Summary
Aims
Exogenous progesterone has been shown to attenuate the rewarding effects of cocaine. However, its effects on provoked drug craving, stress arousal and cognitive performance has not been systematically investigated in cocaine dependent men and women. Thus, we conducted a double-blind placebo-controlled study assessing the efficacy of progesterone in reducing provoked drug craving, stress system arousal and improving cognitive performance in cocaine dependent men and women.
Methods
Forty-two early abstinent treatment-seeking cocaine dependent individuals were randomly assigned to either daily doses of placebo (12M/9F) or micronized progesterone (12M/9F) (400 mg/day), for 7 days. Under experimental conditions, all subjects were exposed to three 5-min personalized guided imagery conditions (stress, cocaine cue, relaxing), one per day, consecutively in a random, counterbalanced order. Subjective craving, mood, hypothalamicpituitary-adrenal (HPA) and cardiovascular output, and a cognitive measure of inhibitory control (Stroop Color Word Task) were assessed pre- and post imagery.
Results
Progesterone relative to placebo significantly decreased cue-induced craving and cortisol responses and increased cue-induced ACTH. In addition, women but not men receiving progesterone reported lower ratings of negative emotion and higher ratings of relaxed mood following stress exposure. Improved Stroop performance was observed in all participants receiving progesterone, across all conditions.
Conclusions
Progesterone was selectively effective in reducing cocaine cue-induced but not stress-related cocaine craving as well as specific measures of the provoked arousal state. Findings suggest that progesterone’s effects on drug craving and arousal are moderated by both the type of environmental cue exposure and gender.
Keywords: Progesterone, Gender, Cocaine dependence, Stress, Drug cue, Stroop
1. Introduction
Cocaine dependence is one of the most common and preventable health care problems in the US, with an estimated 1700 initiates per day (SAMHSA, 2010). Moreover, due to changing socioeconomic environments, the number of women dependent on cocaine is rapidly increasing to similar levels as men (SAMHSA, 2006). Coupled with the fact that women also display greater vulnerability in terms of treatment outcome (Quinones-Jenab, 2006; Anker and Carroll, 2011), the need for an FDA approved medication is critical. In view of this, clinical and preclinical studies have shown that the administration of micronized progesterone mediates the acute subjective and physiological reinforcing effects of cocaine more robustly in females compared with males (Evans et al., 2002; Sofuoglu et al., 1999; Russo et al., 2008, 2010 Feltenstein et al., 2009; Evans and Foltin, 2006, 2010; Hudson and Stamp, 2011; Reed et al., 2011; de Wit, 2011). While progesterone demonstrates some efficacy in male rodents by decreasing locomotor activity (Rupprecht, 2003) and in both healthy (Childs et al., 2010) and substance using males (Sofuoglu et al., 2004, 2007) by attenuating negative mood and cardiovascular response, these changes are relatively minimal compared to those observed in females (Quinones-Jenab and Jenab, 2010). Similarly, with regard to motivation for cocaine use, preclinical studies have indicated that both progesterone and its metabolite allopregnanolone inhibit cocaine-primed reinstatement in female, but not male rats (Anker et al., 2007, 2009). Additionally, outpatient treatment studies in humans have shown that exogenous progesterone is not efficacious in reducing consumption in cocaine dependent methadone-maintained men (Sofuoglu et al., 2007).
Although micronized progesterone shows some potential as a pharmacological treatment for cocaine-abusing women (Reed et al., 2011), previous research has typically assessed either the acute subjective and physiological responses to cocaine at different phases of the menstrual cycle (MC) (Evans et al., 2002; Sofuoglu et al., 2002) or the acute effects of exogenous progesterone administration during the early follicular phase (Justice and de Wit, 1999, 2000; Kouri et al., 2002). Moreover, only a few studies employing these paradigms have compared findings to cocaine dependent men (Sofuoglu et al., 1999, 2004; Mendelson et al., 1999; Evans and Foltin, 2006; Collins et al., 2007). In the current study therefore, we aim to examine whether exogenous micronized progesterone is also able to mediate stress system adaptations known to be related to cocaine craving and relapse (Fox and Sinha, 2009), in both men and women following an abstinence period of four weeks.
During early protracted abstinence from cocaine, dysregulated basal and stress-induced physiological, HPA and emotional changes are strongly associated with increased cocaine craving, cocaine use and relapse (Back et al., 2005; Sinha et al., 2006; Fox et al., 2008a; Fox and Sinha, 2009). These changes include a tonic up-regulation in cortisol and ACTH (Sarnyai et al., 2001; Fox et al., 2008b, 2009) as well as sensitized negative mood, craving, cardiovascular and cortisol output in response to stress and cue (Sinha et al., 2003, 2006; Fox et al., 2005, 2008a, b). As cocaine dependence is a chronic stress state and sex hormones modulate stress system function (Lindheim et al., 1994) exogenous progesterone may mediate components of stress dysregulation during early abstinence.
In support of this hypothesis, previous findings from our own laboratory have highlighted a role for endogenous progesterone in reducing some of the chronic stress system adaptations during early abstinence in women. Findings from one study showed that early abstinent women in the mid luteal phase of their MC (high progesterone) demonstrated significant reductions in stress and cue-induced craving as well as cue-induced anxiety and blood pressure compared with women in the early follicular phase (low progesterone) (Sinha et al., 2007). Similarly, cocaine dependent women in their first month of abstinence demonstrated significantly up-regulated salivary cortisol and negative affect along-side enhanced levels of progesterone across the entire MC, suggesting a potentially compensatory role for endogenous progesterone in response to an enhanced distressed state (Fox et al., 2008b).
In addition to having potent anxiolytic and vascular effects on the peripheral nervous system (Sita and Miller, 1996), progesterone may also strengthen prefrontal regulatory mechanisms. This may be due to the actions of its metabolite allopregnanolone on GABA-activated inhibition of neural firing in dopaminergic regions of the striatum, nucleus accumbens (NAc), hippocampus and pre-frontal cortex (PFC) (Becker, 1999; Becker and Hu, 2008; Russo et al., 2008). As optimal levels of dopamine within these mesocorticolimbic systems reduce “noise” in the PFC by suppressing neuronal processing of irrelevant information (Brennan and Arnsten, 2008), progesterone may be a suitable agent for strengthening regulatory processes integral to treatment outcome (Witkiewitz and Marlatt, 2005).
We therefore investigated the subjective, physiological, HPA and cognitive effects of 400 mg/day of exogenous micronized progesterone versus placebo in early abstinent men and women (p.o, b.i.d) for seven days. Dependent measures were assessed at baseline and following exposure to personalized stress and cocaine cue-related imagery. We hypothesized that the progesterone group would demonstrate reduced cocaine craving, negative mood, and cardiovascular output, as well as normalized HPA axis function and improved Stroop performance following stress and cue, compared with placebos. In view of previous research, we also hypothesized that gender would moderate the effects of exogenous progesterone.
2. Materials and methods
Participants
Forty-two treatment-seeking cocaine dependent individuals (24 M/18F) who responded to local advertisements around the New Haven area participated in the study. Current cocaine dependence was determined using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders IV (SCID IV – First et al., 1997) as well as positive urine toxicology screens for the cocaine metabolite (benzoylecgonine). Exclusion criteria included DSM-IV dependence for any drug other than cocaine, alcohol or nicotine. Participants using prescribed medications for any psychiatric or medical disorders were also excluded, and all individuals underwent stringent medical assessments including electrocardiography and laboratory tests of renal, hepatic, pancreatic, hematopoietic and thyroid function. Women with amenorrhea were also excluded from the study. Written and verbal consent were obtained from all participants and the Human Investigation Committee of the Yale University School of Medicine approved the study.
Design
A placebo-controlled, double-blind experimental design was used in the current study. All subjects were exposed to three personalized guided imagery conditions (stress, drug cue, relaxing) across three consecutive days, one imagery condition per day, in a randomized and counterbalanced order. Research staff was blind to which imagery condition was presented on what day. Subjects also remained blind until imagery presentation. Medication Group, (placebo Vs progesterone) was the between-subjects factor; imagery condition (stress, drug cue, relaxing) and Time-points (repeated assessments) were within-subjects factors. Such a design allowed us to assess inter and intra-individual differences in response to stress and cue provocation relative to a relaxing, or control condition, within each subject and to model the interaction of these factors on response to medication.
General procedures
All participants were admitted to the Clinical Neuroscience Research Unit (CNRU) of the Connecticut Mental Health Center (CMHC) for approximately four weeks of inpatient treatment and study participation. The CNRU is a locked inpatient treatment research facility where drug testing is conducted regularly to ensure abstinence. All subjects participated in four weeks of group counseling for cocaine dependence using the standard 12-Step based Group Drug Counseling Manual as a guide (Mercer et al., 1994). During the first week, participants were administered structured baseline assessments measuring psychiatric and substance use history. In the second week, scripts for the guided imagery induction were developed. The laboratory sessions were conducted over 3 days, approximately 14 days after admission in order to allow for steady state levels of study medication.
Progesterone dosing schedule
All micronized progesterone (Prometrium®) pills (200 mg bid) were purchased from Solvay Pharmaceuticals, Marietta, Georgia, through the study pharmacy located at the CMHC. All active and placebo capsules used for medication administration appeared identical. Placebo pills contained lactose and subjects were randomly assigned to placebo or progesterone conditions by the research pharmacist, experienced in Urn randomization procedures (Stout et al., 1994). Urn randomization is a restricted randomization design, which ensures that treatment assignment in small samples are balanced, but behaves like complete randomization as participant numbers increase (Wei, 1978). This is achieved by probability of assignment fluctuating as a function of the degree of imbalance (Wei and Lachin, 1988), and incorporates both balancing and bias reduction properties.
All female participants were admitted to the inpatient unit approximately one to two weeks before the expected beginning of menstruation (MC day1). The 7 day medication protocol was initiated between days 1 and 3 of their MC when both progesterone and estradiol levels are low and remain stable (approximately <85 pg/ml for estradiol and <1.1 ng/ml for progesterone; Chabbert Buffet et al., 1998). This was to ensure minimal interaction with endogenous sex steroid hormones and for hormone levels to be consistent with those of male participants, as physiological levels of progesterone in men are similar to the follicular phase progesterone levels often observed in women (Zumoff et al., 1990). In the case of male participants, treatment medication was administered after approximately 7–10 days of inpatient stay for consistency. Study medications were administered at 7:30 AM and 9 PM daily for seven days (Progesterone 200 mg, p.o.; b.i.d).
All women were required to complete a detailed MC history questionnaire providing self report information regarding the dates and regularity of their last three MCs, the length of menses each time, and the onset of menses in the current cycle. MC phase was also confirmed by obtaining serum levels of progesterone and estradiol upon admission to the inpatient unit and during the three laboratory testing days. Serum levels and self reported MC details were then compared to ensure accuracy.
Safety and side effects measures
These comprised: (a) sitting and standing vital signs (HR/BP) taken every 15 min for 1 h, every other day while on the medication, (b) assessment of laboratory chemistry at study admission and discharge, including electrocardiography, renal, hepatic, and pancreatic tests, and (c) assessment of any changes in physical and mental functioning every other day while on the medication, in order to evaluate any potential adverse events or side effects.
Imagery script development procedures
imagery script development was conducted in a session one or two days prior to the laboratory sessions. Procedures are based on methods developed by Lang et al. (Lang et al., 1980, 1983; Miller et al., 1987), and further adapted and documented in our previous studies (Sinha et al., 1992, 2000, 2003, 2006, 2009, 2011; Fox et al., 2005, 2007, 2009; Sinha, 2009).
The stress imagery script was based on each individual subjects’ description of a recent personal stressful event that was experienced as most stressful. Most stressful was determined by having each subject rate their perceived stress on a 10-point Likert scale where 1 = not at all stressful and 10 = the most stress they felt recently in their life. Only situations rated as 8 or above were accepted as appropriate for script development. The stress imagery scripts did not include scenarios either relating to or culminating in drug use. The cue imagery script was developed by having subjects identify a recent situation that included cocaine-related stimuli and resulted in subsequent cocaine use (i.e. watching others smoke crack). Drug-related scenarios did not include scenarios that involved stressful events such as being arrested. A relaxation imagery script was also developed from the subjects’ description of a personal non-drug-related relaxing situation, such as a summer beach scene or a relaxing fall afternoon reading. All scripts were then recorded onto an audiotape to be played in the subsequent laboratory sessions.
In addition to the script development, on the day prior to the laboratory sessions, subjects were brought into the testing room in order to acclimatize themselves to specific aspects of the study procedures, including IV insertion, subjective rating forms and training in relaxation and imagery procedures.
Laboratory sessions (conducted across three consecutive days)
All subjects were allowed an initial smoke break at 7:30 AM in order to reduce potential nicotine withdrawal during the session. At 8:00 AM, after settling in a sitting position on a hospital bed, a heparin-treated catheter was inserted by the research nurse in the antecubital region of the subject’s non-preferred arm, to periodically obtain blood samples. A blood pressure cuff was placed on the subject’s preferred arm and a pulse sensor was placed on the subject’s forefinger. This was followed by a 45 min adaptation period during which the subjects were instructed to practice relaxation. At 9:10 AM, subjects were provided headphones and given the following instructions for the 5-min imagery procedure: “Close your eyes and imagine the situation being described, ‘as if’ it were happening right now. Let your body and mind get completely involved in the situation, doing what you would do in the real situation”. All dependent measures were collected at various time-points pre and post imagery (See Table 1). At the end of each session, relaxation instructions were provided to ameliorate any residual effects of imagery exposure. Subjects were then free to leave the testing room and eat breakfast.
Table 1.
Collection time-points for all measures before and after exposure to relaxing, stress and cue-related imagery.
| Time | Time-point | Cocaine craving | Subjective emotion | HR/BP | Cortisol/ACTH | Progesterone | Stroop |
|---|---|---|---|---|---|---|---|
| 08:00 AM | Set-up | X | X | ||||
| Adaptation period (45 mins) | |||||||
| 08:45 AM | Baseline 1 | X | X | X | X | X | |
| 09:05 AM | Baseline 2 (B) a | X | X | X | X | X | X |
| 09:10 AM | 5-min imagery exposure (relaxing/stress/cue) presented one imagery per day | ||||||
| 09:17 AM | Imagery (I) | X | X | X | X | X | |
| 09:22 AM | Recovery (R0) | X | X | ||||
| 09:30 AM | Recovery 1 (R1) | X | X | X | X | ||
| 09:45 AM | Recovery 2 (R2) | X | X | X | X | ||
| 10:00 AM | Recovery 3 (R3) | X | X | X | X | ||
| 10:15 AM | Recovery 4 (R4) | X | X | X | X | ||
| 10:30 AM | Recovery 5 (R5) | X | X | X | X | ||
Baseline used in the current analyses.
2.1. Laboratory assessments
Subjective measures
Cocaine Craving Questionnaire-Brief (CCQ Tiffany et al., 1993): The CCQ (brief) is a shortened adaptation of the 45 item Tiffany Cocaine Craving self-report questionnaire. All 7-items are in the form of a craving-related statement and scores for each item response range from 1 (strongly disagree) to 7 (strongly agree). Differential Emotion Scale (Izard, 1972): The scale comprises 30 adjectives (or items) and participants are required to rate on a 5-point Likert scale the extent to which each word described the way s/he felt at the current time (1 = “not at all” and 5 = “very strongly”). The sub-scales were collapsed into three separate scales: negative emotion: (sub-scales sadness and anger); anxiety and arousal: (sub-scales anxiety and fear); positive mood: (sub-scale relaxed state).
Cognitive measure
Stroop Color/Word Test (Golden, 1976): The Stroop test has been used extensively in both clinical and experimental fields to assess the ability to inhibit incongruent competing conflicts. In the interference trial 100 words are presented, and in all cases, the word (i.e. red) is different from the color it is printed in (i.e. blue. (e.g.“red”). Subjects are given 45 s to read aloud the color of the ink as quickly as possible. The number of raw items read for each trial is recorded and converted to standardized T scores.
Cardiovascular measures
A Critikon Dinamap 120 Patient Monitor (GE Medical Systems, Tampa, FL) was used to assess blood pressure and a pulse sensor was attached to the subject’s finger to provide a continuous measure of pulse.
Blood samples
ACTH and cortisol samples were obtained in heparinized plasma collection tubes and placed on ice immediately after drawing. Samples were then aliquoted after being centrifuged at 4C within 30 min of collection and subsequently stored at −70 C. All plasma samples were processed at the Yale Center for Clinical Investigation Core Laboratories using standard radioimmuno-assay procedures. Blood samples for progesterone levels were obtained in serum collection tubes and kept at room temperature for 2 h prior to being centrifuged. All samples were then shipped to Graham Massey Analytical Lab, Inc (Shelton, CT) for standard immune-assay procedures.
2.2. Statistical analysis
Linear mixed effect (LME) models (Laird and Ware, 1982) were implemented to analyze the data, using SPSS software (version 17). Within-subjects factors of imagery condition (stress, cocaine cue, relaxing), Time-point (varying levels) and the Between-subjects factors of Medication Group (progesterone/placebo) and Gender (males/females) were the fixed effects. Subjects represented the random effect. T-tests and Chi-square analyses were used to examine group differences in demographic variables. The Bonferroni test for multiple comparisons was used to analyze simple effects.
3. Results
All main and simple effects of Medication Group, Imagery Condition and Time-points are displayed in Table 2. Results of 2 and 3-way interactions only are displayed in the text. All figures show differences between medication groups only, and are collapsed across time-points in the case of no significant Time-point interactions, across condition in the case of no significant imagery condition effects and across gender in the case of no significant Gender effects.
Table 2.
Significant main effects and interactions of Medication Group, Gender, Imagery Condition and Time-points (all simple effect contrasts are displayed in the text).
| Dependent variables | Main effects | 2-Way & 3-way interactions |
|---|---|---|
| Cocaine craving (CCQ) | Medication × Condition: [F(2296) = 3.52; p = .03] | |
| Medication × Time-point: [F(2296) = 3.52; p = .03] | ||
| Negative emotion (DES) |
Gender [F(1,38) = 4.4; p = .04] F > M Condition [F(2869) = 75.1; p < .0001] S > R, p < .0001; C > R, p < .0001, S > C, p < .0001 Time-Point [F(7, 869) = 19.0; p < .0001] +77 (peak) TP > all other TPs, p > .0001 |
Medication × Gender × Condition: [F(2869) = 10.65; p < .0001] |
| Anxiety & arousal (DES) | Gender × Condition: [F(2869) = 17.44; p 7lt; .0001] | |
| Positive emotion (DES) |
Medication × Gender × Condition: [F(2869) = 4.23; p 7lt; .02] |
|
| SBP |
Medication × Gender × Condition: [F(2957) = 6.08; p = .002] |
|
| ACTH | Medication × Condition: [F(2514) = 5.79; p = .003] | |
| Cortisol |
Condition [F(2602) = 9.33; p < .0001] C > S, p < .0001 |
Medication × Condition: [F(2,62) = 18.85; p < .0001] Medication × Time-point: [F(6594) = 4.21; p < .0001] |
| Stroop |
Medication Group [F(1,42) = 4.14; p < .05] PG > PLA, p = .05 |
Medication × Time-point: [F(1200) = 4.21; p = .04] |
R = relaxation, S = stress, C = cue; PG = progesterone, PLA = placebo; TP = time-point.
Participants
Both placebo and progesterone medication groups were statistically matched for gender, race, years in education, age, recent prior drug and alcohol use and other clinical characteristics (Table 3).
Table 3.
Demographics and clinical characteristics.
| Placebo n = 21 | Progesterone n = 21 | |
|---|---|---|
| Gender – no. of males | 12 (57%) | 12 (57%) |
| Race | ||
| African American | 15 (71.4%) | 17 (81.0%) |
| Caucasian | 5 (23.8%) | 3 (14.2%) |
| Hispanic | 1 (4.8%) | 1 (4.8%) |
| Agea | 41.6 ± 7.9 | 41.9 ± 5.9 |
| Years spent in educationa | 12 ± 1.6 | 11.9 ± 1.7 |
| No. of regular smokers | 20 (95.2%) | 20 (95.2) % |
| No. of cigarettes per day | 11.8 ± 8.2 | 13.6 ± 8.7 |
| Years of cocaine use a | 15.0 ± 8.6 | 12.8 ± 6.4 |
| No. of days used in past month a | 12.8 ± 8.8 | 13.1 ± 8.7 |
| Years of alcohol use a | 14.1 ± 9.0 | 15.9 ± 10.2 |
| No. of days used in past month a | 10.9 ± 10 | 10.8 ± 9.8 |
| Lifetime depression (%) | 1 (4.8%) | 1 (4.8%) |
| Lifetime anxiety (incl PTSD) (%) | 5 (23.8%) | 6 (28.6%) |
| Lifetime anxiety (without PTSD) (%) | 4 (19%) | 2 (9.5%) |
Data indicate means and standard deviations. All variables: p > .05.
Safety measures
Preliminary findings from both the safety measures and laboratory sessions indicate that progesterone was well-tolerated, with mild levels of transient side effects. Three of the 21 (14.3%) participants in the progesterone group and 6/21 (28.6%) patients in the placebo group reported side-effects during the seven days of medication administration. In the progesterone group 1/21 participants reported headaches (4.8%), nausea (4.8%), dizziness (4.8%) and loss of appetite (4.8%). Two of the 21 participants reported moderate weight gain (9.5%). In the placebo group 1/21 participants reported headaches (4.8%), feeling achy (4.8%), coughing (4.8%), elevated blood pressure (4.8%) chest pain (4.8%) and neck pain (4.8%). Two of the 21 placebo patients reported tiredness (9.5%). All reported symptoms dissipated over time from moderate to mild without further intervention.
3.1. Effects of medication and gender at baseline
A main effect of Medication Group for plasma progesterone [F(1, 34) = 28.2; p < .0001] indicated that, as expected, the progesterone group demonstrated significantly higher levels of plasma progesterone compared with the placebo group across all three conditions (Data not shown). No main effect or interaction with Gender was observed
A main effect of Medication Group for ACTH levels [F(1, 36) = 3.97; p = .05] also showed that the progesterone group demonstrated significantly lower levels of basal (pre-guided imagery) plasma ACTH across all three conditions compared with the placebo group (Data not shown). No other main effects of Medication or Gender, or Medication × Gender interactions were observed at baseline.
No further baseline effects of progesterone were observed with regard to any other dependent measures.
Effects of medication and gender on response to imagery (relaxation, stress, cocaine cue): all main and interaction effects are displayed in Table 2.
3.2. Subjective measures: craving (CCQ-brief)
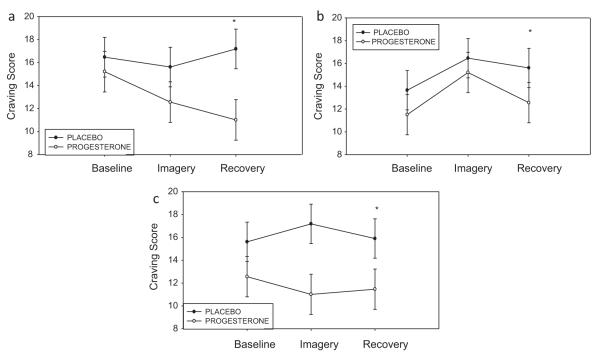
A Medication × Condition effect indicated that the progesterone group reported significantly lower levels of cocaine craving compared with placebo group following exposure to drug cue ( p < .05) irrespective of time-points in both males and females.
A Medication × Time-point effect also indicated that the progesterone group reported significantly lower ratings of cocaine craving at the recovery time-point (R) compared with the placebo group, irrespective of imagery conditions ( p < .05), in both males and females (Fig. 1a–c). Line graphs displayed in Fig. 1a–c shows both these Medication × Condi-Condition and Medication × Time-point interaction effects collapse across gender.
Figure 1.
Differences between the placebo and progesterone groups in cocaine craving before, immediately after and during recovery from, imagery exposure (means and SEMs shown). (a) Relaxation imagery. *Group difference: p < .05; (b) stress imagery. *Group difference: p < .05; (c) cue imagery. *Group difference: p < .05.
3.3. Subjective measures: negative emotion (DES)
A Medication × Gender × Condition effect indicated that progesterone decreased negative emotion in females following stress irrespective of time-points: consistent with previous findings, placebo females reported significantly higher ratings of negative emotion following stress compared with placebo males ( p < .0001). Notably, this sex-specific discrepancy was not observed between the male and female progesterone group ( p = ns) highlighting further the decrease in stress-induced negative emotion in the progesterone-treated females. Progesterone females also reported significantly lower ratings of negative emotion compared with placebo females following exposure to stress imagery ( p = .04). The block graph displayed in Fig. 2, demonstrates this Medication × Gender × Condition effect collapsed across all time-points.
Figure 2.
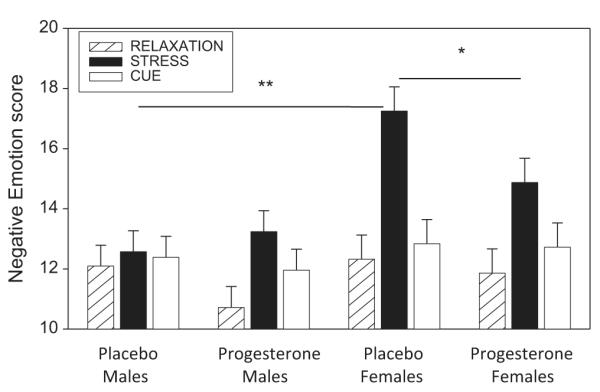
Differences between male and female placebo and progesterone groups in negative emotion following exposure to relaxation, stress and cue-related imagery (means and SEMs shown). *Group difference: p < .05; **group difference: p < .01. Stress > relaxation ( p < .0001) and cue > relaxation ( p < .0001) in progesterone males, progesterone females and placebo females.
A Medication × Gender × Condition effect also indicated that all participants reported significantly higher ratings of negative emotion following exposure to stress relative to the relaxation imagery condition and following exposure to stress relative to the cue condition ( p < .0001, in all cases) with the exception of the placebo males.
3.4. Subjective measures: anxiety and arousal (DES)
A Gender × Condition effect indicated that females reported higher anxiety and arousal following stress compared with males ( p < .02), across all time-points in both the progesterone and placebo groups: The block graph displayed in Fig. 2c, demonstrates this Gender × Condition effect with data collapsed across both medication groups and all time-points.
A Gender × Condition effect also indicated that females reported significantly higher ratings of anxiety and arousal following stress ( p < .0001) and cue ( p < .0001) compared with the relaxing imagery condition, and following stress compared with cue ( p = .01). Males reported significantly higher ratings of anxiety and arousal following cue compared with the relaxing ( p = .002) and stress ( p = .004) imagery (Data not shown).
3.5. Subjective measures: positive emotion (DES)
A Medication × Gender × Condition effect indicated that females receiving placebo reported significantly lower ratings of positive mood following exposure to stress compared with females receiving progesterone ( p = .03), across all time-points. In addition, while both the placebo (R > C, p < .0001) and progesterone (R > C, p = .001) females reported significantly greater positive mood in the relaxation imagery compared with the cue condition, this effect of condition on positive mood was not observed in the males (Data not shown).
3.6. Physiological measures: systolic blood pressure
A Medication × Gender × Condition effect indicated that females receiving progesterone demonstrated a stress-related and cue-related dampening of SBP (R > S, p < .05; R > C, p = .006), across all time-points. Conversely, females receiving placebo demonstrated increases in SBP following exposure to stress compared with the relaxing condition ( p = .04) and following exposure to stress compared with the cue condition ( p = .008).
No significant effects on DBP or heart rate were observed in terms of either Medication or Gender.
3.7. HPA-axis measures: plasma ACTH (change from baseline)
A Medication × Condition effect indicated that the progesterone group demonstrated higher levels of ACTH following cue compared with those receiving placebo ( p = .04), in both males and females across all time-points. Higher ACTH levels were also observed following cue compared with the stress imagery condition in the progesterone group (C > S, p = .004).
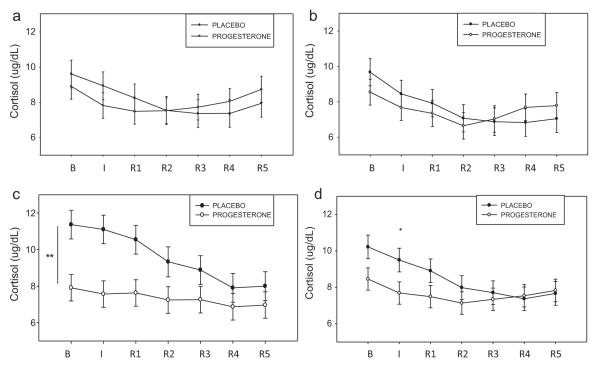
3.8. HPA-axis measures: plasma cortisol
A Medication × Condition effect indicated that the progesterone group demonstrated significantly lower levels of cortisol following exposure to cue compared with the placebo group ( p = .01), across all time-points. In addition, while the placebo group showed increases in cortisol following exposure to cue compared with both the relaxing ( p < .0001) and stress ( p < .0001) imagery conditions, the progesterone group showed only a suppressed cue-related response (R > C, p < .03). The line graphs displayed in Fig. 3a–c shows the Medication × Condition effect collapsed across both males and females.
Figure 3.
Differences between the placebo and progesterone groups in plasma cortisol across all time-points and imagery conditions (means and SEMs shown); (a) relaxation imagery. Data shows diurnal drop in cortisol over the course of the morning; (b) stress imagery. Data shows diurnal drop in cortisol over the course of the morning; (c) cue imagery. **Group difference: p ≤ .01. In placebo group: cue > relaxation, p < .0001; cue > stress, p < .0001. In progesterone group: relaxation > cue, p < .03. Data shows diurnal drop in cortisol over the course of the morning: (d) group difference in cortisol averaged across all three imagery conditions. *Group difference: p ≤ .05. In placebo: I > R2, p = .01; I > R3, p = .001; I > R4, p < .0001; I > R5, p = .001. Data shows diurnal drop in cortisol over the course of the morning.
A Medication × Time-point effect indicated that the progesterone group demonstrated significantly lower levels of cortisol response immediately following exposure to all imagery conditions (imagery (I) time-point) compared with the placebo group ( p < .05), in both males and females. Conversely, the placebo group showed a significant increase in cortisol response immediately following exposure to all imagery conditions (“I” time-point) compared with most recovery time-points (I ≥ R2, p = .01; I ≥ R3, p = .001; 1 ≥ R4, p < .0001; 1 ≥ R5, p = .001). This peak increase in cortisol was not demonstrated in the progesterone group, suggesting an overall dampened peak cortisol response. The line graph displayed in Fig. 3d, shows this Medication × Time-point effect collapsed across both males and females and all imagery conditions.
3.9. Cognitive measures: Stroop
A Medication × Time-point effect indicated that the progesterone group demonstrated significantly higher scores at the post-imagery (P) time-point ( p = .02) compared with the placebo group, across males and females and all three imagery conditions. No differences between groups or gender were seen at the pre-imagery baseline (B) time-point (p = ns). Additionally, a significant improvement in Color/Word scores was seen from the pre- (B) to post- (P) imagery time-points in the progesterone group across males and females and all three conditions ( p = .002). This increased improvement in score was not observed in the placebo group.
3.10. Secondary analysis
Extended correlational analysis showed a significant negative association between basal progesterone levels and basal ACTH (r = −.33, p = .04) across both medication groups.
4. Discussion
In the current study, exogenous progesterone (400 mg/day), administered daily across seven consecutive days was found to be safe and well tolerated by early abstinent cocaine dependent men and women. Furthermore, progesterone was shown to have selective gender and cue-related effects on cocaine craving as well as arousal and in Stroop performance assessed as a measure of inhibitory control known to be associated with self control and relapse risk. Administration of exogenous progesterone resulted in a decrease in basal ACTH tone, as well as decreased plasma cortisol and increased plasma ACTH following exposure to cue in both males and females. Progesterone also reduced negative emotion and systolic blood pressure (SBP) in females, but not males following exposure to stress. In addition, individuals receiving exogenous progesterone demonstrated improved stroop performance compared with those receiving placebo, under both positive and negative provocation conditions. These findings are largely consistent with our previous pilot study in cocaine dependent women studied in the mid-luteal high progesterone period that showed reduced cue-induced cocaine craving, anxiety and blood pressure and lower stress-induced craving, compared to these reponses in those assessed in a low endogenous progesterone phase (Sinha et al., 2007). Overall findings suggest that progesterone demonstrates some selective efficacy with regard to the provoked drug craving and stress arousal state in early abstinent dependent individuals. However, efficacy may be dependent upon moderators such as gender and type of cue.
With regard to subjective and physiological response to stress, progesterone was shown to have a more robust effect in females. Findings complement previous preclinical research (Anker et al., 2007; Hudson and Stamp, 2011; Larson et al., 2007; Russo et al., 2008), although previous human research remains relatively ambiguous due to variations in paradigm, dose and subject sample. While some studies have shown exogenous progesterone to attenuate the subjective effect of acute cocaine administration more robustly in females (Evans and Foltin, 2006), others have clearly demonstrated stress-induced reductions in negative mood in healthy males (Childs et al., 2010).
In the current study, females who were administered progesterone reported less negative emotion (sadness and anger) and greater positive emotion (relaxed state) across all time-points following exposure to the stress imagery condition compared with females administered placebo. Importantly, differences in negative emotion between the male and female placebo group, showed females to report significantly higher stress-induced negative mood compared with the males and is consistent with previous research in both substance abusers and healthy volunteers (Franken-haeuser et al., 1978; Earle et al., 1999; Chaplin et al., 2008; Fox et al., 2008a, 2009). The dampening of a typically heightened negative mood response to stress in cocaine dependent females, may therefore suggest that exogenous progesterone also serves to reduce certain aspects of sensitized emotion following a period of protracted abstinence, as well as following acute cocaine administration (Evans and Foltin, 2006; McCance-Katz et al., 2005). This is particularly salient with respect to the fact that cocaine dependent females are far more likely than cocaine dependent males to attribute frequent drug use to negative situations and relapse to negative emotional states (Waldrop et al., 2007, 2010).
In corroboration with previous research (Chaplin et al., 2008, 2010; Fox et al., 2008a, 2009; Fox and Sinha, 2009), all females in the present study reported significantly higher ratings of anxiety and arousal compared with men following exposure to stress. Unlike its effects on negative mood, however, progesterone administration did not contribute to the reduction of these “vigilance” related negative emotions in cocaine dependent females. One explanation may be due to the fact that negative emotions are associated with discreet bio-physiological and neural systems (Schwartz et al., 1981; Ekman et al., 1990; Ekman and Scherer, 1984) which may, in turn, be selectively modulated by progesterone. While anger has been associated with increased cardiac output, fear has been linked with decreased cardiac output and increased total peripheral resistance in a group of healthy subjects (Montoya et al., 2005). Similarly, while fear and appetitive emotions including anxiety have typically been associated with marked activation of the amygdala (Morris et al., 1998; Whalen et al., 1998), this is less pronounced following stimuli evoking other types of negative emotion (Hariri et al., 2002; Phan et al., 2002). The existence of emotion-specific blood pressure responses and neural system activations in major categories of emotions may therefore underlie progesterone’s emotional specificity.
Significant reductions in SBP following stress and cue relative to the relaxing condition was also observed in females administered progesterone, but not observed in the other groups. Findings support a study assessing the effects of gonadal hormones on cardiovascular response to stress in menopausal women, where the combined administration of Estratab (esterone) plus Prometrium (micronized progesterone) significantly lowered blood pressure following a speech stressor (Matthews et al., 2005). Findings also tentatively corroborate studies that have shown micronized progesterone to provide beneficial effects on ambulatory blood pressure in menopausal women (Lee et al., 2011). From a broader perspective, dampening of stress-related SBP in progesterone females is also fitting in view of the reduction observed in negative emotion, and both may be inter-related in contributing to mechanisms underlying the early abstinent cocaine craving state in dependent females.
In terms of progesterone’s effects on HPA axis markers, no gender variation was observed. However, progesterone-related changes in response were markedly more robust following exposure to cue compared with stress-related imagery. Following exposure to cue, both males and females who had been administered progesterone demonstrated increased levels of plasma ACTH and decreased levels of plasma cortisol compared with those who received placebo. Progesterone is known to attenuate the inhibition of cortisol feedback on ACTH (Keller-Wood et al., 1988; Svec, 1988), and this mechanism may account for the sensitized ACTH stress response observed in the current study. For example, in animal studies, female rats have demonstrated enhanced HPA axis responsivity in proestrus, when progesterone levels are relatively high (Young et al., 2001). Additionally, in humans, increased ACTH secretion has been documented in response to various stressors such as cold pressor task and the Trier Social Stress Test (TSST) during the mid-luteal phase of the MC (Tersman et al., 1991; Kirschbaum et al., 1995, 1999; Altemus et al., 1997; Galliven et al., 1997). While progesterone-induced increases in ACTH following cue may at first appear counter-intuitive to reducing arousal mechanisms associated with cocaine craving (Fox and Sinha, 2009), it is worth noting that progesterone’s therapeutic mechanism may be attributable to cortisol reduction rather than ACTH reduction. In view of this, recent research shows that it is higher cortisol relative to ACTH ratios which appear to be critical in terms of predicting relapse in substance abusing populations (Sinha et al., 2011).
In the current study, exogenous progesterone was also shown to improve stroop performance, irrespective of gender or cue-related provocation. As such, findings may tentatively suggest that progesterone improves inhibitory control and cognitive flexibility as measures by the Stroop task associated with both stress and cue-related arousal, as well as positive arousal systems provoked by the relaxation condition. Possible mechanisms underpinning improved stroop performance may be related to the fact that exogenous progesterone increases functional coupling between the amygdala and regulatory circuits of the brain including the dorsal anterior cingulate following threatening stimuli (van Wingen et al., 2008). In addition, the progesterone metabolite allopregnanolone increases GABA-activated chloride ion currents and inhibits neuronal firing in habit-forming circuitry including the striatum, Nucleus Accumbens (NAc), and hippocampus (Becker, 1999; Becker and Hu, 2008; Russo et al., 2008). Such anxiolytic processes may have important ramifications for improved inhibitory control. However, future research is again encouraged to examine these specific mechanisms more directly.
Study limitations include the fact that although both the progesterone and the placebo group were statistically well-matched in terms of demographics and drug use, both samples were also regular smokers and many also met criteria for alcohol abuse. This is consistent with many experimental and clinical studies which have indicated that the simultaneous abuse of alcohol and nicotine is typical in cocaine dependence (Patkar et al., 2006; McCance-Katz et al., 1999). While findings may not, therefore, reflect the effects of exogenous progesterone on a “pure” dependent cocaine sample, per se, they do reflect the efficacy of progesterone on an ecologically valid sample of individuals who define themselves as being primarily cocaine dependent. In addition, the phasic effects of progesterone on HPA markers appeared to be much more robust following exposure to cue rather than stress imagery, suggesting some remaining vulnerability in terms of efficacy. Current research may also be limited by low subject numbers and the administration of a single 400 mgs/day progesterone dose across only seven days, particularly in view of the fact that progesterone attenuated cue-related cocaine craving only and not stress-related craving, as we previously reported with high endogenous progesterone levels in cocaine dependent women (Sinha et al., 2007). As such, future research assessing how dose moderates response over a longer period of time with a larger subject sample may help to clarify some of these cue-related dissociations. In support of this, previous studies assessing the efficacy of adrenergic agents in reducing a provoked craving state in dependent individuals have shown that higher doses may be required to reduce cocaine craving following stress compared with cue (Fox et al., 2012).
In the present study, exogenous progesterone was shown to reduce stress-related negative affect and blood pressure to a greater extent in females compared with males. While this corroborates some clinical research, it is also important to acknowledge that, in general, findings from previous studies remain equivocal due to methodological issues regarding sample size, genomic variations, dosing pattern, route of administration, subject population and type of stressor (Anker et al., 2012; Fox and Sinha, 2009; Evans and Foltin, 2010). For example, several studies have shown no differences in response to cocaine between males and follicular or luteal phase females, with regard to HR, plasma cocaine levels or feelings of being high (Mendelson et al., 1999; Sofuoglu et al., 1999; Collins et al., 2007). Furthermore, progesterone-induced reductions in blood pressure following acute cocaine administration have been demonstrated in both males and females (Sofuoglu et al., 2004). Most importantly, very few studies have examined the effects of progesterone during early abstinence in both males and females.
Clearly, further gender-related research is warranted to fully determine the effects of progesterone on stress system adaptations in cocaine dependent populations. Future research is particularly encouraged to assess these adaptations during the early protracted withdrawal phase, using extended doses of exogenous progesterone within increased subject samples of both males and females. Despite these limitations, the current study indicates that exogenous progesterone may reduce some of the underlying arousal mechanisms associated with relapse vulnerability across subjective, biophysiological, endocrine and cognitive regulatory domains. Although, subjective and physiological reductions in provoked arousal were more robust in females, cue-related decreases in craving and HPA axis markers were comparable across gender, demonstrating some benefits for males during early recovery from cocaine. As progesterone-related changes are moderated by both gender and cue type, extended research is warranted in order to fully understand the nature of progesterone as an efficacious medication for cocaine dependence in both men and women.
Acknowledgements
We would like to thank the staff at The Clinical Neuroscience Research Unit, The Substance Abuse Center at The Connecticut Mental Health Center and the Yale Center for Clinical Investigation (YCCI) supported by the Yale Clinical and Translational Science Award (Yale CTSA) for their assistance in completing this study.
Role of the funding source This study was supported in part by the National Institute of Health (NIH) Grants 1K01-DA029040 (Fox); R01-DA027130 (Sinha); UL1-DE019586 (Sinha); PL1-DA024859 (Sinha), and and the Connecticut Department of Mental Health and Addiction Services.
Footnotes
Contribution Helen Fox is the first author and wrote the initial draft of the manuscript for publication and also analyzed the data. Keri Tuit managed the literature searches. Mehmet Sofuoglu and Rajita Sinha both acted as consultants for the final version of the manuscript. Peter Morgan was also a consultant as well as the attending physician on the study. All authors contributed to and have approved the final manuscript.
Conflict of interest The authors declare that they have no competing financial interests pertaining to the aims and results of this study.
References
- Altemus M, Redwine L, Leong YM, Yoshikawa T, Yehuda R, Detera-Wadleigh S, Murphy DL. Reduced sensitivity to glucocorticoid feedback and reduced glucocorticoid receptor mRNA expression in the luteal phase of the menstrual cycle. Neuropsychopharmacology. 1997;17:100–109. doi: 10.1016/S0893-133X(97)00039-0. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr. Top. Behav. Neurosci. 2011;8:73–96. doi: 10.1007/7854_2010_93. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Holtz NA, Carroll ME. Effects of progesterone on escalation of intravenous cocaine self-administration in rats selectively bred for high or low saccharin intake. Behav. Pharmacol. 2012;23(2):205–210. doi: 10.1097/FBP.0b013e32834f9e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Holtz NA, Zlebnik N, Carroll ME. Effects of allopregnanolone on the reinstatement of cocaine-seeking behavior in male and female rats. Psychopharmacology (Berl) 2009;203(1):63–72. doi: 10.1007/s00213-008-1371-9. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Larson EB, Gliddon LA, Carroll ME. Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Drug Alcohol Depend. 2007;107:264–267. doi: 10.1037/1064-1297.15.5.472. [DOI] [PubMed] [Google Scholar]
- Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology (Berl) 2005;180:169–176. doi: 10.1007/s00213-004-2129-7. [DOI] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol. Biochem. Behav. 1999;64:803–812. doi: 10.1016/s0091-3057(99)00168-9. Review. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front. Neuroendocrinol. 2008;29:6–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan AR, Arnsten AF. Neuronal mechanisms underlying attention deficit hyperactivity disorder: the influence of arousal on prefrontal cortical function. Ann. N. Y. Acad. Sci. 2008;1129:236–245. doi: 10.1196/annals.1417.007. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabbert Buffet N, Djakoure C, Maitre SC, Bouchard P. Regulation of the human menstrual cycle. Front. Neuroendocrinol. 1998;19:151–186. doi: 10.1006/frne.1998.0167. [DOI] [PubMed] [Google Scholar]
- Chaplin T, Hong K-I, Bergquist K, Sinha R. Gender differences in response to emotional stress: an assessment across subjective, behavioral, and physiological domains and relations to alcohol craving. Alcohol Clin. Exp. Res. 2008;32:1242–1250. doi: 10.1111/j.1530-0277.2008.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin TM, Hong K, Fox HC, Siedlarz KM, Bergquist K, Sinha R. Behavioral arousal in response to stress and drug cue in alcohol and cocaine addicted individuals versus healthy controls. Hum. Psychopharmacol. 2010;25:368–376. doi: 10.1002/hup.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E, Van Dam NT, de Wit H. Effects of acute progesterone administration upon responses to acute psychosocial stress in men. Exp. Clin. Psychopharmacol. 2010;18(1):78–86. doi: 10.1037/a0018060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SL, Evans SM, Foltin RW, Haney M. Intranasal cocaine in humans: effects of sex and menstrual cycle. Pharmacol. Biochem. Behav. 2007;86:117–124. doi: 10.1016/j.pbb.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H. Sex hormones: a new treatment for cocaine abuse? Neuropsychopharmacology. 2011;36(11):2155–2156. doi: 10.1038/npp.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earle TL, Linden W, Weinberg J. Differential effects of harassment on cardiovascular and salivary cortisol stress reactivity and recovery in women and men. J. Psychosom. Res. 1999;46:125–141. doi: 10.1016/s0022-3999(98)00075-0. [DOI] [PubMed] [Google Scholar]
- Ekman P, Davidson RJ, Friesen WV. The Duchenne smile: emotional expression and brain physiology. II. J. Pers. Soc. Psychol. 1990;58:342–353. [PubMed] [Google Scholar]
- Ekman P, Scherer K. Questions about emotion: an introduction. In: Scherer K, Ekman P, editors. Approaches to Emotion. Lawrence Erlbaum; Hillsdale, NJ: 1984. pp. 1–8. [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31:659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Does the response to cocaine differ as a function of sex or hormonal status in human and non-human primates? Horm. Behav. 2010;58:13–21. doi: 10.1016/j.yhbeh.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Byrd EA, Henderson AR, See RE. Attenuation of cocaine-seeking by progesterone treatment in female rats. Psychoneuroendocrinology. 2009;34:343–352. doi: 10.1016/j.psyneuen.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for the DSM-IV Axis I Disorders Patient Edition (SCID-I/P, Version 20, 4/97 Revision) New York State Psychiatric Institute; New York: 1997. [Google Scholar]
- Fox HC, Bergquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol dependent individuals. Alcoholism: Clinical and Experimental Research. 2007;31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong KA, Paliwal P, Morgan PT, Sinha R. Altered levels of sex and stress steroid hormones assessed daily over a 28-day cycle in early abstinent cocaine-dependent females. Psychopharmacology (Berl) 2008b;195:527–536. doi: 10.1007/s00213-007-0936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Hong KA, Siedlarz KM, Sinha R. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine dependent individuals compared to social drinkers. Neuropsychopharmacology. 2008a;33:796–805. doi: 10.1038/sj.npp.1301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Hong KI, Siedlarz K, Bergquist KL, Anderson GM, Kreek MJ, Sinha R. Gender dissociations in autonomic and HPA responses to stress and cues in alcohol dependent patients with cocaine abuse. Alcohol Alcohol. 2009;44:575–585. doi: 10.1093/alcalc/agp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox H, Seo D, Tuit K, Hansen J, Kimmerling A, Morgan PT, Sinha R. Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: preliminary findings. J. Psychopharmacol. 2012 Feb 1; doi: 10.1177/0269881111430746. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Sinha R. Sex differences in drug-related stress system changes: implications for treatment in substance-abusing women. Harv. Rev. Psychiatry. 2009;17:103–119. doi: 10.1080/10673220902899680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Talih M, Malison R, Anderson GM, Kreek MJ, Sinha R. Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and to drug related cues. Psychoneuroendocrinology. 2005;30:880–891. doi: 10.1016/j.psyneuen.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Frankenhaeuser M, von Wright MR, Collins A, von Wright J, Sedvall G, Swahn CG. Sex differences in psychoneuroendocrine reactions to examination stress. Psychosom. Med. 1978;40:334–343. doi: 10.1097/00006842-197806000-00006. [DOI] [PubMed] [Google Scholar]
- Galliven EA, Singh A, Michelson D, Bina S, Gold PW, Deuster PA. Hormonal and metabolic responses to exercise across time of day and menstrual cycle phase. J. Appl. Physiol. 1997;83:1822–1831. doi: 10.1152/jappl.1997.83.6.1822. [DOI] [PubMed] [Google Scholar]
- Golden CJ. Identification of brain disorders by the Stroop Color and Word Test. J. Clin. Psychol. 1976;32:654–658. doi: 10.1002/1097-4679(197607)32:3<654::aid-jclp2270320336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Hudson A, Stamp JA. Ovarian hormones and propensity to drug relapse: a review. Neurosci. Biobehav. Rev. 2011;35:427–436. doi: 10.1016/j.neubiorev.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Izard C. Patterns of Emotions: A New Analysis of Anxiety and Depression. Academic Press; New York: 1972. [Google Scholar]
- Justice AJ, de Wit H. Acute doses of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 1999;145:67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of d-amphetamine during the early and late follicular phases of the menstrual cycle in women. Pharmacol. Biochem. Behav. 2000;66:509–515. doi: 10.1016/s0091-3057(00)00218-5. [DOI] [PubMed] [Google Scholar]
- Keller-Wood M, Silbiger J, Wood CE. Progesterone attenuates the inhibition of adrenocorticotropin responses by cortisol in nonpregnant ewes. Endocrinology. 1988;123:647–651. doi: 10.1210/endo-123-1-647. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus–pituitary–adrenal axis. Psychosom. Med. 1999;6:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. Preliminary evidence for reduced cortisol responsivity to psychological stress in women using oral contraceptive medication. Psychoneuroendocrinology. 1995;20:509–514. doi: 10.1016/0306-4530(94)00078-o. [DOI] [PubMed] [Google Scholar]
- Kouri EM, Lundahl LH, Borden KN, McNeil JF, Lukas SE. Effects of oral contraceptives on acute cocaine response in female volunteers. Pharmacol. Biochem. Behav. 2002;74:173–180. doi: 10.1016/s0091-3057(02)00992-9. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Lang PJ, Kozak MJ, Miller GA, Levin DN, McLean A., Jr. Emotional imagery: conceptual structure and pattern of somato-visceral response. Psychophysiology. 1980;17:179–192. doi: 10.1111/j.1469-8986.1980.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Levin DN, Miller GA, Kozak MJ. Fear behavior, fear imagery, and the psychophysiology of emotion: the problem of affective response integration. J. Abnorm. Psychol. 1983;92:276–306. doi: 10.1037//0021-843x.92.3.276. [DOI] [PubMed] [Google Scholar]
- Larson EB, Anker JJ, Gliddon LA, Fons KL, Carroll ME. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp. Clin. Psychopharmacol. 2007;15:461–471. doi: 10.1037/1064-1297.15.5.461. [DOI] [PubMed] [Google Scholar]
- Lee DY, Kim J-Y, Kim J-H, Choi D-S, Kim D-K, Koh KK, Yoon B-k. Effects of hormone therapy on ambulatory blood pressure in postmenopausal Korean women. Climacteric. 2011;14:92–99. doi: 10.3109/13697137.2010.491924. [DOI] [PubMed] [Google Scholar]
- Lindheim SR, Legro RS, Morris RS, Wong IL, Tran DQ, Vijod MA, Stanczyk FZ, Lobo RA. The effect of progestins on behavioral stress responses in postmenopausal women. J. Soc. Gynecol. Investig. 1994;1(1):79–83. doi: 10.1177/107155769400100116. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Owens JF, Salomon K, Harris KF, Berga SL. Influence of hormone therapy on the cardiovascular responses to stress of postmenopausal women. Biol. Psychol. 2005;69:39–56. doi: 10.1016/j.biopsycho.2004.11.004. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Carroll KM, Rounsaville BJ. Gender differences in treatment-seeking cocaine abusers – implications for treatment and prognosis. Am. J. Addict. 1999;8:300–311. doi: 10.1080/105504999305703. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Hart CL, Boyarsky B, Kosten T, Jatlow P. Gender effects following repeated administration of cocaine and alcohol in humans. Subst. Use Misuse. 2005;40(4):511–528. doi: 10.1081/ja-200030693. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Kaufman MJ, Levin JM. Cocaine pharmacokinetics in men and in women during the follicular and luteal phases of the menstrual cycle. Neuropsychopharmacology. 1999;21:294–303. doi: 10.1016/S0893-133X(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Mercer D, Carpenter G, Daley D, Patterson C, Volpicelli J. Group Drug Counseling Manual. University of Pennsylvania; 1994. [Google Scholar]
- Miller GA, Levi D, Kozak M, Cook EW, McLean A, Lang PJ. Individual differences in imagery and the psychophysiology of emotion. Cogn. Emot. 1987;1:367–390. [Google Scholar]
- Montoya P, Campos JJ, Schandry R. See red? Turn pale? Unveiling emotions through cardiovascular and hemodynamic changes. Span. J. Psychol. 2005;8:79–85. doi: 10.1017/s1138741600004984. [DOI] [PubMed] [Google Scholar]
- Morris JS, Öhman A, Dolan R. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393:467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Mannelli P, Peindl K, Murray HW, Meier B, Leone FT. Changes in tobacco smoking following treatment for cocaine dependence. Am. J. Drug Alcohol Abuse. 2006;32:135–148. doi: 10.1080/00952990500479209. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Quinones-Jenab V. Why are women from venus and men from mars when they abuse cocaine? Brain Res. 2006;1126:200–203. doi: 10.1016/j.brainres.2006.08.109. [DOI] [PubMed] [Google Scholar]
- Quinones-Jenab V, Jenab S. Progesterone attenuates cocaine-induced responses. Horm. Behav. 2010;58(1):22–32. doi: 10.1016/j.yhbeh.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Reed SC, Evans SE, Bedi G, Rubin E, Foltin R. The effects of oral micorinized progesterone on smoked cocaine self-administration in women. Horm. Behav. 2011;59:227–235. doi: 10.1016/j.yhbeh.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht R. Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology. 2003;28(2):139–168. doi: 10.1016/s0306-4530(02)00064-1. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Sun WL, Minerley AC, Weierstall K, Nazarian A, Festa ED, Niyomchai T, Akhavan A, Jenab S, Quiñones-Jenab V. Progesterone does not affect cocaine-induced conditioned place preference or locomotor activity in male rats. Ethn. Dis. 2010;20(Suppl. 1):S1–73. 77. [PubMed] [Google Scholar]
- Russo SJ, Sun WL, Minerly AC, Weierstall K, Nazarian A, Festa ED, Niyomchai T, Akhavan A, Luine V, Jenab S, Quiñones-Jenab V. Progesterone attenuates cocaine-induced conditioned place preference in female rats. Brain Res. 2008;1189:229–235. doi: 10.1016/j.brainres.2007.10.057. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Overview of findings from the 2006 National Survey on Drug Use and Health [Office of Applied Studies, NHSAA Series H-21; DHHS pub. no. SMA 03-3774] SAMHSA; Rockville, MD: 2007. [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings (Office of Applied Studies, NSDUH Series H-38A, HHS Publication No. SMA 10-4586 Findings) Rockville, MD: 2010. [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of cortocotropin-releasing factor in drug addiction. Pharmocol. Rev. 2001;53:209–243. [PubMed] [Google Scholar]
- Schwartz GE, Weinberger DA, Singer JA. Cardiovascular differentiation of happiness, sadness, anger, and fear following imagery and exercise. Psychosom. Med. 1981;43:343–364. doi: 10.1097/00006842-198108000-00007. [DOI] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict. Biol. 2009;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch. Gen. Psychiatry. 2011;68:942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Paliwal P, Hong KA, Morgan PT, Bergquist KL. Sex steroid hormones, stress response and drug craving in cocaine dependent women: implications for relapse susceptibility. Exp. Clin. Psychopharmacol. 2007;15:445–452. doi: 10.1037/1064-1297.15.5.445. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist KL, Bhagwagar Z, Siedlarz K. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch. Gen. Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R, Lovallo WR, Parsons OA. Cardiovascular differentiation of emotions. Psychosom. Med. 1992;54:422–435. doi: 10.1097/00006842-199207000-00005. [DOI] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. Hypothalamic-pituitary-ACDrenal axis and sympatho-ACDreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Sita A, Miller SB. Estradiol, progesterone and cardiovascular response to stress. Psychoneuroendocrinology. 1996;21:339–346. doi: 10.1016/0306-4530(95)00053-4. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharmacol. Biochem. Behav. 2002;72:431–435. doi: 10.1016/s0091-3057(02)00716-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp. Clin. Psychopharmacol. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Kosten TR. Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacol. Biochem. Behav. 2004;78:699–705. doi: 10.1016/j.pbb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Poling J, Gonzalez G, Gonsai K, Oliveto A, Kosten TR. Progesterone effects on cocaine use in male cocaine users maintained on methadone: a randomized, doubleblind, pilot study. Exp. Clin. Psychopharmacol. 2007;15(5):453–460. doi: 10.1037/1064-1297.15.5.453. [DOI] [PubMed] [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J. Stud. Alcohol. Suppl. 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Svec F. The biopotency of dexamethasone at causing hepatic glucocorticoid receptor down-regulation in the intact mouse. Biochim. Biophys. Acta. 1988;970(1):90–95. doi: 10.1016/0167-4889(88)90226-1. [DOI] [PubMed] [Google Scholar]
- Tersman Z, Collins A, Eneroth P. Cardiovascular responses to psychological and physiological stressors during the menstrual cycle. Psychosom. Med. 1991;53:185–197. doi: 10.1097/00006842-199103000-00008. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of the cocaine craving questionnaire. Drug Alcohol Depend. 1993;34:19–28. doi: 10.1016/0376-8716(93)90042-o. [DOI] [PubMed] [Google Scholar]
- van Wingen GA, van Broekhoven F, Verkes RJ, Petersson KM, Bäckström T, Buitelaar JK, Fernández G. Progesterone selectively increases amygdala reactivity in women. Mol. Psychiatry. 2008;13(3):325–333. doi: 10.1038/sj.mp.4002030. [DOI] [PubMed] [Google Scholar]
- Waldrop AE, Back SE, Brady KT, Upadhyaya HP, McRae AL, Saladin ME. Daily stressor sensitivity, abuse effects, and cocaine use in cocaine dependence. Addict. Behav. 2007;32:3015–3025. doi: 10.1016/j.addbeh.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrop AE, Price KL, Desantis SM, Simpson AN, Back SE, McRae AL, Spratt EG, Kreek MJ, Brady KT. Community-dwelling cocaine-dependent men and women respond differently to social stressors versus cocaine cues. Psychoneuroendocrinology. 2010;35:798–806. doi: 10.1016/j.psyneuen.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei LJ. The adaptive biased-coin design for sequential experiments. Ann. Stat. 1978;6:92–100. [Google Scholar]
- Wei LJ, Lachin JM. Properties of the urn randomization in clinical trials. Control. Clin. Trials. 1988 Dec;9(4):345–364. doi: 10.1016/0197-2456(88)90048-7. 1988. Erratum in: Controlled Clin Trials 10(1):following 126. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Marlatt GA. Emphasis on interpersonal factors in a dynamic model of relapse. Am. Psychol. 2005;60(4):341–342. doi: 10.1037/0003-066X.60.4.341. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J. Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Altemus M, Parkison V, Shastry S. Effects of estrogen antagonists and agonists on the ACTH response to restraint stress in female rats. Neuropsychopharmacology. 2001;25:881–891. doi: 10.1016/S0893-133X(01)00301-3. [DOI] [PubMed] [Google Scholar]
- Zumoff B, Miller L, Levin J, Levit CD, Miller EH, Heinz U, Kalin M, Denman H, Jandorek R, Rosenfeld RS. Follicular-phase serum progesterone levels of nonsmoking women do not differ from the levels of nonsmoking men. Steroids. 1990;55:557–559. doi: 10.1016/0039-128x(90)90052-d. [DOI] [PubMed] [Google Scholar]