Abstract
Background
The aim of the study was to evaluate the predictive value of genes involved in the action of cisplatin-etoposide in Small Cell Lung Cancer (SCLC).
Methods
184 SCLC patients’ primary tumour samples were analyzed for ERCCI, BRCA1, ATP7B, PKM2 TOPOI, TOPOIIA, TOPOIIB and C-MYC mRNA expression. All patients were treated with cisplatin-etoposide.
Results
The patients’ median age was 63 years and 120 (65%) had extended stage, 75 (41%) had increased LDH serum levels and 131 (71%) an ECOG performance status was 0-1. Patients with limited stage, whose tumours expressed high ERCC1 (p=0.028), PKM2 (p=0.046), TOPOI (p=0.008), TOPOIIA (p=0.002) and TOPOIIB (p<0.001) mRNA had a shorter Progression Free Survival (PFS). In limited stage patients, high expression of ERCC1 (p=0.014), PKM2 (p=0.026), TOPOIIA (p=0.021) and TOPOIIB (p=0.019) was correlated with decreased median overall survival (mOS) while in patients with extended stage, only high TOPOIIB expression had a negative impact on Os (p=0.035). The favorable expression signature expression signature (low expression of ERCC1, PKM2, TOPOIIA and TOPOIIB) was correlated with significantly better PFS and Os in both LS-SCLC (p<0.001 and p=0.007, respectively) and ES-SCLC (p=0.007 and (p=0.011, respectively) group. The unfavorable expression signature was an independent predictor for poor PFS (HR: 3.18; p=0.002 and HR: 3.14; p=0.021) and Os (HR: 4.35; p=0.001and HR: 3.32; p=0.019) in both limited and extended stage, respectively.
Conclusions
Single gene’s expression analysis as well as the integrated analysis of ERCC1, PKM2, TOPOIIA and TOPOIIB may predict treatment outcome in patients with SCLC. These findings should be further validated in a prospective study.
Introduction
Small cell lung cancer (SCLC) is a biologically complex malignancy with treatment that has remained largely unchanged over the last 30 years [1]. Although the proportion of patients with SCLC has been decreased, it still accounts for approximately 15% of all lung cancer cases [2]. SCLC is characterized by early dissemination, and 70%–80% of patients have metastatic disease at diagnosis (extensive stage, ES-SCLC) while the rest present with disease limited to the thorax (limited-stage, LS-SCLC) [3]. The standard of care for SCLC is platinum-based combination chemotherapy, using either etoposide or irinotecan, in association with radiotherapy in limited stage disease (LS-SCLC). There is a typical disease trajectory with an initial good clinical response followed by rapid relapse of chemoresistant tumour and death [4]. Because surgical resection is not part of standard care, SCLC research using primary tumour tissue is limited to small diagnostic samples and, thus, the therapeutic benefits from the molecular analysis of this tumours are limited during the last 30 years [5].
Theoretically, the molecular characteristics obtained from the primary tumours have the potential to affect therapeutic decisions and to allow clinicians to select chemotherapy drugs that will give patients maximal benefit while simultaneously minimize toxicity [6]. Primary and/or acquired resistance to chemotherapy or radiotherapy is the main cause of poor outcome in patients with lung cancer [7,8]. Over the last years a growing body of evidence, from gene expression, mutational and proteomic profiling studies, as well as from in vitro models led to the identification of molecular markers related with resistance to platinum analogs and topoisomerase inhibitors [9].
The Excision Repair Cross Complementation group 1 (ERCC1) is an enzyme which plays a key role in the GG-Nucleotide Excision Repair (GG-NER) pathway which repairs DNA adducts and other DNA helix-distorting lesions including those associated with cisplatin administration [10]. The majority of studies on ERCC1 and cisplatin, in NonSmall Cell Lung Cancer (NSCLC) as well as in other solid tumours, share in common the finding that low-ERCC1 expression is associated with a better response to platinum-based chemotherapy [11-13]. On the other hand, the results of in vitro studies have suggested the superiority of TC-NER pathway, in which breast cancer susceptibility gene 1 (BRCA1) protein is involved, to GG-NER pathway in predicting platinum resistance [14]. Modulation of BRCA1 expression leads to modification of TC-NER and, hence, to radio- and chemo-resistance [15,16]. BRCA1 is also involved in homologous recombination repair (HRR) and non-homologous end joining (NHEJ), in response to DNA damage and may be a regulator of mitotic spindle assembly, as BRCA1 and b-tubulin co-localize to the microtubules of the mitotic spindle and to the centrosomes [17,18]. In addition, recent data have shown that import and export transporters involved in maintenance of copper homeostasis are also involved in the transport of cisplatin [19]. Especially, overexpression of the P-type transporter ATP7B conferred cisplatin resistance associated with decreased intracellular accumulation of cisplatin and carboplatin [20]. Also, the role of pyruvate kinase isoform M2 (PKM2) in resistance to platinum analogs is recently under extensive investigation [21,22]; PKM2 replaces the specific isoforms (type L, R, and M1) during tumourigenesis and substitution is under the control of the transcription factor C-MYC [23,24]. The overexpression of MYC proteins in SCLC is largely a result of gene amplification and leads to more rapid proliferation and loss of terminal differentiation [25]. C-MYC overexpression occurs in 16–32% of SCLC and in 40% of cell lines established from patients whose disease progressed after chemotherapy [26,27]. Finally, several mechanisms of resistance have been suggested to involve DNA topoisomerase I and II (TOPOI and TOPOII), which are the target of several compounds, including topoisomerase-I inhibitors (such as irinotecan and topotecan) as well as topoisomerase-II inhibitors (such as etoposide and doxorubicin) [7,28,29].
This study retrospectively analyzes the predictive value on both response to treatment and survival of BRCA1, ERCC1, ATP7B, PKM2, TOPOI, TOPΟ-IIA, TOPOIIB and C-MYC mRNA levels, as they are detected by real-time PCR in SCLC patients treated with platinum-based combination chemotherapy with or without chest radiotherapy.
Materials and Methods
Patients
From the database of histologically confirmed SCLC patients, treated with cisplatin and etoposide, in the Department of Medical Oncology in the University Hospital of Heraklion, we retrospectively collected all available tissue samples and the corresponding clinical data. In total, 184 formalin-fixed paraffin-embedded (FFPE) bronchoscopic or fine needle aspiration (FNA) biopsies from the primary tumours were collected. The study was approved by the institutional review board and all patients had signed informed consent for molecular analysis at the time of diagnosis. Main inclusion criteria were: histologically confirmed SCLC; first-line treatment with cisplatin or carboplatin and etoposide with or without radiotherapy and available tumour tissue. The study has been approved from the ethics and scientific committee of University Hospital of Heraklion (approval number 4456/14-5-2010) and all patients sign informed consent for their participation in the study.
Pathological evaluation, micro-dissection and RNA extraction
All paraffin-embedded tumours were reviewed by two independent pathologists to ensure the validity of the specimen and to select the proper area for microdissection. Sections of 5µm thickness were prepared and after staining with nuclear Fast Red (Sigma-Aldrich, St Louis, MO USA) cancer cells were procured using an Eppendorf piezoelectric microdissector (Eppendorf, Hamburg, Germany). RNA extraction was performed using the trizol LS method (Invitrogen, Carlsbad, CA, USA) and 50ng of total RNA for each gene was needed to prepare cDNA, using SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA, USA) [30,31]. Relative cDNA quantification was performed using the ABI Prism 7900HT Sequence Detection System (AB). Comparative Ct method was used for gene expression quantification using β-actin and PGK1 as internal reference genes and commercial RNA (Stratagene, La Jolla, CA, USA) as calibrators. Final expression values were determined as follows: 2-(ΔCt sample-ΔCt calibrator), where ΔCt values of the sample and the calibrators are estimated by subtracting the Ct value of the target gene from the median of the housekeeping genes values. In all experiments, only triplicates with a standard deviation of the Ct value <0.25 were accepted. In addition, genomic DNA contamination was excluded by including non-reverse transcribed RNA as a control for all 184 patients’ samples.
Primers and probes for gene expression analysis were designed using Primer Express 2.0 Software (AB) on the basis of their Ref Seq in http://www.ncbi.nlm.nih.gov/sites/entrez?db=gene designed. The detailed sequence and hybridization data are provided in table S1.
Statistical Considerations
Gene expression levels were quantified as continuous variable. The Pearson’s correlation coefficient analysis was used to determine correlation among different genes. Progression free survival (PFS) was calculated from the diagnosis of disease to demonstrated radiological progression or death from any cause. Overall survival (OS) was calculated from the diagnosis of disease to death from any cause. Median PFS and OS were estimated using the Kaplan-Meier curves and comparisons have been made using two-sided log-rank test. A univariate Cox regression analysis, with hazard ratios and 95% confindence intervals (CIs), was used to assess the association between each potential predictive factor and survival or PFS. These factors were then included in a multivariate Cox proportional hazards regression model with a stepwise procedure (both forward and backward) to evaluate the independent significance of different variables on survival and PFS. All results were considered as statistically significant with p<0.05 (two-sided test).
Results
Patients’ characteristics and clinico-pathological features
The main demographics and clinical characteristics of the study population are summarized in Table 1. Patients were predominately males (87%), with median age of 63 years and good performance status (PS; European Cooperative Oncology Group-ECOG 0-1:71%); about one third had LS-SCLC and 41% presented elevated serum levels of LDH at the time of initial diagnosis. The treatment protocol consisted of four to six three-week cycles of 100mg of eoposide per square meter on days 1, 2 and 3 and 80mg of cisplatin per square meter or carboplatin AUC 5-6 on day 1. All regimens required hydration and administration of antiemetic drugs. Sixty of LS-SCLC patients (60 out of 64; 94%) received curative radiotherapy which was started with cycle 2 of chemotherapy.
Table 1. Patients’ and tumours’ characteristics.
| Feature | Ν | % |
|---|---|---|
| 184 | ||
| Median Age (Range) years | 63 (33-78) | |
| ≤ 65 years | 98 | 54 |
| Gender | ||
| Male | 160 | 87 |
| Female | 24 | 13 |
| Stage | ||
| Limited | 64 | 35 |
| Extended | 120 | 65 |
| ECOG PS | ||
| 0-1 | 131 | 71 |
| 2 | 53 | 29 |
| LDH @ Diagnosis | ||
| Normal | 109 | 59 |
| Elevated | 75 | 41 |
| Curative Radiotherapy | ||
| Limited | 60 | 33 |
| Extended | 0 | 0 |
| Relapse | ||
| Yes | 171 | 93 |
| No | 13 | 7 |
| Second line Chemotherapy | ||
| Yes | 145 | 79 |
| No | 39 | 11 |
| Survival Status | ||
| Death | 169 | 92 |
| Alive | 15 | 8 |
| Median Progression Free Survival (Range) | Months | |
| Extended | 4.0 (0.3-16) | |
| Limited | 8.0 (1.3-121) | |
| Median Overall Survival (Range) | Months | |
| Extended | 7.6 (2.3-26.0) | |
| Limited | 15.0 (4.6-156.0) | |
Patients’ outcome was typical for SCLC. At the time of analysis and after a median follow-up of 9.1 months (min-max: 0.3-156 months), 171 disease relapses and 169 deaths have been recorded. The median OS was 15 months for patients with LS-SCLC and 8 months for those with ES-SCLC and the median PFS was 8 and 4 months, respectively (Table 1). The response to first line treatment is presented in Table 2.
Table 2. Response to treatment.
| CR n (%) | PR n (%) | SD n (%) | PD n (%) | NE n (%) | |
|---|---|---|---|---|---|
| LS-SCLC (n=64) | 13 (20) | 30 (47) | 7 (11) | 11 (17) | 3 (5) |
| ORR: 43 (67) | |||||
| ES-SCLC (n=120) | 1 (0.8) | 42 (35) | 18 (15) | 54 (45) | 5 (4.2) |
| ORR: 43 (35.8) | |||||
CR: Complete Response, PR; Partial Response, SD: Stable Disease,
PD: Progressive Disease, ORR: Overall Response Rate
Genes’ mRNA expression and correlations
The median expression values for each gene are presented in Table S2. The correlation among several genes’ expression confirmed the biological model at the basis of the selection of the analyzed genes. Significant correlations were observed between PKM2 and C-MYC (r= 0.21; p= 0.015), BRCA1 and ERCC1 (r= 0.65; p<0.001), TOPOI and TOPΟIIA (r= 0.40; p<0.001), TOPOI and TOPOIIB (r= 0.26; p= 0.002), and between TOPΟ-IIA and TOPOIIB (r= 0.67; p<0.001). There was no any significant correlation between the studied genes and the main clinical features such as disease stage, gender, PS and LDH levels.
Genes’ expression and patients’ outcome
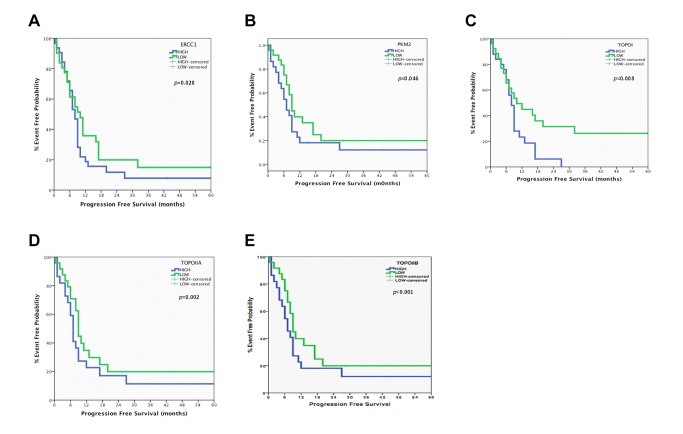
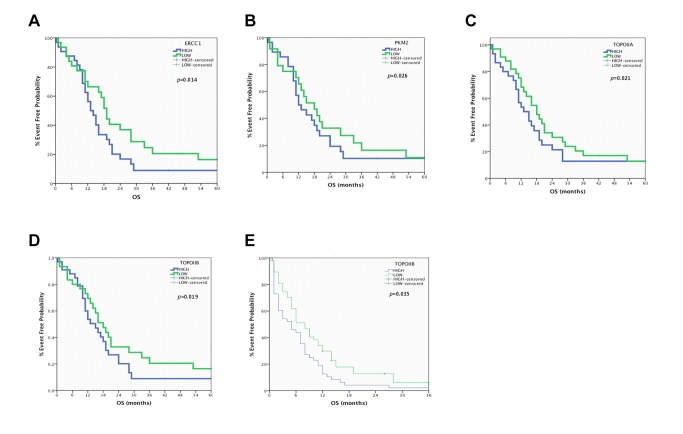
When the whole patients’ population was analyzed no significant correlation has been observed regarding median PFS or OS (Table S3). In contrast, when we examined separately LS-SCLC and ES-SCLC patients’ significant associations have been revealed. Indeed, in the LS-SCLC group, shorter median PFS was observed in patients with high mRNA expression of ERCC1 (7.9 vs. 10.1 months; p = 0.028), PKM2 (7.1 vs. 9.0 months; p = 0.046), TOPOI (7.8 vs. 10.2 months; p = 0.008), TOPOIIA (7 vs. 9.3 months; p = 0.002) and TOPOIIB (6.6 vs. 9.1 months; p <0.001) in comparison with those with low expression of these genes (Table S4 and Figure 1). Moreover, median OS was significantly decreased in patients with high mRNA expression of ERCC1 (13.2 vs. 19.1 months; p = 0.014), PKM2 (12 vs. 18.0 months; p = 0.026), TOPOIIA (13.0 vs. 18.3 months; p = 0.021) and TOPOIIB (12.8 vs. 18.4 months; p = 0.019) in comparison with those with low expression of these genes (Table S4 and Figure 2). No significant correlation has been observed between median PFS and OS and mRNA expression of any of the studied genes, with the exception of TOPOIIB, in patients with ES-SCLC (Table S5). More specifically, high mRNA expression of TOPOIIB was significantly associated with decreased median OS (5.0 vs. 9.2 months; p = 0.035) (Table S5 and Figure 2E)
Figure 1. Progression Free Survival in Limited Stage-SCLC.
A. ERCC1 mRNA levels and Progression Free Survival in Limited Stage-SCLC. B. PKM2 mRNA levels and Progression Free Survival in Limited Stage-SCLC. C. TOPOI mRNA levels and Progression Free Survival in Limited Stage-SCLC. D. TOPOIIA mRNA levels and Progression Free Survival in Limited Stage-SCLC. E. TOPOIIB mRNA levels and Progression Free Survival in Limited Stage-SCLC.
Figure 2. Overall Survival in Limited Stage-SCLC.
A. ERCC1 mRNA levels and Overall Survival in Limited Stage-SCLC. B. PKM2 mRNA levels and Overall Free Survival in Limited Stage-SCLC. C. TOPOIIA mRNA levels and Overall Free Survival in Limited Stage-SCLC. D. TOPOIIB mRNA levels and Overall Free Survival in Limited Stage-SCLC. E. TOPOIIB mRNA levels and Overall Free Survival in Extended Stage-SCLC.
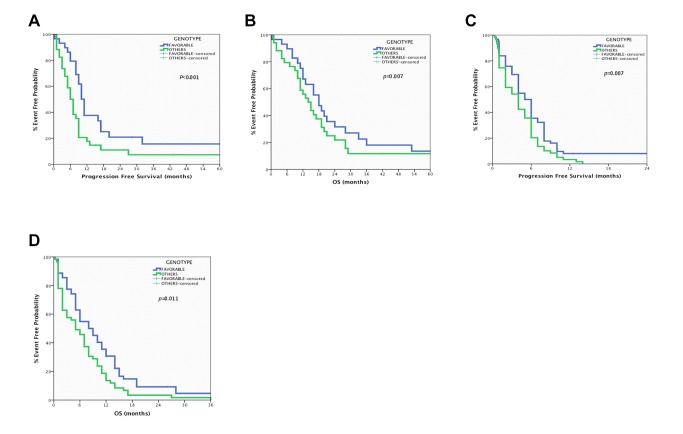
When the expression of ERCC1, PKM2 (sensitivity to platinum analogs) and TOPOIIA and TOPOIIB (sensitivity to etoposide) were combined significant correlations in both patients’ populations have been observed. The unfavorable expression signature (high mRNA expression of ERCC1, PKM2, TOPOIIA and TOPOIIB) in patients with LS-SCLC (n=26) was a significant predictor of shorter median PFS (6 vs. 11 months; p<0.001) and OS (13 vs. 18 months; p=0.007) when compared with those with favorable expression signature (low mRNA expression of ERCC1, PKM2, TOPOIIA and TOPOIIB; n=25) (Figure 3A and 3B). Similarly, in the ES-SCLC group, the patients with the unfavorable expression signature (n=53) presented significantly decreased PFS (3.8 vs. 6.1 months; p = 0.007) and OS (4.2 vs. 8.4 months; p = 0.011) in comparison with those with the favorable expression signature (n=51; Figure 3C and D)
Figure 3. Predictive value of the expression signature (favorable: low ERCC1, PKM2, TOPOIIA and TOPOIIB mRNA levels) in SCLC.
A. Correlation of expression signature with Progression Free Survival in Limited Stage-SCLC. B. Correlation of expression signature with Overall Survival in Limited Stage-SCLC. C. Correlation of expression signature with Progression Free Survival in Extended Stage-SCLC. D. Correlation of expression signature with Overall Survival in Extended Stage-SCLC.
Multivariate analysis for PFS and OS
Univariate analysis demonstrated that in patients with LS-SCLC high ERCC1 (HR: 1.71, 95% CI: 1.24-2.91; p=0.01), PKM2 (HR: 1.64, 95% CI: 1.17-2.42; p=0.031), TOPOI (HR: 1.41, 95% CI: 1.26-1.98; p=0.044), TOPOIIA (HR: 1.74, 95% CI: 1.33-3.1; p=0.009) and TOPOIIB (HR: 1.82, 95% CI: 1.46-3.18; p=0.004) mRNA expression, the unfavourable expression signature (HR: 4.97, 95% CI: 2.74-7.61; p=0.001), as well as PS of 2 (HR: 1.46, 95% CI: 1.22-2.81; p=0.017) and elevated serum levels of LDH (HR: 1.52, 95% CI: 1.21-2.78; p=0.021) were significantly associated with decreased PFS (Table 3). Also, high ERCC1 (HR: 1.63, 95% CI: 1.13-2.27; p=0.027), PKM2 (HR: 1.49, 95% CI: 1.06-1.84; p=0.046), TOPOIIA (HR: 1.69, 95% CI: 1.2-2.34; p=0.018), TOPOIIB (HR: 1.8, 95% CI: 1.43-3.11; p=0.006) mRNA expression as well as the unfavourable expression signature (HR: 5.34, 95% CI: 3.85-7.62; p<0.0001), PS of 2 (HR: 1.58, 95% CI: 1.29-2.9; p=0.013) and elevated serum levels of LDH levels (HR: 1.56, 95% CI: 1.22-2.94; p=0.027) were associated with decreased median OS. In ES-SCLC group, only the unfavourable expression signature (HR: 3.56, 95% CI: 1.89-6.46; p=0.001), PS of 2 (HR: 1. 81, 95% CI: 1.17-2.83; p=0.023) and elevated serum levels of LDH (HR: 1.93, 95% CI: 1.43-2.94; p=0.019) were associated with decreased PFS, while TOPOIIB (HR: 1.53, 95% CI: 1.09-1.89; p=0.046) mRNA expression, the unfavourable expression signature (HR: 4.14, 95% CI: 1.95-6.58; p=0.001), PS of 2 (HR: 1.94, 95% CI: 1.62-2.58; p=0.017) and elevated serum levels of LDH (HR: 2.01, 95% CI: 1.61-2.64; p=0.013) were correlated with shorter median OS (Table 4).
Table 3. Univariate analysis for Progression Free Survival and Overall Survival.
|
ProgressionFreeSurvival
|
OverallSurvival
|
|||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p-value | p-value | |||
| Limited-StageSCLC(n=64) | ||||||
| ERCC1 expression (High vs Low) | 1.71 | 1.24-2.91 | 0.01 | 1.63 | 1.13-2.27 | 0.027 |
| PKM2 expression (High vs Low) | 1. 64 | 1.17-2.42 | 0.031 | 1.49 | 1.06-1.84 | 0.046 |
| TOPOI expression (High vs Low) | 1.41 | 1.26-1.98 | 0.044 | 1.37 | 0.96-1.51 | 0.106 |
| TOPOIIA expression (High vs Low) | 1.74 | 1.33-3.1 | 0.009 | 1.69 | 1.2-2.34 | 0.018 |
| TOPOIIB expression (High vs Low) | 1.82 | 1.46-3.18 | 0.004 | 1.8 | 1.43-3.11 | 0.006 |
| Expression signature (unfavourable vs. favourable) | 4.97 | 2.74-7.61 | 0.001 | 5.34 | 3.85-7.62 | <0.001 |
| PS (2 vs. 0-1) | 1.46 | 1.22-2. 81 | 0.017 | 1.58 | 1.29-2.9 | 0.013 |
| LDH (elevated vs. normal) | 1.52 | 1.21-2.78 | 0.021 | 1.56 | 1.22-2.94 | 0.027 |
| Extended-StageSCLC(n=120) | ||||||
| TOPOIIB expression (High vs. Low) | 1.25 | 0.89-1.80 | 0.186 | 1.53 | 1.09-1.89 | 0.046 |
| Expression signature (unfavourable vs. favourable) | 3.56 | 1.89-6.46 | 0.001 | 4.14 | 1.95-6.58 | 0.001 |
| PS (2 vs. 0-1) | 1.81 | 1.17-2.83 | 0.023 | 1.94 | 1.62-2.58 | 0.017 |
| LDH (elevated vs. normal) | 1.93 | 1.43–2.94 | 0.019 | 2.01 | 1.61-2.64 | 0.0.13 |
Table 4. Multivariate analysis for Progression Free Survival and Overall Survival.
|
ProgressionFreeSurvival
|
OverallSurvival
|
|||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p-value | p-value | |||
| Limited- Stage SCLC (n=64) | ||||||
| ERCC1 expression (High vs Low) | 1.34 | 0.96-1.89 | 0.091 | 1.33 | 0.93-1.77 | 0.104 |
| PKM2 expression (High vs Low) | 1. 32 | 0.93-1.82 | 0.111 | 1.39 | 0.96-1.83 | 0.086 |
| TOPOI expression (High vs Low) | 1.29 | 0.86-1.78 | 0.178 | 1.26 | 0.81-1.61 | 0.215 |
| TOPOIIA expression (High vs Low) | 1.62 | 1.13-2.41 | 0.039 | 1.49 | 0.97-2.12 | 0.081 |
| TOPOIIB expression (High vs Low) | 1.79 | 1.40-2. 88 | 0.022 | 1.77 | 1.41-2.91 | 0.016 |
| Expression signature (unfavourable vs. favourable) | 3.18 | 1.94-4.85 | 0.002 | 4.35 | 2.76-6.08 | 0.001 |
| PS (2 vs. 0-1) | 1.38 | 1.14-2. 63 | 0.02 | 1.45 | 1.22-2.64 | 0.016 |
| LDH (elevated vs. normal) | 1.6 | 1.32-2.94 | 0.017 | 1.63 | 1.29-3.01 | 0.011 |
| Extended-StageSCLC(n=120) | ||||||
| TOPOIIB expression (High vs Low) | 1.11 | 0.74-1.63 | 0.29 | 1.44 | 0.98-1.83 | 0.064 |
| Expression signature (unfavourable vs. favourable) | 3.14 | 1.76-4.82 | 0.021 | 3.32 | 1.91-5.32 | 0.019 |
| PS (2 vs. 0-1) | 1.76 | 1.19-2.78 | 0.031 | 1.88 | 1.58-2.47 | 0.024 |
| LDH (elevated vs. normal) | 1.84 | 1.41–2.77 | 0.022 | 1.92 | 1.56-2.44 | 0.0.19 |
Cox proportional hazard analysis revealed that in patients with LS-SCLC high mRNA expression of TOPOIIA (HR: 1.62, 95% CI: 1.13-2.41; p=0.039) and TOPOIIB (HR: 1.79, 95% CI: 1.40-2.88; p=0.022) as well as unfavourable expression signature (HR: 3.18, 95% CI: 1.94-4.85; p=0.002), PS of 2 (HR: 1. 38, 95% CI: 1.14-2.63; p=0.02) and elevated serum levels of LDH (HR: 1.6, 95% CI: 1.32-2.94; p=0.017) emerged as independent factors associated with decreased PFS (Table 4). Similarly, TOPOIIB (HR: 1.77, 95% CI: 1.41-2.91; p=0.016) mRNA expression, unfavourable expression signature (HR: 4.35, 95% CI: 2.76-6.08; p=0.001) ), PS of 2 (HR: 1. 45, 95% CI: 1.22-2.64; p=0.016) and elevated serum levels of LDH levels (HR: 1.63, 95% CI: 1.29-3.01; p=0.011) were emerged as independent factors correlated with decreased median OS in the same group of patients (Table 4). In the group of patients with ES-SCLC only the unfavourable expression signature (HR: 3.14, 95% CI: 1.76-4.82; p=0.021), PS of 2 (HR: 1.76, 95% CI: 1.19-2.78; p=0.031) and elevated serum levels of LDH (HR: 1.84, 95% CI: 1.41-2.77; p=0.022) emerged as independent factors associated with decreased PFS (Table 4). Similarly, unfavourable expression signature (HR: 3.32, 95% CI: 1.91-5.32; p=0.019), PS of 2 (HR: 1. 88, 95% CI: 1.58-2.47; p=0.024) and elevated serum levels of LDH (HR: 1.92, 95% CI: 1.56-2.44; p=0.019), as well as TOPOIIB mRNA expression (HR: 1.44, 95% CI: 0.98-1.83; p=0.064) were revealed as independent factors associated with shorter median OS (Table 4).
Discussion
Over the past decades, there has been a modest improvement in survival of patients with SCLC, but the percentage of long-term survivors remains dismal. Due to the difficulty in obtaining sufficient and adequate tumour material, only few small studies have thus far been performed in SCLC and this disease remains an important target for treatment and research. In the present study which, in the best of our knowledge is the largest one, we analyzed the expression of several related genes with treatment response and we found a potential predictive model in SCLC patients treated with cisplatin and etoposide.
For the first time in 1999, new biological predictive markers were observed to have an impact on response to chemotherapy and survival of patients with SCLC. Consistent with our findings, Dingemans et al reported that TOPOIIB expression was predictive for response to chemotherapy, with higher response rates in patients with low TOPOIIB levels while high TOPOIIA expression predicted for shorter survival [7]. In agreement with these findings, we observed that LS-SCLC patients with low mRNA expression levels of TOPOIIA and TOPOIIB had significantly better PFS (p=0.002 and p<0.001, respectively) and OS (p=0.021 and p=0.019, respectively), while TOPOIIB was correlated with decreased median OS in ES-SCLC (p=0.035). The most of the in vitro studies have reported a close correlation between the expression of TOPOIIA and sensitivity to drugs. Also, the higher expression of this enzyme in SCLC has been reported as a possible reason for the higher chemosensitivity [32]. In contrast our results are in the opposite direction and this discrepancy may be attributed to methodological issues (protein versus mRNA expression, cell lines versus human cancer samples etc); in addition, it might be partially explained by the fact that, in our study, TOPOII inhibitors were administered together with cisplatin. The findings regarding the expression of TOPOIIB are also supported by other studies in SCLC [33].
In addition, TOPOI overexpression has been correlated with decreased PFS (HR: 1.41; p=0.044). Indeed, augmented expression of TOPOI has been described in various types of tumours [34]. For instance, ovarian cancer cases treated with platinum-based drugs but not with topotecan with increased expression of TOPOIA demonstrated a significant shorter overall survival, indicating that on cases with augmented expression of TOPOIA application of topotecan should be considered as it might change the outcome in this group of patients [35], but the biological rationale is lacking.
The current study also demonstrated that LS-SCLC patients with high expression of ERCC1 and PKM2 had shorter median PFS (p=0.028 and p=0.046, respectively) and OS (p=0.014 and p=0.026, respectively), while patients with low levels of TOPOI presented longer PFS (p=0.008) but not OS (p=0.41) compared with those with high mRNA expression. These findings are in agreement with those from recently reported studies which demonstrated that the protein or mRNA expression of ERCC1 and TOPOI in SCLC is predictive of treatment efficacy [33,36]. Sereno et al have demonstrated that TOPOI mRNA analysis can predict cispaltin response and prognosis in SCLC patients [36]. Simultaneously, since expression of TOPOIA has been documented as a significant predictive index in camptothecin based therapy, tumours with high expression of TOPOI may be more amenable to this type of treatment [35]. Also, Ceppi et al, quantified ERCC1, RRM1, and TOPOII mRNA expression in 85 SCLC patients treated with platinum/etoposide [33]; they found that TOPOII expression was associated with better response in LS-SCLC patients, while patients of the same stage of disease and low ERCC1 mRNA levels had significantly longer survival. Τhe multivariate analysis demonstrated that ERCC1 expression was an independent prognostic factor for survival in LS-SCLC [33]. Similarly, Lee et al, reported that the protein expression of ERCC1 in 77 SCLC patients treated with platinum-based doublets, was associated with poor OS, especially in patients with LS-SCLC indicating that high expression of ERCC1 can be a prognostic biomarker for this group of patients [37]. Finally, Chiappori et al, using an immunofluorescence-based automated quantitative technique, scoring RRM1, ERCC1 and TOPOII levels in tumour specimens, reported that TOPOII mRNA expression predicted for better response while ERCC1 mRNA expression was the only independent prognostic factor for survival; conversely, there was no prognostic or predictive role for any of these genes in ES-SCLC [38]. These findings strongly suggest that LS-SCLC seems to represent a disease which may be biologically distinct from ES-SCLC based on the affected molecular mechanism.
Another important finding in the present study is that the integrated analysis of ERCC1, PKM2 (related with resistance to cisplatin), TOPOIIA and TOPOIIB (related with resistance to etoposide) was able to confirm that the group of patients with the favorable expression signature (low expression of all genes) presented an improved OS and PFS when compared with those with the unfavorable expression signature. In fact, in LS-SCLC patients with the favorable expression signature, a PFS of almost one year (p<0.001) and an OS of 18 months (p=0.007) was observed, whereas ES-SCLC patients with low expression of the four genes achieved a PFS of 6.1 months (p=0.007) and an OS of 8.4 months (p=0.011) which were significantly higher compared to patients with the unfavorable expression signature (3.8 months and 4.2 for PFS and OS, respectively).
Despite that, the promising results of the present study should be interpreted with caution due to the limitation of this type of research and not definitive conclusions could be made. The study was retrospective, lacks a validation group and although has included the larger number of patients’ samples reported ever, the total number of enrolled patients remains relatively small. However, we consider that this type of research may identify subgroups of patients with substantial benefit from the standard chemotherapy and radiotherapy for SCLC and may lead to the design of a prospective clinical trial in SCLC, where the predictive powers of these biomarker expression levels will be tested and validated prospectively. Finally, this study indicates that even in malignancies with limitations in tissue availability, such as SCLC, combinatory efforts may provide adequate samples for molecular analysis and contribute by that to the goal of “individualized” treatment.
Supporting Information
Sequence of the primers and probes of all reference and target genes.
(DOC)
Genes’ expression values.
(DOC)
Whole patients’ population: Correlation of genes’ expression value with Progression Free Survival and Overall Survival.
(DOCX)
LS-SCLC#: Correlation of genes’ expression value and Progression Free Survival and Overall Survival.
(DOCX)
ES-SCLC#: Correlation of genes’ expression value and Progression Free Survival and Overall Survival.
(DOCX)
Funding Statement
This study was partially supported by grants from the Cretan Association for Biomedical Research (CABR) and the Hellenic Society of Medical Oncology (HeSMO). NK is a recipient of a HeSMO fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
References
- 1. Metro G, Duranti S, Fischer MJ, Cappuzzo F, Crino L (2012) Emerging drugs for small cell lung cancer--an update. Expert Opin Emerg Drugs 17: 31-36. doi:10.1517/14728214.2012.656588. PubMed: 22288522. [DOI] [PubMed] [Google Scholar]
- 2. Jänne PA, Freidlin B, Saxman S, Johnson DH, Livingston RB et al. (2002) Twenty-five years of clinical research for patients with limited-stage small cell lung carcinoma in North America. Cancer 95: 1528-1538. doi:10.1002/cncr.10841. PubMed: 12237922. [DOI] [PubMed] [Google Scholar]
- 3. Govindan R, Page N, Morgensztern D, Read W, Tierney R et al. (2006) Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 24: 4539-4544. doi:10.1200/JCO.2005.04.4859. PubMed: 17008692. [DOI] [PubMed] [Google Scholar]
- 4. Amarasena IU, Walters JA, Wood-Baker R, Fong K (2008) Platinum versus non-platinum chemotherapy regimens for small cell lung cancer. Cochrane Database Syst Rev CD: 006849 PubMed: 18843733. [DOI] [PubMed] [Google Scholar]
- 5. Lawson MH, Cummings NM, Rassl DM, Russell R, Brenton JD et al. (2011) Two novel determinants of etoposide resistance in small cell lung cancer. Cancer Res 71: 4877-4887. doi:10.1158/0008-5472.CAN-11-0080. PubMed: 21642373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bepler G (2005) Using translational research to tailor the use of chemotherapy in the treatment of NSCLC. Lung Cancer 50 Suppl 1: S13-S14. doi:10.1016/S0169-5002(05)81553-3. PubMed: 16291424. [DOI] [PubMed] [Google Scholar]
- 7. Dingemans AM, Witlox MA, Stallaert RA, van der Valk P, Postmus PE et al. (1999) Expression of DNA topoisomerase IIalpha and topoisomerase IIbeta genes predicts survival and response to chemotherapy in patients with small cell lung cancer. Clin Cancer Res 5: 2048-2058. PubMed: 10473085. [PubMed] [Google Scholar]
- 8. Viktorsson K, De Petris L, Lewensohn R (2005) The role of p53 in treatment responses of lung cancer. Biochem Biophys Res Commun 331: 868-880. doi:10.1016/j.bbrc.2005.03.192. PubMed: 15865943. [DOI] [PubMed] [Google Scholar]
- 9. Bonanno L, Favaretto A, Rugge M, Taron M, Rosell R (2011) Role of genotyping in non-small cell lung cancer treatment: current status. Drugs 71: 2231-2246. doi:10.2165/11597700-000000000-00000. PubMed: 22085382. [DOI] [PubMed] [Google Scholar]
- 10. de Laat WL, Jaspers NG, Hoeijmakers JH (1999) Molecular mechanism of nucleotide excision repair. Genes Dev 13: 768-785. doi:10.1101/gad.13.7.768. PubMed: 10197977. [DOI] [PubMed] [Google Scholar]
- 11. Cobo M, Isla D, Massuti B, Montes A, Sanchez JM et al. (2007) Customizing cisplatin based on quantitative excision repair cross-complementing 1 mRNA expression: a phase III trial in non-small-cell lung cancer. J Clin Oncol 25: 2747-2754. doi:10.1200/JCO.2006.09.7915. PubMed: 17602080. [DOI] [PubMed] [Google Scholar]
- 12. Papadaki C, Sfakianaki M, Ioannidis G, Lagoudaki E, Trypaki M et al. (2012) ERCC1 and BRAC1 mRNA expression levels in the primary tumor could predict the effectiveness of the second-line cisplatin-based chemotherapy in pretreated patients with metastatic non-small cell lung cancer. J Thorac Oncol 7: 663-671. doi:10.1097/JTO.0b013e318244bdd4. PubMed: 22425915. [DOI] [PubMed] [Google Scholar]
- 13. Olaussen KA, Dunant A, Fouret P, Brambilla E, André F et al. (2006) DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med 355: 983-991. doi:10.1056/NEJMoa060570. PubMed: 16957145. [DOI] [PubMed] [Google Scholar]
- 14. Furuta T, Ueda T, Aune G, Sarasin A, Kraemer KH et al. (2002) Transcription-coupled nucleotide excision repair as a determinant of cisplatin sensitivity of human cells. Cancer Res 62: 4899-4902. PubMed: 12208738. [PubMed] [Google Scholar]
- 15. Boukovinas I, Papadaki C, Mendez P, Taron M, Mavroudis D et al. (2008) Tumor BRCA1, RRM1 and RRM2 mRNA expression levels and clinical response to first-line gemcitabine plus docetaxel in non-small-cell lung cancer patients. PLOS ONE 3: e3695. doi:10.1371/journal.pone.0003695. PubMed: 19002265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taron M, Rosell R, Felip E, Mendez P, Souglakos J et al. (2004) BRCA1 mRNA expression levels as an indicator of chemoresistance in lung cancer. Hum Mol Genet 13: 2443-2449. doi:10.1093/hmg/ddh260. PubMed: 15317748. [DOI] [PubMed] [Google Scholar]
- 17. Lotti LV, Ottini L, D’Amico C, Gradini R, Cama A et al. (2002) Subcellular localization of the BRCA1 gene product in mitotic cells. Genes Chromosomes Cancer 35: 193-203. doi:10.1002/gcc.10105. PubMed: 12353262. [DOI] [PubMed] [Google Scholar]
- 18. Mullan PB, Quinn JE, Gilmore PM, McWilliams S, Andrews H et al. (2001) BRCA1 and GADD45 mediated G2/M cell cycle arrest in response to antimicrotubule agents. Oncogene 20: 6123-6131. doi:10.1038/sj.onc.1204712. PubMed: 11593420. [DOI] [PubMed] [Google Scholar]
- 19. Kuo MT, Chen HH, Song IS, Savaraj N, Ishikawa T (2007) The roles of copper transporters in cisplatin resistance. Cancer Metastasis Rev 26: 71-83. doi:10.1007/s10555-007-9045-3. PubMed: 17318448. [DOI] [PubMed] [Google Scholar]
- 20. Nakagawa T, Inoue Y, Kodama H, Yamazaki H, Kawai K et al. (2008) Expression of copper-transporting P-type adenosine triphosphatase (ATP7B) correlates with cisplatin resistance in human non-small cell lung cancer xenografts. Oncol Rep 20: 265-270. PubMed: 18636185. [PubMed] [Google Scholar]
- 21. Guo W, Zhang Y, Chen T, Wang Y, Xue J et al. (2011) Efficacy of RNAi targeting of pyruvate kinase M2 combined with cisplatin in a lung cancer model. J Cancer Res Clin Oncol 137: 65-72. doi:10.1007/s00432-010-0860-5. PubMed: 20336315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martinez-Balibrea E, Plasencia C, Ginés A, Martinez-Cardús A, Musulén E et al. (2009) A proteomic approach links decreased pyruvate kinase M2 expression to oxaliplatin resistance in patients with colorectal cancer and in human cell lines. Mol Cancer Ther 8: 771-778. doi:10.1158/1535-7163.MCT-08-0882. PubMed: 19372549. [DOI] [PubMed] [Google Scholar]
- 23. Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE et al. (2008) The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452: 230-233. doi:10.1038/nature06734. PubMed: 18337823. [DOI] [PubMed] [Google Scholar]
- 24. David CJ, Chen M, Assanah M, Canoll P, Manley JL (2010) HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature 463: 364-368. doi:10.1038/nature08697. PubMed: 20010808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prins J, De Vries EG, Mulder NH (1993) The myc family of oncogenes and their presence and importance in small-cell lung carcinoma and other tumour types. Anticancer Res 13: 1373-1385. PubMed: 8239508. [PubMed] [Google Scholar]
- 26. Johnson BE, Makuch RW, Simmons AD, Gazdar AF, Burch D et al. (1988) myc family DNA amplification in small cell lung cancer patients’ tumors and corresponding cell lines. Cancer Res 48: 5163-5166. PubMed: 2842046. [PubMed] [Google Scholar]
- 27. Meyer N, Penn LZ (2008) Reflecting on 25 years with MYC. Nat Rev Cancer 8: 976-990. doi:10.1038/nrc2231. PubMed: 19029958. [DOI] [PubMed] [Google Scholar]
- 28. Kaufmann SH, Karp JE, Jones RJ, Miller CB, Schneider E et al. (1994) Topoisomerase II levels and drug sensitivity in adult acute myelogenous leukemia. Blood 83: 517-530. PubMed: 7904487. [PubMed] [Google Scholar]
- 29. Wang JC, Caron PR, Kim RA (1990) The role of DNA topoisomerases in recombination and genome stability: a double-edged sword? Cell 62: 403-406. doi:10.1016/0092-8674(90)90002-V. PubMed: 2165864. [DOI] [PubMed] [Google Scholar]
- 30. Papadaki C, Mylonaki A et al. (2009) Predictive significance of BRCA1, TXR1 and TSP1 tumoral expression in patients with metastatic breast cancer treated with taxanes-based 1st line chemotherapy. Impact Congress , Abstract RT [Google Scholar]
- 31. Papadaki C, Tsaroucha E, Kaklamanis L, Lagoudaki E, Trypaki M et al. (2011) Correlation of BRCA1, TXR1 and TSP1 mRNA expression with treatment outcome to docetaxel-based first-line chemotherapy in patients with advanced/metastatic non-small-cell lung cancer. Br J Cancer 104: 316-323. doi:10.1038/sj.bjc.6606027. PubMed: 21157449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guinee DG Jr., Holden JA, Benfield JR, Woodward ML, Przygodzki RM et al. (1996) Comparison of DNA topoisomerase II alpha expression in small cell and nonsmall cell carcinoma of the lung. In search of a mechanism of chemotherapeutic response. Cancer 78: 729-735. doi:10.1002/(SICI)1097-0142(19960815)78:4. PubMed: 8756364. [DOI] [PubMed] [Google Scholar]
- 33. Ceppi P, Longo M, Volante M, Novello S, Cappia S et al. (2008) Excision repair cross complementing-1 and topoisomerase IIalpha gene expression in small-cell lung cancer patients treated with platinum and etoposide: a retrospective study. J Thorac Oncol 3: 583-589. doi:10.1097/JTO.0b013e3181734f24. PubMed: 18520795. [DOI] [PubMed] [Google Scholar]
- 34. Husain I, Mohler JL, Seigler HF, Besterman JM (1994) Elevation of topoisomerase I messenger RNA, protein, and catalytic activity in human tumors: demonstration of tumor-type specificity and implications for cancer chemotherapy. Cancer Res 54: 539-546. PubMed: 8275492. [PubMed] [Google Scholar]
- 35. Surowiak P, Materna V, Kaplenko I, Spaczynski M, Dietel M et al. (2006) Topoisomerase 1A, HER/2neu and Ki67 expression in paired primary and relapse ovarian cancer tissue samples. Histol Histopathol 21: 713-720. PubMed: 16598670. [DOI] [PubMed] [Google Scholar]
- 36. Sereno M, Cejas P, Moreno V, Belda-Iniesta C, López R et al. (2012) ERCC1 and topoisomerase I expression in small cell lung cancer: prognostic and predictive implications. Int J Oncol 40: 2104-2110. PubMed: 22344449. [DOI] [PubMed] [Google Scholar]
- 37. Lee HW, Han JH, Kim JH, Lee MH, Jeong SH et al. (2008) Expression of excision repair cross-complementation group 1 protein predicts poor outcome in patients with small cell lung cancer. Lung Cancer 59: 95-104. doi:10.1016/j.lungcan.2007.07.023. PubMed: 17889401. [DOI] [PubMed] [Google Scholar]
- 38. Chiappori AA, Zheng Z, Chen T, Rawal B, Schell MJ et al. (2010) Features of potentially predictive biomarkers of chemotherapeutic efficacy in small cell lung cancer. J Thorac Oncol 5: 484-490. doi:10.1097/01.JTO.0000391377.17179.6b. PubMed: 20107425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequence of the primers and probes of all reference and target genes.
(DOC)
Genes’ expression values.
(DOC)
Whole patients’ population: Correlation of genes’ expression value with Progression Free Survival and Overall Survival.
(DOCX)
LS-SCLC#: Correlation of genes’ expression value and Progression Free Survival and Overall Survival.
(DOCX)
ES-SCLC#: Correlation of genes’ expression value and Progression Free Survival and Overall Survival.
(DOCX)