Abstract
Background
Whether and how sex and age affect bariatric-surgery outcome is poorly understood. Estrogens regulate body composition in women and animals and increased weight loss in a rodent model of gastric bypass, suggesting that premenopausal women may lose more weight following bariatric surgery.
Methods
1,356 female gastric-bypass or gastric-banding patients were retrospectively grouped as 20-45 y old (presumptively premenopausal; n = 1199) and 55-65 y old (presumptively postmenopausal; n = 157). Mixed-model ANCOVA followed by Bonferroni-corrected t-tests were used to categorically test the effect of age on percent excess body weight loss (%EBWL) at 1 and 2 y post-surgery, controlling for preoperative EBW and surgery type. Age effects were also tested dimensionally in all women and in 289 male patients.
Results
20-45 y-old women showed greater %EBWL 1 and 2 y post-surgery than 55-65 y-old women (p’s < 0.0005). No age effect was detected in 20-25 vs. 30-35, 30-35 vs. 40-45, or 20-25 vs. 40-45 y-old women (p’s > 0.2) This age effect was detected only after gastric banding, with 20-45 y-old women losing ~7 kg more than 55-65 y-old women after 2 y. Dimensional analysis confirmed a significant inverse effect of age on bariatric surgery outcome in women, but did not detect any effect in men.
Conclusions
Results indicate that 55-65 y-old women lose less weight than 20-45 y-old women in the initial 2 y after bariatric surgery, especially gastric banding; this may be mediated by age- or menopause-associated changes in physical activity, energy expenditure, or energy intake.
Keywords: estrogen, menopause, gastric bypass, gastric banding, RYGB
Introduction
Bariatric surgery is currently the only effective means to reverse morbid obesity, and its use continues to increase worldwide [1, 2]. Although it is not widely discussed, there is a marked disparity in the number of men and women who elect bariatric surgery. In the USA, for example, more than 80% of bariatric surgery patients are women [3, 4]. This difference, together with many data in humans and animals indicating that female reproductive-axis function potently influences eating and body-weight regulation [5, 6], suggests the importance of determining whether female reproductive-axis function influences bariatric-surgery outcome. To our knowledge, however, no such study has been reported.
A prominent example of the influence of reproductive physiology on body-weight regulation is the increase in adiposity that accompanies menopause. Studies that statistically segregate the effects of aging per se and menopausal status indicate that menopause is associated with a ~5-10% increase in adiposity together with a somewhat smaller loss in lean body mass [7-11]. Furthermore, this increase seems to occur predominately in intra-abdominal adiposity, which has more deleterious metabolic effects than subcutaneous adiposity [12,13]. The effect of menopause on bariatric surgery outcome has not been studied, but in ovariectomized rats estradiol treatment increased gastric-bypass induced weight loss [14]. Therefore, we analyzed the changes in excess body weight 1-2 y following gastric-bypass and gastric-banding surgery in a series of women classified by age as presumptively pre- or post-menopausal.
Materials and Methods
Participants
The overall sample consisted of 1,787 women and 289 men, 18-65 y of age, who underwent either laparoscopic Roux-en-Y gastric bypass (RYGB) or laparoscopic gastric banding between May 1, 2001 to May 1, 2011 at the Center for Bariatric Surgery and Metabolic Diseases at St Luke’s Hospital, NY, NY, a level 1A center for excellence in bariatric surgery. All patients met the criteria for bariatric surgery proposed by the National Institutes of Health Consensus Panel in 1991 [15]. Because of the lack of specific data identifying the onset of menopause in our sample, based on epidemiological data indicating that ~90% of women undergo natural menopause between 45 and 55 y of age [16-19], women were classified as presumptively premenopausal (< 45 y of age) or presumptively postmenopausal (> 55 y). This yielded 1,199 women between 18-45 y old in the presumptively premenopausal group and 157 women between 55-65 y old in the presumptively postmenopausal group. Men and patients between 45 and 55 y of age were not included in categorical analyses. Participant baseline characteristics are described in Table 1.
Table 1.
Sample Demographic and Body Weight Data
| Dimensional Men |
Dimensional Women |
Categorical Women* |
Categorical Women Bypass |
Categorical Women Banding |
|
|---|---|---|---|---|---|
| N | 289 | 1787 | 1356 | 1031 | 325 |
| Age (y) | 38.8 ± 10.1 (18-65) |
38.3 ± 11.4 (18-65) |
36.6 ± 10.5 (18-65) |
36.1 ± 10.1 (18-65) |
38.3 ± 11.4 (18-65) |
| Ethnicity (%) | |||||
| Hispanic | 39.2 | 42.7 | 44.7 | 47.8 | 34.8 |
| African | 24.3 | 29.5 | 27.6 | 26.8 | 30.1 |
| American | |||||
| Non-Hispanic | 32.6 | 24.5 | 24.1 | 22.7 | 28.5 |
| White | |||||
| Asian | 2.1 | 0.3 | 0.4 | 0.3 | 0.6 |
| Other | 1.7 | 3.0 | 3.2 | 2.4 | 6.0 |
|
Pre-op weight
(kg) |
155.6 ± 32.7 (90-320) |
125.1 ± 10.1 (81-244) |
125.8 ± 23.8 (82-244) |
127.9 ± 24.7 (86-244) |
119.0 ± 19.0 (82-192) |
|
Pre-op BMI
(kg/m2) |
50.6 ± 9.4 (35-96) |
47.2 ± 7.6 (35-88) |
47.3 ± 7.6 (35-88) |
48.0 ± 7.9 (35-88) |
45.2 ± 6.3 (35-70) |
|
Pre-op EBW
(kg) |
88.0 ± 30.2 (34-247) |
66.8 ± 21.3 (30-180) |
67.3 ± 21.6 (30-180) |
69.3 ± 22.5 (34-180) |
61.1 ± 17.1 (30-127) |
Primary sample
Age and weight are presented as mean ± SD and (range)
Neither age nor ethnicity varied significantly between pre- vs. post- menopausal-age participants or banding vs. bypass patients.
Design
Excess body weights were computed at 1 wk pre-surgery, and 12 and 24 mo post-surgery, and retrospective categorical and dimensional analyses of percent excess body weight loss (%EBWL) were conducted by sex, age and surgery type. Excess body weight was defined as measured body weight minus the body weight that would result in a body mass index (BMI) of 25 kg/m2, the upper limit of the normal range [20]. This study was approved by the St Luke’s-Roosevelt Hospital Institutional Review Board.
Statistical Analyses
Data were analyzed using mixed-model analyses of covariance (ANCOVA) followed by Bonferroni-corrected pairwise comparisons, with time (baseline, 12 and 24 mo post-surgery) included as a within-groups factor and menopausal age (pre vs. post) as a between-groups factor. ANCOVAs were conducted controlling for preoperative excess body weight and surgery type (RYGB, gastric banding). Surgery type was included as a between-subjects factor in order to test whether the effect of menopausal age on postoperative weight loss varied by procedure type.
In order to test the specificity of the effect of age group, ANCOVAs were repeated with different age groupings (20-25 vs. 30-35 y-old women, 30-35 vs. 40-45 y-old women, and 20-25 vs. 40-45 y-old women) as the between-groups factors. Although there was insufficient power to assess an effect of age in men categorically (n = 218 in the < 45 y-old group and n = 18 in the > 55 y-old group, yielding only 11% power), there was adequate power to test an effect of age dimensionally in both men (n = 289) and women (n = 1787). Age was regressed on %EBWL, with preoperative excess body weight and procedure type included in the model as covariates. All tests were two-tailed, with α = 0.05 and multiple imputation [21,22] used for missing values.
Results
Holding procedure type and preoperative percent excess body weight constant, 20-45 y-old, presumptively premenopausal women showed greater %EBWL than 55-65 y-old, presumptively postmenopausal women at both 12 and 24 mo after bariatric surgery (overall F1,1355 = 6.9, p = 0.001; 12 and 24 mo ps < 0.0005).
To determine whether the effect of age stratification on bariatric-surgery outcome was specific to the 55-65 y-old women, similar analyses were done contrasting 20-25 vs. 30-35 y-old women, 30-35 vs. 40-45 y-old women, and 20-25 vs. 40-45 y-old women. None of these contrasts were significant (all ps > 0.2). Testing dimensionally, while controlling for procedure, age inversely predicted %EBWL in women at 12 mo (t1783 = −3.6; p < 0.0001) and 24 mo (t285 = −2.5; p = 0.012) post-surgery. However, age did not predict %EBWL in men at 12 or 24 mo post-surgery (both ps > 0.3).
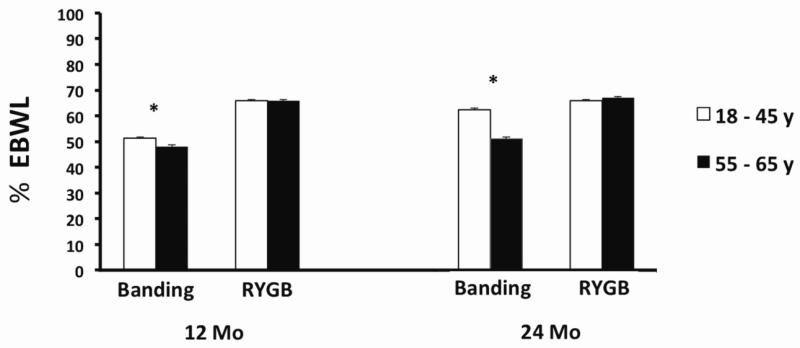
A three-way interaction was observed between age, follow-up time and surgery type, prompting individual (corrected) comparisons of RYGB and gastric-banding patients. Percent excess body-weight loss was greater in 20-45 vs. 55-65 y-old women undergoing gastric banding both 12 and 24 mo post-surgery (overall F1,323 = 4.2, p = 0.016; Figure 1), which translated into about a 7 kg increase in weight loss in 20-45 vs. 55-65 y-old women at 24 mo post-surgery. However, this effect was not detected in women undergoing RYGB (overall p = 0.24; Figure 1).
Figure 1. Percent excess body weight loss (%EBWL) 12 and 24 mo after gastric banding or Roux-en-Y gastric-bypass surgery, controlling for Pre-op %EBW in premenopausal-aged women and postmenopausal-aged women. By 24 mo after gastric banding, premenopausal-age women (n= 271) lost about 10% more weight than postmenopausal-age women (n = 54); there was no significant difference in premenopausal-age (n = 928) and postmenopausal-age (n = 103) women after RYGB. * p < 0.01
Results did not depend on the choice of relevant dependent or control variables. The same pattern of effects was detected when analyses were conducted with preoperative %EBW, weight or BMI included as covariates. Similarly, results did not differ when using total body-weight loss (TBWL) or %TBWL as the outcome variable.
Discussion
We report categorical analyses indicating that, at 12 and 24 mo, post-bariatric surgery, weight loss is significantly reduced in women aged 55-65 y relative to women aged 20-45 y, as well as dimensional analyses indicating that there is an inverse relationship between age and bariatric-surgery outcome in women, but not in men. Both age [23-29] and sex [29-31] have been previously reported to affect post-bariatric surgery weight loss; however, to our knowledge, this is the first report of a sex-specific effect of aging. Scozzari et al. [28] recently reported a decreased post-RYGB weight loss in the oldest quartile of their sample of 489 patients (i.e., patients ≥ 52 y old lost about 1.5 BMI units less than patients < 52 y old 1-2 y postoperatively). They did not detect a sex-specific effect but, as they began with only 113 male patients (of whom only about 80-84% were available for follow-up), it is unclear whether their analysis had sufficient statistical power to do so. Thus, as in many studies of the effects of age in bariatric-surgery outcome, the relative paucity of men in the samples may obscure sex-specific effects.
Our categorical analysis was designed to detect a menopause-associated effect, based on epidemiological data on the timing of natural menopause [16-19]. This yielded novel evidence consistent with our hypothesis that menopausal status affects bariatric surgery outcome. Specifically, the failure to find effects of age in women < 45 y of age or in men in adequately powered analyses suggests that the difference between 25-45 y-old women and 55-65 y-old women was related to menopausal status rather than chronological age. However, we emphasize that this hypothesis is speculative and that alternative factors, such as the age-related decreases in metabolic rate, functional status, and physical activity need to be explicitly ruled out in future studies.
Surprisingly, the apparent effect of menopausal age on outcome appeared to depend on surgery type; significant effects were detected in women undergoing gastric banding, but not RYGB. The failure to detect a significant menopausal-age effect in RYGB patients may have been due to the difference in postoperative weight loss between RYGB and gastric banding (i.e., %EBWL was greater after RYGB than after gastric banding both 12 and 24 mo post-surgery).
The current data are consistent with our hypothesis that menopausal status accounts for some of the variance in postoperative weight loss following at least one form of bariatric surgery in women. Our observation that estradiol treatment increased weight loss in ovariectomized rats undergoing gastric bypass [14] suggests the menopause-related reduction in circulating estrogens may be a causative factor. Thus, identification of an influence of menopausal status or the use of hormone replacement therapy on bariatric-surgery outcome might help inform patients’ choice of bariatric-surgery type. It would also be clinically useful given the high degree of variation in bariatric-surgery outcome, which is currently not well understood. For example, in Sjostrom’s [32] summary of the Swedish obesity study, postoperative weight loss at 10 y post-surgery ranged from −61 kg (i.e., 61 kg weight gain) to 106 kg weight loss.
Future work should address several weaknesses of our study. Most importantly, because we used a surrogate measure of menopausal status, it is necessary for future studies to statistically segregate the effects of menopausal status and aging. Ideally, such studies should investigate aging-related changes that can affect body weight and composition, such as decreases in metabolic rate, physical activity, and functional status. In this context, it is important to note some of these factors, in particular metabolic rate [6], appear to mediate the effects of both menopause and aging. For example, in a landmark 4 y longitudinal study of perimenopausal women, Lovejoy et al. [11] found that although aging per se was associated with a decrease in sleeping energy expenditure, the decrease was 50% greater in women who became postmenopausal during the study than in those who did not. In addition, fat oxidation rate decreased only in women who became postmenopausal. Daily caloric intake also appeared to decrease across the menopausal transition, but insufficiently to prevent increases in body weight and adipose tissue. In contrast, aging decreased physical activity, measured by 24 h tri-axial accelerometry, similarly in both groups.
Future studies should also determine whether menopausal status affects gastric-banding outcome more than RYGB outcome, as our data suggest. Because these two procedures differ in efficacy for weight loss [33-36], it could be that the effect of menopause is related to degree of weight loss rather than surgery type. It will also be important to determine whether menopausal status truly interacts with bariatric surgery to affect weight loss or metabolic health or whether the two effects simply add. Our data suggest that there may be a true interaction because the effect of menopause on body weight appeared rather small, suggesting that the gain in adipose tissue may be more or less balanced by a loss in lean body mass [7-10]. Finally, it will be important to consider a number of important variables that we were not able to include, such as insulin resistance, metabolic syndrome, body composition, physical and psychiatric comorbidities, functional status, physical activity level, hormone-replacement therapy (HRT) and surgical menopause [29,37-40].
Interestingly, the effects that we detected increased in magnitude during the second year post-surgery. The range of reported postoperative weight change from surgery to 12 mo post-surgery is usually relatively small, as the vast majority of patients lose significant weight in a relatively linear fashion during the first year [32,33]. In contrast, large individual differences in weight loss emerge during the second year postoperatively. Thus, identifying factors that influence surgery outcome in this and later periods is especially important.
It should be noted that the potential measurement error introduced by the stratification of women by menopausal age rather than documented onset of menopause would serve to reduce the apparent effect of menopausal status on bariatric-surgery outcome. First, because about 5% of women enter natural menopause before 45 y of age and 5% enter menopause after 55 y of age [16-19], we may have misclassified the menopausal status of ~10% of our sample. Second, a substantial percentage of women, about 10% of women < 45 y of age in one recent study [41], undergo surgical menopause; such patients would also have been misclassified. Third, because estrogens appear to mediate the effects of reproductive-axis function on weight regulation, women who elected to receive postmenopausal HRT should not have been considered together with women who did not elect HRT. Thus, if there is an effect of menopausal status on bariatric-surgery outcome, it is likely larger than that suggested in this study.
Footnotes
Conflict of Interest Disclosures
Christopher Ochner, PhD: no conflict of interest
Julio Teixeira, MD: no conflict of interest
Nori Geary, PhD: no conflict of interest
Lori Asarian, PhD: no conflict of interest
References
- 1.Buchwald H, Williams SE. Bariatric surgery worldwide 2003. Obes Surg. 2004 Oct;14:1157–1164. doi: 10.1381/0960892042387057. [DOI] [PubMed] [Google Scholar]
- 2.Still CD, Benotti P, Wood GC, et al. Outcomes of preoperative weight loss in high-risk patients undergoing gastric bypass surgery. Arch Surg. 2007 Oct;142:994–998. doi: 10.1001/archsurg.142.10.994. [DOI] [PubMed] [Google Scholar]
- 3.Samuel I, Mason EE, Renquist KE, et al. Bariatric surgery trends: an 18-year report from the International Bariatric Surgery Registry. The American journal of surgery. 2006;192:657–662. doi: 10.1016/j.amjsurg.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Pratt GMLC, Hughes GD, Clark BL, et al. Demographics and outcomes at american society for metabolic and bariatric surgery centers of excellence. Surgery Endosc. 2009;23:795–799. doi: 10.1007/s00464-008-0077-8. [DOI] [PubMed] [Google Scholar]
- 5.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci. 2006 Jul;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geary N, Lovejoy J. Sex differences in energy metabolism, obesity and eating behavior. In: Becker J, Berkley K, Geary N, Hampson E, Hearman I, Young E, editors. Sex on the Brain: From Genes to Behavior. Oxford University Press; New York: 2008. [Google Scholar]
- 7.Ley CJ, Lees B, Stevenson JC. Sex- and menopause-associated changes in body-fat distribution. Am J Clin Nutr. 1992 May;55:950–954. doi: 10.1093/ajcn/55.5.950. [DOI] [PubMed] [Google Scholar]
- 8.Panotopoulos G, Ruiz JC, Raison J, et al. Menopause, fat and lean distribution in obese women. Maturitas. 1996 Aug;25:11–19. doi: 10.1016/0378-5122(96)01119-x. [DOI] [PubMed] [Google Scholar]
- 9.Phillips GB, Jing T, Heymsfield SB. Does insulin resistance, visceral adiposity, or a sex hormone alteration underlie the metabolic syndrome? Studies in women. Metabolism. 2008 Jun;57:838–844. doi: 10.1016/j.metabol.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toth MJ, Tchernof A, Sites CK, et al. Effect of menopausal status on body composition and abdominal fat distribution. Int J Obes Relat Metab Disord. 2000 Feb;24:226–231. doi: 10.1038/sj.ijo.0801118. [DOI] [PubMed] [Google Scholar]
- 11.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 2008;32:949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLaughlin T, Lamendola C, Liu A, et al. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab. 2011 Nov;96:E1756–1760. doi: 10.1210/jc.2011-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ESHRE Capri Workshop Group Perimenopausal risk factors and future health. Hum Reprod Update. 2011;17:706–717. doi: 10.1093/humupd/dmr020. [DOI] [PubMed] [Google Scholar]
- 14.Asarian L, Abegg K, Geary N, et al. Estradiol increases body-weight loss and gut-peptide satiation after Roux-en-Y gastric bypass in ovariectomized rats. Gastroenterology. doi: 10.1053/j.gastro.2012.05.008. In press. [DOI] [PubMed] [Google Scholar]
- 15.NIH Consensus Statement Gastrointestinal Surgery for Severe Obesity. 1991 Mar 25-27;9(1):1–20. [PubMed] [Google Scholar]
- 16.Henderson KD, Bernstein L, Henderson B, et al. Predictors of the timing of natural menopause in the Multiethnic Cohort Study. Am J Epidemiol. 2008 Jun;167:1287–1294. doi: 10.1093/aje/kwn046. [DOI] [PubMed] [Google Scholar]
- 17.Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001 May;153:865–874. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 18.Sammel MD, Freemen EW, Liu Z, Lin H, Guo W. Factors that influence entry into stages of the menopausal transition. Menopause. 2009;16:1218–1227. doi: 10.1097/gme.0b013e3181a8f62b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farahmand M, Tehrani FR, Pourrajabi L, Najafi M, Azizi F. Factors associated with menopausal age in Iranian women: Tehran Lipid and Glucose Study. J Obstet Gynaecol Res. 2012 doi: 10.1111/j.1447-0756.2012.02050.x. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Bray GA, Bouchard C, Church TS, et al. Is it time to change the way we report and discuss weight loss? Obesity (Silver Spring) 2009 Apr;17:619–621. doi: 10.1038/oby.2008.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham JWHS. Modeling longitudinal and multilevel data. Lawrence Erlbaum; Mahwah, NJ: 2000. Multiple imputation in multivariate research. [Google Scholar]
- 22.Little R, Ruben D. Statistical Analysis With Missing Data. 2nd ed Wiley and Sons; 2002. [Google Scholar]
- 23.Bobbioni-Harsch E, Huber O, Morel P, Chassot G, Lehmann T, Volery M, et al. Factors influencing energy intake and body weight loss after gastric bypass. Eur J Clin Nutr. 2002;56:551–556. doi: 10.1038/sj.ejcn.1601357. [DOI] [PubMed] [Google Scholar]
- 24.Dunkle-Blatter SE, St Jean MR, Whitehead C, Strodel W, 3rd, Benotti PN, Still C, et al. Outcomes among elderly bariatric patients at a high-volume center. Surg Obes Relat Dis. 2007;3:163–169. doi: 10.1016/j.soard.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Printen KJ, Mason EE. Gastric bypass for morbid obesity in patients more than fifty years of age. Surg Gynecol Obstet. 1977;144:192–194. [PubMed] [Google Scholar]
- 26.Sjøberg EJ, Andersen E, Hoel R, Reinertsen S, Søreide O. Gastric banding in the treatment of morbid obesity. Factors influencing immediate and long-term results. Acta Chir Scand. 1989;155:31–34. [PubMed] [Google Scholar]
- 27.Stefanidis D, Kuwada TS, Gersin KS. The importance of the length of the limbs for gastric bypass patients--an evidence-based review. Obes Surg. 2011 Jan;21:119–124. doi: 10.1007/s11695-010-0239-3. [DOI] [PubMed] [Google Scholar]
- 28.Scozzari G, Passera R, Benvenga R, Toppino M, Morino M. Age as a long-term prognostic factor in bariatric surgery. Ann Surg. 2012 Nov;256:724–728. doi: 10.1097/SLA.0b013e3182734113. [DOI] [PubMed] [Google Scholar]
- 29.Ma Y, Pagoto SL, Olendzki BC, Hafner AR, Perugini RA, Mason R, Kelly JJ. Predictors of weight status following laparoscopic gastric bypass. Obes Surg. 2006 Sep;16:1227–1231. doi: 10.1381/096089206778392284. [DOI] [PubMed] [Google Scholar]
- 30.Melton GB, Steele KE, Schweitzer MA, Lidor AO, Magnuson TH. Suboptimal weight loss after gastric bypass surgery: correlation of demographics, comorbidities, and insurance status with outcomes. J Gastrointest Surg. 2008;12:250–255. doi: 10.1007/s11605-007-0427-1. [DOI] [PubMed] [Google Scholar]
- 31.Dallal RM, Quebbemann BB, Hunt LH, Braitman LE. Analysis of weight loss after bariatric surgery using mixed-effects linear modeling. Obes Surg. 2009 Jun;19(6):732–737. doi: 10.1007/s11695-009-9816-8. [DOI] [PubMed] [Google Scholar]
- 32.Sjöström CD, Lystig T, Lindroos AK. Impact of weight change, secular trends and ageing on cardiovascular risk factors: 10-year experiences from the SOS study. Int J Obes (Lond) 2011 Nov;35:1413–1420. doi: 10.1038/ijo.2010.282. [DOI] [PubMed] [Google Scholar]
- 33.Butner KL, Nickols-Richardson SM, Clark SF, et al. A review of weight loss following Roux-en-Y gastric bypass vs restrictive bariatric surgery: impact on adiponectin and insulin. Obes Surg. 2010 May;20:559–568. doi: 10.1007/s11695-010-0089-z. [DOI] [PubMed] [Google Scholar]
- 34.Kalfarentzos F, Skroubis G, Kehagias I, et al. A prospective comparison of vertical banded gastroplasty and Roux-en-Y gastric bypass in a non-superobese population. Obes Surg. 2006 Feb;16:151–158. doi: 10.1381/096089206775565096. [DOI] [PubMed] [Google Scholar]
- 35.Tice JA, Karliner L, Walsh J, et al. Gastric banding or bypass? A systematic review comparing the two most popular bariatric procedures. Am J Med. 2008 Oct;121:885–893. doi: 10.1016/j.amjmed.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 36.Dorman RB, Serrot FJ, Miller CJ, et al. Case-Matched Outcomes in Bariatric Surgery for Treatment of Type 2 Diabetes in the Morbidly Obese Patient. Ann Surg. 2011 Oct; doi: 10.1097/SLA.0b013e318232b033. [DOI] [PubMed] [Google Scholar]
- 37.Hsu LK, Benotti PN, Dwyer J, et al. Nonsurgical factors that influence the outcome of bariatric surgery: a review. Psychosom Med. 1998 May-Jun;60:338–346. doi: 10.1097/00006842-199805000-00021. 1998. [DOI] [PubMed] [Google Scholar]
- 38.Bocchieri-Ricciardi LE, Chen EY, Munoz D, et al. Pre-surgery binge eating status: effect on eating behavior and weight outcome after gastric bypass. Obes Surg. 2006 Sep;16:1198–1204. doi: 10.1381/096089206778392194. [DOI] [PubMed] [Google Scholar]
- 39.Legenbauer T, De Zwaan M, Benecke A, et al. Depression and anxiety: their predictive function for weight loss in obese individuals. Obes Facts. 2009;2:227–234. doi: 10.1159/000226278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Legenbauer T, Petrak F, de Zwaan M, et al. Influence of depressive and eating disorders on short- and long-term course of weight after surgical and nonsurgical weight loss treatment. Compr Psychiatry. 2011 May-Jun;52:301–311. doi: 10.1016/j.comppsych.2010.06.012. 2011. [DOI] [PubMed] [Google Scholar]
- 41.Henderson KD, Bernstein L, Henderson B, et al. Predictors of the timing of natural menopause in the Multiethnic Cohort Study. Am J Epidemiol. 2008 Jun;167:1287–1294. doi: 10.1093/aje/kwn046. [DOI] [PubMed] [Google Scholar]