Abstract
Although it is generally accepted that the medial temporal lobe (MTL) is critical for episodic memory, the contributions of cortical regions in the MTL, such as the perirhinal (PRc) and parahippocampal (PHc) cortices, remain unresolved. Recent studies have asserted that the PRc supports the processing of object and face information, whereas the PHc supports the processing of scene information. These findings have been used to characterize the PRc and PHc as being important for the memory of objects and scenes, respectively. However, these results are also consistent with the idea that these MTL regions are critical for the memory of stimuli that are processed as either items or contexts. It has been difficult to differentiate between these two accounts given that in most studies, item and context are operationalized as different types of memoranda (e.g., memory for objects compared to memory for background scenes). Here, we tested the extent to which different MTL regions are involved in the retrieval of item or context information when the material type is held constant. Participants encoded pairs of fractal images and were oriented to encode one fractal as an item and the other as a context. At test, they were cued with previously studied item or context fractals and asked to retrieve the corresponding associate. Results indicated that on test trials, PRc activity was increased during recall of fractals that were encoded as items, whereas PHc activity was greater during recall of fractals that were encoded as contexts. These results provide direct evidence that, even when stimulus type is held constant, the PRc and PHc are preferentially involved in supporting memory for item and context information, respectively.
Keywords: perirhinal cortex, parahippocampal cortex, episodic memory, context, cued recall, medial temporal lobes
Episodic memory reflects the ability to remember that an object or item was previously presented in a specific temporal or spatial context, such as remembering when or where a person was earlier encountered. Considerable evidence indicates that the hippocampus plays a critical role in episodic memory (for reviews, see Diana, Yonelinas, & Ranganath, 2007; Eichenbaum, Yonelinas, & Ranganath, 2007). However, controversy persists over the roles of cortical areas (i.e., the perirhinal [PRc] and parahippocampal [PHc] cortices) in the medial temporal lobes (MTL). Convergent results from studies of tract tracing in animals (Burwell & Amaral, 1998; Suzuki & Amaral, 1994; Deacon, Eichenbaum, Rosenberg, & Eckmann, 1983; Furtak, Wei, Agster, & Burwell, 2007) and functional connectivity in humans (Libby, Ekstrom, Ragland, & Ranganath, 2012) indicate that the PRc primarily receives higher-order sensory input from several association cortices involved in object identification, whereas the PHc has greater reciprocal connections with the retrosplenial cortex – a region important for spatial memory. Consistent with these findings, there is accumulating evidence to suggest that the relative involvement of these two regions in memory processes may depend on the category of material that is to be remembered (for reviews, see Graham, Barense, & Lee, 2010; Manns & Eichenbaum, 2006). That is, the PRc appears to be preferentially engaged in processing object and face information, whereas the PHc appears to be preferentially engaged in processing visual scene information (Awipi & Davachi, 2008; Lee, Scahill, & Graham, 2008; Liang, Wagner, & Preston, 2013; Litman, Awipi, & Davachi, 2009; Pihlajamaki et al., 2004; Staresina, Duncan, & Davachi, 2011; but see Buffalo, Bellgowan, & Martin, 2006; Preston et al., 2010; Pustina, Gizewski, Forsting, Daum, & Suchan, 2012).
An alternative account for these results is provided by the Binding of Items and Contexts (BIC) theory, which proposes that the PRc and PHc are differentially involved in the processing of item and context information, respectively (Diana et al., 2007; Eichenbaum et al., 2007; Ranganath, 2010; also see Davachi, 2006; Eacott & Gaffan, 2005; Eichenbaum, Sauvage, Fortin, Komorowski, & Lipton, 2012; Montaldi & Mayes, 2010; Ranganath & Ritchey, 2012). Studies linking the PRc and PHc to memory for objects and scenes are compatible with this view, as objects are essentially items, and scenes are likely to be processed as spatial contexts in which items may appear. However, a key difference between the two views is that the BIC theory proposes that there are other variables that may determine whether a stimulus is encoded as an item or as a context. For instance, according to the BIC and related theories, contexts are typically stable across time, extend across space, are not task relevant, and presented in the background, whereas items are, by comparison, transient, focal, task relevant, and foregrounded (Fuhs & Touretzky, 2007; Ranganath, 2010; Yeh & Barsalou, 2006).
Unfortunately, it has been difficult to adjudicate between the two views described above because most previous studies have operationalized item and context using different types of memoranda. For example, item memory is typically assessed as memory for words, objects, or faces, whereas context memory is typically measured as memory for the background scene, the background color, or the orientation task conducted by the participant at encoding (Ranganath, 2010). In these studies, it has been unclear whether the functional dissociations between the PRc and PHc were driven by differences in item and context processing or simply because of stimulus differences (e.g., objects vs. scenes, or words vs. encoding tasks). A better test for the BIC model would be to experimentally manipulate the extent to which a stimulus is processed as an item or as a context, while holding the material type constant.
The current experiment addressed this question by examining memory for meaningless visual fractal stimuli pairs that could be treated as either items or background contexts. We oriented participants to process one fractal in each pair as an item and the other as a context and examined memory for the arbitrary associations between these pairs of fractal stimuli. As schematically depicted in Figure 1a, each context fractal was shown in a relatively large size and remained on the screen in the background across four consecutive trials. In contrast, each item fractal was transiently presented in the foreground, and these items were the focus of the orienting task. Participants were then scanned during a cued-recall test in which they were presented with a fractal and asked to recall paired fractals that had been studied either as items or as contexts. We predicted that if the PRc supports the retrieval of item information then PRc activation should be greater when participants recall fractals that had been encoded as items. Conversely, if the PHc supports the retrieval of context information then PHc activation should be increased when participants recall fractals that had been studied as contexts. Alternatively, if the two regions support similar episodic memory processes, then we would expect that activity in both areas should be sensitive to the retrieval of both item and context fractals.
Figure 1.
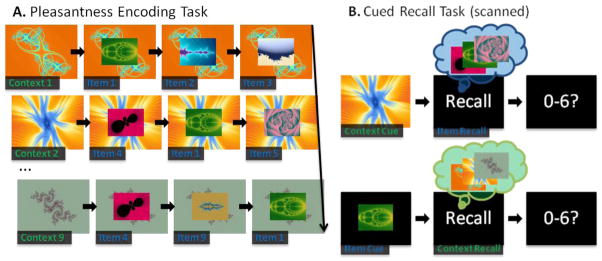
Schematic depiction of the experimental procedure. (A) A representative trial sequence for an encoding block. Each context fractal was paired with three different item fractals and each item fractal was paired with three different context fractals. During each encoding trial, participants made pleasantness judgments on the foreground fractal. (B) Examples of item and context recall trials. Following an item or context fractal cue, participants covertly recalled the associated fractals for the given cue and then reported the strength of their recall using a seven-point response scale.
Methods
Participants
A total of 17 right handed healthy young adults (age M = 22.53, SD = 3.64; M education = 15.47, SD = 2.27; 10 female) were recruited from the University of California, Davis and surrounding communities. One participant was excluded due to equipment malfunction. All participants were paid for their participation and all procedures were approved by the University of California, Davis human participants review board.
Materials
A total of 216 fractals were generated, and for each participant, they were randomly assigned to one of four conditions such that 81 served as contexts, 81 served as items, 27 served as new context lures, and 27 served as new item lures. An additional set of 24 fractals were used in the practice phase. Context fractals were presented at a visual angle of approximately 8.60° x 6.45° and item fractals were presented at a visual angle of approximately 4.30° x 3.22°.
Design and Procedure
Following consent and a practice phase, participants completed nine study-test blocks in the MR scanner; fMRI data were collected only during the test phases. Each study block consisted of nine context fractals and nine item fractals presented in a series of nine trial sequences (Fig. 1a). Within each sequence, a context fractal was first presented for 5,000 ms to familiarize participants with the fractal, and then a series of three different item fractals were successively superimposed over the context. Each item fractal was shown for 4,000 ms, with a 1,000 ms ISI following each item, wherein the context remained on the screen. To ensure adequate processing of each stimulus, participants were instructed to make a pleasantness judgment on a six point scale for the context fractal at the onset of the trial sequence and each of the three superimposed items. Each item was presented in three different sequences, and thus was paired with three different contexts. This allowed us to equate both the familiarity of and the number of associates for each item and context fractal. The order of presentation and pairings of fractals was randomized across participants.
Immediately following each study block, participants were presented with a test block that consisted of 24 cued-recall trials. In each trial, participants were cued with either an item or a context fractal for 1,500 ms. Following this, the word “Recall” was presented on the screen for 5,500 ms; participants were instructed to covertly recall the three fractals associated with the cue during this time. After the covert recall, participants were given 1,500 ms to rate, on a seven point scale, the strength of recall for the associated fractals during that trial (Fig. 1b). A “0” response was to be given for a cue that was not studied, whereas responses between “1” and “6” served as a scale from very weak recall to very strong recall. Participants were instructed to make these judgments based on both the number of targets recalled and the vividness of their recall. There was a variable 1,500–9,500 ms (M= 5,000 ms) fixation ISI between trials. Of the 24 trials in each test block, there were nine context, nine item, three context lure, and three item lure cued recall trials, and the presentation of these fractal cues was randomized across participants.
fMRI Acquisition and Analysis
Imaging data was collected on a 3T Siemens Trio scanner. Functional images were acquired with a gradient echoplanar imaging (EPI) sequence (repetition time = 2,000 ms, echo time = 25 ms, field of view = 220 mm, flip angle = 90°; matrix size = 64 x 64). Each test block consisted of 164 volumes, and each volume contained 34 axial slices, with a voxel size of 3.4375 x 3.4375 x 3.4 mm. Additionally, high resolution (1 x 1 x 1 mm) T1 coplanar images (MPRAGE) were also acquired for each participant. The EPI data were preprocessed with Statistical Parametric Mapping 8 (SPM8) software (Wellcome Trust Centre for Neuroimaging). After the first four scans of each test block were discarded, the data were slice-time corrected using sinc interpolation, realigned using a six-parameter, rigid-body transformation, spatially normalized to the MNI template, resliced into 3 mm isotropic voxels, and spatially smoothed with an isotropic 8 mm full-width at half-maximum Gaussian filter. Statistical analyses were performed in SPM8 using the general linear model (GLM). A high-pass filter and grand mean scaling were applied to the data. The nine test blocks were concatenated and session constant regressors modeling each block were included as nuisance factors along with an intercept. Trials were modeled with a first-level fixed-effects analysis using a canonical hemodynamic response function with the fractal cues serving as the event onsets. In a second-level random-effects analysis, a one-sample t-test contrast of recall trials, collapsed across recall type (item and context) and strength (high and medium), greater than baseline was examined to identify significant clusters in the PRc and PHc, from which parameter estimates for each participant were extracted and entered into a three-way within-subjects ANOVA with recall type (item and context), recall strength (high and medium), and region (PRc and PHc) as factors, using the SPSS software package. Additionally, targeted contrast images of high (“6” and “5”) greater than medium (“4” and “3”) recall were modeled separately for item and context recall in a one-sample t-test in a second-level random-effects analysis.
For both analyses, the family-wise error (FWE) rate was corrected for multiple comparisons to p < .05 using Monte Carlo simulations with the 3dClustSim program in the AFNI software package (http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html). Significant clusters within separate PRc and PHc masks were identified using a voxel-wise threshold of p < .005 and a cluster size of seven contiguous voxels. To construct the masks, the PRc and PHc were first manually traced on each participant’s normalized MPRAGE based on anatomical criteria outlined in Duvernoy &Bourgouin, (1998), Insausti et al. (1998), and Zeineh et al. (2001). The respective masks were then created from the union of every participant’s traced PRc or PHc. All reported p-values in the behavioral analyses are two-tailed and unless otherwise noted, all reported p-values in the fMRI analyses are one-tailed.
Results
Behavioral Results
The cued recall scores for the item and context conditions are presented in Figure 2. The figure shows that participants were able to distinguish between old and new fractals, in the sense that the majority of new fractal trials elicited “0” (i.e., unstudied) ratings, whereas the majority of studied fractals elicited high or medium strength recall ratings for both item and context recall conditions. Consistent with this impression, the proportion of high (“6” and “5”) and medium (“4” and “3”) strength ratings was significantly greater for studied fractals than for new fractals (high context: t[15] = 12.10; medium context: t[15] = 7.50; high item: t[15] = 9.50; medium item: t[15] = 6.95; ps < .0001), whereas no significant differences were observed in the proportion of low (“2” and “1”) strength recall ratings between old and new trials (low context: t[15] = 1.39; low item: t[15] = .19; ps > .18). In addition, recall rates were matched across the critical item and context retrieval conditions where participants could distinguish between old and new fractals. That is, memory discriminability, as measured by the difference between correct and false recall, did not significantly differ between the item and context recall trials either for high (t[15] = .71) or medium (t[15] = 1.51, p = .15) strength ratings.
Figure 2.
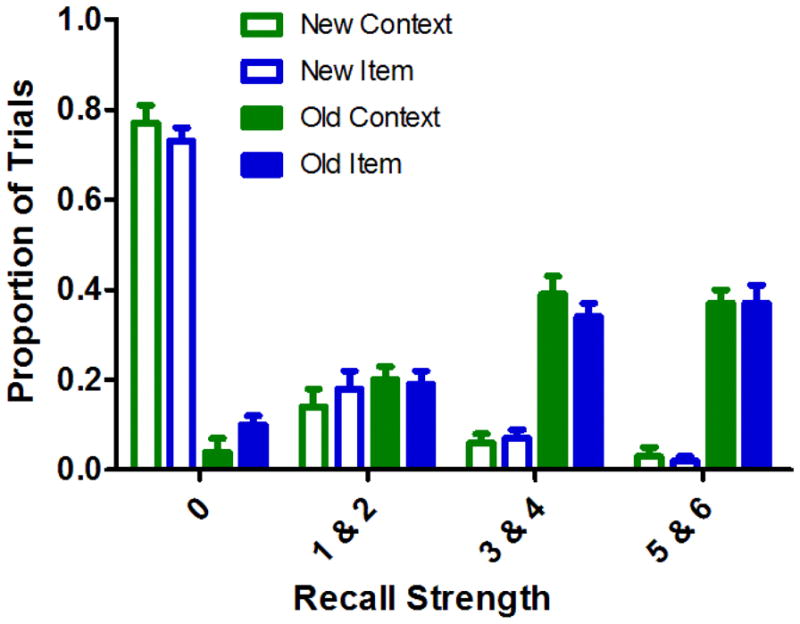
Cued-recall ratings for studied and unstudied cues in the item and context retrieval conditions. Error bars depict the standard error of the mean.
fMRI Results
Targeted analyses focused on medium and high item and context recall trials given that in these conditions, recall discriminability was matched with negligible rates of false recall. In addition, there were too few low confidence responses in many of the subjects to include those trials (e.g., 6 of the 16 subjects had cells with fewer than 10 low confidence trials). In order to identify regions in the PRc and PHc involved in recall, we first contrasted task-related recall activity, collapsed across recall type (item and context) and recall strength (high and medium), greater than baseline within PRc and PHc masks. This contrast revealed one cluster in the right PRc (Montreal Neurological Institute [MNI] coordinates: x = 30, y = −4, z = −41 mm; t[15] = 3.68), and another in the right PHc (MNI: x = 30, y =−25, z = −29 mm; t[15] = 3.71). For each cluster, we then extracted the mean parameter estimates from each participant in order to assess whether the effect of item and context recall differentially influenced these two MTL regions. A three-way within-subjects ANOVA (recall type x recall strength x region) of these parameter estimates revealed a significant three-way interaction (F[1,15] = 7.10, p < .05). Figure 3 shows the pattern of activity in the PRc and PHc regions that led to this three-way interaction. The PHc exhibited increased activation during high relative to medium context recall (t[15] = 1.80, p < .05; Fig. 3a), with no significant difference between high and medium item recall (t[15] = .34). Conversely, the PRc showed a trend for more activity during high relative to medium item recall (t[15] = 1.63, p = .06; Fig. 3b), whereas high and medium context recall were not significantly different (t[15] = .51).
Figure 3.
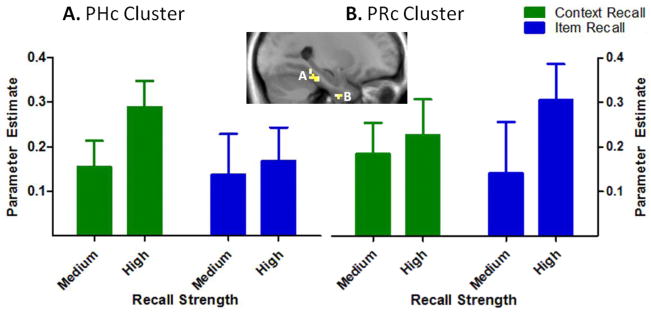
Differential recruitment of the perirhinal (PRc) and parahippocampal (PHc) cortices during item and context recall. Mean parameter estimates, indexing activation in (A) a region of right PHc and (B) right PRc that exhibited greater activation during recall trials relative to baseline. Error bars depict the standard error of the mean.
To more fully characterize responsiveness of the PRc and PHc to item and context recall, we also ran voxel-based analyses, contrasting activity within PRc and PHc masks between high and medium recall trials, separately for item and context recall conditions. Consistent with the three-way interaction, activity in a region in right PHc was greater for high context recall trials relative to medium context recall trials (MNI: x = 27, y = −46, z = −17 mm; t[15] = 3.36; Fig. 4a), and activity in a region in left PRc1 was greater on high item recall trials relative to medium item recall trials (MNI: x = −21, y = −7, z = −32 mm; t[15] = 3.66; Fig. 4b). Analyses of successful context recall revealed no suprathreshold voxels in PRc, and analyses of successful item recall revealed no suprathreshold voxels in PHc.
Figure 4.
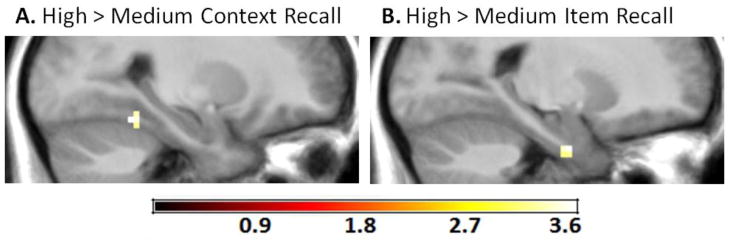
Retrieval strength effects for context recall and for item recall. (A) A cluster in the right PHc exhibited greater activity during high relative to medium context recall. (B) A cluster in the left PRc exhibited greater activity during high relative to medium item recall.
Discussion
The current study tested the hypothesis that subregions within the MTL are differentially sensitive to item and context processing. That is, we expected that PRc activity should be related to recall of item information, whereas PHc activity should be related to recall of context information. We found that activity in both the PRc and the PHc was related to increases in recall strength, and these effects were determined entirely by whether the retrieved information had been encoded as an item or as a context. As expected, the PRc was sensitive to successful item recall, whereas the PHc was sensitive to successful context recall.
Prior studies have reported category-specific dissociations between the PRc and the PHc, with the PRc being sensitive to objects and faces, and the PHc being sensitive to scenes (Awipi & Davachi, 2008; Lee et al., 2008; Liang et al., 2013; Litman et al., 2009; Pihlajamaki et al., 2004; Staresina et al., 2011; but see Buffalo et al., 2006; Preston et al., 2010; Pustina et al., 2012). However, given the item properties (e.g., foregrounded) of objects and faces, and the contextual properties (e.g., backgrounded) of scenes, these dissociations could also be interpreted as item and context processing in the PRc and PHc, respectively. For example, Staresina and colleagues (2011) recently reported that PRc and posterior PHc activity at encoding was related to the subsequent recollection of adjectives that were studied with objects or scenes, respectively. An alternative explanation, however, is that the encoding task (i.e., imagine the object or scene described by the adjective) biased participants to create mnemonic representations that were either items (i.e., a single focal object) or contexts (i.e., a spatial environment). Other studies have shown that the PRc is sensitive to item memory (words or objects) and that the PHc is sensitive to source (i.e., experimental contexts such as the orienting task) memory (Awipi & Davachi, 2008; Diana, Yonelinas, & Ranganath, 2010; 2012; Gaffan, Healey, Eacott, 2004; Norman & Eacott, 2005; for reviews see Diana et al., 2007; Ranganath & Ritchey, 2012), another effect that could reflect differences in the relative roles of the two regions in memory for item and context information.
In addition to differentiating the roles of PRc and PHc in item and context processing, the current results add to support the idea that PRc activity is not simply related to item familiarity, but that it can also support the recollection of item details (Diana et al., 2010; Hannula, Libby, Yonelinas, & Ranganath, 2013; Staresina & Davachi, 2006; 2008; 2010). Our results also accord with previous studies demonstrating that PHC activity is sensitive to memory tests wherein objects (i.e., items) are used to cue the retrieval of an associated scene (i.e., context) (Hannula, Libby, Yonelinas, & Ranganath, 2013; Staresina, Henson, Kriegeskorte, & Alink, 2012). Additionally, PHc activity is also sensitive to non-spatial contextual features, such as items that contain strong associations with particular situational (Bar & Aminoff, 2003; Bar, Aminoff, Ishai, 2008; Bar, Aminoff, & Schacter, 2008), temporal (Jenkins & Ranganath, 2010; Tubridy & Davachi, 2011), categorical (Diana, Yonelinas, & Ranganath, 2008), and semantic (Diana et al., 2012) contexts.
In the current study, rather than using different stimulus categories that have preexisting item or context features, we manipulated the way each stimulus was presented in order to encourage the formation of separate item and context representations. Context fractals were presented in the background and larger, and they were temporally contiguous across several trials, whereas item fractals were presented in the foreground and smaller, they were task-relevant, and they changed across consecutive trials. An interesting question for future research will be to determine which specific characteristics of item and context processing are most critical in driving the observed differences in PRc/PHc activity. For example, the item fractals in the current study were transiently presented in the foreground and were the focus of the orienting task, whereas the context fractals were shown in a relatively large size and remained on the screen in the background across three consecutive trials. How each of these factors contributes to the overall context effect, however, is currently unknown.
One recent study suggested that the PHc is sensitive to both the real-world and retinal size of objects, with larger objects yielding more activation than smaller objects (Konkle & Oliva, 2012). A PHc preference for larger stimuli can’t explain the current crossover effects we observed between PRc and PHc, but overall size may be one factor that determines whether stimuli are processed as items or contexts. We currently favor the idea that all of the characteristics manipulated here (e.g., the focus of the orienting task, stimulus size, foreground/background, transient/continuous presentation) are important in determining whether a stimulus is treated as an item or a context. However, even if only some of these variables (e.g., size) matter, it would not necessarily support category specificity accounts (e.g., Graham, Barense, & Lee, 2010; Manns & Eichenbaum, 2006), as none of those models, to our knowledge, clearly specifies the critical stimulus characteristics that differentiate representation of objects and scenes within the PRc and PHc. Thus, future work should seek to elucidate which of these factors were most important in determining the perception of item and context. It is likely that, just as multiple, possibly interacting factors determine the segregation of object and spatial properties within a visual scene (e.g., Nasr & Tootell, 2012; Oliva & Torralba, 2007; Levy, Hasson, Avidan, Hendler, & Malach, 2001; Malach, Levy, & Hasson, 2002), the factors manipulated here might interact to determine the perception of item and context. Thus, it might be overly simplistic to assume that one can reduce the perception of item or context features to one or two variables. It might seem surprising that we did not observe significant activations in the hippocampus in relation to item and context recall. Direct comparisons of the conditions of interest did not yield any significant hippocampal clusters, although this null result should be interpreted with caution. That is, it is possible that participants were recollecting noncriterial details during item and context recall trials (Yonelinas & Jacoby, 1996). In the current paradigm, medium strength recall trials, by definition, indicate that task-relevant qualitative information was recollected (i.e., the fractals paired with the cue) (Mandler, 1980). To the extent that participants also recollected noncriterial information (e.g., the pleasantness ratings given to the task-relevant fractals) during medium strength trials, it might have obscured our ability to detect hippocampal involvement when comparing high and medium strength recall trials.
Another potential criticism of our findings is that they are contingent on a subjective measure of recall strength, and thus may not accurately capture processes engaged by item and context retrieval. We are confident that this is not the case, given that participants were highly effective in rejecting new cues as unstudied, whereas the majority of old cues elicited ratings of medium or high strength recall. The distribution of these subjective judgments of recall strength indicated that participants were able to accurately discriminate between old and new fractal cues. Additionally, subjective measures of recognition, such as the remember/know method, have been used in many previous studies to discriminate between MTL subregions contributing to recollection and familiarity, respectively (for review, see Diana et al., 2007), and ratings of subjective recollective strength have been previously linked with increased source memory accuracy and increased MTL activation (Hannula, Libby, Yonelinas, & Ranganath, 2013; Vilberg & Rugg, 2007; 2009; Yu, Johnson, & Rugg, 2012). Finally, it is worth mentioning that even if one chooses to dismiss the validity of participants subjective judgments of recall strength, any alternate explanation for the present results must account for why PRc activity was preferentially related to subjective ratings of item recall strength, whereas PHc activity was preferentially related to subjective ratings of context recall strength. Any such account would most likely lead to the conclusion that these two MTL regions are differentially involved in the retrieval of item and context information.
Taken together, these results demonstrate the surprising result that, by manipulating the perception of item and context information during learning, one can observe different patterns of MTL activation during associative retrieval of these items and contexts, even when controlling for both the type and the familiarity of the stimuli. As predicted, the recall of fractals that were initially processed as items was associated with PRc activity, whereas the recall of fractals that were initially processed as contexts was associated with PHc activity. Accordingly, this finding supports the view that MTL involvement in memory retrieval is sensitive to experimental manipulations that can affect what is experienced and encoded as item or context. By fully considering these experimental factors, one can better understand the role of the PRc and PHc in memory and perception beyond category-specific dissociations that may reflect the underlying item and context properties inherent in certain types of stimuli.
Research Highlights.
Fractal pairs were encoded with one as an item and the other as a context.
PRc activity was greater during recall of fractals encoded as items.
PHc activity was greater during recall of fractals encoded as contexts.
Indicates that PRc and PHc are involved in item and context memory, respectively.
Results cannot be explained by category-specificity; stimulus type was held constant.
Acknowledgments
This study was supported by grants R01 MH083734 and F31 MH096346 from the NIMH.
Footnotes
Note that the task>baseline analysis revealed significant right PRc activity whereas the voxel-based analysis revealed significant left PRc activity. These are independent analyses, with the former being sensitive to recall-related activity and the latter being sensitive to the difference in activity between conditions, and this apparent hemispheric difference is likely due to a statistical thresholding effect. (e.g., lowering the threshold in the voxel-based analysis revealed a cluster in the right PRc overlapping with the task>baseline analysis).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Awipi T, Davachi L. Content-specific source encoding in the human medial temporal lobe. Journal of Experimental Psychology: Learning, Memory and Cognition. 2008;34(4):769–779. doi: 10.1037/0278-7393.34.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38(2):347–358. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- Bar M, Aminoff E, Ishai A. Famous faces activate contextual associations in the parahippocampal cortex. Cerebral Cortex. 2008;18(6):1233–1238. doi: 10.1093/cercor/bhm170. [DOI] [PubMed] [Google Scholar]
- Bar M, Aminoff E, Schacter DL. Scenes unseen: the parahippocampal cortex intrinsically subserves contextual associations, not scenes or places per se. Journal of Neuroscience. 2008;28(34):8539–8544. doi: 10.1523/JNEUROSCI.0987-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalo EA, Bellgowan PS, Martin A. Distinct roles for medial temporal lobe structures in memory for objects and their locations. Learning & Memory. 2006;13(5):638–643. doi: 10.1101/lm.251906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. The cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. Journal of Comparative Neurology. 1998;398(2):179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology. 2006;16(6):693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Deacon TW, Eichenbaum H, Rosenberg P, Eckmann KW. Afferent connections of the perirhinal cortex in the rat. Journal of Comparative Neurology. 1983;220(2):168–190. doi: 10.1002/cne.902200205. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends in Cognitive Sciences. 2007;11(9):379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. High-resolution multi-voxel pattern analysis of category selectivity in the medial temporal lobes. Hippocampus. 2008;18(6):536–541. doi: 10.1002/hipo.20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Medial temporal lobe activity during source retrieval reflects information type, not memory strength. Journal of Cognitive Neuroscience. 2010;22(8):1808–1818. doi: 10.1162/jocn.2009.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Adaptation to cognitive context and item information in the medial temporal lobes. Neuropsychologia. 2012;50(13):3062–3069. doi: 10.1016/j.neuropsychologia.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM, Bourgouin P. The human hippocampus: functional anatomy, vascularization and serial sections with MRI. 2. New York: Springer; 1998. [Google Scholar]
- Eacott MJ, Gaffan EA. The roles of perirhinal cortex, postrhinal cortex, and the fornix in memory for objects, contexts, and events in the rat. Quarterly Journal of Experimental Psychology. 2005;58B(3–4):202–217. doi: 10.1080/02724990444000203. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Sauvage M, Fortin N, Komorowski R, Lipton P. Towards a functional organization of episodic memory in the medial temporal lobe. Neuroscience & Biobehavioral Reviews. 2012;36(7):1597–1608. doi: 10.1016/j.neubiorev.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhs MC, Touretzky DS. Context learning in the rodent hippocampus. Neural Computation. 2007;19(12):3173–3215. doi: 10.1162/neco.2007.19.12.3173. [DOI] [PubMed] [Google Scholar]
- Graham KS, Barense MD, Lee AC. Going beyond LTM in the MTL: a synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia. 2010;48(4):831–853. doi: 10.1016/j.neuropsychologia.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Furtak SC, Wei SM, Agster KL, Burwell RD. Functional neuroanatomy of the parahippocampal region in the rat: the perirhinal and postrhinal cortices. Hippocampus. 2007;17(9):709–722. doi: 10.1002/hipo.20314. [DOI] [PubMed] [Google Scholar]
- Gaffan EA, Healey AN, Eacott MJ. Objects and positions in visual scenes: effects of perirhinal and postrhinal cortex lesions in the rat. Behavioral Neuroscience. 2004;118(5):992–1010. doi: 10.1037/0735-7044.118.5.992. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Libby LA, Yonelinas AP, Ranganath C. Medial temporal lobe contributions to cued recall of items and context. Neuropsychologia. 2013 doi: 10.1016/j.neuropsychologia.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkänen A. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. American Journal of Neuroradiology. 1998;19(4):659–71. [PMC free article] [PubMed] [Google Scholar]
- Jenkins LJ, Ranganath C. Prefrontal and medial temporal lobe activity at encoding predicts temporal context memory. Journal of Neuroscience. 2010;30(46):15558–15565. doi: 10.1523/JNEUROSCI.1337-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkle T, Oliva A. A Real-World Size Organization of Object Responses in Occipitotemporal Cortex. Neuron. 2012;74(6):1114–1124. doi: 10.1016/j.neuron.2012.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AC, Scahill VL, Graham KS. Activating the medial temporal lobe during oddity judgment for faces and scenes. Cerebral Cortex. 2008;18(3):683–696. doi: 10.1093/cercor/bhm104. [DOI] [PubMed] [Google Scholar]
- Levy I, Hasson U, Avidan G, Hendler T, Malach R. Center-periphery organization of human object areas. Nature Neuroscience. 2001;4(5):533–539. doi: 10.1038/87490. [DOI] [PubMed] [Google Scholar]
- Liang JC, Wagner AD, Preston AR. Content representation in the human medial temporal lobe. Cerebral Cortex. 2013;23(1):80–96. doi: 10.1093/cercor/bhr379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby LA, Ekstrom AD, Ragland JD, Ranganath C. Differential connectivity of perirhinal and parahippocampal cortices within human hippocampal subregions revealed by high-resolution functional imaging. Journal of Neuroscience. 2012;32(19):6550–6560. doi: 10.1523/JNEUROSCI.3711-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman L, Awipi T, Davachi L. Category-specificity in the human medial temporal lobe cortex. Hippocampus. 2009;19(3):308–319. doi: 10.1002/hipo.20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malach R, Levy I, Hasson U. The topography of high-order human object areas. Trends in Cognitive Sciences. 2002;6(4):176–184. doi: 10.1016/s1364-6613(02)01870-3. [DOI] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. Evolution of declarative memory. Hippocampus. 2006;16(9):795–808. doi: 10.1002/hipo.20205. [DOI] [PubMed] [Google Scholar]
- Mandler G. Recognizing: The judgment of previous occurrence. Psychological Review. 1980;87(3):252–271. [Google Scholar]
- Montaldi D, Mayes AR. The role of recollection and familiarity in the functional differentiation of the medial temporal lobes. Hippocampus. 2010;20(11):1291–1314. doi: 10.1002/hipo.20853. [DOI] [PubMed] [Google Scholar]
- Nasr S, Tootell RBH. A cardinal orientation bias in scene-selective visual cortex. Journal of Neuroscience. 2012;32(43):14921–14926. doi: 10.1523/JNEUROSCI.2036-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman G, Eacott MJ. Dissociable effects of lesions to the perirhinal cortex and the postrhinal cortex on memory for context and objects in rats. Behavioral Neuroscience. 2005;119(2):557–566. doi: 10.1037/0735-7044.119.2.557. [DOI] [PubMed] [Google Scholar]
- Oliva A, Torralba A. The role of context in object recognition. Trends in Cognitive Sciences. 2007;11(12):520–527. doi: 10.1016/j.tics.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Pihlajamaki M, Tanila H, Kononen M, Hanninen T, Hamalainen A, Soininen H, Aronen HJ. Visual presentation of novel objects and new spatial arrangements of objects differentially activates the medial temporal lobe subareas in humans. European Journal of Neuroscience. 2004;19(7):1939–1949. doi: 10.1111/j.1460-9568.2004.03282.x. [DOI] [PubMed] [Google Scholar]
- Preston AR, Bornstein AM, Hutchinson JB, Gaare ME, Glover GH, Wagner AD. High-resolution fMRI of content-sensitive subsequent memory responses in human medial temporal lobe. Journal of Cognitive Neuroscience. 2010;22(1):156–173. doi: 10.1162/jocn.2009.21195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nature Reviews Neuroscience. 2012;13(10):713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- Ranganath C. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus. 2010;20(11):1263–1290. doi: 10.1002/hipo.20852. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Differential encoding mechanisms for subsequent associative recognition and free recall. Journal of Neuroscience. 2006;26(36):9162–9172. doi: 10.1523/JNEUROSCI.2877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Selective and shared contributions of the hippocampus and perirhinal cortex to episodic item and associative encoding. Journal of Cognitive Neuroscience. 2008;20(8):1–12. doi: 10.1162/jocn.2008.20104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Object unitization and associative memory formation are supported by distinct brain regions. Journal of Neuroscience. 2010;30(29):9890–9897. doi: 10.1523/JNEUROSCI.0826-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Duncan KD, Davachi L. Perirhinal and parahippocampal cortices differentially contribute to later recollection of object-and scene-related event details. Journal of Neuroscience. 2011;32(24):8739–8747. doi: 10.1523/JNEUROSCI.4978-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Henson RN, Kriegeskorte N, Alink A. Episodic reinstatement in the medial temporal lobe. The Journal of Neuroscience. 2012;32(50):18150–18156. doi: 10.1523/JNEUROSCI.4156-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. The perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. Journal of Comparative Neurology. 1994;350(4):497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Tubridy S, Davachi L. Medial temporal lobe contributions to episodic sequence encoding. Cerebral Cortex. 2011;21(2):272–280. doi: 10.1093/cercor/bhq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Dissociation of the neural correlates of recognition memory according to familiarity, recollection, and amount of recollected information. Neuropsychologia. 2007;45(10):2216–2225. doi: 10.1016/j.neuropsychologia.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Left parietal cortex is modulated by amount of recollected verbal information. Neuroreport. 2009;20(14):1295–1299. doi: 10.1097/WNR.0b013e3283306798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh W, Barsalou LW. The situated nature of concepts. American Journal of Psychology. 2006;119(3):349–384. [PubMed] [Google Scholar]
- Yu SS, Johnson JD, Rugg MD. Hippocampal activity during recognition memory co-varies with the accuracy and confidence of source memory judgments. Hippocampus. 2012;22(6):1429–1437. doi: 10.1002/hipo.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Jacoby LJ. Noncriterial recollection: Familiarity as automatic irrelevant recollection. Consciousness and Cognition. 1996;5(1–2):131–141. doi: 10.1006/ccog.1996.0008. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Unfolding the human hippocampus with high resolution structural and functional MRI. The Anatomical Record. 2001;265(2):111–120. doi: 10.1002/ar.1061. [DOI] [PubMed] [Google Scholar]


