Abstract
Despite increases in the frequency and intensity of disturbances on coral reefs over the past few decades, the response of subtropical coral assemblages to climate change is poorly understood. To address this knowledge gap on Australian reefs and provide a baseline for future comparisons, we quantified spatial (10-100’s of kilometres) and temporal (decadal) patterns of benthic assemblages across a latitudinal gradient along the east Australian coastline (23.5° S to 31.5° S). Benthic community composition was quantified at six locations from the southern Great Barrier Reef, Queensland (Heron Reef, 23.5° S, 152° E) to northern New South Wales (31° S, 153.1° E) and at Lord Howe Island (31.5° S, 159.1° E). Our results indicate significant latitudinal differences in benthic assemblages, while community composition at some sites was more similar to those hundreds of kilometres away than to that of neighbouring reefs. A general trend was observed with decreasing cover of Acroporidae with increasing latitude, corresponding with an increasing cover of Pocilloporidae and Dendrophylliidae. Heron Reef comprised a high proportion of Acropora corals (43% total coral cover) and coralline algae (44%). In contrast, high-latitude reefs were dominated by mixed coral assemblages (0-52%) and high macroalgal cover (16-27%). Decadal comparisons of high-latitude reefs showed regional stability of benthic assemblages (9 out of 11 assemblages remained stable at > 75% similarity), during a period of warming oceans (0.15-0.24°C per decade). Such temporal stability suggests that eastern Australian subtropical communities may be more resistant than tropical reef communities that have experienced assembly shifts caused by perturbations associated with recent global climate change. Despite the clear differences in the structure of coral assemblages evident in our spatial surveys, we suggest that the temporal stability of high-latitude reefs may provide a limited refuge for tropical coral populations in an increasingly uncertain future.
Introduction
Scleractinian coral communities are exposed to a range of disturbances from both natural and human induced processes that can range from small-scale localised mortality events (e.g. predation and competition) to region-wide mortality events (e.g. disease outbreaks and coral bleaching) [1,2,3,4]. Recent large-scale loss in hard coral cover has led to a plethora of studies on the resilience of hard corals to a range of recent climate change associated perturbations at tropical locations [5,6,7]; however, there is a dearth of studies that detail the ability of corals to persist on marginal, high-latitude subtropical reefs under future climate change trajectories.
Coral assemblages along the eastern Australian continent extend from 11° S in the northern Great Barrier Reef (GBR) to as far south as 31° S along the coastline of New South Wales (NSW). On subtropical reefs adjacent to the NSW mainland, corals generally do not form accreting reef structures; rather colonies form a thin veneer attached to rocky substratum [8]. An exception are the high-latitude subtropical coral reefs found along the Lord Howe Rise, located 600 km east of mainland Australia, where active reef accretion is observed on the western side of Lord Howe Island [9,10]. In general, coral communities south of the GBR comprise a suite of tropical, subtropical and temperate species, with species richness declining with increasing latitude [11]. While low in diversity, coral cover at some sites is comparable to that of the GBR [12], with up to 45% cover observed at some locations [8,10,13,14,15].
In the past 30 years, the east coast of Australia has experienced significant warming trends of between 0.1-0.5°C per decade [16], raising concern about the future of tropical coral assemblages on the GBR [12]. Under future climate change scenarios, it has been suggested that high-latitude marginal reefs may provide refuge for tropical coral species [6,17,18]. This has been the case in historic times of climate change; in the Pleistocene, equatorial declines in reef corals were accompanied by range shifts and expansion towards subtropical latitudes [19,20]. However, subtropical eastern Australia marginal reefs are characterised by high macroalgal cover and low rates of coral recruitment [21], which may render these low resilient environments highly susceptible to future disturbances such as rising seawater temperature.
To date, several studies on eastern Australia high-latitude reefs have characterised coral communities over small spatial scales [8,10,13,14]. High-latitude reefs are typically characterised by an absence of reef building taxa (e.g. staghorn Acropora and massive Porites) that are commonly found at lower latitudes on the GBR, and higher levels of macroalgal cover [22]. However, a recent study has documented the increase in frequency of tropical species in high-latitude environments (e.g. Acroporamicroclados [18]). The observation of tropical species ‘appearing’ in marginal locations potentially indicates a change in species ranges driven by changes in ocean temperatures following climate change. Despite increases in disturbance events in subtropical reefs over the past few decades [23,24,25,26,27] and the potential for range shifts in coral taxa from lower latitudes, little is known about the temporal stability of high-latitude refugia, and few baselines exist with which to compare future shifts in coral community structure.
Through detailed surveys of subtropical reefs across a latitudinal gradient on the eastern Australian coastline (23.5° S to 31.5° S), we aimed to quantify coral assemblages across a range of spatial scales (local - regional scales). Additionally, a subset of our data is compared with earlier studies [13,14,15,28] to quantify temporal changes in subtropical environments. Specifically, we aimed to: 1) determine spatial patterns of benthic community structure among subtropical eastern Australia reefs; 2) contrast benthic assemblages across a latitudinal gradient exceeding 1000 km (southern GBR to northern NSW); and 3) quantify decadal trends in benthic community structure among eastern Australian subtropical reefs.
Materials and Methods
Study Sites
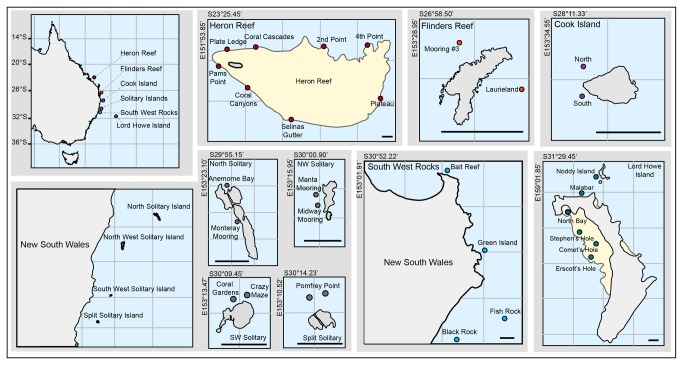
Subtropical benthic community composition data were collected from five locations along the east coast of Australia in 2006 (Figure 1): Flinders Reef (FR); Cook Island (CI); Solitary Islands Marine Park (SIMP); South West Rocks (SWR); and Lord Howe Island Marine Park (LHIMP). These surveys were conducted in conjunction with coral health assessment investigations and therefore sampling was done in areas of highest coral cover. Location details for SIMP, SWR, and LHIMP sites have been described previously [26]. Sites at FR were located on the northwest and northeast side of a small emergent sandstone outcrop of approximately 500 m long and 250 m wide. Two sites were investigated on the western side of CI. To allow comparisons between subtropical and tropical locations, benthic assemblage data was gathered from eight sites around Heron Reef (HERON) in the southern GBR [29]. Surveys were conducted at depths where coral cover was highest (8-14 m), with the exception of the four LHIMP lagoon sites (2-5 m depth) and HERON Reef slope (4-6 m). All surveys were conducted in accordance with the NSW Department of Primary Industry scientific research permit (P06/0064).
Figure 1. Map of study sites on the eastern Australian coastline.
Site specific locations inset.
Benthic community structure
Consistent with previous research on eastern Australian subtropical reefs [30], we used video transects and in-situ assessments of coral taxa to identify and quantify benthic community structure. Five 30-m transects were randomly positioned at each site and, using a Sony mini-dv camera in a waterproof housing, a SCUBA diver swam along each transect at a constant speed (0.1 m s-1), approximately 50 cm above the substratum. Two transects from each site were used to compile a comprehensive list of benthic categories across all sites. These categories complemented classifications previously used at these locations [13,14,15], thus enabling direct comparisons. From the video footage, coral cover and community structure were quantified for each transect using Coral ,Point Count (CPCe [31]). To enable comparison between data collected from Heron Reef and from surveys conducted in the 1990s at subtropical reefs, benthic categories were merged to the highest common taxonomic level possible, thus standardising variables over larger spatial and temporal scales.
Spatial patterns of community structure among subtropical reefs
Benthic and coral community data from all sites were square-root transformed prior to analysis, which reduced the skewness in the data and improved homogeneity of variances [32]. A resemblance matrix of similarities was calculated using the Bray-Curtis coefficient and the resulting resemblance matrix visualised using Principal Coordinates Analysis (PCO). To test for differences in benthic assemblage over small (hundreds of metres to kilometres) and large (> 100 km) spatial scales, a Permutational Analysis of Variance (PERMANOVA [33]) was performed with location as a fixed factor and sites nested within location. Both a priori and post-hoc comparisons tested for overall and site differences, with significance values determined from 9999 and 999 unrestricted permutations, respectively. All univariate and multivariate analyse were preformed using PRIMER 6 / PERMANOVA+ software.
Community structure comparisons between subtropical reefs and the southern GBR
To compare patterns of community structure between subtropical locations and southern GBR, coral community structure data from high-latitude sites (FR, CI, SIMP, SWR, LHIMP) were aggregated to Family taxonomic level (i.e., Acroporidae, Pocilloporidae, Faviidae, Dendrophylliidae) and compared with southern GBR data (HERON [29]). Abundance data were square-root transformed and PCO biplots visually displayed the resemblance data. To determine which taxonomic groups contributed most to patterns of community structure, eigenvectors of the variables (taxonomic groups) were overlaid onto the resultant PCO biplot.
Decadal patterns of change in subtropical benthic assemblages
Subtropical benthic and coral assemblage data were compared with data collected during the 1990s [13,14,15,28] at each of the five subtropical locations (FR, CI, SIMP, SWR, LHIMP) using multivariate procedures. Data collected during this study were combined to common taxa groups, thus enabling direct comparison. PCO was used to visually display the underlying Bray-Curtis resemblance matrix (as detailed in the previous section), and trajectories were overlaid to show relative changes in assemblage composition between decades within each site.
Results
Spatial patterns of benthic and coral community structure
Surveys of subtropical eastern Australian reefs and southern GBR showed that scleractinian corals were present at all locations, with the exception of two sites surveyed at SWR. Mean hard coral cover ranged between 0% at SWR sites and 52.3 ± 5.2% (± SE) at LHIMP (Figure 2). Macroalgal cover was relatively high across all subtropical reefs (16.1 ± 1.9% - 26.8 ± 4.5%), yet almost absent from surveys at the southern GBR sites (0.6 ± 0.1%, HERON). Clear latitudinal trends were observed with Acropora spp. declining with increasing latitude on reefs adjacent to the mainland (Heron to SWR) but not at LHIMP, where Acropora was the dominant coral genus. Pocillopora displayed a clear trend of increasing relative abundance with increasing latitude (Figure 2). In contrast, dendrophyliid species dominated nearshore (< 1 km) and midshelf (< 5 km) reefs.
Figure 2. Latitudinal gradient of sites on the eastern Australian coastline.
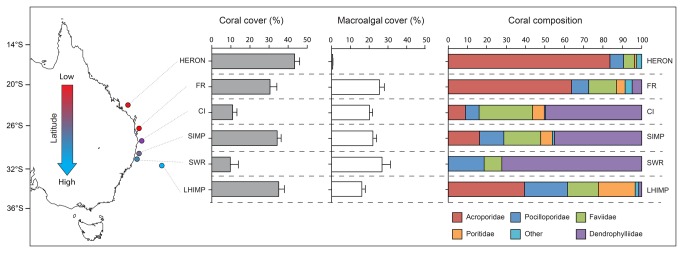
Corresponding latitudinal trends of coral cover, macroalgae and coral composition from sites.
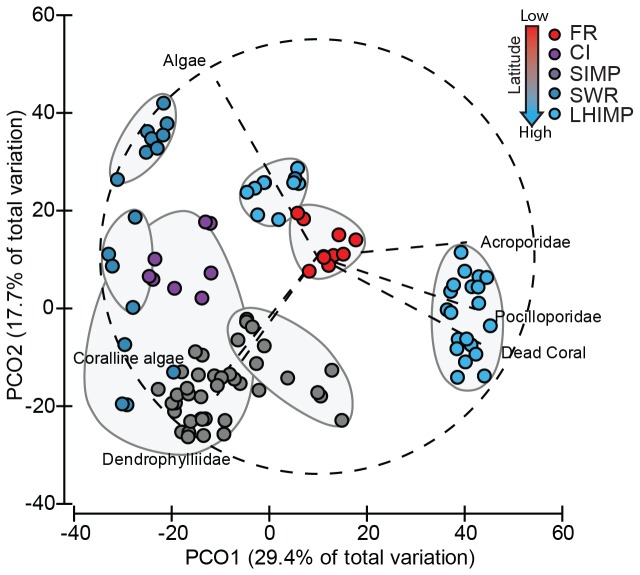
The principal coordinates analysis displayed a clear pattern of benthic community structure among subtropical locations, with the first and second axes explaining 29.4% and 17.7% of the total variation, respectively (Figure 3). Hierarchical cluster analysis of benthic taxa revealed strong clustering in six distinct groups, separated at the 65% similarity level. The coral Families Acroporidae and Pocilloporidae dominated LHIMP lagoon sites. Flinders Reef comprised a suite of coral species, including Isopora spp. , Pocilloporadamicornis and Poritesheronensis , thus orientating these sites toward the centre of the ordination. Bait Reef and Green Island (SWR) appeared distinct in the PCO; this dissimilarity was driven by the presence of high macroalgae cover (Figure 3). In contrast, Black Rock (SWR), most sites within the SIMP and CI grouped towards the bottom of the ordination and were distinguished from the other groups by the dominance of dendrophylliid species. Two-way PERMANOVA indicated that there was a highly significant difference in benthic assemblage between location (F4, 103 = 4.39, P = 0.001, ECV = 38.42%) and between sites within location (F17, 103 = 12.16, P = 0.001, ECV = 43.15%), suggesting that significant variability in community types exists both between and among high-latitude locations.
Figure 3. Principal coordinates analysis of benthic community composition from eastern Australia subtropical locations.
Sites grouped by >65% similarity and Pearson correlation vectors overlaid to display benthic categories that contributed to greater than 70% correlation.
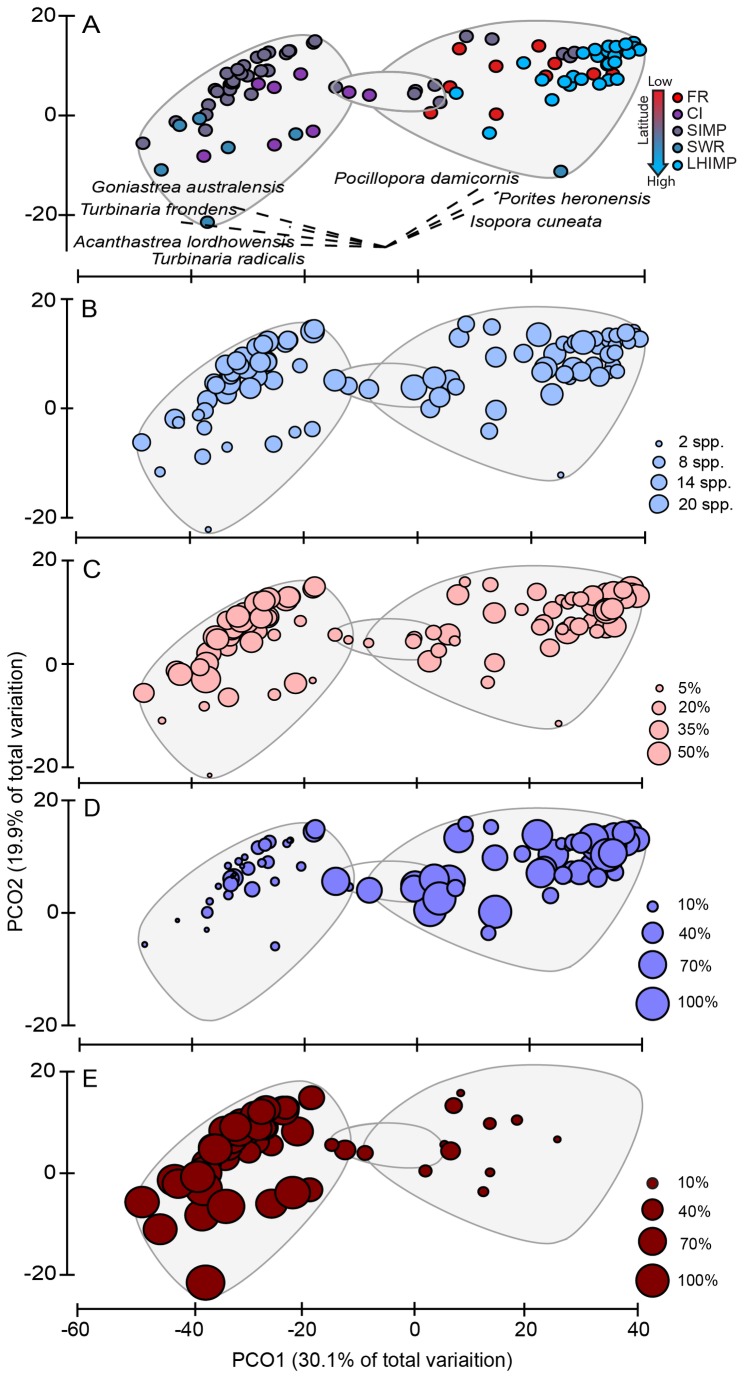
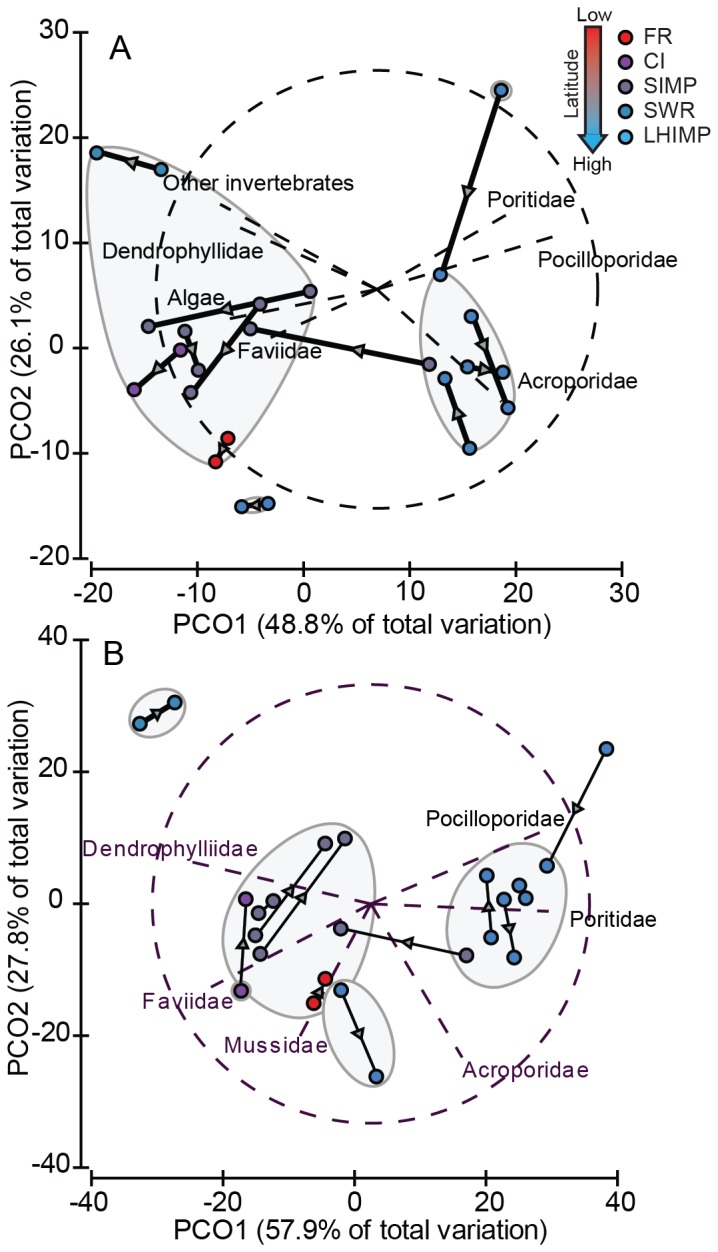
Clear patterns of coral community structure among locations were evident from the PCO (Figure 4A). Similarity cluster analysis of coral taxa showed a strong clustering in four distinct groups. Sites at LHIMP and FR were dominated by Pocilloporadamicornis , Isopora spp. and Poritesheronensis , while CI and SWR were dominated by Turbinaria spp., Goniastreaaustralensis and Acanthastrea species. A third cluster containing transects from CI and SIMP comprised an intermediate mix of coral taxa. Coral community structure at SIMP sites was highly variable, occurring in all three groups. No clear pattern was present within groups for coral generic richness (Figure 4B) nor coral cover (Figure 4C), in that groups with high and low diversity were present across all clusters, despite strong differences in the composition of taxa forming these clusters (Figure 4D, E). Acroporidae dominated the total coral cover at FR and LHIMP lagoon sites (right cluster, Figure 4D), while Dendrophylliidae comprised a high proportion of the coral assemblage in the sites that grouped to the left of the PCO (Figure 4E). Overall there were significant location (F4, 103 = 4.956, P = 0.001, ECV = 40.06%) and highly significant sites within location (F17, 103 = 8.78, P = 0.001, ECV = 37.18%) differences amongst the coral assemblage found on these reefs. Post-hoc analysis revealed significant variability in coral composition between SIMP and all other locations except CI; and between LHIMP and CI and SWR (Table 1).
Figure 4. Principal coordinates analysis (PCO) of subtropical coral community composition along eastern Australia (A).
Bubble plots overlaid onto PCO displaying B) mean coral taxa richness, C) mean percent coral cover, and D) proportion of Acroporidae and E) Dendrophylliidae cover of the total coral cover.
Table 1. Post-hoc results from PERMANOVA, which compared coral community structure between subtropical locations.
| FR | CI | SIMP | SWR | LHIMP | |
|---|---|---|---|---|---|
| FR | X | - | - | - | - |
| CI | ns | X | - | - | - |
| SIMP | * | ns | X | - | - |
| SWR | ns | ns | ** | X | - |
| LHIMP | ns | * | *** | *** | X |
X = no test, ns = not significant, * p < 0.05, ** p < 0.01, *** p < 0.001.
A weak but significant trend across all sites indicated that coral generic richness increased with percent coral cover (R2 = 0.168, F1, 20 = 4.037, p = 0.048; Figure 5). Sites at FR were characterised by high coral cover and mix of low/high diversity. SIMP sites were highly variable in coral cover and diversity. LHIMP lagoon sites tended to be high in coral cover with low diversity, while the LHIMP two deeper sites were characterised by low cover/high diversity and low cover/low diversity (Figure 5). CI and SWR sites were generally low in coral cover and diversity.
Figure 5. The relationship between relative coral taxa richness and coral cover recorded from replicate video transects recorded from subtropical reefs.
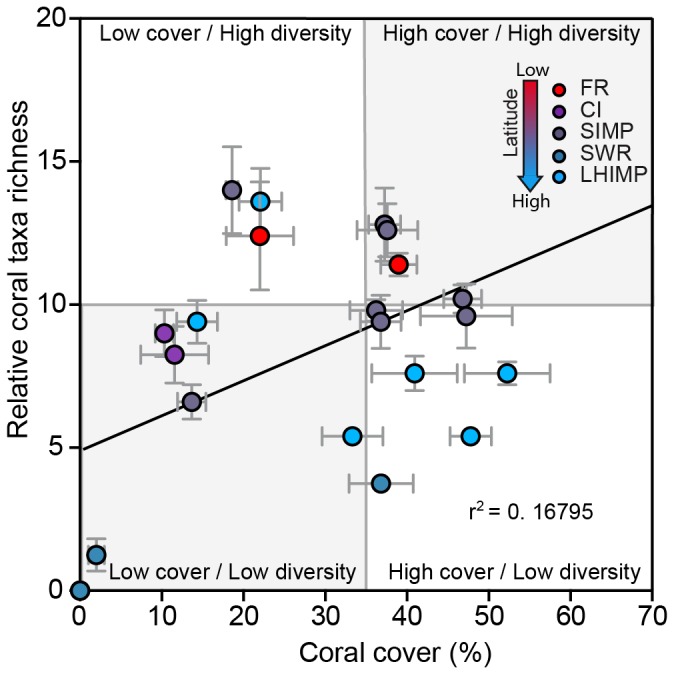
Line represents a goodness-of-fit relationship based on linear regression model.
Community structure comparisons between subtropical reefs and the southern GBR
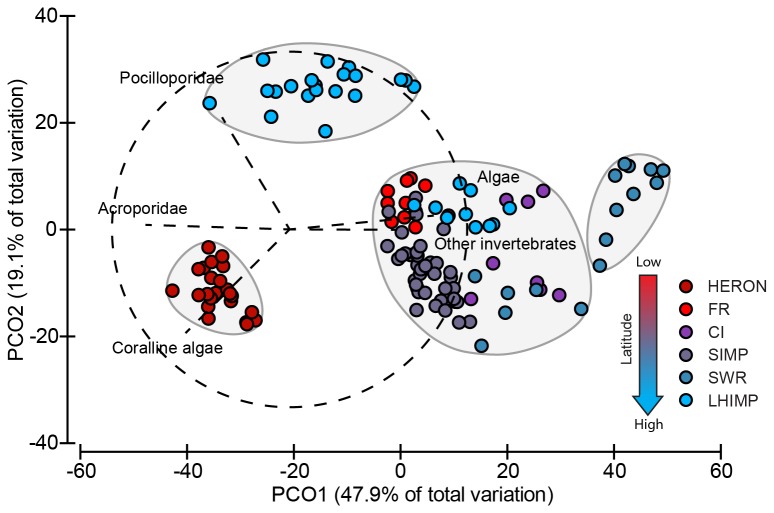
The first axis of the PCO for the benthic community resemblance matrix (Figure 6) explained 47.9% of the total variation, while the second axis explained 19.1%. Hierarchical cluster analysis revealed four distinct groups at the 65% resemblance level (Figure 6), separating HERON, LHIMP lagoon sites and SWR. HERON groups clustered towards vectors of coralline algae and Acroporidae, whereas LHIMP lagoon sites orientation demonstrated a dominance of Pocilloporidae. In contrast, SWR clustered strongly to the opposite side, dominated by other invertebrates and algae. The remaining subtropical sites formed the fourth group representing a similar centroid mix of community types.
Figure 6. Principal coordinates analysis of the benthic community resemblance matrix compiled from reefs extending from southern GBR (Heron Reef) to subtropical (FR, CI, SIMP, SWR and LHIMP) locations.
Main taxa vectors (dotted lines) that contributed greater than 75% correlation displayed and sites replicates grouped according to 75% similarity.
PERMANOVA analysis indicated a significant effect for location and sites nested within location, which together explained the largest component of variance (28.76% and 26.89% ECV, respectively). Distance from mainland (18.35%) and latitude (17.71%) were also significant, but contributed to a lesser extent (Table 2). Post-hoc analysis of location indicates that HERON was significantly different from all subtropical sites (Table 3). SIMP was also significantly different from all other sites (Table 3). FR, CI and SWR were not significantly different from each other. LHIMP was significantly different from all sites except FR (Table 3).
Table 2. PERMANOVA results of benthic community structure.
| Source | df | SS | MS | Pseudo-F | P(perm) | perms | ECV |
|---|---|---|---|---|---|---|---|
| Latitude | 1 | 31104 | 31104 | 22.55 | 0.001 | 999 | 17.71 |
| Distance | 1 | 27264 | 27264 | 15.017 | 0.001 | 999 | 18.35 |
| Location | 5 | 25344 | 5068.8 | 3.2763 | 0.002 | 999 | 28.76 |
| Sites (Location) | 23 | 35904 | 1561.1 | 14.254 | 0.001 | 999 | 26.89 |
| Res | 96 | 10514 | 109.52 | 8.29 | |||
| Total | 126 | 1.30E+05 |
Latitude and distance from mainland as covariates, site as a random factor nested in location, location as a fixed factor.
Table 3. Post-hoc results from PERMANOVA of benthic community structure between locations.
| HR | FR | CI | SIMP | SWR | LHIMP | |
|---|---|---|---|---|---|---|
| HR | X | - | - | - | - | - |
| FR | * | X | - | - | - | - |
| CI | *** | ns | X | - | - | - |
| SIMP | *** | * | * | X | - | - |
| SWR | *** | ns | ns | *** | X | - |
| LHIMP | *** | ns | * | *** | *** | X |
X = no test, ns = not significant, * p < 0.05, ** p < 0.01, *** p < 0.001.
Decadal patterns in subtropical community structure
The principle coordinates analysis of decadal changes in benthic and coral community composition resemblance matrices revealed that the first two eigenvectors accounted for 75% and 86% of the total similarities, respectively (Figure 7A, B). Cluster analysis indicated four distinct groups at the 75% similarity level for both data sets, indicating few differences among sites. PERMANOVA also revealed no significant decadal effect on both benthic and coral pattern of assemblage, during a period when seawater temperatures have risen between 0.15 and 0.24°C per decade over the past 30 years (Table S1). As shown by the site trajectories, only two sites deviated from the 75% similarity level (Figure 7A, B). One LHIMP site moved from a Poritidae/Pocilloporidae dominance to an Acroporidae dominance, while one SIMP site moved from Acroporidae dominance to macroalgae/Faviidae dominance. The remaining sites remained stable through time.
Figure 7. Decadal patterns of change in A) benthic and B) coral assemblages at five subtropical locations between the 1990s and 2000s.
Sites compared included: FR – data pooled from two sites, Mooring 3 and Laurieland; CI – data pooled from two sites, North and South; SIMP – North, North West, South West and Split Solitary islands; SWR – Black Rock; and LHIMP – North Bay, Stephen’s Hole, Comet’s Hole, Erscott’s Hole and Malabar Reef.
Discussion
Latitudinal variation in benthic community composition
Our results revealed significant regional differences in benthic community composition across a large latitudinal scale ranging from tropical to subtropical reef communities. Significant difference in community composition was found over small spatial scales (< 1 km), but our results also indicate remarkable similarities between locations that were separated by hundreds of kilometres. For example, the benthic community at FR (high cover of Acropora spp.) was more similar to some LHIMP sites than the geographically closer CI assemblage. In contrast, the benthic assemblage at CI was dominated by marine algae and was similar to LHIMP deeper and SIMP sites. This resemblance may be due to a number of physical factors including wave exposure and nutrient availability at these locations.
Benthic communities at CI, which is located approximately 4 km south east of a large coastal river (Tweed River), are regularly exposed to terrigenous discharge following storm events. The direction of the freshwater plume into the marine environment is determined by the strength of local wind, wave action and current direction, particularly the East Australian Current (EAC), which generally deflects riverine waters from the Tweed River to the south [34,35]. Additionally, the combination of the southward flowing EAC and the narrowing of the NSW continental shelf results in the formation of thermal fronts along the nearshore zone adjacent to Tweed Heads, Cape Byron and South West Rocks [36,37]. These fronts regularly transport cool upwelled, nutrient-rich waters in a northward direction, resulting in highly productive regions, and facilitate phytoplankton and macroalgal growth. Similarly, exposed reef communities at LHIMP are regularly subjected to dominant south to south-easterly sea conditions. This generates a dynamic environment with a constant supply of nutrients from northward flowing waters and upwellings associated with the Lord Howe Seamount [38].
Harriott and Banks [11] suggested that increased nutrients from periodic pulses of terrigenous discharge and upwelling events, stimulate algal growth along the NSW coast. Collectively, these factors may limit coral settlement while enhancing the growth of macroalgae and other competing sessile invertebrates. These competing organisms potentially affect coral calcification and growth (through bioerosion), limit coral recruitment (competition for space) and reduce the survival of new coral recruits, producing sub-optimal conditions for coral and reef growth in high-latitude reef environments on the eastern Australian coastline.
In high-latitude environments, exposure and wave energy play a significant role in structuring coral assemblages [39,40]. Along the northern NSW coast, wave height of the dominant south easterly seas can often exceed 6 m during unstable climatic conditions, with a maximum height of > 13 m being recorded in the past 10 years [41]. Previous studies have demonstrated a strong correlation between wave energy and coral distribution [42,43]. Jokiel et al. [44] demonstrated that coral cover, diversity and species richness in Hawaii were all negatively correlated with maximum wave height and wave direction. Considering the potential effects of such extreme wave exposure, further studies of small-scale wave exposure gradients would enable a clearer understanding of disturbance regimes and the distribution of benthic communities on subtropical reefs in eastern Australia.
Subtropical reef coral community composition
Our results indicated that between location variability in coral community composition (40.06%) was greater than the variation within locations (37.18%). Such local-scale variability indicates considerable heterogeneity in high-latitude coral assemblages. For example, significant differences in community structure were observed among sites at SWR, despite the spatial separation of less than 4 km. The benthic community at Black Rock (SWR) was characterised by relatively high coral cover and diversity, which is more similar to sites at SIMP (70-100 km away) than it is to nearby sites within the same location (SWR). This may reflect local-scale variability in recruitment patterns, or the effects of small-scale variability in exposure and wave energy in structuring coral assemblages in high-latitude reefs. Our results also indicated that coral species richness at SWR was significantly lower than at all other locations. This abrupt decline in coral cover in combination with a significant reduction in species richness south of the SIMP may indicate the limited or sporadic connection between SWR and northern locations or represents the current biogeographical environmental limits of many subtropical and tropical coral species [11].
The higher abundance of Acropora spp. at NSI compared to other reefs in the SIMP may be due to the increased influence of the southward flowing EAC, which has been hypothesised to carry broadcast spawning Acropora coral larvae from northern reefs such as FR [45]. Seawater temperatures at NSI tends to be 1-2°C above those recorded at the midshelf islands of SIMP [46], which suggests a greater influence of the EAC and potentially provides a more suitable recruitment temperature for Acropora species arriving from northerly reefs. Wilson and Harrison [47] observed asynchronous spawning of 24 coral species at the northern islands in the SIMP and suggested the potential for successful recruitment of planulae larvae during favourable oceanic conditions. However, they indicated that recruitment at high-latitude reefs is restricted by the limited release of gametes, reduced fertilisation rates, and the sporadic nature of the dominant currents, which may restrict settlement of NSI spawned corals further south.
Community structure comparisons between subtropical reefs and the southern GBR
Our results indicate clear differences in high-latitude subtropical community structure compared to the southern GBR site (Heron Reef). Multivariate correlation analysis showed a significant difference between southern GBR and high-latitude sites, with latitude, distance from shore, location and sites within location all having significant influence. That site nested within location was significant, even with latitude as a covariate within our model, suggests that other site-specific factors independent from latitude may be controlling the pattern of benthic community structure across sites. Our results indicate that location contributed greatest to the percent estimated components of variation, suggesting a strong difference between locations and a break in assemblage composition between southern GBR and locations further south.
Heron Reef sites comprised a high coral cover (mainly branching and tabular Acropora spp.) and coralline algae, with a virtual absence of macroalgae, which is typical of Indo-Pacific coral reefs [48]. The high cover of coralline algal cover recorded at Heron Reef may promote coral recruitment at this location [49], while the high rates of herbivory controls the levels of macroalgae and turf algae cover [50]. In contrast, high-latitude locations further south were characterised by lower coral cover and high macroalgal cover, with low abundance of coralline algae. Such high levels of macroalgal cover are likely to have a significant negative effect on the recruitment of corals, as macroalgae strongly suppress coral recruitment rates (e.g. [51,52]). Indeed, studies of coral recruitment at high-latitude sites (e.g. SIMP) recorded up to 35 times lower rates of coral recruitment compared to sites on the GBR [53]. This difference was primarily attributed to reduced light levels, low herbivore abundance, high macroalgal cover, and greater competition for settlement space with other benthic organisms that are more abundant at temperate locations (e.g. sponges and ascidians). Such consistently high levels of macroalgal cover and low rates of coral recruitment at high-latitude sites will likely have significant negative implications for the potential of these reefs to function as coral refugia (particularly for tropical coral species) under future climate change scenarios.
Decadal patterns in subtropical community structure
Comparisons between this study and research completed during the early 1990s revealed that benthic and coral assemblages have remained stable in recent times, during a period when high-latitude reefs have been exposed to disease epizootics, bleaching conditions and storm events [25,26,54,55], and increases in sea surface temperatures of 0.19-0.24 °C per decade (Table S1). Despite high thermal stress at the locations recorded in the present study during the study period [24,56], benthic assemblages remained remarkably stable. Environmental factors governing high-latitude locations such as upwelling, strong currents, cloud cover, and light attenuation with increasing depth, have been reported to reduce the impact of thermal and light stress during bleaching events. These extrinsic factors appear to be acting independently or synergistically to mitigate conditions that induce thermal stress in high-latitude coral communities. This hypothesis is supported by a study of two subtropical regions (Bahamas and South Africa [57]), where cooler upwelled water minimised the effects of thermal stress on coral communities during the 1998 global mass bleaching event. Additionally, the authors proposed that adjacent deeper reefs would also provide a local larval supply to replenish damaged shallow areas following bleaching events [57].
Local upwelling and deep reefs are common features of benthic habitats along the coast of northern NSW, particularly throughout the SIMP. Recent swath mapping of seabed habitats has found reefs extending from mainland Australia to the continental shelf, and hard corals are a dominant component of midshelf exposed reefs between depths of 10-25 metres at 30° S [58]. Further genetics work on the connectivity of this region is needed to determine whether these deeper reefs may provide a refugium by acting as a local larval source for coral recruitment to shallower reefs following acute disturbances. Despite such potential for refugia during times of stress, intrinsic factors, such as limited genetic diversity in subtropical coral populations [59,60,61], reduced local reproductive capacity [47,62,63] and sporadic long-distance recruitment [53] may reduce the ability of subtropical coral communities to successfully recover from future chronic or catastrophic disturbances that result in a significant loss of coral cover.
The relative stability of subtropical coral communities at the sites studied contrasts the current trend of coral decline at tropical locations; approximately 20% and 80% of hard coral cover loss has occurred on Indo-Pacific and the Caribbean reefs, respectively [4,64,65]. This consistency of coral cover over the past 10-15 years on subtropical eastern Australian reefs, despite increases in sea surface temperature and the frequency of extreme warm events (Table S1 [16]) may indicate that these subtropical communities are resilient to perturbations associated with global climate change (e.g. bleaching and disease outbreaks), or that environmental change associated with climate change has not yet led to a decline in coral cover within this region. This subtropical region represents an area where a history of naturally variable environmental conditions (e.g. constant temperature fluctuation) may have led to greater resistance to elevated temperatures over geological time, and further investigation into these marginal coral communities is warranted.
Supporting Information
Average changes in coastal sea surface temperatures (SST) between 1982 and 2010 at study locations (data for Gladstone, Flinders Reef, Cook Island, Solitary Islands, South West Rocks from Lima & Wethey 2012). Average SST change per decade for Lord Howe Island was calculated from NOAA OI 1⁄4 Degree Daily SST Analysis data following the methods of Lima & Wethey (2012).
(DOCX)
Acknowledgments
We would like to thank all the people who contributed to data collection, particularly, Mathew Harrison, Andrew Carroll and Scott Godwin. Finally, thanks are extended to Steve Smith who provided access to past benthic data and gave constructive comments on early drafts. We thank the anonymous reviewers for providing comments that have improved the manuscript.
Funding Statement
Funding for data collection was provided by the first author's university (University of New England) during his PhD candidature, with some contribution by the Northern Rivers Catchment Management Authority, NSW. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Carpenter KE, Abrar M, Aeby G, Aronson RB, Banks S et al. (2008) One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science 321: 560-562. doi:10.1126/science.1159196. PubMed: 18653892. [DOI] [PubMed] [Google Scholar]
- 2. Baker AC, Glynn PW, Riegl B (2008) Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook. Estuarine Coast Shelf Sci 80: 435-471. doi:10.1016/j.ecss.2008.09.003. [Google Scholar]
- 3. Glynn PW (1984) Widespread coral mortality and the 1982-1983 El Nino warming event. Environ Conserv 11: 133-146. doi:10.1017/S0376892900013825. [Google Scholar]
- 4. Bruno JF, Selig ER (2007) Regional Decline of Coral Cover in the Indo-Pacific: Timing, Extent, and Subregional Comparisons. PLOS ONE 2: e711. doi:10.1371/journal.pone.0000711. PubMed: 17684557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mumby PJ, Hastings A, Edwards HJ (2007) Thresholds and the resilience of Caribbean coral reefs. Nature 450: 98-101. doi:10.1038/nature06252. PubMed: 17972885. [DOI] [PubMed] [Google Scholar]
- 6. Hughes TP, Graham NAJ, Jackson JBC, Mumby PJ, Steneck RS (2010) Rising to the challenge of sustaining coral reef resilience. Trends Ecol Evol 25: 633-642. doi:10.1016/j.tree.2010.07.011. PubMed: 20800316. [DOI] [PubMed] [Google Scholar]
- 7. Obura DO (2005) Resilience and climate change: lessons from coral reefs and bleaching in the Western Indian Ocean. Estuarine Coast Shelf Sci 63: 353-372. doi:10.1016/j.ecss.2004.11.010. [Google Scholar]
- 8. Veron JEN, How RA, Done TJ, Zell LD, Dodkin MJ et al. (1974) Corals of the Solitary Islands, Central New South Wales. Aust J Mar Freshw Res 25: 193-208. doi:10.1071/MF9740193. [Google Scholar]
- 9. Woodroffe CD, Kennedy DM, Dickson ME (2006) Geomorphological evolution of Lord Howe Island and carbonate production at the latitudinal limit to reef growth. J Coast Res 22: 188-201. [Google Scholar]
- 10. Veron JEN, Done TJ (1979) Corals and coral communities of Lord Howe Island. Australian Journal of Freshwater Research 30: 203-236. doi:10.1071/MF9790203. [Google Scholar]
- 11. Harriott VJ, Banks SA (2002) Latitudinal variation in coral communities in eastern Australia: a quantitative biophysical model of factors regulating coral reefs. Coral Reefs 21: 83-94. [Google Scholar]
- 12. De’ath G, Fabricius KE, Sweatman H, Puotinen M (2012) The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc Natl Acad Sci USA 109: 17995-17999. doi:10.1073/pnas.1208909109. PubMed: 23027961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harriott VJ, Harrison PL, Banks SA (1995) The coral communities of Lord Howe Island. Mar Freshw Res 46: 457-465. doi:10.1071/MF9950457. [Google Scholar]
- 14. Harriott VJ, Smith SDA, Harrison PL (1994) Patterns of coral community structure of subtropical reefs in the Solitary Islands Marine, Reserve, eastern Australia. Mar Ecol Prog S 109: 67-76. doi:10.3354/meps109067. [Google Scholar]
- 15. Harrison PL, Harriott VJ, Banks SA, Holmes NJ (1998) The coral communities of Flinders Reef and Myora Reef in the Moreton Bay Marine Park, Queensland, Australia; Tibbetts LR, Hall NJ, Dennison WC. Brisbane: The University of Queensland. [Google Scholar]
- 16. Lima FP, Wethey DS (2012) Three decades of high-resolution coastal sea surface temperatures reveal more than warming. Nature Communications 3: 704 PubMed: 22426225. [DOI] [PubMed] [Google Scholar]
- 17. Beger M, Babcock R, Booth DJ, Bucher D, Condie SA et al. (2011) Research challenges to improve the management and conservation of subtropical reefs to tackle climate change threats. Ecol Manag Restor 12: e7-e10. doi:10.1111/j.1442-8903.2011.00573.x. [Google Scholar]
- 18. Baird AH, Sommer B, Madin JS (2012) Pole-ward range expansion of Acropora spp. along the east coast of Australia. Coral Reefs 31: 1063. doi:10.1007/s00338-012-0928-6. [Google Scholar]
- 19. Greenstein BJ, Pandolfi JM (2008) Escaping the heat: range shifts of reef coral taxa in coastal Western Australia. Glob Change Biol 14: 513-528. doi:10.1111/j.1365-2486.2007.01506.x. [Google Scholar]
- 20. Kiessling W, Simpson C, Beck B, Mewis H, Pandolfi JM (2012) Equatorial decline of reef corals during the last Pleistocene interglacial. Proc Natl Acad Sci USA 109: 21378-21383. doi:10.1073/pnas.1214037110. PubMed: 23236154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoey AS, Pratchett MS, Cvitanovic C (2011) High macroalgal cover and low coral recruitment undermines the potential resilience of the world’s southernmost coral reef assemblages. PLOS ONE 6: e25824. doi:10.1371/journal.pone.0025824. PubMed: 21991366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wallace CC, Rosen BR (2006) Diverse staghorn corals (Acropora) in high-latitude Eocene assemblages: implications for the evolution of modern diversity patterns of reef corals. Proceedings of the Royal Society B: Biological Sciences 273: 975-982. doi:10.1098/rspb.2005.3307. PubMed: 16627283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harriott VJ (1995) Is the crown-of-thorns starfish a threat to the reefs of Lord Howe Island? Aquat Conserv Mar Freshw Ecosyst 5: 179-190. doi:10.1002/aqc.3270050303. [Google Scholar]
- 24. Dalton SJ, Carroll AG (2011) Monitoring coral health to determine coral bleaching response at high latitude eastern Australian reefs: an applied model for a changing climate. Diversity 3: 592-610. doi:10.3390/d3040592. [Google Scholar]
- 25. Dalton SJ, Godwin S, Smith SDA, Pereg L (2010) Australian subtropical white syndrome: a transmissible, temperature-dependent coral disease. Mar Freshw Res 61: 342-350. doi:10.1071/MF09060. [Google Scholar]
- 26. Dalton SJ, Harrison M, Carroll AG, Smith SDA, Pereg L et al. (2010) Spatial and temporal patterns of Australian subtropical white syndrome at eastern Australian reefs: host range, prevalence and progression of tissue necrosis. In: Albin H, Borg G. Handbook of disease outbreaks: prevention, detection and control. New York: Nova Science Publishers Inc. pp. 187-210. [Google Scholar]
- 27. Harrison PL, Dalton SJ, Carroll AG (2011) Extensive coral bleaching on the world’s southernmost coral reef at Lord Howe Island, Australia. Coral Reefs 30: 775. doi:10.1007/s00338-011-0778-7. [Google Scholar]
- 28. Harriott VJ, Banks SA, Mau RL, Richardson D, Roberts LG (1999) Ecological and conservation significance of the subtidal rocky reef communities of northern New South Wales, Australia. Mar Freshw Res 50: 299-306. doi:10.1071/MF98042. [Google Scholar]
- 29. Roff G, Kvennefors ECE, Fine M, Ortiz J, Davy JE et al. (2011) The ecology of "Acroporid White Syndrome", a coral disease from the southern Great Barrier Reef. PLOS ONE 6: e26829. doi:10.1371/journal.pone.0026829. PubMed: 22163267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harriott VJ, Harrison PL, Banks SA (1995) The coral communities of Lord Howe Island. Mar Freshw Res 46: 457-465. doi:10.1071/MF9950457. [Google Scholar]
- 31. Kohler KE, Gill SM (2006) Coral ,Point Count with Excel extensions (CPCe): A Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comput Geosci 32: 1259-1269. doi:10.1016/j.cageo.2005.11.009. [Google Scholar]
- 32. Clarke KR, Warwick RM (2001) Changes in marine communities: An approach to statistical analysis. 2nd edition UK: PRIMER-E Ltd. [Google Scholar]
- 33. Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: Guide to software and statistical methods. Plymouth, UK: PRIMER-E.. [Google Scholar]
- 34. Davies PL (2005) An evaluation of applications of satellite imagery in the study of coastal ecosystems [Ph.D]. Lismore: Southern Cross University. 261pp. [Google Scholar]
- 35. Rule MJ, Jordan A, McIlgorm A (2007) The marine environment of northern New South Wales: A review of current knowledge and existing datasets. Coffs Harbour: National Marine Science Centre. [Google Scholar]
- 36. Oke PR, Middleton JH (2000) Topographically induced upwelling off eastern Australia. J Phys Oceanogr 30: 512-531. doi:10.1175/1520-0485(2000)030. [Google Scholar]
- 37. Roughan M, Middleton JH (2004) On the East Australian Current: Variability, encroachment, and upwelling. J Geophys Res Oceans 109: C07003. doi:10.1029/2003JE002224. [Google Scholar]
- 38. Environment Australia (2002) Report on the state of conservation of Lord Howe Island Canberra: Environment Australia; 28 p. [Google Scholar]
- 39. Jokiel PL, Brown EK (2004) Global warming, regional trends and inshore environmental conditions influence coral bleaching in Hawaii. Glob Change Biol 10: 1627-1641. doi:10.1111/j.1365-2486.2004.00836.x. [Google Scholar]
- 40. Dollar SJ (1982) Wave stress and coral communities structure in Hawaii. Coral Reefs 1: 71-81. doi:10.1007/BF00301688. [Google Scholar]
- 41. Manly Hydraulics Laboratory (2007) NSW ocean wave data collection program NSW Department of Commerce. Available: http://mhl.nsw.gov.au/www/owave.htmlx. Accessed 17 April 2007.
- 42. Dollar SJ, Grigg RW (2004) Anthropogenic and natural stresses on selected coral reefs in Hawaii: A multidecade synthesis of impact and recovery. Pac Sci 58: 281-304. doi:10.1353/psc.2004.0016. [Google Scholar]
- 43. Storlazzi CD, Brown EK, Field ME, Rodgers K, Jokiel PL (2005) A model for wave control on coral breakage and species distribution in the Hawaiian Islands. Coral Reefs 24: 43-55. doi:10.1007/s00338-004-0430-x. [Google Scholar]
- 44. Jokiel PL, Brown EK, Friedlander A, Ku’ulei Rodgers S, Smith WR (2004) Hawaii coral reef assessment and monitoring program: spatial patterns and temporal dynamics in reef coral communities. Pac Sci 58: 159-174. doi:10.1353/psc.2004.0018. [Google Scholar]
- 45. Wilson JR, Harrison PL (1998) Settlement-competency periods of larvae of three species of scleractinian corals. Mar Biol Berl 131: 339-345. doi:10.1007/s002270050327. [Google Scholar]
- 46. Dalton SJ (2009) Stressors of eastern Australian subtropical corals: Australian subtropical white syndrome and coral bleaching [Ph.D.]. Armidale, NSW: University of New England. 191pp. [Google Scholar]
- 47. Wilson JR, Harrison PL (2003) Spawning patterns of scleractinian corals at the Solitary Islands - a high latitude coral community in eastern Australia. Mar Ecol Prog S 260: 115-123. doi:10.3354/meps260115. [Google Scholar]
- 48. Roff G, Mumby PJ (2012) Global disparity in the resilience of coral reefs. Trends Ecol Evol 27: 404-413. doi:10.1016/j.tree.2012.04.007. PubMed: 22658876. [DOI] [PubMed] [Google Scholar]
- 49. Connell J, Hughes TP, Wallace CC (1997) A 30-year study of coral abundance, recruitment, and disturbance at several scales in space and time. Ecol Monogr 67: 461-488. doi:10.1890/0012-9615(1997)067[0461:AYSOCA]2.0.CO;2. [Google Scholar]
- 50. Marshell PA, Mumby PJ (2012) Revisiting the functional roles of the surgeonfish Acanthurus nigrofuscus and Ctenochaetus striatus . Coral Reefs 31: 1093-1101. doi:10.1007/s00338-012-0931-y. [Google Scholar]
- 51. Birrell CL, McCook LJ, Willis BL (2005) Effects of algal turfs and sediment on coral settlement. Mar Pollut Bull 51: 408-414. doi:10.1016/j.marpolbul.2004.10.022. PubMed: 15757739. [DOI] [PubMed] [Google Scholar]
- 52. Birrell CL, McCook LJ, Willis BL (2008) Chemical effects of macroalgae on larval settlement of the broadcast spawning coral Acropora millepora . Mar Ecol Prog S 362: 129-137. doi:10.3354/meps07524. [Google Scholar]
- 53. Harriott VJ, Banks SA (1995) Recruitment of scleractinian corals in the Solitary Islands Marine, Reserve, a high latitude coral-dominated community in eastern Australia. Mar Ecol Prog S 123: 155-161. doi:10.3354/meps123155. [Google Scholar]
- 54. Dalton SJ, Smith SDA (2006) Coral disease dynamics at a subtropical location, Solitary Islands Marine, Park, eastern Australia. Coral Reefs 25: 37-45. doi:10.1007/s00338-005-0039-8. [Google Scholar]
- 55. Edgar RJ, Malcolm H, Dalton S (2003) Coral bleaching in the Solitary Islands Marine, Park, NSW. Technical data report. Coffs Harbour NSW: NSW Marine Parks Authority. 41pp. [Google Scholar]
- 56. Malcolm HA, Davies PL, Jordan A, Smith SDA (2011) Variation in sea temperature and the East Australian Current in the Solitary Islands region between 2001-2008. Deep Sea Res II Topical Stud Oceanogr 58: 616-627. doi:10.1016/j.dsr2.2010.09.030. [Google Scholar]
- 57. Riegl B, Piller WE (2003) Possible refugia for reefs in times of environmental stress. Int J Earth Sci 92: 520-531. doi:10.1007/s00531-003-0328-9. [Google Scholar]
- 58. NSWMPA (2010) Seabed habitat mapping in the Solitary Islands Marine Park and Jervis Bay Marine Park. Hurstville: 58 p. [Google Scholar]
- 59. Ayre DJ, Hughes TP (2004) Climate change, genotypic diversity and gene flow in reef-building corals. Ecol Lett 7: 273-278. doi:10.1111/j.1461-0248.2004.00585.x. [Google Scholar]
- 60. Miller KJ, Ayre DJ (2008) Protection of genetic diversity and maintenance of connectivity among reef corals within marine protected areas. Conserv Biol 22: 1245-1254. doi:10.1111/j.1523-1739.2008.00985.x. PubMed: 18637917. [DOI] [PubMed] [Google Scholar]
- 61. Noreen AME, Harrison PL, Van Oppen MJH (2009) Genetic diversity and connectivity in a brooding reef coral at the limit of its distribution. Proc R Soc Lond B 276: 3927-3935. doi:10.1098/rspb.2009.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wilson JR, Harrison PL (2005) Post-settlement mortality and growth of newly settled reef corals in a subtropical environment. Coral Reefs 24: 418-421. doi:10.1007/s00338-005-0033-1. [Google Scholar]
- 63. Harriott VJ (1992) Recruitment patterns of scleractinian corals in an isolated sub-tropical reef system. Coral Reefs 11: 215-219. doi:10.1007/BF00301996. [Google Scholar]
- 64. Côté IM, Gill JA, Gardner TA, Watkinson AR (2005) Measuring coral reef decline through meta-analyses. Philosophical. Transactions of the Royal Society of London Biological Sciences 360 pp. 385-395. PubMed: 15814352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gardner TA, Côté IM, Gill JA, Grant A, Watkinson AR (2003) Long-term region-wide declines in Caribbaean corals. Science 301: 958-960. doi:10.1126/science.1086050. PubMed: 12869698. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Average changes in coastal sea surface temperatures (SST) between 1982 and 2010 at study locations (data for Gladstone, Flinders Reef, Cook Island, Solitary Islands, South West Rocks from Lima & Wethey 2012). Average SST change per decade for Lord Howe Island was calculated from NOAA OI 1⁄4 Degree Daily SST Analysis data following the methods of Lima & Wethey (2012).
(DOCX)