Abstract
Background
Aclidinium bromide is a new long-acting muscarinic antagonist (LAMA) indicated for maintenance bronchodilator treatment of chronic obstructive pulmonary disease (COPD). The efficacy of aclidinium was compared with tiotropium and glycopyrronium, using a network meta-analysis (NMA) of randomized controlled trials (RCTs) in moderate-to-severe COPD patients.
Methods
A systematic review was performed to identify RCTs evaluating aclidinium 400 μg twice daily (BID), glycopyrronium 50 μg once daily (OD), tiotropium 18 μg OD, or tiotropium 5 μg OD in adults with moderate-to-severe COPD. The outcomes of interest were: trough forced expiratory volume in 1 second (FEV1); St George’s Respiratory Questionnaire (SGRQ) total score and proportion of patients achieving ≥4 unit change; Transition Dyspnea Index (TDI) focal score and proportion of patients achieving ≥1 point change. The results were synthesized by means of a Bayesian NMA.
Results
Twenty-one studies (22,542 patients) were included: aclidinium 400 μg BID (three studies); tiotropium 5 μg OD (three studies); tiotropium 18 μg OD (13 studies); and glycopyrronium 50 μg OD (two studies). Regarding trough FEV1 at 24 weeks, aclidinium demonstrated comparable efficacy to tiotropium 5 μg (difference in change from baseline [CFB]), (0.02 L [95% credible interval CrI −0.05, 0.09]); tiotropium 18 μg (0.02 L [95% CrI −0.05, 0.08]); and glycopyrronium (0.00 L [95% CrI −0.07, 0.07]). Aclidinium resulted in higher improvement in SGRQ score at 24 weeks, compared to tiotropium 5 μg (difference in CFB, −2.44 [95% CrI −4.82, −0.05]); and comparable results to tiotropium 18 μg (−1.80 [95% CrI −4.52, 0.14]) and glycopyrronium (−1.52 [95% CrI −4.08, 1.03]). Improvements in TDI score were comparable for all treatments.
Conclusion
Maintenance treatment with aclidinium 400 μg BID is expected to produce similar improvements in lung function, health-related quality of life, and dyspnea compared to tiotropium 5 μg OD; tiotropium 18 μg OD; and glycopyrronium 50 μg OD.
Keywords: COPD, aclidinium, tiotropium, glycopyrronium, systematic review, network meta-analysis
Introduction
Chronic obstructive pulmonary disease (COPD) treatments aim to prevent and control symptoms, reduce exacerbations, and improve health status. Current Global Initiative for Chronic Obstructive Lung Disease (GOLD)1 and the National Institute for Health and Care Excellence (NICE) guidelines2 recommend the use of long-acting bronchodilators, including long-acting muscarinic antagonists (LAMA), as they are more effective at producing maintained symptom relief than short-acting bronchodilators.
Until 2012, the only LAMA marketed for the treatment of COPD was tiotropium bromide. Tiotropium bromide is a once-daily (OD) LAMA and a widely prescribed medication for COPD. Inhaled tiotropium is available as a powder and in solution as a mist. The dose of the powder formulation is 18 μg and 5 μg for the mist.3,4
Aclidinium bromide is a new LAMA that was recently approved in Europe and the United States as a maintenance bronchodilator treatment in adult patients with COPD.5,6 The recommended dose is one inhalation of aclidinium 400 μg bromide twice-daily (BID), equivalent to 322 μg of active treatment. Aclidinium bromide is administered by inhalation through a multidose dry powder inhaler device.7 Glycopyrronium bromide was recently approved in Europe for maintenance bronchodilator treatment of COPD.8 The recommended dose is one inhalation of 50 μg once daily, equivalent to glycopyrronium 44 μg.
The recent availability of aclidinium bromide poses the question of what its long-term relative efficacy would be in comparison to other LAMA treatment options. Only short-term (<12 weeks), randomized controlled trials (RCTs) comparing aclidinium to tiotropium are available,9,10 which have shown comparability of the two treatments. There are no head-to-head RCTs comparing aclidinium to glycopyrronium.
To address the need for treatment comparisons, a systematic literature review was undertaken to identify long-term RCTs (≥12 weeks) while the data were synthesized by means of a network meta-analysis (NMA). A NMA allows for indirect comparisons in the absence of trials involving a direct comparison of interventions, and it can provide useful evidence of the relative treatment effects between competing interventions.
The relative efficacy of aclidinium 400 μg BID, tiotropium 5 μg OD, tiotropium 18 μg OD, and glycopyrronium 50 μg OD as maintenance bronchodilator treatment to relieve symptoms in patients with moderate-to-severe COPD was assessed in terms of lung function, health status, and dyspnea.
Materials and methods
Study identification and selection
Using a predefined strategy (Table S1), MEDLINE, MEDLINE in Process, EMBASE (using OVID), and Cochrane Controlled Trials Registry databases were searched for the period of July 1989 to October 2012. To capture advance online publications ahead of print that are not yet available on EMBASE or MEDLINE, a PubMed search was performed restricted to 2012 and excluding articles indexed for MEDLINE or PubMed In Process. Search terms included a combination of free text and thesaurus terms relevant to COPD, aclidinium bromide, tiotropium bromide, glycopyrronium bromide, and RCTs. Additional targeted searches were performed in clinicaltrials.gov database (Table S1). Conference abstracts dating back 2 years were included in the screening process. Abstracts and full-text articles in a language other than English were excluded.
The inclusion criteria for population, intervention, comparators, outcomes, and study design (PICOS) are described below.
Population of interest: Adults with COPD, as defined by GOLD guidelines.1 Studies with high proportions (>30%) of mild and/or very severe patients were excluded.
Interventions: aclidinium 400 μg BID, glycopyrronium 50 μg OD, tiotropium 18 μg OD, or tiotropium 5 μg OD, administered using any inhalation device.
Comparators: Studies that compare any of the interventions against each other or placebo.
Outcomes: Outcomes of interest included the following: trough Forced Expiratory Volume in 1 second (FEV1) at 12 weeks and 24 weeks; St George’s Respiratory Questionnaire (SGRQ) total score at 12 weeks and 24 weeks; the proportion of patients within each group achieving a clinically meaningful change (at least four units) in SGRQ total score at 12 weeks and 24 weeks; Transition Dyspnea Index (TDI) total score at 12 weeks and 24 weeks; the proportion of patients within each group achieving a clinically meaningful change (at least one unit) in TDI focal score at 12 weeks and 24 weeks. Studies reporting outcomes within 2 weeks of the time point of interest, ie, between 10–14 weeks and 22–26 weeks, were included and the outcomes were grouped as 12 and 24 weeks, respectively.
Study design: RCTs with study duration ≥10 weeks.
Data collection and validity assessment
Two reviewers were involved in a three-step approach for data collection. All three steps were performed independently and in duplicate. First, titles and abstracts of the identified citations were assessed according to the research question and PICOS criteria. In a second screening step, potentially relevant articles were screened as full texts, using the same PICOS criteria. As a third step, for identified trials that met the selection criteria, the reviewers conducted extraction of data relating to study design, population characteristics, interventions, and the outcomes of interest, using a standardized prepiloted form. Any disagreement was resolved by consensus.
The following study characteristics were extracted: author; publication year; drug dose and administration; inhalation device; number of patients randomized and intention-to-treat (ITT) population; trial design; inclusion criteria; background treatments; trial location; and duration. Additionally, the following patient characteristics were extracted in order to evaluate the comparability of the patients: proportion of males; mean age; mean FEV1; FEV1 percentage predicted; mean forced vital capacity (FVC); mean FEV1/FVC percentage; proportion of current smokers; mean duration of COPD; mean smoking history in pack-years; concomitant use of long-acting β-agonist (LABA); percentage of patients with concomitant use of inhaled corticosteroids (ICS); number of exacerbations in previous year; percentage reversibility; and race/ethnicity.
For the continuous outcomes (trough FEV1, SGRQ total score, TDI focal score), the mean difference in change from baseline (CFB) versus placebo (or difference at follow-up, adjusted for baseline characteristics) and its standard error (SE) were extracted, where available. If not available, difference in CFB were calculated based on the CFB (or the CFB adjusted for baseline characteristics) per treatment arm. The SE, if not reported, was estimated based on the uncertainty or variation reported (eg, confidence intervals). For the dichotomous outcomes (% of SGRQ and TDI responders), the number of responders was extracted, if reported, or calculated, based on the reported percentage and the ITT population. If the necessary data were not reported in the text or the tables of the publication but in graphs, these were digitalized, and then the software DigitizeIt version 1.5 (Digitize It, Braunschweig, Germany) was used to extract them.
The methodological and reporting quality of the trials included were assessed by means of the Jadad checklist for RCTs.11 The risk of bias at the study level was assessed, based on the adequacy of the following factors: randomization; allocation concealment; blinding of patients and investigators; and complete and nonselective results reporting. The risk of bias at the outcome level was assessed, based on the adequacy of the following factors: application of the ITT principle; blinding of the outcome assessor; statistical evaluation; and complete and nonselective results reporting.
Publication bias of primary outcomes, trough FEV1, SGRQ total score, and TDI focal score was evaluated by visual inspection of funnel plots.
Data synthesis
The relative efficacy of the study drugs was evaluated using a NMA within a Bayesian framework.12–15 For all continuous outcomes, a generalized linear model with identity link and a normal likelihood distribution was used,16,17 while a logit link with binomial likelihood distribution was used for dichotomous outcomes.
For each outcome, fixed and random effects models were evaluated. The goodness of fit of each model to the data was assessed using the Deviance Information Criterion (DIC),16 and the model with the lower DIC value was selected.
Vague (flat) priors were used for all calculations. A normal distribution with zero mean and variance equal to 104 was used for treatment effects and a uniform distribution with range zero to 5 for the between-trial standard deviation.
The posterior densities for the unknown parameters were estimated using Markov chain Monte Carlo (MCMC) simulations for each model. All results are based on 80,000 iterations on three chains, with a burn-in of 20,000 iterations. Convergence assessment was based on visual inspection of trace and autocorrelation plots and on the Gelman–Rubin–Brooks diagnostic (R < 1.2). The accuracy of the posterior estimates was assessed, using the Monte Carlo error for each parameter (Monte Carlo error <5% of the posterior standard deviation).
Differences in study design and patient characteristics across trials that could affect the relative treatment effect introduce bias to the analysis. Based on clinical experience and the results of published systematic reviews,18,19 the percentage of current smokers, the severity level (% severe–% very severe patients), the FEV1 percentage predicted at baseline, the percentage of patients with concomitant use of ICS, and the concomitant use of LABA were identified as potential factors that could modify the treatment effect. To address this risk, adjustment by treatment-by-covariate meta-regression models,20 when feasible, was used for the former four while a sensitivity analysis, excluding LABA-allowing studies, was used to address the latter. For all analyses, WinBUGS version 1.4.3 (MRC Biostatistics Unit, Cambridge, UK) was used,21 while the regression models were based on those reported by Dias et al.17
The results of the NMA are presented as differences in CFB and odds ratios (OR). Point estimates of both were derived from the median of the posterior distribution while their 95% credible intervals (CrI) were estimated from the 2.5th and 97.5th percentiles of the posterior distribution. When adjusted for covariates, the treatment effects obtained were estimated at the mean covariate value. At each endpoint, the probability that aclidinium is the better treatment is presented.
Results
Study selection
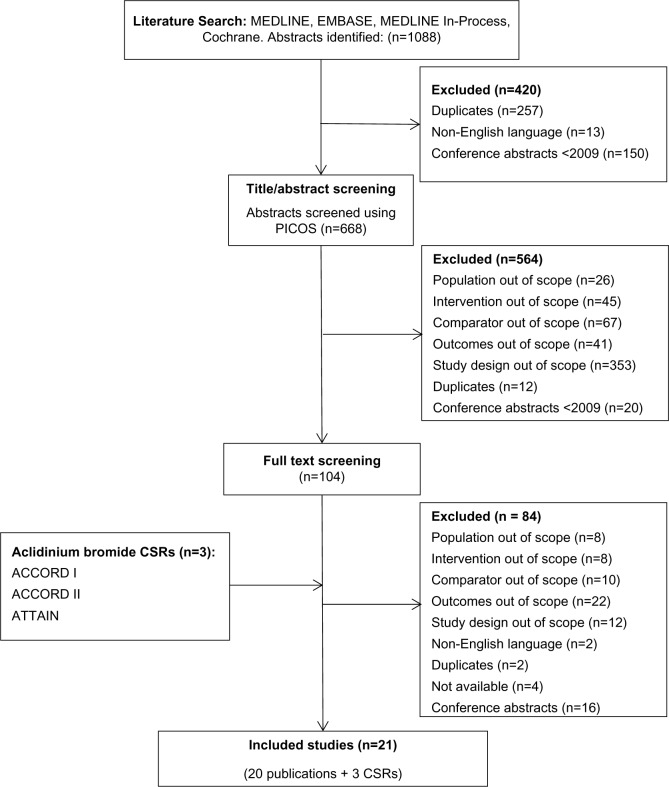
An overview of the study selection process is presented in Figure 1. The search in MEDLINE, MEDLINE In-Process, EMBASE, and Cochrane Controlled Trials Registry databases identified 1,088 potentially relevant abstracts. After removing duplicates, abstracts not in English and conference proceedings predating 2009, a total of 668 abstracts were screened using PICOS criteria. The abstract and title screening excluded 564 abstracts due to trial design (63%), or because the comparators (12%), interventions (8%), outcomes (7%), or population were out of scope (5%). An additional 20 conference proceedings were found to predate 2009, and twelve abstracts were identified as duplicates and removed from the database. For the remaining 104 abstracts, full-text publications were obtained and screened. An additional 84 publications were excluded due to outcomes (26%), study design (14%), comparators (12%), population (10%), or interventions not of interest (10%), language (2), duplicates (2), and not available (4). Furthermore, 16 conference abstracts were excluded as they were superseded by full-text articles, and no additional data were reported.
Figure 1.
Flow diagram of study selection process.
Note: Both a publication and CSR were available for the ATTAIN and ACCORD I study.
Abbreviations: PICOS, population, intervention, comparators, outcomes, and study design; CSRs, clinical study reports; ACCORD, AClidinium in Chronic Obstructive Respiratory Disease COPD; ATTAIN, Aclidinium To Treat Airway obstruction IN COPD patients.
Three relevant Clinical Study Reports (CSR) were provided by Almirall and Forest on aclidinium ([AClidinium in Chronic Obstructive Respiratory Disease COPD], ACCORD I,22,23 ACCORD II,24 and [Aclidinium To Treat Airway obstruction In COPD patieNts] ATTAIN25,26). Ultimately, 20 publications27–44 and three clinical study reports,22,24,25 comprising 21 different trials, were identified from the systematic literature review and were included in the NMA.
Study characteristics
Tables 1 and 2 present an overview of the study design and patient characteristics of the selected studies. Overall, the 21 studies included had randomized 22,542 patients to either one of the interventions or placebo. Three studies compared aclidinium 400 μg with placebo; three studies compared tiotropium 5 μg with placebo; 13 studies compared tiotropium 18 μg with placebo; one study compared glycopyrronium 50 μg to placebo; and one study compared glycopyrronium 50 μg, tiotropium 18 μg, and placebo.
Table 1.
Characteristics of included studies
| Source | Trial design | Treatment | Number of patients | Location | Inclusion/exclusion criteria | Background treatment | Study duration | Jadad score |
|---|---|---|---|---|---|---|---|---|
| Bateman27 | RCT, PC, DB, MC | Tiotropium, 5 μg, OD; Tiotropium, 10 μg, OD; Placebo | 670 667 653 |
South Africa, Europe, Canada | ≥ 40 years age; FEV1 ≤ 60%; FEV1 ≤ 70% of FVC; ≥ 10 pack-years; diagnosis of COPD and stable, moderate-to-severe airway obstruction | Allowed: oral and inhaled corticosteroids, theophylline, mucolytic agents and antileukotrienes, salbutamol. Patients on long-acting β-adrenergics and inhaled corticosteroids were switched to a monoproduct inhaled corticosteroid prior to run-in | 48 weeks | 3 |
| Bateman28 | RCT, PC, DB, PG, MC | Tiotropium, 5 μg, OD; Placebo | 1 952 1 965 |
336 centers; 31 countries | FEV1 ≤ 60% prebronchodilator; FEV1/FVC ≤ 70% prebronchodilator; ≥40 years age; ≥ 10 pack-years | Allowed: salbutamol pMDI; all respiratory medications were permitted Not allowed: inhaled anticholinergics |
48 weeks + 1 week run-in | 5 |
| Voshaar42 | RCT, PC, DB, MC | Tiotropium, 5 μg, OD; Tiotropium, 10 μg, OD; Ipratropium, 36 μg, OD; Placebo | 180 180 178 181 |
64 centers; Europe, South Africa, USA, Canada | FEV1 ≤ 60% prebronchodilator; FEV1/FVC ≤ 70%; ≥ 10 pack-years | Allowed: salbutamol, oral corticosteroids, orally inhaled corticosteroids, theophyllines, and mucolytics Not allowed: anticholinergics, inhaled β-adrenergics other than salbutamol, fixed combination inhalers |
12 weeks | 3 |
| Brusasco25 | RCT, PC, DB, MC, DD | Tiotropium, 18 μg, OD; Salmeterol, 50 μg, BID; Placebo | 402 405 400 |
18 countries | FEV1 ≤ 65%; FEV1/FVC ≤ 70%; > 40 years of age; > 10 pack-years | NR | 24 weeks | 4 |
| Casaburi30 | RCT, PC, DB, MC | Tiotropium, 18 μg, OD; Placebo | 279 191 |
25 centers; USA | FEV1 ≤ 65%; FEV1/FVC ≤ 70%; ≥40 years of age; diagnosis of COPD defined by ATS; smoking history of > 10 pack-years | Allowed: stable doses of theophylline, ICS, oral prednisone Not allowed: other inhaled or oral bronchodilators |
13 weeks | 3 |
| Casaburi31 | 2 RCTs, PC, DB, MC | Tiotropium; 18 μg; OD; Placebo | 550 371 |
50 centers | FEV1 ≤ 65%; FEV1/FVC ≤ 70% | Allowed: stable doses of theophylline, ICS, oral prednisone | 56 weeks | 3 |
| Chan32 | RCT, PC, DB, MC | Tiotropium, 18 μg, OD; Placebo | 608 305 |
101 centers; Canada | FEV1 ≤ 65%; FEV1/FVC ≤ 70%; ≥ 1 exacerbation previous year but not 6 weeks prior to randomization | Allowed: stable dose oral corticosteroids, ICS, theophylline preparations, mucolytic preparations (not containing bronchodilators), LABAs | 48 weeks | 4 |
| Covelli33 | RCT, PC, DB, MC | Tiotropium, 18 μg, OD; Placebo | 100 96 |
12 centers; USA | FEV1 ≤ 60%; FEV1/FVC ≤ 70% excluded if exacerbation in prior 6 weeks | Allowed: ICS, LABAs and theophyllines Not allowed: cromones, leukotriene antagonists, and inhaled anticholinergics |
12 weeks | 4 |
| Donohue34 | RCT, PC, DB, MC, DD | Tiotropium, 18 μg, OD; Salmeterol, 50 μg, BID; Placebo | 209 213 201 |
39 countries; 12 countries | FEV1 ≤ 60%; FEV1/FVC ≤ 70%; ≥40 years of age; smoking history of > 10 pack-years | Allowed: usual ICS and oral steroids Not allowed: Inhaled anticholinergic LABAs |
24 weeks | 4 |
| Donohue35 | RCT, PC, DB,** MC, DD | Indacaterol, 150 μg, OD; Indacaterol, 300 μg, OD; Tiotropium, 18 μg, OD; Placebo | 420 418 420 425 |
USA, Europe | FEV1 30%–80% postbronchodilator; FEV1/FVC < 70%; ≥40 years age; ≥20 pack-years; diagnosis of moderate-to-severe COPD (GOLD criteria) | ICS monotherapy, albuterol, treatment with anticholinergic bronchodilators or [β2-agonists was discontinued with appropriate washout; patients receiving fixed-combination [β2-agonist/ICS were switched to ICS monotherapy at an equivalent dose | 26 weeks | 4 |
| Moita36 | RCT, PC, DB, MC | Tiotropium, 18 μg, OD; Placebo | 147 164 |
31 centers; Portugal | FEV1 ≤ 70%; FEV1/FVC ≤ 70%, excluded if ≥3 exacerbations previous year or exacerbation in 6 weeks prior | Allowed: LABAs, theophylline, mucolytics, ICS, stable doses oral corticosteroids. Temporary increases in theophylline or oral steroids for exacerbations; Not allowed: theophylline 24-hour preparations |
12 weeks | 4 |
| Niewoehner37 | RCT, PC, DB, MC | Tiotropium, 18 μg, OD; Placebo | 914 915 |
26 centers; USA | FEV1 ≤ 60%; FEV1/FVC ≤ 70% excluded if not recovered from exacerbation ≥30 days prior | Allowed: all other respiratory medications (including ICS and LABAs) Not allowed: open-label anticholinergic bronchodilator |
24 weeks | 4 |
| Tashkin (UPLIFT)38 | RCT, PC, DB, MC | Tiotropium, 18 μg, OD; Placebo | 2 987 3 006 |
490 centers; 37 countries | FEV1 ≤ 70%; FEV1/FVC ≤ 70%; excluded if exacerbation 4 weeks prior | Allowed: all respiratory medications, except other inhaled anticholinergic drugs | 4 years | 5 |
| Tonnel (TIPHON)35 | RCT, PC, DB, MC | Tiotropium, 18 μg, OD; Placebo | 266 288 |
123 centers; France | FEV1 20%–70%; FEV1/SVC ≤ 70% | Allowed: stable doses of theophylline preparations (excluding 24-hour preparations), mucolytics, ICS, and oral steroids | 36 weeks | 5 |
| Verkindre40 | RCT, PC, DB, MC | Tiotropium; 18 μg, OD; Placebo | 46 54 |
10 centers; France | FEV1 ≤ 50%; FEV1/FVC ≤ 70%; residual volume ≥ 125%; excluded if unstable doses oral corticosteroid 6 weeks prior | Allowed: stable doses oral corticosteroids, ICS, theophylline preparations, mucolytic agents Not allowed: use of SABAs, oral ß2-agonists, or LABAs |
12 weeks | 3 |
| Vogelmeier41 | RCT, PC, DB,* MC | Formoterol, 12 meg BID + Tiotropium, 18 meg, OD; Formoterol, 12 μg, BID; Tiotropium, 18 μg, OD; Placebo | 207 210 221 209 |
86 centers; 8 countries Europe | FEV1 < 70%; FEV1/FVC < 70%; stable COPD; aged 40 years at COPD onset; ≥ 10 pack-years | Allowed: salbutamol, ICS monotherapy | 24 weeks | 3 |
| CSR ACCORD I22 | RCT, MC, PC, DB | Aclidinium, 200 μg, BID; Aclidinium, 400 μg, BID; Placebo | 185 190 187 |
106 centers; USA, Canada | ≥40 years of age, ≥ 10 pack-years; stable, moderate-to-severe COPD as defined by criteria of GOLD; FEV1 30%–80% postsalbutamol; FEV1/FVC < 70% Excluded: patients with asthma; respiratory tract infection or COPD exacerbation in the 6 weeks before visit 1 |
ICS allowed; LABA discontinued | 12 weeks | 4 |
| CSR ACCORD II24 | RCT, MC, PC, DB | Aclidinium, 200 μg, BID; Aclidinium, 400 μg, BID; Placebo | 184 178 182 |
103 centers; USA Canada | ≥40 years of age, ≥ 10 pack-years; stable, moderate-to-severe COPD as defined by criteria of GOLD; FEV1 30%–80% postsalbutamol; FEV1/FVC < 70% Excluded: patients with asthma; respiratory tract infection or COPD exacerbation in the 6 weeks before visit 1 |
ICS allowed; LABA discontinued | 12 weeks | 4 |
| CSR ATTAIN25 | RCT, MC, PC, DB | Aclidinium, 200 μg, BID; Aclidinium, 400 μg, BID; Placebo | 277 269 273 |
103; Europe, South America, Russia, South Africa | ≥ 10 pack-years; FEV1 30%–80% postsalbutamol; FEV1/FVC < 70% | NR | 24 weeks | 4 |
| GLOW 143 | RCT, DB, PC, MC | Glycopyrronium, 50 meg, OD; Placebo | 552 270 | 128; US, Europe, Australia, SE Asia, South America | postbronchodilator FEV1 ≥ 30% and < 80%; postbronchodilator FEV1/FVC < 0.7; ≥ 10 pack-years | Allowed: ICS monotherapy, short acting β2-agonists as required Not allowed: LABAs, LAMAs, theophylline |
26 weeks | 3 |
| GLOW 244 | RCT, MC, PC, DB** | Glycopyrronium, 50 μg, OD; Tiotropium, 18 μg, OD; Placebo | 529 268 269 |
NR | Men and woman aged ≥40 years, smoking history ≥10 pack-years, moderate-to-severe stable COPD (2008 GOLD guidelines), postbronchodilator FEV1 ≥ 30% and < 80% of the predicted normal, and postbronchodilator FEV1/FVC < 0.70 were enrolled | Allowed: concomitant medications (inhaled or intranasal corticosteroids and H1 antagonists) and salbutamol/albuterol inhaler to be used as rescue medication during the study Not allowed: LABA LAMA |
52 weeks | 3 |
Notes:
Tiotropium versus placebo is not DB;
open label for tiotropium arm. + indicates that the first two lines of this cell (Formoterol and Tiotropium) were administered simultaneously.
Abbreviations: BID, twice daily; COPD, chronic obstructive pulmonary disease; CSR, clinical study report; UPLIFT, Understanding Potential Long-term Impacts on Function with Tiotropium; TIPHON, Tiotropium: Influence sur la Perception de l’amelioration des activites Habituelles Objectivee par une echelle Numerique; ACCORD, ACIidinium in Chronic Obstructive Respiratory Disease COPD; ATTAIN, Aclidinium To Treat Airway obstruction IN COPD patients; GLOW, GLycopyrroniurn bromide in COPD airways clinical study 1; DB, double blind; DD, double dummy; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global initiative for chronic Obstructive Lung Disease; ICS, inhaled corticosteroids; LABA, long acting β-agonist; LAMA, long-acting muscarinic antagonist; MC, multicenter; NR, not reported; OD, once daily; OL, open label; PC, placebo controlled; RCT, randomized controlled trial; S, south; SE, southeast; USA, United States of America; pMDI, pressurized metered-dose inhaler; ATS, American Thoracic Society.
Table 2.
Patient characteristics at baseline for the included studies (only arms of interest)
| Author | Treatment | Male (%) | Mean age (years) | Current smokers (%) | Patients in severe or very severe COPD states (%) | Patients with concomitant ICS use (%) | Mean pack-years | Mean FEV1% pred |
|---|---|---|---|---|---|---|---|---|
| Bateman27 | Tiotropium, 5 μg, OD; | 73 | 65 | 38 | NR | 49 | NR | 38 |
| Placebo | 75 | 65 | 36 | NR | 55 | NR | 38 | |
| Bateman28 | Tiotropium, 5 μg, OD; | 78 | 65 | 36 | NR | 56 | 46 | 40 |
| Placebo | 77 | 65 | 36 | NR | 56 | 45 | 40 | |
| Voshaar42 | Tiotropium, 5 μg, OD; | 69 | 64 | 37 | 59 | 48 | 52 | 40 |
| Placebo | 69 | 63 | 43 | 47 | 52 | 51 | 42 | |
| Brusasco29 | Tiotropium, 18 μg, OD; | 77 | 64 | NR | 82 | NR | 44 | 39 |
| Placebo | 76 | 65 | NR | 82 | NR | 42 | 39 | |
| Casaburi30 | Tiotropium, 18 μg, OD; | 67 | 65 | NR | NR | NR | 65 | 39 |
| Placebo | 63 | 65 | NR | NR | NR | 61 | 38 | |
| Casaburi31 | Tiotropium, 18 μg, OD; | 67 | 65 | NR | NR | 43 | 63 | 39 |
| Placebo | 63 | 65 | NR | NR | 40 | 59 | 38 | |
| Chan32 | Tiotropium, 18 μg, OD; | 59 | 67 | 32 | NR | 66 | 50 | 39 |
| Placebo | 61 | 67 | 30 | NR | 71 | 51 | 39 | |
| Covelli33 | Tiotropium, 18 μg, OD; | 66 | 66 | 40 | NR | 54 | 66 | 40 |
| Placebo | 49 | 63 | 37 | NR | 58 | 65 | 39 | |
| Donohue34 | Tiotropium, 18 μg, OD; | 74 | 65 | 42 | 59 | 66 | 47 | 41 |
| Placebo | 75 | 66 | 42 | 60 | 66 | 46 | 41 | |
| Donohue35 | Tiotropium, 18 μg, OD; | 65 | 64 | NR | NR | 35 | 50 | 54 |
| Placebo | 61 | 63 | NR | NR | 40 | 50 | 56 | |
| Moita36 | Tiotropium, 18 μg, OD; | NR | NR | 28 | NR | NR | Per | Per |
| Placebo | NR | NR | 25 | NR | NR | subgroup | subgroup | |
| Niewoehner37 | Tiotropium, 18 μg, OD; | 98 | 68 | 29 | NR | 61 | 67 | 36 |
| Placebo | 99 | 68 | 30 | NR | 58 | 69 | 36 | |
| Tashkin | Tiotropium, 18 μg, OD; | 75 | 65 | 29 | 52 | 62 | 49 | 40 |
| (UPLIFT)38 | Placebo | 74 | 65 | 30 | 53 | 62 | 48 | 39 |
| Tonnel | Tiotropium, 18 μg, OD; | 87 | 65 | 24 | 57 | 38 | 44 | 47 |
| (TIPHON)39 | Placebo | 85 | 64 | 30 | 62 | 36 | 43 | 46 |
| Verkindre40 | Tiotropium, 18 μg, OD; | 94 | 61 | 24 | NR | NR | 46 | 35 |
| Placebo | 94 | 60 | 33 | NR | NR | 42 | 36 | |
| Vogelmeier41 | Tiotropium, 18 μg, OD; | 79 | 63 | NR | 44 | NR | 39 | 52 |
| Placebo | 78 | 62 | NR | 46 | NR | 40 | 51 | |
| CSR | Aclidinium, 400 μg, BID; | 53 | 65 | 42 | 36 | 47 | 57 | 48 |
| ACCORD I22 | Placebo | 52 | 65 | 47 | 37 | 45 | 53 | 48 |
| CSR | Aclidinium, 400 μg, BID; | 50 | 63 | 50 | 54 | 39 | 54 | 44 |
| ACCORD II24 | Placebo | 55 | 62 | 56 | 37 | 42 | 53 | 49 |
| CSR | Aclidinium, 400 μg, BID; | 68 | 63 | 55 | 31 | 51 | 42 | 51 |
| ATTAIN25 | Placebo | 69 | 62 | 53 | 34 | 58 | 39 | 52 |
| GLOW 143 | Glycopyrronium, 50 μg, OD; | 83 | 64 | 33 | 40 | 55 | 45 | NR |
| Placebo | 81 | 64 | 34 | 38 | 51 | 45 | NR | |
| GLOW 244 | Glycopyrronium, 50 μg, OD; | 65 | 64 | 45 | 37 | 56 | 49 | NR |
| Tiotropium, 18 μg, OD; | 63 | 64 | 44 | 35 | 52 | 50 | NR | |
| Placebo | 65 | 64 | 46 | 35 | 51 | 48 | NR |
Abbreviations: BID, twice daily; COPD, chronic obstructive pulmonary disease; CSR, clinical study report; UPLIFT, Understanding Potential Long-term Impacts on Function with Tiotropium; TIPHON, Tiotropium: Influence sur la Perception de l’amelioration des activites Habituelles Objectivee par une echelle Numerique; ACCORD, AClidinium in Chronic Obstructive Respiratory Disease COPD; ATTAIN, Aclidinium To Treat Airway obstruction IN COPD patients; GLOW, GLycopyrronium bromide in COPD airways clinical study; FEV1% pred, forced expiratory volume in 1 second percentage predicted at baseline; ICS, inhaled corticosteroids; OD, once daily; NR, not reported.
The results of the methodological quality assessment for the included studies, by means of Jadad score, are presented in Table 1. All studies scored at least 3 out of 5, indicating good-quality RCTs.
All studies included were parallel-group, placebo controlled, and randomized. Eighteen studies were double-blind; three studies were open-label.35,41,44 All studies were multicenter in design. The average sample size was 1,242, ranging from 10040 to 5,99338 patients. Patients were permitted to use short-acting bronchodilators for symptom relief. The use of ICSs was permitted in most trials, although Brusasco in 200329 did not report on ICS use. Differences were observed in the concomitant use of LABAs during the trial period: six trials allowed LABA use;28,32,33,36–38 two trials did not report on the use of LABAs;29,31 and the remaining trials forbade the use of LABAs.
Enrolled patients were adults with a diagnosis of COPD, and average disease duration of 8.7 years. Patients were predominantly male (between 49% and 99% of patients), and mean age ranged from 60–68 years. All patients were current or exsmokers, with most studies including patients with a smoking history of at least 10 pack-years. Most trials included patients with an FEV1/FVC of ≤70%, and an FEV1% predicted that ranged from <80% to <50%. Baseline FEV1 ranged from 0.96 L–1.51 L. Spirometry measurements for mean FEV1%, predicted at baseline, ranged from 38%–40% in tiotropium 5 μg; 36%–54% in tiotropium 18 μg; 44%–51% in aclidinium studies; and it was not reported in both glycopyrronium studies. The proportion of patients taking concomitant-inhaled corticosteroids ranged from 36%–66% in tiotropium 18 μg, 39%–51% in aclidinium, and 55%–56% in glycopyrronium studies. The use of meta-regression models can reduce the impact of bias due to inconsistencies and between-study heterogeneity.20 For this reason, the results of the NMA were adjusted for the baseline FEV1% predicted and the ICS use by means of treatment-by-covariate meta-regression.
Despite some differences identified across the studies in terms of study design, patient characteristics or outcome definitions, 20 studies are considered to be broadly comparable and, therefore, were included in the base case analysis. One study, ACCORD II,24 was excluded from the base case analysis due to a chance imbalance in patients’ baseline characteristics in favor of the placebo group – despite randomization. The impact of this study on the indirect treatment comparison results was by including it in a scenario analysis.
A visual inspection of the funnel plots of FEV1, SGRQ total score, and TDI focal score did not reveal any profound asymmetries, suggesting the absence of publication bias. Given the low number of studies per outcome and time point, this assessment should be interpreted with caution.
Network meta-analysis
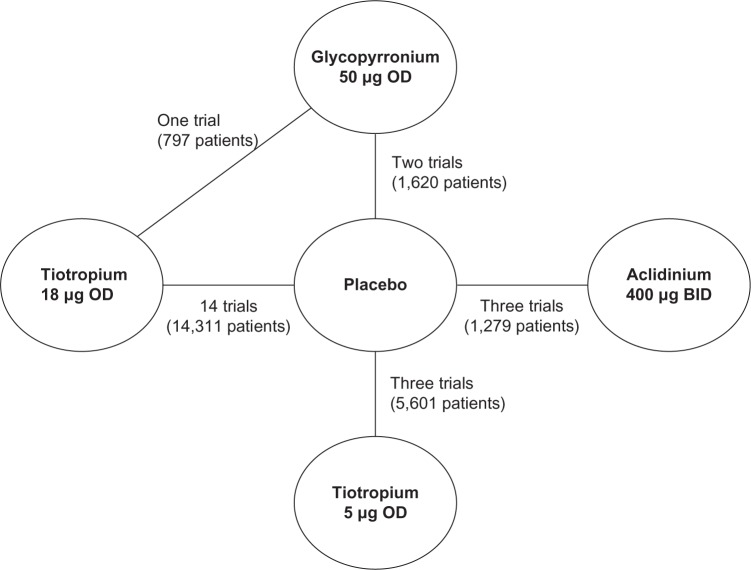
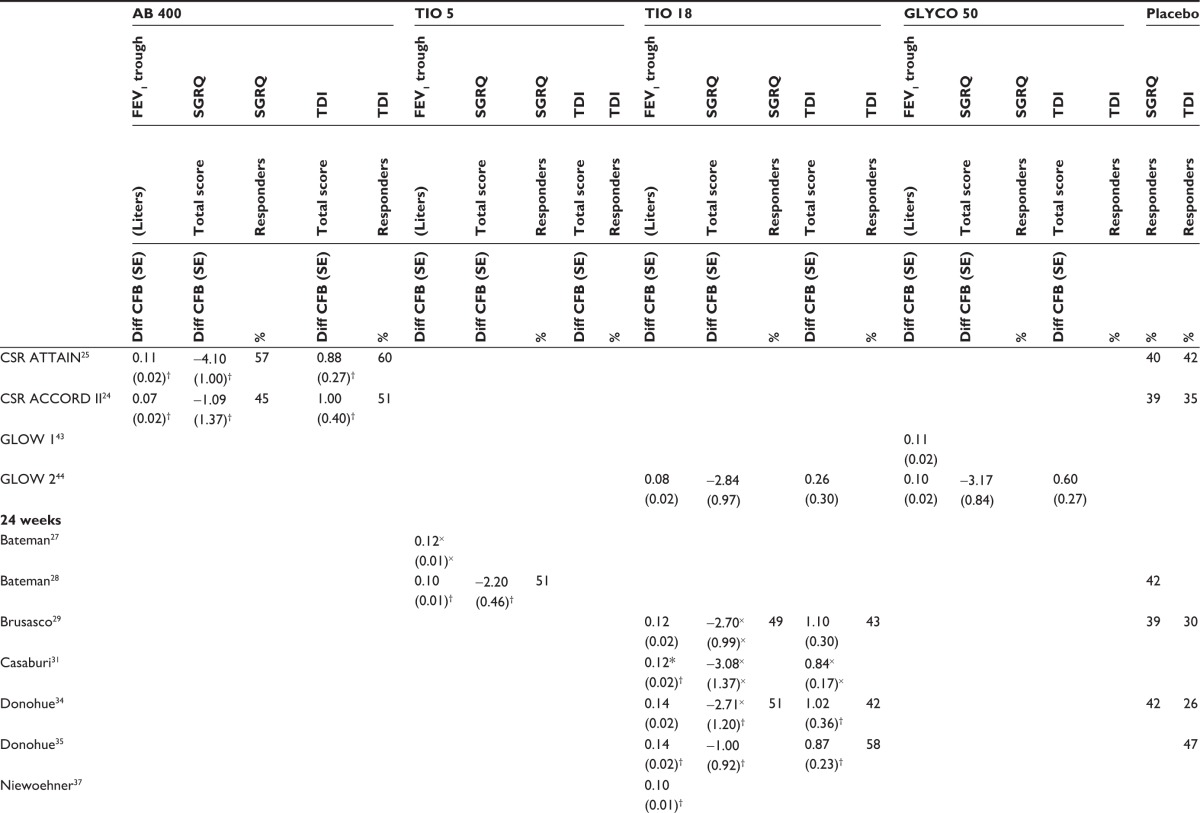
Figure 2 presents the network diagram based on the 21 studies identified in the review that were included in the NMA, showing a total of 26 connections between the comparators. There is one closed loop, providing indirect evidence. As all studies were placebo-controlled, placebo is used as the reference treatment. The individual study results reported in the 21 trials included in the NMA are presented in Table 3. Mean and standard error, as extracted or estimated, are presented for the continuous outcomes, while percentages of responders for placebo and active treatment arms are presented for the dichotomous outcomes.
Figure 2.
Network formed by interventions and their direct comparisons included in the analyses.
Note: The GLOW2 trial compares glycopyrronium to tiotropium 18 μg and placebo and is therefore included three times in this figure.
Abbreviations: GLOW, GLycopyrronium bromide in COPD airways clinical study; OD, once daily; BID, twice a day.
Table 3.
Individual studies results included in the network meta-analysis
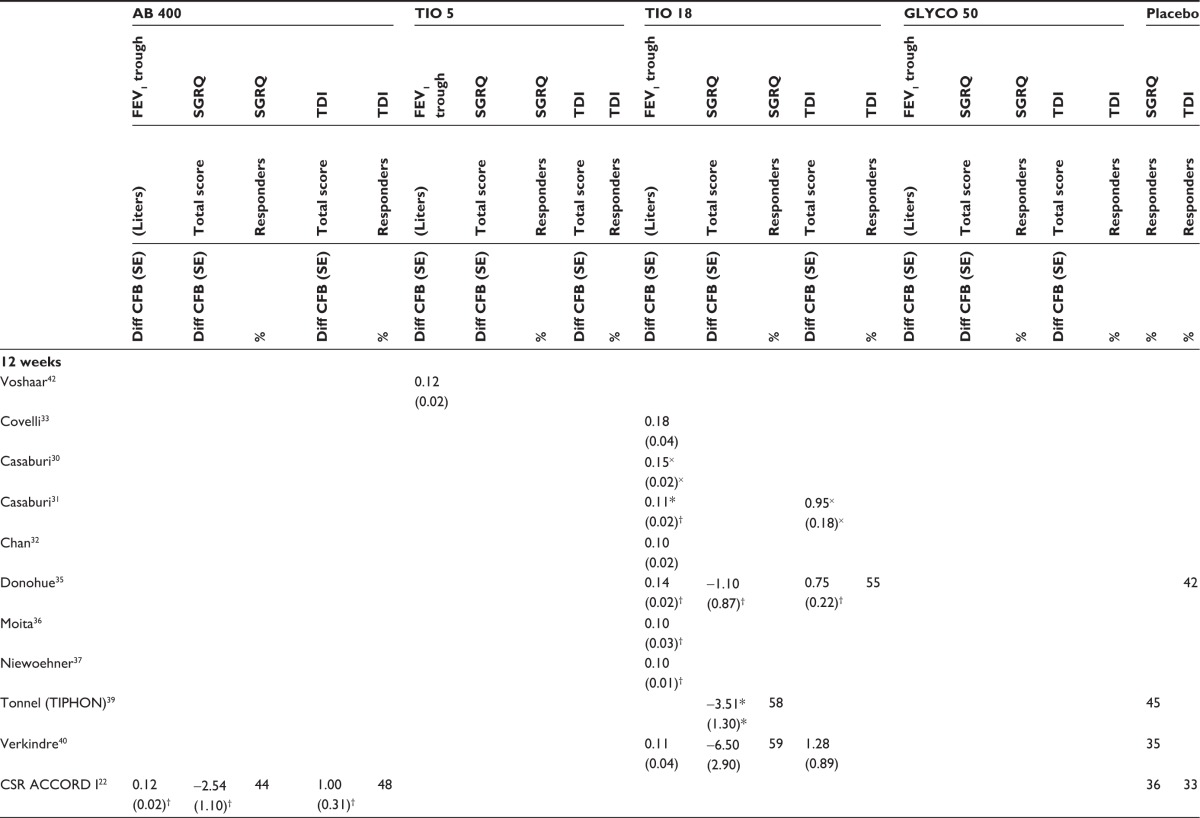
|
|
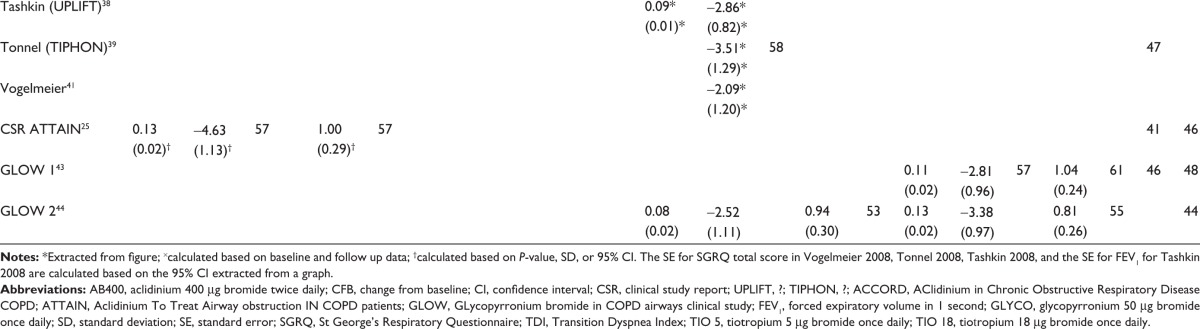
|
The data were synthesized in three series of NMAs. The base case analysis is based on 20 trials, excluding the ACCORD II study.24 In a sensitivity analysis, all studies reporting concomitant use of LABA treatment28,32,33,36–38 were excluded (Scenario 1). In a second scenario analysis, results including ACCORD II are presented (Scenario 2). Furthermore, a covariates analysis was performed by adjusting the results for ICS concomitant use and the FEV1% predicted at baseline.
The results of the NMA, as differences in CFB or OR with the corresponding 95% CrI for base case for all treatments versus placebo (reference treatment of the NMA) are summarized in Table 4. The comparative efficacy of aclidinium versus placebo and alternative active treatments is presented for the base case in Figure 3. Base case results, adjusted for percentage of ICS use and FEV1%-predicted covariates – together with the results of Scenarios 1 and 2 – are presented in Table 5.
Table 4.
Base case: results of the NMA for all treatments versus placebo
| Trough FEV1
|
SGRQ total score
|
SGRQ % responders
|
TDI focal score
|
TDI % responders
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 12 weeks | 24 weeks | 12 weeks | 24 weeks | 12 weeks | 24 weeks | 12 weeks | 24 weeks | 12 weeks | 24 weeks | |
|
|
|
|
|
|
||||||
| Diff CFB (95% Crl) | Diff CFB (95% Crl) | Diff CFB (95% Crl) | Diff CFB (95% Crl) | Odds ratio | Odds ratio | Diff CFB (95% Crl) | Diff CFB (95% Crl) | Odds ratio | Odds ratio | |
| TIO 5 | 0.12 (0.07,0.16) |
0.1 1 (0.08,0.15) |
−2.20 (−3.10,−1.30) |
1.44 (1.27, 1.63) |
||||||
| TIO 18 | 0.11 (0.10,0.12) |
0.1 1 (0.09,0.13) |
−2.37 (−3.48,−1.25) |
−2.45 (−3.18,−1.71) |
1.82 (1.32,2.53) |
1.50 (1.24, 1.83) |
0.77 (0.53, 1.02) |
0.92 (0.71, 1.13) |
1.69 (1.29, 2.23) |
1.67 (1.42, 1.97) |
| GLYCO | 0.11 (0.08,0.13) |
0.13 (0.09,0.17) |
−2.99 (−4.53,−1.45) |
−3.11 (−4.38,−1.84) |
1.56 (1.16,2.09) |
0.80 (0.32, 1.27) |
0.92 (0.59, 1.25) |
1.69 (1.38, 2.07) |
||
| AB400 | 0.11 (0.08,0.14) |
0.13 (0.07,0.19) |
−3.38 (−4.82,−1.95) |
−4.63 (−6.85, −2.42) |
1.75 (1.34,2.27) |
1.94 (1.38,2.73) |
0.93 (0.53, 1.33) |
1.00 (0.43, 1.57) |
1.99 (1.53, 2.60) |
1.58 (1.13, 2.33) |
Abbreviations: AB 400, aclidinium 400 μg bromide twice daily; CFB, change from baseline; Crl, credible interval; FEV1, forced expiratory volume in 1 second; GLYCO, glycopyrronium 50 μg bromide once daily; NMA, network meta-analysis; SGRQ, St George’s Respiratory Questionnaire; TDI, Transition Dyspnea Index; TIO 5, tiotropium 5 μg bromide once daily; TIO 18, tiotropium 18 μg bromide once daily.
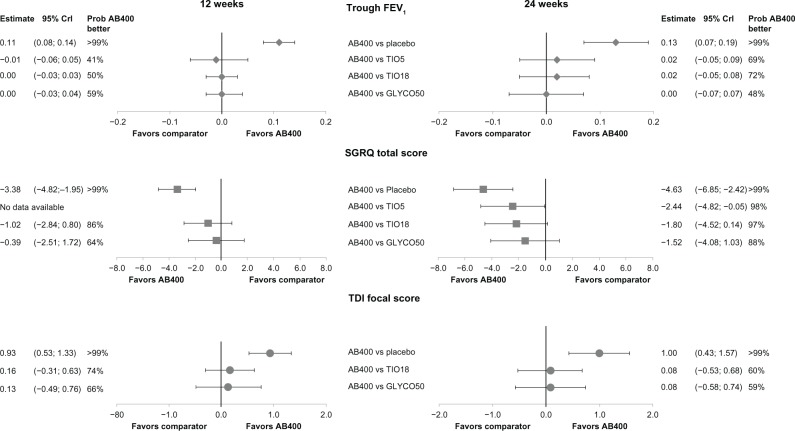
Figure 3.
Forest plot of base case network meta-analysis results for aclidinium.
Note: Difference in change from baseline (CFB), 95% credible intervals (95% Crl) and probability that aclidinium is better than the comparator at 12 weeks and 24 weeks.
Abbreviations: AB400, aclidinium 400 μg twice daily; TI05, tiotropium 5 μg bromide once daily; TI018, tiotropium 18 μg bromide once daily; GLYC050, glycopyrronium 50 μg once daily; FEV, forced expiratory volume in 1 second; SGRQ, St George’s Respiratory Questionnaire; TDI, Transition Dyspnea Index.
Table 5.
Results of the network meta-analysis for AB400 versus all other treatments
| Trough FEV1
|
SGRQ
|
TDI
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 weeks
|
24 weeks
|
12 weeks
|
24 weeks
|
12 weeks
|
24 weeks
|
|||||||
| Diff CFB (95% Crl) | Probability AB400 better | Diff CFB (95% Crl) | Probability AB400 better | Diff CFB (95% Crl) | Probability AB400 better | Diff CFB (95% Crl) | Probability AB400 better | Diff CFB (95% Crl) | Probability AB400 better | Diff CFB (95% Crl) | Probability AB400 better | |
| Scenario 1: excluding LABA allowing studies | ||||||||||||
| TIO 5 | −0.01 (−0.06, 0.05) |
41% | 0.00 (−0.05, 0.06) |
57% | ||||||||
| TIO 18 | −0.01 (−0.04, 0.02) |
32% | 0.00 (−0.04, 0.05) |
58% | −2.29 (−4.66, 0.07) |
97% | ||||||
| GLYCO | 0.00 (−0.03, 0.04) |
55% | 0.00 (−0.05, 0.05) |
46% | −1.55 (−4.12, 1.00) |
88% | ||||||
| Scenario II: including ACCORD II | ||||||||||||
| TIO 5 | −0.02 (−0.09, 0.05) |
28% | ||||||||||
| TIO 18 | −0.02 (−0.05, 0.02) |
19% | −0.50 (−2.20, 1.18) |
72% | 0.17 (−0.26, 0.60) |
78% | ||||||
| GLYCO | −0.01 (−0.06, 0.04) |
33% | 0.12 (−1.89, 2.11) |
45% | 0.15 (−0.45, 0.74) |
69% | ||||||
| Base case: adjusted for ICS | ||||||||||||
| TIO 5 | −0.01 (−0.06, 0.05) |
40% | 0.02 (−0.03, 0.06) |
75% | −2.53 (−4.94, −0.12) |
98% | ||||||
| TIO 18 | 0.00 (−0.04, 0.03) |
38% | 0.02 (−0.03, 0.06) |
78% | −0.47 (−2.45, 1.51) |
68% | −2.15 (−4.49, 0.17) |
97% | 0.34 (−0.20, 0.89) |
89% | 0.02 (−0.60, 0.65) |
53% |
| GLYCO | 0.00 (−0.04, 0.04) |
49% | 0.00 (−0.05, 0.05) |
47% | −0.44 (−2.55, 1.68) |
66% | −1.54 (−4.10, 1.01) |
88% | 0.14 (−0.48, 0.76) |
67% | 0.08 (−0.58, 0.74) |
59% |
| Base case: adjusted for FEV1% predicted | ||||||||||||
| TIO 5 | 0.00 (−0.08, 0.08) |
45% | 0.00 (−0.08, 0.07) |
44% | −3.47 (−6.21, −0.74) |
>99% | ||||||
| TIO 18 | 0.00 (−0.05, 0.05) |
46% | 0.01 (−0.07, 0.07) |
51% | −1.12 (−3.95, 0.70) |
89% | −2.83 (−5.30, −0.37) |
99% | 0.20 (−0.29, 0.69) |
79% | 0.10 (−0.56, 0.76) |
62% |
| GLYCO | 0.00 (−0.06, 0.06) |
53% | 0.00 (–0.09, 0.06) |
30% | −1.05 (−3.30, 1.20) |
82% | −2.30 (−5.03, 0.43) |
95% | 0.19 (−0.46, 0.84) |
71% | 0.11 (−0.61,0.82) |
61% |
Abbreviations: AB400, aclidinium 400 μg bromide twice daily; CFB, change from baseline; Crl, credible interval; FEV1 forced expiratory volume in 1 second; FEV1 pred, FEV1 percentage predicted at baseline; GLYCO, glycopyrronium 50 μg bromide once daily; ICS, inhaled corticosteroids; LABA, long-acting β-agonist; ACCORD, ACIidinium in Chronic Obstructive Respiratory Disease COPD; SGRQ, St George’s Respiratory Questionnaire; TDI, Transition Dyspnea Index; TIO 5, tiotropium 5 μg bromide once daily; TIO 18, tiotropium 18 μg bromide once daily.
Adjustment of the base case results for percentage of current smokers (results not presented in this paper) suggested that they are not likely to be affected, in line with the published results of similar studies.18 Similarly, a scenario analysis excluding studies with a gender imbalance, ie, GLycopyrronium bromide in COPD airWays clinical study 1 (GLOW1), ATTAIN, and Covelli,33 showed minor effects in the NMA results; eg, <0.01 L in CFB difference for trough FEV1 at 12 weeks, and are not presented in this paper. The proportion of patients with severe or very severe COPD was not reported in ten out of 16 studies (Table 2). Therefore, the authors decided not to address this source of inhomogeneity (eg, by imputing data or excluding the nonreporting studies).
Lung function
Data on lung function measured by means of trough FEV1 were reported by 19 studies22,24,25,27–40,42,44 (including 21,558 patients). All treatments were more efficacious than placebo, with a point estimate above the Minimal Clinically Important Difference (MCID) of 100 mL (Table 4).
In the base case, after 12 weeks, aclidinium demonstrated comparable results versus tiotropium 5 μg (difference in CFB −0.01 L [95% CrI −0.06, 0.05]) and no difference versus tiotropium 18 μg (difference in CFB 0.00 L [95% CrI −0.03, 0.03]) and glycopyrronium (difference in CFB 0.00 L [95% CrI −0.03, 0.04]) (Figure 3). The corresponding probabilities of aclidinium being a better treatment range from 41%–59% (Figure 3). After 24 weeks, aclidinium showed a numerically higher difference in terms of trough FEV1 versus tiotropium 5 μg (difference in CFB 0.02 L [95% CrI −0.05, 0.09]) and tiotropium 18 μg (difference in CFB 0.02 L [95% CrI −0.05, 0.08]) with the probabilities of aclidinium being a better treatment at 69% and 72%, respectively. At the same time point, there was no difference versus glycopyrronium (difference in CFB 0.00 L [95% CrI −0.07, 0.07]), and the probability of aclidinium being a better treatment was at 48%.
Although the point estimates showed minimal changes, the results were not sensitive to scenario and covariate analyses (Table 5). In all cases, the point estimate of the differences in CFB between the regimens was in the range of −0.02 L to 0.02 L (Figure 3; Table 5).
Health status
In total, 14 studies22,24,25,28,29,31,34,35,38–41,43,44 (including 17,140 patients) reported on health status using SGRQ as the assessment tool. All active treatments improved CFB of SGRQ total score versus placebo (a lower SGRQ score represents improvement) at both time points (Table 4). Aclidinium demonstrated an improvement of 4.63 units (95% CrI −6.85, −2.42) after 24 weeks as compared to placebo, for which the point estimate is above the clinically significant improvement of four units (Figure 3).
In the base case, aclidinium resulted in comparable results at 12 weeks versus tiotropium 18 μg (difference in CFB −1.02 [95% CrI −2.84, 0.8]) and glycopyrronium (difference in CFB −0.39 [95% CrI −2.51; 1.72]), with the probabilities of aclidinium being a better treatment at 86% and 64%, respectively (Figure 3). At 24 weeks, aclidinium is expected to improve health-related quality of life more than tiotropium 5 μg (difference in CFB −2.44 [95% CrI −4.82, −0.05]) and demonstrate a trend toward lower (better) SGRQ scores compared to tiotropium 18 μg (difference in CFB −1.80 [95% CrI −4.52, 0.14]) and glycopyrronium (difference in CFB −1.52 [95% CrI −4.08, 1.03]). The probabilities of aclidinium being a better treatment are reflecting these results, ranging from 88%–98% (Figure 3). The results for both scenarios and for the covariate analyses were consistent with the base case (Table 5). No studies allowing LABA use reported SGRQ data at 12 weeks. At 24 weeks, in the scenario where studies that allowed LABA concomitant treatment were excluded, aclidinium showed improved SGRQ total scores over tiotropium 18 μg as well.
The proportion of patients achieving the MCID in SGRQ total score of >4 units was reported in nine studies22,24,25,28,29,34,39,40,43 (including 7,886 patients). In line with the NMA results for the CFB of total SGRQ score, a greater proportion of patients achieved the MCID in SGRQ total score with active treatments than with placebo (Table 4). For aclidinium, the OR versus placebo was 1.75 (95% CrI 1.34, 2.27) after 12 weeks and 1.94 (95% CrI 1.38, 2.73) after 24 weeks.
Dyspnea
Relief from dyspnea, assessed by the Transitional Dyspnea Index (TDI), was reported in ten studies22,24,25,29,31,34,35,40,43,44 (including 6,248 patients). All treatments improved dyspnea versus placebo (a higher TDI score represents improvement) with a difference in CFB for TDI focal score close to the MCID of one unit (Table 4). No tiotropium 5 μg studies reported this outcome. Aclidinium demonstrated favorable results versus tiotropium 18 μg and glycopyrronium, with the probabilities of aclidinium being a better treatment ranging from 59%–74% (Figure 3). When adjusting for covariates, differences tended to become more pronounced in favor of aclidinium (Table 5). In all analyses performed, the drugs showed comparable efficacy in improving TDI focal score (all credible intervals include zero), although aclidinium showed a numerically higher mean effect. None of the studies reporting this outcome allowed for LABA concomitant treatment.
The proportion of patients achieving the MCID in TDI focal score of >1 unit than placebo was examined in eight studies22,24,25,29,34,35,43,44 (including 5,224 patients); the NMA showed that all active drugs produced a greater improvement of the TDI focal score than placebo (Table 4). The OR for aclidinium versus placebo was 1.99 (95% CrI 1.53, 2.60) after 12 weeks and 1.58 (95% CrI 1.13, 2.23) after 24 weeks.
Discussion
The aim of this study was to assess the relative effectiveness of aclidinium 400 μg bromide BID compared to tiotropium 18 μg OD, tiotropium 5 μg OD, and glycopyrronium 50 μg OD in patients with moderate-to-severe COPD in terms of lung function, health-related quality of life, and dyspnea.
This NMA suggests that aclidinium is expected to be comparable to all active treatments and better than placebo with respect to all outcomes assessed at 12 and 24 weeks. The meta-regression adjustment for percentage of concomitant use of ICS, and FEV1% predicted at baseline did not change the main findings. This was also the case for two scenario analyses undertaken, ie, including ACCORD II – excluding LABA-allowing studies.
The outcomes assessed in this study are of key importance in maintenance treatments for COPD. FEV1 was the primary endpoint in all of the studies, as spirometry reflects an important prognostic factor that is used to define severity for COPD. Although spirometry is clinically important, patient-centered outcomes, such as health status and dyspnea, may better reflect the effectiveness of a particular pharmacotherapy.45 SGRQ represents a key patient-reported outcome that provides direct insight into the overall health status of patients, while dyspnea is a common and troublesome manifestation of COPD, and relief from dyspnea is an important goal of pharmacotherapy.
Other meta-analyses assessing the efficacy and safety of tiotropium have previously been published.18,46,47 The results of these studies are consistent with the current NMA with respect to the comparison of tiotropium 18 μg, aclidinium 400 μg, and placebo. The current NMA extends those findings by including other LAMAs in the analysis. To our knowledge, there are no systematic literature reviews published on the relative efficacy of LAMAs.
Limitations
As with any systematic review, the quality of the trials included present a limitation of the current study. Overall, the RCTs were of high quality. A potential limitation of the evidence base is the perceived imbalance in patient severity between the treatments compared in the ACCORD II aclidinium study. For this reason, the study was excluded from the base case and was included only as a scenario analysis. Although in the scenario analysis the results for all outcomes were slightly less favorable for aclidinium, it did not change the conclusion of the current study that the active treatments are comparable. Another potential limitation of the evidence base is the open-label evaluation of tiotropium in three studies,35,41,44 although there is no evidence that the treatment effect is different.35 Furthermore, our review was limited to studies published in the English language.
In many cases, the data required for the analysis (eg, standard error) were not reported, and an estimation based on the available data (eg, confidence interval) was performed, thus restricting the accuracy. Furthermore, when not reported in the text or tables, values were estimated from figures which could also limit the accuracy.
Another inherent limitation of systematic reviews is the presence of heterogeneity. The degree of heterogeneity between studies included in the NMA was evaluated during the validity assessment step of the current study. Differences were identified in terms of the proportion of ICS use and FEV1% predicted at baseline, and adjustment of the analyses for these differences using a constant treatment-by-covariate interaction led to consistent interpretation. Results adjusted for differences identified in the study design or patient characteristics had only a marginal impact on the effect estimates (by changing the estimated mean difference in change from baseline or the odds ratio or by increasing the uncertainty) and are, therefore, not believed to be a likely source of bias in the unadjusted analysis.
Although the meta-regression analysis suggests that the results of the NMA are not likely to be greatly affected by similarity and consistency violations, it was not possible to evaluate or adjust for all potential effect modifiers. In some cases, there was insufficient information reported across the studies to fully evaluate the study or patient characteristics. For example, the concomitant treatments permitted during the study were not always clearly reported, and the proportion of patients receiving alternative concomitant treatments was inconsistently reported across the studies. Similarly, the proportion of patients with severe or very severe COPD was not always reported. In the case of ethnicity, it was assumed that this factor was not a treatment effect modifier, although limited information regarding the breakdown of this information was available.
Conclusion
Based on a NMA of the available RCTs reporting on efficacy outcomes in terms of bronchodilator (trough FEV1), health status (as assessed by SGRQ total score and proportion of responders with at least four-point improvement), and dyspnea (as assessed by TDI focal score and proportion of responders with at least one point improvement), aclidinium 400 μg bromide BID is expected to be at least comparable to tiotropium 18 μg OD, tiotropium 5 μg OD, and glycopyrronium 50 μg OD at 12 and 24 weeks. Compared to tiotropium 5 μg, at 24 weeks, aclidinium is expected to be more efficacious in the SGRQ total score in all scenarios.
Supplementary table
Search strategy
| Databases: Embase, MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations |
| Search engine: Ovid |
| Time period: 1989 to October 2012 |
| Search date: October 26th, 2012 |
| 1. Tiotropium bromide/or aclidinium bromide/or glycopyrronium bromide/(6235) |
| 2. (Tiotropium or spiriva or aclidinium bromide or Eklira or Glycopyrronium or NVA-237 or NVA237 or (NVA adj “237”) or glycopyrronium bromide or glycopyrrolate).ti,ab. (3378) |
| 3. (COPD or chronic obstructive pulmonary disease or COAD or chronic obstructive airway disease or chronic obstructive lung disease or chronic bronchitis or emphysema).ab. or (COPD or chronic obstructive pulmonary disease or COAD or chronic obstructive airway disease or chronic obstructive lung disease or chronic bronchitis or emphysema).ti. (112741) |
| 4. Exp Pulmonary Disease, Chronic Obstructive/or exp Chronic obstructive lung disease/(74383) |
| 5. (Randomised or randomized or randomly or placebo or trial).ab. or (randomised or randomized or randomly or placebo or trial).ti. (1503026) |
| 6. Exp RANDOMIZED CONTROLLED TRIAL/(654235) |
| 7. Exp controlled clinical trial/(530101) |
| 8. (1 or 2) and (3 or 4) and (5 or 6 or 7) (1004) |
| 9. (Animals not humans).sh. (3705463) |
| 10. 8 not 9 (1004) |
| 11. 10 (1004) |
| 12. Limit 11 to English language (944) |
| 13. Limit 12 to yr = “1989-Current” (944) |
| 14. Remove duplicates from 13 (632) |
| Database: Cochrane |
| Search date: October 26th, 2012 |
| 1. MeSH descriptor Pulmonary Disease, Chronic Obstructive explode all trees (1834) |
| 2. (COPD or chronic obstructive pulmonary disease or COAD or chronic obstructive airway disease or chronic obstructive lung disease or chronic bronchitis or emphysema) (9973) |
| 3. Tiotropium or spiriva or aclidinium bromide or Eklira or Glycopyrronium or NVA-237 or NVA237 or (NVA adj “237”) or glycopyrronium bromide or glycopyrrolate (953) |
| 4. ((#1 OR #2) AND #3) (520) |
| 5. (#4), from 1989 to 2012 limit to trials (446) |
| Database: clinicaltrials.gov |
| Search date: October 26th, 2012 |
| NVA-237 or NVA237 or (NVA adj “237”) or glycopyrronium bromide or glycopyrrolate [INTERVENTION] |
| AND copd [CONDITION] |
| AND (“Phase II” OR “Phase III” OR “Phase IV”) [PHASE] |
| Database: Pubmed |
| Search date: October 26th, 2012 |
| 1. Search tiotropium OR spiriva OR aclidinium bromide OR Eklira OR Glycopyrronium OR NVA-237 OR NVA237 OR glycopyrronium bromide OR glycopyrrolate [Title/Abstract] (1782) |
| 2. Search COPD OR chronic obstructive pulmonary disease OR COAD OR chronic obstructive airway disease OR chronic obstructive lung disease OR chronic bronchitis OR emphysema [Title/Abstract] (65084) |
| 3. Search randomised OR randomized OR randomly OR placebo OR trial [Title/Abstract] (846800) |
| 4. Search ((#3) AND #2) AND #1 (366) |
| 5. Search ((“Glycopyrrolate” [Mesh]) OR “tiotropium” [Supplementary Concept]) OR “(3R)-3-((hydroxy(di-2-thienyl)acetyl)oxy)-1-(3-phenoxypropyl)-1-azoniabicyclo(2.2.2)octane bromide” [Supplementary Concept] Filters: Publication date from 2012/01/01 to 2013/12/31 (55) |
| 6. Search (#4) NOT #5 Filters: Publication date from 2012/01/01 to 2013/12/31 (32) |
Acknowledgments
The authors would like to acknowledge Francesc Peris, Mercedes Prior (Almirall), and Gert Bergman (MAPI Consultancy) for their feedback on the results of this study; Walter Bouwmeester (MAPI Consultancy) for his help in the NMA part; and Shamika de Silva (MAPI Consultancy) for editorial assistance in drafting the manuscript. Assistance with editing and formatting of the manuscript for submission by Prescott Medical Communications Group (Chicago, IL, USA) was made possible by funding from Forest Research Institute, Inc (Jersey City, NJ, USA), a wholly owned subsidiary of Forest Laboratories, Inc (New York, NY, USA).
Footnotes
Disclosure
This study was conducted by MAPI Consultancy on behalf of Almirall SA (Barcelona, Spain) and Forest Research Institute (FRI; Jersey City, NJ, USA), who funded the study and the writing of this manuscript. All authors participated in the design and conduct of the study, as well as drafting and revising the manuscript. Leandro Lindner is an employee of Almirall SA. Michelle Mocarski is an employee of FRI. Andreas Karabis and Eline Huisman are employees of MAPI Consultancy and served as paid consultants to Almirall and FRI during the conduct of this study and the preparation of this manuscript. Andrew Greening has no confict of interest to declare in this work.
References
- 1.Global Initiative for Chronic Obstructive Lung D Global Strategy for the Diagnosis, Management and Prevention of COPD (GOLD) Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdfAccessed February 25, 2013
- 2.National Institute for Health and Clinical Excellence Chronic obstructive pulmonary disease: Management of chronic obstructive pulmonary disease in adults in primary and secondary care (partial update) National Institute for Health and Care Excellence; 2013[updated May 8, 2013] Available from: http://guidance.nice.org.uk/cg101Accessed May 13, 2013 [Google Scholar]
- 3.United States Food and Drug Administration Prescribing Information: TUDORZA PRESSAIR Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202450s000lbl.pdfAccessed February 25, 2013
- 4.Boehringer Ingelheim Limited [homepage on the Internet] Summary of product characteristics: Spiriva® Respimat® 2.5 microgram, Solution for Inhalation Boehringer Ingelheim Limited; [updated June 27, 2013. Available from: http://www.medicines.org.uk/emc/medicine/20134Accessed July 2, 2013 [Google Scholar]
- 5.European Medicines Agency Summary of Product Characteristics: Eklira Genuair 322 micrograms inhalation powder Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002211/WC500132661.pdfAccessed February 25, 2013
- 6.United States Food and Drug Administration Highlights of Prescribing Information for TUDORZA™ PRESSAIR™ (aclidinium bromide inhalation powder) 2012St Louis, MO, USA: Forest Pharmaceuticals Inc; Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202450s000lbl.pdfAccessed February 25, 2013 [Google Scholar]
- 7.van der Palen J, Ginko T, Kroker A, et al. Preference, satisfaction and critical errors with Genuair® and HandiHaler® in patients with COPD [abstract] Eur Respir J. 2012;40(Suppl 56):389s. [Google Scholar]
- 8.European Medicines Agency Summary of Opinion: Glycopyrronium Bromide London: European Medicines Agency; 2012Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/002691/WC500129032.pdfAccessed February 25, 2013 [Google Scholar]
- 9.Beier J, Kirsten AM, Mroz R, et al. S51 Efficacy of aclidinium bromide compared with tiotropium and placebo in patients with moderate to severe COPD: A phase IIIb study. Thorax. 2012;67(Suppl 2):A26–A27. doi: 10.3109/15412555.2013.814626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuhr R, Magnussen H, Sarem K, et al. Efficacy of aclidinium bromide 400 μg twice daily compared with placebo and tiotropium in patients with moderate to severe COPD. Chest. 2012;141(3):745–752. doi: 10.1378/chest.11-0406. [DOI] [PubMed] [Google Scholar]
- 11.National Institute for Health and Care Excellence The Guidelines Manual 2009. Appendix D: Methodology Checklist: Randomised Controlled Trials London: National Institute for Health and Care Excellence; 2009Available from: http://www.nice.org.uk/media/633/21/The_guidelines_manual_2009_-_Appendix_D_Methodology_checklist_-_randomised_controlled_trials.pdfAccessed February 25, 2013 [Google Scholar]
- 12.Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331(7521):897–900. doi: 10.1136/bmj.331.7521.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23(20):3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 14.Jansen JP, Crawford B, Bergman G, Stam W. Bayesian meta-analysis of multiple treatment comparisons: an introduction to mixed treatment comparisons. Value Health. 2008;11(5):956–964. doi: 10.1111/j.1524-4733.2008.00347.x. [DOI] [PubMed] [Google Scholar]
- 15.Dempster AP. The direct use of likelihood for significance testing. Statistics and Computing. 1997;7(4):247–252. [Google Scholar]
- 16.Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc Series B Stat Methodol. 2002;64(4):583–639. [Google Scholar]
- 17.Dias S, Welton NJ, Sutton AJ, Ades AE.NICE DSU Technical Support Document 2: A generalised linear modelling framework for pair-wise and network meta-analysis of randomised controlled trials Available from: http://www.nicedsu.org.uk/TSD2%20General%20meta%20analysis%20corrected%20Mar2013.pdfAccessed October 9, 2012 [PubMed] [Google Scholar]
- 18.Cope S, Zhang J, Williams J, Jansen JP. Efficacy of once-daily indacaterol 75 μg relative to alternative bronchodilators in COPD: a study level and a patient level network meta-analysis. BMC Pulm Med. 2012;12:29. doi: 10.1186/1471-2466-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;7:CD009285. doi: 10.1002/14651858.CD009285.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Cooper NJ, Sutton AJ, Morris D, Ades AE, Welton NJ. Addressing between-study heterogeneity and inconsistency in mixed treatment comparisons: Application to stroke prevention treatments in individuals with non-rheumatic atrial fibrillation. Stat Med. 2009;28(14):1861–1881. doi: 10.1002/sim.3594. [DOI] [PubMed] [Google Scholar]
- 21.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGs – A Bayesian modelling framework: Concepts, structure, and extensibility. Statistics and Computing. 2000;10(4):325–337. [Google Scholar]
- 22.Forest Research Institute, Inc Clinical Study Report LAS-MD-33: Efficacy and Safety of Aclidinium Bromide at Two Dose Levels (200 μg Twice Daily, 400 μg Twice Daily) vs Placebo When Administered to Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease. Clinical Trial Registry [ NCT00891462] Available from: http://clinicaltrials.gov/ct2/show/NCT00891462.
- 23.Kerwin EM, D’Urzo AD, Gelb AF, Lakkis H, Garcia GE, Caracta CF, ACCORD I study investigators Efficacy and safety of a 12-week treatment with twice-daily aclidinium bromide in COPD patients (ACCORD COPD I) COPD. 2012;9(2):90–101. doi: 10.3109/15412555.2012.661492. [DOI] [PubMed] [Google Scholar]
- 24.Forest Research Institute, Inc Clinical Study Report LAS-MD-38: A Randomized, Double Blind, Placebo-Controlled Study Evaluating the Efficacy, Safety and Tolerability of 2 Doses of Aclidinium Bromide Compared With Placebo for 12 Weeks in Patients with Moderate to Severe Stable Chronic Obtructive Pulmonary Disease Followed by a 40-week Evaluation of the Higher Bromide Dose. Clinical Trial Registry [ NCT01045161] Available from: http://clinicaltrials.gov/ct2/show/NCT01045161.
- 25.Almirall SA. Clinical Study Report LAS-MD-34: Efficacy and Safety of Aclidinium Bromide at Two Dose Levels vs Placebo When Administered to Patients With Moderate to Severe Chronic Obstructive Pulmonary Disease (COPD) Clinical Trial Registry [ NCT01001494] Available from: http://clinicaltrials.gov/show/NCT01001494.
- 26.Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J. 2012;40(4):830–836. doi: 10.1183/09031936.00225511. [DOI] [PubMed] [Google Scholar]
- 27.Bateman E, Singh D, Smith D, et al. Efficacy and safety of tiotropium Respimat SMI in COPD in two 1-year randomized studies. Int J Chron Obstruct Pulmon Dis. 2010;5:197–208. [PMC free article] [PubMed] [Google Scholar]
- 28.Bateman ED, Tashkin D, Siafakas N, et al. A one-year trial of tiotropium Respimat plus usual therapy in COPD patients. Respir Med. 2010;104(10):1460–1472. doi: 10.1016/j.rmed.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. [Erratum appears in Thorax. Feb 2005;60(2):105] Thorax. 2003;58(5):399–404. doi: 10.1136/thorax.58.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casaburi R, Briggs DD, Jr, Donohue JF, Serby CW, Menjoge SS, Witek TJ., Jr The spirometric efficacy of once-daily dosing with tiotropium in stable COPD: a 13-week multicenter trial. The US Tiotropium Study Group. Chest. 2000;118(5):1294–1302. doi: 10.1378/chest.118.5.1294. [DOI] [PubMed] [Google Scholar]
- 31.Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19(2):217–224. doi: 10.1183/09031936.02.00269802. [DOI] [PubMed] [Google Scholar]
- 32.Chan CK, Maltais F, Sigouin C, Haddon JM, Ford GT, SAFE Study Group A randomized controlled trial to assess the efficacy of tiotropium in Canadian patients with chronic obstructive pulmonary disease. Can Respir J. 2007;14(8):465–472. doi: 10.1155/2007/192961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Covelli H, Bhattacharya S, Cassino C, Conoscenti C, Kesten S. Absence of electrocardiographic findings and improved function with once-daily tiotropium in patients with chronic obstructive pulmonary disease. Pharmacotherapy. 2005;25(12):1708–1718. doi: 10.1592/phco.2005.25.12.1708. [DOI] [PubMed] [Google Scholar]
- 34.Donohue JF, van Noord JA, Bateman ED, et al. A 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest. 2002;122(1):47–55. doi: 10.1378/chest.122.1.47. [DOI] [PubMed] [Google Scholar]
- 35.Donohue JF, Fogarty C, Lötvall J, et al. INHANCE Study Investigators Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182(2):155–162. doi: 10.1164/rccm.200910-1500OC. [DOI] [PubMed] [Google Scholar]
- 36.Moita J, Bárbara C, Cardoso J, et al. Tiotropium improves FEV1 in patients with COPD irrespective of smoking status. Pulm Pharmacol Ther. 2008;21(1):146–151. doi: 10.1016/j.pupt.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Niewoehner DE, Rice K, Cote C, et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med. 2005;143(5):317–326. doi: 10.7326/0003-4819-143-5-200509060-00007. [DOI] [PubMed] [Google Scholar]
- 38.Tashkin DP, Celli B, Senn S, et al. UPLIFT Study Investigators A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 39.Tonnel AB, Perez T, Grosbois JM, Verkindre C, Bravo ML, Brun M, TIPHON study group Effect of tiotropium on health-related quality of life as a primary efficacy endpoint in COPD. Int J Chron Obstruct Pulmon Dis. 2008;3(2):301–310. doi: 10.2147/copd.s2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verkindre C, Bart F, Aguilaniu B, et al. The effect of tiotropium on hyperinflation and exercise capacity in chronic obstructive pulmonary disease. Respiration. 2006;73(4):420–427. doi: 10.1159/000089655. [DOI] [PubMed] [Google Scholar]
- 41.Vogelmeier C, Kardos P, Harari S, Gans SJ, Stenglein S, Thirlwell J. Formoterol mono- and combination therapy with tiotropium in patients with COPD: a 6-month study. Respir Med. 2008;102(11):1511–1520. doi: 10.1016/j.rmed.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 42.Voshaar T, Lapidus R, Maleki-Yazdi R, et al. A randomized study of tiotropium Respimat Soft Mist inhaler vs ipratropium pMDI in COPD. Respir Med. 2008;102(1):32–41. doi: 10.1016/j.rmed.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 43.D’Urzo A, Ferguson GT, van Noord JA, et al. Efficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: the GLOW1 trial. Respir Res. 2011;12:156. doi: 10.1186/1465-9921-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kerwin E, Hébert J, Gallagher N, et al. Efficacy and safety of NVA237 versus placebo and tiotropium in patients with COPD: the GLOW2 study. Eur Respir J. 2012;40(5):1106–1114. doi: 10.1183/09031936.00040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Molen T, Cazzola M. Beyond lung function in COPD management: effectiveness of LABA/LAMA combination therapy on patient-centred outcomes. Prim Care Respir J. 2012;21(1):101–108. doi: 10.4104/pcrj.2011.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karabis A, Lindner L, Mocarski M, et al. Comparative efficacy of aclidinium bromide 400 Mcg bid versus tiotropium 18 Mcg and 5 Mcg QD as maintenance bronchodilator treatment to relieve symptoms in adult patients with Chronic Obstructive Pulmonary Disease (COPD): a network meta-analysis. Value Health. 2012;15(7):A560. [Google Scholar]
- 47.Yohannes AM, Willgoss TG, Vestbo J. Tiotropium for treatment of stable COPD: a meta-analysis of clinically relevant outcomes. Respir Res. 2011;56(4):477–487. doi: 10.4187/respcare.00852. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy
| Databases: Embase, MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations |
| Search engine: Ovid |
| Time period: 1989 to October 2012 |
| Search date: October 26th, 2012 |
| 1. Tiotropium bromide/or aclidinium bromide/or glycopyrronium bromide/(6235) |
| 2. (Tiotropium or spiriva or aclidinium bromide or Eklira or Glycopyrronium or NVA-237 or NVA237 or (NVA adj “237”) or glycopyrronium bromide or glycopyrrolate).ti,ab. (3378) |
| 3. (COPD or chronic obstructive pulmonary disease or COAD or chronic obstructive airway disease or chronic obstructive lung disease or chronic bronchitis or emphysema).ab. or (COPD or chronic obstructive pulmonary disease or COAD or chronic obstructive airway disease or chronic obstructive lung disease or chronic bronchitis or emphysema).ti. (112741) |
| 4. Exp Pulmonary Disease, Chronic Obstructive/or exp Chronic obstructive lung disease/(74383) |
| 5. (Randomised or randomized or randomly or placebo or trial).ab. or (randomised or randomized or randomly or placebo or trial).ti. (1503026) |
| 6. Exp RANDOMIZED CONTROLLED TRIAL/(654235) |
| 7. Exp controlled clinical trial/(530101) |
| 8. (1 or 2) and (3 or 4) and (5 or 6 or 7) (1004) |
| 9. (Animals not humans).sh. (3705463) |
| 10. 8 not 9 (1004) |
| 11. 10 (1004) |
| 12. Limit 11 to English language (944) |
| 13. Limit 12 to yr = “1989-Current” (944) |
| 14. Remove duplicates from 13 (632) |
| Database: Cochrane |
| Search date: October 26th, 2012 |
| 1. MeSH descriptor Pulmonary Disease, Chronic Obstructive explode all trees (1834) |
| 2. (COPD or chronic obstructive pulmonary disease or COAD or chronic obstructive airway disease or chronic obstructive lung disease or chronic bronchitis or emphysema) (9973) |
| 3. Tiotropium or spiriva or aclidinium bromide or Eklira or Glycopyrronium or NVA-237 or NVA237 or (NVA adj “237”) or glycopyrronium bromide or glycopyrrolate (953) |
| 4. ((#1 OR #2) AND #3) (520) |
| 5. (#4), from 1989 to 2012 limit to trials (446) |
| Database: clinicaltrials.gov |
| Search date: October 26th, 2012 |
| NVA-237 or NVA237 or (NVA adj “237”) or glycopyrronium bromide or glycopyrrolate [INTERVENTION] |
| AND copd [CONDITION] |
| AND (“Phase II” OR “Phase III” OR “Phase IV”) [PHASE] |
| Database: Pubmed |
| Search date: October 26th, 2012 |
| 1. Search tiotropium OR spiriva OR aclidinium bromide OR Eklira OR Glycopyrronium OR NVA-237 OR NVA237 OR glycopyrronium bromide OR glycopyrrolate [Title/Abstract] (1782) |
| 2. Search COPD OR chronic obstructive pulmonary disease OR COAD OR chronic obstructive airway disease OR chronic obstructive lung disease OR chronic bronchitis OR emphysema [Title/Abstract] (65084) |
| 3. Search randomised OR randomized OR randomly OR placebo OR trial [Title/Abstract] (846800) |
| 4. Search ((#3) AND #2) AND #1 (366) |
| 5. Search ((“Glycopyrrolate” [Mesh]) OR “tiotropium” [Supplementary Concept]) OR “(3R)-3-((hydroxy(di-2-thienyl)acetyl)oxy)-1-(3-phenoxypropyl)-1-azoniabicyclo(2.2.2)octane bromide” [Supplementary Concept] Filters: Publication date from 2012/01/01 to 2013/12/31 (55) |
| 6. Search (#4) NOT #5 Filters: Publication date from 2012/01/01 to 2013/12/31 (32) |