Abstract
The E5 proteins are short transmembrane proteins encoded by many animal and human papillomaviruses. These proteins display transforming activity in cultured cells and animals, and they presumably also play a role in the productive virus life cycle. The E5 proteins are thought to act by modulating the activity of cellular proteins. Here, we describe the biological activities of the best-studied E5 proteins and discuss the evidence implicating specific protein targets and pathways in mediating these activities. The primary target of the 44-amino acid BPV1 E5 is the PDGF β receptor, whereas the EGF receptor appears to be an important target of the 83-amino acid HPV16 E5 protein. Both E5 proteins also bind to the vacuolar ATPase and affect MHC class I expression and cell-cell communication. Continued studies of the E5 proteins will elucidate important aspects of transmembrane protein-protein interactions, cellular signal transduction, cell biology, virus replication, and tumorigenesis.
Keywords: papillomaviruses, cervical cancer, transmembrane proteins, PDGF receptor, vacuolar ATPase, EGF receptor, HPV, traptamers
Introduction
Papillomaviruses undergo productive infection in epithelial cells in their natural animal hosts. Infection initially occurs in the basal epithelial cells, but no progeny virus is produced in these cells. Rather, as the cells migrate away from the basal layer toward the surface of the epithelium and undergo terminal keratinocyte differentiation, vegetative viral DNA replication occurs and the viral late genes are expressed, leading to the production of infectious virus. All conserved viral genes presumably play a role in the productive viral replication cycle. Some papillomavirus proteins, such as the E1 major viral DNA replication protein and the L1 major capsid protein, play direct roles in viral replication, but other viral proteins generate a cellular environment conducive to high level viral DNA replication or late gene expression, for example by driving cells into S-phase or blocking differentiation. In cultured cells, these latter activities can induce a non-productive form of infection known as cell transformation, in which no progeny virus particles are produced and only a subset of viral genes is expressed. Transformed cells display many features of tumor cells, and the viral genes that transform cells are the oncogenes responsible for tumor formation in vivo. Thus, study of viral oncogenes and cell transformation will provide insights not only into aspects of carcinogenesis but also into the underlying biochemical activities of the viral proteins that evolved to support productive infection.
Many but not all papillomaviruses encode E5 proteins, short membrane-associated proteins with transforming activity but poorly defined roles in productive infection. The E5 gene is located at the 3’ end of the early region of the viral genome and is expressed from a spliced mRNA that initiates upstream of the E2 gene. Ranging in size from approximately 40 to 85 amino acids, the E5 proteins are rich in hydrophobic amino acids clustered into one or more putative transmembrane domains. The E5 proteins are not thought to have intrinsic enzymatic activity, but rather act by modulating the activity of a variety of cellular proteins (Table 1). However, because of their small size and largely hydrophobic nature, the E5 proteins lack the large soluble, globular domains that typically mediate specific protein-protein interactions. Rather, the E5 proteins utilize an alternative mechanism to engage their target proteins. Short proteins and peptides are often unstructured in solution, but the hydrophobic composition of the E5 proteins enforces a stable conformation that provides a novel interface for protein interactions. In the hydrophobic membrane environment, the main-chain amide and carboxyl groups of transmembrane domains form hydrogen bonds with each other to minimize the energetic cost of membrane insertion, resulting in the formation of an α–helix. Thus, despite their small size and unusual composition, the E5 proteins adopt an energetically favorable, well-formed structure in cell membranes in which the amino acid side-chains are radially displayed on the outside of the helical axis. Thus disposed, these side-chains can interact within the membrane in a sequence-specific manner with other molecules of the E5 protein, thereby undergoing homo-oligomerization, or with the transmembrane segments of cellular proteins, forming protein complexes.
Table 1.
Cellular targets of papillomavirus E5 proteins
| Cellular targeta | Origin of E5 protein |
|---|---|
| PDGF β receptorb | BPV1 |
| pp60src | |
| Phosphoinositol 3’ kinase | |
| p38 MAP kinase | |
| EGF receptorc | HPV16 |
| Erk1/2 | |
| AKT | |
| Cyclo-oxygenase-2 | |
| VEGF | |
| c-Cbl | |
| 16K vacuolar-ATPase subunit | BPV1, BPV4, HPV16, HPV6, HPV11 |
| Immune evasion | |
| MHC class I antigens | BPV1, BPV4, HPV16 |
| MHC class II antigens | HPV16 |
| CD1d | HPV16, HPV6 |
| Cell-cell communication | |
| Connexin 26 | BPV1 |
| Connexin 43 | HPV16 |
| Apoptosis | |
| Fas | HPV16 |
| Bcl-2 | HPV16 |
| Bax | BPV1, HPV16 |
| Cyclin dependent kinase inhibitors | |
| p21 | HPV16 |
| p27 | BPV4, HPV16 |
| NFκB | BPV1 |
| Arachidonic acid metabolism | BPV1 |
| Tissue metalloproteases | BPV1 |
| Calpain 3 | BPV1 |
| p38 MAP kinase | HPV16 |
| Phospholipase C-γ1 | HPV16 |
| ErbB4 | HPV16 |
| c-jun, c-fos | HPV16 |
| EVER1 and EVER2 | HPV16 |
| ZnT-1 | HPV16 |
| Bap31 | HPV16, HPV31 |
| Calnexin | HPV16 |
| Calpactin 1 | HPV16 |
| Karyopherin β3 | HPV16 |
| Endothelin-1 receptor | HPV16 |
| Cyclin A | BPV4 |
Some targets appear to be binding partners of the E5 protein; for others there is functional evidence that the E5 protein directly or indirectly modulates the target.
BPV1 E5 may also activate pp60src and PI3’ kinase independently of the PDGF β receptor
HPV16 E5 may also activate Erk1/2 independently of the EGF receptor
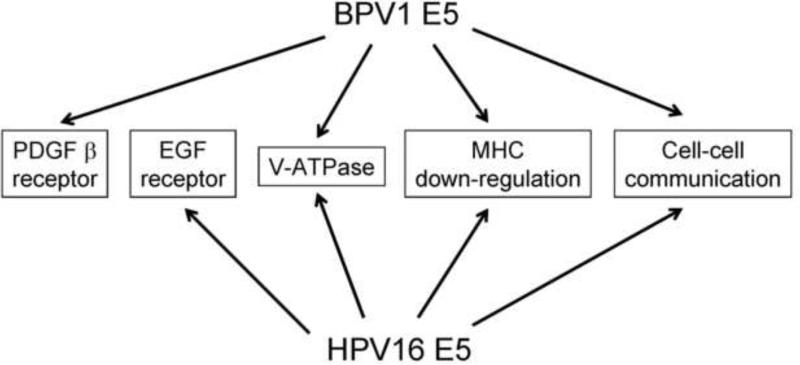
Because only two E5 proteins have been studied in detail, those encoded by bovine papillomavirus type 1 (BPV1), a fibropapillomavirus, and human papillomavirus type 16 (HPV16), a high-risk genital papillomavirus, this review focuses primarily on these proteins. Although BPV1 and HPV16 E5 show minimal sequence similarity with each other, they both transform cells and display overlapping targets and activities (Figure 1). A more detailed description of the E5 proteins and their activities is available in the excellent review by Venuti et al. (Venuti et al., 2011).
Figure 1.
Major targets and activities of BPV1 and HPV16 E5 proteins.
Fibropapillomavirus E5 proteins
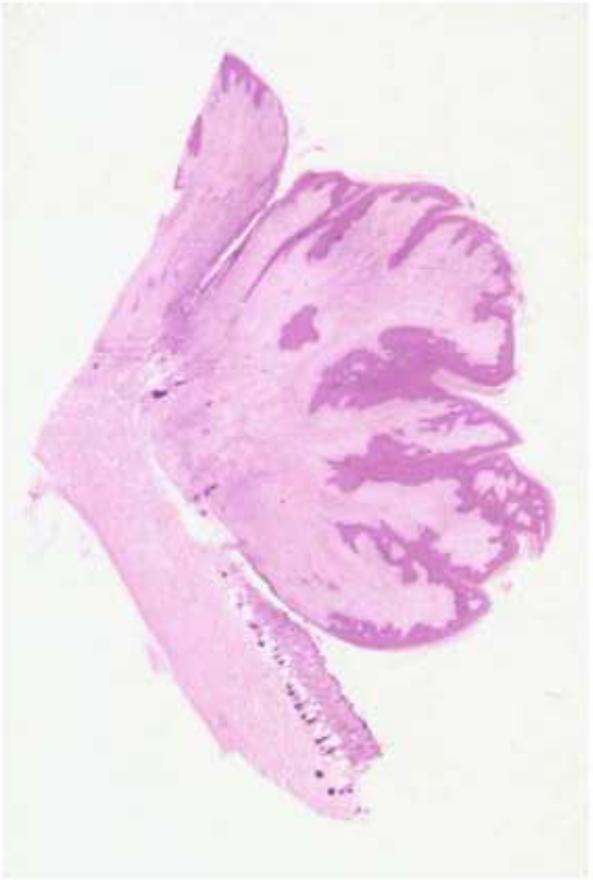
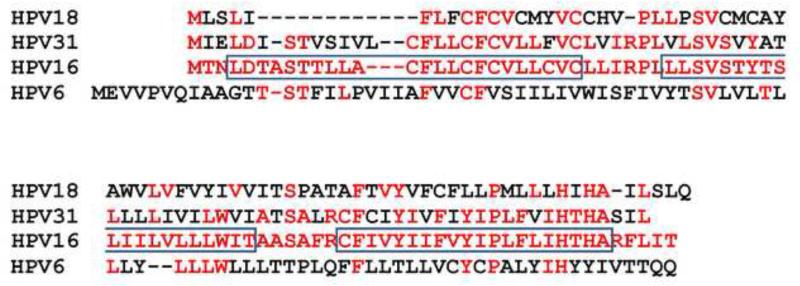
The most highly conserved E5 proteins are encoded by the fibropapillomaviruses, a clade of closely related papillomaviruses designated the delta papillomaviruses (van Doorslaer, 2013). These viruses infect ungulates, such as cattle, moose, and deer, resulting in the formation of warts characterized by a prominent dermal fibroblast proliferative component as well as an overlying epithelial papilloma (Figure 2). In contrast, the papillomas caused by other papillomaviruses (such as the human papillomaviruses, which express distinct E5 proteins or no E5 protein at all) consist exclusively of epithelial keratinocytes. The best understood E5 protein is encoded by BPV1, a fibropapillomavirus. In bovine cutaneous warts caused by BPV1, the 44-amino acid E5 protein is localized to the basal keratinocytes (where it might stimulate cell proliferation) and to highly-differentiated keratinocytes in superficial epithelial layers where virus production occurs (Burnett, Jareborg, and DiMaio, 1992), and it is assumed that E5 is also expressed in the proliferative dermal fibroblasts. The E5 protein is also expressed in other BPV1-induced tumors including bovine urinary bladder tumors, fibropapillomas in water buffalos, and equine sarcoids, a fibroblastic tumor of horses and related species caused by BPV1 and the nearly identical BPV2 (Carr et al., 2001; Silvestre et al., 2009). In addition to their overall hydrophobic composition and similar sizes, all fibropapillomavirus E5 proteins contain a glutamine at position 17, an aspartic acid or glutamic acid at position 33, and cysteines at positions 37 and 39 (Figure 3). As noted below, all four of these amino acids are required for biological activity of BPV1 E5 in cultured fibroblasts.
Figure 2.
Bovine fibropapilloma. The photomicrograph shows a cross section of a bovine cutaneous fibropapilloma. The dermal fibroblast component is stained pink, and the overlying epithelial cell component is stained purple.
Figure 3.
Sequence comparison of fibropapillomavirus E5 proteins. The essential gln17, asp33, cys37 and cys39 in BPV1 and related E5 proteins are shown in red. The ovine and red deer papillomaviruses E5 genes have an in-frame methionine codon a short distance upstream of the one encoding the methionine shown at the left, but it is not known where translation initiates. All fibropapillomaviruses belong to the delta papillomavirus clade (van Doorslaer, 2013). As a measure of similarity among the fibropapillomaviruses, the L1 proteins of the fibropapillomaviruses shown in the figure are between 73% and 77% identical to BPV1 L1; in comparison, the HPV18 L1 protein is only 48% identical to BPV1 L1.
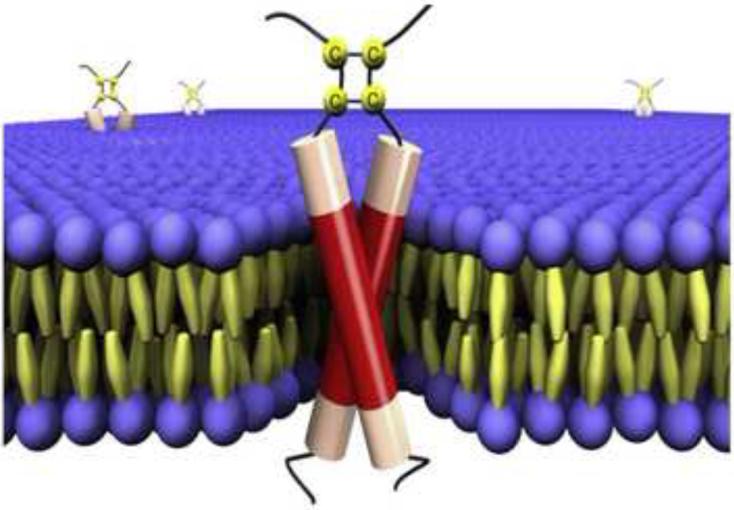
The BPV1 E5 protein exists in cells primarily as a dimer localized to membranes of the endoplasmic reticulum (ER) and Golgi apparatus (Schlegel et al., 1986). Since E5 dimers are converted to monomers by reducing agents in the presence of SDS or by mutation of the C-terminal cysteine residues, it is thought that covalent dimerization is mediated by disulfide bonds involving these cysteines (Horwitz et al., 1988; Schlegel et al., 1986). This suggests that the E5 protein is oriented with its C-terminus in the lumen of the ER or Golgi, which is a non-reducing environment, and not in the reducing environment of the cytoplasm, which would disrupt the disulfide bonds. This orientation was also inferred from the results of antibody binding experiments (Burkhardt et al., 1989). Thus, the C-terminus of the E5 protein points away from the cytoplasm, classifying it as a type II transmembrane protein. Because of its small size, the E5 protein can be considered an isolated transmembrane domain, which is thought to span the membrane a single time (Figure 4).
Figure 4.
BPV1 E5 protein. The figure shows a schematic representation of BPV1 E5 dimers imbedded in the cell membrane, with the carboxy-terminus at the top. Reprinted from Biophys. J. with permission from Elsevier (Windisch et al., 2010).
Transforming activity of BPV1 E5
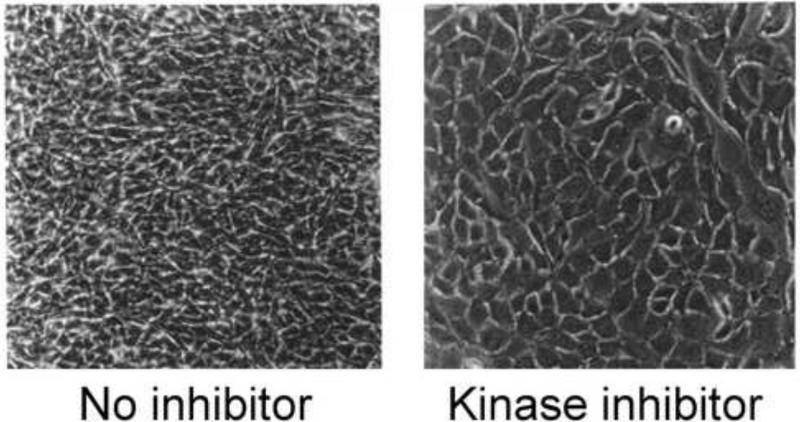
The BPV1 E5 protein displays robust transforming activity in cultured fibroblasts. In the absence of other viral or cellular genes, BPV1 E5 can transform cultured murine NIH3T3 and C127 fibroblast cell lines as well as non-immortalized human foreskin fibroblasts (Bergman et al., 1988; Burkhardt, DiMaio, and Schlegel, 1987; DiMaio, Guralski, and Schiller, 1986; Petti, Nilson, and DiMaio, 1991; Petti and Ray, 2000; Schiller et al., 1986). In contrast to the cobblestone appearance of a monolayer of untransformed cells, BPV1 E5-transformed cells acquire a transformed morphology consisting of a piled-up, highly-refractile and spindle-shaped appearance (Figure 5). Transformed cells also escape from contact inhibition, resulting in DNA synthesis at confluence, higher saturation density, and formation of transformed foci on a monolayer of untransformed cells; lose anchorage dependence and acquire the ability to form cell colonies in semisolid medium; and display tumorigenicity. Upon extended incubation at confluence, E5-transformed human fibroblasts undergo apoptosis, which appears to be due to a secretion of soluble factor that activates the cellular pro-apoptotic protein, Bax (Zhang, Lehman, and Petti, 2002). The E5 protein also activates NF-κB and modulates arachadonic acid metabolism and expression of tissue metalloproteases, which may affect cell invasion and motility (Kilk et al., 1996; Tsirimonaki et al., 2006; Vali, Kilk, and Ustav, 2001; Yuan et al., 2011a). The BPV1 genome also contains two other oncogenes, E6 and E7, which display weaker focus forming activity than E5 in cultured fibroblasts (Neary and DiMaio, 1989). In contrast, E6 and E7 are the primary HPV oncogenes. This difference may be due at least in part to the cells in which transformation is measured, typically fibroblasts for BPV1 and keratinocytes for HPV.
Figure 5.
BPV1 E5 transformed cells. Left panel shows a photomicrograph of mouse C127 cell fibroblasts transformed by BPV1 E5 and maintained at confluence, demonstrating the refractile, piled-up appearance of transformed cells. Right panel shows transformed cells treated with a PDGF receptor kinase inhibitor, which causes the cells to revert to flat, untransformed morphology.
The high conservation of the fibropapillomavirus E5 proteins since the divergence of their animal hosts millions of years ago suggests that, in addition to their ability to transform cells, these proteins play a role in some essential aspect of virus replication. The strict correlation between the presence of a BPV1-like E5 gene with potent transforming activity in cultured fibroblasts and the ability of these viruses to form fibropapillomas in infected animals suggests that the E5 protein is responsible for the unique ability of this group of viruses to induce warts with a fibroblastic component. However, since the vast majority of papillomaviruses replicate in the absence of fibroblast proliferation, the contribution of dermal fibroblasts to wart formation and virus replication is unclear. Indeed, organotypic cell culture experiments demonstrated that E5-transformed fibroblasts are not required for virion production by BPV1-infected bovine keratinocytes (McBride, Dlugosz, and Baker, 2000). This implies that the E5 protein plays a role in infected keratinocytes to support virus replication, presumably to generate the proper cellular environment for his process. However, the precise nature of the contribution of E5 to virus replication is obscure, as is its relationship to the ability of the E5 protein to transform cells.
Role of PDGF β receptor in E5 transformation
In cultured cells and in animals, the transforming activity of BPV1 E5 is tightly linked to its ability to bind to and activate the cellular platelet-derived growth factor (PDGF) β receptor (Table 2). The ~1000-amino acid PDGF β receptor is a receptor tyrosine kinase consisting of an extracellular ligand-binding domain, a single transmembrane domain, and a cytoplasmic catalytic domain. In the absence of ligand, the PDGF β receptor is monomeric and unphosphorylated. Binding of PDGF to the extracellular domain of the receptor induces receptor dimerization, which in turn results in autophosphorylation of the intracellular domain of the receptor on tyrosine residues and recruitment and activation of cellular signaling proteins, resulting in mitogenesis (Figure 6). Sustained mitogenic signaling by the PDGF receptor, for example in response to expression of the v-sis oncogene (which encodes a homologue of PDGF), results in tumorigenic transformation.
Table 2.
Cellular systems demonstrating a physical or functional interaction between BPV1 E5 and PDGF β receptor
| Cultured cellsa |
| Transformed mouse fibroblast cell lines |
| Transformed mortal human fibroblasts |
| Growth factor independent mouse cellsb |
| Naturally occurring tumorsc |
| Bovine cutaneous fibropapillomas |
| Bovine bladder fibropapillomas |
| Bubaline fibropapillomasd |
| Equine sarcoids |
| Other cells types in animalsc |
| Bovine placenta |
In cultured cells, E5 exists in a stable complex with PDGF β receptor and causes receptor activation.
These are mouse cells stably expressing an exogenous human or murine PDGF β receptor.
In these cells, E5-induced activation of the endogenous PDGF β receptor or colocalization between E5 and the receptor has been observed; complex formation has been detected in bovine fibropapillomas.
Bubaline refers to water buffaloes.
Figure 6.
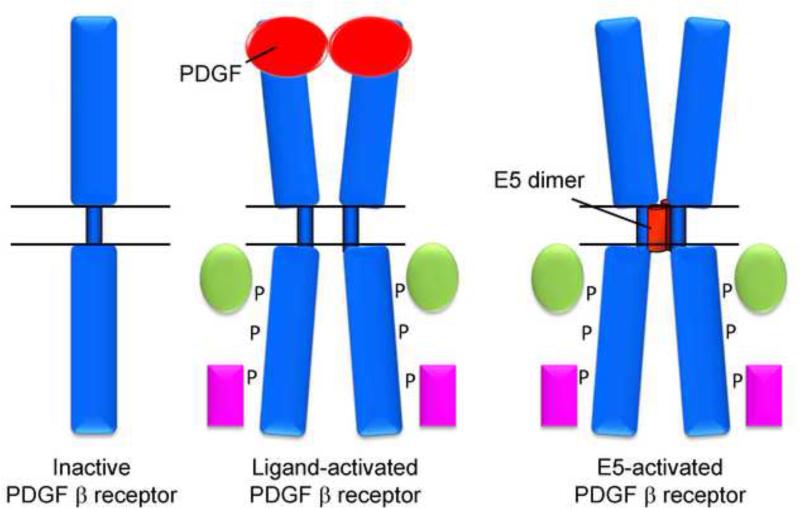
Model for E5 interaction with the PDGF β receptor. A monomer of inactive PDGF β receptor is shown in left. The middle shows PDGF β receptor activated by binding of PDGF to the extracellular domain. This results in receptor dimerization, tyrosine phosphorylation in the intracellular domain, and recruitment of cellular signaling substrates shown in green and purple. The right diagram shows PDGF β receptor activated by binding of a dimer of the BPV1 E5 protein to the transmembrane domain of the receptor. The horizontal lines represent the cell membrane, with the cytoplasm beneath.
Like v-sis, the BPV1 E5 protein causes sustained activation of the cellular PDGF β receptor, as assessed by increased receptor dimerization, tyrosine phosphorylation, in vitro tyrosine kinase activity, and assembly into complexes with known cytoplasmic mediators of PDGF receptor signaling (Drummond-Barbosa et al., 1995; Goldstein et al., 1992; Goldstein et al., 1994; Lai, Henningson, and DiMaio, 1998; Lai, Henningson, and DiMaio, 2000; Petti, Nilson, and DiMaio, 1991). The importance of PDGF β receptor in E5-induced transformation was shown most clearly in gene transfer experiments. In these studies, the E5 protein was inert in epithelial and hematopoietic cells lacking PDGF β receptor, but could induce tumorigenic transformation or growth factor independence in these cells after transfer of the receptor gene (Drummond-Barbosa et al., 1995; Goldstein et al., 1994; Nilson and DiMaio, 1993). These experiments also demonstrated that transformation required PDGF β receptor kinase activity (Drummond-Barbosa et al., 1995). In addition, transformation of fibroblasts was blocked by pharmacologic inhibition of PDGF receptor enzymatic activity or by genetic manipulations that inhibited expression or activity of the PDGF β receptor or its signaling pathway (Figure 5) (Klein et al., 1998; Lai, Edwards, and DiMaio, 2005; Petti and Ray, 2000; Riese and DiMaio, 1995; Yuan et al., 2011a; Yuan et al., 2011b).
These gene transfer studies also showed that the functional interaction between the E5 protein and the PDGF β receptor was highly specific. Although the E5 protein can induce growth factor independence in cooperation with the PDGF β receptor, it does not cooperate with the PDGF α receptor, a closely related receptor tyrosine kinase, which also binds PDGF (Goldstein et al., 1994; Petti and DiMaio, 1994; Staebler et al., 1995). Similarly, some single amino acid substitutions in and around the transmembrane domain of the PDGF β receptor prevented cooperation, also implying that productive interaction with the PDGF β receptor was highly specific (Nappi and Petti, 2002; Nappi, Schaefer, and Petti, 2002; Petti et al., 1997). On the other hand, deletion of the extracellular, ligand-binding domain of the PDGF β receptor did not inhibit cooperation (Drummond-Barbosa et al., 1995; Staebler et al., 1995). Because these extracellular deletions prevented the receptor from responding to PDGF but did not affect E5 activity, this result demonstrated that the E5 protein activates the PDGF β receptor in a ligand-independent manner.
The ligand independence of E5-mediated PDGF β receptor activation implied that the viral protein utilized a novel mechanism of activation. Indeed, co-immunoprecipitation analysis from detergent extracts of transformed cells demonstrated that the E5 protein and the tyrosine-phosphorylated PDGF β receptor exist in a stable complex in these cells (Goldstein et al., 1992; Petti and DiMaio, 1992). Complex formation occurred with PDGF β receptor mutants lacking the extracellular binding domain, and studies of receptor mutants and chimeras mapped binding to the transmembrane domain of the receptor (Figure 6) (Cohen et al., 1993; Drummond-Barbosa et al., 1995; Goldstein et al., 1992; Petti et al., 1997; Staebler et al., 1995). Indeed, the E5 protein was able to form a complex with a short segment of the PDGF β receptor consisting largely of its transmembrane domain, demonstrating that this domain was sufficient for complex formation (Nappi, Schaefer, and Petti, 2002). Mutational analysis summarized in the next section demonstrated a strong correlation between PDGF β receptor binding and activation and cell transformation.
PDGF β receptor activation also appears responsible for the tumorigenic activity of BPV1 in vivo. The transformed dermal fibroblasts in fibropapillomas and equine sarcoids are rich in PDGF β receptor. Animals experimentally infected with BPV1 develop tumors of mesenchymal cells such as meningiomas, sarcomas, and chondromas, which express abundant PDGF β receptor, suggesting that this receptor is also a prime target of the E5 protein in vivo.
Borzacchiello and colleagues have conducted a comprehensive analysis of E5 expression and activity in naturally occurring animal tumors {reviewed in (Corteggio et al., 2013)}. They found that the PDGF β receptor and its downstream signaling pathways are activated in skin and urinary bladder fibropapillomas and in equine sarcoids (Table 2) (Bocaneti et al., 2012; Borzacchiello et al., 2009; Borzacchiello et al., 2006; Corteggio et al., 2011; Corteggio et al., 2010; Roperto et al., 2013). Most strikingly, the PDGF β receptor is colocalized or physically associated with the E5 protein in these lesions. E5 expression may also cause a number of other biochemical abnormalities in tumor cells, including effects on connexin 26 (which may affect cell-cell communication) and calpain 3 (Roperto et al., 2010; Silva et al., 2012). These studies also revealed expression of E5 in unexpected places, including white blood cells and the placentas of cows suffering from BPV2-induced bladder tumors (Brandt et al., 2008; Roperto et al., 2012; Roperto et al., 2011; Roperto et al., 2010). In the placentas, the E5 protein was associated with activated PDGF β receptor.
Taken together, these experiments indicated that the E5 protein binds stably to the transmembrane region of the PDGF β receptor, resulting in receptor dimerization and activation as well as transformation in cultured cells and in animals (Figure 6). These results not only uncovered a novel mechanism of viral transformation, but demonstrated that receptor tyrosine kinases can be activated by proteins unrelated to their normal ligands. Furthermore, these experiments highlighted the ability of transmembrane domains to undergo highly specific, biologically relevant interactions.
Mutational analysis of the BPV1 E5 protein
The BPV1 E5 protein has been subjected to extensive mutational analysis. In general, hydrophobic substitutions in the transmembrane segment of the E5 protein (approximately spanning residues 10 to 32) do not inhibit transforming activity (Horwitz et al., 1988). Indeed, the E5 protein can tolerate wholesale substitutions of hydrophobic amino acids, particularly if glutamine or a related hydrophilic amino acid is retained at position 17 (Horwitz, Weinstat, and DiMaio, 1989; Kulke et al., 1992; Meyer et al., 1994). In contrast, many substitutions at Gln17 and Asp33 interfered with activity (Klein et al., 1999; Klein et al., 1998; Sparkowski, Anders, and Schlegel, 1994). Replacement of the cysteines in E5 with serine also inhibits dimer formation, transformation, and complex formation with the PDGF β receptor, suggesting that E5 dimerization is required for activity (Horwitz et al., 1988; Nilson et al., 1995). Consistent with this interpretation, a heterologous dimerization domain can compensate for the absence of the cysteines (Mattoon et al., 2001). The strict conservation of these four required residues (Gln17, Asp33, Cys37, and Cys39) in fibropapillomavirus E5 proteins suggests that the ability of E5 to activate the PDGF β receptor has been conserved throughout fibropapillomavirus evolution (Figure 3). In general, E5 mutations coordinately affect PDGF β receptor activation and focus formation, providing genetic evidence that activation is required for cell transformation (Klein et al., 1999; Klein et al., 1998; Nilson et al., 1995; Sparkowski et al., 1996).
The PDGF β receptor is a type I transmembrane protein, i.e., with its N-terminus extending away from the cytoplasm. In contrast, as noted above, the E5 protein appears to adopt the opposite orientation. Thus, if the E5 protein and the transmembrane domain of the PDGF β receptor contact one another directly, they align in an anti-parallel orientation. This would place Asp33 in E5 at the extracellular membrane interface near the juxtamembrane Lys499 in the PDGF β receptor. Because Asp33 and Lys499 have opposite charges, they could form a salt bridge to stabilize the E5/PDGF β receptor complex (Meyer et al., 1994; Surti et al., 1998). Similarly, Gln17 of E5 could form a hydrogen bond with Thr513 in the PDGF β receptor transmembrane domain (or with a main-chain polar group in the vicinity of Thr513). The results of mutational analysis are consistent with this arrangement: a negatively-charged amino acid is required at position 33 and an amino acid capable of hydrogen bond formation is required at position 17 (Adduci and Schlegel, 1999; Klein et al., 1999; Klein et al., 1998; Sparkowski, Anders, and Schlegel, 1994). In addition, mutations at positions 499 and 513 PDGF β receptor (as well as at some additional positions) also disrupt complex formation and inhibit transforming activity (Nappi and Petti, 2002; Nappi, Schaefer, and Petti, 2002; Petti et al., 1997). In most but not all studies, mutations coordinately affect complex formation and PDGF β receptor activation, suggesting that complex formation is required for receptor activation.
A novel genetic approach was used to map the interface of the two E5 monomers within the E5 dimer (Mattoon et al., 2001). Varying extents of the E5 protein were fused to Put3, a dimeric yeast protein. In essence, this forced the E5 monomers to adopt different rotational orientations relative to one another in each of the constructs. Testing the different constructs in C127 cell focus forming assays identified a single one with robust activity. Based on the known structure of Put3, the active construct was predicted to have an E5 dimer interface consisting of E5 amino acids Ala14, Gln17, Leu21, Leu24, and Phe28. This interface is consistent with molecular modeling studies summarized below. On the other hand, alanine scanning mutations along the length of the E5 protein produced a more complicated picture; some mutations differentially affected the ability of the E5 protein to form dimers and higher order oligomers, bind the PDGF β receptor, activate the receptor, and transform cells. On the basis of these studies, Adduci and Schlegel (Adduci and Schlegel, 1999) proposed that the E5 protein could adopt alternative homo-oligomeric interfaces (neither of which corresponded to the 14/17/21/24/28 interface described above). These latter experiments also identified a face on the E5 protein proposed to bind to the PDGF β receptor. The basis for these discrepancies is not clear, and a full understanding of the structure of the E5 dimer and its association with the PDGF β receptor will require high-resolution structural studies.
Studies of the E5 protein and its mutants may also provide insight into PDGF β receptor itself. Some mutants containing substitutions in the carboxy-terminal end of the E5 protein increased the saturation density of mortal human fibroblasts but, unlike wild-type E5 or v-sis, did not induce focus formation (Nilson et al., 1995; Petti et al., 2008). In cells expressing these mutants, PDGF β receptor recruited certain signaling substrates and not others, and a receptor signaling pathway involving SHP2-p66shc-p190BRhoGAP correlated specifically with focus formation and not with other transformed phenotypes (Petti et al., 2008). Thus, it appears that these E5 mutants cause partial activation of the PDGF β receptor, presumably by causing phosphorylation of only a subset of the ten or more tyrosines in the intracellular domain of the receptor that are phosphorylated in response to ligand treatment. Further analysis of these and other E5 mutants may reveal unexpected features of PDGF receptor signaling.
Biophysical characterization of BPV1 E5
The BPV1 E5 protein has also been subjected to biophysical analysis. Spectroscopic studies on synthetic E5 peptides and recombinant proteins in lipid bilayers indicated that the protein adopted a transmembrane orientation with an overall α-helical configuration (Surti et al., 1998; Windisch et al., 2010). Analytical centrifugation and nuclear magnetic resonance (NMR) experiments demonstrated that the E5 transmembrane domain has intrinsic dimerization potential in the absence of the C-terminal disulfide bonds, suggesting that transmembrane domain interactions nucleate E5 dimer formation, which is subsequently stabilized by disulfide bond formation (Oates et al., 2008; Windisch et al., 2010).
Molecular modeling conducted by molecular dynamics and energy minimalization approaches identified a potential low-energy model of the E5 dimer in which the monomers form a symmetric, left-handed coiled-coil with the dimer interface comprised of amino acids Ala14, Gln17, Leu21, Leu24, Phe 28, with Gln17 hydrogen bonding to its counterpart on the opposite helix (Surti et al., 1998). NMR analysis of E5 peptides is also consistent with the symmetric 14/17/21/24/28 dimer interface (King et al., 2011). In this model, which is congruent with the model derived from the Put3 experiments, the essential Gln17 from one E5 monomer and Asp33 from the other are on the same face of the dimer where they are in a position to interact directly with one molecule of the PDGF β receptor (Surti et al., 1998). This model thus proposes that dimerization of the E5 protein generates the PDGF β receptor binding site, explaining why E5 dimerization is required for complex formation with the PDGF β receptor. Because the E5 dimer has two such faces, this model also provides a simple explanation for the ability of the E5 protein to induce dimerization of the PDGF β receptor. More recent molecular modeling docked the PDGF β receptor transmembrane domain onto the E5 dimer in a manner consistent with these assignments (King et al., 2011). However, it should be noted that these modeling exercises did not account for the effects of the lipid bilayer on E5 structure and, as noted above, no high-resolution information yet exists.
Alternative E5 activities and models for E5 action
Although the BPV1 E5 protein can transform fibroblasts by activating the PDGF β receptor, in some situations the E5 protein may utilize a different mechanism to transform cells. In particular, Schlegel and colleagues reported the existence of E5 substitution mutants that had lost the ability to bind or activate the PDGF β receptor yet retained transforming activity (Sparkowski et al., 1996; Suprynowicz et al., 2002; Suprynowicz et al., 2000). They furthermore showed that these mutants activated intracellular signaling pathways involving the c-src proto-oncogene product and PI3'kinase, and that src signaling was required for transformation by one of these mutants. However, our group reported that several of these anomalous E5 mutants did in fact activate the PDGF β receptor (Lai, Edwards, and DiMaio, 2005), and src and PI3'kinase are known components of the PDGF β receptor signaling network, so it remains unclear if these mutants truly utilize an alternative mechanism of transformation, or if these apparent differences rather reflect technical differences in measuring PDGF β receptor binding and activation.
The BPV1 E5 protein (and other E5 proteins, see below) also forms a stable complex with the 16kDa, transmembrane subunit c (designated 16K) of the vacuolar ATPase (V-ATPase), a multi-subunit protein complex that normally causes acidification of intracellular organelles such as the Golgi apparatus (Goldstein et al., 1992; Goldstein et al., 1991). This interaction is thought to involve Gln17 in the E5 protein and a glutamic acid in the fourth transmembrane domain of the 16K protein, and it appears to inhibit V-ATPase activity, resulting in alkalinization and swelling of the Golgi apparatus and other vesicular structures (Andresson et al., 1995; Schapiro et al., 2000; Tsirimonaki et al., 2006). It has been proposed that the 16K/E5 interaction may affect transformation (Andresson et al., 1995; Schapiro et al., 2000), but the role of this interaction in E5-mediated transformation has not been studied in detail.
In addition to its transforming activity, the BPV1 E5 protein decreases cell-surface expression of major histocompatibility complex class I (MHCI) antigens (Ashrafi et al., 2002), an activity shared with other E5 proteins (see below). BPV E5 proteins use multiple mechanisms to down-regulate MHCI expression, including transcriptional repression and inhibition of intracellular trafficking (Ashrafi et al., 2006a; Marchetti et al., 2002). MHCI down-regulation may contribute to immune evasion by inhibiting recognition of infected cells by cytotoxic T lymphocytes.
Reprogramming the BPV1 E5 protein
If specific amino acids in the transmembrane domain of the E5 protein bind directly to the transmembrane domain of the PDGF β receptor, it may be possible to identify other transmembrane sequences that also recognize the PDGF β receptor. To accomplish this, we constructed libraries expressing hundreds of thousands of artificial proteins modeled on the E5 protein, but containing randomized sequences of hydrophobic amino acids in place of the transmembrane domain of the native E5 protein. Genetic techniques were then used to select rare, biologically active proteins from these libraries. We used this approach to isolate many different transmembrane proteins, some as small as 32 amino acids, that activated the PDGF β receptor and transformed cells (Freeman-Cook et al., 2004; Freeman-Cook et al., 2005; Ptacek et al., 2007; Talbert-Slagle et al., 2009) (e.g., TM36-4 in Figure 7). The transmembrane domain sequences of these novel oncogene products are strikingly different from each other and from the E5 protein, indicating that multiple divergent transmembrane sequences can accommodate the transmembrane domain of the PDGF β receptor. Furthermore, none of the tested proteins activated the PDGF α receptor, suggesting that the specificity of the E5 protein for the PDGF β receptor did not arise because of natural selection against a viral protein that activated the PDGF α receptor, but rather that these small transmembrane proteins are intrinsically specific.
Figure 7.

Biologically active artificial proteins modeled on the BPV1 E5 protein. The top line shows the sequence of the BPV1 E5 protein. The blue amino acids in the other lines show the randomized transmembrane segment. BPV1 E5 and pTM36-4 activate the PDGF β receptor; TC2-3 activates the human erythropoietin receptor; and BY1PC2 down-regulates the HIV co-receptor, CCR5.
We have also isolated similar small transmembrane proteins that functionally interact with different transmembrane targets. For example, we selected a 44-amino acid transmembrane protein, TC2-3, that activates the human erythropoietin receptor (EPOR) but not the PDGF β receptor and induces erythroid differentiation of human hematopoietic progenitor cells (Figure 7) (Cammett et al., 2010). Strikingly, TC2-3 does not activate the murine EPOR, highlighting the exquisite specificity of small transmembrane proteins. Similarly, we have isolated multiple small traptamers (e.g., BY1PC2 in Figure 7) with diverse transmembrane domains that specifically inhibit expression of a multipass transmembrane protein, CCR5 (Scheideman et al., 2012). Because CCR5 is an essential cell surface receptor for human immunodeficiency virus, these proteins selectively inhibit infection by HIV types that require CCR5 for cell entry. The transmembrane sequences of TC2-3 and BY1PC2 do not resemble the E5 protein (Figure 7). Thus, the BPV1 E5 protein has been used as a model to construct artificial, transmembrane proteins with novel biological activities.
Human papillomavirus E5 proteins
Studies of the BPV1 E5 protein paved the way for similar analysis of HPV E5 proteins. Although the HPV E5 proteins are very hydrophobic, they share no sequence similarity with BPV1 E5 and in the case of the high-risk HPV are about twice as large. Not all HPVs encode an E5 protein, and several open reading frames designated E5 in various HPV types are likely to be spurious (Bravo and Alonso, 2004). Comprehensive sequence and phylogenetic analysis of the HPV E5 region identified several different classes of E5 proteins (Bravo and Alonso, 2004). The high-risk HPVs infecting genital mucosa, including HPV16, the most prevalent HPV type in cervical, other anogenital, and oropharyngeal cancers (zur Hausen, 2009), encode a conserved ~80-amino acid protein (designated E5α) with weak transforming activity in cultured cells and animals (Figure 8). The 83-amino acid HPV16 E5 protein (16E5) has been subjected to extensive analysis, but the molecular basis for its biological activities is still not well understood. The low-risk genital HPVs such as HPV6 and HPV11, which cause genital warts, encode two E5 proteins (designated E5A and E5B or E5γ and E5δ). These E5 proteins are only distantly related to 16E5 (Figure 8), and E5A displays weak transforming activity in limited studies. The sequences of the E5 proteins from the HPVs and related papillomaviruses are shown in Figure 9. Based on the presence of a conserved E5 gene in many HPVs, it is likely that HPV E5 plays a significant role in the virus replicative life cycle. Strikingly, the HPV E5 genes appear to have coevolved with the major HPV oncogenes E6 and E7, and their presence in the viral genome correlates with risk of cancer, suggesting that the E5 proteins may contribute to human carcinogenesis (Bravo and Alonso, 2004; Schiffman et al., 2005).
Figure 8.
Sequence comparison of representative human papillomavirus E5 proteins. The putative transmembrane domains of HPV16 E5 are shown in boxes. Amino acids identical to those in HPV16 E5 are shown in red. The HPV6 sequence is E5A.
Figure 9.
This figure shows an alignment of all recognized HPV E5 proteins, as well as E5 proteins from related animal alpha papillomaviruses. Acidic amino acids are shown in red, basic amino acids in blue, and hydrophilic amino acids in green. All HPV and related animal papillomavirus E5 sequences present in the PaVE database (http://pave.niaid.nih.gov/#home) were extracted and aligned using the ClustalW module of Geneious v6 created by Biomatters (http://www.geneious.com/) (courtesy of A. McBride).
Oncogenic activity of HPV E5
The major transforming activity of the HPVs resides in the E6 and E7 genes, which can immortalize primary human keratinocytes in culture and display potent oncogenic activity in transgenic mice. In cultured cells, HPV E5 proteins are weakly transforming when expressed alone and require a cofactor for more robust activity. The first HPV E5 gene shown to have transforming activity is encoded by HPV6 (Chen and Mounts, 1990), which can induce focus formation and anchorage independence in murine fibroblasts, but virtually all subsequent studies focused on 16E5. At physiological levels of expression in human foreskin keratinocytes, 16E5 localizes primarily to the ER, while at higher expression levels is it present in the membranes of the Golgi apparatus, plasma membrane and nuclear envelope as well (Conrad, Bubb, and Schlegel, 1993; Disbrow, Hanover, and Schlegel, 2005; Hu and Ceresa, 2009; Krawczyk et al., 2010; Lewis et al., 2008; Oetke et al., 2000; Suprynowicz et al., 2008). There are conflicting reports regarding the orientation of 16E5 in the membrane (Hu and Ceresa, 2009; Hu et al., 2009; Krawczyk et al., 2010).
16E5 acts synergistically with epidermal growth factor (EGF) in murine fibroblasts to induce anchorage independent growth and growth in low serum (Leechanachai et al., 1992; Pim, Collins, and Banks, 1992; Straight et al., 1993), as well as to enhance DNA synthesis in primary human keratinocytes (Straight et al., 1993). Furthermore, both 6E5 and 16E5 cooperate with HPV E7 to induce the proliferation of primary rodent epithelial cells (Bouvard et al., 1994; Vallee and Banks, 1995). 16E5 displays additional transforming activities, including the ability to render mouse cell lines tumorigenic (Leechanachai et al., 1992; Leptak et al., 1991), enhance the ability of E6 and E7 to immortalize primary human keratinocytes (Stoppler et al., 1996), and increase the motility and invasiveness of a human keratinocyte cell line (Barbaresi et al., 2010; Kivi et al., 2008). The N-terminal hydrophobic domain of 16E5 is required for its ability to induce anchorage independent growth and invasiveness of human keratinocytes (Barbaresi et al., 2010; Lewis et al., 2008). 16E5 does not induce focus formation in fibroblasts (Chen and Mounts, 1990; Disbrow et al., 2003; Leptak et al., 1991; Suprynowicz et al., 2005), a signature activity of BPV1 E5.
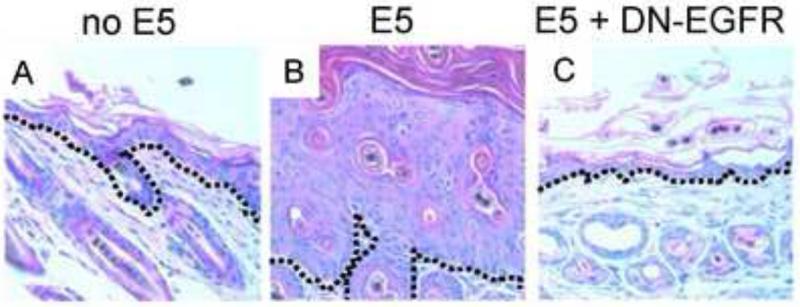
Despite its weak transforming activity in vitro, high-risk HPV E5 may play an important role in carcinogenesis. Analysis of natural variants of 16E5 revealed that those with the greatest mitogenic activity in vitro were derived from HPV16 strains most commonly associated with cervical neoplasia (Nath et al., 2006), and as noted above, the presence of an E5 gene in the viral genome is correlated with carcinogenic potential (Bravo and Alonso, 2004; Schiffman et al., 2005). Importantly, expression of 16E5 in stratified squamous epithelia in transgenic mice causes epidermal hyperplasia and a higher frequency of spontaneous skin tumors (Genther-Williams et al., 2005). Genetic studies demonstrated that EGF receptor (EGFR) signaling is required for E5-induced epidermal hyperplasia (Figure 10). Prolonged estrogen treatment of 16E5 transgenic mice caused cervical cancer, although E5 is less active in this assay than E6 or E7 (Genther-Williams et al., 2005; Maufort et al., 2010). In human cervical cancers, the HPV18 E5 gene is often deleted upon integration of the viral DNA into the host cell genome (Schwarz et al., 1985), and detection of the E5 protein in cervical cancers has proven difficult. However, the viral genome persists as a plasmid in some HPV16-positive cervical carcinomas (Choo et al., 1987; Cullen et al., 1991; Matsukura, Koi, and Sugase, 1989), and the 16E5 protein has been detected in precancerous and malignant HPV16-positive cervical lesions (Chang et al., 2001; Kell et al., 1994; Sahab et al., 2012), suggesting that HPV E5 may contribute to malignant progression. Such a role is supported by the finding that 16E5 contributes to both the promotion and progression stages of skin carcinogenesis in transgenic mice (Maufort et al., 2007).
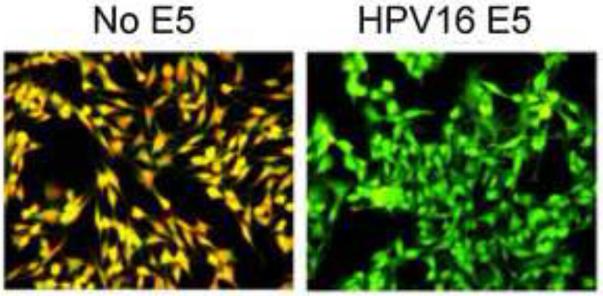
Figure 10.
HPV16 E5-induced epithelial hyperplasia. Left panel shows a photomicrograph of the skin of a non-transgenic mouse, with the dashed black line indicating the position of the basement membrane and the purple area above being the epithelial layer. Middle panel shows a photomicrograph of transgenic mouse skin expressing HPV16 E5, demonstrating the thickened layer of hyperplastic epithelial cells. Right panel shows a photomicrograph of transgenic mouse skin co-expressing HPV16 E5 and a dominant negative form of the EGF receptor (DN-EGFR). All pictures taken at the same magnification. Adapted by permission from the authors and the American Association for Cancer Research.
Role of the EGFR in the oncogenic activity of HPV E5
Several lines of evidence implicate the EGFR, a transmembrane receptor tyrosine kinase, as a key mediator of the transforming effects of 16E5. As mentioned above, EGF stimulates the transforming and mitogenic activity of 16E5 in cultured cells (Leechanachai et al., 1992; Pim, Collins, and Banks, 1992; Straight et al., 1993), and EGFR signaling is required for E5-induced epithelial hyperplasia in transgenic mice (Genther-Williams et al., 2005). Importantly, numerous studies demonstrated that 16E5 increases the levels of EGFR at the cell surface, thereby sensitizing cells to EGF (Crusius et al., 1998; Pim, Collins, and Banks, 1992; Straight et al., 1993; Tomakidi et al., 2000b). Thus, unlike BPV1 E5, 16E5 does not activate its target receptor in a ligand-independent manner.
16E5 also stimulates EGFR signaling pathways. It promotes prolonged activation of ERK1/2 and AKT in response to EGF (Crusius, Auvinen, and Alonso, 1997; Gu and Matlashewski, 1995; Pim, Collins, and Banks, 1992; Zhang, Spandau, and Roman, 2002). 16E5 also stimulates expression of the c-jun and c-fos oncogenes by acting through the NF1 regulatory element (Chen et al., 1996b; Chen et al., 1996c), and it increases the expression of cyclooxygenase-2 (COX-2) and vascular endothelial growth factor (VEGF) via multiple EGFR-dependent pathways (Kim et al., 2006; Kim et al., 2009). The up-regulation of COX-2 generates a positive feedback loop of prostaglandin E2 (PGE2) signaling, resulting in up-regulation of the PGE2 receptor Ep4, which appears to promote VEGF expression and contribute to anchorage independent growth (Oh et al., 2009). Finally, 16E5 down-modulates the cell cycle inhibitors p21 and p27 in fibroblasts and keratinocytes by both transcriptional and post-translational mechanisms (Pedroza-Saavedra et al., 2010; Tsao et al., 1996). In contrast, 16E5 appears to inhibit the action of ErbB4, a different member of the EGFR family (Chen et al., 2007).
The molecular mechanism by which 16E5 affects EGFR levels or signaling is unresolved. It has been reported that 16E5 can form a stable complex with the EGFR in cells overexpressing the receptor (Hwang, Nottoli, and DiMaio, 1995), but a second group failed to demonstrate this interaction (Conrad et al., 1994), and an interaction between an HPV E5 protein and endogenous EGFR has not been reported. 16E5 may increase EGFR levels indirectly by expediting the transport of the receptor from endosomes to the cell surface (Straight et al., 1993). It was initially reported that 16E5 reduced endosomal acidification and thereby inhibited lysosomal degradation of internalized EGFR, resulting in increased receptor recycling to the cell surface (Straight, Herman, and McCance, 1995). These results are consistent with subsequent findings that the C-terminus of 16E5 is required for enhancing ligand-dependent EGFR activation as well as increasing endosomal pH (Disbrow, Hanover, and Schlegel, 2005; Rodriguez, Finbow, and Alonso, 2000). 16E5 may modulate endosome acidification via its ion channel activity or its inhibition of the V-ATPase (see below). However, other studies suggested that 16E5 influences EGFR transport by altering vesicular trafficking in a pH-independent manner, by affecting reorganization of the actin cytoskeleton, or by inhibiting vesicle fusion (Suprynowicz et al., 2010; Thomsen et al., 2000). Alternatively, 16E5 may inhibit ubiquitination and subsequent proteasomal degradation of the EGFR by disrupting the binding of Cbl, an E3 ubiquitin ligase (Zhang et al., 2005). Finally, 16E5 may modify the lipid composition and dynamics of cell membranes (Bravo, Crusius, and Alonso, 2005). This is consistent with the observation that 16E5 increases the plasma membrane expression of caveolin-1 and the ganglioside GM1, both components of lipid rafts (Suprynowicz et al., 2008). These changes in membrane chemistry could affect vesicular trafficking and/or cell signaling. For example, higher levels of GM1 on the cell surface can augment the proliferative response to EGF (Nishio et al., 2005).
Alternative mechanisms of HPV E5 oncogenic activity
16E5 may also affect EGFR-independent pathways. 16E5 can stimulate ERK1/2, p38 MAP kinases, and phospholipase Cγ-1 independently of the EGFR in human keratinocytes (Crusius et al., 1999; Crusius, Rodriguez, and Alonso, 2000). Venuti et al. reported that 16E5 enhanced endothelin-1-induced mitogenic signaling, resulting in the growth of primary human keratinocytes (Venuti et al., 1998). In addition, 16E5 binds to ZnT-1, a zinc transporter, and to EVER1 and EVER2, transmembrane channel-like proteins, which are mutated in patients with epidermodysplasia verruciformis, an inherited predisposition to skin carcinomas associated with HPV5 and HPV8 infections (Lazarczyk et al., 2008). 16E5 binding impairs the ability of these proteins to inhibit c-jun and zinc-regulated transcription factors.
High-risk HPV E5 proteins may also contribute to transformation by increasing cellular DNA content. Hu and coworkers showed that 16E5 promotes cell-cell fusion, thereby inducing the formation of binucleated cells (Hu et al., 2009). A fraction of 16E5 must be expressed on plasma membrane of both cells for fusion to occur (Hu and Ceresa, 2009), suggesting that E5 molecules expressed by adjacent cells interact with each other. Another report showed that 16E5 causes a doubling of the number of chromosomes via endoreplication (Hu et al., 2010). Polyploidy resulting from E5-induced cell-cell fusion or endoreplication was proposed to lead to chromosomal instability during subsequent rounds of cell division, thereby increasing the likelihood of cell transformation and neoplastic progression (Gao and Zheng, 2010; Hu and Ceresa, 2009; Hu et al., 2009; Hu et al., 2010).
Role of the 16-kDa subunit of the V-ATPase in HPV E5 activity
Like BPV1 E5, 6E5 and 16E5 physically interact with the 16K subunit of the V-ATPase under conditions of overexpression (Conrad, Bubb, and Schlegel, 1993). Different groups have reported that different segments of 16E5 mediate this interaction (Adam, Briggs, and McCance, 2000; Rodriguez, Finbow, and Alonso, 2000). In yeast, 16E5 interacts with 16K and may reduce the assembly and stability of the V-ATPase complex (Adam, Briggs, and McCance, 2000; Ashby et al., 2001; Briggs, Adam, and McCance, 2001). In addition, 16E5 inhibits acidification of endosomes in human keratinocytes and melanocytes (Di Domenico et al., 2009; Straight, Herman, and McCance, 1995) (Figure 11). HPV11 E5 also binds 16K, but there is no correlation between the ability of E5 mutants to bind 16K and induce anchorage independent growth (Chen et al., 1996a).
Figure 11.
HPV16 E5 increases intracellular pH. Control human FRM melanoma cells (left panel) or cells expressing HPV16 E5 (right panel) were treated with the pH-sensitive dye acridine orange and visualized by fluorescence microscopy. Loss of orange color indicates increased pH. Adapted from J. Exp. Clin. Cancer Res. with permission (Di Domenico et al., 2009).
Although it was hypothesized that the binding of 16E5 to 16K impairs V-ATPase function, which leads to decreased acidification of the endosomes followed by increased recycling of the EGFR to the cell surface and heightened receptor activation in response to EGF (Disbrow, Hanover, and Schlegel, 2005; Straight, Herman, and McCance, 1995; Straight et al., 1993), subsequent studies are not consistent with this model. In immortalized human keratinocytes, the ability of 16E5 to interact with 16K could be dissociated from its ability to enhance ligand-dependent EGFR activation or affect V-ATPase function (Adam, Briggs, and McCance, 2000; Rodriguez, Finbow, and Alonso, 2000). Moreover, several lines of evidence suggest that 16E5 plays role in endocytic trafficking rather than (or in addition to) endosome acidification. First, 16E5 perturbs endocytic trafficking without inhibiting endosomal acidification in mouse fibroblasts (Thomsen et al., 2000). Second, 16E5 specifically alters EGFR trafficking by inhibiting the fusion of endosomal vesicles in a 16K-independent manner (Suprynowicz et al., 2010). Third, one report failed to observe an effect of 16E5 on V-ATPase function in yeast (Ashby et al., 2001). Finally, an interaction of 16E5 with endogenous 16K in primary human keratinocytes was detected only at a low level (Suprynowicz et al., 2010). Therefore, complex formation between 16E5 and 16K may affect a process other than EGFR transport, such as cell-cell communication. Consistent with this view, 16E5 impairs cell-cell communication in human keratinocytes, an effect attributed to decreased phosphorylation and expression of connexin 43, a major component of gap junctions (Oelze et al., 1995; Tomakidi et al., 2000a). Since 16K is also a component of gap junctions, an interaction between 16E5 and 16K could affect connexin 43 or some other gap junctional protein. 16E5-induced loss of gap junctions might render HPV-infected cells less sensitive to growth inhibitory signals emanating from surrounding uninfected cells (Tomakidi et al., 2000a). Regardless of the consequences of 16K binding, it is likely that the 16K/E5 interaction plays an important role in the virus life cycle because it has been observed for several different E5 proteins.
Role of HPV E5 in evading the immune response and apoptosis
Like BPV1 E5, 16E5 appears to play a role in immune evasion by down-regulating the cell-surface expression of cellular proteins involved in antigen presentation. 16E5 physically interacts with the heavy chain component of the human MHCI antigen (HLA-I) and retains HLA-I in the Golgi apparatus and ER, thereby impeding its transport to the cell surface (Ashrafi et al., 2006b; Ashrafi et al., 2005). The di-leucine motifs within the N-terminal hydrophobic domain of 16E5 and HPV31 E5 are required for binding to and down-regulating cell surface HLA-I (Ashrafi et al., 2006b; Cortese, Ashrafi, and Campo, 2010). This segment of 16E5 is also required for direct binding to Bap31, an integral ER membrane protein, which also binds 31E5 and can act as a MHCl chaperone (Regan and Laimins, 2008). Calnexin, a molecular chaperone involved in HLA-I transport to the cell surface, also physically interacts with this domain of 16E5 and is required for intracellular retention of HLA-I (Gruener et al., 2007). 16E5 also perturbs MHC class II maturation by inhibiting endosomal processing of the invariant chain component of MHCII, thereby blocking peptide loading and subsequent transport of MHCII to the cell surface (Zhang et al., 2003). Finally, both 6E5 and 16E5 promote proteasomal degradation of CD1d, an HLA-I-like glycoprotein, and abrogate CD1d-mediated cytokine production (Miura et al., 2010). Possibly reflecting these E5 activities, HLA-1 expression is reduced in human cervical pre-cancerous and cancerous lesions (Cromme et al., 1993; Ritz et al., 2001). The development of multiple mechanisms to down-regulate MHC antigens emphasizes the evolutionary importance of this activity to the papillomaviruses, presumably to evade the host immune response during productive infection. Consistent with this hypothesis, 16E5-expressing cells are poorly recognized by CD8+ T cells (Campo et al., 2010), implying that down-regulation of HLA-I by 16E5 inhibits immune presentation of viral peptides and decreases the adaptive immune response to virally infected cells.
The high-risk HPV E5 proteins also inhibit apoptosis via multiple mechanisms. 16E5 protects human foreskin keratinocytes from ultraviolet B-irradiation-induced apoptosis via EGF-dependent PI3 kinase and ERK1/2 signaling (Zhang, Spandau, and Roman, 2002). It also inhibits hydrogen peroxide-induced apoptosis of human cervical cancer cells by stimulating ubiquitination and proteasomal degradation of the pro-apoptotic protein Bax through a COX-2-PGE-2-protein kinase A-dependent pathway (Oh et al., 2010). 16E5 also protects cultures of human keratinocytes from apoptosis induced by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) or Fas ligand (FasL) (Kabsch and Alonso, 2002; Kabsch et al., 2004). In the latter case, this appeared to be due to the ability of 16E5 to down-regulate cell-surface expression of Fas. Auvenin et al. showed that 16E5 colocalizes with the anti-apoptotic factor, Bcl-2, at intracellular membranes (Auvinen, Alonso, and Auvinen, 2004). Bap31 can also regulate apoptosis, so the interaction of E5 with Bap31 may also influence cell survival. E5-mediated inhibition of apoptosis by one or more of these mechanisms may well promote carcinogenesis by allowing cell survival in inappropriate settings.
Role of HPV E5 in virus replication
The conservation of high-risk HPV E5 genes suggests that they are required for the productive stage of the virus life cycle. E5 mRNA is expressed at high levels in the differentiated suprabasal cells of HPV-infected stratified epithelia (Longworth and Laimins, 2004). In cultures of differentiating human keratinocytes harboring HPV16 and HPV31 genomes, E5 expression is required for full viral genome amplification, activation of late gene expression, and colony formation following methylcellulose-induced differentiation (Fehrmann, Klumpp, and Laimins, 2003; Genther et al., 2003). Furthermore, genetic studies indicated that the E5/Bap31 interaction is required for colony formation in differentiating keratinocytes (Regan and Laimins, 2008). Thus, high-risk E5 proteins enable differentiating keratinocytes to retain their proliferative capacity, which presumably maintains these cells in a state conducive to viral DNA replication. In addition, in immortal human keratinocytes 16E5 inhibits DNA synthesis in response to activation of the keratinocyte growth factor receptor/fibroblast growth factor receptor 2b (KGFR/FGFR2b) (Belleudi et al., 2011). Since KGFR/FGFR2b is expressed primarily in the suprabasal cells of the epithelium, the effects of 16E5 on this receptor might perturb keratinocyte differentiation.
HPV E5 has also been hypothesized to play a role in viral egress (Krawczyk et al., 2011; Krawczyk et al., 2008b). Both low-risk and high-risk HPV E5 proteins cooperate with HPV E6 to induce the formation of perinuclear vacuoles that resemble koilocytotic vacuoles, a diagnostic feature of HPV infection in stratified epithelium (Krawczyk et al., 2008b). Vacuolization may result in increased cell fragility, which in turn may facilitate viral egress (Krawczyk et al., 2011). In addition, the C-terminal domain of 16E5 binds calpactin I and redirects it to the perinuclear region, where it may facilitate the formation of koilocytotic vacuoles (Krawczyk et al., 2011). This domain of 16E5 also binds to karyopherin β3, a member of the nuclear import receptor family (Krawczyk et al., 2008a), but it remains to be determined if any of these interactions play a role in koilocytosis.
It is striking that some HPV (e.g., the β-HPV) appear to lack an E5 gene, yet obviously propagate in vivo. Either the E5 gene is so divergent in these HPV types that it is unrecognizable, the essential E5 function is subsumed by some other viral protein, or the virus has devised some other strategy to dispense with the E5 requirement.
Structure of HPV16 E5
16E5 contains three hydrophobic domains predicted to serve as membrane-spanning segments (Bubb, McCance, and Schlegel, 1988; Halbert and Galloway, 1988). Molecular modeling predicts that 16E5 is a three-pass transmembrane protein (Figures 8 and 12) (Nath et al., 2006; Wetherill et al., 2012). 16E5 appears to self-associate via non-covalent interactions between its transmembrane domains (Gieswein, Sharom, and Wildeman, 2003; Wetherill et al., 2012; Yang, Wildeman, and Sharom, 2003) and possibly via disulfide bonds as well (Disbrow et al., 2003). Using biochemical analysis, transmission electron microscopy, and molecular modeling, Wetherill et al. recently showed that recombinant, epitope-tagged 16E5 oligomerized with hexameric stoichiometry into a ring-like structure in the presence of detergents (Wetherill et al., 2012) (Figure 12). Furthermore, hexameric 16E5 displayed pH-dependent ion channel activity in liposomes, and a small molecule that inhibited of 16E5 ion channel activity reduced the EGF-dependent cell signaling effects of 16E5. If these results are confirmed, they would represent a breakthrough in our understanding of the HPV E5 proteins.
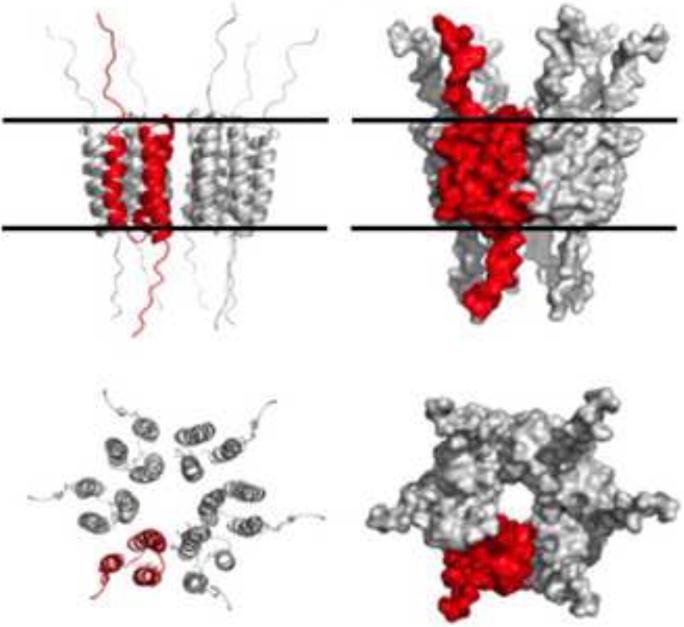
Figure 12.
Model of a hexameric ion channel generated by the HPV16 E5 protein in vitro. Top illustrations show a side view of the hexameric channel in the ribbon (left) or space-filling (right) representation. A 16E5 monomer with three membrane-spanning segments is shown in red. Presumed position of the membrane surface is shown by the horizontal lines. Bottom illustrations show an axial view in the same representations. Adapted from J. Virol. with permission from ASM Press (Wetherill et al., 2012).
Bovine papillomavirus type 4 E5
Bovine papillomavirus type 4 infects mucosal surfaces and induces epithelial warts. BPV4 has also been implicated in alimentary tract carcinomas in conjunction with the ingestion of bracken fern, which contains chemical carcinogens (Campo et al., 2010). BPV4 contains a short open reading frame in the 5’ end of the early region, at the genomic position of the E6 gene in other papillomavirus genomes. Previously named ORF E8, this gene has the potential to encode a 42-amino acid hydrophobic protein and has been designated the BPV4 E5 gene by Campo and colleagues. Because of its unique genome location, the origin of BPV4 E5 and its evolutionary relationship to the other recognized E5 genes is not clear. Nevertheless, this protein shares several biological properties with the canonical E5 proteins, including the ability to transform primary cells in cooperation with other oncogenes. BPV4 E5 does not seem to activate growth factor receptors but rather to affect expression of cell cycle regulatory factors (Grindlay, Campo, and O'Brien, 2005; O'Brien et al., 1999; O'Brien, Grindlay, and Campo, 2001; O'Brien and Campo, 1998). Like other E5 proteins, BPV4 E5 localizes primarily to intracellular membranes, retains MHCI in the Golgi apparatus, associates with the 16K subunit of V-ATPase, and inhibits cell-cell communication (Ashrafi et al., 2000; Ashrafi et al., 2002; Faccini et al., 1996). Although BPV4 E5 is expressed in fibropapillomas in the alimentary canal (Anderson and Akkina, 2007), its role in gastrointestinal tumorigenesis is not known.
Conclusions
In summary, papillomavirus E5 proteins have diverse effects on cell behavior, which may contribute to transformation/carcinogenesis and the productive phase of the virus life cycle. The biological activities of the E5 proteins presumably flow from their interactions with various cellular protein targets (Table 1). An interesting unresolved question concerns the mechanism by which the E5 proteins engage these multiple protein targets. Does the E5 protein bind directly to numerous different targets, and if so how does such a short protein display such binding behavior? Most of the interactions involving the E5 proteins were detected by methods such as co-immunoprecipitation, which show that two proteins are present in a complex, not necessarily that they contact one another directly. Thus, is it possible that each E5 protein has only one or two direct targets, which in turn mediate numerous indirect interactions responsible for its diverse biological activities. The recently described ion channel activity of HPV16 E5 provides another possible mechanism how an E5 protein may be able to influence multiple biological activities without binding directly to numerous targets.
BPV1 E5 appears to act primarily via a unique mechanism involving direct transmembrane activation of the PDGF β receptor, although interactions with other targets including the V-ATPase may contribute to its biological activities. The BPV1 E5/PDGF β receptor interaction has provided new insights into the specificity of transmembrane protein-protein interactions and has inspired a novel approach to modulate cell behavior with artificial transmembrane proteins that have never arisen in nature. Because up to 30% of all cellular proteins are thought to be transmembrane proteins and thus potential targets for E5-like proteins, this approach may have wide applicability.
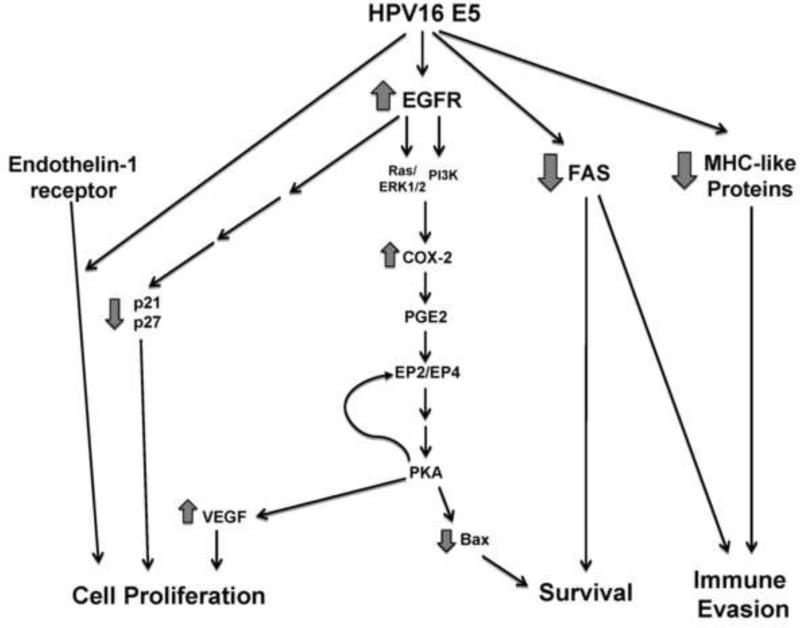
Although a unifying theory of 16E5 action is still elusive, a common theme appears to be its ability to modulate trafficking of membrane proteins, resulting in alterations in the cell surface levels or activity of several different receptors, including those involved in cell proliferation (EGFR), immune recognition (MHC and related proteins) and apoptosis (Fas), thereby influencing a variety of pathways that could contribute to carcinogenesis (Figure 13). Several 16E5 properties may contribute to these pleiotropic effects: its fusogenic and channel forming activity, its ability to bind and modulate the V-ATPase, or its interactions with chaperone-like proteins such as calnexin, Bap31, and calpactin. Genetic and biochemical studies that characterize 16E5 protein complexes should provide further insight into the molecular basis for the various activities of this enigmatic viral oncoprotein and may suggest interventions to prevent or treat HPV-associated malignancies.
Figure 13.
Schematic diagram of the major signaling pathways implicated in HPV16 E5-mediated transformation.
E5 proteins are short transmembrane proteins encoded by many papillomaviruses
The E5 proteins display transforming activity in cultured cells and animals
The E5 proteins modulate the activity of cellular proteins
The BPV1 E5 activates the cellular PDGF β receptor in a ligand independent fashion
The EGF receptor appears to be an important target of the HPV16 E5 protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Permissions
Figure 4. Reprinted from Biophys. J., 99(6), Windisch, D., Hoffmann, S., Afonin, S., Vollmer, S., Benamira, S., Langer, B., Burck, J., Muhle-Goll, C., and Ulrich, A.S., Structural role of the conserved cysteines in the dimerization of the viral transmembrane oncoprotein E5, pp. 1764-1772, 2010, with permission from Elsevier, PMID: 20858420.
Figure 10. Adapted by permission from the authors and the American Association for Cancer Research: Genther-Williams, S.M., Disbrow, G.L., Schlegel, R., Lee, D., Threadgill, D.W., and Lambert, P.F. Requirement of epidermal growth factor receptor for hyperplasia induced by E5, a high-risk human papillomavirus oncogene, Cancer Res., 2005, 65:6534-6543, PMID: 16061632
Figure 11. Adapted from J. Exp. Clin. Cancer Res., 28:4, Di Domenico, F., Foppoli, C., Blarzino, C., Perluigi, M., Paolini, F., Morici, S., Coccia, R., Cini, C., and De Marco, F. Expression of human papilloma virus type 16 E5 protein in amelanotic melanoma cells regulates endo-cellular pH and restores tyrosinase activity, doi:10.1186/1756-9966-28-4, 2009, with permission, PMID: 19133143.
Figure 12. Adapted from J. Virol., 86(9), Wetherill, L.F., Holmes, K.K., Verow, M., Muller, M., Howell, G., Harris, M., Fishwick, C., Stonehouse, N., Foster, R., Blair, G.E., Griffin, S., and Macdonald, A. High-risk human papillomavirus E5 oncoprotein displays channel-forming activity sensitive to small-molecule inhibitors, pp. 5341-5351, 2012, with permission from ASM Press, PMID: 22357280. .
References
- Adam JL, Briggs MW, McCance DJ. A mutagenic analysis of the E5 protein of human papillomavirus type 16 reveals that E5 binding to the vacuolar H+-ATPase is not sufficient for biological activity, using mammalian and yeast expression systems. Virology. 2000;272:315–325. doi: 10.1006/viro.2000.0376. [DOI] [PubMed] [Google Scholar]
- Adduci AJ, Schlegel R. The transmembrane domain of the E5 oncoprotein contains functionally discrete helical faces. J. Biol. Chem. 1999;274:10249–10258. doi: 10.1074/jbc.274.15.10249. [DOI] [PubMed] [Google Scholar]
- Anderson J, Akkina R. Complete knockdown of CCR5 by lentiviral vector-expressed siRNAs and protection of transgenic macrophages against HIV-1 infection. Gene Therapy. 2007;14:1287–1297. doi: 10.1038/sj.gt.3302958. [DOI] [PubMed] [Google Scholar]
- Andresson T, Sparkowski J, Goldstein DJ, Schlegel R. Vacuolar H+-ATPase mutants transform cells and define a binding site for the papillomavirus E5 oncoprotein. J. Biol. Chem. 1995;270:6830–6837. doi: 10.1074/jbc.270.12.6830. [DOI] [PubMed] [Google Scholar]
- Ashby ADM, Meagher L, Campo MS, Finbow ME. E5 transforming proteins of papillomaviruses do not disturb the activity of the vacuolar H+-ATPase. J. Gen. Virol. 2001;82:2353–2362. doi: 10.1099/0022-1317-82-10-2353. [DOI] [PubMed] [Google Scholar]
- Ashrafi GH, Brown DR, Fife KH, Campo MS. Down-regulation of MHC class I is a property common to papillomavirus E5 proteins. Virus Res. 2006a;120(1-2):208–11. doi: 10.1016/j.virusres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Ashrafi GH, Haghshenas M, Marchetti B, Campo MS. E5 protein of human papillomavirus 16 downregulates HLA class I and interacts with the heavy chain via its first hydrophobic domain. Intl. J. Cancer. 2006b;119:2105–2112. doi: 10.1002/ijc.22089. [DOI] [PubMed] [Google Scholar]
- Ashrafi GH, Haghshenas MR, Marchetti B, O'Brien PM, Campo MS. E5 protein of human papillomavirus type 16 selectively downregulates surface HLA class I. Int. J. Cancer. 2005;113:276–283. doi: 10.1002/ijc.20558. [DOI] [PubMed] [Google Scholar]
- Ashrafi GH, Pitts JD, Faccini A, McLean P, O'Brien PM, Finbow ME, Campo MS. Binding of bovine papillomavirus type 4 E8 to ductin (16K proteolipid), down-regulation of gap junction intercellular communication and full cell transformation are independent events. J. Gen. Virol. 2000;81:689–694. doi: 10.1099/0022-1317-81-3-689. [DOI] [PubMed] [Google Scholar]
- Ashrafi GH, Tsirimonaki E, Marchetti B, O'Brien PM, Sibbet GJ, Andrew L, Campo MS. Down-regulation of MHC class I by bovine papillomavirus E5 oncoproteins. Oncogene. 2002;21:248–259. doi: 10.1038/sj.onc.1205008. [DOI] [PubMed] [Google Scholar]
- Auvinen E, Alonso A, Auvinen P. Human papillomavirus type 16 E5 protein colocalizes with the antiapoptotic Bcl-2 protein. Arch. Virol. 2004;149:1745–1759. doi: 10.1007/s00705-004-0325-8. [DOI] [PubMed] [Google Scholar]
- Barbaresi S, Cortese MS, Quinn J, Ashrafi GH, Graham SV, Campo MS. Effects of human papillomavirus type 16 E5 deletion mutants on epithelial morphology: functional characterization of each transmembrane domain. J Gen Virol. 2010;91(Pt 2):521–30. doi: 10.1099/vir.0.016295-0. [DOI] [PubMed] [Google Scholar]
- Belleudi F, Leone L, Purpura V, Cannella F, Scrofani C, Torrisi MR. HPV16 E5 affects the KGFR/FGFR2b-mediated epithelial growth through alteration of the receptor expression, signaling and endocytic traffic. Oncogene. 2011;30(50):4963–76. doi: 10.1038/onc.2011.203. [DOI] [PubMed] [Google Scholar]
- Bergman P, Ustav M, Sedman J, Moreno-Lopez J, Vennstrom B, Pettersson U. The E5 gene of bovine papillomavirus type 1 is sufficient for complete oncogenic transformation of mouse fibroblasts. Oncogene. 1988;2:453–459. [PubMed] [Google Scholar]
- Bocaneti F, Altamura G, Corteggio A, Martano M, Roperto F, Velescu E, Borzacchiello G. Expression of platelet derived growth factor beta receptor, its activation and downstream signals in bovine cutaneous fibropapillomas. Res Vet Sci. 2012 doi: 10.1016/j.rvsc.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Borzacchiello G, Mogavero S, De Vita G, Roperto S, Della Salda L, Roperto F. Activated platelet-derived growth factor beta receptor expression, PI3K-AKT pathway molecular analysis, and transforming signals in equine sarcoids. Vet Pathol. 2009;46(4):589–97. doi: 10.1354/vp.08-VP-0191-B-FL. [DOI] [PubMed] [Google Scholar]
- Borzacchiello G, Russo V, Gentile F, roperto F, Venuti A, Nitsch L, Campo MS, Roperto S. Bovine papillomavirus E5 oncoprotein binds to the activated form of the platelet-derived growth factor beta receptor in naturally occurring bovine urinary blader tumours. Oncogene. 2006;25:1251–1260. doi: 10.1038/sj.onc.1209152. [DOI] [PubMed] [Google Scholar]
- Bouvard V, Matlashewski G, Gu Z-M, Storey A, Banks L. The human papillomavirus type 16 E5 gene cooperates with the E7 gene to stimulate proliferation of primary cells and increases viral gene expression. Virology. 1994;203:73–80. doi: 10.1006/viro.1994.1456. [DOI] [PubMed] [Google Scholar]
- Brandt S, Haralambus R, Schoster A, Kirnbauer R, Stanek C. Peripheral blood mononuclear cells represent a reservoir of bovine papillomavirus DNA in sarcoid-affected equines. J Gen Virol. 2008;89(Pt 6):1390–5. doi: 10.1099/vir.0.83568-0. [DOI] [PubMed] [Google Scholar]
- Bravo IG, Alonso A. Mucosal human papillomaviruses encode four different E5 proteins whose chemistry and phylogeny correlate with malignant or benign growth. J. Virol. 2004;78:13613–13626. doi: 10.1128/JVI.78.24.13613-13626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo IG, Crusius K, Alonso A. The E5 protein of the human papillomavirus type 16 modulates composition and dynamics of membrane lipids in keratinocytes. Arch. Virol. 2005;150:231–246. doi: 10.1007/s00705-004-0420-x. [DOI] [PubMed] [Google Scholar]
- Briggs MW, Adam JL, McCance DJ. The human papillomavirus type 16 E5 protein alters vacuolar H+-ATPase function and stability in Saccharomyces cerevisiae. Virology. 2001;280:169–175. doi: 10.1006/viro.2000.0783. [DOI] [PubMed] [Google Scholar]
- Bubb V, McCance DJ, Schlegel R. DNA sequence of the HPV-16 E5 ORF and the structural conservation of its encoded protein. Virology. 1988;163(1):243–6. doi: 10.1016/0042-6822(88)90259-0. [DOI] [PubMed] [Google Scholar]
- Burkhardt A, DiMaio D, Schlegel R. Genetic and biochemical definition of the bovine papillomavirus E5 transforming protein. Embo J. 1987;6(8):2381–5. doi: 10.1002/j.1460-2075.1987.tb02515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt A, Willingham M, Gay C, Jeang K-T, Schlegel R. The E5 oncoprotein of bovine papillomavirus is oriented asymmetrically in Golgi and plasma membranes. Virology. 1989;170:334–339. doi: 10.1016/0042-6822(89)90391-7. [DOI] [PubMed] [Google Scholar]
- Burnett S, Jareborg N, DiMaio D. Localization of bovine papillomavirus type 1 E5 protein to transformed basal keratinocytes and permissive differentiated cells in fibropapilloma tissue. Proc Natl Acad Sci U S A. 1992;89(12):5665–9. doi: 10.1073/pnas.89.12.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammett TJ, Jun SJ, Cohen EB, Barrera FN, Engelman DM, DiMaio D. Construction and genetic selection of small transmembrane proteins that activate the human erythropoietin receptor. Proc. Natl. Acad. Sci USA. 2010;107:3447–3452. doi: 10.1073/pnas.0915057107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo MS, Graham SV, Cortese MS, Ashrafi GH, Araibi EH, Dornan ES, Miners K, Nunes C, Man S. HPV-16 E5 down-regulates expression of surface HLA class I and reduces recognition by CD8 T cells. Virology. 2010;407(1):137–42. doi: 10.1016/j.virol.2010.07.044. [DOI] [PubMed] [Google Scholar]
- Carr EA, Theon AP, Madewell BR, Hitchcock ME, Schlegel R, Schiller JT. Expression of a transforming gene (E5) of bovine papillomavirus in sarcoids obtained from horses. Am J Vet Res. 2001;62(8):1212–7. doi: 10.2460/ajvr.2001.62.1212. [DOI] [PubMed] [Google Scholar]
- Chang JL, Tsao YP, Liu DW, Huang SJ, Lee WH, Chen SL. The expression of HPV-16 E5 protein in squamous neoplastic changes in the uterine cervix. J Biomed Sci. 2001;8(2):206–13. doi: 10.1007/BF02256414. [DOI] [PubMed] [Google Scholar]
- Chen S-L, Tsai T-C, Han C-P, Tsao Y-P. Mutational analysis of human papillomavirus type 11 E5a oncoprotein. J. Virol. 1996a;70:3502–3508. doi: 10.1128/jvi.70.6.3502-3508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SL, Huang CH, Tsai TC, Lu KY, Tsao YP. The regulation mechanism of c-jun and junB by human papillomavirus type 16 E5 oncoprotein. Arch. Virol. 1996b;141:791–800. doi: 10.1007/BF01718155. [DOI] [PubMed] [Google Scholar]
- Chen SL, Lin ST, Tsai TC, Hsiao WC, Tsao YP. ErbB4 (JM-b/CYT-1)-induced expression and phosphorylation of c-Jun is abrogated by human papillomavirus type 16 E5 protein. Oncogene. 2007;26(1):42–53. doi: 10.1038/sj.onc.1209768. [DOI] [PubMed] [Google Scholar]
- Chen SL, Lin YK, Li LY, Tsao YP, Lo HY, Wang WB, Tsai TC. E5 proteins of human papillomavirus types 11 and 16 transactivate the c-fos promoter through the NF1 binding element. J Virol. 1996c;70(12):8558–63. doi: 10.1128/jvi.70.12.8558-8563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SL, Mounts P. Transforming activity of E5a protein of human papillomavirus type 6 in NIH 3T3 and C127 cells. J. Virol. 1990;64:3226–3233. doi: 10.1128/jvi.64.7.3226-3233.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo KB, Pan CC, Liu MS, Ng HT, Chen CP, Lee YN, Chao CF, Meng CL, Yeh MY, Han SH. Presence of episomal and integrated human papillomavirus DNA sequences in cervical carcinoma. J Med Virol. 1987;21(2):101–7. doi: 10.1002/jmv.1890210202. [DOI] [PubMed] [Google Scholar]
- Cohen BD, Goldstein DJ, Rutledge L, Vass WC, Lowy DR, Schlegel R, Schiller JT. Transformation-specific interaction of the bovine papillomavirus E5 oncoprotein with the platelet-derived growth factor receptor transmembrane domain and the epidermal growth factor receptor cytoplasmic domain. J. Virol. 1993;67:5303–5311. doi: 10.1128/jvi.67.9.5303-5311.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad M, Bubb VJ, Schlegel R. The human papillomavirus type 6 and 16 E5 proteins are membrane-associated proteins which associate with the 16-kilodalton pore-forming protein. J. Virol. 1993;67:6170–6178. doi: 10.1128/jvi.67.10.6170-6178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad M, Goldstein D, Andresson T, Schlegel R. The E5 protein of HPV-6, but not HPV-16, associates efficiently with cellular growth factor receptors. Virology. 1994;200:796–800. doi: 10.1006/viro.1994.1244. [DOI] [PubMed] [Google Scholar]
- Corteggio A, Altamura G, Roperto F, Borzacchiello G. Bovine papillomavirus E5 and E7 oncoproteins in naturally occurring tumors: are two better than one? Infect Agent Cancer. 2013;8(1):1. doi: 10.1186/1750-9378-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corteggio A, Di Geronimo O, Roperto S, Roperto F, Borzacchiello G. Activated platelet-derived growth factor beta receptor and Ras-mitogen-activated protein kinase pathway in natural bovine urinary bladder carcinomas. Vet J. 2011;191(3):393–5. doi: 10.1016/j.tvjl.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Corteggio A, Urraro C, Roperto S, Roperto F, Borzacchiello G. Phosphatidylinositol-3-kinase-AKT pathway, phospho-JUN and phospho-JNK expression in spontaneously arising bovine urinary bladder tumours. J Comp Pathol. 2010;143(2-3):173–8. doi: 10.1016/j.jcpa.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Cortese MS, Ashrafi GH, Campo MS. All 4 di-leucine motifs in the first hydrophobic domain of the E5 oncoprotein of human papillomavirus type 16 are essential for surface MHC class I downregulation activity and E5 endomembrane localization. Intl. J. Cancer. 2010;126:1675–1682. doi: 10.1002/ijc.25004. [DOI] [PubMed] [Google Scholar]
- Cromme FV, Meijer CJ, Snijders PJ, Uyterlinde A, Kenemans P, Helmerhorst T, Stern PL, van den Brule AJ, Walboomers JM. Analysis of MHC class I and II expression in relation to presence of HPV genotypes in premalignant and malignant cervical lesions. Br J Cancer. 1993;67(6):1372–80. doi: 10.1038/bjc.1993.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crusius K, Auvinen E, Alonso A. Enhancement of EGF- and PMA-mediated MAP kinase activation in cells expressing the human papillomavirus type 16 E5 protein. Oncogene. 1997;15:1437–1444. doi: 10.1038/sj.onc.1201312. [DOI] [PubMed] [Google Scholar]
- Crusius K, Auvinen E, Steuer B, Gaissert H, Alonso A. The human papillomavirus type 16 E5-protein modulates ligand-dependent activation of the EGF receptor family in the human epithelial cell line HaCaT. Exp. Cell Res. 1998;241:76–83. doi: 10.1006/excr.1998.4024. [DOI] [PubMed] [Google Scholar]
- Crusius K, Kaszkin M, Kinzel V, Alonso A. The human papillomavirus type 16 E5 protein modulates phospholipase C-g-1 activity and phosphatidyl inositol turnover in mouse fibroblasts. Oncogene. 1999;18:6714–6718. doi: 10.1038/sj.onc.1203075. [DOI] [PubMed] [Google Scholar]
- Crusius K, Rodriguez I, Alonso A. The human papillomavirus type 16 E5 protein modulates ERK1/2 and p38 MAP kinase activation by an EGFR-independent process in stressed human keratinocytes. Virus Genes. 2000;20:65–69. doi: 10.1023/a:1008112207824. [DOI] [PubMed] [Google Scholar]
- Cullen AP, Reid R, Campion M, Lorincz AT. Analysis of the physical state of different human papillomavirus DNAs in intraepithelial and invasive cervical neoplasm. J Virol. 1991;65(2):606–12. doi: 10.1128/jvi.65.2.606-612.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Domenico F, Foppoli C, Blarzino C, Perluigi M, Paolini F, Morici S, Coccia R, Cini C, De Marco F. Expression of human papilloma virus type 16 E5 protein in amelanotic melanoma cells regulates endo-cellular pH and restores tyrosinase activity. J Exp Clin Cancer Res. 2009;28:4. doi: 10.1186/1756-9966-28-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaio D, Guralski D, Schiller JT. Translation of open reading frame E5 of bovine papillomavirus is required for its transforming activity. Proc Natl Acad Sci U S A. 1986;83(6):1797–801. doi: 10.1073/pnas.83.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disbrow GL, Hanover JA, Schlegel R. Endoplasmic reticulum-localized human papillomavirus type 16 E5 protein alters endosomal pH but not trans-Golgi pH. J. Virol. 2005;79:5839–5846. doi: 10.1128/JVI.79.9.5839-5846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disbrow GL, Sunitha I, Baker CC, Hanover J, Schlegel R. Codon optimization of the HPV-16 E5 gene enhances protein expression. Virology. 2003;311:105–114. doi: 10.1016/s0042-6822(03)00129-6. [DOI] [PubMed] [Google Scholar]
- Drummond-Barbosa DA, Vaillancourt RR, Kazlauskas A, DiMaio D. Ligand-independent activation of the platelet-derived growth factor beta receptor: requirements for bovine papillomavirus E5-induced mitogenic signaling. Mol. Cell. Biol. 1995;15(5):2570–2581. doi: 10.1128/mcb.15.5.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccini AM, Cairney M, Ashrafi GH, Finbow ME, Campo MS, Pitts JD. The bovine papillomavirus type 4 E8 protein binds to ductin and causes loss of gap junctional intercellular communication in primary fibroblasts. J. Virol. 1996;70:9041–9045. doi: 10.1128/jvi.70.12.9041-9045.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrmann F, Klumpp DJ, Laimins LA. Human papillomavirus type 31 E5 protein supports cell cycle progression and activates late viral functions upon epithelial differentiation. J. Virol. 2003;77:2819–2831. doi: 10.1128/JVI.77.5.2819-2831.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman-Cook L, Dixon AM, Frank JB, Xia Y, Ely L, Gerstein M, Engelman DM, DiMaio D. Selection and characterization of small random transmembrane proteins that bind and activate the platelet-derived growth factor b receptor. J. Mol. Biol. 2004;338:907–920. doi: 10.1016/j.jmb.2004.03.044. [DOI] [PubMed] [Google Scholar]
- Freeman-Cook LL, Edwards APB, Dixon AM, Yates KE, Ely L, Engelman DM, DiMaio D. Specific locations of hydrophilic amino acids in constructed transmembrane ligands of the platelet-derived growth factor b receptor. J. Mol. Biol. 2005;345:907–921. doi: 10.1016/j.jmb.2004.10.072. [DOI] [PubMed] [Google Scholar]
- Gao P, Zheng J. High-risk HPV E5-induced cell fusion: a critical initiating event in the early stage of HPV-associated cervical cancer. Virol J. 2010;7:238. doi: 10.1186/1743-422X-7-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genther-Williams SM, Disbrow GL, Schlegel R, Lee D, Threadgill DW, Lambert PF. Requirement of epidermal growth factor receptor for hyperplasia induced by E5, a high-risk human papillomavirus oncogene. Cancer Res. 2005;65:6534–6542. doi: 10.1158/0008-5472.CAN-05-0083. [DOI] [PubMed] [Google Scholar]
- Genther SM, Sterling S, Duensing S, Munger K, Sattler C, Lambert PF. Quantitative role of the human papillomavirus type 16 E5 gene during the productive stage of the viral life cycle. J. Virol. 2003;77:2832–2842. doi: 10.1128/JVI.77.5.2832-2842.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieswein CE, Sharom FJ, Wildeman AG. Oligomerization of the E5 protein of human papillomavirus type 16 occurs through multiple hydrophobic regions. Virology. 2003;313:415–426. doi: 10.1016/s0042-6822(03)00296-4. [DOI] [PubMed] [Google Scholar]
- Goldstein DJ, Andresson T, Sparkowski JJ, Schlegel R. The BPV-1 E5 protein, the 16 kDa membrane pore-forming protein and the PDGF receptor exist in a complex that is dependent on hydrophobic transmembrane interactions. EMBO J. 1992;11:4851–4859. doi: 10.1002/j.1460-2075.1992.tb05591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DJ, Finbow ME, Andresson T, McLean P, Smith K, Bubb V, Schlegel R. Bovine papillomavirus E5 oncoprotein binds to the 16K component of vacuolar H(+)-ATPases. Nature. 1991;352:347–349. doi: 10.1038/352347a0. [DOI] [PubMed] [Google Scholar]
- Goldstein DJ, Li W, Wang L-M, Heidaran MA, Aaronson SA, Shinn R, Schlegel R, Pierce JH. The bovine papillomavirus type 1 E5 transforming protein specifically binds and activates the beta-type receptor for platelet-derived growth factor but not other tyrosine kinase-containing receptors to induce cellular transformation. J. Virol. 1994;68:4432–4441. doi: 10.1128/jvi.68.7.4432-4441.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindlay GJ, Campo MS, O'Brien V. Transactivation of the cyclin A promoter by bovine papillomavirus type 4 E5 protein. Virus Res. 2005;108:29–38. doi: 10.1016/j.virusres.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Gruener M, Bravo IG, Momburg F, Alonso A, Tomakidi P. The E5 protein of the human papillomavirus type 16 down-regulates HLA-I surface expression in calnexin-expressing but not in calnexin-deficient cells. Virol J. 2007;4:116. doi: 10.1186/1743-422X-4-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z-M, Matlashewski G. Effect of human papillomavirus type 16 oncogenes on MAP kinase activity. J. Virol. 1995;69:8051–8056. doi: 10.1128/jvi.69.12.8051-8056.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert CL, Galloway DA. Identification of the E5 open reading frame of human papillomavirus type 16. J Virol. 1988;62(3):1071–5. doi: 10.1128/jvi.62.3.1071-1075.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz BH, Burkhardt AL, Schlegel R, DiMaio D. 44-amino-acid E5 transforming protein of bovine papillomavirus requires a hydrophobic core and specific carboxyl-terminal amino acids. Mol. Cell. Biol. 1988;8(10):4071–8. doi: 10.1128/mcb.8.10.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz BH, Weinstat DL, DiMaio D. Transforming activity of a 16-amino-acid segment of the bovine papillomavirus E5 protein linked to random sequences of hydrophobic amino acids. J. Virol. 1989;63(11):4515–9. doi: 10.1128/jvi.63.11.4515-4519.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Ceresa BP. Characterization of the plasma membrane localization and orientation of HPV16 E5 for cell-cell fusion. Virology. 2009;393(1):135–43. doi: 10.1016/j.virol.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Plafker K, Vorozhko V, Zuna RE, Hanigan MH, Gorbsky GJ, Plafker SM, Angeletti PC, Ceresa BP. Human papillomavirus 16 E5 induces bi-nucleated cell formation by cell-cell fusion. Virology. 2009;384(1):125–34. doi: 10.1016/j.virol.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Potapova TA, Li S, Rankin S, Gorbsky GJ, Angeletti PC, Ceresa BP. Expression of HPV16 E5 produces enlarged nuclei and polyploidy through endoreplication. Virology. 2010;405(2):342–51. doi: 10.1016/j.virol.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang ES, Nottoli T, DiMaio D. The HPV16 E5 protein: expression, detection, and stable complex formation with transmembrane proteins in COS cells. Virology. 1995;211(1):227–33. doi: 10.1006/viro.1995.1395. [DOI] [PubMed] [Google Scholar]
- Kabsch K, Alonso A. The human papillomavirus type 16 E5 protein impairs TRAIL- and FasL-mediated apoptosis in HaCaT cells by different mechanisms. J. Virol. 2002;76:12162–12172. doi: 10.1128/JVI.76.23.12162-12172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch K, Mossadegh N, Kohl A, Komposch G, Schenkel J, Alonso A, Tomakidi P. The HPV-16 E5 protein inhibits TRAIL- and FasL-mediated apoptosis in human keratinocyte raft cultures. Intervirology. 2004;47:48–56. doi: 10.1159/000076642. [DOI] [PubMed] [Google Scholar]
- Kell B, Jewers RJ, Cason J, Pakarian F, Kaye JN, Best JM. Detection of E5 oncoprotein in human papillomavirus type 16-positive cervical scrapes using antibodies raised to synthetic peptides. J Gen Virol. 1994;75(Pt 9):2451–6. doi: 10.1099/0022-1317-75-9-2451. [DOI] [PubMed] [Google Scholar]
- Kilk A, Talpsepp T, Vali U, Ustav M. Bovine papillomavirus oncoprotein E5 induces the NF kappa B activation through superoxide radicals. Biochem Mol Biol Int. 1996;40(4):689–97. [PubMed] [Google Scholar]
- Kim SH, Juhnn YS, Kang S, Park SW, Sung MW, Bang YJ, Song YS. Human papillomavirus 16 E5 up-regulates the expression of vascular endothelial growth factor through the activation of epidermal growth factor receptor, MEK/ ERK1,2 and PI3K/Akt. Cell Mol Life Sci. 2006;63(7-8):930–8. doi: 10.1007/s00018-005-5561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Oh JM, No JH, Bang YJ, Juhnn YS, Song YS. Involvement of NF-kappaB and AP-1 in COX-2 upregulation by human papillomavirus 16 E5 oncoprotein. Carcinogenesis. 2009;30(5):753–7. doi: 10.1093/carcin/bgp066. [DOI] [PubMed] [Google Scholar]
- King G, Oates J, Patel D, van den Berg HA, Dixon AM. Towards a structural understanding of the smallest known oncoprotein: investigation of the bovine papillomavirus E5 protein using solution-state NMR. Biochim Biophys Acta. 2011;1808(6):1493–501. doi: 10.1016/j.bbamem.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Kivi N, Greco D, Auvinen P, Auvinen E. Genes involved in cell adhesion, cell motility and mitogenic signaling are altered due to HPV 16 E5 protein expression. Oncogene. 2008;27(18):2532–41. doi: 10.1038/sj.onc.1210916. [DOI] [PubMed] [Google Scholar]
- Klein O, Kegler-Ebo D, Su J, Smith S, DiMaio D. The bovine papillomavirus E5 protein requires a juxtamembrane negative charge for activation of the platelet-derived growth factor beta receptor and transformation of C127 cells. J Virol. 1999;73(4):3264–72. doi: 10.1128/jvi.73.4.3264-3272.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein O, Polack GW, Surti T, Kegler-Ebo D, Smith SO, DiMaio D. Role of glutamine 17 of the bovine papillomavirus E5 protein in platelet-derived growth factor beta receptor activation and cell transformation. J. Virol. 1998;72(11):8921–8932. doi: 10.1128/jvi.72.11.8921-8932.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk E, Hanover JA, Schlegel R, Suprynowicz FA. Karyopherin beta3: a new cellular target for the HPV-16 E5 oncoprotein. Biochem Biophys Res Commun. 2008a;371(4):684–688. doi: 10.1016/j.bbrc.2008.04.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk E, Suprynowicz FA, Hebert JD, Kamonjoh CM, Schlegel R. The human papillomavirus type 16 E5 oncoprotein translocates calpactin I to the perinuclear region. J Virol. 2011;85(21):10968–75. doi: 10.1128/JVI.00706-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk E, Suprynowicz FA, Liu X, Dai Y, Hartmann DP, Hanover J, Schlegel R. Koilocytosis: a cooperative interaction between the human papillomavirus E5 and E6 oncoproteins. Am J Pathol. 2008b;173(3):682–8. doi: 10.2353/ajpath.2008.080280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk E, Suprynowicz FA, Sudarshan SR, Schlegel R. Membrane orientation of the human papillomavirus type 16 E5 oncoprotein. J Virol. 2010;84(4):1696–703. doi: 10.1128/JVI.01968-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulke R, Horwitz BH, Zibello T, DiMaio D. The central hydrophobic domain of the bovine papillomavirus E5 transforming protein can be functionally replaced by many hydrophobic amino acid sequences containing a glutamine. J Virol. 1992;66(1):505–11. doi: 10.1128/jvi.66.1.505-511.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C-C, Edwards APB, DiMaio D. Productive interaction between transmembrane mutants of the bovine papillomavirus E5 protein and the platelet-derived growth factor b receptor. J. Virol. 2005;79:1924–1929. doi: 10.1128/JVI.79.3.1924-1929.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CC, Henningson C, DiMaio D. Bovine papillomavirus E5 protein induces oligomerization and trans- phosphorylation of the platelet-derived growth factor b receptor. Proc. Natl. Acad. Sci. U S A. 1998;95:15241–15246. doi: 10.1073/pnas.95.26.15241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CC, Henningson C, DiMaio D. Bovine papillomavirus E5 protein induces the formation of signal transduction complexes containing dimeric activated platelet-derived growth factor b receptor and associated signaling proteins. J. Biol. Chem. 2000;275:9832–9840. doi: 10.1074/jbc.275.13.9832. [DOI] [PubMed] [Google Scholar]
- Lazarczyk M, Pons C, Mendoza JA, Cassonnet P, Jacob Y, Favre M. Regulation of cellular zinc balance as a potential mechanism of EVER-mediated protection against pathogenesis by cutaneous oncogenic human papillomaviruses. J Exp Med. 2008;205(1):35–42. doi: 10.1084/jem.20071311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leechanachai P, Banks L, Moreau F, Matlashewski G. The E5 gene from human papillomavirus type 16 is an oncogene which enhances growth factor-mediated signal transduction to the nucleus. Oncogene. 1992;7:19–25. [PubMed] [Google Scholar]
- Leptak C, Ramon y Cajal S, Kulke R, Horwitz BH, Riese DJ, 2nd, Dotto GP, DiMaio D. Tumorigenic transformation of murine keratinocytes by the E5 genes of bovine papillomavirus type 1 and human papillomavirus type 16. J Virol. 1991;65(12):7078–83. doi: 10.1128/jvi.65.12.7078-7083.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C, Baro MF, Marques M, Gruner M, Alonso A, Bravo IG. The first hydrophobic region of the HPV16 E5 protein determines protein cellular location and facilitates anchorage-independent growth. Virol J. 2008;5:30. doi: 10.1186/1743-422X-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longworth MS, Laimins LA. Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol Mol Biol Rev. 2004;68(2):362–72. doi: 10.1128/MMBR.68.2.362-372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti B, Ashrafi GH, Tsirimonaki E, O'Brien PM, Campo MS. The bovine papillomavirus oncoprotein E5 retains MHC class I molecules in the Golgi apparatus and prevents their transport to the cell surface. Oncogene. 2002;21:7808–7816. doi: 10.1038/sj.onc.1205885. [DOI] [PubMed] [Google Scholar]
- Matsukura T, Koi S, Sugase M. Both episomal and integrated forms of human papillomavirus type 16 are involved in invasive cervical cancers. Virology. 1989;172(1):63–72. doi: 10.1016/0042-6822(89)90107-4. [DOI] [PubMed] [Google Scholar]
- Mattoon D, Gupta K, Doyon J, Loll PJ, DiMaio D. Identification of the transmembrane dimer interface of the bovine papillomavirus E5 protein. Oncogene. 2001;20:3824–3834. doi: 10.1038/sj.onc.1204523. [DOI] [PubMed] [Google Scholar]
- Maufort JP, Shai A, Pitot HC, Lambert PF. A role for HPV16 E5 in cervical carcinogenesis. Cancer Res. 2010;70(7):2924–31. doi: 10.1158/0008-5472.CAN-09-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maufort JP, Williams SM, Pitot HC, Lambert PF. Human papillomavirus 16 E5 oncogene contributes to two stages of skin carcinogenesis. Cancer Res. 2007;67(13):6106–12. doi: 10.1158/0008-5472.CAN-07-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride AA, Dlugosz A, Baker CC. Production of infectious bovine papillomavirus from cloned viral DNA by using an organotypic raft/xenograft technique. Proc Natl Acad Sci U S A. 2000;97(10):5534–9. doi: 10.1073/pnas.97.10.5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AN, Xu Y-F, Webster MK, Smith AS, Donoghue DJ. Cellular transformation by a transmembrane peptide: structural requirements for the bovine papillomavirus E5 oncoprotein. Proc. Natl. Acad. Sci. USA. 1994;91:4634–4638. doi: 10.1073/pnas.91.11.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S, Kawana K, Schust DJ, Fujii T, Yokoyama T, Iwasawa Y, Nagamatsu T, Adachi K, Tomio A, Tomio K, Kojima S, Yasugi T, Kozuma S, Taketani Y. CD1d, a sentinel molecule bridging innate and adaptive immunity, is downregulated by the human papillomavirus (HPV) E5 protein: a possible mechanism for immune evasion by HPV. J Virol. 2010;84(22):11614–23. doi: 10.1128/JVI.01053-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nappi VM, Petti LM. Multiple transmembrane amino acid requirements suggest a highly specific interaction between the bovine papillomavirus E5 oncoprotein and the platelet-derived growth factor beta receptor. J. Virol. 2002;76:7976–7986. doi: 10.1128/JVI.76.16.7976-7986.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nappi VM, Schaefer JA, Petti LM. Molecular examination of the transmembrane requirements of the platelet-derived growth factor beta receptor for a productive interaction with the bovine papillomavirus E5 oncoprotein. J. Biol. Chem. 2002;277:47149–47159. doi: 10.1074/jbc.M209582200. [DOI] [PubMed] [Google Scholar]
- Nath R, Mant CA, Kell B, Cason J, Bible JM. Analyses of variant human papillomavirus type-16 E5 proteins for their ability to induce mitogenesis of murine fibroblasts. Cancer Cell Int. 2006;6:19. doi: 10.1186/1475-2867-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary K, DiMaio D. Open reading frames E6 and E7 of bovine papillomavirus type 1 are both required for full transformation of mouse C127 cells. J Virol. 1989;63(1):259–66. doi: 10.1128/jvi.63.1.259-266.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilson LA, DiMaio D. Platelet-derived growth factor receptor can mediate tumorigenic transformation by the bovine papillomavirus E5 protein. Mol Cell Biol. 1993;13(7):4137–45. doi: 10.1128/mcb.13.7.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilson LA, Gottlieb RL, Polack GW, DiMaio D. Mutational analysis of the interaction between the bovine papillomavirus E5 transforming protein and the endogenous beta receptor for platelet-derived growth factor in mouse C127 cells. J Virol. 1995;69(9):5869–74. doi: 10.1128/jvi.69.9.5869-5874.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio M, Tajima O, Furukawa K, Urano T. Over-expression of GM1 enhances cell proliferation with epidermal growth factor without affecting the receptor localization in the microdomain in PC12 cells. Int J Oncol. 2005;26(1):191–9. [PubMed] [Google Scholar]
- O'Brien PM, Ashrafi GH, Grindlay GJ, Anderson R, Campo MS. A mutational analysis of the transforming functions of the E8 protein of bovine papillomavirus type 4. Virology. 1999;255:385–394. doi: 10.1006/viro.1998.9563. [DOI] [PubMed] [Google Scholar]
- O'Brien PM, Grindlay GJ, Campo MS. Cell transformation by the E5/E8 protein of bovine papillomavirus type 4. J. Biol. Chem. 2001;276:33861–33868. doi: 10.1074/jbc.M100958200. [DOI] [PubMed] [Google Scholar]
- O'Brien V, Campo MS. BPV-4 E8 transforms NIH 3T3 cells, up-regulates cyclin A and cyclin A-associated kinase activity and de-regulates expression of the cdk inhibitor p27KIP1. Oncogene. 1998;17:293–301. doi: 10.1038/sj.onc.1201937. [DOI] [PubMed] [Google Scholar]
- Oates J, Hicks M, Dafforn TR, DiMaio D, Dixon AM. In vitro dimerization of the bovine papillomavirus E5 protein transmembrane domain. Biochemistry. 2008;47:8985–8992. doi: 10.1021/bi8006252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelze I, Kartenbeck J, Crusius K, Alonso A. Human papillomavirus type 16 E5 protein affects cell-cell communication in an epithelial cell line. J. Virol. 1995;69:4489–4494. doi: 10.1128/jvi.69.7.4489-4494.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oetke C, Auvinen E, Pawlita M, Alonso A. Human papillomavirus type 16 E5 protein localizes to the Golgi apparatus but does not grossly affect cellular glycosylation. Arch Virol. 2000;145(10):2183–91. doi: 10.1007/s007050070048. [DOI] [PubMed] [Google Scholar]
- Oh JM, Kim SH, Cho EA, Song YS, Kim WH, Juhnn YS. Human papillomavirus type 16 E5 protein inhibits hydrogen-peroxide-induced apoptosis by stimulating ubiquitin-proteasome-mediated degradation of Bax in human cervical cancer cells. Carcinogenesis. 2010;31(3):402–10. doi: 10.1093/carcin/bgp318. [DOI] [PubMed] [Google Scholar]
- Oh JM, Kim SH, Lee YI, Seo M, Kim SY, Song YS, Kim WH, Juhnn YS. Human papillomavirus E5 protein induces expression of the EP4 subtype of prostaglandin E2 receptor in cyclic AMP response element-dependent pathways in cervical cancer cells. Carcinogenesis. 2009;30(1):141–9. doi: 10.1093/carcin/bgn236. [DOI] [PubMed] [Google Scholar]
- Pedroza-Saavedra A, Lam EW, Esquivel-Guadarrama F, Gutierrez-Xicotencatl L. The human papillomavirus type 16 E5 oncoprotein synergizes with EGF-receptor signaling to enhance cell cycle progression and the down-regulation of p27(Kip1). Virology. 2010;400(1):44–52. doi: 10.1016/j.virol.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Petti L, DiMaio D. Stable association between the bovine papillomavirus E5 transforming protein and activated platelet-derived growth factor receptor in transformed mouse cells. Proc. Natl. Acad. Sci USA. 1992;89(15):6736–6740. doi: 10.1073/pnas.89.15.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petti L, DiMaio D. Specific interaction between the bovine papillomavirus E5 transforming protein and the beta receptor for platelet-derived growth factor in stably transformed and acutely transfected cells. J. Virol. 1994;68(6):3582–3592. doi: 10.1128/jvi.68.6.3582-3592.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petti L, Nilson LA, DiMaio D. Activation of the platelet-derived growth factor receptor by the bovine papillomavirus E5 transforming protein. EMBO J. 1991;10(4):845–855. doi: 10.1002/j.1460-2075.1991.tb08017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petti LM, Ray FA. Transformation of mortal human fibroblasts and activation of a growth inhibitory pathway by the bovine papillomavirus E5 oncoprotein. Cell Growth Differ. 2000;11:395–408. [PubMed] [Google Scholar]
- Petti LM, Reddy V, Smith SO, DiMaio D. Identification of amino acids in the transmembrane and juxtamembrane domains of the platelet-derived growth factor receptor required for productive interaction with the bovine papillomavirus E5 protein. J. Virol. 1997;71(10):7318–7327. doi: 10.1128/jvi.71.10.7318-7327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petti LM, Ricciardi EC, Page HJ, Porter KA. Transforming signals resulting from sustained activation of the PDGF b receptor in mortal human fibroblasts. J. Cell Sci. 2008;121:1172–1182. doi: 10.1242/jcs.018713. [DOI] [PubMed] [Google Scholar]
- Pim D, Collins M, Banks L. Human papillomavirus type 16 E5 gene stimulates the transforming activity of the epidermal growth factor receptor. Oncogene. 1992;7:27–32. [PubMed] [Google Scholar]
- Ptacek JB, Edwards APB, Freeman-Cook LL, DiMaio D. Packing contacts can mediate highly specific interactions between artificial transmembrane proteins and the PDGF b receptor. Proc. Natl. Acad. Sci USA. 2007;104:11945–11950. doi: 10.1073/pnas.0704348104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan JA, Laimins LA. Bap31 is a novel target of the human papillomavirus E5 protein. J Virol. 2008;82(20):10042–51. doi: 10.1128/JVI.01240-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riese DJ, II, DiMaio D. An intact PDGF signaling pathway is required for efficient growth transformation of mouse C127 cells by the bovine papillomavirus E5 protein. Oncogene. 1995;10(7):1431–1439. [PubMed] [Google Scholar]
- Ritz U, Momburg F, Pilch H, Huber C, Maeurer MJ, Seliger B. Deficient expression of components of the MHC class I antigen processing machinery in human cervical carcinoma. Int J Oncol. 2001;19(6):1211–20. doi: 10.3892/ijo.19.6.1211. [DOI] [PubMed] [Google Scholar]
- Rodriguez MI, Finbow ME, Alonso A. Binding of human papillomavirus 16 E5 to the 16 kDa subunit c (proteolipid) of the vacuolar H+-ATPase can be dissociated from the E5-mediated epidermal growth fator receptor overactivation. Oncogene. 2000;19:3727–3732. doi: 10.1038/sj.onc.1203718. [DOI] [PubMed] [Google Scholar]
- Roperto S, Borzacchiello G, Esposito I, Riccardi M, Urraro C, Luca R, Corteggio A, Tate R, Cermola M, Paciello O, Roperto F. Productive infection of bovine papillomavirus type 2 in the placenta of pregnant cows affected with urinary bladder tumors. PLoS One. 2012;7(3):e33569. doi: 10.1371/journal.pone.0033569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roperto S, Comazzi S, Ciusani E, Paolini F, Borzacchiello G, Esposito I, Luca R, Russo V, Urraro C, Venuti A, Roperto F. PBMCs are additional sites of productive infection of bovine papillomavirus type 2. J Gen Virol. 2011;92(Pt 8):1787–94. doi: 10.1099/vir.0.031740-0. [DOI] [PubMed] [Google Scholar]
- Roperto S, De Tullio R, Raso C, Stifanese R, Russo V, Gaspari M, Borzacchiello G, Averna M, Paciello O, Cuda G, Roperto F. Calpain3 is expressed in a proteolitically active form in papillomavirus-associated urothelial tumors of the urinary bladder in cattle. PLoS One. 2010;5(4):e10299. doi: 10.1371/journal.pone.0010299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roperto S, Russo V, Ozkul A, Sepici-Dincel A, Maiolino P, Borzacchiello G, Marcus I, Esposito I, Riccardi MG, Roperto F. Bovine papillomavirus type 2 infects the urinary bladder of water buffalo (Bubalus bubalis) and plays a crucial role in bubaline urothelial carcinogenesis. J Gen Virol. 2013;94(Pt 2):403–8. doi: 10.1099/vir.0.047662-0. [DOI] [PubMed] [Google Scholar]
- Sahab Z, Sudarshan SR, Liu X, Zhang Y, Kirilyuk A, Kamonjoh CM, Simic V, Dai Y, Byers SW, Doorbar J, Suprynowicz FA, Schlegel R. Quantitative measurement of human papillomavirus type 16 E5 oncoprotein levels in epithelial cell lines by mass spectrometry. J Virol. 2012;86(17):9465–73. doi: 10.1128/JVI.01032-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro F, Sparkowski J, Adduci A, Suprynowicz FA, Schlegel R, Grinstein S. Golgi alkalinization by the papillomavirus E5 oncoprotein. J. Cell. Biol. 2000;148:305–315. doi: 10.1083/jcb.148.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheideman EH, Marlatt SA, Xie Y, Hu Y, Sutton RE, DiMaio D. Transmembrane protein aptamers that inhibit CCR5 expression and HIV coreceptor function. J Virol. 2012;86(19):10281–92. doi: 10.1128/JVI.00910-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman M, Herrero R, Desalle R, Hildesheim A, Wacholder S, Rodriguez AC, Bratti MC, Sherman ME, Morales J, Guillen D, Alfaro M, Hutchinson M, Wright TC, Solomon D, Chen Z, Schussler J, Castle PE, Burk RD. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology. 2005;337:76–84. doi: 10.1016/j.virol.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Schiller JT, Vass WC, Vousden KH, Lowy DR. E5 open reading frame of bovine papillomavirus type 1 encodes a transforming gene. J. Virol. 1986;57:1–6. doi: 10.1128/jvi.57.1.1-6.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R, Wade-Glass M, Rabson MS, Yang Y-C. The E5 transforming gene of bovine papillomavirus encodes a small hydrophobic protein. Science. 1986;233:464–467. doi: 10.1126/science.3014660. [DOI] [PubMed] [Google Scholar]
- Schwarz E, Freese UK, Gissmann L, Mayer W, Roggenbuck B, Stremlau A, zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985;314(6006):111–4. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- Silva MA, Silva KM, Jesus AL, Barros LO, Corteggio A, Altamura G, Borzacchiello G, Freitas AC. The Presence and Gene Expression of Bovine Papillomavirus in the Peripheral Blood and Semen of Healthy Horses. Transbound Emerg Dis. 2012 doi: 10.1111/tbed.12036. [DOI] [PubMed] [Google Scholar]
- Silvestre O, Borzacchiello G, Nava D, Iovane G, Russo V, Vecchio D, D'Ausilio F, Gault EA, Campo MS, Paciello O. Bovine papillomavirus type 1 DNA and E5 oncoprotein expression in water buffalo fibropapillomas. Vet Pathol. 2009;46(4):636–41. doi: 10.1354/vp.08-VP-0222-P-FL. [DOI] [PubMed] [Google Scholar]
- Sparkowski J, Anders J, Schlegel R. Mutation of the bovine papillomavirus E5 oncoprotein at amino acid 17 generates both high- and low-transforming variants. J. Virol. 1994;68:6120–6123. doi: 10.1128/jvi.68.9.6120-6123.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkowski J, Mense M, Anders M, Schlegel R. E5 oncoprotein transmembrane mutants dissociate fibroblast transforming activity from 16-kilodalton protein binding and platelet-derived growth factor receptor binding and phosphorylation. J. Virol. 1996;70:2420–2430. doi: 10.1128/jvi.70.4.2420-2430.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staebler A, Pierce JH, Brazinski S, Heidaran MA, Li W, Schlegel R, Goldstein DJ. Mutational analysis of the beta-type platelet-derived growth factor receptor defines the site of interaction with the bovine papillomavirus type 1 E5 transforming protein. J. Virol. 1995;69:6507–6517. doi: 10.1128/jvi.69.10.6507-6517.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppler MC, Straight SW, Tsao G, Schlegel R, McCance DJ. The E5 gene of HPV-16 enhances keratinocyte immortalization by full-length DNA. Virology. 1996;223(1):251–4. doi: 10.1006/viro.1996.0475. [DOI] [PubMed] [Google Scholar]
- Straight SW, Herman B, McCance DJ. The E5 oncoprotein of human papillomavirus type 16 inhibits the acidification of endosomes in human keratinocytes. J. Virol. 1995;69:3185–3192. doi: 10.1128/jvi.69.5.3185-3192.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight SW, Hinkle PM, Jewers RJ, McCance DJ. The E5 oncoprotein of human papillomavirus type 16 transforms fibroblasts and effects the downregulation of the epidermal growth factor receptor in keratinocytes. J. Virol. 1993;67:4521–4532. doi: 10.1128/jvi.67.8.4521-4532.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suprynowicz FA, Baege A, Sunitha I, Schlegel R. c-Src activation by the E5 oncoprotein enables transformation independently of PDGF receptor activation. Oncogene. 2002;21:1695–1706. doi: 10.1038/sj.onc.1205223. [DOI] [PubMed] [Google Scholar]
- Suprynowicz FA, Disbrow GL, Krawczyk E, Simic V, Lantzky K, Schlegel R. HPV-16 E5 oncoprotein upregulates lipid raft components caveolin-1 and ganglioside GM1 at the plasma membrane of cervical cells. Oncogene. 2008;27(8):1071–8. doi: 10.1038/sj.onc.1210725. [DOI] [PubMed] [Google Scholar]
- Suprynowicz FA, Disbrow GL, Simic V, Schlegel R. Are transforming properties of the bovine papillomavirus E5 protein shared by E5 from high-risk human papillomavirus type 16? Virology. 2005;332:102–113. doi: 10.1016/j.virol.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Suprynowicz FA, Krawczyk E, Hebert JD, Sudarshan SR, Simic V, Kamonjoh CM, Schlegel R. The human papillomavirus type 16 E5 oncoprotein inhibits epidermal growth factor trafficking independently of endosome acidification. J Virol. 2010;84(20):10619–29. doi: 10.1128/JVI.00831-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suprynowicz FA, Sparkowski J, Baege A, Schlegel R. E5 oncoprotein mutants activate phosphoinositide 3′-kinase independently of platelet-derived growth factor receptor activation. J. Biol. Chem. 2000;275:5111–5119. doi: 10.1074/jbc.275.7.5111. [DOI] [PubMed] [Google Scholar]
- Surti T, Klein O, Aschheim K, DiMaio D, Smith SO. Structural models of the bovine papillomavirus E5 protein. Proteins. 1998;33(4):601–612. [PubMed] [Google Scholar]
- Talbert-Slagle K, Marlatt S, Barrera FN, Khurana E, Oates J, Gerstein M, Engelman DM, DiMaio D. Artificial transmembrane oncoproteins smaller than the bovine papillomavirus E5 protein redefine the sequence requirements for activation of the PDGF b receptor J. Virol. 2009;83:9773–9785. doi: 10.1128/JVI.00946-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen P, van Deurs B, Norrild B, Kayser L. The HPV16 E5 oncogene inhibits endocytic trafficking. Oncogene. 2000;19:6023–6032. doi: 10.1038/sj.onc.1204010. [DOI] [PubMed] [Google Scholar]
- Tomakidi P, Cheng H, Kohl A, Komposch G, Alonso A. Connexin 43 expression is downregulated in raft cultures of human keratinocytes expressing the human papillomavirus type 16 E5 protein. Cell Tissue Res. 2000a;301:323–327. doi: 10.1007/s004410000231. [DOI] [PubMed] [Google Scholar]
- Tomakidi P, Cheng H, Kohl A, Komposch G, Alonso A. Modulation of the epidermal growth factor receptor by the human papillomavirus type 16 E5 protein in raft cultures of human keratinocytes. Eur. J. Cell Biol. 2000b;79:407–412. doi: 10.1078/0171-9335-00060. [DOI] [PubMed] [Google Scholar]
- Tsao Y-P, Li L-Y, Tsai T-C, Chen S-L. Human papillomavirus type 11 and 16 E5 represses p21WafI/SdiI/CipI gene expression in fibroblasts and keratinocytes. J. Virol. 1996;70:7535–7539. doi: 10.1128/jvi.70.11.7535-7539.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsirimonaki E, Ullah R, Marchetti B, Ashrafi GH, McGarry L, Ozanne B, Campo MS. Similarities and differences between the E5 oncoproteins of bovine papillomaviruses type 1 and type 4: cytoskeleton, motility and invasiveness in E5-transformed bovine and mouse cells. Virus Res. 2006;115(2):158–68. doi: 10.1016/j.virusres.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Vali U, Kilk A, Ustav M. Bovine papillomavirus oncoprotein E5 affects the arachidonic acid metabolism in cells. Intl J Biochem Cell Biol. 2001;33:227–235. doi: 10.1016/s1357-2725(01)00015-2. [DOI] [PubMed] [Google Scholar]
- Vallee GF, Banks L. The human papillomavirus (HPV)-6 and HPV-16 proteins co-operate with HPV-16 E7 in the transformation of primary rodent cells. J. Gen. Virol. 1995;76:1239–1245. doi: 10.1099/0022-1317-76-5-1239. [DOI] [PubMed] [Google Scholar]
- van Doorslaer K. Evolution of the papillomaviridae. Virology. 2013 doi: 10.1016/j.virol.2013.05.012. (In press) [DOI] [PubMed] [Google Scholar]
- Venuti A, Paolini F, Nasir L, Corteggio A, Roperto S, Campo MS, Borzacchiello G. Papillomavirus E5: the smallest oncoprotein with many functions. Mol. Cancer. 2011;10:140. doi: 10.1186/1476-4598-10-140. doi: 10.1186/1476-4598-10-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venuti A, Salani D, Poggiali F, Manni V, Bagnato A. The E5 oncoprotein of human papillomavirus type 16 enhances endothelin-1-induced keratinocyte growth. Virology. 1998;248:1–5. doi: 10.1006/viro.1998.9227. [DOI] [PubMed] [Google Scholar]
- Wetherill LF, Holmes KK, Verow M, Muller M, Howell G, Harris M, Fishwick C, Stonehouse N, Foster R, Blair GE, Griffin S, Macdonald A. High-risk human papillomavirus E5 oncoprotein displays channel-forming activity sensitive to small-molecule inhibitors. J Virol. 2012;86(9):5341–51. doi: 10.1128/JVI.06243-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windisch D, Hoffmann S, Afonin S, Vollmer S, Benamira S, Langer B, Burck J, Muhle-Goll C, Ulrich AS. Structural role of the conserved cysteines in the dimerization of the viral transmembrane oncoprotein E5. Biophys J. 2010;99(6):1764–72. doi: 10.1016/j.bpj.2010.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DH, Wildeman AG, Sharom FJ. Overexpression, purification, and structural analysis of the hydrophobic E5 protein from human papillomavirus type 16. Protein Expr Purif. 2003;30(1):1–10. doi: 10.1016/s1046-5928(03)00049-4. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Gault EA, Campo MS, Nasir L. Different contribution of bovine papillomavirus type 1 oncoproteins to the transformation of equine fibroblasts. J Gen Virol. 2011a;92(Pt 4):773–83. doi: 10.1099/vir.0.028191-0. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Gault EA, Campo MS, Nasir L. p38 mitogen-activated protein kinase is crucial for bovine papillomavirus type-1 transformation of equine fibroblasts. J Gen Virol. 2011b;92(Pt 8):1778–86. doi: 10.1099/vir.0.031526-0. [DOI] [PubMed] [Google Scholar]
- Zhang B, Li P, Wang E, Brahmi Z, Dunn KW, Blum JS, Roman A. The E5 protein of human papillomavirus type 16 perturbs MHC class II antigen maturation in human foreskin keratinocytes treated with interferon-g. Virology. 2003;310:100–108. doi: 10.1016/s0042-6822(03)00103-x. [DOI] [PubMed] [Google Scholar]
- Zhang B, Spandau DF, Roman A. E5 protein of human papillomavirus type 16 protects human foreskin keratinocytes from UV B-irradiation-induced apoptosis. J. Virol. 2002;76:220–231. doi: 10.1128/JVI.76.1.220-231.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Srirangam A, Potter DA, Roman A. HPV16 E5 protein disrupts the c-Cbl-EGFR interaction and EGFR ubiquitination in human foreskin keratinocytes. Oncogene. 2005 doi: 10.1038/sj.onc.1208453. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lehman JM, Petti LM. Apoptosis of mortal human fibroblasts transformed by the bovine papillomavirus E5 oncoprotein. Mol. Cancer Res. 2002;1:122–136. [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology. 2009;384(2):260–5. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]