Abstract
Racetubes, a conventional system employing hollow glass tubes, are typically used for monitoring circadian rhythms from the model filamentous fungus, Neurospora crassa. However, a major technical limitation in using a conventional system is that racetubes are not amenable for real-time gas perturbations. In this work, we demonstrate a simple microfluidic device combined with real-time gas perturbations for monitoring circadian rhythms in Neurospora crassa using bioluminescence assays. The developed platform is a useful toolbox for investigating molecular responses under various gas conditions for Neurospora and can also be applied to other microorganisms.
INTRODUCTION
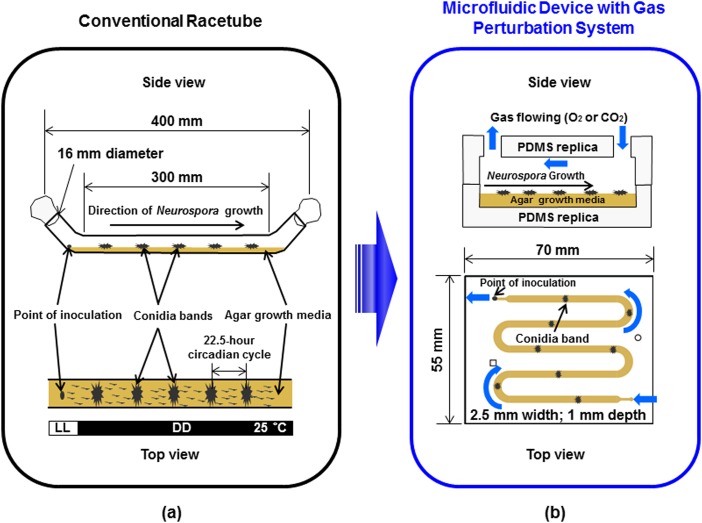
Circadian rhythms are physiological events that recur with a period of about 24 h. The molecular machinery of circadian rhythms provides temporal information to other cellular processes and regulates a variety of metabolic and physiological functions including sleep/wake cycles.1, 2, 3, 4, 5 Disruption of circadian rhythms leads to increased incidence of many diseases such as metabolic disorders and cancer.2, 4, 6Neurospora crassa is a model organism to investigate circadian rhythms, which helped to elucidate many of the basic mechanisms that underlie circadian rhythms. For circadian studies, Neurospora is typically grown in racetubes5, 7 (1.6 × 35 cm) with defined solid agar media as shown in Figure 1a. Neurospora undergo daily asexual differentiations in constant darkness (DD) resulting in aerial hyphae for conidiation, which appear as banding. The rhythmic phenotype of Neurospora are observed and analyzed by tracking bands of conidia and/or molecular components employing bioluminescence reporters. A bioluminescence reporter is constructed by fusing the codon-optimized firefly luciferase gene with a promoter of interest.7 We use a frq-luciferase reporter where luciferase is fused with the promoter of one of the core circadian clock components, frequency (frq) gene, as shown in Gooch et al., 2008.7 This macro-scale setup does not allow real-time gas perturbations, which influence cellular growth conditions, due to technical limitations in using racetubes enclosed with cotton-plugs that are not favorable for gas exchange or flow control. Thus, it is highly desirable to develop the new platform described in Figure 1b that enables real-time gas perturbations and monitoring of circadian rhythms using bioluminescence assays.
Figure 1.
Illustration of the system for monitoring circadian rhythms of Neurospora: (a) conventional macro-scale racetube system; our objective is to translate this macro-scale set up into microfluidic platform and (b) microfluidic device with gas perturbation system. LL is constant light and DD is constant darkness condition.
Microfluidic lab-on-a-chip (LOC) technology has impacted various fields in chemistry, biology, engineering, and biomedical and pharmaceutical research over the past decades.8, 9, 10 Recently, microfluidic cell culture platforms that are capable of controlling oxygen perturbations or gradients on cells have been reported,11, 12, 13, 14 including oxygen gradients using spatially confined chemical reactions,11 fine temporal control of gas content and acidity,12 and high-resolution imaging of a three-dimensional cell culture.14 These devices may be used to better understand cellular behavior in hypoxia,15 which is the pathological condition of oxygen deficiency. Intriguingly, hypoxia is a critical regulatory factor in a tumor's microenvironment, resulting in various clinical events such as respiratory disorders and anti-tumor drug resistance.16, 17 However, there have not been any microfluidic devices to investigate temporal variations of circadian rhythms in Neurospora under different gas conditions even though there was a development of microfluidic devices that studied the growth dynamics of Neurospora.18 Therefore, it is desirable to develop a microfluidic platform that allows real-time gas perturbations for monitoring variations of circadian rhythms in Neurospora crassa.
In this report, we demonstrate a simple and efficient microfluidic device for controlling real-time gas perturbations and monitoring circadian rhythms in Neurospora crassa using bioluminescence assays. This platform successfully overcomes one of the technical limitations of conventional racetube systems, which is not being amenable for real-time gas perturbations. A microfluidic device using Polydimethylsiloxane (PDMS) is fabricated and the agar growth media is manually pipetted into the developed device. Then, Neurospora spore suspension is inoculated onto the device and its growth is observed with a gas control system developed for real-time gas perturbations.
EXPERIMENTAL DETAILS
Design and microfabrication
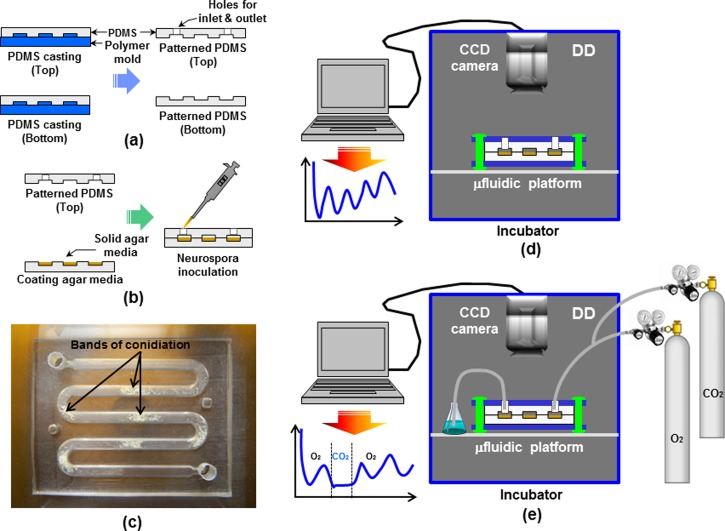
Figure 2 summarizes the fabrication process of the microfluidic device for Neurospora growth and the experimental setup for monitoring the circadian rhythms of Neurospora. Fabrication of the master mold is one of the important steps for the microfluidic device. A polymer master mold containing microchannels was fabricated using a previously developed method in our lab.19, 20 Using a patterned transparency film mask, a commercially available printing press plate with a 1.05 mm thick photosensitive polymer (WA210, Flint Group, OH) was exposed to diffuse ultraviolet (UV) light (1 J/cm2, 365 nm). Then, the exposed printing plate was developed in a hard polymer plate developer and then post-exposed to UV light to yield the printing plate mold. Finally, the polymer master mold for the microfluidic device was obtained. To fabricate the microfluidic device, microchannels were constructed in PDMS by conventional soft lithography.8 PDMS (Sylgard 184, Dow Corning, WI), as an elastomer was mixed with a curing agent in a 10:1 (w/w) ratio. After mixing, the PDMS mixture was degassed for 2 h in a vacuum chamber for best performance. The PDMS mixture was poured, cast onto the polymer master mold, and cured on a hot plate at 80 °C for approximately 2 h as described in Figure 2a. The cured PDMS replica was peeled from the master mold, cut as desired, and combined with the other part (top or bottom) for the microfluidic device.
Figure 2.
Microfabrication procedures for the microfluidic device: (a) PDMS casting; (b) agar media coating and Neurospora inoculation into the microfluidic platform; (c) fabricated microfluidic device with grown Neurospora crassa; experimental setup for monitoring the circadian rhythms of Neurospora crassa in (d) microfluidic device; and (e) microfluidic device setup with gas perturbation system.
Experimental setup
Autoclaved liquid phase of agar-based growth medium (1X Vogel's medium with 0.1% glucose, 0.17% arginine, 50 ng/ml biotin, and 1.5% agar) was mixed with luciferin (12.5 μM final concentration) before injection into the microchannel. The bottom part of the PDMS microfluidic device was put onto the hot plate set to 70 °C to avoid solidification of the agar-based growth medium, and 1 ml of the growth medium was manually pipetted onto the microchannel. Then, the injected media was mildly flown through the microchannel using a pipette tip. Top and bottom parts of the device were put together and 2 μl of Neurospora conidia suspension (ras-1bd; frq-luc::his-3)7 was inoculated at the inlet of the device described in Figure 2b. Neurospora in the device was grown overnight in constant light (LL) condition and then transferred to DD condition at 25 °C where the circadian clock resets to subjective dusk. The fabricated microfluidic device with Neurospora crassa was shown in Figure 2c. The growth of Neurospora and the activity of the frq-luciferase reporter in the microfluidic device were monitored in real-time using a highly sensitive charge coupled device (CCD) camera (PIXIS camera, Princeton Instruments, NJ) in DD as shown in Figure 2d. Photons emitted from the frq-luciferase reporter activity are collected and integrated for 10 min every hour for the entire time-course, which reports a period from the entire population of Neurospora in the device. Figure 2e shows the CCD camera setup for real-time monitoring of Neurospora growth in a microfluidic device with gas perturbations. The inoculated device was sandwiched with two plastic plates with four holes that enable clamping of the top and bottom part of the device to prevent gas leaks (Figure 2e). Two gases O2 (99.98% pre-pure) and CO2 (99.5% technical grade) were used for the gas perturbation system. The flow rate (10 sccm) of the two gases for the microfluidic device with gas perturbation system was controlled by the flowmeter (Dwyer, IN) and needle valves (Swagelok, OH) connected with the gas cylinders (Wright Brothers, Inc., OH). It is important to note that the agar media dries out about three days after the continuous flow of dry gas and Neurospora does not grow once the agar media dries out. A 250 ml Erlenmeyer flask filled with 75 ml of autoclaved water was connected to the inlet port of the device (Fig. 2e) for detecting air bubbles introduced by O2 or CO2. More information is available in the supplementary material23 or on the Internet at http://honglab.wordpress.com.
RESULTS AND DISCUSSION
Figures 3a, 3b, 3c, 3d, 3e, 3f show snapshots of Neurospora growing on the microfluidic platform captured by the CCD camera over time. A frq-luciferase bioluminescence reporter provides a highly quantitative and automated measurement of rhythmic gene expression. The bioluminescence reaction catalyzed by firefly luciferase is based on the two steps below21, 22
| (1) |
| (2) |
Figure 3.
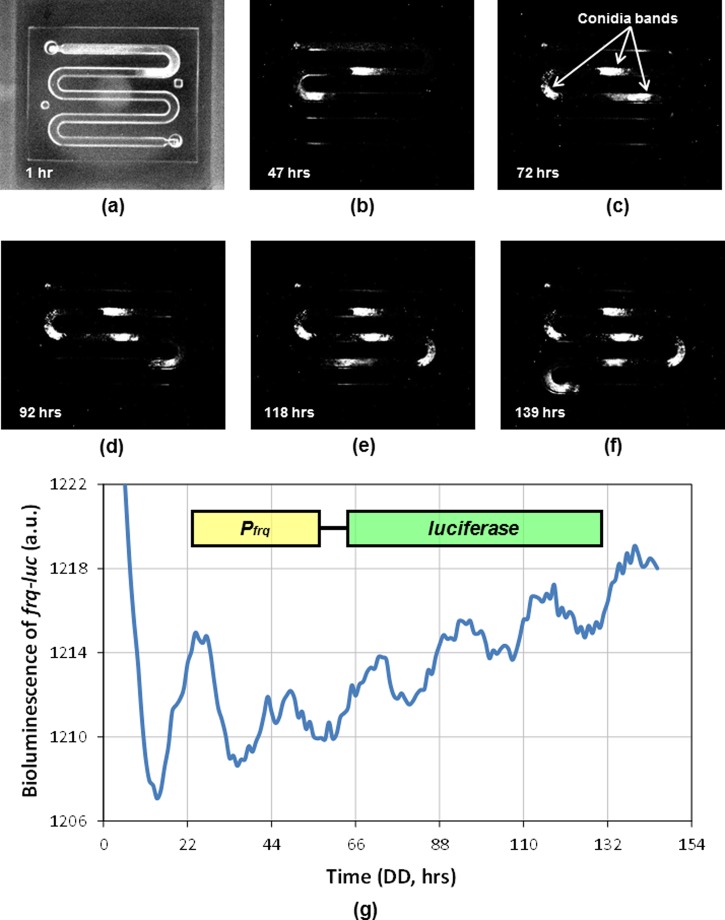
Recorded pictures taken with a CCD camera system to capture bioluminescence in Neurospora crassa from a PDMS microfluidic platform: (a) 1 h; (b) 47 h; (c) 72 h; (d) 92 h; (e) 118 h; and (f) 139 h. Representative conidia bands are indicated. Neurospora is grown in LL overnight before turning fluorescent lights off inside the incubator to synchronize the circadian clock. Neurospora grows at about 1.25 mm/h. (g) Bioluminescence data from a Neurospora strain containing frq-luciferase reporter in the microfluidic platform. Bioluminescence data is collected in DD with 1h resolution.
Light can be produced while the reaction forms electronically excited oxyluciferin. A photon of light is released through the reaction when oxyluciferin returns to the ground state. Rhythmic banding patterns of Neurospora are observed by bioluminescence described in Figure 3 in the microfluidic platform. The bioluminescence signals from the entire microfluidic device were detected by the CCD camera, collected via the WinView/32 software program, and analyzed using Microsoft Excel. The time-course data of the frq-luciferase gene expression shows robust circadian rhythms in Neurospora growing in the microfluidic platform (Figure 3g). Typically, Neurospora grows and covers the entire surface area of a given agar media platform. The total surface area for Neurospora growth is about 4800 mm2 in a conventional racetube versus about 640 mm2 in the proposed microfluidic device. These robust rhythms in a microfluidic device indicate that a small mass of Neurospora in this platform is sufficient to produce measurable bioluminescence and the implementation of the microfluidic device for real-time perturbation study is plausible. This is important to highlight because the intensity of light is proportional to the mass of Neurospora.
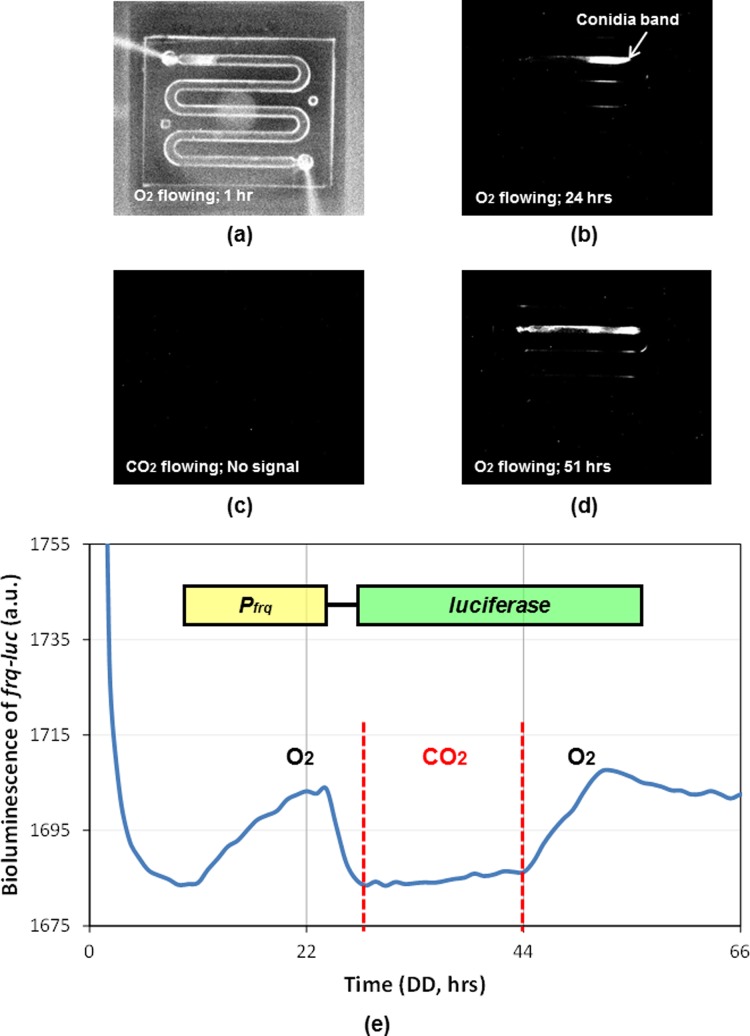
Figures 4a, 4b, 4c, 4d shows pictures of Neurospora growing in the microfluidic device with gas perturbation system captured by the CCD camera over time. O2 gas (10 sccm flow rate) was introduced into the outlet port of the microfluidic device while Neurospora was initiated to grow from the inlet port of the device as shown in Figure 2e. Neurospora was grown in the presence of O2 gas (99.98% pre-pure) for 27 h, and then switched to CO2 gas (99.5% technical grade) conditions for 17 h. O2 gas was re-introduced after 17 h of CO2 gas conditions as indicated in Figure 4e. The growth of Neurospora was not affected by O2 gas and the frq-luciferase reporter activity demonstrated similar oscillation and phase information as in Figure 3g, which is accompanied by a rhythmic banding (Figure 4b). In contrast, the growth of Neurospora was severely decreased under CO2 gas conditions, and the bioluminescence signal of the frq-luciferase reporter was abolished (Figures 4c, 4e). Neurospora did not die under the above gas conditions because it started to grow and emit bioluminescence signals again once CO2 gas was switched back to O2 gas conditions (Figures 4d, 4e). These observations indicate that the proposed microfluidic device is an optimum platform for real-time gas perturbations in studying the molecular responses from Neurospora crassa.
Figure 4.
Recorded pictures taken with CCD camera system to capture bioluminescence in Neurospora crassa from a PDMS microfluidic device with gas perturbation system: (a) 1 h (O2); (b) 24 h (O2); (c) 33 h (CO2; no light signal); and (d) 51 h (O2). Representative conidia band is indicated. The growth rates were measured: ∼1.17 mm/h in O2 gas; ∼0.38 mm/h in CO2 gas. (e) Bioluminescence data from a Neurospora strain containing frq-luciferase reporter in the microfluidic device with a real-time gas perturbation system.
CONCLUSIONS
In conclusion, a simple PDMS-based microfluidic device combined with a gas perturbation system has been designed, fabricated, and applied for real-time monitoring of the frq-luciferase reporter activities in Neurospora crassa. Robust rhythms obtained in a microfluidic device show that a smaller mass of Neurospora covering less than 640 mm2 in the proposed platform is sufficient to produce a measurable bioluminescence compared with that in a conventional racetube system. We discover that Neurospora grows and demonstrates circadian rhythms under O2 gas conditions, and that gas perturbations may reset the phase of circadian rhythms. Further experiments are necessary to prove that gas perturbations may advance or delay the circadian clock. The variations of growth and bioluminescence during the CO2 gas perturbation in a microfluidic device present that the proposed microfluidic device is an ideal platform for real-time gas perturbations in studying the molecular responses of Neurospora crassa. Furthermore, this gas perturbation system may be adapted to investigate molecular mechanisms that have a faster time scale than circadian rhythms such as cell division cycles in lower eukaryotic systems.
ACKNOWLEDGMENTS
This work was supported from the start-up funds of C. Hong in the Department of Molecular and Cellular Physiology, University of Cincinnati. We thank J. Shim for his technical assistance. And we would like to thank J. Dunlap and J. Loros for providing frq-luciferase reporter strain (ras-1bd; frq-luc::his-3).
References
- Loros J. J. and Dunlap J. C., Annu. Rev. Physiol. 63, 757 (2001). 10.1146/annurev.physiol.63.1.757 [DOI] [PubMed] [Google Scholar]
- Gachon F., Nagoshi E., Brown S. A., Ripperger J., and Schibler U., Chromosoma 113, 103 (2004). 10.1007/s00412-004-0296-2 [DOI] [PubMed] [Google Scholar]
- Wijnen H. and Young M. W., Annu. Rev. Genet. 40, 409 (2006). 10.1146/annurev.genet.40.110405.090603 [DOI] [PubMed] [Google Scholar]
- Sahar S. and Sassone-Corsi P., Nat. Rev. Cancer 9, 886 (2009). 10.1038/nrc2747 [DOI] [PubMed] [Google Scholar]
- Baker C. L., Loros J. J., and Dunlap J. C., FEMS Microbiol. Rev. 36, 95 (2012). 10.1111/j.1574-6976.2011.00288.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis R. C., Allen D. S., Fentiman I. S., and Key T. J., J. Natl. Cancer Inst. 96, 475 (2004). 10.1093/jnci/djh077 [DOI] [PubMed] [Google Scholar]
- Gooch V. D., Mehra A., Larrondo L. F., Fox J., and Touroutoutoudis M., Eukaryot Cell 7(1), 28 (2008). 10.1128/EC.00257-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y. and Whitesides G. M., Annu. Rev. Mater. Sci. 28, 153 (1998). 10.1146/annurev.matsci.28.1.153 [DOI] [Google Scholar]
- Craighead H., Nature 442, 387 (2006). 10.1038/nature05061 [DOI] [PubMed] [Google Scholar]
- El-Ali J., Sorger P. K., and Jensen K. F., Nature 442, 403 (2006). 10.1038/nature05063 [DOI] [PubMed] [Google Scholar]
- Park J., Bansal T., Pinelis M., and Maharbiz M. M., Lab Chip 6, 611 (2006). 10.1039/b516483d [DOI] [PubMed] [Google Scholar]
- Polinkovsky M., Gutierrez E., Levchenko A., and Groisman A., Lab Chip 9, 1073 (2009). 10.1039/b816191g [DOI] [PubMed] [Google Scholar]
- Sinkala E. and Eddington D. T., Lab Chip 10, 3291 (2010). 10.1039/c0lc00244e [DOI] [PubMed] [Google Scholar]
- Funamoto K., Zervantonakis I. K., Liu Y., Ochs C. J., Kim C., and Kamm R. D., Lab Chip 12, 485 (2012). 10.1039/c2lc40306d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenz A., Homann D., and Eltzschig H. K., Antioxid. Redox Sign. 15, 2221 (2011). 10.1089/ars.2010.3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. L., Nat. Rev. Cancer 2, 38 (2002). 10.1038/nrc704 [DOI] [PubMed] [Google Scholar]
- Trédan O., Galmarini C. M., Patel K., and Tannock I. F., J. Natl. Cancer Inst. 99, 1441 (2007). 10.1093/jnci/djm135 [DOI] [PubMed] [Google Scholar]
- Held M., Edwards C., and Nicolau D. V., Fungal Biol. 115, 493 (2011). 10.1016/j.funbio.2011.02.003 [DOI] [PubMed] [Google Scholar]
- Browne A. W., Rust M. J., Jung W., Lee S. H., and Ahn C. H., Lab Chip 9, 2941 (2009). 10.1039/b903755a [DOI] [PubMed] [Google Scholar]
- Jang A., Zou Z., Lee K. K., Ahn C. H., and Bishop P. L., Talanta 83(1), 1 (2010). 10.1016/j.talanta.2010.07.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- See http://en.wikipedia.org/wiki/Luciferase for the bioluminescence reaction catalyzed by firefly luciferase. [DOI] [PubMed]
- Fraga H., Fernandes D., Novotny J., Fontes R., and Esteves da Silva J. C., Chem. Bio. Chem. 7(6), 929 (2006). 10.1002/cbic.200500443 [DOI] [PubMed] [Google Scholar]
- See supplementary material at http://dx.doi.org/10.1063/1.4819478 for information on setting up the microfluidic device with a gas perturbation system.