Abstract
Background
Advanced glycation end products (AGEs) have been shown to be a predictor of cardiovascular risk in Caucasian subjects. In this study we examine whether the existing reference values are useable for non-Caucasian ethnicities. Furthermore, we assessed whether gender and smoking affect AGEs.
Methods
AGEs were determined by a non-invasive method of skin auto-fluorescence (AF). AF was measured in 200 Arabs, 99 South Asians, 35 Filipinos and 14 subjects of other/mixed ethnicity in the Qatar Metabolomics Study on Diabetes (QMDiab). Using multivariate linear regression analysis and adjusting for age and type 2 diabetes, we assessed whether ethnicity, gender and smoking were associated with AF.
Results
The mean AF was 2.27 arbitrary units (AU) (SD: 0.63). Arabs and Filipinos had a significant higher AF than the South Asian population (0.25 arbitrary units (AU) (95% CI: 0.11‒0.39), p = 0.001 and 0.34 (95% CI: 0.13‒0.55), p = 0.001 respectively). Also, AF was significantly higher in females (0.41 AU (95% CI: 0.29‒0.53), p < 0.001). AF associated with smoking (0.21 AU (95% CI: 0.01‒0.41), p = 0.04) and increased with the number of pack-years smoked (p = 0.02).
Conclusions
This study suggests that the existing reference values should take ethnicity, gender and smoking into account. Larger studies in specific ethnicities are necessary to create ethnic- and gender-specific reference values.
Keywords: skin auto-fluorescence, advanced glycation endproducts, type 2 diabetes, gender differences, ethnicity, smoking, epidemiology
Introduction
Advanced Glycation End products (AGEs) accumulate in tissues as a result of aging,1 and have been found to increase more rapidly in patients with conditions such as type 2 diabetes (T2D) and cardiovascular disease.2-5 Where HbA1c is a “summary” of the average sugar levels over the past 3–6 mo, AGEs in the skin can serve as a marker of the average sugar level of the past 5‒10 y.6
The AGE Reader is a noninvasive device that measures tissue accumulation of Advanced Glycation End products (AGEs) by skin auto-fluorescence (AF).7,8 Based on the amount of accumulated AGEs, the AGE Reader may provide an immediate vascular risk prediction. The AGE Reader is a relatively new instrument that is currently widely used in research on diabetes and its complications. Several studies have already shown the usefulness of skin AF as a new marker in predicting diabetic complications,7,9,10 and it turned out to be a stronger predictor than HbA1c.10 For the cardiovascular risk prediction, the AGE Reader uses a single reference curve, identical for both females and males, obtained from Caucasian subjects.11 In the initial reference curve it was found that gender and smoking had a limited effect at most.11 Therefore, clinical evaluations are based on this single reference curve, without taking other factors such as ethnicity, gender or smoking into account.
The prevalence of T2D in Qatar and the Gulf States is among the highest in the world and is growing rapidly.12 Clinical studies in this region are increasingly focusing on noninvasive techniques for early detection and complication prevention of T2D. The AGE Reader could be a practical adjuvant in clinical practice in the Arab population. The aim of this study is to examine whether the existing reference curve also applies to the largest ethnic populations seen in Qatar. Furthermore, we will assess whether gender and smoking affect skin AF in this particular population.
Results
For analysis 348 subjects (173 males and 175 females) were included. The subject characteristics are shown in Tables 1, 2, and 3. The median age was 47.7 y (90% range: 25.6–66.4). Skin AF is given in arbitrary units (AU) and the mean AF was 2.27 AU (SD: 0.63).
Table 1. Subject characteristics.
| Characteristics | Type 2 Diabetes n = 178 |
Non Type 2 Diabetes n = 170 |
|---|---|---|
| Age (years) |
54.0 (34.8–70.7) |
38.5 (23.3–62.5) |
| Gender (% female) |
75 (44.1%) |
98 (55.1%) |
| Ethnicity |
|
|
| Arab (%) |
85 (50.0%) |
115 (64.6%) |
| South Asian (%) |
65 (38.2%) |
34 (19.1%) |
| Filipino (%) |
13 (7.6%) |
22 (12.4%) |
| Other or mix (%) |
7 (4.1%) |
7 (3.9%) |
| Current smoking (% smoking) |
15 (8.8%) |
15 (8.4%) |
| Skin auto-fluorescence (AU) |
2.32 (1.57–3.72) |
2.05 (1.45–3.16) |
| Reflectance (%) | 8.81 (6.28–17.67) | 9.72 (6.46–18.31) |
Arab: Bahrain, Egypt, Iraq, Jordan, Kuwait, Lebanon, Morocco, Oman, Palestine, Qatar, Saudi Arabia, Somalia, Sudan, Syria, Tunisia, United Arab Emirates and Yemen South Asian: India, Bangladesh, Nepal, Pakistan, Sri Lanka Values represent median (90% range) or number of subjects (%) AU: Arbitrary Units, i.e., the output units of the AGE reader.
Table 2. Subject characteristics stratified by ethnicity.
| Characteristics | Arab n = 200 |
South Asian n = 99 |
Filipino n = 35 |
|||
|---|---|---|---|---|---|---|
| Type 2 Diabetes n = 85 |
Non Type 2 Diabetes n = 115 |
Type 2 Diabetes n = 65 |
Non Type 2 Diabetes n = 34 |
Type 2 Diabetes n = 13 |
Non Type 2 Diabetes n = 22 |
|
| Age (years) |
53.9 (34.2–71.2) |
39.1 (22.6–64.4) |
52.6 (35.2–69.1) |
39.0 (25.0–57.6) |
49.3 (37.8–63.0) |
37.2 (23.2–57.8) |
| Gender (% female) |
51 (60.0%) |
70 (60.9%) |
11 (16.9%) |
13 (38.2%) |
11 (84.6%) |
13 (59.1%) |
| Smoking (%) |
8 (9.4%) |
10 (8.7%) |
6 (9.2%) |
2 (5.9%) |
1 (7.7%) |
2 (9.1%) |
| Skin AF (AU) |
2.64 (1.67–4.02) |
2.09 (1.47–3.31) |
1.90 (1.43–3.31) |
1.80 (1.36–2.59) |
2.56 (1.97–3.58) |
2.18 (1.62–3.37) |
| Reflectance (%) | 10.43 (6.47–22.02) | 10.54 (6.84–20.42) | 7.13 (6.21–10.54) | 7.82 (6.05–15.85) | 10.81 (8.16–15.05) | 9.41 (6.51–14.96) |
Arab: Bahrain, Egypt, Iraq, Jordan, Kuwait, Lebanon, Morocco, Oman, Palestine, Qatar, Saudi Arabia, Somalia, Sudan, Syria, Tunisia, United Arab Emirates and Yemen South Asian: India, Bangladesh, Nepal, Pakistan, Sri Lanka. Values represent median (90% range) or number of subjects (%) AU: Arbitrary Units, i.e., the output units of the AGE reader.
Table 3. Subject characteristics stratified by gender.
| Characteristics | Female n = 173 |
Male n = 175 |
||
|---|---|---|---|---|
| Type 2 Diabetes n = 75 |
Non Type 2 Diabetes n = 98 |
Type 2 Diabetes n = 95 |
Non Type 2 Diabetes n = 80 |
|
| Age (years) |
52.6 (33.7–70.6) |
36.5 (19.5–61.2) |
54.4 (34.9–71.1) |
41.7 (25.9–64.3) |
| Ethnicity |
|
|
|
|
| Arab (%) |
51 (68.0%) |
70 (71.4%) |
34 (35.8%) |
45 (56.3%) |
| South Asian (%) |
11 (14.7%) |
13 (13.3%) |
54 (56.8%) |
21 (26.3%) |
| Filipino (%) |
11 (14.7%) |
13 (13.3%) |
2 (2.1%) |
9 (11.3%) |
| Other or mix (%) |
2 (2.7%) |
2 (2.0%) |
5 (5.3%) |
5 (6.3%) |
| Smoking (%) |
1 (1.3%) |
4 (4.1%) |
14 (14.7%) |
11 (13.8%) |
| Skin AF (AU) |
2.64 (1.77–3.95) |
2.11 (1.60–3.41) |
2.02 (1.49–3.51) |
1.85 (1.37–2.60) |
| Reflectance (%) | 9.91 (6.40–22.89) | 10.49 (6.73–22.85) | 7.90 (6.26–14.59) | 9.00 (6.28–14.51) |
Arab: Bahrain, Egypt, Iraq, Jordan, Kuwait, Lebanon, Morocco, Oman, Palestine, Qatar, Saudi Arabia, Somalia, Sudan, Syria, Tunisia, United Arab Emirates and Yemen. South Asian: India, Bangladesh, Nepal, Pakistan, Sri Lanka. Values represent median (90% range) or number of subjects (%). AU: Arbitrary Units, i.e., the output units of the AGE reader.
Results from the multivariate linear regression model show that the Arabs and Filipinos had a significant higher skin AF then the South Asian population (0.25 AU (95% CI: 0.11‒0.39), p = 0.001 and 0.34 AU (95% CI: 0.13‒0.54), p = 0.001 respectively) (Table 4 and Fig. 1). These differences were found among both subjects with type 2 diabetes and controls (Table 4), and among both females and males (Table 5). There was no significant difference in skin AF between Arabs and Filipinos.
Table 4. Factors related to skin auto-fluorescence (in arbitrary units) using multivariate analyses in all subjects and stratified by type 2 diabetes state.
| Risk Factor | Effect size (CI) |
P-value |
Effect size (CI) |
P-value |
Effect size (CI) |
P-value |
|---|---|---|---|---|---|---|
| All subjects, n = 348 | Type 2 Diabetes, n = 178 | Non Type 2 Diabetes, n = 170 | ||||
| Age (years) |
0.021 (0.016, 0.026) |
< 0.001 |
0.023 (0.014, 0.033) |
< 0.001 |
0.019 (0.014, 0.025) |
< 0.001 |
| Gender (Female = reference) |
-0.41 (-0.53, -0.29) |
< 0.001 |
-0.43 (-0.65, -0.21) |
< 0.001 |
-0.39 (-0.53, -0.25) |
< 0.001 |
| Ethnicity (South Asians = reference) |
|
|
|
|
|
|
| Arabs |
0.25 (0.11, 0.39 |
0.001 |
0.30 (0.07, 0.52) |
0.01 |
0.20 (0.02, 0.37) |
0.03 |
| Filipinos |
0.34 (0.13, 0.55) |
0.001 |
0.31 (-0.08, 0.69) |
0.11 |
0.34 (0.11, 0.58) |
0.005 |
| Smoking (non-smoking = reference) |
0.21 (0.01, 0.41) |
0.04 |
0.30 (-0.03, 0.63) |
0.07 |
0.17 (-0.07, 0.42) |
0.16 |
| Diabetes (non-diabetic = reference) |
0.14 (0.01, 0.26) |
0.03 |
N/A |
N/A |
N/A |
N/A |
| Reflectance (%) | 0.84 (-0.59, 2.26) | 0.25 | -0.20 (-2.53, 2.13) | 0.87 | 1.79 (0.02, 3.56) | 0.05 |
Arab: Bahrain, Egypt, Iraq, Jordan, Kuwait, Lebanon, Morocco, Oman, Palestine, Qatar, Saudi Arabia, Somalia, Sudan, Syria, Tunisia, United Arab Emirates and Yemen. South Asian: India, Bangladesh, Nepal, Pakistan, Sri Lanka. Values represent regression coefficients (95% confidence interval) and their corresponding p-values. For categorical or dichotomous variables, the effect estimates represent the difference in skin AF compared with the reference group.
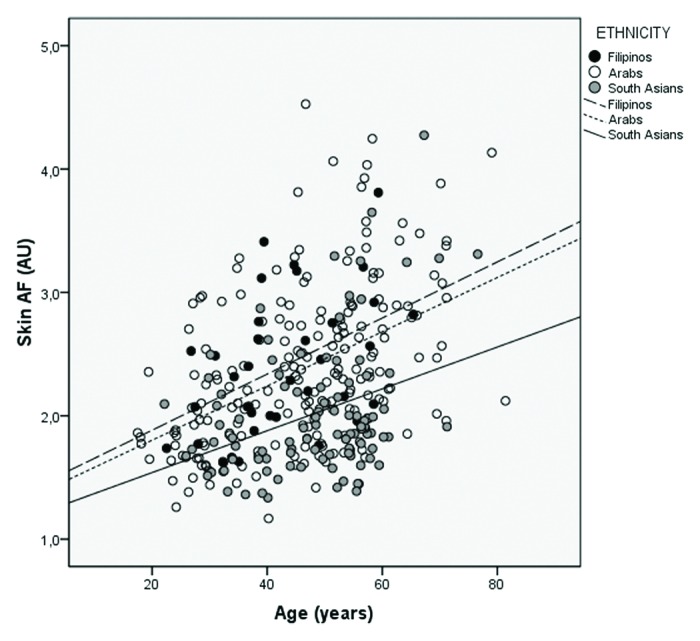
Figure 1. Scatterplot of skin auto-fluorescence by age, stratified by ethnicity.
Table 5. Factors related to skin auto-fluorescence (in arbitrary units) using multivariate analyses stratified gender.
| Risk Factor | Effect size (CI) |
P-value |
Effect size (CI) |
P-value |
|---|---|---|---|---|
| Males, n = 175 | Females, n = 173 | |||
| Age (years) |
0.023 (0.016, 0.033) |
< 0.001 |
0.019 (0.012, 0.027) |
< 0.001 |
| Ethnicity (South Asians = reference) |
|
|
|
|
| Arabs |
0.15 (-0.04, 0.33) |
0.11 |
0.30 (0.51, 0.55) |
0.02 |
| Filipinos |
0.35 (0.02, 0.68) |
0.04 |
0.33 (0.21, 0.65) |
0.04 |
| Smoking (non-smoking = reference) |
0.21 (0.00, 0.41) |
0.05 |
0.32 (-0.18, 0.81) |
0.21 |
| Diabetes (non-diabetic = reference) |
0.13 (-0.04, 0.30) |
0.13 |
0.16 (-0.04, 0.35) |
0.11 |
| Reflectance (%) | 3.42 (0.57, 6.27) | 0.02 | 0.15 (-1.59, 1.89) | 0.86 |
Arab: Bahrain, Egypt, Iraq, Jordan, Kuwait, Lebanon, Morocco, Oman, Palestine, Qatar, Saudi Arabia, Somalia, Sudan, Syria, Tunisia, United Arab Emirates and Yemen. South Asian: India, Bangladesh, Nepal, Pakistan, Sri Lanka. Values represent regression coefficients (95% confidence interval) and their corresponding p-values. For categorical or dichotomous variables, the effect estimates represent the difference in skin AF compared with the reference group.
Skin AF was significantly higher in women compared with men [0.42 AU (95% CI: 0.30–0.54), p < 0.001] (Table 4 and Fig. 2). Stratified analyses showed that these differences were comparable among subjects with and without type 2 diabetes (Table 4) and among South Asians, Arabs and Filipinos (Table 6).
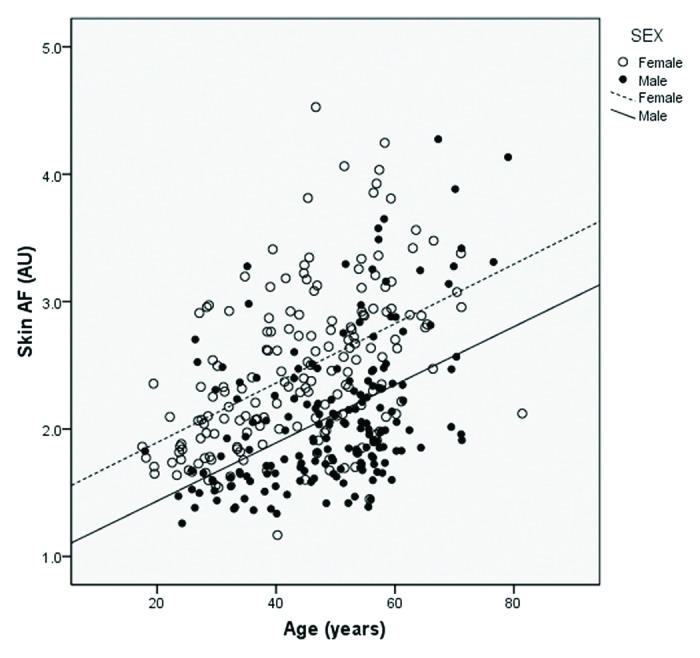
Figure 2. Scatterplot of skin auto-fluorescence by age, stratified by gender.
Table 6. Factors related to skin auto-fluorescence (in arbitrary units) using multivariate analyses stratified by ethnicity.
| Risk Factor | Effect size (CI) | P-value | Effect size (CI) | P-value | Effect size (CI) | P-value |
|---|---|---|---|---|---|---|
|
South Asians, n = 99 |
Arabs, n = 200 |
Filipinos, n = 35 |
||||
| Age (years) |
0.016 (0.005, 0.026) |
< 0.001 |
0.022 (0.016, 0.029) |
< 0.001 |
0.018 (-0.001, 0.038) |
0.06 |
| Gender (Female = reference) |
-0.26 (-0.51, -0.01) |
0.05 |
-0.46 (-0.63, -0.29) |
< 0.001 |
-0.31 (-0.70, 0.08) |
0.11 |
| Smoking (non-smoking = reference) |
0.30 (-0.06, 0.65) |
0.10 |
0.23 (-0.05, 0.50) |
0.10 |
N/A |
N/A |
| Diabetes (non-diabetic = reference) |
0.18 (-0.05, 0.42) |
0.13 |
0.15 (-0.03, 0.32) |
0.10 |
0.05 (-0.39, 0.49) |
0.82 |
| Reflectance (%) | 6.59 (2.01, 11.17) | 0.005 | -0.03 (-1.69, 1.64) | 0.97 | 3.84 (-3.26, 10.93) | 0.28 |
Arab: Bahrain, Egypt, Iraq, Jordan, Kuwait, Lebanon, Morocco, Oman, Palestine, Qatar, Saudi Arabia, Somalia, Sudan, Syria, Tunisia, United Arab Emirates and Yemen. South Asian: India, Bangladesh, Nepal, Pakistan, Sri Lanka. Values represent regression coefficients (95% confidence interval) and their corresponding p-values. For categorical or dichotomous variables, the effect estimates represent the difference in skin AF compared with the reference group. In the Filipino group there were only three smokers and therefore the variable was removed from the analyses.
Current smoking was positively associated with skin AF [0.21 AU (95% CI: 0.01–0.41), p = 0.04] compared with the non-smoking or past smoking. The effect of smoking was similar among males and females, and among Arabs and South Asians (Table 5 and 6). We found that the skin AF was positively associated with the number of pack-years smoked (p = 0.02). Furthermore, those subjects with 15 or more pack-years had a significantly higher AF than those with less than 2.5 pack years [0.34 AU (95% CI: 0.04‒0.64), p = 0.03].
Discussion
This study showed that overall skin AF is higher in Arabs and Filipinos compared with South Asians. In this specific population, females had a higher skin AF than males. Furthermore, we demonstrated that smoking and pack-years were positively associated with a higher skin AF.
To our knowledge this is the largest study performed so far that uses the AGE reader in South Asians, Filipinos and Arabs. Several methodological issues need to be discussed. First of all, we did not have a Caucasian reference group in our study. Also, we had a relatively high percentage of subjects with type 2 diabetes in our population, but the findings regarding gender and ethnicity were consistent both the type 2 diabetes subjects and controls. Regarding other potential confounding factor, we also found significant differences in BMI, serum creatinine and HbA1c between the three ethnicity groups. South Asians had a higher serum creatinine and HbA1c levels and Arabs had higher BMI compared with the other two groups. However, AF was not associated to these potential confounders and adjusting for these variables in the model did not materially change the effect sizes (data not shown). We also found significant differences between males and females in BMI (females being higher) and HbA1c (males being higher), however the effect size did not change after adjusting for these variables in the model (data not shown). Finally, AF was not associated with skin color, i.e., reflectance, and adjusting for this factor did not alter the effect estimates. We thus conclude that it is unlikely that the associations found with ethnicity and gender are due to residual confounding.
Limited data exists on skin AF in patients with different ethnicity other than Caucasian and no prior data exist on skin AF in South Asian, Arab or Filipinos. Most studies in non-Caucasian population were performed in East Asian (Chinese and Japanese) subjects.13-16 Yue et al. validated the reference values in a Chinese population and found that these values were similar with those found in Caucasians.13 A study on skin AF and gestational diabetes found that women with ethnicity other than white European had a significant higher skin AF, however the paper did not report which other ethnicities were included.17 Several other studies have been performed in non-Caucasian ethnicities, but were assessing cardiovascular risk of a particular disease in comparison to controls.14-16 Therefore, these studies did not apply any reference curve. In the current study, the South Asian population followed a very similar line as the reference curve created by Koetsier et al. in Caucasians.11 In contrast, Arabs and Filipinos had a significant higher skin AF compared with South Asians. These findings were independent of the presence of type 2 diabetes or gender, and suggest that multi-ethnic studies should take these ethnic differences into account. Furthermore, the manufacturer of the AGE reader describes clinical cut-offs in skin AF for various levels of cardiovascular risk. These cut-offs would need to be validated for non-Caucasian ethnicities.
Regarding gender, this study is in contrast with previous findings that gender has no effect on skin AF.11,13,14 Koetsier et al. found a small, non-significant gender differences in certain sub-populations within current smokers with women having a higher AF.11 We found a strong effect of gender on skin AF, with females having higher skin AF compared with men. This is the first time a substantial gender difference in skin AF has been found. This finding was robust, among both subjects with type 2 diabetes and controls, and across all ethnicities. Cardiovascular mortality among males and females in this region is similar.18 The increase skin AF found in women is therefore unlikely to be a true representation of an increased cardiovascular risk. Most importantly, the use of body lotions and skin creams on the forearm has been shown to strongly increase skin AF.19 This increase in skin AF can return to normal after cleaning, but in some cases can remain up to two weeks after usage.19 We requested all subjects that had used creams or lotions to wash their forearm thoroughly with soap prior to measurement. However, it cannot be excluded that women in Qatar may use more or other lotions and creams than men, thus increasing the value measured by skin AF.
Regarding smoking, this study further supports the earlier finding that smoking results in increased AGE accumulation.11,20 Previous studies found this increase only in particular sub-groups (e.g., females). We showed that a smoking dosage of more than 15 pack-years increases AF. Also, current smokers had a higher AF than past and non-smokers. We found no evidence for a gender-smoking interaction, but this also may be due to a lack of statistical power.
In conclusion, we found evidence for relatively large differences between ethnicities and gender for AF. Epidemiological studies on cardiovascular risks using the AGE reader need to take ethnicity and gender into account in their analyses as covariates. Finally, larger studies in specific ethnicities are necessary to create ethnic-specific reference values.
Methods
Study design
This study was embedded in the Qatar Metabolomics Study on Diabetes (QMDiab), a cross-sectional case-control study with 374 subjects. The study was realized by collaboration between the Dermatology Department of Hamad Medical Corporation and Weill Cornell Medical College Qatar. Patients were asked to enroll between February 2012 and June 2012. The study has been approved by the Institutional Review Board (IRB) of Hamad Medical Corporation and Weill Cornell Medical College Qatar and is accordance with the Helsinki Declaration of 1975. Written informed consent was obtained from all participants.
Population for analyses
Inclusion criteria for was having age above 18 y and sufficient knowledge of Arabic or English. No other exclusion criteria were implemented. Of the 374 subjects, 348 had a valid measurement recorded. The AGE Reader measurement failed in 26 subjects as a result of skin darkness. The AGE Reader is not able to perform a valid measurement in skin with a reflection below 6% [usually skin type V and VI (Fitzpatrick Scale)].
AGE reader
The skin AF results were obtained with the noninvasive AGE Reader (Diagnoptics, the Netherlands). The method has been described in detail previously.8 In brief, the assessment of the tissue AGEs is based on a technique that enlists the fluorescence properties of certain AGEs. When the measurement is performed, the excitation light source emits UV light with a peak wavelength of 370 nm and illuminates 4cm2 of the skin. The emission and reflected excitation light is detected by an optical fiber and transmitted to the spectrometer. Skin AF is calculated as the ratio between the total emission intensity (420‒600 nm) and the total excitation intensity (300‒420 nm). Skin AF is expressed in arbitrary units (AU) and the mean skin AF in this study was 2.27 AU (standard deviation: 0.63). Before the measurement, subjects were asked whether they had used creams and/or lotions on their forearm. If so, they were requested to wash their arms thoroughly with soap prior to measurement. For each subject the skin AF was measured by the AGE Reader on the volar side of the dominant forearm. We performed a triple measurement (the Pearson correlation coefficients between the three measurements were between 0.97 and 0.99) and the mean from these three assessments was used for the analyses.
Questionnaires
Information regarding health and lifestyle (including the presence of type 2 diabetes) and socio-demographics was collected by questionnaires. Subjects were asked to provide details regarding the country of birth of their parents and grandparents. Based on the country of birth of their parents and grandparents, we determined the ethnicity of the subject. We divided the ethnicities into three groups; Arabs (Bahrain, Egypt, Iraq, Jordan, Kuwait, Lebanon, Morocco, Oman, Palestine, Qatar, Saudi Arabia, Somalia, Sudan, Syria, Tunisia, United Arab Emirates and Yemen), South Asians (India, Bangladesh, Nepal, Pakistan, Sri Lanka), and Filipinos. Smoking was reported as “current smoker,” “past smoker” and “non-smoker.” We computed pack-years to describe the individual smoking load. Pack-years were calculated as average number of packs of cigarettes smoked per day multiplied by the number of years the person has smoked.
Statistical analysis
First, using multivariate linear regression analysis and adjusting for age and T2D, we assessed whether ethnicity, gender, smoking and skin reflectance were associated with skin AF. Ethnicity was divided into three groups; Arabs, South Asians, and Filipinos. South Asians were chosen as reference, since they were closest to the reference curve obtained in Caucasians.11 Gender was stratified into males and females (reference group) and smoking was stratified into current smokers and current non-smokers (reference group). Then, we performed the same multivariate analyses to assess the effect of ethnicity and gender stratified for presence of type 2 diabetes. We also examined the effect of ethnicity on skin AF stratified for gender and vice versa. Finally, multivariate linear regression analysis (adjusting for age, ethnicity, gender and type 2 diabetes) was used to assess the effect of smoking load on AF. For 95 subjects (current and past smokers) pack-years were created to describe the individual smoking load. Subgroups were stratified into tertiles as follows: less than 2.5 pack years; between 2.5 and 15 pack years; and 15 pack years or more. All statistical analyses were performed using the Statistical Package of Social Sciences version 20 (SPSS Inc., Chicago, IL, USA).
Acknowledgments
We would like to thank all the participants of this study. This study was supported by “Biomedical Research Program” funds at Weill Cornell Medical College in Qatar, a program funded by the Qatar Foundation. Support for some of the experiments was provided by the Weill Cornell Medical College in Qatar (WCMC-Q) bioinformatics and virtual metabolomics core which is funded by the Qatar Foundation. The statements made herein are solely the responsibility of the authors. We express our appreciation to Zeinab El-Din and Walaa El Maraghy for their assistance in data collection. Futhermore, we thank all the support staff at the dermatology department of Hamad Medical Center.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/26046
References
- 1.Dunn JA, McCance DR, Thorpe SR, Lyons TJ, Baynes JW. Age-dependent accumulation of N epsilon-(carboxymethyl)lysine and N epsilon-(carboxymethyl)hydroxylysine in human skin collagen. Biochemistry. 1991;30:1205–10. doi: 10.1021/bi00219a007. [DOI] [PubMed] [Google Scholar]
- 2.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 3.Dyer DG, Dunn JA, Thorpe SR, Bailie KE, Lyons TJ, McCance DR, Baynes JW. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J Clin Invest. 1993;91:2463–9. doi: 10.1172/JCI116481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCance DR, Dyer DG, Dunn JA, Bailie KE, Thorpe SR, Baynes JW, Lyons TJ. Maillard reaction products and their relation to complications in insulin-dependent diabetes mellitus. J Clin Invest. 1993;91:2470–8. doi: 10.1172/JCI116482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monnier VM, Vishwanath V, Frank KE, Elmets CA, Dauchot P, Kohn RR. Relation between complications of type I diabetes mellitus and collagen-linked fluorescence. N Engl J Med. 1986;314:403–8. doi: 10.1056/NEJM198602133140702. [DOI] [PubMed] [Google Scholar]
- 6.Genuth S, Sun W, Cleary P, Sell DR, Dahms W, Malone J, Sivitz W, Monnier VM, DCCT Skin Collagen Ancillary Study Group Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes. 2005;54:3103–11. doi: 10.2337/diabetes.54.11.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lutgers HL, Gerrits EG, Graaff R, Links TP, Sluiter WJ, Gans RO, Bilo HJ, Smit AJ. Skin autofluorescence provides additional information to the UK Prospective Diabetes Study (UKPDS) risk score for the estimation of cardiovascular prognosis in type 2 diabetes mellitus. Diabetologia. 2009;52:789–97. doi: 10.1007/s00125-009-1308-9. [DOI] [PubMed] [Google Scholar]
- 8.Meerwaldt R, Graaff R, Oomen PH, Links TP, Jager JJ, Alderson NL, Thorpe SR, Baynes JW, Gans RO, Smit AJ. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia. 2004;47:1324–30. doi: 10.1007/s00125-004-1451-2. [DOI] [PubMed] [Google Scholar]
- 9.Gerrits EG, Lutgers HL, Kleefstra N, Graaff R, Groenier KH, Smit AJ, Gans RO, Bilo HJ. Skin autofluorescence: a tool to identify type 2 diabetic patients at risk for developing microvascular complications. Diabetes Care. 2008;31:517–21. doi: 10.2337/dc07-1755. [DOI] [PubMed] [Google Scholar]
- 10.Meerwaldt R, Lutgers HL, Links TP, Graaff R, Baynes JW, Gans RO, Smit AJ. Skin autofluorescence is a strong predictor of cardiac mortality in diabetes. Diabetes Care. 2007;30:107–12. doi: 10.2337/dc06-1391. [DOI] [PubMed] [Google Scholar]
- 11.Koetsier M, Lutgers HL, de Jonge C, Links TP, Smit AJ, Graaff R. Reference values of skin autofluorescence. Diabetes Technol Ther. 2010;12:399–403. doi: 10.1089/dia.2009.0113. [DOI] [PubMed] [Google Scholar]
- 12.Scully T. Diabetes in numbers. Nature. 2012;485:S2–3. doi: 10.1038/485S2a. [DOI] [PubMed] [Google Scholar]
- 13.Yue X, Hu H, Koetsier M, Graaff R, Han C. Reference values for the Chinese population of skin autofluorescence as a marker of advanced glycation end products accumulated in tissue. Diabet Med. 2011;28:818–23. doi: 10.1111/j.1464-5491.2010.03217.x. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka K, Tani Y, Asai J, Nemoto F, Kusano Y, Suzuki H, Hayashi Y, Asahi K, Nakayama M, Miyata T, et al. Skin autofluorescence is associated with severity of vascular complications in Japanese patients with Type 2 diabetes. Diabet Med. 2012;29:492–500. doi: 10.1111/j.1464-5491.2011.03448.x. [DOI] [PubMed] [Google Scholar]
- 15.Ohnuki Y, Nagano R, Takizawa S, Takagi S, Miyata T. Advanced glycation end products in patients with cerebral infarction. Intern Med. 2009;48:587–91. doi: 10.2169/internalmedicine.48.1390. [DOI] [PubMed] [Google Scholar]
- 16.Jiang J, Chen P, Chen J, Yu X, Xie D, Mei C, Xiong F, Shi W, Zhou W, Liu X, et al. Accumulation of tissue advanced glycation end products correlated with glucose exposure dose and associated with cardiovascular morbidity in patients on peritoneal dialysis. Atherosclerosis. 2012;224:187–94. doi: 10.1016/j.atherosclerosis.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 17.de Ranitz-Greven WL, Bos DC, Poucki WK, Visser GH, Beulens JW, Biesma DH, de Valk HW. Advanced glycation end products, measured as skin autofluorescence, at diagnosis in gestational diabetes mellitus compared with normal pregnancy. Diabetes Technol Ther. 2012;14:43–9. doi: 10.1089/dia.2011.0105. [DOI] [PubMed] [Google Scholar]
- 18.The World Health Organization. Causes of death 2008 Health statistics and informatics Department 2011.
- 19.Noordzij MJ, Lefrandt JD, Graaff R, Smit AJ. Dermal factors influencing measurement of skin autofluorescence. Diabetes Technol Ther. 2011;13:165–70. doi: 10.1089/dia.2010.0123. [DOI] [PubMed] [Google Scholar]
- 20.Cerami C, Founds H, Nicholl I, Mitsuhashi T, Giordano D, Vanpatten S, Lee A, Al-Abed Y, Vlassara H, Bucala R, et al. Tobacco smoke is a source of toxic reactive glycation products. Proc Natl Acad Sci U S A. 1997;94:13915–20. doi: 10.1073/pnas.94.25.13915. [DOI] [PMC free article] [PubMed] [Google Scholar]


