBC. Unfortunately, FC guided TUR could not significantly decrease the rate of progression into muscle invasive bladder cancer.
Introduction
Transurethral resection (TUR) with cystoscopy is the current main treatment for non-muscle-invasive bladder cancer (NMIBC), but residual tumour was found in 30%–44% patients after initial treatment [1]. This rate could exceed 70% for high-grade tumours such as carcinoma in situ (CIS) [2]. Furthermore, the probability for recurrence of NMIBC at 1 and 5 years had been reported as 15%-61% and 31%–78%, respectively, whereas the rates for progression at 1 and 5 years had been reported as <1%–17% and <1%-45%, respectively [3]. Therefore, tumour recurrence in patients with NMIBC was a common problem.
Residual tumours that were undetected or overlooked during initial TUR may contribute to recurrence. White light cystoscopy (WLC) was considered the current standard method for detecting tumours during TUR, however, its sensitivity and specificity was not entirely satisfactory [4]. Small papillary bladder tumours and CIS were very difficult to detect using WLC; therefore, this method was associated with a potential risk of recurrence [5]. Therefore, there was an urgent need for improving the sensitivity of cystoscopy.
Fluorescence cystoscopy (FC) had been introduced for NMIBC diagnosis and treatment [6]. Using this method, photoactive porphyrins such as 5-aminolaevulinic acid (5-ALA) or hexylaminolevulinate (HAL) were used to instill into the bladder and emitted red fluorescence under blue light. This method, also known as photodynamic diagnosis, had been studied extensively in recent years.
Several studies [7,8] had demonstrated that FC was more sensitive than WLC in detecting small papillary bladder tumours and CIS, thus improving tumour detection rates and decreasing residual tumour rates. Furthermore, no significant adverse effects related to the use of this method had been reported till date. FC had received approval for use in the detection of bladder cancer in several countries. However, debate continues about the applicability because of cost-effectiveness of FC, it may be used only for suspected high-grade tumours according to the guidelines proposed by the European Association of Urology (EAU) [9]. No overall consensus has been reached in Europe or other regions concerning the use of this technique in all patients or only selected patients.
Three previously conducted meta-analyses demonstrated the superiority of FC over WLC in the detection of bladder tumours, especially CIS [7,8,10]. According to the results of these studies, complete resection was more often achieved with FC, thus demonstrating the diagnostic accuracy of FC in patients with NMIBC. However, insufficient data concerning the therapeutic outcome of FC in patients with NMIBC were available. Evidence regarding tumour recurrence and progression was still lacking.
This study aimed to conduct a meta-analysis of evidence from randomized controlled trials (RCTs) to assess the therapeutic outcome of FC guided TUR in patients with NMIBC.
Materials and Methods
Study selection
In accordance with a pre-specified study protocol, an electronic database search of Medline, Embase and the Cochrane database was systematically undertaken to identify studies conducted between 1996 and October 2012. The search terms belonged to the Medical Subject Headings database and included fluorescence cystoscopy, photodynamic diagnoses, bladder cancer/tumour, white light cystoscopy, randomized controlled trial. These terms were searched individually and in combination. The proceedings of the American Society of Clinical Oncology, the European Association of Urology and the American Urological Association were also manually searched. Moreover, the reference lists of all included studies were scanned to identify additional potentially relevant studies. Searches were restricted to English language publications. The search was independently conducted by two authors (HCY, JGQ). Any discrepancies were resolved in consultation with the third author (PH).
Inclusion criteria
RCTs that assessed the clinical efficacy of FC and compared it with that of WLC in patients with suspected or proven NMIBC were included. These studies included at least one outcome of interest to the current study. When two or more studies reported on a group of patients at the same institution during an overlapping time period, the study with the longest follow-up period was included.
Outcomes of interest
The following outcome data were extracted from the included studies:
-
1
Recurrence rate: the number of bladder cancer recurrences after initial TUR
-
2
Recurrence-free survival rate at 1 year
-
3
Recurrence-free survival rate at 2 years
-
4
Time to first recurrence, defined as the time until bladder cancer recurrence after initial TUR
-
5
Progression rate, defined as the number of patients with disease progression into muscle invasive bladder cancer during the follow-up period.
Study quality assessment
The quality of the literature was assessed separately by two authors (HCY and JGQ) using the 6 items of the Jadad scale score [11]. Scores of 0 to 8 were allocated to each study. Studies with scores of 5 points or more were defined as high-quality studies. Those with scores of 3 or 4 were designated as moderate quality, and those that scored 2 points or less were of low quality.
Data extraction and statistical analysis
The meta-analysis was performed in line with the recommendations of the Cochrane Collaboration [12]. Two reviewers (HCY and JGQ) reviewed the selected studies and independently extracted the following information: study design, year of publication, study population characteristics and relevant outcome data. Any discrepancy was resolved in consultation with the third author (PH). Statistical analysis of dichotomous variables was performed using the odds ratio (OR) as the summary statistic, while continuous variables were analyzed using the weighted mean difference (MD). For both variables, 95% confidence intervals (CI) were reported. When the outcome data involved comparison of two survival curves such as those for recurrence-free survival (RFS), the log hazard ratio statistic was used. In some studies, only the mean and p-values for the time of first recurrence, the p-value derived in log-rank tests for RFS and the events observed in each group were reported, and therefore the standard deviation (SD) and hazard ratio (HR) were estimated using the statistical methods12.
Heterogeneity was assessed using the I2 statistic. The Mantel–Haenszel chi-squared test for heterogeneity was performed. I2 values of <25% were defined as low heterogeneity, those between 25% and 50% as moderate heterogeneity and those >50% as high heterogeneity. In case of lack of heterogeneity, fixed-effects model was used for the meta-analysis, or else random-effects model was used. When data were reliable and sufficient, subgroup analysis was introduced by grouping the trials to determine possible heterogeneity and bias. For these tests, a p-value of <0.05 was considered statistically significant. The analysis was conducted using Review Manager Version RevMan 5.0.
Results
Search results and reporting quality
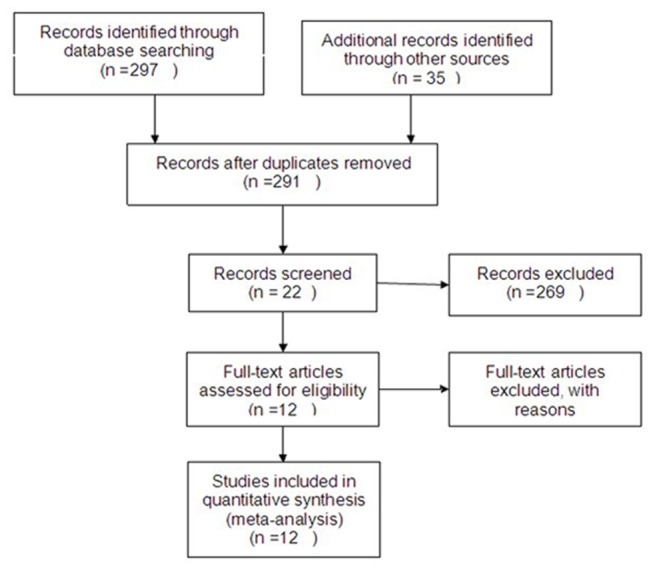
Using the search strategy described previously, after study assessment there were 12 RCTs [13-24] for analysis in this review. Of these RCTs, two [19,20] were from the same authors or institution, but the extracted outcome data were different. These studies included 2258 patients in whom FC or WLC had been performed for NMIBC. The FC group was divided into two subgroups: the 5-ALA group and HAL group. Baseline information was comparable between the FC and WLC groups. The baseline characteristics and quality assessment of the included studies are summarized in Table 1. Flow diagram of evidence acquisition is illustrated in Figure 1.
Table 1. Baseline characteristics and quality assessment of the Included Studies.
| Study | Age(year) | Patient sex (M/F) | FA | Jadad scale score (6 Items) | Study type | Cases | follow-up (months) |
|---|---|---|---|---|---|---|---|
| Riedl CR 2001 | - | - | 5-ALA | 6 | RCT | 51/51 | - |
| Kriegmair M 2002 | 69.3/69.6 | 53:12/45:19 | 5-ALA | 5 | RCT | 65/64 | - |
| Filbeck T 2002 | 70/68 | - | 5-ALA | 6 | RCT | 88/103 | 21.2/20.5 |
| Babjuk M 2005 | 69.8/67.9 | 39:23/43:17 | 5-ALA | 5 | RCT | 60/62 | 20.7/22.4 |
| Schumacher CM 2010 | 68.9/70.1 | 104:34/103:38 | 5-ALA | 6 | RCT | 138/141 | 12/12 |
| Stenzl A 2011 | - | - | 5-ALA | 7 | RCT | 183/187 | 12/12 |
| Stenzl A 2010 | 68/69.6 | 212:59/223:57 | HAL | 5 | RCT | 271/280 | 12/12 |
| Grossman HB 2012 | |||||||
| Dragoescu.O 2011 | 62/58 | 16:6/18:4 | HAL | 5 | RCT | 22/22 | 9/9 |
| Geavlete B 2012 | - | - | HAL | 7 | RCT | 114/125 | 24/24 |
| Hermann GG 2011 | 69/71 | 58:19/51:17 | HAL | 5 | RCT | 77/68 | 12/12 |
| Karaolides T 2012 | 63.8/66.2 | 40:5/33:8 | HAL | 6 | RCT | 45/41 | 18/18 |
M = male; F = female; 5-ALA = 5-aminolevulinic acid; HAL = hexylaminolaevulinic acid; CIS = carcinoma in situ; FA = fluorescence agent; n = number of patients; RCT = randomized controlled trials.
Figure 1. Flow diagram of evidence acquisition.
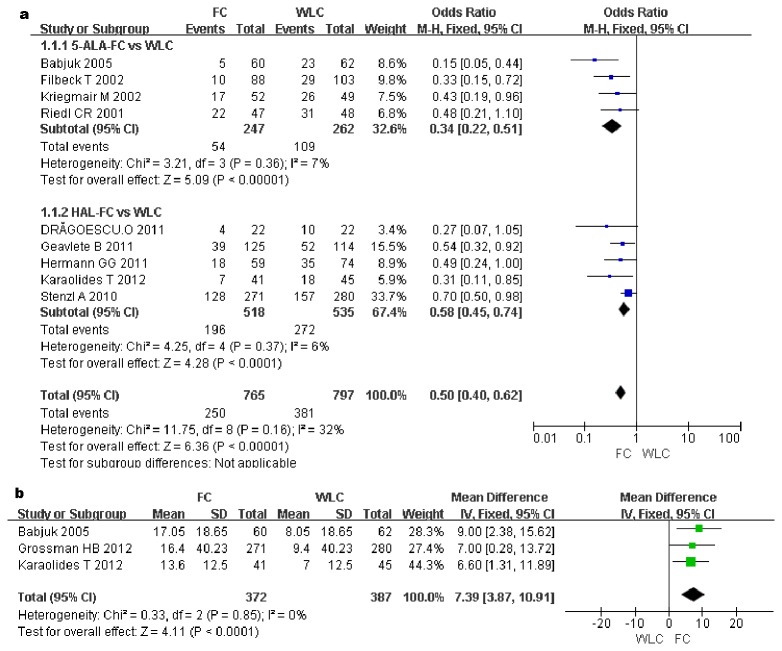
Recurrence rate
The recurrence rate was measured in nine studies including 1562 patients. The recurrence rate was significantly lower in the FC group than in the WLC group (OR, 0.5; 95%CI, 0.4–0.62; p<0.00001). Subgroup analysis revealed a statistically significant difference between the WLC group and the 5-ALA group (OR, 0.34; 95%CI, 0.22–0.51; p<0.00001) and between the WLC group and the HAL group (OR, 0.58; 95%CI, 0.45–0.74; p<0.0001) (Figure 2a).
Figure 2. Forest plot of FC vs. WLC for recurrence rate (a).
Forest plot of FC vs. WLC for the time to first recurrence (week) (b).
The Time to first recurrence (weeks)
The time to first recurrence was reported in three RCTs involving 759 patients. The pooled estimates of these studies showed a statistically significant difference between the FC and WLC groups (MD, 7.39 week; 95%CI, 3.87–10.91; p<0.0001). This result indicates that the time of first recurrence in the FC group was delayed significantly (7.39 weeks) compared with that in the WLC group (Figure 2b).
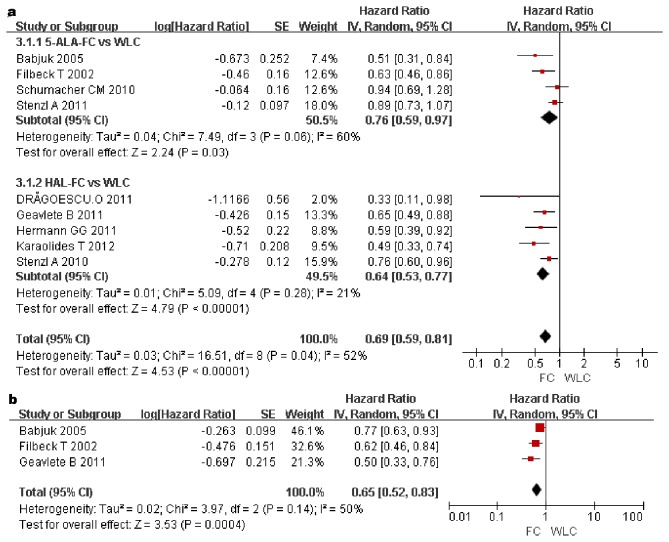
RFS rates at 1 and 2 years
Nine studies involving 2027 patients and three studies involving 552 patients reported RFS rates at 1 and 2 years. In the pooled estimates, a statistically significant difference in favour of FC was observed at 1 (HR, 0.69; 95%CI, 0.59–0.81; p<0.00001) and 2 years (HR, 0.65; 95%CI, 0.52–0.83; p=0.0004) (Figure 3a and 3b). Subgroup analysis also detected a statistically significant difference between the WLC and 5-ALA group at 1 year (HR, 0.76; 95%CI, 0.59–0.97; p = 0.03) and between the WLC and HAL groups at 1 year (HR, 0.64; 95%CI, 0.53–0.77; p<0.00001) (Figure 3a). Therefore, RFS rates at 1 and 2 years were higher in the FC group than in the WLC group.
Figure 3. Forest plot of FC vs. WLC for RFS rate at 1 year (a).
Forest plot of FC vs. WLC for RFS rate at 2 years (b).
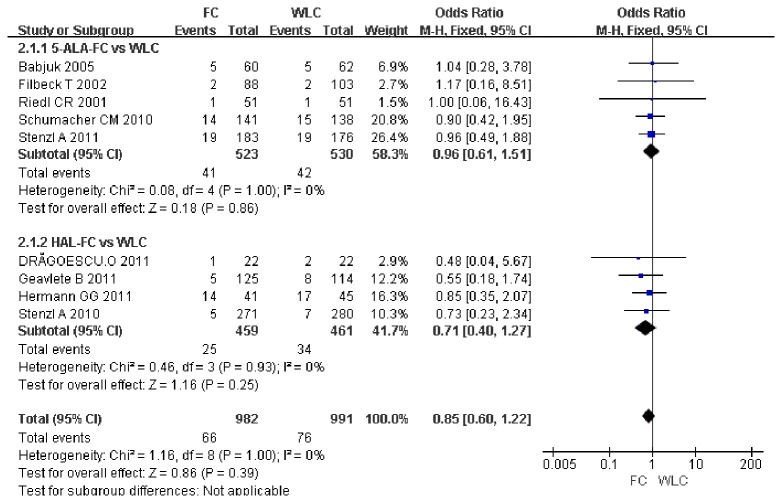
Progression rate
Nine studies including 1973 patients reported on the rate of progression. A meta-analysis of these studies showed that no significant difference was observed in the rate of progression into muscle invasive bladder cancer between the FC group and the WLC group (OR, 0.85; 95% CI, 0.6–1.22; p = 0.39). Subgroup analysis found no significant difference in the rate of progression between the WLC and 5-ALA group (OR, 0.96; 95%CI, 0.61–1.51; p = 0.86) and between the WLC and HAL group (OR, 0.71; 95% CI, 0.40–1.27; p= 0.25) (Figure 4).
Figure 4. Forest plot of FC vs. WLC for progression rate.
Discussion
The benefit for FC which could detect more bladder tumours and reduce residual tumours had been proved previously by another systematic review and meta-analysis [7,8]. The findings in these studies indicated that this technique may result in more complete tumour resection and decreased recurrence rates. This conclusion was also supported by the results of the current study that showed that the recurrence rate was significantly lower, the time to first recurrence after initial TUR was prolonged and RFS rate at 1 and 2 years was improved when FC-guided TUR was used than when WLC was used. However, there was no significant decrease in the rate of progression into muscle invasive bladder cancer in patients who underwent FC.
Three previously meta-analyses showed the superiority of FC over WLC in the detection of bladder tumours, especially CIS [7,8,10], which demonstrate the diagnostic accuracy of FC in patients with NMIBC. However, the objective of our meta-analysis was to evaluate the effect of FC in patients with NMIBC. Another meta-analysis of the use of FC in patients with NMIBC, that of Shen et al [25], found no significant difference in tumour detection rate and RFS between the FC and WLC groups. Their study found no advantage of FC over conventional WLC in terms of diagnostic accuracy and therapeutic outcome. In their study, 14 RCTs were included. However, one of these studies was a retrospective study, and 3 had been published repeatedly. These factors may have caused a publication bias. The heterogeneity of the pooled results was also very high and the method of data extraction for the RFS rate was unreasonable. Therefore, these results should be interpreted with caution as a result of the potential risk of bias.
We also found that differences in RFS rates between patients in the WLC group and those in the FC subgroups were not coinciding. Babjuk et al [16], Geavlete et al [22] and Karaolides et al [24] reported no statistically significant difference in RFS at 1 year between the FC and WLC groups when solitary tumours were treated (p = 0.74, 0.064 and 0.352, respectively); however, a statistically significant difference in RFS was observed between the FC and WLC groups when multifocal tumours were treated (p = 0.001, 0.001 and <0.001, respectively). The superiority of FC was particularly obvious in the intermediate-risk (p = 0.02) and high-risk (p = 0.05) groups, but this advantage did not achieve statistical significance in the low-risk group (p = 0.25) [15]. Data for these subgroups were insufficient for inclusion in the current meta-analysis.
The results of this study also showed that although the recurrence rate was significantly decreased and time to recurrence was longer in patients who underwent FC, these improvements did not translate into a decrease in the rate of progression into muscle invasive bladder cancer. We considered that one factor underlying this finding may be that patients with high-risk tumours should be treated with adjuvant therapy such as bacillus Calmette–Guérin instillation, regardless of whether CIS was easily found. Another factor may be that the sensitivity of FC, especially for CIS, is as yet uncertain [26]. Some studies have reported that FC may result in a higher incidence of false-positive results compared with WLC. However, with increased experience with and technical improvements in FC, false-positive rates are dropping. One study showed that only a 1% difference in the rate of false positives is existed between the FC and WLC groups [27].
All meta-analyses are inherently limited by the quality of the primary studies. Fortunately, all included studies in the current meta-analysis were RCTs, and most were of high quality. However, the statistical data in some included studies were incomplete, detailed data could not be acquired by contacting the relevant authors, so these data were lost. In addition, some parameters important to this meta-analysis were only measured in certain studies. These factors may be a possible source of bias. Subgroup analysis such as solitary vs multifocal tumors or low vs high-risk tumor could not be conducted, which may be reveal more useful information, but few study perform a relative subgroup study, we cannot extract sufficient data to conduct the subgroup analysis. This was another limitation of the current study. Moreover, some studies [13-19] used 5-ALA as a photoactive porphyrin, while others [19-24] used HAL. The factor may have introduced heterogeneity in the present results. However, the heterogeneity of all of the pooled estimates in this review was acceptable. Therefore, the results of this study are realistic because they were based on available data and high-quality RCTs.
Bladder cancer is an expensive cancer to treat. The lifetime cost per patient has been estimated at up to $200,000, which puts an enormous economic stress on the medical system [28]. Most costs are incurred in association with NMIBC, which has a life-long tendency to recur in most cases and requires repeated endoscopic surgery. FC is gradually becoming accepted as a useful tool for the diagnosis and management of NMIBC. Furthermore, the EAU guidelines [9] also provide recommendations for the use of FC in patients with NMIBC.
The results of this study showed that the use of FC during initial TUR-BT can facilitate more complete tumour resection, resulting in a decreased recurrence rate, prolonged the time of first recurrence and improved RFS at 1 and 2 years. Therefore, FC can change the therapeutic strategy for patients with NMIBC, longer intervals to follow-up with cystoscopy, fewer TUR-BT procedures and less adjuvant therapy will become possible for the treatment of NMIBC in patients who have undergone FC. Burger et al [29] reported that a single extra cost of €135 for FC resulted in savings of €187 per patient per year over a follow-up period of more than 7 years among patients who underwent FC. Therefore, improved prognostic outcome of bladder cancer in patients who undergo FC can decrease long-term costs of care and follow-up, despite the additional costs for fluorescent agents and learning of this modified technique [29,30]. This finding suggested that the use of FC could result in savings. Further studies evaluating the use of FC from an economic point of view are needed. However, FC is likely to have a positive effect not only on prognosis but also on patient quality of life [30].
Recently, flexible cystoscopy was widely used in detecting bladder tumors and in the follow-up of bladder cancer patients [31,32]. Some studies reported that PDD-guided flexible cystoscopy could identifies smaller, more papillary tumors or flat CIS lesions in anaesthetized patients in the operating room than white light cystoscopy and rigid cystoscopes [33,34]. Hermann GG et al [35] reported that PDD-guided flexible cystoscopy can be performed in an outpatients department setting and that simultaneous biopsies are able to give a reliable histological diagnosis of bladder cancer. If CIS and high-risk non-muscle-invasive bladder tumours were followed in the outpatients department with flexible cystoscopes under local anaesthesia instead of anaesthesia in the operating room, many resources could be spared for patients and the health system. But Flex biopsies are not sufficiently reliable to identify muscle invasive bladder cancer because of a low presentation of muscularis propria in the biopsies and thus a risk of overlooking, therefore, patients with high-grade disease should be referred to the operating room for cystoscopy and biopsy. However, further research should be performed to assess the effect of PDD-guided flexible cystoscopy.
Further research focusing on larger, multicentre RCTs and comparing subgroups of patients such as solitary vs multifocal tumors or low vs high-risk tumor with NMIBC are required. Such research may provide urologists with more comprehensive and detailed recommendations concerning the management of patients with NMIBC.
Conclusions
Compared with WLC, FC guided TUR could significantly decrease recurrence rates, prolong the time to first recurrence after initial TUR and improve RFS at 1 and 2 years. Therefore FC was demonstrated to be an effective procedure for delaying recurrence of NMIBC. The benefits of FC may be result in savings and decrease the burden of the cost of caring for patients with NMIBC on the health care economy. Unfortunately, FC guided TUR could not significantly decrease the rate of progression into muscle invasive bladder cancer. Further studies are required to explore possible reasons.
Funding Statement
The study was financially supported by Natural Science Foundation of China (81200551, and 81270841). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Daniltchenko D, Riedl CR, Sachs MD, Koenig F, Daha KL et al. (2005) Long-term benefit of 5-aminolevulinic acid fluorescence assisted transurethral resection of superficial bladder cancer: 5-year results of a prospective randomized study. J Urol 174: 2129–2133. doi:10.1097/01.ju.0000181814.73466.14. [DOI] [PubMed] [Google Scholar]
- 2. Al ’- Shukri SKh, Danil’chenko DI, Kënig F, Shnorr D (2000) ALA fluorescent diagnosis of bladder cancer. Urologiia 5: 48–50. [PubMed] [Google Scholar]
- 3. Sylvester RJ, vander Meijden APM, Oosterlinck W, Witjes JA, Bouffioux C et al. (2006) Predicting recurrence and progression in individual patients with stage Ta T1bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 49: 466–475. doi:10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 4. Jocham D, Witjes F, Wagner S, Zeylemaker B, van Moorselaar J et al. (2005) Improved detection and treatment of bladder cancer using hexaminolevulinate imaging: a prospective, phase III multicenter study. J Urol 174: 862–866. doi:10.1097/01.ju.0000169257.19841.2a. [DOI] [PubMed] [Google Scholar]
- 5. Witjes JA (2004) Bladder carcinoma in situ in 2003: state of the art. Eur Urol 45: 142–146. doi:10.1016/j.eururo.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 6. Kriegmair M, Baumgartner R, Knuechel R, Steinbach P, Ehsan A et al. (1994) Fluorescence photodetection of neoplastic urothelial lesions follow-ing intravesical instillation of 5-aminolevulinic acid. Urology 44: 836–841. doi:10.1016/S0090-4295(94)80167-3. [DOI] [PubMed] [Google Scholar]
- 7. Kausch I, Sommerauer M, Montorsi FS, Stenzl A, Jacqmin D et al. (2010) Photodynamic Diagnosis in Non–Muscle-Invasive Bladder Cancer: A Systematic Review and Cumulative Analysis of Prospective Studies. Eur Urol 57: 595–606. doi:10.1016/j.eururo.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 8. Lerner SP, Liu H, Wu MF, Thomas YK, Witjes JA (2012) Fluorescence and white light cystoscopy for detection of carcinoma in situ of the urinary bladder. Urol Oncol 30: 285-289. doi:10.1016/j.urolonc.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 9. Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Böhle A et al. (2011) EAU guidelines on non-muscle-inva-sive urothelial carcinoma of the bladder, the 2011 update. Eur Urol 59: 997–1008. doi:10.1016/j.eururo.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 10. Mowatt G, N’Dow J, Vale L, Nabi G, Boachie C et al. (2011) Photodynamic diagnosis of bladder cancer compared with white light cystoscopy: Systematic review and meta-analysis. Int J Technol Assess Health Care 27: 3-10. doi:10.1017/S0266462310001364. [DOI] [PubMed] [Google Scholar]
- 11. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ et al. (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17: 1–12. doi:10.1016/S0197-2456(96)90740-0. [DOI] [PubMed] [Google Scholar]
- 12. Higgins JPT, Green S (2009) Cochrane handbook for systematic reviews of interventions, version 5.0.2 updated September 2009. The; Cochrane: Collaboration. Available: http://cochrane.org/resources/handbook/. [Google Scholar]
- 13. Riedl CR, Daniltchenko D, Koenig F, Simak R, Loening SA et al. (2001) Fluorescence endoscopy with 5-aminolevulinic acid reduces early recurrence rate in superficial bladder cancer. J Urol 165: 1121-1123. doi:10.1016/S0022-5347(05)66442-7. [PubMed] [Google Scholar]
- 14. Kriegmair M, Zaak D, Rothenberger KH, Rassweiler J, Jocham D et al. (2002) Transurethral resection for bladder cancer using 5-aminolevulinic acid induced fluorescence endoscopy versus white light endoscopy. J Urol 168: 475-478. doi:10.1016/S0022-5347(05)64661-7. [PubMed] [Google Scholar]
- 15. Filbeck T, Pichlmeier U, Knuechel R, Wieland WF, Roessler W (2002) clinically relevant improvement of recurrence-free survival with 5-aminolevulinic acid induced fluorescence diagnosis in patients with superficial bladder tumors. J Urol 168: 67-71. doi:10.1016/S0022-5347(05)64833-1. [PubMed] [Google Scholar]
- 16. Babjuk M, Soukup V, Petrík R, Jirsa M, Dvorácek J (2005) 5-aminolaevulinic acid-induced fluorescence cystoscopy during transurethral resection reduces the risk of recurrence in stage Ta/T1 bladder cancer. BJU Int 96: 798-802. doi:10.1111/j.1464-410X.2004.05715.x. PubMed: 16153204. [DOI] [PubMed] [Google Scholar]
- 17. Schumacher MC, Holmäng S, Davidsson T, Friedrich B, Pedersen J et al. (2010) Transurethral resection of non-muscle-invasive bladder transitional cell cancers with or without 5-aminolevulinic Acid under visible and fluorescent light: results of a prospective, randomised, multicentre study. Eur Urol 57: 293-299. doi:10.1016/j.eururo.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 18. Stenzl A, Penkoff H, Dajc-Sommerer E, Zumbraegel A, Hoeltl L et al. (2011) Detection and clinical outcome of urinary bladder cancer with 5-aminolevulinic acid-induced fluorescence cystoscopy: A multicenter randomized, double-blind, placebo-controlled trial. Cancer 117: 938-947. doi:10.1002/cncr.25523. [DOI] [PubMed] [Google Scholar]
- 19. Stenzl A, Burger M, Fradet Y, Mynderse LA, Soloway MS et al. (2010) Hexaminolevulinate guided fluorescence cystoscopy reduces recurrence in patients with nonmuscle invasive bladder cancer. J Urol 184: 1907-1913. doi:10.1016/j.juro.2010.06.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grossman HB, Stenzl A, Fradet Y, Mynderse LA, Kriegmair M et al. (2012) Long-term decrease in bladder cancer recurrence with hexaminolevulinate enabled fluorescence cystoscopy. J Urol 188: 58-62. doi:10.1016/j.juro.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Drăgoescu O, Tomescu P, Pănuş A, Enache M, Maria C et al. (2011) Photodynamic diagnosis of non-muscle invasive bladder cancer using hexaminolevulinic acid. Rom J Morphol Embryol 52: 123-127. [PubMed] [Google Scholar]
- 22. Geavlete B, Multescu R, Georgescu D, Jecu M, Stanescu F et al. (2012) Treatment changes and long-term recurrence rates after hexaminolevulinate (HAL) fluorescence cystoscopy: does it really make a difference in patients with non-muscle-invasive bladder cancer (NMIBC)? BJU Int 109: 549-556. doi:10.1111/j.1464-410X.2011.10374.x. [DOI] [PubMed] [Google Scholar]
- 23. Hermann GG, Mogensen K, Carlsson S, Marcussen N, Duun S (2011) Fluorescence-guided transurethral resection of bladder tumours reduces bladder tumour recurrence due to less residual tumour tissue in Ta/T1 patients: a randomized two-centre study. BJU Int 108: E297-E303. doi:10.1111/j.1464-410X.2011.10090.x. [DOI] [PubMed] [Google Scholar]
- 24. Karaolides T, Skolarikos A, Bourdoumis A, Konandreas A, Mygdalis V et al. (2012) Hexaminolevulinate -induced fluorescence versus white light during transurethral resection of noninvasive bladder tumor: does it reduce recurrences? Urology 80: 354-359. doi:10.1016/j.urology.2012.03.067. [DOI] [PubMed] [Google Scholar]
- 25. Shen P, Yang J, Wei W, Li Y, Li D et al. (2012) Effects of fluorescent light-guided transurethral resection on non-muscle-invasive bladder cancer: a systematic review and meta-analysis. BJU Int 110: E209-E215. doi:10.1111/j.1464-410X.2011.10892.x. [DOI] [PubMed] [Google Scholar]
- 26. Isfoss BL (2011) The sensitivity of fluorescent-light cystoscopy for the detection of carcinoma in situ (CIS) of the bladder: a meta-analysis with comments on gold standard. BJU Int 108: 1703-1707. doi:10.1111/j.1464-410X.2011.10485.x. [DOI] [PubMed] [Google Scholar]
- 27. Jocham D, Witjes F, Wagner S, Zeylemaker B, van Moorselaar J et al. (2005) Improved detection and treatment of bladder cancer using hexaminolevulinate imaging:a prospective, phase III multicenter study. J Urol 174: 862–866. doi:10.1097/01.ju.0000169257.19841.2a. [DOI] [PubMed] [Google Scholar]
- 28. Botteman MF, Pashos CL, Redaelli A, Zeylemaker B, van Moorselaar J et al. (2003) The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics 21: 1315–1330. doi:10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 29. Burger M, Zaak D, Stief CG, Filbeck T, Wieland WF et al. (2007) Photodynamic diagnostics and noninvasive bladder cancer: is it cost-effective in long-term application? A Germany based cost analysis. Eur Urol 52: 142-147. doi:10.1016/j.eururo.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 30. Malmström PU, Hedelin H, Thomas YK, Thompson GJ, Durrant H et al. (2009) Fluorescence-guided transurethral resection of bladder cancer using hexaminolevulinate: analysis of health economic impact in Sweden. Scand J Urol Nephrol 43: 192–198. doi:10.1080/00365590902808541. PubMed: 19330681. [DOI] [PubMed] [Google Scholar]
- 31. Herr HW (1990) Outpatient flexible cystoscopy and fulguration of recurrent superficial bladder tumors. J Urol 144: 1365–1366. [DOI] [PubMed] [Google Scholar]
- 32. Grossman HB, Gomella LG, Fradet Y, Presti JC Jr, Ritenour CWM et al. (2004) The use of Hexvix and fluorescence cystoscopy as an adjunct in the diagnosis of stage Ta/T1 urothelial cancer in the urinary bladder. J Urol 171: 69. [Google Scholar]
- 33. Loidl W, Schmidbauer J, Susani M, Marberger M (2005) Flexible cystoscopy assisted by hexaminolevulinate induced fluores-cence: a new approach for bladder cancer detection and surveillance? Eur Urol 47: 323–326. doi:10.1016/j.eururo.2004.10.025. PubMed: 15716195. [DOI] [PubMed] [Google Scholar]
- 34. Witjes JA, Moonen PM, van der Heijden AG (2005) Comparison of hexaminolevulinate based flexible and rigid fluorescence cystoscopy with rigid white light cystoscopy in bladder can-cer: results of a prospective Phase II study. Eur Urol 47: 319–322. doi:10.1016/j.eururo.2004.09.017. PubMed: 15716194. [DOI] [PubMed] [Google Scholar]
- 35. Hermann GG, Mogensen K, Toft BG, Glenthøj A, Pedersen HM (2012) Outpatient diagnostic of bladder tumours in flexible cystoscopes: evaluation of fluorescence-guided flexible cystoscopy and bladder biopsies. Scand J Urol Nephrol 46: 31-36. doi:10.3109/00365599.2011.637954. [DOI] [PubMed] [Google Scholar]