Abstract
In plants, nitrogen is the most important nutritional factor limiting the yield of cultivated crops. Since nitrogen is essential for synthesis of nucleotides, amino acids and proteins, studies on gene expression in plants cultivated under different nitrogen availability require particularly careful selection of suitable reference genes which are not affected by nitrogen limitation. Therefore, the objective of this study was to select the most reliable reference genes for qPCR analysis of target cucumber genes under varying nitrogen source and availability. Among twelve candidate cucumber genes used in this study, five are highly homologous to the commonly used internal controls, whereas seven novel candidates were previously identified through the query of the cucumber genome. The expression of putative reference genes and the target CsNRT1.1 gene was analyzed in roots, stems and leaves of cucumbers grown under nitrogen deprivation, varying nitrate availability or different sources of nitrogen (glutamate, glutamine or NH3). The stability of candidate genes expression significantly varied depending on the tissue type and nitrogen supply. However, in most of the outputs genes encoding CACS, TIP41, F-box protein and EFα proved to be the most suitable for normalization of CsNRT1.1 expression. In addition, our results suggest the inclusion of 3 or 4 references to obtain highly reliable results of target genes expression in all cucumber organs under nitrogen-related stress.
Introduction
Real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR) is currently the method of choice for mRNA transcription studies, since it provides outputs with high sensitivity, specificity and capacity [1], [2]. However, for accurate gene expression quantification, it is essential to normalize real-time PCR data to a fixed reference. Reference genes are commonly referred to as genes of highly reliable expression, which is not affected by various experimental settings and is stable in different types of tissues and organs used in the assay [3]. The most widely used internal controls include the genes encoding: actin and tubulin (alpha/beta), cytoskeletal proteins; glyceraldehyde 3-phosphate dehydrogenase (GAPDH), involved in glycolysis; ubiquitins (UBQs), involved in the degradation of cellular proteins; 18S RNA, a part of the ribosomal functional core; RNA polymerase II (RPII or POLR2A), catalyzing the synthesis of the precursors of mRNA, most snRNA and microRNA; elongation factor 1-alpha (EF1α), which facilitates translational elongation; tyrosine-3 monooxygenase/tryptophan-5 monooxygenase activation protein; zeta polypeptide (YWHAZ), and hypoxanthine phosphoribosyl transferase 1 (HPRT1), which has a central role in the generation of purine nucleotides [4]–[13]. Nevertheless, growing evidence clearly shows that the expression of genes commonly used as reliable internal controls is often significantly affected during different experimental conditions [14]. Consequently, the systematic evaluation of common and novel reference genes derived from genome-wide analyses is becoming an essential component of real-time reverse transcription–PCR analysis so as to improve the reliability of published results [3], [15], [16]. Recently, a number of statistical algorithms have been developed to evaluate the expression stabilities of candidate reference genes in order to select the most reliable reference for a particular experimental assay. GeNorm [14], NormFinder [17] and BestKeeper [18] are free VBA (Visual Basic Applets) applets for Microsoft Excel that have been commonly used to determine the expression stability of candidate reference genes in plants such as potato [19]; grapevine [20]; rice [21], [22]; tomato [23], [24]; soybean [25]; citrus [26]–[28]; coffee [29]; brachiaria grass [30]; peach [31]; cotton [32]; eucalyptus [33]; chicory [34]; cucumber [35], [36]; petunia [37]; banana and plantains [38]; and peanut [39]. As a result, some suitable references were selected for studying gene expression analyses in a variety of differentially developed plant tissues and organs or in plants grown under biotic and abiotic stresses. However, there is currently little knowledge about the expression stability of commonly used reference genes or novel references under varying nitrate availability or different sources of nitrogen in the environment. Nitrogen is an important constituent of the majority of essential structural, genetic and metabolic compounds in plant cells [40], [41]. It is a structural component of all amino acids, the building elements of structural and enzyme proteins, and chlorophyll, the pigment essential for photosynthesis. In addition, nitrogen builds the energy-transfer compounds, such as ATP, electron-transfer molecules, such as NAD(P) or FAD, and the genetic material essential for growth and reproduction (nucleic acids DNA and RNA). Hence lack or deficiency of the available nitrogen in soil solution results in severe disturbances in the synthesis and action of key cellular biomolecules, causing a detrimental effect on plant growth and development [40]–[42]. In soil solution, the majority of plant-available nitrogen exists in the inorganic NH4 + and NO3 − forms, but most crop plants require mainly nitrate in large quantities to sustain high yields [43], [44]. In contrast to ammonium, the level of nitrate in soils is highly variable, since NO3 − ions do not bind to soil complexes and thus leach easily when excess water percolates through the soil [45]. Numerous studies have been performed to reveal mechanisms that underlie the plant response to nitrogen or nitrate deprivation or varying availability. The estimation of patterns of expressed genes may provide insight into complex regulatory networks and help to identify genes involved in the signaling and metabolic pathways underlying developmental and cellular processes in plants grown under different nitrogen nutrition.
Since the accurate quantification of the expression of genes involved in nitrogen transport or metabolism requires reliable internal controls with highly stable expression independent of nitrogen or nitrate supply and concentration, the selection of suitable candidate genes is highly desired. We have recently identified seven novel candidates for reference genes in cucumber encoding :CACS (clathrin adaptor complex subunit), PDF2 (protein phosphatase 2), TIP41 (PPA2 activator), GW881873 (expressed protein), F-box, HEL (helicase), YSL8 (mitosis protein) and analyzed the expression of novel genes as well as the five commonly used references: ACT (actin), TUA (tubulin), CYP (cyclophilin), UBI-1 (ubiquitin), EFα (elongation factor α) under abiotic stress and plant growth regulators [36]. In this work, we present for the first time the genomic organization of all 12 analyzed reference genes in cucumber whole genome contigs and validate their expression stability under varying nitrate supply and various nitrogen sources. Based on the obtained results, we propose reliable internal controls for studies of the expression of cucumber genes involved in nitrogen transport and metabolism.
Materials and Methods
Plant material
Cucumber plants were grown essentially as described earlier [46], with slight modification of nitrogen source and availability in nutrition media. The roots, stems or leaves were collected from 2-week-old plants grown under different source of nitrogen and from 4-week-old plants grown upon different nitrate provision. Two-week-old plants were grown for the first week in media containing 5 mM KNO3, and for the second week they were transferred to the fresh N-free nutrient solution containing K2CO3 instead of KNO3. Following nitrogen starvation, plants were transferred to the fresh N-free medium or to the media enriched in 5 mM KNO3, 5 mM NH4Cl, 5 mM glutamine (Gln) or 5 mM glutamic acid (Glu) for 6 or 12 hours. Part of the 4-week-old plants were grown on N-free medium or media containing 0.5 mM or 10 mM KNO3 for 4 weeks. Some of the plants were transferred for one week into 0.5 mM KNO3 following 3 weeks of growth in N-free medium (temporary nitrate provision). Other plants were grown for the first 2 weeks in N-free medium, then for one week in 0.5 mM KNO3-containing medium and for the last week again in N-free medium (temporary nitrate starvation). Last part of the seedlings was grown as follows: plants grown for the first week in N-free medium and for the second week in 0,5 mM nitrate were again put into N-free medium for the third week and transferred into fresh medium containing 0,5 mM nitrate for the last (fourth) week (temporary nitrate re-supply). All nutrient solutions were aerated and changed three times a week. For each treatment four samples (50 mg) of each tissue from four different plants were collected and stored at −80°C until use.
Total RNA isolation and cDNA synthesis
Total RNA was extracted using the TRI-Reagent (Sigma) according to the protocol provided by manufacturer. RNA quantity and quality was assessed spectrophotometrically (Nanodrop) and the samples showing A260/A280 ratio of 1.8–2.0 and an A260/A230 ratio of 2.0–2.2 were used for subsequent analysis. RNA integrity was assessed on an Agilent 2100 Bioanalyzer using the RNA 6000 pico labchip Kit (Agilent Technologies). All samples were further treated with RNase-free DNase (Fermentas), according to the manufacturer's instructions. First strand cDNA was synthesized by reverse transcribing 2 µg of total RNA with high-capacity cDNA Reverse Transcription kit (Applied Biosystems, USA) in a 20 µl reaction using random primers and MultiScribe™ Reverse Transcriptase (50 U) according to manufacturer's instructions. Reverse transcription was performed at 37°C for 1 hour followed by 85°C for 5 min. cDNA was diluted 8 times for the use of real-time qRT-PCR reaction. All cDNA were stored at −20°C until use.
The organization and validation of reference genes
We have previously selected 12 putative reference genes and validated their expression stability in cucumber roots or roots, stems and leaves under abiotic stress (heavy metals, salt, osmotic and oxidative stress) and different plant growth regulators [36]. They included five references commonly used in the studies on plant genes expression (actin, tubulin, cyclophilin, ubiquitin or elongation factor) as well as seven putative candidates homologous to novel reference genes selected in A. thaliana [3]: clathrin adaptor complex subunit CACS (At5g46630), expressed protein At33380, TIP41(At54g34270), helicase (At1g58050), phoshpolipase 2 PDF2 (Atg13320), mitosis protein YSL8 (At5g08290) and F-box (At5g15710). The full sequences of all cucumber genes were identified in the whole-cucumber genome shotgun reads using the queries of A. thaliana cDNAs, Blastn [47] and FGENESH or FGENESH+ [48] softwares. The genomic organization and putative function of all selected candidate genes are presented in table 1. The gene encoding cucumber nitrate transporter NRT1.1 was used as the target for the normalization of expression data. Primer pairs on the selected reference and target gene sequences (Table S1) were designed using the Lightcycler Probe Design software (Roche), with the conditions of 154–290 base pairs (bp) as the PCR amplicon length and 60°C as the optimal Tm (melting temperature).
Table 1. Description of cucumber candidate reference genes based on the comparison with their Arabidopsis orthologs.
| Gene symbol and lenght | NCBI contig accession no | Gene position within contig | NCBI EST accession no | Arabidopsis ortholog locus | Arabidopsis ortholog description | Function |
| ACT 1134 bp | ACHR01010658 | 7140–8587 | AB010922 | At5g09810 | Actin 7 | Structural constituent of cytoskeleton, protein binding |
| TUA 1467 bp | ACHR01012753 | 92843–95078 | AJ715498 | At4g14960 | Tubulin alpha-6 | Structural constituent of cytoskeleton, protein folding |
| EFα 1344 bp | ACHR01002194 | 9646–11514 | EF446145 | At5g60390 | Elongation factor 1-alpha | Translational elongation |
| CYP 519 bp | ACHR01007623 | 9364–9882 | AY942800 | At2g16600 | Peptidyl-prolyl cis-trans isomerase CYP19-1 | Protein folding, signal transduction |
| CACS 1278 bp | ACHR01010524 | 38791– 44675 | GW881874 | At5g46630 | AP-2 complex subunit mu-1 (Clathrin adaptor complexes medium subunit family protein) | Intracellular protein transport, vesicle-mediated transport |
| HEL 1014 bp | ACHR01008789 | 1228–5426 | GW881869 | At1g58050 | RNA helicase family protein | Helicase activity |
| TIP41 873 bp | ACHR01001003 | 28762–31836 | GW881871 | At4g34270 | TIP41-like family protein | PP2A phosphatase activator |
| UBI-1 306 bp | ACHR01007578 | 26294–27611 | AF104391 | At5g57860 | Ubiquitin family protein | Protein binding, protein modification |
| F-box 1378 bp | ACHR01005017 | 38102–39873 | GW881870 | At5g15710 | F-box/kelch-repeat protein | Unknown |
| YSL8 429 bp | ACHR01006572 | 23112–24359 | GW881872 | At5g08290 | Yellow-Leaf-Specific gene 8, YLS8 | Vesicle-mediated transport, mitosis, vacuole and Golgi organization |
| GW881873 984 bp | ACHR01016153 | 59983–63343 | GW881873 | At4g33380 | Expressed protein | Unknown |
| PDF2 2103 bp | ACHR01000299 | 57230–62778 | GW881868 | At1g13320 | Protein Phosphatase 2A Subunit A3 | N-terminal protein myristoylation, regulation of phosphorylation |
Seven of the twelve candidate cucumber reference genes (CACS, TIP41, PDF2, GW881873, YSL8, HEL) have been recently retrieved from the whole cucumber genome sequence [36] using the novel reference genes identified in Arabidopsis as the query sequences [3]. The commonly used remaining five genes (ACT, TUA, UBI-1, EFα, CYP) were previously available in the Genbank database as partial cDNAs. The full cDNAs and exon/intron organization of all 12 candidate genes were established using BlastN, and FGENESH or FGENESH+.
Amplification of gene transcripts
The expression study was performed using a 96 well plate on an Lightcycler 480 (Roche) with 2× SYBR Green Mix B (A&A Biotechnology). The reactions were performed according to the manufacturer's instructions: the PCR program was initiated at 95°C for 10 min to activate Taq DNA polymerase, followed by 45 thermal cycles of 10 seconds at 94°C, 10 seconds at 60°C and 15 seconds at 72°C. Melting curve analysis was performed immediately after the real-time PCR. The temperature range used for the melting curve generation was from 65°C to 95°C. All assays were performed using three technical and biological replicates, a non-template control and a non-RT control. The standard curves were generated by amplifying at least seven dilution series of cDNA (Table S1). The correlation coefficient (R2) and PCR efficiency were calculated using the slopes of the standard curves (Figure S2). The linear R2 for all the primers ranged between 0.978–0.999, whereas PCR efficiencies of primers ranged from 95%–105% (Figure S2, Table S1). To confirm the PCR products size, the reactions were subjected to electrophoresis on 2.0% agarose gels stained with ethidium bromide following Real-time PCR assay. The determination of the crossing amplification point (Cp) as well as the relative quantification analysis (ΔΔCT-method) were performed using the Lightcycler 480 software 1.5. The amplification of non-template controls generated Cp values above 45 or was not detectable. The non-normalized expression data were analyzed by geNorm v3.5 and NormFinder version 2 whereas the raw Cp values were imported into BestKeeper version 1.
The evaluation of reference gene expression stability
Considering the heterogeneity of treatments, the biological samples from 2-week-old plants and 4-week-old plants were analyzed separately. For each analysis of stability of gene expression, four subsets were established based on the organ used, including roots, stems, leaves and all organs of cucumber plants. At first, the reliability of all twelve cucumber candidate genes was evaluated using two different statistical algorithms, geNorm [14] and NormFinder [17]. Based on the their outputs, the two worst references were removed and the expression stability of the remaining ten genes was further validated using BestKeeper [18]. All three Visual Basic applets for Microsoft Excel base on different principles. The geNorm calculates an internal control gene-stability measure M as the average pairwise variation of each gene with other candidate genes and select two ideal references through the sequential exclusion of genes with the lowest stability of expression [14]. The lower the M value, the higher stability of the expression of particular gene. In addition, geNorm calculates the optimal number of genes required for normalization of target gene expression based on the pairwise variation (Vn/n+1) between normalization factors established for defined number of reference genes. The inclusion of additional reference is not required if the V value is below the 0,15 [14]. Contrary to geNorm, the NormFinder calculates and ranks the stability value for each gene investigated based on the comparison between the intra-group and inter-group variations of candidate genes expression [17]. The lowest stability value correspond to the highest expression stability, but a minimum of 3 genes and a minimum of 2 samples per group are required for the analysis. Similar to geNorm and Normfinder, the Bestkeeper estimates the most appropriate reference by using the geometric mean of the expression of the candidate cDNA, however, it takes into account the raw data instead of the data converted in relative quantity. In addition, a maximum 10 candidate genes may be analyzed using this applet. BestKeeper calculates a pairwise correlation coefficient between each gene and the BestKeeper index (BI) and a standard deviation (SD) of the Cp-values between the whole data set. The gene with the most stable expression should have the highest coefficient of correlation with the BI indicates [18].
Results
The genomic organization and expression of cucumber reference genes
12 candidate reference genes were identified from two sources: traditional housekeeping genes actin, ubiqutin, cyclophilin, tubulin, elongation factor α, frequently used for transcript normalization in cucumber were found in the GenBank database whereas cucumber homologues to the superior reference genes selected from Arabidopsis transcriptome microarray data [3]: clathrin adaptor complex subunit CACS, expressed protein GW881873, TIP41, helicase, phoshpolipase 2 PDF2, mitosis protein YSL8 and F-box, were found within the cucumber whole-genome contigs available in GenBank under the accession number ACHR0100000. The accession numbers of genes and contigs, gene names and length as well as the functions of putative proteins according to The Arabidopsis Initiative Resource (TAIR) are listed in table 1. Real-time PCR analysis of the transcript abundance revealed that the particular candidate reference genes displayed different expression ranges across the full set of cucumber samples assayed (Figure S1). While the avarage Cp values varied from 17 with a SD±1.5 for EFα gene to 26 with SD±2.0 for PDF2, most of the genes analyzed have shown an expression rate between 19 and 24. The genes encoding CYP, YSL8 and TUA showed the most variations in expression between all samples assayed, whereas the Cp values of CACS, EFα and F-box genes were more uniformly expressed. Taken together, all twelve genes displayed a relatively wide range of expression levels in cucumber roots, stems and leaves.
Expression stability analysis
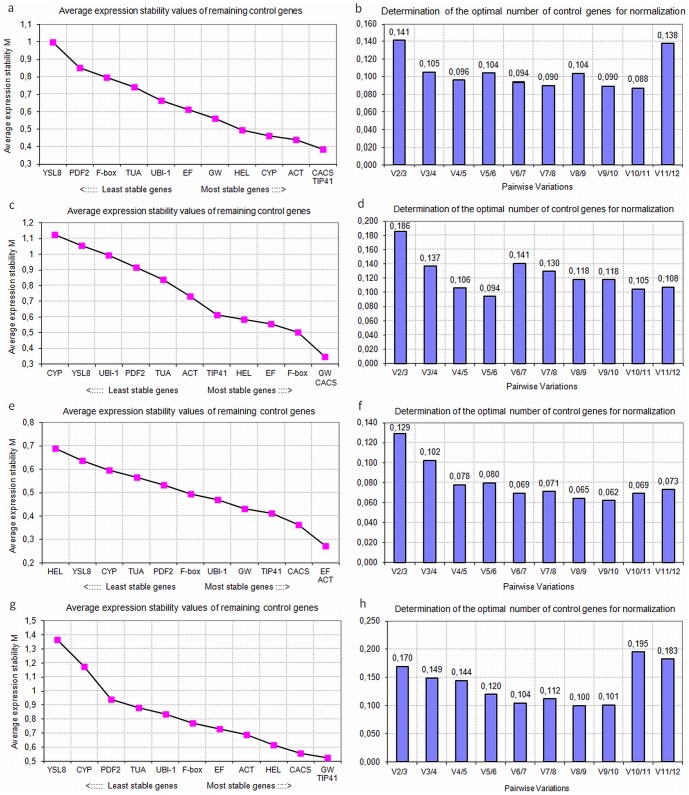
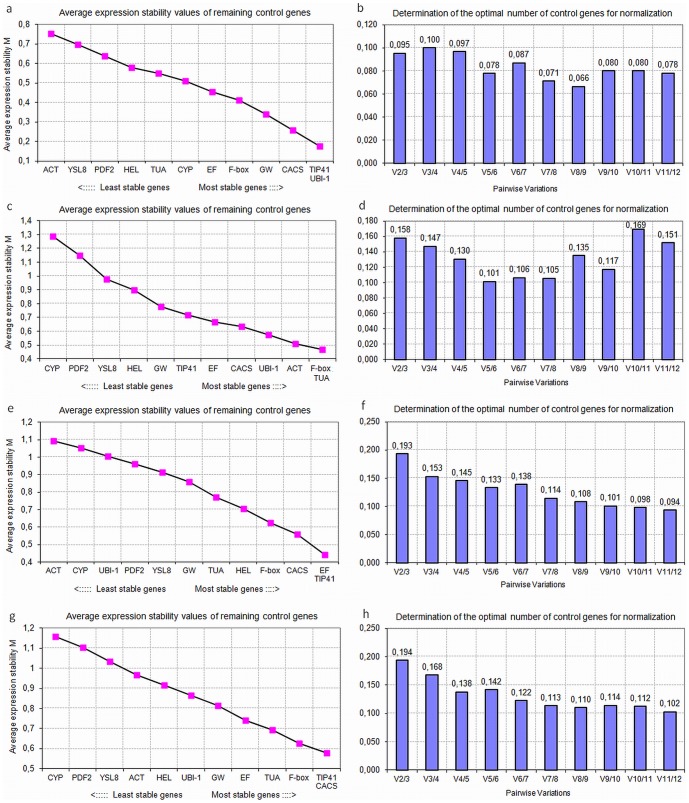
In order to find the most suitable internal control for cucumber RT-qPCR normalization, we assessed the stability of expression of 12 candidate genes using the pairwise variation in expression stability implemented in geNorm v3.5 as well as the NormFinder, which estimates the stability of gene expression based on the comparison between inter- and intra-group variability. The samples from 2-week-old plants and 4-week-old plants were analyzed separately with regard to the different developmental stage and differential treatment. Figures 1 and 2 show the M values of reference genes examined by geNorm when the samples were considered separately as roots, stems and leaves or together, as all organs. The outputs revealed some significant differences between particular candidate genes expression stability in individual cucumber organs and under different nitrogen nutrition. In roots, CACS, TIP41, ACT and CYP showed the most stable expression upon different nitrogen sources, whereas YSL8 and PDF2 ranked at the worst positions (Figure 1a and 2a). In contrast, ACT was the least stable gene in roots grown under varying nitrate, where in turn, beside CACS and TIP41, the expression of UBI-1 gene was very constant (Figure 2a). More significant variations were observed in stems, were CACS, GW881873 and F-box or ACT, F-box and TUA were ranked at top positions in samples from plants grown under different nitrogen source or varying nitrate availability, respectively (Figure 1c and 2c). On the contrary, CYP, YSL8 and PDF2 were ranked poorly in stem tissues regardless the nutritional treatment, whereas UBI-1 showed an uniform expression under differential nitrogen treatment (M<0.6) but was less stable under varying NO3 − supply (M∼1) (Figure 1c and 2c). The differential treatment of plants also affected the ranking of gene expression in leaves, where EFα, TIP41 and CACS were the most reliable genes but either HEL, YSL8 and CYP or ACT, CYP and UBI-1 showed the least stable expression under varying nitrogen source or nitrate supply, respectively (Figure 1e and 2e). Like in roots, in leaves ACT was ranked as the best gene upon differential nitrogen nutrition and as the worst gene under varying nitrate supply (Figure 1e and 2e). Despite some apparent differences in all rankings generated by geNorm, the overall analysis of candidate genes expression in all samples from roots, stems and leaves confirmed that TIP41 and CACS ranked at top positions whereas YSL8, PDF2 and CYP ranked as the most variable genes regardless the heterogeneity of plant treatment (Figure 1g and 2g). Though all of the 12 genes showed acceptable expression stabilities (M≤1.5), according to Vandesompele et. al. [14], the M values were general higher when samples from all organs were analyzed together (Figure 1 and 2). In addition, geNorm also calculated the optimal number of reference genes required for a more reliable normalization (Vn/n+1). Taking into account the entire dataset from 2-week-old plants and considering a cut-off (Vn/n+1≤0.15), the pairwise value for two genes (V2/3) was 0.17, while for three genes (V3/4) was 0.149 (Fig. 1h). Therefore, the third reference gene should be included for normalization to improve further gene expression evaluations in cucumbers grown under different nitrogen compounds. The overall analysis of gene expression in 4-week-old plants revealed the V2/3 and V3/4 values of 0.194 and 0.168, respectively, whereas the pairwise value for four genes (V4/5) was 0.138 (Figure 2h). Based on the analysis, a minimum of four references would be necessary for accurate analysis of target genes expression in plants grown under varying nitrate supply. Similarly to geNorm, NormFinder determined TIP41, CACS, EFα, or F-box as the most stable reference genes in the two entire datasets, whereas CYP, PDF2 or YSL8 were usually ranked as the most variable (Table 2). Commonly used actin was again ranked in the last positions when samples from roots or all organs from plants grown on varying nitrate were considered, however, it displayed relatively stable expression in roots and leaves of plants grown in different nitrogen compounds (Table 2). The highest variability in candidate gene expression reflected by the highest stability values calculated by NormFinder was observed when all organs from plants grown in different nitrogen source were analyzed together (Table 2). Further evaluation of the ten most stable reference genes in BestKeeper confirmed that CACS, TIP41, and F-box were ranked in the highest positions in samples from 2-week-old plants (Table 3). CACS was also the most reliable gene in stems, leaves and all organs taken together in plants grown on varying nitrate, whereas EFα was better in roots (Table 4). Although the results obtained by the three algorithms seem to be a bit divergent in the rankings of candidate genes, they show that at least four sufficiently reliable reference genes (CACS, TIP41, F-box, EFα) could be suitable for normalizing all cucumber sample sets. Moreover, the geNorm-based overall analysis of the expression of twelve candidate genes in all samples collected from 2-week-old and 4-week-old cucumbers grown under different nitrogen source and varying nitrate availability revealed, that CACS, TIP41, F-box and EFα show the highest expression stability not only in conditions of various nitrogen nutrition but also at different developmental stages of cucumber plants (Figure S3).
Figure 1. GeNorm based evaluation of candidate gene expression in samples from plants grown in different nitrogen compounds.
Average expression stability values (M) of the remaining candidate cucumber reference genes during stepwise exclusion of the least stable reference gene in roots (a), stems (c), leaves (e) and all cucumbers organs taken together (g). The lowest the M values indicate the most stable expression of candidate cucumber genes. Determination of optimal number of reference genes based on pairwise variation (V) analysis of normalization factors of the candidate reference genes in roots (b), stems (d), leaves (f) and all cucumber organs taken together (h). The Vn/n+1 value was calculated for every comparison between two of the twelve consecutive candidate reference genes. According to [14], additional (n+1)th reference gene should be included into analysis whenever the Vn/n+1 value drops below the 0.15 threshold.
Figure 2. GeNorm based evaluation of candidate gene expression in samples from plants grown in different nitrate supply.
Average expression stability values (M) of the remaining candidate cucumber reference genes during stepwise exclusion of the least stable reference gene in roots (a), stems (c), leaves (e) and all cucumbers organs taken together (g). The lowest the M values indicate the most stable expression of candidate cucumber genes. Determination of optimal number of reference genes based on pairwise variation (V) analysis of normalization factors of the candidate reference genes in roots (b), stems (d), leaves (f) and all cucumber organs taken together (h). The Vn/n+1 value was calculated for every comparison between two of the twelve consecutive candidate reference genes. According to [14], additional (n+1)th reference gene should be included into analysis whenever the Vn/n+1 value drops below the 0.15 threshold.
Table 2. Candidate cucumber genes ranked according to their expression stability as determined by NormFinder.
| Ranking order | Plants grown under different source of nitrogen* | Plants grown under varying availability of nitrate** | ||||||
| Roots | Stems | Leaves | All organs | Roots | Stems | Leaves | All organs | |
| 1 | TIP41 | TIP41 | EF | CACS | EF | CACS | CACS | CACS |
| 0.016 | 0.114 | 0.147 | 0.248 | 0.160 | 0.268 | 0.074 | 0.180 | |
| 2 | CACS | F-box | CACS | TIP41 | CACS | Tubulin | EF | EF |
| 0.086 | 0.144 | 0.169 | 0.253 | 0.171 | 0.326 | 0.121 | 0.273 | |
| 3 | Actin | CACS | Actin | F-box | F-box | UBQ | TIP41 | TIP41 |
| 0.094 | 0.156 | 0.192 | 0.281 | 0.197 | 0.346 | 0.122 | 0.289 | |
| 4 | F-box | GW881873 | UBQ | EF | TIP41 | TIP41 | Tubulin | GW881873 |
| 0.102 | 0.166 | 0.193 | 0.331 | 0.198 | 0.359 | 0.225 | 0.311 | |
| 5 | EF | EF | GW881873 | GW881873 | UBQ | GW881873 | F-box | F-box |
| 0.130 | .182 | 0.210 | 0.367 | 0.209 | 0.362 | 0.243 | 0.341 | |
| 6 | GW881873 | Helicase | TIP41 | Actin | GW881873 | EF | Actin | UBQ |
| 0.169 | 0.193 | 0.241 | 0.385 | 0.235 | 0.366 | 0.323 | 0.342 | |
| 7 | Cyclophilin | TUA | PDF2 | UBQ | Cyclophilin | F-box | Helicase | Tubulin |
| 0.186 | 0.228 | 0.249 | 0.433 | 0.247 | 0.376 | 0.324 | 0.378 | |
| 8 | UBQ | Actin | F-box | PDF2 | Tubulin | Helicase | YSL8 | Helicase |
| 0.208 | 0.257 | 0.266 | 0.455 | 0.295 | 0.378 | 0.331 | 0.391 | |
| 9 | Tubulin | Cyclophilin | Cyclophilin | Helicase | Helicase | YSL8 | UBQ | PDF2 |
| 0.264 | 0.315 | 0.351 | 0.459 | 0.298 | 0.383 | 0.341 | 0.416 | |
| 10 | Helicase | PDF2 | Tubulin | Tubulin | YSL8 | Actin | PDF2 | YSL8 |
| 0.295 | 0.334 | 0.380 | 0.615 | 0.334 | 0.430 | 0.361 | 0.451 | |
| 11 | PDF2 | YSL8 | Helicase | YSL8 | PDF2 | Cyclophilin | GW881873 | Actin |
| 0.326 | 0.335 | 0.412 | 1.158 | 0.380 | 0.655 | 0.384 | 0.452 | |
| 12 | YSL8 | UBQ | YSL8 | Cyclophilin | Actin | PDF2 | Cyclophilin | Cyclophilin |
| 0.521 | 0.351 | 0.444 | 1.269 | 0.426 | 0.741 | 0.479 | 0.479 | |
Stability values are listed from the most stable to the least stable gene.
Samples from two-week-old plants grown under nitrate, ammonia, glutamine or glutamate for 4 or 12 hours.
Samples from 4-week-old plants grown under nitrogen deficiency, 0.5 mM nitrate, 10 mM nitrate, temporary nitrate provision, temporary nitrate starvation or temporary nitrate re-supply.
Table 3. BestKeeper based evaluation of reference genes stability in cucumber plants grown in different nitrogen compounds.
| Gene | UBQ | EF | ACT | GW881873 | F-BOX | CYP | TIP41 | CACS | HEL | TUA | PDF2 | YSL8 |
| Roots | 0,819 | 0,890 | 0,894 | 0,837 | 0,929 | 0,934 | 0,981 | 0,951 | 0,834 | 0,820 | - | - |
| Stems | - | 0,980 | 0,964 | 0,965 | 0,989 | 0,614 | 0,979 | 0,972 | 0,972 | 0,861 | 0,931 | - |
| Leaves | 0,969 | 0,983 | 0,974 | 0,976 | 0,950 | 0,939 | 0,966 | 0,985 | - | 0,873 | 0,981 | - |
| All organs | 0,855 | 0,969 | 0,930 | 0,954 | 0,904 | - | 0,981 | 0,973 | 0,916 | 0,817 | 0,776 | - |
The stability values were calculated based on the pairwise correlation between genes and BI (BestKeeper Index). The highest Person coefficient values representing the most stable genes are marked in bold. Genes ranked at the lowest positions by geNorm and NormFinder for each set of analyzed samples were not included (-) in BestKeeper evaluation.
Table 4. BestKeeper based evaluation of reference genes stability in cucumber plants grown in varying NO3 − supply.
| Gene | UBQ | EF | ACT | GW881873 | F-BOX | CYP | TIP41 | CACS | HEL | TUA | PDF2 | YSL8 |
| Roots | 0,706 | 0,989 | - | 0,908 | 0,940 | 0,919 | 0,893 | 0,986 | 0,919 | 0,924 | - | 0,835 |
| Stems | 0,810 | 0,949 | 0,760 | 0,963 | 0,986 | - | 0,983 | 0,992 | 0,941 | 0,989 | - | 0,982 |
| Leaves | 0,889 | 0,994 | - | 0,942 | 0,986 | - | 0,990 | 0,996 | 0,970 | 0,952 | 0,931 | 0,923 |
| All organs | 0,701 | 0,779 | - | 0,886 | 0,790 | - | 0,956 | 0,988 | 0,824 | 0,740 | 0,963 | 0,950 |
The stability values were calculated based on the pairwise correlation between genes and BI (BestKeeper Index). The highest Person coefficient values representing the most stable genes are marked in bold. Genes ranked at the lowest positions by geNorm and NormFinder for each set of analyzed samples were not included (-) in BestKeeper evaluation.
Discussion
Investigation of the expression level of genes encoding enzymes and proteins involved in nitrogen transport and metabolism is the crucial step in understanding the mechanisms underlying plant response to nitrogen supply, which could help breeders to improve crop fertilization and production. The availability of cucumber genomic resources [49], [50] allows for identification of the whole families of genes involved in nitrogen metabolism and nitrogen compounds' uptake, transport and assimilation in this plant. To date, only two studies relying on RT-qPCR analysis in cucumber have validated candidate reference genes for transcript normalization. These studies included a few test conditions such as abiotic stresses (salinity, drought, osmotic and oxidative stress, heat, cold) and plant growth regulators [35], [36]. Still, the evaluation of reference genes expression in cucumber grown under different nutritional conditions is lacking.
Here, we evaluated the stability of expression of seven novel and five traditional reference genes in roots, stems and leaves of cucumbers grown under different nitrogen regimes. Initial analysis in geNorm and NormFinder showed some differences in the particular rankings of candidate genes; however, both applets consistently selected similar genes as showing the most stable or unstable expression patterns. The slight divergences probably result from different basic assumptions of the geNorm and NormFinder models. The NormFinder estimates both the intra- and inter-group variation to calculate a stability value for each candidate gene. The lower the inter- and intra-group variations, the higher the position of the gene in the ranking. In contrast, geNorm selects the best two internal control genes taking into account only similar intergroup variation, which may be problematic in the case of co-regulated genes [51]. Differences between NormFinder and geNorm outputs were also demonstrated by other studies [28], [29], [35], [36], [52], [53]. The best 10 reference genes were further analyzed by BestKeeper, which calculated the coefficient of variance of each putative reference gene as a percentage of the average Cp level. Based on the three different outputs, all algorithms seem to be relevant to elect internal controls for each experimental set. In all cases, the best reference genes recommended by one program were also highly ranked by the two others. Our results demonstrated that CACS, TIP41, EFα, and F-box were the most stably expressed reference genes in most samples and subsets studied. Nevertheless, the best combination of genes varied significantly depending on experimental condition and organ assayed. This observation confirms that the validation of the stability of candidate genes expression is a prerequisite for reliable normalization in specific biological samples and assays. Among top ranked genes, TIP41 and EFα were identified as the most stable genes in roots, whereas EFα and CACS were uniformly expressed in leaves of cucumber plants. Beside F-box, CACS and TIP41 were also highly ranked in stems. Our results support the previous study on cucumber candidate reference genes, demonstrating that CACS, TIP41, F-box and EFα showed the most stable transcript accumulation under heavy metal, salt, osmotic or oxidative stress and upon application of growth regulators [36]. Wan et.al. [35] also demonstrated that EFα expression in cucumber was highly stable in different tissues and under abiotic and biotic stress. In addition, Czechowski et.al. [3] also observed that genes homologous to the cucumber top references EFα, CACS and F-box were stably expressed in Arabidopsis roots under abiotic stress. Moreover, CACS, TIP41, F-box and EFα were among the most reliable candidate references in samples from different developmental stages, organs, tissues and genotypes of Arabidopsis plants (M≤0.5), as calculated by geNorm software [3]. Interestingly, EFα expression was significantly affected by S, P or sugar starvation in Arabidopsis [3] whereas its cucumber homolog was stably expressed upon nitrogen starvation. The gene encoding elongation factor was also top ranked during nitrogen starvation in tomato [24]. It may be cautiously concluded that the expression of genes encoding elongation factors is not significantly altered upon nitrogen deficiency, so they could serve as suitable internal controls in conditions of nitrogen-related stress. In the case of F-box, beside Arabidopsis [3], [54] and cucumber [36], this gene was also considered a good candidate gene for normalizing a wide range of tissue from soybean and citrus or floral organs in cotton [25], [28], [32]. To date, F-box proteins have been shown to participate in ubiquitination of the proteins targeted for degradation, signal transduction and cell cycle regulation [55]. Such basic physiological functions are usually maintained by constitutively expressed housekeeping genes. Although the F-box protein identified in cucumber has not been functionally characterized yet, it seems to be a constitutively expressed gene in the whole plant regardless of nitrogen availability and source, and thus could be recommended as a reliable reference for studying target genes expression under nitrogen-related stress. Similarly to F-box, YSL8 was also ranked among the top reference genes in various differentially developed organs of Arabidopsis and in roots and leaves of thale cress treated with elevated Cu and Cd [3], [54]. In contrast, YSL8 was ranked among the least stably expressed candidate genes under different nitrogen nutrition (Figure 1 and 2, Table 2). Similarly, the gene was usually ranked in a lower position in roots or in roots, stems and leaves of cucumbers grown under abiotic stress and phytohormones [36]. Hence, we may conclude that YSL8 may not be a suitable reference gene in studies of cucumber gene expression. Similarly to YSL8, Clathrin adaptor complex subunit is also involved in intracellular and vesicle-mediated transport; however, CACS was ranked in the top positions in all analyses in our study, regardless of cucumber organs identity or treatment. Clathrin adaptor proteins link clathrin to their receptors in vesicles, forming a coat, which is important for cargo selection and direction of the vesicle transport [56]. Endocytosis and exocytosis of vesicles are performed by cells to take up nutrients, to import signaling receptors or to mediate export of toxic compounds [57]. Perhaps the expression of CACS remains constitutive regardless of nutritional condition because the protein encoded by this gene participates in such basic, intracellular transport processes. The other novel cucumber candidate reference was PDF2, which was uniformly expressed in the Arabidopsis organs at various developmental stages [3]. However, in the previous studies on cucumber, PDF2 displayed intermediate or low stability values in plants grown with application of phytohormones and abiotic stress [36]. In the current analyses PDF2 along with YSL8 was considered the least stable candidate gene under different nitrogen nutrition. PDF2 is one of three genes encoding the 65 kDa regulatory subunit of protein phosphatase 2A (PP2A), which plays crucial roles in the regulation of growth and development [58]. Altered PP2A activity in plants was associated with disturbances in hormone homeostasis and signaling, defense responses, cell division, morphogenesis, and reproduction [59]. Since the growth and development of plant is significantly affected by nitrogen availability, such a regulatory protein may not be a suitable reference for normalization of organ samples from plants grown under a varying nitrogen source or supply. The last candidate reference among novel cucumber genes was HEL, which was found to be very stable in a series of developmental samples and different organs in Arabidopsis [3]. In cucumber, HEL was ranked in intermediate positions when samples from plants grown under abiotic stress, phytohormones [36] or nitrogen-related stress (Figure 1 and 2, Table 2) were analyzed. Therefore, a better reference could be suggested for studies of target genes expression in cucumber.
Genes commonly referred to as housekeeping genes, such as tubulins, actins, cyclophilins or ubiquitins, have often been used as internal controls in target genes expression studies. Validation of expression stability of these genes in plants has brought contradictory results. Czechowski et. al. [3] found ACT2 to be the least stably expressed gene in Arabidopsis among the 27 samples from different stages, organs and conditions. ACT was also considered unreliable during flax development [53] and citrus grown in drought stress [28]. In cucumber, the expression of ACT7 homolog was found to be significantly affected during abiotic stress and phytohormone treatment [36]. Though the gene was ranked generally in lower positions during nitrogen-related stress, it was found among the most reliable genes in roots or leaves of plants grown in different nitrogen sources (Figure 1, Table 2). Actins were also considered highly stable genes in cucumber grown in cold or heat (ACT3, ACT2, ACT1), drought or salt (ACT2, ACT3), hormones (ACT2) or when the samples from different tissues or treatments were taken together (ACT, ACT3) [35].
Similarly to ACT, TUB was also ranked in the last position for different genotypes of citrus analyzed in various experimental conditions [27], [28] and during flax development [53]. In cucumber, TUA was considered an inadequate reference gene under heavy metals, oxidative, salt and osmotic stress [36] as well as under high or low temperature [35]. However, the expression of the gene was highly stable when different cucumber tissues or samples from plants treated only with three different hormones – ABA (abscisic acid), SA (salicylic acid) and MeJA (methyl jasmonic acid) – were analyzed [35]. In our study, TUA was ranked mostly in an intermediate position in all subsets analyzed, except for the stems from plants grown in varying nitrate supply, where it displayed significantly higher expression stability. Given the observations, both actins and tubulins can be considered adequate internal controls for expression studies on target genes in cucumber in particular conditions.
Similarly to YSL8 and PDF2, CYP was ranked among the least stable candidate genes in cucumber grown under different nitrogen nutrition. It was also considered an unreliable internal control in cucumbers grown under abiotic stress and phytohormones [35], [36]. The expression of CYP also significantly varied in different tissues of peach and maize, in potato grown in abiotic and biotic stress, in grapevine during berry development and during wheat endosperm development [19], [20], [31], [60]. Although this gene was stably expressed in wheat flag leaves sampled in organic and conventional fields [61], it may not be a reliable internal control for expression analyses in cucumber plants.
The last common reference gene evaluated in cucumber was UBI-1, encoding the putative ubiquitin peptide which marks proteins to ensure their proper localization or degradation, as reviewed by [62]. Though generally ranked in intermediate positions, UBI-1 demonstrated highly stable expression in roots and stems of plants grown under varying nitrate supply. UBI-1 was also previously ranked among the top 5 candidate reference genes in cucumbers grown under heavy metals, oxidative, salt and osmotic stress [36] and subject to cold, heat or phytohormones [35]. Nevertheless, UBI-1 expression appears to be less stable when compared to other candidates for reference genes.
In summary, despite slight differences found in different subsets, we concluded that at least four genes – CACS, TIP41, EFα and F-box – appear to be good reference genes for normalizing a wide range of organ samples of cucumber in different experimental conditions, even though the molecular and biological function of three of the putative proteins encoded by these genes (CACS, TIP41 and F-box) remains unclear. Moreover, our study suggests that more than two reliable internal controls should be used to normalize target genes expression in all cucumber organs under different nitrogen nutrition. In contrast, two reference genes were suitable for analysis of samples taken from plants grown under abiotic stress and phytohormones [35], [36]. Commonly used reference genes like actins, tubulins or cyclophilins and ubiquitins should be carefully evaluated for each experimental condition tested, since their expression may be significantly affected depending on organ identity or experimental assay. This work constitutes the first systematic study in cucumber to validate optimal reference genes for RT-qPCR normalization with consideration of different organs, various nitrogen source and varying NO3 − availability.
Supporting Information
The average expression levels with SD of candidate reference genes in roots, stems and leaves of cucumber plants grown under different nitrogen nutrition.
(DOC)
The parameters of real-time PCR amplification of candidate reference genes.
(DOC)
GeNorm based evaluation of candidate gene expression in samples from plants grown in different nitrogen compounds or under varying nitrate availability. A. Average expression stability values (M) of the remaining candidate cucumber reference genes during stepwise exclusion of the least stable reference gene all cucumbers organs. The lowest the M values indicate the most stable expression of candidate cucumber genes. B. Determination of optimal number of reference genes based on pairwise variation (V) analysis of normalization factors of the candidate reference genes all cucumber organs. The Vn/n+1 value was calculated for every comparison between two of the twelve consecutive candidate reference genes. According to [14], additional (n+1)th reference gene should be included into analysis whenever the Vn/n+1 value drops below the 0.15 threshold.
(DOC)
Primer sequences used to quantify the expression of the selected traditional and novel cucumber reference genes by real-time PCR.
(DOC)
Acknowledgments
We would like to express our sincere gratitude and our highest appreciation to Ewelina Posyniak for technical assistance during the realization of this project.
Funding Statement
The research was mainly supported by the Polish National Science Centre (grant no. N N303 818740). AW was financially supported by the fellowship for young PhD researchers granted by European Social Fund of European Union. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bustin SA, Benes V, Nolan T, Pfaffl MW (2005) Quantitative real-time RT-PCR – a perspective. J Mol Endocrinol 34: 597–601. [DOI] [PubMed] [Google Scholar]
- 2. Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, et al. (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55: 611–622. [DOI] [PubMed] [Google Scholar]
- 3. Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139: 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thellin O, Zorzi W, Lakaye B, De Borman B, Coumans B, et al. (1999) Housekeeping genes as internal standards: use and limits. J Biotechnol 75: 291–295. [DOI] [PubMed] [Google Scholar]
- 5. Catalan V, Gomez-Ambrosi J, Rotellar F, Silva C, Rodriguez A, et al. (2007) Validation of endogenous control genes in human adipose tissue: relevance to obesity and obesity-associated type 2 diabetes mellitus. Horm Metab Res 39: 495–500. [DOI] [PubMed] [Google Scholar]
- 6. de Jonge HJ, Fehrmann RS, de Bont ES, Hofstra RM, Gerbens F, et al. (2007) Evidence based selection of housekeeping genes. PLoS One 2: e898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maroufi A, Bockstaele EV, Loose MD (2010) Validation of reference genes for gene expression analysis in chicory (Cichorium intybus) using quantitative real-time PCR. BMC Mol Biol 11: 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mehta R, Birerdinc A, Hossain N, Afendy A, Chandhoke V, et al. (2010) Validation of endogenous reference genes for qRT-PCR analysis of human visceral adipose samples. BMC Mol Biol 11: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tea M, Michael MZ, Brereton HM, Williams KA (2013) Stability of small non-coding RNA reference gene expression in the rat retina during exposure to cyclic hyperoxia. Mol Vis 19: 501–508. [PMC free article] [PubMed] [Google Scholar]
- 10. Hu R, Fan C, Li H, Zhang Q, Fu YF (2009) Evaluation of putative reference genes for gene expression normalization in soybean by quantitative real-time RT-PCR. BMC Mol Biol 10: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garg R, Sahoo A, Tyagi AK, Jain M (2010) Validation of internal control genes for quantitative gene expression studies in chickpea (Cicer arietinum L.). Biochem Biophys Res Commun 2: 283–288. [DOI] [PubMed] [Google Scholar]
- 12. Uddin MJ, Cinar MU, Tesfaye D, Looft C, Tholen E, et al. (2011) Age-related changes in relative expression stability of commonly used housekeeping genes in selected porcine tissues. BMC Research Notes 4: 441–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gu YR, Li MZ, Zhang K, Chen L, Jiang AA, et al. (2011) Evaluation of endogenous control genes for gene expression studies across multiple tissues and in the specific sets of fat- and muscle-type samples of the pig. J Anim Breed Genet 128: 319–325. [DOI] [PubMed] [Google Scholar]
- 14. Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, et al. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Faccioli P, Ciceri GP, Provero P, Stanca AM, Morcia C, et al. (2007) A combined strategy of “in silico” transcriptome analysis and web search engine optimization allows an agile identification of reference genes suitable for normalization in gene expression studies. Plant Mol Biol 63: 679–688. [DOI] [PubMed] [Google Scholar]
- 16. Paolacci AR, Tanzarella OA, Porceddu E, Ciaffi M (2009) Identification and validation of reference genes for quantitative RT-PCR normalization in wheat. BMC Mol Biol 10: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andersen CL, Jensen JL, Orntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64: 5245–5250. [DOI] [PubMed] [Google Scholar]
- 18. Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP (2004) Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnol Lett 26: 509–515. [DOI] [PubMed] [Google Scholar]
- 19. Nicot N, Hausman JF, Hoffmann L, Evers D (2005) Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J Exp Bot 56: 2907–2914. [DOI] [PubMed] [Google Scholar]
- 20. Reid KE, Olsson N, Schlosser J, Peng F, Lund ST (2006) An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biol 6: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jain M, Nijhawan A, Tyagi AK, Khurana JP (2006) Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem Biophys Res Commun 345: 646–651. [DOI] [PubMed] [Google Scholar]
- 22. Li QF, Sun SSM, Yuan DY, Yu HX, Gu M, et al. (2010) Validation of candidate reference genes for the accurate normalization of real-time quantitative RT-PCR data in rice during seed development. Plant Mol Biol Rep 28: 49–57. [Google Scholar]
- 23. Exposito-Rodriguez M, Borges AA, Borges-Perez A, Perez JA (2008) Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. BMC Plant Biol 8: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lovdal T, Lillo C (2009) Reference gene selection for quantitative real-time PCR normalization in tomato subjected to nitrogen, cold, and light stress. Anal Biochem 387: 238–242. [DOI] [PubMed] [Google Scholar]
- 25. Libault M, Thibivilliers S, Bilgin DD, Radwan O, Benitez M (2008) Identification of four soybean reference genes for gene expression normalization. Plant Genome 1: 44–54. [Google Scholar]
- 26. Liu Q, Zhu A, Chai L, Zhou W, Yu K, et al. (2009) Transcriptome analysis of a spontaneous mutant in sweet orange [Citrus sinensis (L.) Osbeck] during fruit development. J Exp Bot 60: 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carvalho K, de Campos MK, Pereira LF, Vieira LG (2010) Reference gene selection for real-time quantitative polymerase chain reaction normalization in “Swingle” citrumelo under drought stress. Anal Biochem 402: 197–199. [DOI] [PubMed] [Google Scholar]
- 28. Mafra V, Kubo KS, Alves-Ferreira M, Ribeiro-Alves M, Stuart RM, et al. (2012) Reference genes for accurate transcript normalization in citrus genotypes under different experimental conditions. PLoS One 7: e31263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cruz F, Kalaoun S, Nobile P, Colombo C, Almeida J, et al. (2009) Evaluation of coffee reference genes for relative expression studies by quantitative real-time RT-PCR. Mol Breeding 23: 607–616. [Google Scholar]
- 30. Silveira ED, Alves-Ferreira M, Guimaraes LA, da Silva FR, Carneiro VT (2009) Selection of reference genes for quantitative real-time PCR expression studies in the apomictic and sexual grass Brachiaria brizantha. BMC Plant Biol 9: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tong Z, Gao Z, Wang F, Zhou J, Zhang Z (2009) Selection of reliable reference genes for gene expression studies in peach using real-time PCR. BMC Mol Biol 10: 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Artico S, Nardeli SM, Brilhante O, Grossi-de-Sa MF, Alves-Ferreira M (2010) Identification and evaluation of new reference genes in Gossypium hirsutum for accurate normalization of real-time quantitative RT-PCR data. BMC Plant Biol 10: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boava LP, Laia ML, Jacob TR, Dabbas KM, Goncalves JF, et al. (2010) Selection of endogenous genes for gene expression studies in Eucalyptus under biotic (Puccinia psidii) and abiotic (acibenzolar-S-methyl) stresses using RT-qPCR. BMC Res Notes 3: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maroufi A, Van Bockstaele E, De Loose M (2010) Validation of reference genes for gene expression analysis in chicory (Cichorium intybus) using quantitative real-time PCR. BMC Mol Biol 11: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wan H, Zhao Z, Qian C, Sui Y, Malik AA, et al. (2010) Selection of appropriate reference genes for gene expression studies by quantitative real-time polymerase chain reaction in cucumber. Anal Biochem 399: 257–261. [DOI] [PubMed] [Google Scholar]
- 36. Migocka M, Papierniak A (2011) Identification of suitable reference genes for studying gene expression in cucumber plants subjected to abiotic stress and growth regulators. Mol Breeding 28: 343–357. [Google Scholar]
- 37. Mallona I, Lischewski S, Weiss J, Hause B, Egea-Cortines M (2010) Validation of reference genes for quantitative real-time PCR during leaf and flower development in Petunia hybrida. BMC Plant Biol 10: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Podevin N, Krauss A, Henry I, Swennen R, Remy S (2012) Selection and validation of reference genes for quantitative RT-PCR expression studies of the non-model crop Musa. Mol Breed 30: 1237–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Morgante CV, Guimaraes PM, Martins AC, Araujo AC, Leal-Bertioli SC, et al. (2011) Reference genes for quantitative reverse transcription-polymerase chain reaction expression studies in wild and cultivated peanut. BMC Res Notes 4: 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marschner H (1995) Mineral nutrition of higher plants. London: Academic Press: 889.
- 41.Epstein E, Bloom A (2005) Mineral nutrition of plants: principles and perspectives. 2nd edn Sunderland, MA: Sinauer Associates.
- 42. Galloway JN, Cowling EB (2002) Reactive nitrogen and the world: 200 years of change. Ambio 31: 64–71. [DOI] [PubMed] [Google Scholar]
- 43. Forde BG, Clarkson DT (1999) Nitrate and ammonium nutrition of plants: physiological and molecular perspectives. Advances in Botanical Research 30: 1–90. [Google Scholar]
- 44. Maathuis F (2009) Physiological functions of mineral nutrients. Current Opinion in Plant Biology 12: 250–258. [DOI] [PubMed] [Google Scholar]
- 45. Crawford NM, Glass ADM (1998) Molecular and physiological aspects of nitrate uptake in plants. Trends in Plant Science 3: 389–395. [Google Scholar]
- 46. Migocka M, Warzybok A, Kłobus G (2013) The genomic organization and transcriptional pattern of genes encoding nitrate transporters 1 (NRT1) in cucumber. Plant Soil 364: 245–260. [Google Scholar]
- 47. Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, et al. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Salamov AA, Solovyev VV (2000) Ab initio gene finding in Drosophila genomic DNA. Genome Res 10: 516–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Huang S, Li R, Zhang Z, Li L, Gu X, et al. (2009) The genome of the cucumber, Cucumis sativus L. Nat Genet 41: 1275–1281. [DOI] [PubMed] [Google Scholar]
- 50. Woycicki R, Witkowicz J, Gawronski P, Dabrowska J, Lomsadze A, et al. (2011) The genome sequence of the North-European cucumber (Cucumis sativus L.) unravels evolutionary adaptation mechanisms in plants. PLoS One 6: e22728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Matta BP, Bitner-Mathe BC, Alves-Ferreira M (2011) Getting real with real-time qPCR: a case study of reference gene selection for morphological variation in Drosophila melanogaster wings. Dev Genes Evol 221: 49–57. [DOI] [PubMed] [Google Scholar]
- 52. Hong SY, Seo PJ, Yang MS, Xiang F, Park CM (2008) Exploring valid reference genes for gene expression studies in Brachypodium distachyon by real-time PCR. BMC Plant Biol 8: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Huis R, Hawkins S, Neutelings G (2010) Selection of reference genes for quantitative gene expression normalization in flax (Linum usitatissimum L.). BMC Plant Biol 10: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Remans T, Smeets K, Opdenakker K, Mathijsen D, Vangronsveld J, et al. (2008) Normalisation of real-time RT-PCR gene expression measurements in Arabidopsis thaliana exposed to increased metal concentrations. Planta 227: 1343–1349. [DOI] [PubMed] [Google Scholar]
- 55. Craig KL, Tyers M (1999) The F-box: a new motif for ubiquitin dependent proteolysis in cell cycle regulation and signal transduction. Prog Biophys Mol Biol 72: 299–328. [DOI] [PubMed] [Google Scholar]
- 56. McMahon HT, Mills IG (2004) COP and clathrin-coated vesicle budding: different pathways, common approaches. Curr Opin Cell Biol 16: 379–391. [DOI] [PubMed] [Google Scholar]
- 57.Alberts B, Johnson A, Lewis J (2002) Transport into the Cell from the Plasma Membrane: Endocytosis. Molecular Biology of the Cell 4th edition New York: Garland Science.
- 58. Ahn CS, Han JA, Lee HS, Lee S, Pai HS (2011) The PP2A regulatory subunit Tap46, a component of the TOR signaling pathway, modulates growth and metabolism in plants. Plant Cell 23: 185–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. DeLong A (2006) Switching the flip: protein phosphatase roles in signaling pathways. Curr Opin Plant Biol 9: 470–477. [DOI] [PubMed] [Google Scholar]
- 60. Dheda K, Huggett JF, Bustin SA, Johnson MA, Rook G, et al. (2004) Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques 37: 112–114, 116, 118–119. [DOI] [PubMed] [Google Scholar]
- 61. Tenea GN, Peres Bota A, Cordeiro Raposo F, Maquet A (2011) Reference genes for gene expression studies in wheat flag leaves grown under different farming conditions. BMC Res Notes 4: 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hochstrasser M (1996) Protein degradation or regulation: Ub the judge. Cell 84: 813–815. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The average expression levels with SD of candidate reference genes in roots, stems and leaves of cucumber plants grown under different nitrogen nutrition.
(DOC)
The parameters of real-time PCR amplification of candidate reference genes.
(DOC)
GeNorm based evaluation of candidate gene expression in samples from plants grown in different nitrogen compounds or under varying nitrate availability. A. Average expression stability values (M) of the remaining candidate cucumber reference genes during stepwise exclusion of the least stable reference gene all cucumbers organs. The lowest the M values indicate the most stable expression of candidate cucumber genes. B. Determination of optimal number of reference genes based on pairwise variation (V) analysis of normalization factors of the candidate reference genes all cucumber organs. The Vn/n+1 value was calculated for every comparison between two of the twelve consecutive candidate reference genes. According to [14], additional (n+1)th reference gene should be included into analysis whenever the Vn/n+1 value drops below the 0.15 threshold.
(DOC)
Primer sequences used to quantify the expression of the selected traditional and novel cucumber reference genes by real-time PCR.
(DOC)