Abstract
In this article we present arguments that the “antidiabetic” drug metformin could be useful as an add-on therapy to methotrexate for the treatment of psoriasis and, perhaps, for rheumatoid arthritis as well. Biochemical data suggest that both drugs may share a common cellular target, the AMP-activated protein kinase (AMPK). This enzyme is a master regulator of metabolism and controls a number of downstream targets, e.g., important for cellular growth or function in many tissues including T-lymphocytes. Clinical observations as well as experimental results argue for anti-inflammatory, antineoplastic and antiproliferative activities of metformin and a case-control study suggests that the drug reduces the risk for psoriasis.
Patients with psoriasis have higher risk of metabolic syndrome, type 2 diabetes and cardiovascular mortality. Metformin has proven efficacy in the treatment of prediabetes and leads to a pronounced and sustained weight loss in overweight individuals. We expect that addition of metformin to methotrexate can lead to positive effects with respect to the PASI score, reduction of the weekly methotrexate dose and of elevated cardiovascular risk factors in patients with metabolic syndrome and psoriasis. For reasons explained later we suggest that only male, overweight patients are to be included in a pilot trial. On the other side of the coin are concerns that the gastrointestinal side effects of metformin are intolerable for patients under low dose, intermittent methotrexate therapy. Metformin has another side effect, namely interference with vitamin B12 and folate metabolism, leading to elevated homocysteine serum levels. As patients must receive folate supplementation and will be controlled with respect to their B12 status increased hematological toxicity is unlikely to result.
Keywords: psoriasis, metformin, methotrexate, AMP-activated protein kinase, anti-inflammatory
Introduction
It is an undeniable fact that there is a paradigmatic change occurring for one of the most common “skin diseases,” psoriasis. It is now more and more recognized as a systemic disorder.1 Being asked to prepare state-of-the-art review talks (pharmacological and dermatological, respectively) for a conference held in Vienna on behalf of the 50th anniversary of methotrexate we discovered a case-control study indicating that risk for psoriasis was lowered by using either glitazones or metformin.2 Risk reduction for metformin was only observed in males. The authors suggested that, perhaps a common mechanism, namely activation of AMP-activated protein kinase (AMPK) was a possible explanation. Although the glitazones can stimulate AMPK in cellular systems after binding to a mitochondrial receptor (mitoNEET)3 AMPK activation was never (in contrast to metformin) demonstrated ex vivo in humans. Association studies do not prove causality but can stimulate further research. Disregarding the glitazones for reasons not detailed here, we concentrate on metformin to collect arguments for a pilot trial adding this “antidiabetic” as an “anti-inflammatory” agent to the low-dose, weekly methotrexate regimen.
Dermatologists are familiar with methotrexate but less so with metformin, therefore the focus is more on the latter. Endocrinologists on the other hand may be less informed about the biochemical mysteries of the most favored “anti-diabetic” drug. Metformin was introduced in Europe during the same decade when Gubner et al.4 published their results with aminopterin for the treatment of psoriasis and rheumatoid arthritis. First we discuss pharmacodynamics and molecular and cellular activities. A brief overview on the cellular and organismal distribution and disposition-kinetics of the two drugs is also given. We subsequently present biochemical data, animal experiments and clinical observations for our proposal that an “add-on” pilot trial with male psoriasis patients having metabolic syndrome is a rational approach and ethically justified. It must be emphasized that this is not a review article on anti-inflammatory activities of metformin (although we heavily rely on existing evidence) or of methotrexate. Discussions of complex intracellular pathways (including signaling of cytokines, protein kinase cascades, etc.) are beyond our scope. For readers interested in more molecular details we cite key review articles and publications. It is hoped—although both drugs are old (but by no means outdated)—that what was selected from the most recent and long forgotten literature can be fascinating. Perhaps we can stimulate interest of researchers and clinicians in dermatology for metformin and related compounds as systemic (as proposed here for psoriasis) or even as topical drugs.
Methods
We searched the literature via pubmed, Google Scholar, Web of Science with key phrases alone and (more) often in combination: biguanide(s), phenformin, metformin, AICAR, AICAr, methotrexate, T-lymphocytes, monocytes, macrophages, anti-inflammatory, metabolic syndrome, psoriasis, AMP-activated protein kinase, AMPK, reactive oxygen species, complex I (inhibitors), inflammation, inflammatory biomarkers, clinical trials, organic cation transporters. We also approached some of the leading researchers in the field to supply us with further information including publications in press.
Results
Methotrexate and metformin are not only very old drugs but have a remarkable history of an expanding pharmacodynamic profile
Metformin: From an anti-diabetic to anti-inflammatory, anti-aging, rejuvenating, antidote, anti-neoplastic and “anti-androgen” drug
The history of the biguanidines as drugs can be traced back to the Middle Ages. The herb Galega officinalis (French lilac, Goat’s rue) was used among other for symptoms of diabetes mellitus in humans or to increase milk production in farmed animals. The active principles of the toxic (or poisonous) plant are guanidine and isoamylene guanidine (galegine) recognized as hypoglycemic principles early in the 20th century.5 Galegine was used in doses of 150 mg/day with success in more than 3 dozen patients, lacking the abdominal discomfort of synthetic synthalin A (decamethylene-guanidine) and B (dodecamethylene diguanidine).6 The synthalins remained in the pharmaceutical armamentarium of Germany for many years despite their known toxicity. Under the suggestive name of flumamine, injected (most likely i.m. in a dose of 32.5 mg!) metformin enjoyed a brief episode in 1949 as an extremely potent, fast-acting antipyretic and analgesic for viral influenza.7,8 Although this miracle cure was never reproduced, the author suggested that flumamine acts on malaria parasites and promised to report on his malaria cases later. His idea that metformin (and related biguanidines) may be active on malaria parasites is supported by the structural similarity of RJF 00719 [2-(4-cyclohexylphenyl)-1-diaminomethylidene-guanidine)] and RJF 01059 (phenylbiguanide) to phenformin. Both compounds (especially RJF 00719 in the 20 micromolar range) are inhibitors of the Plasmodium falciparum bi-functional thymidilate synthase-dihydrofolate reductase.9
In any event, the publication8 from the Philippines, mentioning glucose lowering effects of substituted biguanidines in the discussion (but not for his treated patients), stimulated a French researcher (Jean Sterne) 1957 to try “flumamine” for diabetics. Jean Sterne was (luckily) unaware of data published a quarter of a century earlier in the at that time most important pharmacological journal of the world.10 The latter German authors, who investigated the antipyretic (sic) activity of biguanide and its chemically modified analogs, actually strongly warned against a human trial for diabetes: 1, 1-dimethylbiguanide (now metformin) was extremely toxic in their animal experiments. In any event, the above mentioned “miracle” cure was the first hint that metformin and structurally related antidiabetics such as phenformin can exhibit “anti-inflammatory” activity. Indeed, in the pre-methotrexate era phenformin was tried with some success for rheumatoid arthritis because it demonstrated “fibrinolytic” activity.11-13 After the discontinuation of phenformin and buformin in many (but not all) countries, only metformin (introduced in Europe in 1957) survived and is—with respect to tons of drug consumed—the world leader. For example, the influent concentration in 5 German wastewater treatment plants varied from 18 to 105 μg/L,14 which is equivalent to an estimated metformin consumption in Germany of 370–897 tons per year. Metformin is an environmental contaminant, ends up in some plants, can be even highly enriched in seeds15 and may be present in concentration of 1 to 3 μg/L in our drinking water. As a caloric restriction mimetic metformin increases life span in some laboratory animals and the worm C.elegans.16 It is gaining experimental popularity as rejuvenating drug or gerosuppressant, being able to stimulate neurogenesis.17 It acts as an antidote since it completely prevents gentamycin kidney toxicity in rats.18 Highly significant in the context of this article, metformin ameliorates hepatic methotrexate toxicity in experimental animals.19 Of equal interest for dermatology, metformin as a topical (or systemic) treatment blocks UV-B induced tumor formation in hairless mice20 and reduces growth of squamous cell carcinoma.21 As an adjunct to conventional cancer therapy or to prevent relapse more than 40 human trials with metformin are listed in ClinicalTrials.gov (www.clinicaltrials.gov accessed November 7, 2012). There is, however, an ongoing debate, if metformin acts via a systemic effect solely by decreased insulin levels (and/or less obesity) or has direct actions on tumor cells as is observed experimentally.22 A detailed discussion of metformin and related biguanidines for cancer prevention and treatment is beyond the scope of this article and the reader is referred to excellent reviews (see refs. 148 and 149).
Metformin was first used for women with (and later without) diabetes to increase insulin sensitivity, promote ovulation and decrease androgen production from the ovaries, by lowering the “hyperinsulinemic” status (see ref. 23). However, adrenal cells in vitro are highly sensitive to metformin,24 suggesting that subtle alterations of adrenal steroid metabolism may occur by direct action. Adrenal glands and especially the cortex due to the presence of transporters accumulate the drug similar to small intestine, liver, skeletal muscle and kidney.25 As will be outlined later metformin appears to have some beneficial effects only in males, which may be related to differential effects on steroid hormones. Metformin’s status to improve fertility in PCOS has not been convincingly demonstrated,23 but it certainly ameliorates symptoms of this highly prevalent condition. In the context of co-morbidities for psoriasis it is remarkable that PCOS women also have an increased risk of cardiovascular events and diabetes.26
Methotrexate: From an anti-neoplastic (anti-metabolic) to anti-inflammatory, vasculoprotective and “antidiabetic” drug
Methotrexate and its more toxic precursor aminopterin were first introduced as anti-neoplastic agents. More by chance the anti-folates, especially low-dose, once weekly methotrexate, found entry into dermatology, not only for treatment of psoriasis but for numerous other skin conditions.27 Methotrexate is still the most effective disease modifying antirheumatic drug for rheumatoid arthritis and related disorders. More recently, based on data from observational studies with low-dose methotrexate for rheumatoid arthritis or psoriasis indicating lower cardiovascular risk,28 it was suggested to employ methotrexate as “vasculoprotective drug” for patients with stable cardiovascular disease and elevated hsCRP.29 Our concept, namely that part of the action of methotrexate is based on activation of AMPK via AICAR (ZMP), is supported by experiments with genetically diabetic (db+/db+) mice, where glucose and insulin levels are significantly lowered after only 4 weekly i.p. methotrexate doses (0.5 mg/kg).30 Unfortunately, the authors failed to demonstrate that either AMPK or own of its downstream effectors such as acetyl-Coenzyme A -carboxylase were phosphorylated.
Metformin and methotrexate have some common features
Uptake via carriers or transporters, elimination via the kidney and “deep compartement”
Both drugs are taken up into cells (including enterocytes of the intestine) by specific carrier/transporter systems. Methotrexate enters as a “blind passenger” via folate uptake mechanisms and is trapped intracellularly by polyglutamination.27 Metformin is taken up via organic cation- (OCTs)31 and plasma membrane monamine transporters.32 Secretion of metformin by kidney tubules and liver luminal membranes is driven by multidrug and toxin extrusion (MATE) proteins.33 Much is known about the pharmacogenetics of metformin especially for naturally occurring variants of OCT1 (import) and OCT2 (export by the kidney) but less so for methotrexate. For both drugs, systemic bioavailability after oral administration is dose-dependent and limited in capacity: the higher the dose the less percentage of it is adsorbed. Both are (mainly) excreted via the kidney albeit via different transporters. Therefore plasma clearance is depending on kidney function. Under conditions of weekly (MTX) or daily dosing (metformin) a “deep” compartement is building up. Higher polyglutamated (MTX-PGs) species slowly increase, if red blood cell MTX-PG content is taken as proxy for tissue (or target cell, “site of action”) residence. A steady-state with respect to the composition of red blood cell MTX-PGs is only reached after several months,34 often paralleling clinical improvement.35 Although on a different time scale (hours and days), the distribution volume for metformin increases from about 1.6 L/kg after a single dose36 to about 4 L/kg25 under continuous dosing. This indicates a large tissue reservoir, mainly but not only dominated by expression and activity of the various transporters. Similar to MTX (and the MTX-PGs), metformin accumulates in red blood cells37 reflecting “deep compartements” elsewhere. Existence of such compartements is also apparent by late phases in plasma or urine elimination kinetics after long-term daily dosing.38 Metformin has a pK of 11.5 and is highly water soluble. According to first principles in pharmacology, as a singly positively charged molecule at almost all pH values in the body, it prefers to reside in lower pH in preference to higher pH. Therefore metformin can also distribute via simple diffusion but on a much prolonged time-scale in contrast to rapid transport via OCTs. The average metformin concentrations at steady-state and daily doses of 2–3 g in plasma of humans are around 1 mg/L (8 micromolar) with an upper limit of 2.4 mg/L.25 After oral intake peak levels in portal blood exposing circulating blood cells and, later, hepatocytes are likely to be much higher.39 Within cells 1000-fold higher concentrations compared with the cytosol are calculated to exist in the intermembrane space of respiring, phosphorylating mitochondria with a high membrane potential.40 Similar arguments may hold for lysosomes, endosomes, caveolae, Golgi and other acidified organelles. Surprisingly little attention has been paid to the fact that the transporters involved in uptake and extrusion of metformin were not designed by nature for drugs but mainly for endogenous substrates. The Km values for metformin are ~1 mM for OCT141 and 0.24–2 mM for MATE1 and MATE2, respectively.33 Considering that experiments with cells are often performed with 1 or 10 mM metformin for many (24–48–72) h (see refs. 42–44), one wonders if metformin as a competing substrate did not change import and export of endogenous metabolites or nutrients. One metabolite which is substrate for both importers and exporters is the essential amino acid tryptophan. The Km values for this amino acid for OCT1 or OCT2 are in the 5–10 mM range ~ten times higher than for metformin. Plasma concentrations of tryptophan are ~60 μM45 and those for metformin are up to 20–30 μM, making competition very likely. Indeed, upon chronic dosing with metformin plasma or serum levels of tryptophan in patients increase.46,47 Tryptophan concentrations in urine have recently proposed as markers to predict patients pharmacokinetic parameters for metformin determined by the naturally occurring human variants of OCT2.48 Thus, it cannot be excluded that part of the metabolic and gene expression changes observed after organ perfusion, cellular experiments and in patients are a consequence of substrate competition with transporters. It is interesting to note that competition of the hypoglycemic biguanidines with mitochondrial (not plasma membrane) cation transporters was suggested as early as 1979.42
The mechanism by which methotrexate and metformin act is still not fully understood
Methotrexate: Are adenosine, AICAR or reactive oxygen species (ROS) mediators of the anti-inflammatory activity?
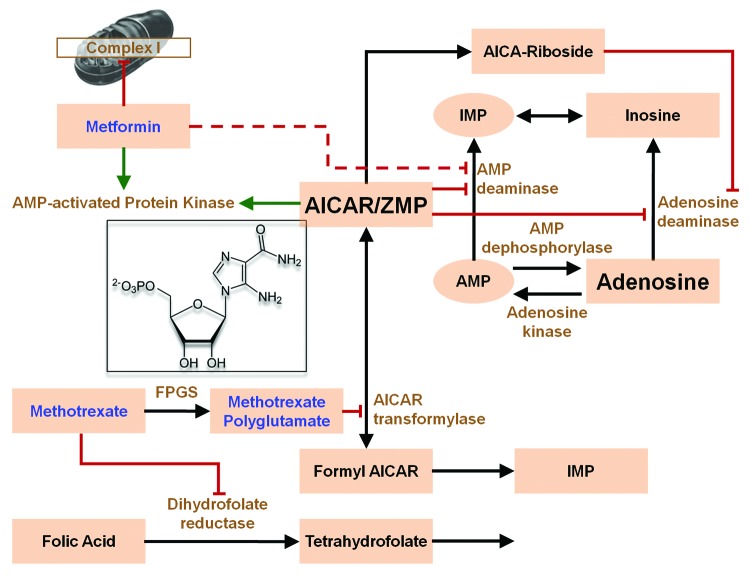
In Figure 1 we show a highly simplified version of the mechanism by which methotrexate is proposed to act as an “anti-inflammatory agent,”27,43 namely by increasing adenosine efflux. Enhanced urinary secretion of AICA (5- aminoimidazole-4-carboxamide), a degradation product of AICAR and of adenosine, was first observed in patients with psoriasis treated with methotrexate,44 pointing to an inhibitory action of MTX (or MTX-PGs) at the level of AICAR transformylase (AICART). The importance of adenosine as an anti-inflammatory mediator was questioned53,54 and could not been convincingly demonstrated in later experiments.55 It is, however, generally accepted that AICAR (ZMP) increases after methotrexate56—either by direct inhibition of AICART or alternatively, favoring the reverse reaction by increasing dihydrofolate via blockade of dihydrofolate reductase.52 (For more details, see the legend of Fig. 1) Another inhibitor of thymidylate synthase and AICART, pemetrexed, increases ZMP and activates AMPK.57-59 In summary, experimental evidence in cell culture, animal experiments30 and observations in patients support the view that methotrexate and related compounds can increase AICAR production and activate AMPK in addition to their inhibitory effects on nucleotide synthesis, which is still the claimed basis for immune-suppressive activity. Nevertheless, the molecular mechanisms underlying the “anti-inflammatory” activity and the postulated targets cells (T-lymphocytes in psoriasis, macrophages and monocytes in rheumatoid arthritis) are still not completely understood. The increased AICAR production and appearance of its degradation product AICA in the general circulation after methotrexate are intriguing. Does the release indicate rapid apoptosis of target cells or a continuous excretory mechanism? In any event, AICA and perhaps the corresponding riboside could act as a kind of “second messenger” to neighboring cells or elsewhere. The riboside may be taken up by the ubiquitous adenosine transporter and phosphorylated by adenosine kinase to ZMP. The purine base AICA may be taken up by nucleobase transporters60 and converted via adenine-phosphoribosyl-transferase to ZMP. That AICA (and the riboside) could be the mediators or signals for clinical efficacy was already suggested by.55 Reactive oxygen species (ROS) as mediators for methotrexate were postulated,54,61 but this was not supported by subsequent research.
Figure 1. Metformin and methotrexate may interact at the cellular level via AMP-activated protein kinase. Simplified scheme43,49 of the proposed “anti-inflammatory” activity of methotrexate. Increase of AICAR (structure shown in the inset) by inhibition of the bifunctional enzyme AICAR transformylase (AICART or ATIC)50 is suggested to inhibit AMP and adenosine deaminase, leading to increased release of adenosine. Although it is generally postulated that the inhibition of AICART is by direct action of methotrexate polyglutamates, there are alternative explanations. These are based on the reversibility of the reaction (indicated by the double-headed arrow) and that AICART has rather low affinity for the polyglutamates. As an exception to all other reactions in mammalian one-carbon metabolism51 10-formyl-7, 8-dihydrofolate and dihydrofolate can be substrate and product, respectively.52 Increase of the product by blockade of the dihydrofolate reductase will slow the reaction and lead to increase in AICAR. FPGS, Folyl-Poly-Glutamyl- Synthase. Green arrows indicate activation; red lines with masthead indicate inhibition, broken line: disputed activity.
AICAr is often employed as an experimental tool in animal models for disease (see Table 1) or given i.v. in human studies (as acadesine)63,64 to activate AMPK as an “AMP mimetic.” Despite the expectation that the increased glucose uptake of human skeletal muscle after AICAr infusion was mediated by AMPK activation no increased phosphorylation of the master kinase was observed. Instead the extracellular signal-regulated kinase 1/2 (ERK1/2) was phosphorylated.65 A word of caution is appropriate here, as some of the experiments claiming AMPK activation as a result of AICAr treatment fail to rigorously prove the claim as direct activators (A-769662) have differential effects on pyrimidine metabolism and the purine salvage pathway compared with AICAr.66 Notably, however, is that AICAr can influence human glucose metabolism in vivo, underlining the hypothesis that it can serve as a signal55 not necessarily via AMPK.
Table 1. Metformin and AICar exhibit anti-inflammatory and immunosuppressive activity in experimental systems.
| Experimental system | Outcome/results | Reference |
|---|---|---|
| Induced autoimmune encephalomyelitis (EAE)in female mice |
Metformin (100 mg/kg body weight per day either i.p. or orally in 3 doses) improved disease score; inhibits immune cell infiltration into CNS, lowered increased levels of IL-17,IL-1β, IFNγ,TNFα and raised AMPK |
112 |
| Macrophage cell line Primary Macrophages |
Metformin and AICAr block LPS-induced secretion of TNFα, IL-6 and IFNγ. AMPK is activated |
112 |
| Naïve CD 3-positive T cells |
Metformin (1–10 mM) inhibits T-cell proliferation and IFNγ, IL-17 secretion upon stimulation |
112 |
| Mouse model for acute and relapsing colitis induced by 2,4,6 trinitrobenzene (TNBS) |
AICAr (500 mg/kg body weight daily i.p.) attenuates weight loss and improves colon inflammation. Levels of TNFα, IFNγ and IL-17 are significantly lowered in colon homogenates. AICAr increased pAMPK (Thr 172), decreased iNOS and elevated pNFΚ B p65. |
113 |
| Mouse model for chronic asthma induced by immunization with ovalbumin and fungal associated allergenic protease |
Metformin (250 mg/kg body weight) or AICAr (100 mg/kg) applied 30 min before each intranasal challenge twice a week decreased airway inflammation score, lymphocyte and eosinophile cell counts in bronchoalveolar lavage. Metformin (but not AICAr) decreased ovalbumin-specific IgG 1. |
114 |
| Experimental autoimmune uveitis in female mice, induced by human interphotoreceptor retinoid binding protein | AICAr (200 mg/kg body weight, injected daily i.p.) reduced severity of EAU (clinically and in histopathology, even 8 d after immunization) | 147 |
Metformin: A drug in search of a primary target
Mitochondrial complex I inhibition, the mitochondrial permeability pore, the metformin-copper hypothesis and phosphatase inhibition
Ever since guanidines were reported to inhibit energy transfer reactions of mitochondria in 1963,67 interest has focused on their effects mitochondrial function, including transport of divalent cations,68 the still unexplained reversal of respiratory inhibition by uncouplers or fatty acids69 and much more. Accepted today as a prime (not primary!) target is complex I. Complex I (NADH-ubichinone-reductase) is part of the respiratory chain located in the inner mitochondrial membrane. This huge multi-subunit complex (around 950kDa) pumps four protons out of the matrix into the inter-membrane space tightly coupled to the reduction of ubichinone and oxidation of NADH +H+. Metformin has inhibitory action (only) on complex I of mitochondria40,70 and can thereby change the cellular ATP production via oxidative phosphorylation. The mystery is that the inhibitory effect (which is never higher than about 40% of the maximal activity) can only be observed when metformin acts on “intact” isolated cells (permeabilized cells are unresponsive) or after organ perfusion with the drug. Mitochondria isolated from such pretreated cells appear irreversibly impregnated, as if metformin somehow, passing plasma cell membranes, acquired a novel property which cannot be mimicked by mixing cytosol and/or plasma membrane particles in the presence of metformin with naive mitochondria. This has led one group of the original discoverers to the suggestion that metformin somehow changed the mitochondrial machinery via phosphorylation/ dephosphorylation or proteolysis.70 One argument was that the degree of inhibition of complex I in isolated intact hepatocytes but not its kinetics was a clear function of the temperature, being optimal at 37°C and almost absent at 15°C. However, it was not known at the time that transport of metformin via OCT1 is highly temperature dependent.41 In any event, inhibition of complex I and the resulting changes of the AMP/ATP ratios are believed to be responsible for the activation of AMPK71 (see below). It should be noted that in contrast to AMPK activation, demonstrated in humans after chronic dosing with metformin, complex I inhibition of isolated mitochondria from skeletal muscle biopsies was not observed.72
Inhibition of complex I by metformin (similar and additive to blockade of cyclophylin D via cyclosporine A) sensitizes the permeability transition pore (PTP) in the inner mitochondrial membrane to attain a state of lower open probability.73,74 The pore plays an important role in cell death induced by free radicals, UV radiation, oxidative stress or ischemia. Results with metformin as a “protecting agent”18,20 suggest that there may be benefits of the drug beyond AMPK activation as explained in the following paragraph.
Inhibition of complex I of mitochondria isolated from cells pretreated with metformin leads to a block in “reverse electron flux”-induced superoxide anion formation.75 Superoxide anions may be formed by escaping electrons at complex I and III. When there is, in simple words, some kind of block downstream of complex I, electrons can flow back, reduce NAD to NADH, increase the ratio of ubiquinol to ubiquinone and contribute to superoxide formation. Superoxide anion cannot pass the inner mitochondrial membrane and is converted to hydrogen peroxide by manganese superoxide dismutase. In the cytosol H2O2 can activate redox-dependent transcription factors and is an indispensable signal (as is calcium) for T-cell activation.76,77 Thus, metformin by reducing ROS, can profoundly change T-cell responses in experimental systems.
Metformin and related biguanidines are excellent chelators (e.g., zinc and copper).78,79 Metformin can form stable complexes80 with copper (association constant around 1016 M−1). A more apolar (hydrophobic) dimeric metformin Cu 2+ complex, which must pass the plasma membrane, slowly forms inside cells and accumulates in mitochondria is now suggested to be the key mediator.81,82 If so, metformin could be a “prodrug,” but the final activity may be not via simple copper chelation.
Other biguanido metal complexes are very potent inhibitors of protein phosphatases83 suggesting that copper or other metal proteins could be among the long sought “primary” targets. Metformin can inhibit tyrosine phosphatases (such as recombinant human protein phosphatase 1 B) in vitro at very low concentrations (8 μM) but only if plasma membrane fragments (from oocytes) are added.84 One wonders about this truly magic potentiation, but perhaps, formation of an active “intermediate” with a phospholipid, a metal ion or a metallo protein occurred.
AMPK activation
Metformin activates a master regulator in metabolism, the AMPK,77,85 via increased phosphorylation of its catalytic α-subunit in position threonine-172, mediated by LKB1. This drug-induced activation is proven by animal experiments,86 in experimental cell systems71 as well as in humans after chronic dosage.87,88 AMPK consists of three subunits where the regulatory gamma subunit is functioning as a cellular AMP or ADP sensor, which changes its conformation upon binding AMP or ZMP or other activators such as salicylate89 and increases the availability of the α subunit to be substrate of upstream protein kinases. Metformin binds to the gamma subunit of AMPK in vitro90 but in contrast to direct activators such as A-769662,91 salicylate, ZMP, AMP or OSU-5392 activation of the enzyme in cell free systems has never been reported. Metformin cannot activate AMPK in cells where the gamma subunit of the enzyme is manipulated to be unresponsive to AMP. Consequently it is generally accepted71 that by lowering the cellular energy status and changing the ATP /AMP ratio via mitochondrial inhibition AMPK is activated. An alternative view, namely that metformin acts by inhibiting AMP deaminase93 is questioned.85 It is not necessary here to discuss the many downstream targets of AMPK, its connections to the mTOR signaling pathway94 or its role in the regulation of sirtuins.95 We also omitted that increased intracellular calcium (via calmodulin-dependent protein kinase β) can phosphorylate and activate AMPK, as no evidence so far was found that metformin acts through this pathway.
The physiological role of the AMPK is mainly to control metabolism, depending on nutrient supply. Thus it is natural to assume its main action in diabetics is on liver, adipose tissue and skeletal muscle. However, there is good evidence that this master protein kinase is also involved in the regulation of inflammatory responses and has controlling activity in dendritic cells, T-lymphocytes, macrophages, endothelial cells and monocytes. This is no surprise, as often these cells must either rapidly proliferate (and switch to more glycolysis96,97) or synthesize, e.g., cytokines for secretion and other mediators, which require major changes in metabolism and import of nutrients.
AMPK independent activities of metformin
Inhibition of mTORC1 (MammalianTarget of Rapamycin Complex I) or activation of protein phosphatase 2A by metformin (and phenformin) were shown to be independent from AMPK,98-100 as well as most of the stimulation of glucose uptake in myotubes.101
Is metformin a methotrexate mimetic?
Methotrexate increases plasma homocysteine levels in patients with psoriasis.102,103 Metformin (upon long-term treatment) can lower plasma vitamin B12104 and increases plasma homocysteine levels.105,106 The underlying assumption is that metformin impairs the intestinal reabsorption of vitamin B12. However, short-term studies in diabetic patients,107 non-diabetic males108 and patients with PCOS109 indicate that the increase in homocysteine levels is not correlated with vitamin B12 deficiency. Plasma levels of the most sensitive indicator of vitamin B12 deficiency, methymalonic acid, are not changed. Furthermore, the increase in homocysteine can be completely prevented or corrected by administration of folate and conventional doses of oral vitamin B12 are unable to attain “normal levels” in metformin treated patients.104 The reversal by folate (and not by vitamin B12) indicates that metformin inhibits target(s) in tetrahydrofolate-dependent metabolic pathways. These observations stimulated a study in which metformin was employed at rather high concentrations in several breast cancer cell lines. Here it was found, by analysis of metabolic changes, that metformin dramatically increased the intracellular levels of 5-formiminotetrahydrofolate.110 This metabolite is the product of histidine catabolism to glutamate and can be converted by two subsequent enzymatic steps to N,5 N10-methylene-tetrahydrofolate. This is the essential substrate (not cofactor) for thymidilate synthase. The authors did not speculate how metformin acts as an “antifolate” but as expected from the ability of metformin to block transport of endogenous substrates (see the section “Uptake via carriers or transporters, elimination via the kidney and “deep compartement”) they observed a marked decrease in intracellular tryptophan, which delivers formate to tetrahydrofolate in the pathway to kynurenines and is precursor for NAD+. Metformin also decreased glutathione which was confirmed by others.111
Consult Table 2 for a brief summary of the accepted targets for metformin and methotrexate.
Table 2. Molecular mechanisms and targets for metformin and methotrexate.
| Whereas for most of the drugs commonly used “ primary targets” (e.g., drug receptors) responsible for therapeutics can be identified,62 no such primary target is identified for metformin. It is therefore doubtful if drug agencies would accept metformin today, as a defined target is often conditional for approval. |
| Possible primary targets for metformin are the transporters. It may compete with the in-and efflux or excretion of endogenous substrates or nutrients and other drugs. If this results in so-called “off-target” effects, or is of no consequence or even contributes to therapeutic efficacy is yet not known. |
| Established “secondary” targets of metformin, important for therapeutics, are mitochondrial complex I (inhibition), also believed to contribute to lactic acidosis after toxic doses/plasma concentrations and AMPK (activation). |
| Accepted “primary targets” of methotrexate and its polyglutamates are tetrahydrofolate-dependent enzymes in nucleotide biosynthesis. A secondary target may be AMPK (activation) via increase of AICAR. |
Metformin is an anti-inflammatory drug
Evidence that metformin or/and AMPK activation via AICAr exhibit anti-inflammatory and immunosuppressive activity in cellular and animal experiments
In Table 1, we present key publications that support the view that metformin (and AICAr as an AMP-mimetic) is anti-inflammatory and immunosuppressive in various experimental mouse models of autoimmune diseases. The key role of AMPK in macrophages was demonstrated by Sag et al.115 They observed that anti-inflammatory stimuli such as IL-10 or TGFβ increase levels of phosphorylated AMPK within minutes, whereas stimulation of Toll-like receptors (TLR4) by lipopolysacccaride (LPS) decreases AMPK activation. TNFα, IL-6 secretion after LPS was increased by stably transfecting with a dominant negative AMPK but IL-10 decreased. Exactly opposite changes were observed by transfection with a constitutively active AMPK. In dendritic cells the anti-inflammatory cytokine IL-10 partially blocked the hypophosphorylation of AMPK by LPS and AICAr blocked the LPS induced maturation.116 The role of AMPK in T-lymphocytes is reviewed by ref. 117. All human white blood cells (neutrophils > monocytes > lymphocytes) express OCT-1 and can transport metformin.118 Human monocytes in vitro are highly sensitive to metformin. Concentrations observed in patients (10 mM) blocked tissue factor activity increase and TNF release after exposing cells to oxidized LDL or LPS.119 T-lymphocytes play a key role in psoriasis and other inflammatory processes therefore inhibition of complex I by metformin77 and reduced ROS formation are possible beneficial actions independent from AMPK.
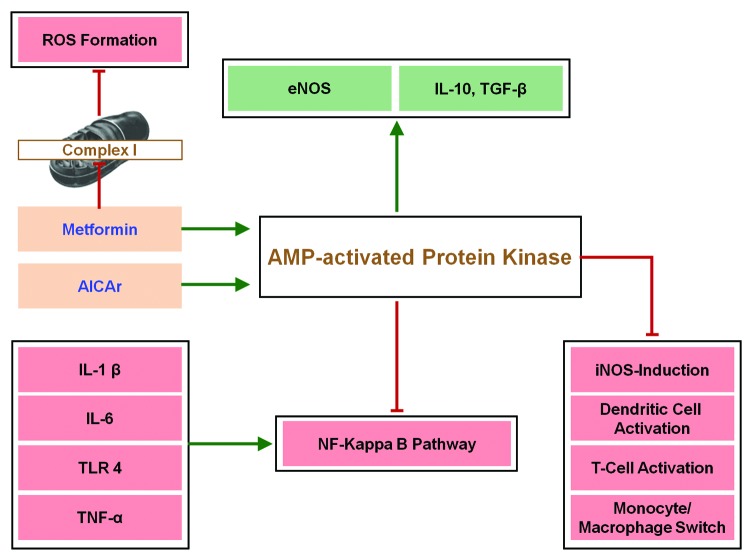
In Figure 2, we show a highly simplified scheme, how metformin and AICAr may exert anti-inflammatory and immunosuppressive activity. (For more molecular details, see ref. 121.)
Figure 2. Simplified scheme how metformin and AICAr act as anti-inflammatory or immunosuppressive agents. Green arrows indicate activation or stimulation; Red lines with masthead indicate inhibition. Metformin may not only stimulate AMPK but block excessive reactive oxygen species (ROS) formation by mitochondria, which can activate the Nuclear Factor (NF)–ΚB pathway (not shown). For details on the role of AMPK in macrophages, see ref. 115. Molecular detailed pathways are found in ref. 120.
Evidence that metformin decreases inflammatory markers in patients
In the first section of our Results, we mentioned briefly that patients with rheumatoid arthritis were successfully treated with phenformin. The intervention was based on the hypothesis that phenformin acts as a fibrinolytic drug. Indeed clinical symptoms of inflammation improved in some patients and the elevated erythrocyte sedimentation rate (ESR), substituted now by CRP, fell significantly. The same authors treated 27 patients with coronary-artery disease for four months with 1.500 mg metformin/day, followed by two months placebo. Twenty-one patients (there were seven drop-outs) demonstrated an increase of plasminogen activator and lower cholesterol. They also noted that the effect was greatest in patients with lowest fibrinolytic activity and that upon cessation of the drug it took a month or more until the starting level was reached.122 Significant decreases of tissue-type plasminogen activator inhibitor and of von Willebrand factor were later confirmed in a randomized double-blind controlled trial with obese subjects but no overt diabetes.121 Other symptoms of the metabolic syndrome, such as higher systolic blood pressure and elevated LDL-cholesterol also improved.123 In non-obese patients with type 2 diabetes, comparing metformin with repaglinide, markers of endothelial dysfunction (plasma plasminogen activator inhibitor-1antigen, von Willebrand factor) and TNFα were significantly decreased by metformin.124 A recent placebo-controlled study in which subjects with impaired fasting glucose (and other symptoms of metabolic syndrome) were treated for 3 mo with simvastatin received metformin as “add-on” experimental treatment. Here it was observed that even in the background of statin-induced “anti-inflammatory” activity, metformin inhibited lymphocyte cytokine release (TNF α, Interferon γ, Interleukin 2).117 There are a few studies with metformin in patients with PCOS. A randomized comparative study proves a significant decrease of IL-6,125 which is confirmed by observations in Taiwan.126 Others found significant decreases of hsCRP127,128 after metformin. With the possible exception of the early discovery of the anti-inflammatory and anti-fibrinolytic activities of the biguanides, all other studies with metformin were performed in patients or subjects with elevated basal insulin levels or increased insulin resistance. The generally accepted interpretation is that the decrease of inflammatory markers is mediated by increased insulin sensitivity or lowering of insulin levels in PCOS and weight loss. However, the animal models listed in Table 1 argue that anti-inflammatory effects of metformin are observable under experimental conditions where insulin and/or obesity are not drivers of inflammation.
Psoriasis and metabolic syndrome
Inflammatory signaling mechanisms play an important role in psoriasis129 and may contribute for developing so-called co-morbidities, such as higher cardiovascular risk or type 2 diabetes.130 Indeed—depending on severity—psoriasis is associated with metabolic syndrome131-133 and diabetes.134,135 Cohort studies prove an association of psoriasis with increased, mainly cardiovascular mortality.136 Interrogation of the psoriasis transcriptome in comparison to healthy controls revealed links to pathways involved in atherosclerosis, fatty acid metabolism, diabetes and hypertension.137 A recent meta-analysis including all results from this and other studies, employing ingenuity pathway analysis, found overrepresented canonical pathways for atherosclerosis, cancer and cardiovascular disease. Interestingly, pathways identified in the pathogenesis of multiple sclerosis (see Table 1) were also among the top five.138 Needless to say, that the same pathways are in the focus of current research with metformin.
Expanded intra-abdominal adipose tissue is suggested to play an important pro-inflammatory role in obese patients with metabolic syndrome and psoriasis. Correcting extreme obesity with bariatric surgery indeed improves psoriasis,139 and one study suggests that weight loss per se had an additive effect to improve PASI scores.140 As will be discussed below metformin is able to prevent progression of patients with metabolic syndrome to overt diabetes and can induce significant and permanent weight loss solely due to reduction of adipose tissue.
Metformin is the only drug with a favorable benefit/risk profile to prevent progression to type 2 diabetes for patients with metabolic syndrome and/or reduced glucose tolerance
Although glitazones and α-glucosidase inhibitors may prevent progression of prediabetes141 or favor regression142 to normoglycemia we will not discuss these drugs. Instead a few results of the metformin and lifestyle intervention study, DPP (diabetes prevention program)143 and its open label follow-up study144 are interesting in the context of our proposal. Metformin reduced incidence of metabolic syndrome and favored its regression but only in males.145 The authors speculated that hormonal alterations are responsible for this highly unexpected result. Weight loss is highly correlated with adherence to metformin and solely due to loss of adipose tissue.146
Conclusion
Metformin is a logical add-on therapy for overweight male patients with psoriasis and metabolic syndrome treated with methotrexate
Several of the newly introduced, highly expensive drugs for psoriasis (e.g., anti-cytokines) are “targeted therapeutics.” In contrast, although approved as an antidiabetic or employed to lower hyperinsulinemic conditions (PCOS) a primary target of the long off-patent and cheap metformin is not (yet) known. This probably explains complete lack of clinical observations not to mention human trials, based on its ability to act, among other, as a powerful anti-inflammatory and immunosuppressive drug. Without a doubt, metformin is—next to lifestyle intervention and for example, statins—a most logical choice for male overweight psoriatric patients with metabolic syndrome. This is ethically justified because evidence exists in the literature that it can prevent further detoriation of prediabetes or regress symptoms of metabolic syndrome.
We collected arguments to support the hypothesis that there may be additive anti-inflammatory effects of metformin for methotrexate-treated patients, which can be first tested in a prospective, open-label clinical pilot trial. Perhaps of equal importance, metformin decreases hepatotoxicity of methotrexate in animal experiments. This observation alone, taken up as a lead, could stimulate clinicians first to check retrospectively if metformin, once started, improved signs for hepatic injury after methotrexate.
Fifty years ago, researchers who treated patients with rheumatoid arthritis (successfully) and coronary artery disease with phenformin or metformin, respectively, could measure only a few laboratory parameters.
Today’s diagnostic armentarium (including the OMICS world) is much greater and in addition to for example, the PASI score, biomarkers systemically and locally can be followed. Both drugs have the same limiting factor, namely kidney function. A major concern is abdominal discomfort after metformin,20,† which may be reduced by starting with low doses (e.g., 0.5 g/day) and retarded formulations, slowly escalating up to the tolerated limit of 2.5 g/day. As a further safety measure, a metformin drug pause is suggested for the day methotrexate is given. Of less importance is the mechanistically unexplained increase of serum or plasma homocysteine after metformin because patients routinely take folate.
If our hypothesis is supported by favorable results in a pilot trial, perhaps one can expect less use of “targeted” drugs in the future. Even if our hypothesis of an additive anti-inflammatory effect is disproven, male, overweight patients with psoriasis and metabolic syndrome, given that metformin is tolerated, will profit from the intervention.
Acknowledgments
The authors would like to thank numerous colleagues who supplied us with additional information including papers in press or reprints. Prof. Alan Dronsfield informed us on the real facts behind the “Magic Cure” with flumamime. Librarians of the University of Innsbruck were helpful providing us with long forgotten, not easily obtainable literature. Paula Olbrich and Johannes Werner supported us in organizing the literature, design of figures and more. We are very grateful for their help.
Glossary
Abbreviations:
- AICA
5-aminoimidazole-4-carboxamide
- AICART
AICAR transformylase
- AICAr
5-aminoimidazole-4-carboxamide ribonucleoside
- AICAR
5-aminoimidazole-4-carboxamide ribonucleotide, synonymous with ZMP
- AICART
AICAR transformylase
- AMPK
AMP-activated protein kinase (Thr/Ser)
- DPP
Diabetes Prevention Program
- IL
interleukin
- LDL
low density lipoprotein
- LPS
lipopolysaccaride
- MATE
multidrug and toxin extrusion proteins
- mTORC1
Mammalian Target of Rapamycin Complex I
- MTX
methotrexate
- MTXPG
methotrexate polyglutamate
- OCT
organic cation transporter
- PASI
Psoriasis Area and Severity index
- PCOS
polycystic ovary syndrome
- PTP
permeability transition pore
- ROS
reactive oxygen species
- TLR
toll like receptor
- TNF
tumor necrosis factor
- PTP
permeability transition pore
Disclosure of Potential Conflicts of Interest
Both authors have received an honorarium by Pfizer for their contributions at a Symposium in Vienna on occasion of the 50th anniversary of methotrexate. No other conflicts of interest exist with respect to the above work.
Footnotes
If abdominal discomfort is a problem and since dermatology is interested in transdermal delivery or local therapy, the reader is referred to www.freepatentsonline.com/y2012/0283332.html.
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/23874
References
- 1.Li W, Han J, Hu FB, Curhan GC, Qureshi AA. Psoriasis and risk of type 2 diabetes among women and men in the United States: a population-based cohort study. J Invest Dermatol. 2012;132:291–8. doi: 10.1038/jid.2011.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brauchli YB, Jick SS, Curtin F, Meier CR. Association between use of thiazolidinediones or other oral antidiabetics and psoriasis: A population based case-control study. J Am Acad Dermatol. 2008;58:421–9. doi: 10.1016/j.jaad.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 3.Wiley SE, Murphy AN, Ross SA, van der Geer P, Dixon JE. MitoNEET is an iron-containing outer mitochondrial membrane protein that regulates oxidative capacity. Proc Natl Acad Sci U S A. 2007;104:5318–23. doi: 10.1073/pnas.0701078104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gubner R, August S, Ginsberg V. Therapeutic suppression of tissue reactivity. II. Effect of aminopterin in rheumatoid arthritis and psoriasis. Am J Med Sci. 1951;221:176–82. doi: 10.1097/00000441-195102000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Bailey CJ, Day C. Metformin:its botanical background. Pract Diabetes Int. 2004;21:115–7. doi: 10.1002/pdi.606. [DOI] [Google Scholar]
- 6.Reinwein H. Ueber die therapeutische Verwendbarkeit des Galegins bei Diabetikern. Munch Med Wochenschr. 1927;74:1794–5. [Google Scholar]
- 7.Dronsfield A, Ellis P. Drug discovery: metformin and the control of diabetes. Education in Chemistry. 2011;185–7. [Google Scholar]
- 8.Garcia EY. Flumamine, a new synthetic analgesic and anti-flu drug. J Philipp Med Assoc. 1950;26:287–93. [PubMed] [Google Scholar]
- 9.Dasgupta T, Chitnumsub P, Kamchonwongpaisan S, Maneeruttanarungroj C, Nichols SE, Lyons TM, et al. Exploiting structural analysis, in silico screening, and serendipity to identify novel inhibitors of drug-resistant falciparum malaria. ACS Chem Biol. 2009;4:29–40. doi: 10.1021/cb8002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hesse G, Taubmann G. Die Wirkung des Biguanids und seiner Dervate auf den Zuckerstoffwechsel. Naunyn-Schmiedebergs Arch Exp Path Pharmacol. 1929;(142):290–308. doi: 10.1007/BF02000097. [DOI] [Google Scholar]
- 11.Fearnley GR, Chakrabarti R. Fibrinolytic treatment of rheumatoid arthritis with phenformin plus ethyloestrenol. Lancet. 1966;2:757–61. doi: 10.1016/S0140-6736(66)90360-6. [DOI] [PubMed] [Google Scholar]
- 12.Fearnley GR, Chakrabarti R, Hocking E. PHENFORMIN IN RHEUMATOID ARTHRITIS A FIBRINOLYTIC APPROACH. Lancet. 1965;285:9–13. doi: 10.1016/S0140-6736(65)90921-9. [DOI] [PubMed] [Google Scholar]
- 13.Kersley GD. Phenformin (Dibotin) in polyarthritis. Ann Rheum Dis. 1968;27:374–6. doi: 10.1136/ard.27.4.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheurer M, Michel A, Brauch H-J, Ruck W, Sacher F. Occurrence and fate of the antidiabetic drug metformin and its metabolite guanylurea in the environment and during drinking water treatment. Water Res. 2012;46:4790–802. doi: 10.1016/j.watres.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Eggen T, Lillo C. Antidiabetic II drug metformin in plants: uptake and translocation to edible parts of cereals, oily seeds, beans, tomato, squash, carrots, and potatoes. J Agric Food Chem. 2012;60:6929–35. doi: 10.1021/jf301267c. [DOI] [PubMed] [Google Scholar]
- 16.Onken B, Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS One. 2010;5:e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menendez JA, Vazquez-Martin A. Rejuvenating regeneration: metformin activates endogenous adult stem cells. Cell Cycle. 2012;11:3521–2. doi: 10.4161/cc.21878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morales AI, Detaille D, Prieto M, Puente A, Briones E, Arévalo M, et al. Metformin prevents experimental gentamicin-induced nephropathy by a mitochondria-dependent pathway. Kidney Int. 2010;77:861–9. doi: 10.1038/ki.2010.11. [DOI] [PubMed] [Google Scholar]
- 19.Hadi NR, Al-Amran FG, Swadi A. Metformin ameliorates methotrexate-induced hepatotoxicity. J Pharmacol Pharmacother. 2012;3:248–53. doi: 10.4103/0976-500X.99426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu CL, Qiang L, Han W, Ming M, Viollet B, He YY. Role of AMPK in UVB-induced DNA damage repair and growth control. Oncogene. 2012;•••:1–8. doi: 10.1038/onc.2012.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaudhary SC, Kurundkar D, Elmets CA, Kopelovich L, Athar M. Metformin, an antidiabetic agent reduces growth of cutaneous squamous cell carcinoma by targeting mTOR signaling pathway. Photochem Photobiol. 2012;88:1149–56. doi: 10.1111/j.1751-1097.2012.01165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin M, Marais R. Metformin: a diabetes drug for cancer, or a cancer drug for diabetics? J Clin Oncol. 2012;30:2698–700. doi: 10.1200/JCO.2012.42.1677. [DOI] [PubMed] [Google Scholar]
- 23.Misso ML, Teede HJ, Hart R, Wong J, Rombauts L, Melder AM, et al. Status of clomiphene citrate and metformin for infertility in PCOS. Trends Endocrinol Metab. 2012;23:533–43. doi: 10.1016/j.tem.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Hirsch A, Hahn D, Kempná P, Hofer G, Nuoffer J-M, Mullis PE, et al. Metformin inhibits human androgen production by regulating steroidogenic enzymes HSD3B2 and CYP17A1 and complex I activity of the respiratory chain. Endocrinology. 2012;153:4354–66. doi: 10.1210/en.2012-1145. [DOI] [PubMed] [Google Scholar]
- 25.Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50:81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Mani H, Levy MJ, Davies MJ, Morris DH, Gray LJ, Bankart J, et al. Diabetes and cardiovascular events in women with polycystic ovary syndrome; a 20 years retrospective cohort study. Clin Endocrinol (Oxf) 2012 doi: 10.1111/cen.12068. In press. [DOI] [PubMed] [Google Scholar]
- 27.Fiehn C. Methotrexate transport mechanisms: the basis for targeted drug delivery and ß-folate-receptor-specific treatment. Clin Exp Rheumatol. 2010;28(Suppl 61):S40–5. [PubMed] [Google Scholar]
- 28.Ahlehoff O, Skov L, Gislason G, Lindhardsen J, Kristensen SL, Iversen L, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J Intern Med. 2013;273:197–204. doi: 10.1111/j.1365-2796.2012.02593.x. [DOI] [PubMed] [Google Scholar]
- 29.Ridker PM. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the cardiovascular inflammation reduction trial (CIRT) J Thromb Haemost. 2009;7(Suppl 1):332–9. doi: 10.1111/j.1538-7836.2009.03404.x. [DOI] [PubMed] [Google Scholar]
- 30.Russo GT, Minutoli L, Bitto A, Altavilla D, Alessi E, Giandalia A, et al. Methotrexate Increases Skeletal Muscle GLUT4 Expression and Improves Metabolic Control in Experimental Diabetes. J Nutr Metab. 2012;2012:132056. doi: 10.1155/2012/132056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zolk O. Disposition of metformin: variability due to polymorphisms of organic cation transporters. Ann Med. 2012;44:119–29. doi: 10.3109/07853890.2010.549144. [DOI] [PubMed] [Google Scholar]
- 32.Zhou M, Xia L, Wang J. Metformin transport by a newly cloned proton-stimulated organic cation transporter (plasma membrane monoamine transporter) expressed in human intestine. Drug Metab Dispos. 2007;35:1956–62. doi: 10.1124/dmd.107.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Damme K, Nies AT, Schaeffeler E, Schwab M. Mammalian MATE (SLC47A) transport proteins: impact on efflux of endogenous substrates and xenobiotics. Drug Metab Rev. 2011;43:499–523. doi: 10.3109/03602532.2011.602687. [DOI] [PubMed] [Google Scholar]
- 34.Dervieux T, Zablocki R, Kremer J. Red blood cell methotrexate polyglutamates emerge as a function of dosage intensity and route of administration during pulse methotrexate therapy in rheumatoid arthritis. Rheumatology (Oxford) 2010;49:2337–45. doi: 10.1093/rheumatology/keq216. [DOI] [PubMed] [Google Scholar]
- 35.van Roon EN, van de Laar MA. Methotrexate bioavailability. Clin Exp Rheumatol. 2010;28(Suppl 61):S27–32. [PubMed] [Google Scholar]
- 36.Chae JW, Baek IH, Lee BY, Cho SK, Kwon KI. Population PK/PD analysis of metformin using the signal transduction model. Br J Clin Pharmacol. 2012;74:815–23. doi: 10.1111/j.1365-2125.2012.04260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robert F, Fendri S, Hary L, Lacroix C, Andréjak M, Lalau JD. Kinetics of plasma and erythrocyte metformin after acute administration in healthy subjects. Diabetes Metab. 2003;29:279–83. doi: 10.1016/S1262-3636(07)70037-X. [DOI] [PubMed] [Google Scholar]
- 38.Sambol NC, Chiang J, O’Conner M, Liu CY, Lin ET, Goodman AM, et al. Pharmacokinetics and pharmacodynamics of metformin in healthy subjects and patients with noninsulin-dependent diabetes mellitus. J Clin Pharmacol. 1996;36:1012–21. doi: 10.1177/009127009603601105. [DOI] [PubMed] [Google Scholar]
- 39.Stepensky D, Friedman M, Raz I, Hoffman A. Pharmacokinetic-pharmacodynamic analysis of the glucose-lowering effect of metformin in diabetic rats reveals first-pass pharmacodynamic effect. Drug Metab Dispos. 2002;30:861–8. doi: 10.1124/dmd.30.8.861. [DOI] [PubMed] [Google Scholar]
- 40.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348:607–14. doi: 10.1042/0264-6021:3480607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sogame Y, Kitamura A, Yabuki M, Komuro S. A comparison of uptake of metformin and phenformin mediated by hOCT1 in human hepatocytes. Biopharm Drug Dispos. 2009;30:476–84. doi: 10.1002/bdd.684. [DOI] [PubMed] [Google Scholar]
- 42.Davidoff F, Haas D. Phenethylbiguanide effect on mitochondrial Ca+ and Mg2+ content. Biochem Pharmacol. 1979;28:3457–63. doi: 10.1016/0006-2952(79)90086-8. [DOI] [PubMed] [Google Scholar]
- 43.Chan ESL, Cronstein BN. Methotrexate--how does it really work? Nat Rev Rheumatol. 2010;6:175–8. doi: 10.1038/nrrheum.2010.5. [DOI] [PubMed] [Google Scholar]
- 44.Baggott JE, Morgan SL, Sams WM, Linden J. Urinary adenosine and aminoimidazolecarboxamide excretion in methotrexate-treated patients with psoriasis. Arch Dermatol. 1999;135:813–7. doi: 10.1001/archderm.135.7.813. [DOI] [PubMed] [Google Scholar]
- 45.Hiratsuka C, Fukuwatari T, Shibata K. Fate of dietary tryptophan in young Japanese women. Int J Tryptophan Res. 2012;5:33–47. doi: 10.4137/IJTR.S10497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huo T, Cai S, Lu X, Sha Y, Yu M, Li F. Metabonomic study of biochemical changes in the serum of type 2 diabetes mellitus patients after the treatment of metformin hydrochloride. J Pharm Biomed Anal. 2009;49:976–82. doi: 10.1016/j.jpba.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 47.Marchetti P, Masiello P, Benzi L, Cecchetti P, Fierabracci V, Giannarelli R, et al. Effects of metformin therapy on plasma amino acid pattern in patients with maturity-onset diabetes. Drugs Exp Clin Res. 1989;15:565–70. [PubMed] [Google Scholar]
- 48.Song IS, Lee Y, Shin M-H, Kim H, Ahn YG, Park I, et al. Pharmacogenetics meets metabolomics: discovery of tryptophan as a new endogenous OCT2 substrate related to metformin disposition. PLoS One. 2012;7:e36637. doi: 10.1371/journal.pone.0036637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen S, O’Brien T, Yap LM, Prince HM, McCormack CJ. The use of methotrexate in dermatology: a review. Australas J Dermatol. 2012;53:1–18. doi: 10.1111/j.1440-0960.2011.00839.x. [DOI] [PubMed] [Google Scholar]
- 50.Greasley SE, Horton P, Ramcharan J, Beardsley GP, Benkovic SJ, Wilson IA. Crystal structure of a bifunctional transformylase and cyclohydrolase enzyme in purine biosynthesis. Nat Struct Biol. 2001;8:402–6. doi: 10.1038/87555. [DOI] [PubMed] [Google Scholar]
- 51.Tibbetts AS, Appling DR. Compartmentalization of Mammalian folate-mediated one-carbon metabolism. Annu Rev Nutr. 2010;30:57–81. doi: 10.1146/annurev.nutr.012809.104810. [DOI] [PubMed] [Google Scholar]
- 52.Baggott JE, Tamura T. Evidence for the hypothesis that 10-formyldihydrofolate is the in vivo substrate for aminoimidazolecarboxamide ribotide transformylase. Exp Biol Med (Maywood) 2010;235:271–7. doi: 10.1258/ebm.2009.009151. [DOI] [PubMed] [Google Scholar]
- 53.Andersson SE, Johansson LH, Lexmüller K, Ekström GM. Anti-arthritic effect of methotrexate: is it really mediated by adenosine? Eur J Pharm Sci. 2000;9:333–43. doi: 10.1016/S0928-0987(99)00073-1. [DOI] [PubMed] [Google Scholar]
- 54.Phillips DC, Woollard KJ, Griffiths HR. The anti-inflammatory actions of methotrexate are critically dependent upon the production of reactive oxygen species. Br J Pharmacol. 2003;138:501–11. doi: 10.1038/sj.bjp.0705054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morgan SL, Oster RA, Lee JY, Alarcón GS, Baggott JE. The effect of folic acid and folinic acid supplements on purine metabolism in methotrexate-treated rheumatoid arthritis. Arthritis Rheum. 2004;50:3104–11. doi: 10.1002/art.20516. [DOI] [PubMed] [Google Scholar]
- 56.Beckers A, Organe S, Timmermans L, Vanderhoydonc F, Deboel L, Derua R, et al. Methotrexate enhances the antianabolic and antiproliferative effects of 5-aminoimidazole-4-carboxamide riboside. Mol Cancer Ther. 2006;5:2211–7. doi: 10.1158/1535-7163.MCT-06-0001. [DOI] [PubMed] [Google Scholar]
- 57.Racanelli AC, Rothbart SB, Heyer CL, Moran RG. Therapeutics by cytotoxic metabolite accumulation: pemetrexed causes ZMP accumulation, AMPK activation, and mammalian target of rapamycin inhibition. Cancer Res. 2009;69:5467–74. doi: 10.1158/0008-5472.CAN-08-4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rothbart SB, Racanelli AC, Moran RG. Pemetrexed indirectly activates the metabolic kinase AMPK in human carcinomas. Cancer Res. 2010;70:10299–309. doi: 10.1158/0008-5472.CAN-10-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuznetsov JN, Leclerc GJ, Leclerc GM, Barredo JC. AMPK and Akt determine apoptotic cell death following perturbations of one-carbon metabolism by regulating ER stress in acute lymphoblastic leukemia. Mol Cancer Ther. 2011;10:437–47. doi: 10.1158/1535-7163.MCT-10-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yao SYM, Ng AML, Cass CE, Baldwin SA, Young JD. Nucleobase transport by human equilibrative nucleoside transporter 1 (hENT1) J Biol Chem. 2011;286:32552–62. doi: 10.1074/jbc.M111.236117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Griffiths HR, Dunston CR, Bennett SJ, Grant MM, Phillips DC, Kitas GD. Free radicals and redox signalling in T-cells during chronic inflammation and ageing. Biochem Soc Trans. 2011;39:1273–8. doi: 10.1042/BST0391273. [DOI] [PubMed] [Google Scholar]
- 62.Gregori-Puigjané E, Setola V, Hert J, Crews BA, Irwin JJ, Lounkine E, et al. Identifying mechanism-of-action targets for drugs and probes. Proc Natl Acad Sci U S A. 2012;109:11178–83. doi: 10.1073/pnas.1204524109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cuthbertson DJ, Babraj JA, Mustard KJW, Towler MC, Green KA, Wackerhage H, et al. 5-aminoimidazole-4-carboxamide 1-beta-D-ribofuranoside acutely stimulates skeletal muscle 2-deoxyglucose uptake in healthy men. Diabetes. 2007;56:2078–84. doi: 10.2337/db06-1716. [DOI] [PubMed] [Google Scholar]
- 64.Boon H, Bosselaar M, Praet SFE, Blaak EE, Saris WHM, Wagenmakers AJM, et al. Intravenous AICAR administration reduces hepatic glucose output and inhibits whole body lipolysis in type 2 diabetic patients. Diabetologia. 2008;51:1893–900. doi: 10.1007/s00125-008-1108-7. [DOI] [PubMed] [Google Scholar]
- 65.Babraj JA, Mustard K, Sutherland C, Towler MC, Chen S, Smith K, et al. Blunting of AICAR-induced human skeletal muscle glucose uptake in type 2 diabetes is dependent on age rather than diabetic status. Am J Physiol Endocrinol Metab. 2009;296:E1042–8. doi: 10.1152/ajpendo.90811.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paglia G, Hrafnsdóttir S, Magnúsdóttir M, Fleming RMT, Thorlacius S, Palsson BØ, et al. Monitoring metabolites consumption and secretion in cultured cells using ultra-performance liquid chromatography quadrupole-time of flight mass spectrometry (UPLC-Q-ToF-MS) Anal Bioanal Chem. 2012;402:1183–98. doi: 10.1007/s00216-011-5556-4. [DOI] [PubMed] [Google Scholar]
- 67.Pressman BC. The Effects Energy of Guanidine and Alkylguanidines Transfer Reactions of Mitochondria. J Biol Chem. 1963;238:401–9. [Google Scholar]
- 68.Davidoff F. Effects of guanidine derivatives on mitochondrial function. Ca2+ uptake and release. J Biol Chem. 1974;249:6406–15. [PubMed] [Google Scholar]
- 69.Davidoff F. Effects of guanidine derivatives on mitochondrial function. II. Reversal of guanidine-derivative inhibiton by free fatty acids. J Clin Invest. 1968;47:2344–58. doi: 10.1172/JCI105919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.El-Mir MY, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223–8. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 71.Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 2012;122:253–70. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Larsen S, Rabøl R, Hansen CN, Madsbad S, Helge JW, Dela F. Metformin-treated patients with type 2 diabetes have normal mitochondrial complex I respiration. Diabetologia. 2012;55:443–9. doi: 10.1007/s00125-011-2340-0. [DOI] [PubMed] [Google Scholar]
- 73.Guigas B, Detaille D, Chauvin C, Batandier C, De Oliveira F, Fontaine E, et al. Metformin inhibits mitochondrial permeability transition and cell death: a pharmacological in vitro study. Biochem J. 2004;382:877–84. doi: 10.1042/BJ20040885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li B, Chauvin C, De Paulis D, De Oliveira F, Gharib A, Vial G, et al. Inhibition of complex I regulates the mitochondrial permeability transition through a phosphate-sensitive inhibitory site masked by cyclophilin D. Biochim Biophys Acta. 2012;1817:1628–34. doi: 10.1016/j.bbabio.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 75.Batandier C, Guigas B, Detaille D, El-Mir MY, Fontaine E, Rigoulet M, et al. The ROS production induced by a reverse-electron flux at respiratory-chain complex 1 is hampered by metformin. J Bioenerg Biomembr. 2006;38:33–42. doi: 10.1007/s10863-006-9003-8. [DOI] [PubMed] [Google Scholar]
- 76.Kaminski MM, Sauer SW, Klemke CD, Süss D, Okun JG, Krammer PH, et al. Mitochondrial reactive oxygen species control T cell activation by regulating IL-2 and IL-4 expression: mechanism of ciprofloxacin-mediated immunosuppression. J Immunol. 2010;184:4827–41. doi: 10.4049/jimmunol.0901662. [DOI] [PubMed] [Google Scholar]
- 77.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–23. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sweeney D, Raymer ML, Lockwood TD. Antidiabetic and antimalarial biguanide drugs are metal-interactive antiproteolytic agents. Biochem Pharmacol. 2003;66:663–77. doi: 10.1016/S0006-2952(03)00338-1. [DOI] [PubMed] [Google Scholar]
- 79.Lockwood TD. The lysosome among targets of metformin: new anti-inflammatory uses for an old drug? Expert Opin Ther Targets. 2010;14:467–78. doi: 10.1517/14728221003774135. [DOI] [PubMed] [Google Scholar]
- 80.Lu LP, Yang P, Qin SD, Zhu ML. Bis[1,1-dimethylbiguanide(1-)-kappa2N2,N5]copper(II) monohydrate. Acta Crystallogr C. 2004;60:m219–20. doi: 10.1107/S0108270104006729. [DOI] [PubMed] [Google Scholar]
- 81.Logie L, Harthill J, Patel K, Bacon S, Hamilton DL, Macrae K, et al. Cellular responses to the metal-binding properties of metformin. Diabetes. 2012;61:1423–33. doi: 10.2337/db11-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rena G, Pearson ER, Sakamoto K. Molecular action and pharmacogenetics of metformin: current understanding of an old drug. Diabetes Management. 2012;2:439–52. doi: 10.2217/dmt.12.42. [DOI] [Google Scholar]
- 83.Lu L, Gao X, Zhu M, Wang S, Wu Q, Xing S, et al. Exploration of biguanido-oxovanadium complexes as potent and selective inhibitors of protein tyrosine phosphatases. Biometals. 2012;25:599–610. doi: 10.1007/s10534-012-9548-4. [DOI] [PubMed] [Google Scholar]
- 84.Holland W, Morrison T, Chang Y, Wiernsperger N, Stith BJ. Metformin (Glucophage) inhibits tyrosine phosphatase activity to stimulate the insulin receptor tyrosine kinase. Biochem Pharmacol. 2004;67:2081–91. doi: 10.1016/j.bcp.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 85.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–62. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074–81. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- 88.Boyle JG, Logan PJ, Jones GC, Small M, Sattar N, Connell JMC, et al. AMP-activated protein kinase is activated in adipose tissue of individuals with type 2 diabetes treated with metformin: a randomised glycaemia-controlled crossover study. Diabetologia. 2011;54:1799–809. doi: 10.1007/s00125-011-2126-4. [DOI] [PubMed] [Google Scholar]
- 89.Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–22. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Y, Wang Y, Bao C, Xu Y, Shen H, Chen J, et al. Metformin interacts with AMPK through binding to γ subunit. Mol Cell Biochem. 2012;368:69–76. doi: 10.1007/s11010-012-1344-5. [DOI] [PubMed] [Google Scholar]
- 91.Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3:403–16. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 92.Lee KH, Hsu EC, Guh JH, Yang HC, Wang D, Kulp SK, et al. Targeting energy metabolic and oncogenic signaling pathways in triple-negative breast cancer by a novel adenosine monophosphate-activated protein kinase (AMPK) activator. J Biol Chem. 2011;286:39247–58. doi: 10.1074/jbc.M111.264598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ouyang J, Parakhia RA, Ochs RS. Metformin activates AMP kinase through inhibition of AMP deaminase. J Biol Chem. 2011;286:1–11. doi: 10.1074/jbc.M110.121806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem J. 2012;441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- 95.Cantó C, Auwerx J. Targeting sirtuin 1 to improve metabolism: all you need is NAD(+)? Pharmacol Rev. 2012;64:166–87. doi: 10.1124/pr.110.003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eigenbrodt E, Glossmann H. Glycolysis–one of the keys to cancer? Trends Pharmacol Sci. 1980;1:240–5. doi: 10.1016/0165-6147(80)90009-7. [DOI] [Google Scholar]
- 97.Kamiński MM, Sauer SW, Kamiński M, Opp S, Ruppert T, Grigaravičius P, et al. T cell activation is driven by an ADP-dependent glucokinase linking enhanced glycolysis with mitochondrial reactive oxygen species generation. Cell Rep. 2012;2:1300–15. doi: 10.1016/j.celrep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 98.Kalender A, Selvaraj A, Kim SY, Gulati P, Brûlé S, Viollet B, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kickstein E, Krauss S, Thornhill P, Rutschow D, Zeller R, Sharkey J, et al. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc Natl Acad Sci U S A. 2010;107:21830–5. doi: 10.1073/pnas.0912793107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ben Sahra I, Regazzetti C, Robert G, Laurent K, Le Marchand-Brustel Y, Auberger P, et al. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011;71:4366–72. doi: 10.1158/0008-5472.CAN-10-1769. [DOI] [PubMed] [Google Scholar]
- 101.Turban S, Stretton C, Drouin O, Green CJ, Watson ML, Gray A, et al. Defining the contribution of AMP-activated protein kinase (AMPK) and protein kinase C (PKC) in regulation of glucose uptake by metformin in skeletal muscle cells. J Biol Chem. 2012;287:20088–99. doi: 10.1074/jbc.M111.330746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Refsum H, Helland S, Ueland PM. Fasting plasma homocysteine as a sensitive parameter of antifolate effect: a study of psoriasis patients receiving low-dose methotrexate treatment. Clin Pharmacol Ther. 1989;46:510–20. doi: 10.1038/clpt.1989.179. [DOI] [PubMed] [Google Scholar]
- 103.Hroch M, Chladek J, Simkova M, Vaneckova J, Grim J, Martinkova J. A pilot study of pharmacokinetically guided dosing of oral methotrexate in the initial phase of psoriasis treatment. J Eur Acad Dermatol Venereol. 2008;22:19–24. doi: 10.1111/j.1468-3083.2007.02264.x. [DOI] [PubMed] [Google Scholar]
- 104.Reinstatler L, Qi YP, Williamson RS, Garn JV, Oakley GP., Jr. Association of biochemical B₁₂ deficiency with metformin therapy and vitamin B₁₂ supplements: the National Health and Nutrition Examination Survey, 1999-2006. Diabetes Care. 2012;35:327–33. doi: 10.2337/dc11-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wulffelé MG, Kooy A, Lehert P, Bets D, Ogterop JC, Borger van der Burg B, et al. Effects of short-term treatment with metformin on serum concentrations of homocysteine, folate and vitamin B12 in type 2 diabetes mellitus: a randomized, placebo-controlled trial. J Intern Med. 2003;254:455–63. doi: 10.1046/j.1365-2796.2003.01213.x. [DOI] [PubMed] [Google Scholar]
- 106.de Jager J, Kooy A, Lehert P, Wulffelé MG, van der Kolk J, Bets D, et al. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial. BMJ. 2010;340:c2181. doi: 10.1136/bmj.c2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aarsand AK, Carlsen SM. Folate administration reduces circulating homocysteine levels in NIDDM patients on long-term metformin treatment. J Intern Med. 1998;244:169–74. doi: 10.1046/j.1365-2796.1998.00361.x. [DOI] [PubMed] [Google Scholar]
- 108.Carlsen SM, Følling I, Grill V, Bjerve KS, Schneede J, Refsum H. Metformin increases total serum homocysteine levels in non-diabetic male patients with coronary heart disease. Scand J Clin Lab Invest. 1997;57:521–7. doi: 10.3109/00365519709084603. [DOI] [PubMed] [Google Scholar]
- 109.Palomba S, Falbo A, Giallauria F, Russo T, Tolino A, Zullo F, et al. Effects of metformin with or without supplementation with folate on homocysteine levels and vascular endothelium of women with polycystic ovary syndrome. Diabetes Care. 2010;33:246–51. doi: 10.2337/dc09-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Corominas-Faja B, Quirantes-Piné R, Oliveras-Ferraros C, Vazquez-Martin A, Cufí S, Martin-Castillo B, et al. Metabolomic fingerprint reveals that metformin impairs one-carbon metabolism in a manner similar to the antifolate class of chemotherapy drugs. Aging (Albany NY) 2012;4:480–98. doi: 10.18632/aging.100472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zakikhani M, Bazile M, Hashemi S, Javeshghani S, Avizonis D, St Pierre J, et al. Alterations in cellular energy metabolism associated with the antiproliferative effects of the ATM inhibitor KU-55933 and with metformin. PLoS One. 2012;7:e49513. doi: 10.1371/journal.pone.0049513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nath N, Khan M, Paintlia MK, Singh I, Hoda MN, Giri S. Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. J Immunol. 2009;182:8005–14. doi: 10.4049/jimmunol.0803563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bai A, Ma AG, Yong M, Weiss CR, Ma Y, Guan Q, et al. AMPK agonist downregulates innate and adaptive immune responses in TNBS-induced murine acute and relapsing colitis. Biochem Pharmacol. 2010;80:1708–17. doi: 10.1016/j.bcp.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 114.Park CS, Bang BR, Kwon HS, Moon KA, Kim TB, Lee KY, et al. Metformin reduces airway inflammation and remodeling via activation of AMP-activated protein kinase. Biochem Pharmacol. 2012;84:1660–70. doi: 10.1016/j.bcp.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 115.Sag D, Carling D, Stout RD, Suttles J. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181:8633–41. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–9. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Krysiak R, Okopien B. Lymphocyte-suppressing and systemic anti-inflammatory effects of high-dose metformin in simvastatin-treated patients with impaired fasting glucose. Atherosclerosis. 2012;225:403–7. doi: 10.1016/j.atherosclerosis.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 118.Engler JR, Zannettino ACW, Bailey CG, Rasko JEJ, Hughes TP, White DL. OCT-1 function varies with cell lineage but is not influenced by BCR-ABL. Haematologica. 2011;96:213–20. doi: 10.3324/haematol.2010.033290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Arai M, Uchiba M, Komura H, Mizuochi Y, Harada N, Okajima K. Metformin, an antidiabetic agent, suppresses the production of tumor necrosis factor and tissue factor by inhibiting early growth response factor-1 expression in human monocytes in vitro. J Pharmacol Exp Ther. 2010;334:206–13. doi: 10.1124/jpet.109.164970. [DOI] [PubMed] [Google Scholar]
- 120.Salt IP, Palmer TM. Exploiting the anti-inflammatory effects of AMP-activated protein kinase activation. Expert Opin Investig Drugs. 2012;21:1155–67. doi: 10.1517/13543784.2012.696609. [DOI] [PubMed] [Google Scholar]
- 121.Charles MA, Morange P, Eschwège E, André P, Vague P, Juhan-Vague I. Effect of weight change and metformin on fibrinolysis and the von Willebrand factor in obese nondiabetic subjects: the BIGPRO1 Study. Biguanides and the Prevention of the Risk of Obesity. Diabetes Care. 1998;21:1967–72. doi: 10.2337/diacare.21.11.1967. [DOI] [PubMed] [Google Scholar]
- 122.Chakrabarti R. Hockin, Elisabeth D, Fearnley GR. Fibrinolytic effect of metformin in coronary-artery disease. Lancet. 1965;286:256–9. doi: 10.1016/S0140-6736(65)92383-4. [DOI] [PubMed] [Google Scholar]
- 123.Fontbonne A, Diouf I, Baccara-Dinet M, Eschwege E, Charles MA. Effects of 1-year treatment with metformin on metabolic and cardiovascular risk factors in non-diabetic upper-body obese subjects with mild glucose anomalies: a post-hoc analysis of the BIGPRO1 trial. Diabetes Metab. 2009;35:385–91. doi: 10.1016/j.diabet.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 124.Lund SS, Tarnow L, Stehouwer CDA, Schalkwijk CG, Teerlink T, Gram J, et al. Impact of metformin versus repaglinide on non-glycaemic cardiovascular risk markers related to inflammation and endothelial dysfunction in non-obese patients with type 2 diabetes. Eur J Endocrinol. 2008;158:631–41. doi: 10.1530/EJE-07-0815. [DOI] [PubMed] [Google Scholar]
- 125.Luque-Ramírez M, Escobar-Morreale HF. Treatment of polycystic ovary syndrome (PCOS) with metformin ameliorates insulin resistance in parallel with the decrease of serum interleukin-6 concentrations. Horm Metab Res. 2010;42:815–20. doi: 10.1055/s-0030-1262855. [DOI] [PubMed] [Google Scholar]
- 126.Lin YS, Tsai SJ, Lin MW, Yang CT, Huang MF, Wu MH. Interleukin-6 as an early chronic inflammatory marker in polycystic ovary syndrome with insulin receptor substrate-2 polymorphism. Am J Reprod Immunol. 2011;66:527–33. doi: 10.1111/j.1600-0897.2011.01059.x. [DOI] [PubMed] [Google Scholar]
- 127.Diamanti-Kandarakis E, Paterakis T, Alexandraki K, Piperi C, Aessopos A, Katsikis I, et al. Indices of low-grade chronic inflammation in polycystic ovary syndrome and the beneficial effect of metformin. Hum Reprod. 2006;21:1426–31. doi: 10.1093/humrep/del003. [DOI] [PubMed] [Google Scholar]
- 128.Banaszewska B, Pawelczyk L, Spaczynski RZ, Duleba AJ. Comparison of simvastatin and metformin in treatment of polycystic ovary syndrome: prospective randomized trial. J Clin Endocrinol Metab. 2009;94:4938–45. doi: 10.1210/jc.2009-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Perera GK, Di Meglio P, Nestle FO. Psoriasis. Ann Rev Pathol. 2012;7:385–422. doi: 10.1146/annurev-pathol-011811-132448. [DOI] [PubMed] [Google Scholar]
- 130.Davidovici BB, Sattar N, Prinz JC, Puig L, Emery P, Barker JN, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130:1785–96. doi: 10.1038/jid.2010.103. [DOI] [PubMed] [Google Scholar]
- 131.Langan SM, Seminara NM, Shin DB, Troxel AB, Kimmel SE, Mehta NN, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556–62. doi: 10.1038/jid.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gisondi P, Tessari G, Conti A, Piaserico S, Schianchi S, Peserico A, et al. Prevalence of metabolic syndrome in patients with psoriasis: a hospital-based case-control study. Br J Dermatol. 2007;157:68–73. doi: 10.1111/j.1365-2133.2007.07986.x. [DOI] [PubMed] [Google Scholar]
- 133.Love TJ, Qureshi AA, Karlson EW, Gelfand JM, Choi HK. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419–24. doi: 10.1001/archdermatol.2010.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ford ES, Zhao G, Tsai J, Li C. Vitamin D and all-cause mortality among adults in USA: findings from the National Health and Nutrition Examination Survey Linked Mortality Study. Int J Epidemiol. 2011;40:998–1005. doi: 10.1093/ije/dyq264. [DOI] [PubMed] [Google Scholar]
- 135.Azfar RS, Seminara NM, Shin DB, Troxel AB, Margolis DJ, Gelfand JM. Increased risk of diabetes mellitus and likelihood of receiving diabetes mellitus treatment in patients with psoriasis. Arch Dermatol. 2012;148:995–1000. doi: 10.1001/archdermatol.2012.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ahlehoff O. Psoriasis and Cardiovascular Disease: epidemiological studies. Dan Med Bull. 2011;58:B4347. [PubMed] [Google Scholar]
- 137.Suárez-Fariñas M, Li K, Fuentes-Duculan J, Hayden K, Brodmerkel C, Krueger JG. Expanding the psoriasis disease profile: interrogation of the skin and serum of patients with moderate-to-severe psoriasis. J Invest Dermatol. 2012;132:2552–64. doi: 10.1038/jid.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tian S, Krueger JG, Li K, Jabbari A, Brodmerkel C, Lowes MA, et al. Meta-analysis derived (MAD) transcriptome of psoriasis defines the “core” pathogenesis of disease. PLoS One. 2012;7:e44274. doi: 10.1371/journal.pone.0044274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Farias MM, Achurra P, Boza C, Vega A, de la Cruz C. Psoriasis following bariatric surgery: clinical evolution and impact on quality of life on 10 patients. Obes Surg. 2012;22:877–80. doi: 10.1007/s11695-012-0646-8. [DOI] [PubMed] [Google Scholar]
- 140.Gisondi P, Del Giglio M, Di Francesco V, Zamboni M, Girolomoni G. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88:1242–7. doi: 10.3945/ajcn.2008.26427. [DOI] [PubMed] [Google Scholar]
- 141.Phung OJ, Sood NA, Sill BE, Coleman CI. Oral anti-diabetic drugs for the prevention of Type 2 diabetes. Diabet Med. 2011;28:948–64. doi: 10.1111/j.1464-5491.2011.03303.x. [DOI] [PubMed] [Google Scholar]
- 142.Phung OJ, Baker WL, Tongbram V, Bhardwaj A, Coleman CI. Oral antidiabetic drugs and regression from prediabetes to normoglycemia: a meta-analysis. Ann Pharmacother. 2012;46:469–76. doi: 10.1345/aph.1Q554. [DOI] [PubMed] [Google Scholar]
- 143.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Diabetes Prevention Program Research Group The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care. 2012;35:723–30. doi: 10.2337/dc11-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, et al. Diabetes Prevention Program Research Group The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med. 2005;142:611–9. doi: 10.7326/0003-4819-142-8-200504190-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Diabetes Prevention Program Research Group Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731–7. doi: 10.2337/dc11-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Suzuki J, Yoshimura T, Simeonova M, Takeuchi K, Murakami Y, Morizane Y, et al. Aminoimidazole carboxamide ribonucleotide ameliorates experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2012;53:4158–69. doi: 10.1167/iovs.11-9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pollak M. Investigating Metformin for Cancer Prevention and Treatment:The End of the Beginning. Cancer Discovery. 2012;2:778–90. doi: 10.1158/2159-8290.CD-12-0263. [DOI] [PubMed] [Google Scholar]
- 149.Emami Riedmaier A, Fisel P, Niles AT, Schaeffeler E, Schwab M. Metformin and cancer:from the old medicine cabinet to pharmacological pitfalls and prospects. Trends Pharmacol Sci. 2013;34:126–35. doi: 10.1016/j.tips.2012.11.005. [DOI] [PubMed] [Google Scholar]