Abstract
P2Y6 receptor in bladder smooth muscle responds to UDP by increasing muscle tone and augmenting bladder contractions. The exact cellular location of the receptor is however unknown. Three commercially available antibodies to P2Y6 receptor gave clean bands on Western blot which were eliminated by specific peptide competition. Two of the three also immunostained bladder smooth muscle cells while leaving adjacent interstitial cells of Cajal unstained. However, attempts to validate the specificity of these antibodies by performing the same assays on bladders from P2Y6 knockout mice were unsuccessful. In Western blots, all three antibodies bound similar proteins in both wild type and P2Y6 knockout tissue. Immunostaining of knockout tissue sections also showed no difference in staining patterns or intensity. We conclude that rigorous controls are required when using commercial reagents to this G-protein coupled receptor and perhaps to other members of the P2Y receptor family.
Introduction
P2Y6 is a G-protein coupled purinergic receptor (GPCR) which is activated by UDP, making it unique in the P2Y receptor family. The rat and human orthologs of P2Y6 were cloned in 1995 and 1996 respectively (Chang et al., 1995, Communi et al., 1996) and were shown to have broad tissue distributions by Northern blot and significant expression in smooth muscle. More recently, the creation of knockout mice lacking the receptor, along with the advent of highly specific pharmacological reagents, has allowed investigators to begin to explore its role in various tissues using state of the art tools (Koizumi et al., 2007, Bar et al., 2008, Jacobson et al., 2009).
Our interest lies in the area of purinergic regulation of bladder motility – both the contractile function required to expel urine as well as the mechanisms of smooth muscle relaxation required to accommodate filling. In the normal setting, mechansosensors in the bladder wall signal to afferent sensory neurons and activate a reflex arc involving the pontine micturition center in the brain. Subsequently, parasympathetic nerves innervating the smooth muscle release acetylcholine and ATP to elicit a voiding contraction. The purinergic component in humans is normally considered minor, but in disease settings such as overactive bladder, neurogenic bladder and interstitial cystitis there is significant upregulation of purinergic pathways (Ruggieri, 2006). Using pharmacological approaches we recently showed that P2Y6 plays an important modulating role in bladder smooth muscle (BSM) contraction in mice. In BSM strips exposed to electrical field stimulation, convincing evidence was obtained for UDP release as a neurotransmitter from parasympathetic neurons with subsequent activation of P2Y6 (Yu et al., 2013).
Although several reports including our own, suggested that detrusor strips express P2Y6, the tissue is composed of several cell types in addition to myocytes. These include fibroblasts, myofibroblasts, neurons and several types of interstitial cell (Koh et al., 2012, Yu et al., 2012). Therefore we wished to precisely identify which cells were expressing the receptor. Surprisingly, when we attempted to investigate the expression of P2Y6 using both Western blot and immunofluorescent localization, we found that three commercially available antibodies, all of which had been used for this purpose and published in various other tissues, failed tests of specificity. Indeed, in our hands they failed the ultimate test in that they were unable to discriminate between wild type and P2Y6 knockout bladder tissue. This brief communication is therefore intended to highlight a specific problem we encountered with the specificity of three commercial reagents to P2Y6, but also to add our observations to the growing body of evidence suggesting that GPCR antibodies widely used by the scientific community are often unreliable and require careful validation. As noted previously in this journal, there appears to be a systemic difficulty in creating antibodies for this important class of signaling protein (Michel et al., 2009).
Methods and Reagents
Animals
P2Y6−/− mice were created from a targeting construct in which the coding exon for P2Y6 was deleted (Bar et al., 2008). Bladders were removed from C57Bl/6J mice after euthanization by CO2 inhalation. Bladders from knockout (KO) mice were either fixed in paraformaldehyde (PFA) and frozen in OCT, or homogenized and dissolved in RIPA buffer for shipment on dry ice to BIDMC in Boston. All experiments were performed in accordance with NIH animal use and care guidelines and with BIDMC IACUC approval.
Antibodies
We chose to test three commercially available antibodies based on two criteria. Firstly, on the grounds that they had been specifically referenced and validated to some extent in the published literature and secondly, because they corresponded to three different regions of the molecular target. A relatively popular P2Y6 antibody from Alomone Labs (Catalog # APR-011) is a rabbit polyclonal reactive to mouse and rat tissue in Western blot and IHC (manufacturer’s information) and directed to the intracellular C-terminal segment. The second from Santa Cruz Biotechnology (Catalog # sc-15215) is a goat polyclonal directed against the N-terminus and rated by the manufacturer for Western blot, immunoprecipitation, ELISA and IHC. The third antibody from MBL International Corp. is a rabbit polyclonal directed against the 2nd cytoplasmic intracellular loop of the human P2Y6 receptor (Catalog # MC-2726). This region is conserved in both mouse and human. Our choice of this product was due to the apparent success of this region in generating antibodies which had successfully detected P2Y6 in both human and mouse samples. Jiang et al. produced their own antibody to this region and showed efficacy in Western blots of P2Y6 transfected CHO cells, immunofluorescence on human mast cells and in flow cytometry of primary human mast cells (Jiang et al., 2009). Thus it appeared to have good potential for specificity and efficacy.
Western blot and immunofluorescence
Tissue preparation, fixation, cryosectioning and immunostaining were performed as described in (Kanasaki et al., 2013). Standard fixation is performed with 4% PFA/100 mM sodium cacodylate buffer pH7.4. However, due to the propensity of some antibodies to ‘see’ antigens under different fixation conditions, we also used cold (−20°C) 100% methanol for 5 min for all tested antibodies. Results were similar under either fixation. Co-immunostained sections were incubated with antibodies to ectonucleoside triphosphate diphosphohydrolase 2 (polyclonal sheep antibody from R&D Systems, Minneapolis, MN, Cat# AF5797)and CD34 (monoclonal rat antibody from Abcam, Cambridge, MA, Cat# MEC14.7) to identify interstitial cells (Yu et al., 2012). Nuclei were visualized with Topro-3-iodide (1:1000) and actin with rhodamine-phalloidin (1:50).
Results
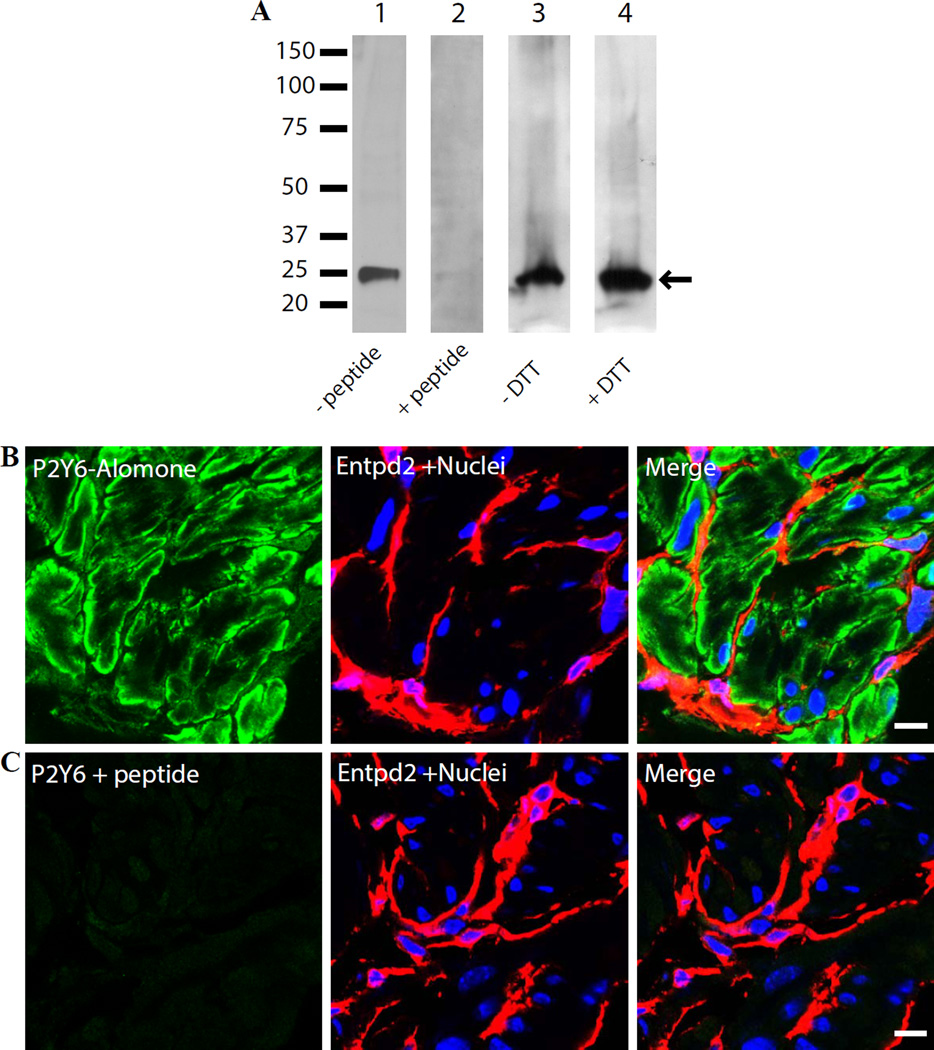
To investigate expression of P2Y6 in detrusor strips from control mice, samples were lysed in 0.5% SDS lysis buffer and cell lysates separated by SDS-PAGE were probed with the Alomone Labs P2Y6 receptor antibody. Western blot analysis revealed a single protein band at a molecular weight of ~25 kDa (Fig. 1A), which is lower than the predicted nominal molecular mass of the protein (~ 37 kDa) and lower than the example shown on the manufacturer’s website which has a band visible at ~100 kDa. In an initial attempt to confirm the specificity of the antibody, we pre-treated it with control peptide, which completely abolished the signal (Fig. 1A). To further investigate the possibility of a higher molecular weight P2Y6 complex in detrusor (e.g. a tetramer could show up at 100 kDa), tissue was lysed by gentle co-immunoprecipitating lysis buffer (no SDS) and probed under reducing (0.1 M dithiothreitol) and non-reducing conditions (without dithiothreitol). Our results showed that only a ~25 kDa protein band could be detected under any of these conditions (Fig. 1A). The antibody robustly identified just a single band in the protein lysate which was competable with cognate peptide. While the lack of coincidence of molecular weights was concerning to us, it has been noted before that GPCRs can migrate to lower molecular weights on SDS-PAGE than the nominal mass would indicate. For example, cell-free expression of GPCRs run on SDS PAGE gel and silver stained, showed four different receptors all running lower than 30 kDa despite their nominal molecular masses being in the range of 36 – 38 kDa (Corin et al., 2011). Furthermore a study of epitope tagged histamine H2 receptors from different species showed a diverse array of bands on Western blots from 27 kDa to high molecular weight, presumed multimers of 150 dKa (Preuss et al., 2007).
Figure 1.
Apparent expression of P2Y6 receptor in BSM using Alomone Labs antibody (APR-011). A). Lysates of mouse detrusor muscle (25 µg protein/lane) were immunoblotted with antibody APR-011. Lanes 1 and 2: P2Y6 antibody preabsorbed with buffer or with immunizing peptide. Lanes 3 and 4: detrusor muscle was lysed with non-denaturing co-immunoprecipitation lysis buffer and resolved under non-reducing (lane3) and reducing (lane 4) conditions by presence or absence of dithiothreitol in sample buffer. B) and C). Cryosections (5µm) of mouse bladders were labeled with antibodies to P2Y6 - green, Entpd2 to label the interstitial cells - red and Topro-3 to label nuclei - blue. Color merged panels are shown on the right. Scale bars = 10 µm.
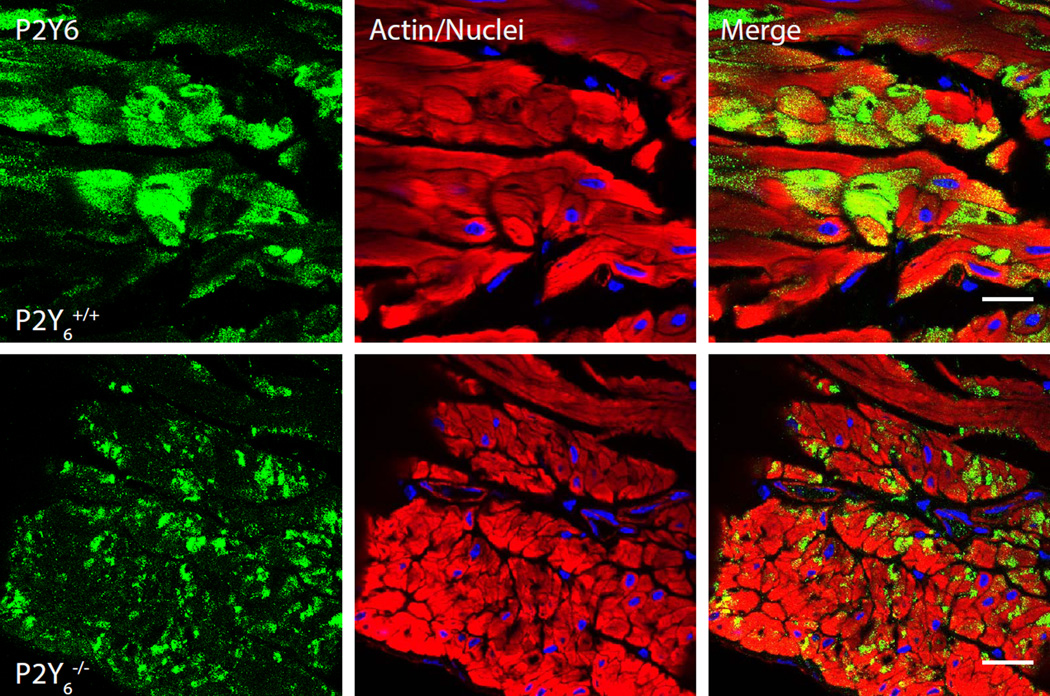
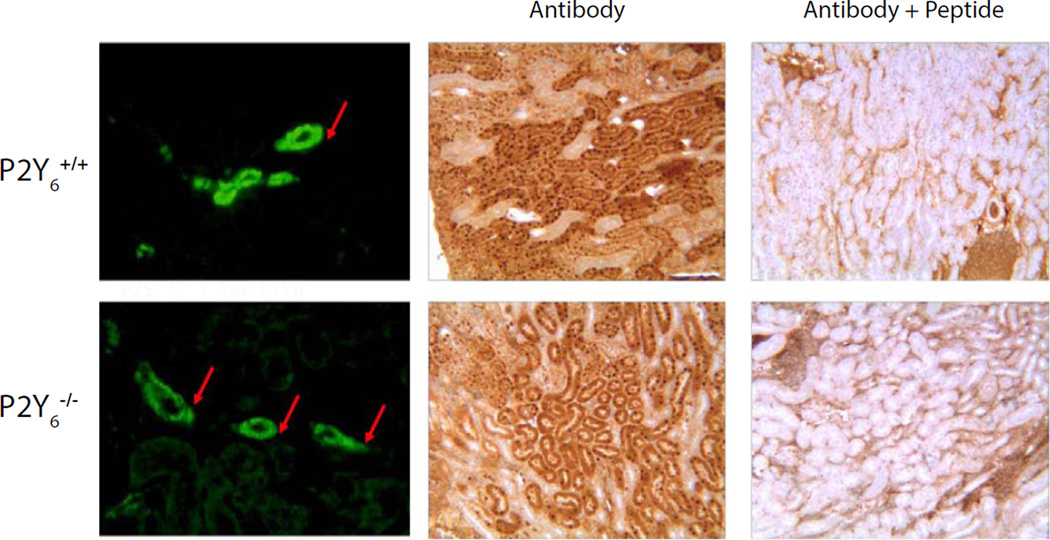
Localization by immunofluorescence staining and confocal imaging on 5 µm cryosections, demonstrated a strong, specific P2Y6 signal in BSM cells and partial staining of lamina propria with positive cells occurring immediately beneath the urothelium (not shown). Staining was completely abrogated by pretreatment of antibody with peptide (Fig. 1B, C). Co-staining of bladder sections with the interstitial cell marker ectonucleoside triphosphate diphosphohydrolase 2 (Entpd2) (Yu et al., 2011b) showed a clear separation between smooth muscle bundles and surrounding interstitial cells of Cajal, encouraging us to believe that P2Y6 was specifically localized to BSM cells. However, when we attempted to show loss of immunostaining in BSM from P2Y6−/− mice, the staining was not different between control mice of the same background and the knockouts (Fig. 2). The green P2Y6 antibody staining in discrete cells within the smooth muscle was identical and since positive cells were found throughout the bladder in the knockout mice, it can only be concluded that the antibody is detecting some other antigen. This lack of specificity was borne out in kidney also with both immunofluorescence and immunoperoxidase staining approaches showing no difference when the Alomone antibody was used on P2Y6 knockout tissue (Fig. 3).
Figure 2.
Immunostaining of bladder cryosections from control (upper panels) and P2Y6−/− bladders (lower panels) using Alomone’s APR-011 antibody. Rhodamine-phalloidin stained smooth muscle actin (red) and Topro-3 identified nuclei (blue). Scale bars = 20 µm.
Figure 3.
Immunostaining of kidney sections from control and knockout mice using APR-011. Left panels show immunofluorescent labeling of tubules (arrows) in both P2Y6 positive and negative tissue. Right panels show peptide competition of the antibody resulting in dramatically diminished binding to kidney sections from both control and KO animals (middle panels).
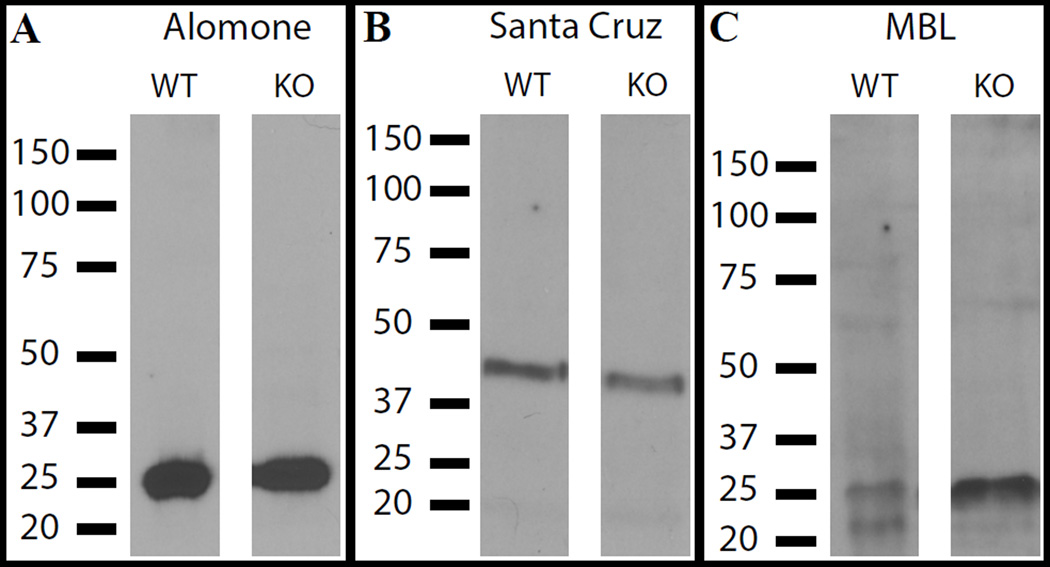
At this point we reevaluated our assumptions and purchased the other two antibodies. We ran Western blots on wild type and P2Y6 KO bladder lysates and immunoprobed with the three different antibodies (Fig. 4). Each detected the same bands in control and in KO tissue. Interestingly, both Alomone and MBL antibodies detected major species at 25 kDa while Santa Cruz detected an epitope closer to the predicted molecular weight, at ~40 kDa. Clearly, none of the antibodies can be considered specific for P2Y6.
Figure 4.
Detection of specific signals by P2Y6 antibodies in Western blots of bladder lysates from both wild type (WT) and P2Y6−/− bladders. Antibodies were used to detect antigen at 1:500 dilution.
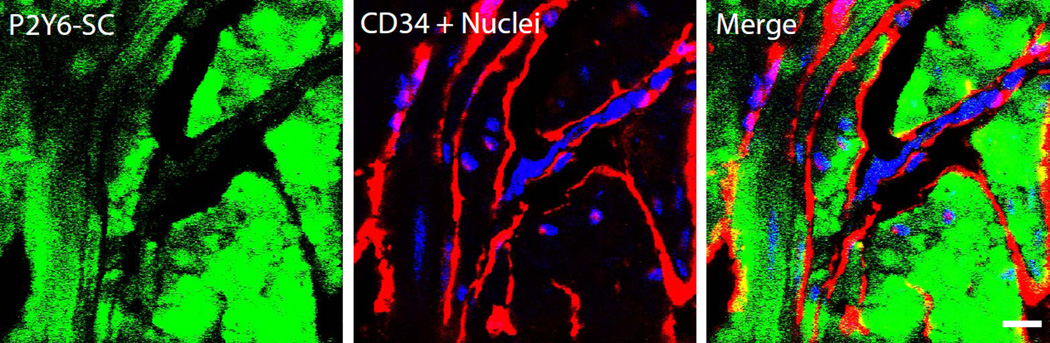
When the Santa Cruz and MBL antibodies were tested for their ability to stain bladder sections, Santa Cruz produced a robust and convincing smooth muscle bundle staining pattern (Fig. 5) which was clearly distinct from interstitial cell marker CD34 (Yu et al., 2012). However, strong staining with this antibody was also seen in KO tissue (not shown). The MBL antibody failed to show a specific signal on tissue sections.
Figure 5.
Apparent expression of P2Y6 receptor in detrusor using the Santa Cruz antibody (sc-15215). Confocal immunofluorescent microscopy of 5 µm bladder cryosections. Sections were costained with antibody to interstitial cell marker CD34 (red). Scale bar = 10 µm.
Discussion
Antibodies in combination with modern imaging techniques and newly developed stable and bright fluorescent reagents have been of enormous benefit in identifying and localizing low abundance proteins in complex tissue. The weak link in these technologies however remains the primary antibodies which are purchased at some expense from commercial suppliers. A common tactic to avoid buying ‘bad’ antibodies is to use the literature to try to identify primary antibodies which have worked for others. For this reason it is concerning to find, as shown here, apparently well validated antibodies which failed when subjected to stringent controls and were unable to discriminate between normal and knockout tissue.
Of the three antibodies we tested, our examination of the literature, indicated that APR-011 from Alomone Labs has been widely used and has been published several times with beautiful images provided in rat retina (Wurm et al., 2009, Brass et al., 2012, Zhang et al., 2012) and duodenum (Mizumori et al., 2009, Brass et al., 2012) and Western blot data provided in cell lines (Lau et al., 2011, Tamaishi et al., 2011). One reason this antibody is so compelling is its ability to discriminate between cell types and produce specific staining patterns within a tissue. This was our experience also in BSM (Figs. 1, 2). However based on its specific staining pattern in tissue containing no P2Y6 we conclude there is another cellular antigen identified by this antibody.
Of the remaining two tested antibodies, the Santa Cruz reagent was used with seemingly good results in mouse skeletal muscle (Chen et al., 2011) and in rat anterior pituitary (Yu et al., 2011a) and for the MBL antibody we could not find specific references of its use, but Jiang et al. demonstrated apparently specific staining with an antibody made to the same epitope (Jiang et al., 2009).
Are there possibly trivial explanations for our results? There have been examples where knockout strategies result in incomplete knockouts which transcribe some portion of the protein coding region, thus making available epitopes for antibodies to ‘see’ (Muller, 1999, Hall et al., 2009). This is unlikely to be the explanation for our data since the P2Y6 knockout was designed around the removal of the entire single coding exon (Bar et al., 2008) and secondly, because the antibodies tested were raised to three entirely different regions of the receptor.
The failure of three commercially available antibodies all of which have been used in published studies on P2Y6, is of concern and reinforces the need for investigators, journals, editors and reviewers to demand appropriately rigorous controls. The gold standard clearly, is to stain similar samples with and without the antigen of interest. This can be accomplished with KO tissue but also, when that option may be unavailable, with heterologous expression of the antigen in cells which lack it (Saper and Sawchenko, 2003). Furthermore the widespread use of the ‘competing peptide control’ as a proof of specificity is actually not a reliable proof of specificity for the holo-protein and perhaps misunderstood by some as to what it indicates (Saper and Sawchenko, 2003).
P2Y6 is a G-protein coupled receptor. Interestingly, there are a growing number of GPCRs which have been described as having poorly validated detection reagents. β(3)-adrenergic receptors have been identified as promising candidates in lower urinary tract for drug interventions which assist with overactivity, urgency and incontinence. However a survey of five commercial antibodies raised against different parts of the protein showed that all were ineffective in Western blot of transfected CHO cells and only two of five appeared to have some degree of specificity in immunocytochemistry (Cernecka et al., 2012). In another study three of four commercial antibodies directed against the GPCR, PAR2, gave non-specific signals to endogenous PAR2 by Western blot (Adams et al., 2012). And an investigation into specificity of histamine H4-receptor antibodies led the authors to conclude that in both Western blot and flow cytometry, three commercial antibodies do not bind to the specified protein, either in heterologous expression systems or in endogenously expressing cells (Beermann et al., 2012). In response to this, Gutzmer et al. disagreed with some of the conclusions since they felt that work from their laboratory using one of the antibodies, had proved its efficacy and that technical differences in the experimental designs were responsible for discrepant results (Gutzmer et al., 2012). While conceding some points, the Beermann group nonetheless strongly defended the primary conclusions (Neumann et al., 2012). This debate emphasizes the difficulty of ‘proving’ that antibodies don’t work but is worth having in an attempt to ‘raise the bar’ overall for what is generally considered satisfactory evidence of specificity.
In conclusion, we feel we can add P2Y6 to the collection of GPCRs which have recently been described as having seriously deficient antibodies. These include dopamine receptors, α and β-adrenergic receptors and muscarinic receptors. As noted in (Michel et al., 2009) the lack of selectivity of GPCR antibodies, appears to be the rule rather than the exception, and antibody based data needs to employ one or more of the rigorous controls described comprehensively in (Seifert et al., 2013).
Acknowledgments
We are indebted to Dr. Bernard Robaye of the Universite Libre de Bruxelles, Gosselies, Belgium for kindly providing tissue from P2Y6 knockout mice. The project described was supported by Grant Number DK083299 from the National Institute of Diabetes and Digestive and Kidney Diseases to WGH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- Adams MN, Pagel CN, Mackie EJ, Hooper JD. Evaluation of antibodies directed against human protease-activated receptor-2. Naunyn Schmiedebergs Arch Pharmacol. 2012;385:861–873. doi: 10.1007/s00210-012-0783-6. [DOI] [PubMed] [Google Scholar]
- Bar I, Guns PJ, Metallo J, Cammarata D, Wilkin F, Boeynams JM, Bult H, Robaye B. Knockout mice reveal a role for P2Y6 receptor in macrophages, endothelial cells, and vascular smooth muscle cells. Mol Pharmacol. 2008;74:777–784. doi: 10.1124/mol.108.046904. [DOI] [PubMed] [Google Scholar]
- Beermann S, Seifert R, Neumann D. Commercially available antibodies against human and murine histamine H(4)-receptor lack specificity. Naunyn Schmiedebergs Arch Pharmacol. 2012;385:125–135. doi: 10.1007/s00210-011-0700-4. [DOI] [PubMed] [Google Scholar]
- Brass D, Grably MR, Bronstein-Sitton N, Gohar O, Meir A. Using antibodies against P2Y and P2X receptors in purinergic signaling research. Purinergic Signal. 2012;8:61–79. doi: 10.1007/s11302-011-9278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernecka H, Ochodnicky P, Lamers WH, Michel MC. Specificity evaluation of antibodies against human beta3-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol. 2012;385:875–882. doi: 10.1007/s00210-012-0767-6. [DOI] [PubMed] [Google Scholar]
- Chang K, Hanaoka K, Kumada M, Takuwa Y. Molecular cloning and functional analysis of a novel P2 nucleotide receptor. J Biol Chem. 1995;270:26152–26158. doi: 10.1074/jbc.270.44.26152. [DOI] [PubMed] [Google Scholar]
- Chen D, Wang W, Guo W, Yu Q, Burnstock G, He C, Xiang Z, Zheng H. Expression of P2Y(6) receptors in the developing mouse skeletal muscle and after injury and repair. J Anat. 2011;218:643–651. doi: 10.1111/j.1469-7580.2011.01364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Communi D, Parmentier M, Boeynaems JM. Cloning, functional expression and tissue distribution of the human P2Y6 receptor. Biochem Biophys Res Commun. 1996;222:303–308. doi: 10.1006/bbrc.1996.0739. [DOI] [PubMed] [Google Scholar]
- Corin K, Baaske P, Ravel DB, Song J, Brown E, Wang X, Geissler S, Wienken CJ, Jerabek-Willemsen M, Duhr S, Braun D, Zhang S. A robust and rapid method of producing soluble, stable, and functional G-protein coupled receptors. PLoS One. 2011;6:e23036. doi: 10.1371/journal.pone.0023036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzmer R, Werfel T, Baumer W, Kietzmann M, Chazot PL, Leurs R. Well characterized antihistamine 4 receptor antibodies contribute to current knowledge of the expression and biology of the human and murine histamine 4 receptor. Naunyn Schmiedebergs Arch Pharmacol. 2012;385:853–854. doi: 10.1007/s00210-012-0744-0. author reply 855–860. [DOI] [PubMed] [Google Scholar]
- Hall B, Limaye A, Kulkarni AB. Overview: generation of gene knockout mice. Curr Protoc Cell Biol. 2009;Chapter 19(Unit 19 12 19 12):11–17. doi: 10.1002/0471143030.cb1912s44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Ivanov AA, de Castro S, Harden TK, Ko H. Development of selective agonists and antagonists of P2Y receptors. Purinergic Signal. 2009;5:75–89. doi: 10.1007/s11302-008-9106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Borrelli L, Bacskai BJ, Kanaoka Y, Boyce JA. P2Y6 receptors require an intact cysteinyl leukotriene synthetic and signaling system to induce survival and activation of mast cells. J Immunol. 2009;182:1129–1137. doi: 10.4049/jimmunol.182.2.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanasaki K, Yu W, von Bodungen M, Larigakis JD, Kanasaki M, Ayala de la Pena F, Kalluri R, Hill WG. Loss of beta1-integrin from urothelium results in overactive bladder and incontinence in mice: a mechanosensory rather than structural phenotype. FASEB J. 2013 doi: 10.1096/fj.12-223404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh BH, Roy R, Hollywood MA, Thornbury KD, McHale NG, Sergeant GP, Hatton WJ, Ward SM, Sanders KM, Koh SD. Platelet-derived growth factor receptor-alpha cells in mouse urinary bladder: a new class of interstitial cells. J Cell Mol Med. 2012;16:691–700. doi: 10.1111/j.1582-4934.2011.01506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 2007;446:1091–1095. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau WK, Chow AW, Au SC, Ko WH. Differential inhibitory effects of CysLT(1) receptor antagonists on P2Y(6) receptor-mediated signaling and ion transport in human bronchial epithelia. PLoS One. 2011;6:e22363. doi: 10.1371/journal.pone.0022363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel MC, Wieland T, Tsujimoto G. How reliable are G-protein-coupled receptor antibodies? Naunyn Schmiedebergs Arch Pharmacol. 2009;379:385–388. doi: 10.1007/s00210-009-0395-y. [DOI] [PubMed] [Google Scholar]
- Mizumori M, Ham M, Guth PH, Engel E, Kaunitz JD, Akiba Y. Intestinal alkaline phosphatase regulates protective surface microclimate pH in rat duodenum. J Physiol. 2009;587:3651–3663. doi: 10.1113/jphysiol.2009.172270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U. Ten years of gene targeting: targeted mouse mutants, from vector design to phenotype analysis. Mech Dev. 1999;82:3–21. doi: 10.1016/s0925-4773(99)00021-0. [DOI] [PubMed] [Google Scholar]
- Neumann D, Beermann S, Mägel L, Jonigk D, Weber-Steffens D, Männel D, Seifert R. Problems associated with the use of commercial and non-commercial antibodies against the histamine H4 receptor. Naunyn-Schmiedeberg's Archives of Pharmacology. 2012;385:855–860. [Google Scholar]
- Preuss H, Ghorai P, Kraus A, Dove S, Buschauer A, Seifert R. Constitutive activity and ligand selectivity of human, guinea pig, rat, and canine histamine H2 receptors. J Pharmacol Exp Ther. 2007;321:983–995. doi: 10.1124/jpet.107.120014. [DOI] [PubMed] [Google Scholar]
- Ruggieri MR., Sr Mechanisms of disease: role of purinergic signaling in the pathophysiology of bladder dysfunction. Nat Clin Pract Urol. 2006;3:206–215. doi: 10.1038/ncpuro0456. [DOI] [PubMed] [Google Scholar]
- Saper CB, Sawchenko PE. Magic peptides, magic antibodies: guidelines for appropriate controls for immunohistochemistry. J Comp Neurol. 2003;465:161–163. doi: 10.1002/cne.10858. [DOI] [PubMed] [Google Scholar]
- Seifert R, Strasser A, Schneider EH, Neumann D, Dove S, Buschauer A. Molecular and cellular analysis of human histamine receptor subtypes. Trends Pharmacol Sci. 2013;34:33–58. doi: 10.1016/j.tips.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaishi N, Tsukimoto M, Kitami A, Kojima S. P2Y6 receptors and ADAM17 mediate low-dose gamma-ray-induced focus formation (activation) of EGF receptor. Radiat Res. 2011;175:193–200. doi: 10.1667/rr2191.1. [DOI] [PubMed] [Google Scholar]
- Wurm A, Erdmann I, Bringmann A, Reichenbach A, Pannicke T. Expression and function of P2Y receptors on Muller cells of the postnatal rat retina. Glia. 2009;57:1680–1690. doi: 10.1002/glia.20883. [DOI] [PubMed] [Google Scholar]
- Yu Q, Guo W, Song X, Liu X, Xiang Z, He C, Burnstock G. Expression of P2Y receptors in the rat anterior pituitary. Purinergic Signal. 2011a;7:207–219. doi: 10.1007/s11302-011-9236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Robson SC, Hill WG. Expression and distribution of ectonucleotidases in mouse urinary bladder. PLoS One. 2011b;6:e18704. doi: 10.1371/journal.pone.0018704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Sun X, Robson SC, Hill WG. Extracellular UDP enhances P2X-mediated bladder smooth muscle contractility via P2Y6 activation of the phospholipase C/inositol trisphosphate pathway. FASEB J. 2013 doi: 10.1096/fj.12-219006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Zeidel ML, Hill WG. Cellular expression profile for interstitial cells of cajal in bladder - a cell often misidentified as myocyte or myofibroblast. PLoS One. 2012;7:e48897. doi: 10.1371/journal.pone.0048897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang PP, Yang XL, Zhong YM. Cellular localization of P2Y(6) receptor in rat retina. Neuroscience. 2012;220:62–69. doi: 10.1016/j.neuroscience.2012.06.032. [DOI] [PubMed] [Google Scholar]